Abstract
We made simultaneous bilateral recordings of unit activity in the nucleus ventroposterior lateralis (VPL) in intact rats and after acute and chronic left thoracic hemisection. We observed an immediate bilateral decline in multireceptive units, reflecting a loss of nociceptive input on the lesion side and a loss of low-threshold inputs contralaterally. Unit properties were restored to normal by 6 wk. Mean spontaneous discharge frequency remained unchanged in left VPL at all intervals. Right VPL displayed a substantial increase in spontaneous discharge frequency at 2 and 4 wk, returning to normal by 6 wk. Activity in left VPL driven by Pinch or Brush of the right limb was unchanged except for an immediate decrease in the response to Pinch, which was reversed by 2 wk despite persistent left hemisection. In right VPL, the response to Pinch or Brush of the left hindlimb was enhanced at 2 and 4 wk but returned to normal by 6 wk. Behaviorally, the same rats displayed increased sensitivity to mechanical stimulation of the left hindlimb, but, unlike VPL activity, there was no significant behavioral recovery. Bursting cells were also observed bilaterally in VPL, but this did not match the restriction of scratches to the hindlimb contralateral to the hemisection considered to be evidence for neuropathic pain. The novel findings include recovery of responsiveness to Pinch on the side ipsilateral to the hemisection despite the lack of spinothalamic input as well as failure for the thalamus contralateral to hemisection to maintain its elevated responsiveness.
Keywords: somatosensory, pain, spinothalamic tract, dorsal columns, spinal cord injury, thalamus
a major problem faced by spinal-injured patients is neuropathic pain. The pain is classified as being at level, if it involves dermatomes supplied by the injured segments, below level, or above level (Siddall et al. 1997). It has been very difficult to identify a specific structure for which the activity triggers the neuropathic pain. The highly connected spinal and supraspinal centers involving both ascending and descending connections that modify projections from nociceptors (reviewed in Mendell 2011) make it very difficult to factor out the multiple contributions to the pain experience.
Here, we have recorded from the somatosensory thalamus in an effort to determine changes that take place after spinal cord injury. There is evidence in rats (Zhang et al. 2006) and humans (Lenz et al. 1998) that activity in this region is related to pain processing, although the mechanisms are not known precisely (see discussion). To improve the power of our determination, we have studied rats at different intervals after the hemisection to determine whether changes in VPL activity, known to occur after spinal injury (see discussion), are correlated over time after hemisection with behavioral changes in the same animals.
We used spinal hemisection as a model of spinal injury. Although this does not reproduce the injury (contusion) that is most often responsible for spinal injury in humans, it has the advantage of causing damage in specified pathways. In particular, the two major ascending spinal pathways projecting input from the peripheral structures on each side to the contralateral nucleus ventroposterior lateralis (VPL) are the dorsal column-medial lemniscal (DCML) system and the spinothalamic tract. The DCML system is uncrossed in the spinal cord and crosses in the medulla to reach the contralateral VPL. The spinothalamic tract is crossed in the spinal cord and projects to VPL ipsilateral to the tract but contralateral to the limb that activates neurons of that tract. Therefore, when the spinal cord is laterally hemisected, it will interrupt input projecting to both sides of the thalamus, the dorsal columns to thalamus contralateral to the hemisection and the spinothalamic system to thalamus ipsilateral to the hemisection. In the rat, the projections from each hindlimb converge on individual cells in the contralateral thalamus via these two pathways (Ma et al. 1987). There is, however, also a small ipsilateral projection of the spinothalamic system (Jones 1985; Willis and Coggeshall 2003; see discussion).
Because of this asymmetry in the spinal portion of the ascending somatosensory pathway resulting from spinal injury, we considered it important to obtain the responses from both sides of the thalamus. To do this in the most unbiased fashion, we recorded simultaneously from VPL on both sides. This enabled us to measure differences in intrinsic excitability on both sides by evaluating spontaneous activity bilaterally over the same time interval.
Animals were studied after acute hemisection (AC) and (in different animals) 2, 4, 6, and 8 wk after hemisection. We classified units as low-threshold (LT), nociceptive-specific (NS), or multireceptive (MR). We found immediate changes in the distribution of unit types that differed on the two sides according to the inputs that were lost due to the hemisection. These changes were not permanent, and by 6 wk the unit types were back to normal despite the persistence of the hemisection. The two sides of the thalamus exhibited very different levels of spontaneous activity after the hemisection. A robust finding was an increase in sensory-evoked activity in the VPL on the uninjured side at 2 and 4 wk after hemisection that did not persist, declining to close to normal values by 6 wk after hemisection. Behavioral changes in response to mechanical and thermal stimuli were only partly correlated with the changes in VPL activity elicited by similar stimuli.
Some preliminary findings have been published in abstract form (Liang et al. 2009).
MATERIALS AND METHODS
At the outset, a total of 53 male Sprague-Dawley rats (375–425 g) were divided randomly into 5 equal groups: Intact rats that were also studied after AC (9 rats), AC (11 rats), and rats that had been hemisected for 2 wk (13 rats), 4 wk (13 rats), 6 wk (8 rats), or 8 wk (8 rats). The number used in this report was determined after assessing the hemisection accuracy (see results). The standard housing condition consisted of 1 rat per cage. All protocols and recording procedures conformed to the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at Stony Brook University.
Spinal Cord Hemisection Injury
The hemisection procedure was carried out with a protocol similar to that used previously in this laboratory (Arvanian et al. 2009). Briefly, rats were deeply anesthetized with isoflurane (Forane; Ohio Medical) at 5% for induction and 3.5% for maintenance with supplemental oxygen (95%). The surgical field was shaved and disinfected with Betadine soap, distilled water, and 75% alcohol. A 1.5- to 2.5-cm longitudinal incision was made exposing several vertebrae centered at T10. After laminectomy, the dura on the left side was cut laterally, and the left side of the spinal cord at T10 was laterally hemisected using a microknife (3-70, Ohyodo-Minami 3-Chome; Feather Safety Razor, Kita-ku, Osaka, Japan), which was guided by a 30-gauge needle inserted vertically through the midline avoiding the posterior spinal vessel or its branches and cord overlesion. A piece of Parafilm (1 × 2 mm) was placed onto the dura incision to minimize cerebrospinal fluid leakage. For chronic lesion groups (i.e., animals to be studied electrophysiologically 2, 4, 6, or 8 wk later), muscle, fascia, and skin were sutured in layers with 4-0 silk thread. Animals were allowed to recover on a 36.5°C heating pad. Postoperative treatments included administration of saline (5.0 ml subcutaneously once daily for ≥3 days) for rehydration, 0.03 mg/kg Buprenex (Reckitt Benckiser Pharmaceuticals, Richmond, VA) as an analgesic agent for the 1st 3 postoperative days, and Baytril (Bayer HealthCare, Animal Health Division, Shawnee Mission, KS) as a prophylactic antibiotic once daily for 7 days at a dose of 0.35 ml/kg im. Following surgery, general health of the animals was carefully monitored. Rats were allowed to eat and drink 6 h after surgery. Weight loss occurred acutely over the 1st 2 postoperative days and was no greater than 5% of preoperative body weight.
