Abstract
Parkinson's disease is known to be associated with abnormal electrical spiking activities of basal ganglia neurons, including changes in firing rate, bursting activities and oscillatory firing patterns and changes in entropy. We explored the relative importance of these measures through optimal feature selection and discrimination analysis methods. We identified key characteristics of basal ganglia activity that predicted whether the neurons were recorded in the normal or parkinsonian state. Starting with 29 features extracted from the spike timing of neurons recorded in normal and parkinsonian monkeys in the internal or external segment of the globus pallidus or the subthalamic nucleus (STN), we used a method that incorporates a support vector machine algorithm to find feature combinations that optimally discriminate between the normal and parkinsonian states. Our results demonstrate that the discrimination power of combinations of specific features is higher than that of single features, or of all features combined, and that the most discriminative feature sets differ substantially between basal ganglia structures. Each nucleus or class of neurons in the basal ganglia may react differently to the parkinsonian condition, and the features used to describe this state should be adapted to the neuron type under study. The feature that was overall most predictive of the parkinsonian state in our data set was a high STN intraburst frequency. Interestingly, this feature was not correlated with parameters describing oscillatory firing properties in recordings made in the normal condition but was significantly correlated with spectral power in specific frequency bands in recordings from the parkinsonian state (specifically with power in the 8–13 Hz band).
Keywords: support vector machine, feature, subthalamic nucleus, internal pallidal segment, external pallidal segment
it is well known that parkinsonism results in firing abnormalities of basal ganglia neurons, specifically in the subthalamic nucleus (STN) and the internal and external pallidal segments (GPi and GPe, respectively). It is very likely that such abnormalities are ultimately linked to the generation of the motor signs of Parkinson's disease, given the remarkable antiparkinsonian effects of pallidotomies and other surgical interventions aimed at the basal ganglia (Baron et al. 2002; Brotchie et al. 1991; Laitinen 1995; Lieberman et al. 1999; Lozano et al. 1995; Vitek et al. 2003).
Early studies of basal ganglia firing abnormalities focused on changes in the mean discharge rate of basal ganglia neurons, with the finding that the average firing rates of GPe neurons were reduced while the average firing rates in GPi and STN were increased in animal models of the disease (e.g., Albin et al. 1989; Bergman et al. 1994; DeLong 1990; Elder and Vitek 2001; Filion and Tremblay 1991; Miller and DeLong 1988; Schneider and Rothblat 1996; Wichmann et al. 1999) as well as patients (Chen et al. 2010; Hutchison et al. 1994; Molnar et al. 2005). Recent optogenetic studies have reinforced the idea that global changes in basal ganglia output contribute to parkinsonism (Kravitz et al. 2010).
More recent studies have emphasized changes in firing patterns. For instance, basal ganglia neurons have a greater than normal tendency to discharge in bursts in the parkinsonian state, as has been shown in animals (Bergman et al. 1994; Breit et al. 2007; Filion and Tremblay 1991; Miller and DeLong 1988; Ni et al. 2000; Vila et al. 2000; Wichmann and Soares 2006) and humans (Hutchison et al. 1994; Magnin et al. 2000). A subset of these bursts may represent rebound (or “low threshold spike”) bursts, presumably driven by activation of T-type calcium channels in the basal ganglia and thalamus. Abnormal bursting in the basal ganglia and thalamus not only may be related to the motor signs of parkinsonism but also may underlie some of the arousal deficits in Parkinson's disease (Barraud et al. 2009; Gatev and Wichmann 2003; Rye et al. 2000; Steriade and Llinas 1988).
Another parkinsonism-related property of spiking activities of basal ganglia neurons is that these neurons often fire in oscillatory firing patterns. These patterns of activity have been documented in the basal ganglia since at least the mid-1980s (Bergman et al. 1994; Gatev et al. 2006; Hammond et al. 2007; Miller and DeLong 1987). After the discovery of strong beta band oscillations in local field potential recordings from the STN in parkinsonian patients (Brown et al. 2001), many current researchers see these oscillations as the predominant electrophysiological abnormality in the parkinsonian brain. Oscillations are primarily identified in STN, GPi, and GPe (Bergman et al. 1994; Levy et al. 2000, 2002a, 2002b; Nini et al. 1995; Raz et al. 1996), although other areas of the basal ganglia-thalamocortical circuitry also show oscillatory discharge (Goldberg et al. 2002; Guehl et al. 2003; Pasquereau and Turner 2011; Pessiglione et al. 2005). The source of these oscillatory activity patterns has not been clarified, but experimental and modeling studies have suggested that they are the product of network interactions rather than being generated at specific basal ganglia locations (Gatev et al. 2006; Hammond et al. 2007; Holgado et al. 2010; Magill et al. 2001, 2004a, 2004b; Plenz and Kitai 1999).