Behavioral Test of Mechanical Sensitivity
Rats were placed individually on an elevated clear plastic cage (21 × 27 × 15 cm) with plastic mesh bottom floor (grid 1.27 × 1.27 cm2) to provide access to the plantar surface of the hindpaws and allowed to acclimate for ≥45 min. Mechanical sensitivity was determined with a graded series of 13 von Frey filaments (Touch Test, Semmes-Weinstein monofilaments; Stoelting, Wood Dale, IL; Brennan et al. 1996). The filaments produced a bending force of 0.07, 0.4, 1.0, 2.0, 4.0, 6.0, 8.0, 10, 15, 26, 60, 100, and 180 g. The 180-g filament was chosen as the cutoff. Stimuli were delivered sequentially to both hindpaws, near the center of the foot pad. In all cases, the stimulation was applied first on the side contralateral to the hemisection (right hindpaw) using the filament producing the lowest force. Each filament was applied three times (3–5 min apart). In each session, the complete series of von Frey filaments was applied in increasing order of force until a well-defined behavioral response such as sharp withdrawal or flinching behavior was triggered. The minimal force applied through von Frey filaments to trigger at least two such responses in three tests was considered as the mechanical response threshold. Tests for mechanical threshold were carried out 1 day before hemisection surgery and at 2-wk intervals until the day before the final terminal electrophysiological procedure.
The method of Hargreaves et al. (1988) was employed to assess paw withdrawal latency to a thermal nociceptive stimulus using a commercial apparatus (Ugo Basile, Comerio, Italy). Rats were unrestrained in individual clear Plexiglas compartments (11 × 17 × 14 cm) on a clear glass plate maintained at room temperature. All rats were allowed to acclimate ≥45 min. A calibrated radiant heat source (i.e., high-intensity projector lamp) at radiant intensity of 68 (arbitrary units) was applied to the middle of the plantar surface of the hindpaw. A photocell automatically stopped the heat source and timer when the rat lifted its paw. Each rat was tested for 10 trials on each hindpaw, with ≥3 min between trials. A 33-s maximum cutoff was established to prevent tissue damage. Latency (in seconds) to withdrawal from the heat source was recorded together with any other behavior indicating attention to the stimulus, including sniffing, licking, or looking at the affected paw or attacking the stimulus. The latency to withdrawal of the paw from the radiant heat source was determined 1 day before and at 2, 4, 6, and 8 wk after hemisection surgery.
Extracellular Recording
Animal preparation.
Electrophysiological recordings of VPL neurons were obtained simultaneously on both sides in both intact and spinally injured rats (see below). After recording VPL activity in intact rats, they were hemisected on the left side at T10, and additional recordings were carried out (AC). Separate groups of rats were recorded at 2, 4, 6, and 8 wk after left spinal cord hemisection at T10. In this terminal procedure, anesthesia was induced with 5% isoflurane (with supplemental 95% O2) and maintained on 3.5% concentration until the trachea was cannulated to allow for artificial ventilation. Then, 25% urethane (1.5 g/kg ip) was administered to maintain anesthesia for the remainder of the experiment. Supplementary doses (¼ of initial dose of 25% urethane) were given as needed to maintain adequate depth of anesthesia as determined by absence of paw withdrawal reflex tested every 15 min (Field et al. 1993). Once cannulated, animals were mounted in a stereotaxic frame and artificially ventilated with 100% O2. Typically, a stroke volume of 2–3 ml and a ventilation rate of 70–90/min were used, which maintained blood oxygen saturation at >95% and end-tidal CO2 concentration at approximately 3.5–4% (SurgiVet V9400 CapnoGraph monitor; Smiths Medical PM, Waukesha, WI). Body temperature was monitored by a rectal thermometer and kept at 37.5°C by a heating pad. The head was fixed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA), and a midline incision was made over the scalp. A fine dental drill was used to remove a 6- × 6-mm area of bone from the center of the rostral 1/3 part of the parietal bone on both sides to allow multiple electrode penetrations. A dam around this area was created with ointment, and the exposed tissues were covered with 0.9% saline.
Simultaneous bilateral recording from VPL neurons.
Extracellular single-unit recordings were made with two low-impedance (∼0.5 MΩ) insulated tungsten microelectrodes (FHC, Bowdoin, ME), which were mounted on separate micropositioners (Model 640-hydraulic and Model 2660-electrical; Kopf Instruments). They were inserted perpendicularly into the brain to target the left and right VPL, respectively, using stereotaxic coordinates [in millimeters (approximately): bregma (−1.8 to −2.4); lateral (2.8–3.5); vertical (4.5–6.5); Fig. 1D; Francis et al. 2008]. Electrical signals were amplified, band-pass filtered (100 Hz to 3 kHz; Model P511; Grass Instruments, Quincy, MA), monitored via a digital oscilloscope workstation (Sigma 60; Nicolet Biomedical, Madison, WI) and an audio amplifier (Grass AM8; Grass Instruments), digitized (20-kHz sampling rate), and processed by a data collection system (CED 1401 interface; Cambridge Electronic Design, Cambridge, United Kingdom) coupled to and stored on a computer with Spike2 software (v6.02; Cambridge Electronic Design). Additional separate channels were used to mark stimulus timing. All event marks were aligned with neuronal activity by time stamps. Spike sorting was performed offline. At the conclusion of recording, direct current (30 μA for 10 s) was passed through the recording electrode to locate the site of the last recorded unit(s).
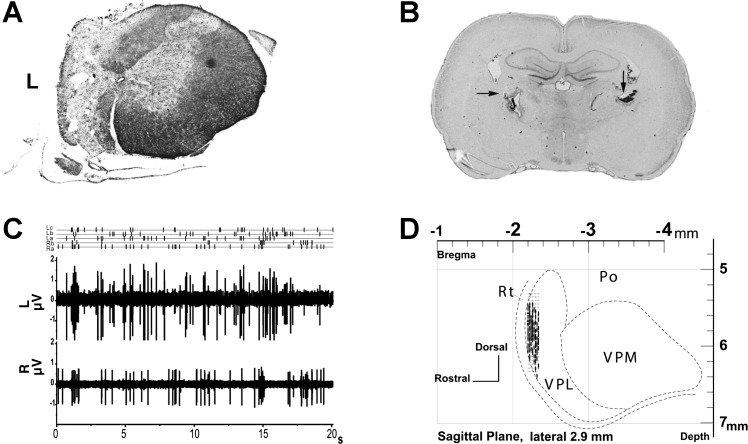
Fig. 1.
A: example of thoracic spinal cord hemisection (4 wk) at T10 (Nissl and myelin stain). B: bilateral electrolytic lesions in nucleus ventroposterior lateralis (VPL) recording sites (arrows). Intact preparation coronal section is shown. C: raw data in dual-channel recording (intact preparation). Three spikes sorted in multiunit recording from left VPL (La, Lb, and Lc) and 2 units sorted in recording from right VPL (Ra and Rb). D: VPL recording sites in sagittal plane for intact preparations. L, left; R, right; VPM, ventroposterior medial nucleus; Po, posterior nucleus; Rt, reticular nucleus.
Initially, cells in the hindlimb area of the left VPL were identified by their response to gentle mechanical stimulation on the right hindlimb. Once a stable recording was obtained from left VPL, the coordinates and depth of that electrode were used to determine the initial right VPL electrode position. The position of the electrode in the right VPL was adjusted as necessary to find a position with well-isolated spikes from neurons with receptive fields (RFs) on the left hindlimb. In the postlesion groups where the left side of the cord was hemisected at T10, neurons in left VPL were mapped by brushing the skin of the right hindlimb, whereas hindlimb neurons in the right VPL were identified primarily by their response to intense mechanical skin stimulation as well as the three coordinates of the left VPL electrode.
Once both VPL electrodes were recording well-isolated spike activity, their RFs were mapped. The RF of the individual neurons was then activated by brushing (0.1 × 10−5 N; Montagne-Clavel and Oliveras 1994), tapping (round-probe, hand-held, strain-gauge-like device, 6.5 × 10−5 N force; Montagne-Clavel and Oliveras 1994), and pinching with a fine forceps producing 35- to 55-N force, which elicited moderate to strong pain on human skin (Kim et al. 2007). These stimuli were delivered to the center of the RF of each cell at ∼1 Hz for brushing and tapping and ∼0.25 Hz for pinching. The stimuli were applied to both hindlimbs individually with the following sequence: Brush (1 min) and Tap (1 min) to the RF of the left hindlimb with a 5- to 10-min interval between brushing and tapping; this was repeated on the right hindlimb. Following this, the RF on the left hindlimb was then subjected to noxious stimulation (pinching) for ∼1 min with 20- to 30-min interval before switching to the right hindlimb to minimize the effect of noxious sensitization (Herrero and Headley 1995). The uniformity of Pinch stimulation with these hand-held stimuli was assessed by examining the mean number of spikes in the response to successive subsets of stimuli recorded from single units in intact preparations and finding no significant difference (ANOVA). Although we cannot rule out some difference in the forces used to evaluate the responses in different units, Brush and Tap were uniformly nonnociceptive on human skin and elicited much smaller thalamic discharges than Pinch, which was always painful when tested on human skin. A sample of dual-channel recording of raw data with spike sorting is shown in Fig. 1C.
Data Analysis and Statistical Analysis
A minimum of 3 min for the spontaneous response and 1 min for each driven response was analyzed on each side in each trial. Data from two or occasionally three neurons were obtained after spike sorting using Spike2 template-matching routines and principal component analysis. Appropriate segments corresponding to stimulation marks were compiled for further analysis with NeuroExplorer 4 software (Nex Technologies, Littleton, MA). Cell discharge statistics were calculated in NeuroExplorer using spike histograms with 1-s bins. For each cell, we calculated the mean frequency for spontaneous activity and the average number of spikes in response to multiple presentations of Brush, Tap, and Pinch stimuli separately. We then averaged these measures over all cells in each state (intact, AC, and chronic hemisection of 2, 4, 6, and 8 wk). Since Tap resulted in data similar to those elicited from Brush, it is not illustrated to simplify the presentation.
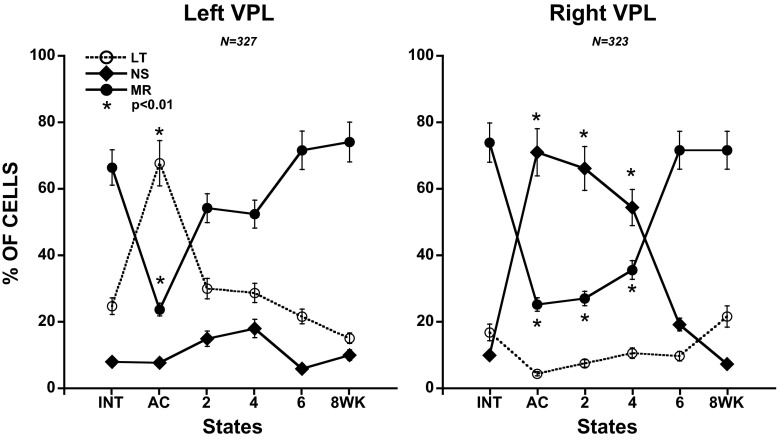
In addition, cells were classified as LT, NS, or MR based on an increase of >10% in discharge frequency from the less intense stimulus (Fig. 2). A LT response was ≥10% higher in mean frequency with Brush stimulation than during spontaneous activity. Similarly, a nociceptive response was ≥10% higher in mean frequency with Pinch than with Brush, and a MR unit (the majority) exhibited increases in mean firing rate to Brush and a further increase to Pinch. These evaluations were done offline using the recorded spike activity and were made separately for cells at each time interval after hemisection (Fig. 3).
Fig. 2.
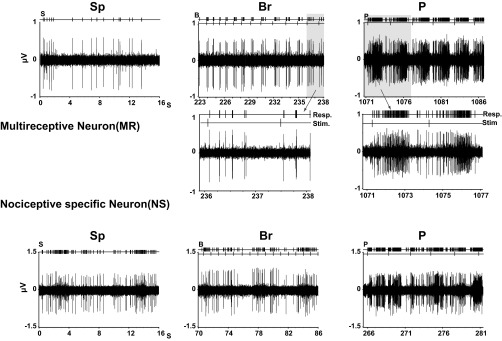
Responses (Resp.) from 2 classes of neuron in VPL. Intact rat (INT): multireceptive neuron (MR; top traces) exhibiting a response to graded mechanical stimulation (Stim.; bottom trace). Insets show higher speed records of the response to individual stimuli. Nociceptive-specific neurons (NS; bottom traces) are unresponsive to gentle cutaneous stimulation but increase their firing to Pinch in the receptive field. Sp, spontaneous; Br, Brush; P, Pinch. Top traces above raw records indicate output of spike discriminator (above) and time of stimulus onset (below).
Fig. 3.
Distribution of low-threshold (LT), NS, and MR neurons before (INT), immediately after [acute hemisection (AC)], and several weeks after left thoracic spinal cord hemisection at T10. In VPL on both sides, there is an immediate loss of multireceptive neurons (AC). In Left VPL, this results from to a loss of nociceptive input such that they become identified as LT. In Right VPL, this results from a loss of LT input such that the cells become classified as NS. The changes are not permanent, with recovery in Left VPL largely complete by 2 wk, whereas in Right VPL, the changes require 6 wk to be evident. In all cases, 1-way ANOVA followed by pairwise analysis and multiple comparisons (Bonferroni) was used to determine the significance of differences in mean number of impulses in the different hemisected groups from intact preparations.
Previous clinical studies show a link between VPL neuron bursting and central neuropathic pain (Lenz et al. 1989). This type of bursting tends to reflect afferent-driven activity as well as the intrinsic properties of the cells (Boraud et al. 2002). To investigate the occurrence of bursts after spinal hemisection, we used the Poisson surprise method (Legendy and Salcman 1985) as implemented in NeuroExplorer 4. The surprise value is defined as the negative natural logarithm of the probability that the intervals of successive spikes in a given time interval are significantly different from what would be expected from a Poisson distribution with the same mean firing rate; this measure is independent of changes in average firing rate. A minimum surprise value of 3 (probability of 1 in 1,000) was required for acceptance. Comparisons of burst parameters between states were made using one-way ANOVA with the Bonferroni post hoc test.