Most recently, several studies have observed that the spiking activity of neurons in the individual basal ganglia nuclei differs in terms of entropy and other nonlinear characteristics of firing (Darbin et al. 2006) and that these changes are affected in parkinsonian patients by treatment with dopamine receptor agonists (Lafreniere-Roula et al. 2010) or by deep brain stimulation in animal models of parkinsonism (Dorval et al. 2008, 2009, 2010).
The relative preponderance and importance of these firing abnormalities in the dopamine-depleted state and their relationship to parkinsonism is not clear, in part because most studies in this field tend to focus on individual changes (such as changes in firing rates, bursts, or oscillations). To develop a better understanding of the relative strength of these changes, we carried out an analysis in which we evaluated the ability of combinations of multiple descriptors of single-cell discharge in the basal ganglia to predict parkinsonism, using recordings in STN, GPe, and GPi from normal and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated (parkinsonian) monkeys.
METHODS
General Methods
Some of the data used in this analysis were also used in previous studies (Soares et al. 2004; Wichmann and Soares 2006). As stated in the other reports, we recorded the activity first in GPe, STN, and GPi in the normal state and then again after the animals had been treated with MPTP to induce parkinsonism. The state of wakefulness was monitored throughout all experiments, and recordings were discarded if the animal showed signs of drowsiness. After completion of the recording sessions, the location of the neurons was verified by histological analysis.
Interspike interval (ISI) data from the recorded cells were then analyzed to measure the firing rate, descriptors of burst discharges, entropy, or oscillatory activity. These analyses resulted in 29 features (listed in Table 1) that were fed into different feature selection algorithms to identify single features, or combinations of them, that would best discriminate between neurons recorded in the normal and parkinsonian states, separately for each of the three structures.
Table 1.
Feature definitions
| Feature | Definition |
|---|---|
| Basic descriptors of firing | |
| 1 | Mean ISI |
| 2 | SD of ISIs |
| 3 | CV of ISIs |
| 4 | Firing rate |
| Integrated spectral power | |
| 5 | 1–3 Hz range |
| 6 | 3–8 Hz range |
| 7 | 8–13 Hz range |
| 8 | 13–30 Hz range |
| 9 | 30–100 Hz range |
| Entropy measures* | |
| 10 | H1 |
| 11 | H2 |
| 12 | H3 |
| 13 | H4 |
| 14 | H5 |
| 15 | zc1 |
| 16 | zc2 |
| 17 | zc3 |
| 18 | zc4 |
| 19 | zc5 |
| Measures describing bursting | |
| 20 | Burst-free rate |
| 21 | Mean spikes per burst |
| 22 | Median spikes per burst |
| 23 | Mean intraburst frequency |
| 24 | Median intraburst frequency |
| 25 | Proportion of time in bursts |
| 26 | Proportion of spikes in bursts |
| 27 | Proportion of rebound bursts |
| 28 | Proportion of LTS bursts |
| 29 | Proportion of rebound LTS bursts |
ISI, interspike interval.
See Dorval et al. (2008, 2010) and explanations in text.
Animals
The two rhesus monkeys (Macaca mulatta, 4–5 kg) that were used for these studies were housed under conditions of protected contact housing with free access to standard primate chow, water, and supplemental fruit and vegetables. Prior to the recording sessions, the animals were adapted to the laboratory environment and trained to sit in a primate chair and permit handling by the experimenter. All experimental protocols were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996), the US Public Health Service “Policy on the Humane Care and Use of Laboratory Animals” (amended 2002), and the American Physiological Society's “Guiding Principles in the Care and Use of Animals” (revised 2000). All experiments were approved by the Institutional Animal Care and Use Committee of Emory University.
Surgical Procedures
After completion of behavioral conditioning, stainless steel chambers for chronic recording (inner diameter 16 mm) were stereotaxically positioned over trephine holes under aseptic conditions and isoflurane inhalation anesthesia (1–3%). Chambers directed at the pallidum (GPe, GPi) were placed at an angle of 50° from the vertical in the coronal plane, and chambers aimed at the STN were placed at an angle of 36° anterior to the vertical in the sagittal plane. The chambers were affixed to the skull with dental acrylic. Stainless steel head holders were also embedded into the acrylic cap to permit stabilization of the head during the recording sessions.
Electrophysiology
All recordings were done with the animal seated in a standard primate chair, with its head restrained. Recordings were only conducted if the animal was fully awake (verified by direct observation). The neuronal activity in GPe, GPi, and STN was recorded extracellularly with tungsten microelectrodes (Frederick Haer, Bowdoin, ME; impedance 0.5–1.0 MΩ at 1 kHz). The microelectrodes were lowered into the brain with a microdrive (MO-95B; Narishige, Tokyo, Japan) and the use of a guide tube that was positioned with its tip barely penetrating the surface of the brain to protect the electrodes as they passed through the dura. The electrical signals were amplified (DAM-80 amplifier; WPI, Sarasota, FL), filtered (400–10,000 Hz; Krohn-Hite, Brockton, MA), displayed on a digital oscilloscope (DL1540; Yokogawa, Tokyo, Japan), made audible via an audio amplifier, and recorded as digital signals with a video recording adapter (model 3000A; Vetter, Rebersburg, PA; sampling rate: 40 kHz). Neurons in the basal ganglia were identified by generally accepted characteristics such as high-frequency discharge with pauses in GPe, tonic high-frequency discharge in GPi, and tonic and regular discharge in an area of high background activity in the STN (see, e.g., DeLong 1971; Wichmann et al. 1994). We did not discriminate between specific functional territories within the basal ganglia in these recordings; thus records from motor and nonmotor areas were included in the analysis.
Administration of MPTP
After completion of recordings in the normal state, the animals received MPTP injected under general isoflurane anesthesia (1–3%) into the right common carotid artery with the external carotid artery occluded, so that the toxin reached the brain via the internal carotid artery (0.5 mg/kg per injection; one monkey received 2 injections separated by 2 wk, while the other received a single injection). Both animals developed similarly obvious signs of moderate parkinsonism (bradykinesia, rigidity, flexed posturing of arm and leg) contralateral to the injections. The animals did not receive any dopaminergic medications throughout these experiments. The recordings in the parkinsonian state started 2 mo after the MPTP injection. Throughout the post-MPTP period, the behavioral state of the animals remained stable, as assessed with routine behavioral observations (Kliem et al. 2010; Soares et al. 2004; Wichmann et al. 2001). After stable parkinsonism was established, electrophysiological recordings resumed on the left side (contralateral to the MPTP administration).
Histology
At the conclusion of the experiments, the monkeys were killed by induction of deep anesthesia with an overdose of pentobarbital sodium, followed by transcardial perfusion with saline and 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2). The brains were removed and cryoprotected in 30% sucrose solution in 0.1 M phosphate buffer. The fixed brain was sectioned in coronal planes (50 μm). One of every four sections was stained with cresyl violet for localization of microelectrode tracks. The results of the histological examinations are documented in our previous publication (Soares et al. 2004).
Data Analysis: Preliminary Steps and Feature Extraction
Preliminary steps.
We included cells in the analysis only if the reconstruction of their location, based on stereotaxic information, micromanipulator readings during the recordings, and the results of postmortem histological analysis, confirmed that they were located within one of the target structures (GPe, GPi, or STN). For inclusion in the analysis, cells also had to be adequately isolated throughout the record, as defined by a signal-to-noise ratio of their signals of 3 or greater.
The recorded activity was processed with a template-matching spike sorting device (Alpha-Omega, Nazareth, Israel) that extracted the timing of spike occurrences. The data were stored as ISIs.
The ISI data were imported into MATLAB (MathWorks, Natick, MA) for further analysis. For confirmation of adequate signal isolation, we constructed ISI distribution histograms for quality control. In addition, raster displays of the spontaneous firing of each cell were carefully examined and episodes of stationary discharge were selected in a blinded fashion. As shown in detail in Table 2, the eventually processed data segments were 546.9 ± 146.4 s long (mean ± SD, range: 63–1,201 s) and included 31,932 ± 16,906 ISIs (range: 2,783–107,684 ISIs).