Averaged spontaneous discharge frequency and the number of spikes in each evoked response in each state (intact, AC, and 2, 4, 6, and 8 wk posthemisection) were compared across the different states using one-way ANOVA. Whenever a significant effect was observed (P < 0.05), further pairwise analysis between two states was carried out using the Bonferroni post hoc test. Tests of factors including pairwise comparisons were performed with either the paired Student's t-test for before-after comparisons or the two-sample Student's t-test to compare two states. Proportions were evaluated using a χ2-test. Data management and statistical analyses were performed using SPSS (v16) and graphed using SigmaPlot (v10.0) and KaleidaGraph (v4.0). Data are presented as means ± SE unless noted.
Histology
Histology was performed to determine the recording sites in VPL as well as the extent of spinal cord hemisection. At the time of death, all surviving animals were anesthetized with overdose 50% urethane (3.0 g/kg ip injection) and perfused intracardially with 0.1 M PBS followed by 4% paraformaldehyde in 0.1 M phosphate buffer. A 1-cm segment of cord centered at the lesion zone and 0.5-cm thickness coronal brain slice centered at recording coordinates were blocked and cryoprotected in 30% sucrose for ≥24 h before cutting. Brain and spinal cord were cut on a cryostat at 40 and 20 μm, respectively. Sections were treated with a Nissl (brain, spinal cord) and Eriochrome Cyanine myelin (spinal cord) stain (Augulis and Sepinwall 1971). All sections were viewed using a Zeiss Axioskop upright microscope. Images were captured using a SPOT RT camera and ImagePro Plus software (Media Cybernetics) as previously described (Arvanian et al. 2006).
RESULTS
Of the total of 53 hemisected rats, 44 survived until the end of the terminal electrophysiological experiment. Of these 44 cords, 17 were considered after gross histological examination to be acceptable as a complete hemisection (39% of completed experiments); data from these animals and 9 intact rats also studied after AC are presented in this report. The criteria were that the entire left side of the cord was hemisected and the right side was largely unaffected, particularly the lateral and ventral white matter. In these selected cases, there was little damage to the contralateral dorsal columns, although under the light microscope it is impossible to rule out at least minimal damage. We also confirmed by reconstruction that both recording electrodes were in the VPL. A sample hemisected cord and the VPL recording sites are shown in Fig. 1, A and B.
During the terminal experiment, we recorded the multiunit extracellular activity of VPL neurons on both sides from rats in multiple electrode penetrations: 3–6 penetrations per side (6–12 per rat) with 4–6 single units per penetration. A total of 650 units collected from intact and hemisected rats were examined in the present study. The control group (from intact animals) consisted of 247 units, whereas 5 spinal hemisected groups (AC and 2, 4, 6, and 8 wk) yielded 49, 107, 141, 53, and 53 units, respectively. An average of 6.5 ± 3.3 units per animal was recorded in left VPL and 6.6 ± 2.2 in right VPL (P > 0.05). All of the cells recorded in the present study had RFs limited to below-level regions, on the hindlimbs or hindpaws and a few on the flank. Here, we report the analysis of response types and firing rates of neurons in both left and right VPL in response to stimulation of its contralateral hindlimb in intact rats and at different times after hemisection.
Electrophysiological Studies
Cell classification.
Recordings were made simultaneously from VPL on both sides of the thalamus. A total of 327 and 323 single units were recorded in 64 and 65 microelectrode penetrations within the left and right VPL, respectively. These neurons were classified as LT (Brush), MR (Brush and Pinch), or NS (Pinch; Wall 1960) based on their response to mechanical stimulation (Fig. 2). In intact animals, we observed that most units (∼70%) could be classified as MR, i.e., they responded to Brush (>10% increase in mean frequency over spontaneous) and increased their discharge frequency by at least a further 10% in response to Pinch stimulation of the RF on the contralateral side. Only ∼20% of the units recorded from intact animals could be activated exclusively by gentle stimulation (Brush), and a further 10% were activated exclusively by nociceptive stimuli with no intermediate response to Brush (Fig. 3).
After left thoracic hemisection, we noted an immediate change in the RF properties of cells in the contralateral right VPL in response to left hindlimb stimulation. The proportion of MR cells fell abruptly after AC (Fig. 3). This was accompanied by an increase in the proportion of NS units. By 2 wk after hemisection, a gradual decline in proportion of NS units had begun. This decline in the proportion of NS units was matched by an increase in the proportion of MR units, both of which reached values characteristic of intact preparations by 6 wk after hemisection. Because changes in the proportion of MR cells mirrored the changes in proportion of NS neurons, it is suggested that the acute increase in percentage of NS neurons was due to MR neurons losing their nonnociceptive (Brush) input and being classified as NS. This conclusion is supported by the acute decline in the percentage of units classified as nonnociceptive (LT).
In the left VPL, we also observed an immediate decline in the proportion of MR neurons, which recovered to values characteristic of intact preparations by 6 wk after hemisection (Fig. 3). In contrast to the findings in right VPL, these changes in the proportion of MR neurons were mirrored by changes in the population of NS neurons responding to Pinch, not to Brush. Thus the initial decline in the proportion of MR cells probably reflects their loss of nociceptive input, causing them to be classified as LT units. The proportion of NS neurons exhibited a small increase at 2 and 4 wk after hemisection but returned to values characteristic of intact preparations by 6 wk.
In summary, VPL on both sides exhibited an immediate loss of cells classified as MR that had reversed by 6 wk after hemisection. On the side opposite the hemisection (right), this was due to a loss of LT input; on the side of the hemisection (left), it was associated with a loss of NS input. This is taken up again in discussion.
Spontaneous activity.
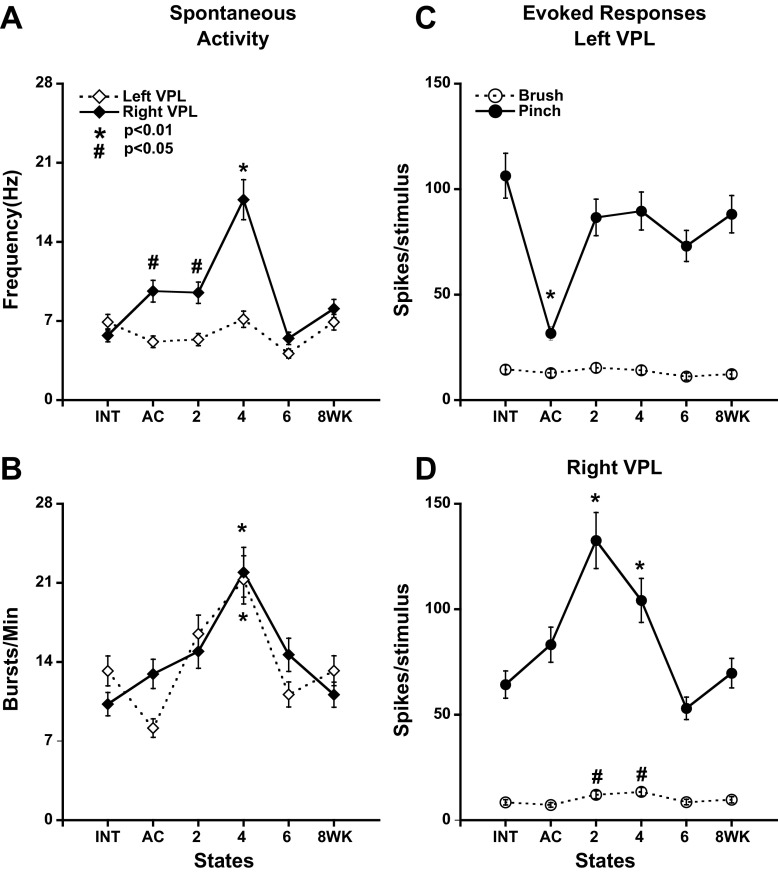
In intact animals, 247 neurons were active spontaneously in the range 0.03–29.8 Hz with mean spontaneous activity of 6.6 ± 8.0 Hz. Spontaneous activity in right and left VPL changed with a different time course after left hemisection (Fig. 4A). It began to increase immediately in right VPL, reaching a peak at 4 wk and then falling to values observed in intact preparations (Fig. 4A). Spontaneous activity in left VPL was largely unchanged (P > 0.05) after left hemisection, recovering close to the level in the intact state (Fig. 4A). A direct comparison of mean spontaneous activity between left and right VPL revealed a significantly higher rate in right VPL immediately after hemisection that lasted until 4 wk postsurgery (Mann-Whitney test, P < 0.01 for AC, 4 wk and P < 0.05 for AC, 2 wk).
Fig. 4.
Activity of bilateral VPL neurons before and after left cord hemisection at T10. Left VPL, dashed; Right VPL, solid. A: spontaneous activity in Right VPL increased significantly between 2 and 4 wk after hemisection. In Left VPL, there was no significant difference in activity over the period examined. B: spontaneous burst rate increased, bilaterally, displaying significant increase only at 4 wk. C and D: average number of spikes in the response per stimulus measured over all Brush or Pinch stimuli delivered to the receptive field on the contralateral limb. A significant decline in the response to Pinch was observed in Left VPL in the acutely hemisected preparation (C). Increased response to Pinch was observed only in Right VPL 2–4 wk after hemisection (D). In all cases, 1-way ANOVA followed by pairwise analysis and multiple comparisons (Bonferroni) was used to determine the significance of differences in mean number of impulses in the different hemisected groups from Intact preparations.
Responses to graded mechanical stimulation.
The results in the posthemisection groups were compared with those in intact animals. In the left VPL (ipsilesional), the mean number of spikes per stimulus in response to nonnoxious stimulation (Brush) of the contralateral RFs (right hindlimb) changed very little in all of the postsurgery groups measured as the mean number of spikes evoked per stimulus (Fig. 4C). The response of these left VPL neurons to noxious stimulation (Pinch) applied to the right hindlimb RF also changed relatively little from values in the intact state after left hemisection except for the acutely hemisected group, which showed a significant decrease in firing, which recovered by 2 wk. In the right VPL (Fig. 4D), the magnitude of responses induced by Brush was little changed except at 2 and 4 wk, when a significant transient increase was observed. The response to Pinch was most elevated at 2 wk after hemisection, but this increase was not maintained at 6 and 8 wk.
The immediate decline in response to Pinch in the left VPL after left hemisection was expected from a lesion of the left spinothalamic tract, but the recovery by 2 wk despite continued interruption of the left spinothalamic tract was not anticipated. Presumably, other pathways were responsible for this recovery (see discussion). The response of right VPL to Brush of the left hindlimb did not decline significantly as might have been expected after cutting the left dorsal columns, but this may also have been due to activity in other pathways as discussed below.
Spontaneous burst activity.
The temporal patterning of spikes following unilateral hemisection lesions was examined using an algorithm that calculates burst prevalence, and other burst-related statistics, based on the relative likelihood of groups of spikes with short interstimulus intervals (see materials and methods). Both left and right VPL exhibited a progressive increase in burst rate (Fig. 4B), reaching a peak at 4 wk. Both then declined sharply from 4 to 6 wk and remained at that level at 8 wk (Fig. 4B). The increase in burst responsiveness of left VPL was notably not parallel to the responsiveness inferred from mean frequency (Fig. 4A).
Behavioral Studies
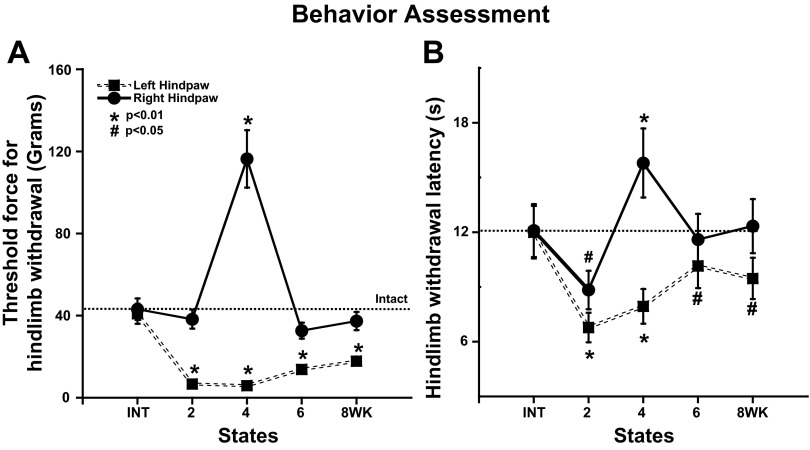
Each rat was tested using simple mechanical (graded von Frey hairs) and thermal stimuli (Hargreaves et al. 1988) at 2-wk intervals and the day before the final electrophysiological experiment (Fig. 5). The threshold from the left hindlimb was notably reduced for both stimulus modalities beginning at 2 wk after hemisection. There was no significant restoration of mechanical sensitivity at 6 and 8 wk in contrast to VPL responses (Fig. 4D). However, there was a small but significant recovery in the thermal threshold between 4 and 6 wk (1-way ANOVA, Bonferroni, P < 0.01; Fig. 5B). Surprisingly, there was little evidence for reduced responses from the right hindpaw despite the left hemisection. The only exception was at 4 wk when an elevated threshold was observed for both mechanical and thermal stimuli.
Fig. 5.
The effect of left T10 hemisection on hindpaw withdrawal force threshold (von Frey) and the thermal withdrawal latency (Hargreaves et al. 1988). A: threshold force for hindpaw withdrawal. Groups of animals were tested on both hindpaws at forces ranging from 0.07 to 180 g (see materials and methods). Right hindpaw (solid) exhibited a significantly increased threshold force only at 4 wk after left spinal cord hemisection (P < 0.01, ANOVA). Threshold for Left hindpaw withdrawal (dashed) was significantly lower than in the intact preparation from 2 to 8 wk after hemisection (P < 0.01). B: thermal hindpaw withdrawal latency test (Hargreaves et al. 1988). Left hindpaw exhibited changes in response paralleling the changes in threshold to mechanical stimuli after left cord hemisection with a significant decrease in withdrawal latency between 2 and 4 wk (P < 0.01). Right hindpaw also exhibited changes generally paralleling the mechanical responsiveness with a significant decrease in withdrawal latency at 2 wk (P < 0.05), which then reversed to a significant increase in latency at 4 wk after hemisection (P < 0.01).