Table 2.
Basic data characteristics
| Structure | State | No. of Cells | No. of Events | Length of Records, s |
|---|---|---|---|---|
| GPe | Normal | 44 | 36,894.2 ± 16,194.1 (5,102–72,182) | 554.3 ± 169.4 (72.1–842.3) |
| MPTP | 40 | 26,157.2 ± 13,572.1 (9,108–71,191) | 547.7 ± 126.0 (286.7–830.5) | |
| GPi | Normal | 33 | 38,073.7 ± 14,633.9 (5,182–61,656) | 523.0 ± 151.7 (63.3–644.8) |
| MPTP | 34 | 44,380.6 ± 17,070.5 (12,689–107,684) | 568.9 ± 159.0 (164.9–1,201.2) | |
| STN | Normal | 15 | 11,260.9 ± 5,379.7 (2,783–23,626) | 498.4 ± 166.1 (73.4–671.7) |
| MPTP | 28 | 21,104.3 ± 7,594.6 (8,054–37,870) | 561.8 ± 95.2 (225.5–640.9) |
Values for number of events and length of records are means ± SD with ranges in parentheses. GPe, external pallidal segment; GPi, internal pallidal segment; STN, subthalamic nucleus; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Feature extraction.
The ISI data were used to extract different descriptors of neuronal discharge. In addition to basic features (average, standard deviation, and coefficient of variation of ISIs, firing rate), we analyzed the oscillatory properties of neuronal discharge within the spectral range of 1–100 Hz, as described in our previous publication (Soares et al. 2004). The power in portions of the spectrum was then integrated over the 1–3 Hz, 3–8 Hz, 8–13 Hz, 13–30 Hz, and 30–100 Hz regions of the spectrum and expressed as a proportion of the total spectral power in the 1–100 Hz range. We also calculated measures of entropy, following the algorithm proposed by Dorval et al. (parameters H1–H5 and zc1–zc5; see Dorval et al. 2008, 2010). In addition, we used the Poisson “surprise” method (Legendy and Salcman 1985) to determine the onset and offsets of bursts in discharge in the recorded data streams, using a surprise value of 3 to identify bursts (Wichmann and Soares 2006). Bursts detected with this method were used to calculate the average and median number of spikes per burst, the average and median intraburst firing rate, the proportion of time that the cell spent bursting, and the proportion of spikes in bursts. We also examined all ISIs that were not part of bursts to determine the cell's “background” (i.e., burst-free) firing rate. Each burst preceded by a long pause (i.e., an ISI that was at least 500 ms long) was classified as a “rebound burst,” and bursts following classic electrophysiological features of T-type calcium channel-dependent bursting (Zirh et al. 1998) were called “LTS” bursts. We then calculated the proportion of rebound bursts that fulfilled LTS burst criteria.
The surprise method can also be used to identify pauses in discharge (Elias et al. 2007). We initially used this fact to calculate the average pause duration and the SD of pause durations, the proportion of spikes that flanked pauses, and the proportion of time a cell spent in pauses. However, these data could not be used for the subsequent classification analysis (below), as many cells did not show pauses in their discharge.
Eventually, 29 features, available from all neurons in all three nuclei, were used for the classification analysis. These features were meant to encompass the characteristics typically used to analyze neuronal discharge, along with a few novel features. As such, the set contains some features that are linearly dependent. For instance, firing rate was included because it is historically the most commonly analyzed feature. However, the mean and standard deviation of ISIs were also included, as well as their combination (CV) to see how the individual features would compare to the combination of the two. The optimal feature selection method in this study allows ranking of the usefulness of combinations of the dependent features for discriminating between recordings from the parkinsonian and normal states.
Data Analysis: Discrimination Between Neural Signals Recorded at Baseline and in the Parkinsonian State
The general goal of the following data analysis steps was to identify combinations of the previously extracted features that would best discriminate between the normal and parkinsonian states. All analyses of the discrimination success were carried out with a support vector machine (SVM) classification method (see below). To find the optimal combination of features for each nucleus, individual features were cumulatively added to the SVM analysis, according to an order that was established by one of four methods that are described (and compared) below. The simplest method was a “naive” feature selection approach in which we first determined the SVM discrimination performance for each feature individually and then added them one at a time in the order of their performance (from best to worst) into the feature set used for the eventual SVM discrimination. The second method ranked features based on their respective “F scores” (Chen and Lin 2006), the third method explicitly considered feature relevance and redundancy, and the fourth was an empirical iterative method that we refer to as the “best n features” method. All feature selection methods were implemented with custom MATLAB scripts.