When we shaved the hindlimbs to prepare them for RF determination in the terminal electrophysiological procedure, we observed signs of scratching or chewing to the hindlimb that could be interpreted as resulting from spontaneous itch or pain (Dey et al. 2005). Overall, 45% of the hemisected animals (n = 44) exhibited evidence of this damage. In 85% of these rats, this damage was restricted to the right hindlimb. In the sample of rats accepted as accurately hemisected based on the histology (n = 17), 47% showed evidence of damage, and in 88% of these it was restricted to the right hindlimb (see discussion). These rats had been in the hemisected state from 4 to 8 wk. We can conclude only that the earliest time that the scratching/chewing began was at 4 wk since it might have ended before the animals were examined at 6 or 8 wk. In one case, the terminal experiment was performed in advance of the planned date because of excessive damage to the right hindlimb.
DISCUSSION
The conventional wisdom for the sensory consequences of a lateral hemisection of the spinal cord, the classic Brown-Séquard lesion, is a loss of pain and temperature sensation contralaterally for dermatomes below the hemisected segment and for light touch and brush ipsilaterally below the lesion. The anatomic basis for this is the crossed spinothalamic pathway carrying nociceptive input and the uncrossed dorsal column system carrying input from LT mechanoreceptors (reviewed in Willis and Coggeshall 2003). More recent electrophysiological investigations raise questions concerning this absolute division since cells projecting through the spinothalamic tract have nonnociceptive as well as nociceptive input, the wide dynamic range cells (Willis 1980). Furthermore, axons receiving nociceptive input in the spinocervical system in rats ascend ipsilaterally in the spinal cord and terminate on cells of the ipsilateral lateral cervical nucleus (Baker and Giesler 1984), which project to the contralateral thalamus. These projections could reduce the sharp division between the sensory modalities carried by ipsilateral and contralateral spinal pathways. In addition, the responses studied in anesthetized preparations are undoubtedly quite different from what occurs in the behaving animal or human where the Brown-Séquard pattern was originally described.
We examined the hindlimb region of the VPL bilaterally in rats with a T10 hemisection. Thus we were examining the targets of spinal neurons located in the lumbar spinal cord caudal to the hemisection. Relay cells in VPL receive input from both the DCML system as well as the spinothalamic tract (Ma et al. 1987). Although we did not positively identify the recorded cells as relay cells by identifying their response to antidromic stimulation of the somatosensory cortex, it has been demonstrated that interneurons, specifically GABAergic interneurons in the rat VPL (Harris and Hendrickson 1987), represent a very small proportion of the cells present in this region, and so we presume that we were recording almost exclusively from relay cells. In terms of the behavioral responses, we were examining below-level responses to noxious and nonnoxious stimulation.
The response of projection neurons in the lumbar cord is enhanced after a more rostral hemisection (Hulsebosch et al. 2009). Numerous mechanisms such as sprouting onto partially denervated cells (Tripp and Wells 1987) or loss of inhibition due to downregulation of the chloride transporter KCC2 (Cramer et al. 2008; Lu et al. 2008) have been advanced as an explanation. In the thalamus, enhanced expression of Nav1.3 has been proposed to contribute to the enhanced activity (Hains et al. 2005, 2006). Others have proposed a loss of GABA-mediated afferent inhibition (Roberts et al. 1992) or inhibition of higher-order thalamic cells from the zona incerta (reviewed in Masri and Keller 2012).
Both the right and left VPL suffered an immediate decline in the proportion of MR cells after left hemisection. However, this decline occurred for different reasons, which illustrates the separation of spinal projection pathways to thalamus (see also Al-Chaer et al. 1996). In the right VPL, the loss of MR cells was due to reduced input from pathways activated by Brush whereby the MR cells became classified as NS neurons. This very likely reflects the loss of left dorsal column input. In left VPL, the immediate decline in the proportion of MR units was due to a loss of nociceptive (Pinch) input, which resulted in classifying these cells as LT units. This occurred as a consequence of cutting the left spinothalamic tract. An important implication of this separation of functional input to left and right thalamus is that the acute hemisections were accurate. It should be noted that the cell classification described here is similar to that reported previously (Guilbaud et al. 1980) where a larger proportion was found to be activated exclusively by nonnoxious stimulation and noxious stimulation with a minority of MR neurons. Apart from differences in anesthesia (2/3 N2O-1/3 O2 + 0.5% halothane vs. urethane in the present experiments), the proportions of different types of cells will depend on the criteria used to consider responses as different.
We report here that cells in VPL contralateral to a thoracic hemisection exhibit an increased responsiveness to intense mechanical stimulation (Pinch) of the contralateral hindpaw (left hemisection; stimulate left hindpaw; record right VPL; Fig. 4). This was measured as the average number of spikes elicited per stimulus. The increase is first observed 2 wk after hemisection, i.e., it is not observed acutely unlike the change in cell classification, which occurs due to the loss of nonnociceptive input (Fig. 3), and it is maintained at 4 wk. Such changes have been reported before (Gwak et al. 2010). What is novel here is the finding that the response declined back to normal levels at 6 and 8 wk, intervals not studied by Gwak et al. (2010). One complication in interpreting this finding as a change in the strength of the input to VPL is the parallel decline in spontaneous activity (Fig. 4A). However, the increase in the Pinch-evoked response in right VPL at 2 wk was much greater than might have been predicted from the minimal change in spontaneous activity at that time suggesting that spontaneous activity level changes cannot fully explain the findings after stimulation.
It is noteworthy that Weng et al. (2003), working in a primate model with spinal cord lesions, also observed that the magnitude of the changes in the thalamus was greater at short than at long intervals after the lesion. In this model, the delays were much longer than those reported here (12–14 wk short and 52–56 wk long vs. 2–4 wk short and 6–8 wk long). This may indicate a slower pace of change in the primate spinal cord after lesions.
The same Pinch stimuli delivered to RFs in the right hindpaw elicited a very different time-dependent profile of activity in left VPL. Immediately after hemisection, there was a significant drop in activity elicited in the thalamus, presumably due to the interruption of the left spinothalamic tract. Surprisingly, there was recovery of activity by 2 wk to levels seen in intact preparations despite the persistence of the hemisection, and this continued throughout the 8-wk period examined. Possibilities for this recovery of responsiveness to Pinch include the action of the ipsilaterally ascending spinocervical or postsynaptic dorsal column systems or the enhanced role of the ipsilateral spinothalamic system (Jones 1985; Willis and Coggeshall 2003) activating the contralateral thalamus via commissural fibers (Glees and Wall 1948); the strength of connections between both sides of the thalamus may be enhanced after spinal injury (Seminowicz et al. 2012). It may be significant that the recovery of normal responsiveness to Pinch in left VPL during the initial 2 wk occurred in parallel with a similar change to levels above normal in the right thalamus (Pinch to left hindlimb). However, there was no secondary decline in responsiveness of left VPL at 6 wk as was observed in right VPL. This may reflect differences between surviving LT pathways ascending via the right dorsal columns projecting to the left VPL and high-threshold pathways ascending via the right spinothalamic tract projecting to the right VPL.
The response of left VPL to nonnoxious (Brush) stimuli delivered to the right hindlimb exhibited no significant change over the 8-wk period. Notably, there was no immediate loss of responsiveness immediately after hemisection as was observed in response to Pinch of the right hindlimb. This difference is consistent with the crossed vs. uncrossed ascending pathways for Pinch and Brush whereby the activity evoked by Brush ascends in the intact right dorsal columns, whereas the activity elicited by Pinch ascends in the left spinothalamic tract, which was interrupted due to left hemisection at T10. The right VPL exhibited relatively little change in the response to Brush of the left hindlimb (above spontaneous activity) despite the interruption in the left dorsal columns. This may reflect activity in MR cells in the left spinal cord projecting via the right spinothalamic tract to the right VPL.
These experiments do not specify the mechanism for the apparent decline of responsiveness in the right VPL at 6 and 8 wk after hemisection. Several general possibilities can be advanced. One of these is the general concept of synaptic homeostasis whereby changes in relative synaptic weights on a cell are altered to achieve the original balance between the different inputs (Marder and Goaillard 2006; Wenner 2011). A second possibility is that the unlesioned white matter contralateral to a chronic hemisection is known to undergo a decline in the ability to transmit impulses beginning ∼1 wk after hemisection due to the action of proteoglycans (Hunanyan et al. 2010). Although there was an increase in VPL responsiveness at the 2- and 4-wk time points after hemisection, it is possible that this was reduced to some extent by a decline in ascending activity in the intact spinothalamic tract.
We found that mechanical and thermal nociceptive sensitivity from the left hindlimb measured behaviorally changed roughly in parallel with the changes in driven activity by Pinch, which should drive the VPL via the right spinothalamic tract. The threshold from the right hindlimb exhibited no change at most intervals as might have been expected from the lack of change in the left VPL responsiveness, although at 4 wk we observed a substantial increase in mechanical and thermal threshold, which was not observed in the discharge of the left VPL. This suggests that VPL discharge was correlated with behavior when its spinothalamic tract input is intact (right VPL) but not when it has been lesioned and VPL develops other inputs (left VPL). The dissociation between nociceptive behavior and VPL activity may also be a consequence of an inhibitory pathway from zona incerta to higher-order cells in thalamus, which sensitizes nociceptive behavior but not VPL activity after spinal damage (Masri and Keller 2012).
The increase in spontaneous activity and particularly the spontaneous burst activity observed in these preparations might underlie the spontaneous bouts of neuropathic pain known to occur after spinal injury (Lenz et al. 1989; Weng et al. 2000). Several animals exhibited skin lesions on their hindpaws often taken as evidence of neuropathic pain. However, it was impossible to link the behavior and electrophysiology because most of the lesions were on the right hindlimb, which would predict increased burst activity in left VPL. Because bursting reached its maximum 4 wk after hemisection and was equally elevated in right and in left VPL, it seems unlikely that VPL bursting activity provided the entire stimulus for the scratching.
Most previous work examining thalamic responses in spinal-injured animals have been undertaken in contused preparations. Using PET scanning, Bruehlmeier et al. (1998) detected increased regional blood flow in thalamus in response to limb stimulation, which was interpreted as increased neural activity in spinal patients. Some of the increase in activity indicated a spread of activated regions from the somatotopically appropriate regions in intact humans. Pattany et al. (2002) used magnetic resonance spectroscopy to demonstrate significant changes in metabolites in the thalamus of spinal patients. Hubscher and Johnson (2006) examined thalamic activity electrophysiologically in contused rats (T8) 30 days after injury and found elevated responsiveness in thalamus to at-level stimulation. The injuries were severe enough to largely eliminate responsiveness to below-level stimulation.
Although contusion is more realistic in terms of modeling the human spinal injury, it is more challenging to arrive at mechanisms because the lesion is more difficult to specify. In a previous study using hemisection, it was reported that the increased activity was in both left and right VPL (Gwak et al. 2010). However, these investigators recorded somewhat more caudal in VPL than we did and did not demonstrate that their hemisections were not over hemisections, which we found in our sample and eliminated from our analysis. In fact, preliminary analysis of these overhemisected preparations suggested that there was a greater tendency for both VPL nuclei to respond to contralateral hindlimb stimulation.
In summary, these studies have revealed a number of new findings related to spinal injuries responsible for the Brown-Séquard syndrome. First, the increase in responsiveness above normal levels after a hemisection occurs in VPL contralateral to the injury. However, there is plasticity in the input to VPL ipsilateral to injury, which restores the response to normal despite the persistent hemisection. Second, the increase in thalamic responsiveness is not permanent. By 6 wk, the response in contralateral VPL is back to normal, suggesting that either the plastic changes are not permanent or other changes such as axonal conduction block (James et al. 2011) or synaptic homeostasis (Cirillo et al. 2012) diminish the effects of the initial plastic changes. Finally, activity in VPL is not an accurate surrogate for the behavioral response. Many previous studies have demonstrated plasticity of descending systems with (Avila-Martin et al. 2011; Barritt et al. 2006) or without treatment (Beattie et al. 1997; Weidner and Tuszynski 2002). It will be important to determine whether the plasticity we have demonstrated here is subject to modification and whether this would provide better control of neuropathic pain in spinal-injured individuals.
GRANTS
This work was supported by the Christopher and Dana Reeve Foundation, by National Institute of Neurological Disorders and Stroke (Grant NS-5-R01-16996), and the William Heiser Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.L. and L.M.M. conception and design of research; L.L. performed experiments; L.L. and L.M.M. analyzed data; L.L. and L.M.M. interpreted results of experiments; L.L. and L.M.M. prepared figures; L.L. and L.M.M. drafted manuscript; L.L. and L.M.M. edited and revised manuscript; L.M.M. approved final version of manuscript.