SVM discrimination.
SVMs optimize the separation between classes of data (such as data generated in the normal and parkinsonian states) in an N-dimensional feature space by choosing a class boundary that maximizes the margin between points from each class that lie closest to the boundary. The fractional correct SVM discrimination (discrimination performance) is then the fraction of data points separated into the correct classes with the chosen boundary. In this study, SVMs were used to optimize class separation for features individually (N = 1), for reduced subsets of features (1 < N < 29), and for the total number of features in the data set (N = 29) in order to compare how different number and composition of features affected the discrimination performance. To reduce the potential for overfitting, the data were divided into training and test sets containing equal proportions of normal and parkinsonian data. The SVM classifier was trained (i.e., the boundary was chosen) with the training data. The discrimination performance was then evaluated by applying the trained classifier to the test data. The method of “5-fold cross-validation” was used for selecting the training and test sets. This method repeatedly divides the data into 5 different subsets of equal size, and each subset is tested with the classifier trained on the remaining 4 subsets (Hsu et al. 2003). Classification of data that are not linearly separable in standard N-dimensional feature space can be performed through use of a nonlinear kernel, such as the radial basis function (RBF; see Theodoridis and Koutroumbas 2008). The RBF kernel was employed for the analysis in this study not only because it allows nonlinear classification but also because it has been successfully used in other studies involving the classification of neuronal discharge (Garrett et al. 2003). Our SVM algorithms were implemented with the LIBSVM library of MATLAB scripts, with α and γ parameters optimized via a search over a set of discrete (α, γ) points in a bounded region (grid search; see Chang and Lin 2001).
F score method.
One of the methods used to generate a rank order of features for the subsequent SVM classification was the F score method. For a given set of features xi, i = 1 … N related to two classes denoted by ′ and *, the F score is defined as
For our study, the data were either recorded in the normal or the parkinsonian state; hence the two classes were “normal” and “parkinsonian” while the features were the i = 1 … 29 features described previously. The numerator of the F score for each feature was computed by summing the square of the difference between the mean of the “normal” feature data and the overall mean of the feature data (from both classes) and the square of the difference between the mean of the “parkinsonian” feature data and the overall mean of the feature data. Similarly, the denominator summed the variances for the “normal” and “parkinsonian” feature data for each feature. The end result was an F score for each feature, indicating how well that feature separated the two classes of data. Higher F scores reflect features with good separation relative to the uncertainty (variance) of the feature. The features were ranked from highest to lowest F score and added in a cumulative manner to the SVM discrimination process.
Relevance and redundancy method.
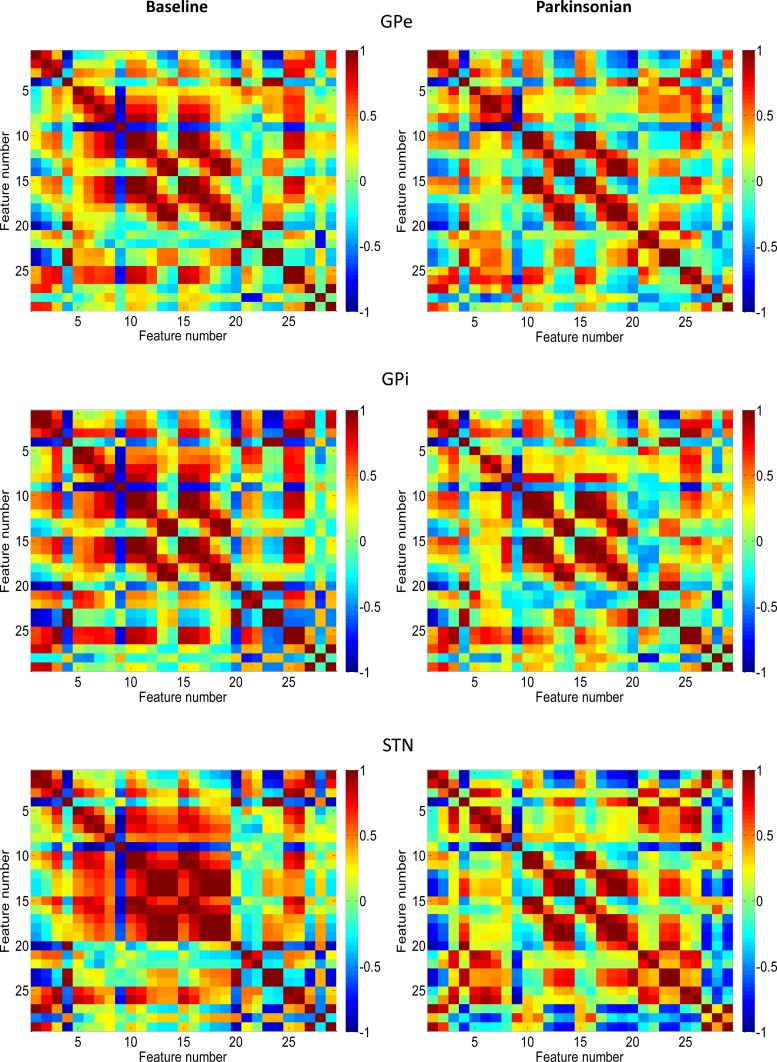