REFERENCES
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol 76: 2661–2674, 1996 [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Bowers WJ, Anderson A, Horner PJ, Federoff HJ, Mendell LM. Combined delivery of neurotrophin-3 and NMDA receptors 2D subunit strengthens synaptic transmission in contused and staggered double hemisected spinal cord of neonatal rat. Exp Neurol 197: 347–352, 2006 [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Schnell L, Lou L, Golshani R, Hunanyan A, Ghosh A, Pearse DD, Robinson JK, Schwab ME, Fawcett JW, Mendell LM. Chronic spinal hemisection in rats induces a progressive decline in transmission in uninjured fibers to motoneurons. Exp Neurol 216: 471–480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augulis V, Sepinwall J. Use of gallocyanin as a myelin stain for brain and spinal cord. Stain Technol 46: 137–143, 1971 [DOI] [PubMed] [Google Scholar]
- Avila-Martin G, Galan-Arriero I, Gomez-Soriano J, Taylor J. Treatment of rat spinal cord injury with the neurotrophic factor albumin-oleic acid: translational application for paralysis, spasticity and pain. PLoS One 6: e26107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ML, Giesler GJ., Jr Anatomical studies of the spinocervical tract of the rat. Somatosens Res 2: 1–18, 1984 [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci 26: 10856–10867, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young W. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol 148: 453–463, 1997 [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross CE. From single extracellular unit recording in experimental and human Parkinsonism to the development of a functional concept of the role played by the basal ganglia in motor control. Prog Neurobiol 66: 265–283, 2002 [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 64: 493–501, 1996 [DOI] [PubMed] [Google Scholar]
- Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur J Neurosci 10: 3918–3922, 1998 [DOI] [PubMed] [Google Scholar]
- Cirillo G, Colangelo AM, Bianco MR, Cavaliere C, Zaccaro L, Sarmientos P, Alberghina L, Papa M. BB14, a nerve growth factor (NGF)-like peptide shown to be effective in reducing reactive astrogliosis and restoring synaptic homeostasis in a rat model of peripheral nerve injury. Biotechnol Adv 30: 223–232, 2012 [DOI] [PubMed] [Google Scholar]
- Cramer SW, Baggott C, Cain J, Tilghman J, Allcock B, Miranpuri G, Rajpal S, Sun D, Resnick D. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Mol Pain 4: 36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey DD, Landrum O, Oaklander AL. Central neuropathic itch from spinal-cord cavernous hemangioma: a human case, a possible animal model, and hypotheses about pathogenesis. Pain 113: 233–237, 2005 [DOI] [PubMed] [Google Scholar]
- Field KJ, White WJ, Lang CM. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim 27: 258–269, 1993 [DOI] [PubMed] [Google Scholar]
- Francis JT, Xu S, Chapin JK. Proprioceptive and cutaneous representations in the rat ventral posterolateral thalamus. J Neurophysiol 99: 2291–2304, 2008 [DOI] [PubMed] [Google Scholar]
- Glees P, Wall PD. Commissural fibers of the macaque thalamus; an experimental study. J Comp Neurol 88: 129–137, 1948 [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Peschanski M, Gautron M, Binder D. Neurones responding to noxious stimulation in VB complex and caudal adjacent regions in the thalamus of the rat. Pain 8: 303–318, 1980 [DOI] [PubMed] [Google Scholar]
- Gwak YS, Kim HK, Kim HY, Leem JW. Bilateral hyperexcitability of thalamic VPL neurons following unilateral spinal injury in rats. J Physiol Sci 60: 59–66, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Waxman SG. Alterations in burst firing of thalamic VPL neurons and reversal by Na(v)1.3 antisense after spinal cord injury. J Neurophysiol 95: 3343–3352, 2006 [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Waxman SG. Changes in electrophysiological properties and sodium channel Nav1.3 expression in thalamic neurons after spinal cord injury. Brain 128: 2359–2371, 2005 [DOI] [PubMed] [Google Scholar]
- Harris RM, Hendrickson AE. Local circuit neurons in the rat ventrobasal thalamus–a GABA immunocytochemical study. Neuroscience 21: 229–236, 1987 [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32: 77–88, 1988 [DOI] [PubMed] [Google Scholar]
- Herrero JF, Headley PM. Sensitization of spinal neurons by non-noxious stimuli in the awake but not anesthetized state. Anesthesiology 82: 267–275, 1995 [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Chronic spinal cord injury induced changes in the responses of thalamic neurons. Exp Neurol 197: 177–188, 2006 [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev 60: 202–213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunanyan AS, Garcia-Alias G, Alessi V, Levine JM, Fawcett JW, Mendell LM, Arvanian VL. Role of chondroitin sulfate proteoglycans in axonal conduction in mammalian spinal cord. J Neurosci 30: 7761–7769, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, Bartus K, Grist J, Bennett DL, McMahon SB, Bradbury EJ. Conduction failure following spinal cord injury: functional and anatomical changes from acute to chronic stages. J Neurosci 31: 18543–18555, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. New York: Plenum Press, 1985 [Google Scholar]
- Kim J, Yao A, Atherley R, Carstens E, Jinks SL, Antognini JF. Neurons in the ventral spinal cord are more depressed by isoflurane, halothane, and propofol than are neurons in the dorsal spinal cord. Anesth Analg 105: 1020–1026, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 53: 926–939, 1985 [DOI] [PubMed] [Google Scholar]
- Lenz FA, Gracely RH, Baker FH, Richardson RT, Dougherty PM. Reorganization of sensory modalities evoked by microstimulation in region of the thalamic principal sensory nucleus in patients with pain due to nervous system injury. J Comp Neurol 399: 125–138, 1998 [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res 496: 357–360, 1989 [DOI] [PubMed] [Google Scholar]
- Liang L, Davila R, Mendell LM. Activity-dependent changes in electrophysiological properties of VPL neurons after acute hemisection injury (Online). In: Neuroscience Meeting Plannered Chicago, IL: Society for Neuroscience abstract no. 857.18, 2009 [Google Scholar]
- Lu Y, Zheng J, Xiong L, Zimmermann M, Yang J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J Physiol 586: 5701–5715, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Peschanski M, Ralston HJ., 3rd The differential synaptic organization of the spinal and lemniscal projections to the ventrobasal complex of the rat thalamus. Evidence for convergence of the two systems upon single thalamic neurons. Neuroscience 22: 925–934, 1987 [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7: 563–574, 2006 [DOI] [PubMed] [Google Scholar]
- Masri R, Keller A. Chronic pain following spinal cord injury. Adv Exp Med Biol 760: 74–88, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM. Computational functions of neurons and circuits signaling injury: relationship to pain behavior. Proc Natl Acad Sci USA 108, Suppl 3: 15596–15601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne-Clavel J, Oliveras JL. Are ventromedial medulla neuronal properties modified by chronic peripheral inflammation? A single-unit study in the awake, freely moving polyarthritic rat. Brain Res 657: 92–104, 1994 [DOI] [PubMed] [Google Scholar]
- Pattany PM, Yezierski RP, Widerstrom-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, Quencer RM. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol 23: 901–905, 2002 [PMC free article] [PubMed] [Google Scholar]
- Roberts WA, Eaton SA, Salt TE. Widely distributed GABA-mediated afferent inhibition processes within the ventrobasal thalamus of rat and their possible relevance to pathological pain states and somatotopic plasticity. Exp Brain Res 89: 363–372, 1992 [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Jiang L, Ji Y, Xu S, Gullapalli RP, Masri R. Thalamocortical asynchrony in conditions of spinal cord injury pain in rats. J Neurosci 32: 15843–15848, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall PJ, Taylor DA, Cousins MJ. Classification of pain following spinal cord injury. Spinal Cord 35: 69–75, 1997 [DOI] [PubMed] [Google Scholar]
- Tripp LN, Wells J. Formation of new synaptic terminals in the somatosensory thalamus of the rat after lesions of the dorsal column nuclei. Brain Res 155: 362–367, 1987 [DOI] [PubMed] [Google Scholar]
- Wall PD. Cord cells responding to touch, damage, and temperature of skin. J Neurophysiol 23: 197–210, 1960 [DOI] [PubMed] [Google Scholar]
- Weidner N, Tuszynski MH. Spontaneous plasticity in the injured spinal cord-implications for repair strategies. Mol Psychiatry 7: 9–11, 2002 [DOI] [PubMed] [Google Scholar]
- Weng HR, Lenz FA, Vierck C, Dougherty PM. Physiological changes in primate somatosensory thalamus induced by deafferentation are dependent on the spinal funiculi that are sectioned and time following injury. Neuroscience 116: 1149–1160, 2003 [DOI] [PubMed] [Google Scholar]
- Wenner P. Mechanisms of GABAergic homeostatic plasticity. Neural Plast 2011: 489470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD. Neurophysiology of nociception and pain in the spinal cord. Res Publ Assoc Res Nerv Ment Dis 58: 77–92, 1980 [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord (3rd ed.). New York: Kluwer, 2003 [Google Scholar]
- Zhang X, Davidson S, Giesler GJ., Jr Thermally identified subgroups of marginal zone neurons project to distinct regions of the ventral posterior lateral nucleus in rats. J Neurosci 26: 5215–5223, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]