The F score approach does not reveal mutual information between features (Chen and Lin 2006), a problem that can lead to incorporation of redundant features in a given feature selection algorithm. To overcome this disadvantage, we tested a feature ranking method that considers the relevance and redundancy (Yu and Liu 2004) of the selected features. For this approach, an SVM classifier was used to estimate the relevance of each feature, along with a cross correlation coefficient measure between features to estimate its redundancy with other features. Visual inspection of the cross correlation map (Fig. 1) to gain insight into the feature selection process was a helpful aspect of this method. The relevance score for each feature was defined as its individual RBF SVM discrimination performance, Pi, between the normal and parkinsonian states. The standard Pearson correlations, rij, were selected as the basis for the redundancy measure, where
Fig. 1.
Pearson correlation between features in normal (left) and parkinsonian (right) states for external pallidal segment (GPe, top), internal pallidal segment (GPi, middle), and subthalamic nucleus (STN, bottom). See Table 1 for definitions for features 1–29. The strengths of the correlation between features on the x-axis with those on the y-axis are color-coded.
The absolute values of the Pearson correlations for each feature versus each of the other features were summed, normalized, and subtracted from 1 to obtain a score ranging from 0 (maximum redundancy) to 1 (no redundancy):
Next, the relevance and redundancy scores were added, and the features were ranked from high to low on the basis of this composite score (Ci = Pi + Ri). The features were then incorporated in a cumulative manner into the SVM discrimination process on the basis of their Ci ranking.
“Best n features” method.
The “best n features” method used an empirical iterative process that selected the individual feature providing the highest fraction of correct SVM discrimination as the first optimal feature. At each subsequent step, an exhaustive search was used to find the j features (singly, j = 1, or pairwise, j = 2) that provided the most improvement in composite SVM discrimination, with features considered in order of their individual SVM discrimination performance. The j features that provided the most improvement at each step were then added to the best feature list, and the process was repeated until all features were included. This required computing the SVM discrimination achieved by incorporating all possible combinations of j features over all m remaining features for each step . After all 29 features were included, the list of ordered features was then truncated to the 1 through n features necessary to reach the point of maximum SVM discrimination, i.e., the “best n features.”
RESULTS
Data Set
The data set consisted of data from GPe, GPi and STN, recorded before and after treatment of the animals with MPTP. The basic data characteristics are shown in Table 2. The Pearson correlation between features (Fig. 1) gives a qualitative overview of the discriminability of the data. In Fig. 1, varying patterns of feature correlation can be seen between the normal (Fig. 1, left) and parkinsonian (Fig. 1, right) states in GPe, GPi, and STN. Note the large contrast between features based on STN data from the normal state compared with features based on STN data from the parkinsonian state, the two classes of data that yielded the best discrimination results in all of the discrimination algorithms tested here (see below).
Discrimination Achieved Using Results of Different Feature Ranking Algorithms
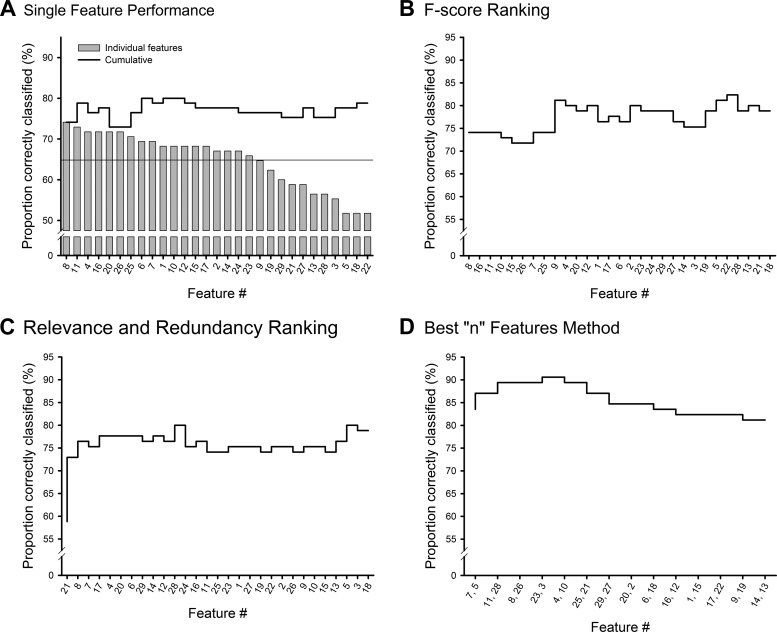
Figure 2 shows the ranking of features obtained by using the four different feature selection processes, applied to data from GPe. For each method in Fig. 2, the x-axis lists the features in the rank order determined by the respective feature selection algorithm and the line shows the performance achieved by incorporating the k best-performing features, for k = 1 … 29. The naive feature selection approach (Fig. 2A) achieved a peak performance of 80% for the GPe data, 79% for the GPi data, and 86% for the STN data. As in all parts of Fig. 2, Fig. 2A shows that the addition of certain features (even those ranked highly by these methods) actually reduced the cumulative discrimination performance.
Fig. 2.
Proportion of GPe data samples correctly identified as originating from either the normal or the parkinsonian state with radial basis function (RBF) support vector machine (SVM) discrimination for single features (bars) and with features cumulatively incorporated from best to worst single feature performance (line) (A); with features cumulatively incorporated by F score ranking (highest to lowest F score) (B); with features cumulatively incorporated by relevance and redundancy ranking (C); and for features cumulatively incorporated by adding the 2 features that increased performance the most at each step (D). The GPe results were chosen as the representative set since they yielded the middle discrimination performance. Results for GPi and STN are similar in appearance.
Figure 2, B and C, illustrate the respective discrimination performance when features were added to the SVM analysis in the rank order as indicated by the F score and the relevance and redundancy methods. For both, the maximal level of discrimination was greater than or equal to that possible with the naive method.
Finally, Fig. 2D demonstrates the results of the “best n features” method (without truncation). Adding iteratively the single feature that gave the most improvement in discrimination increased the discrimination success beyond that achieved with the other methods. Additional increases were seen by bringing in the two best features at each step (pairwise “best n features”). This method resulted in a performance curve that peaked at 91% for the GPe data (10 features), 86% for the GPi data (10 features), and 95% for the STN data (6 features). Note that the performance increased until a maximum was reached and then declined, indicating that no additional discrimination capability was provided by the remaining features. The higher peak and the monotonically increasing and then decreasing nature of the performance curve led us to choose the optimal features selected with this method for further analysis. Table 3 shows the feature sets that yielded the maximum performance with this approach for each nucleus.
Table 3.
Features selected by “best n features” algorithm
| GPe | GPi | STN | |
|---|---|---|---|
| Firing rate/ISI statistics | CV of ISIs, firing rate | CV of ISIs | |
| Fractional spectral power | 1–3 Hz, 8–13 Hz, 13–30 Hz | 1–3 Hz, 13–30 Hz | 1–3 Hz, 8–13 Hz, 30–100 Hz |
| Entropy measures | H1, H2 | H1, zc1 | |
| Burst statistics | Mean intraburst frequency, proportion of spikes in bursts, proportion of LTS bursts | Median intraburst frequency, mean spikes per burst, median spikes per burst, proportion of spikes in bursts, proportion of LTS bursts | Median intraburst frequency, proportion of rebound bursts, proportion of rebound LTS bursts |
Top selected features are in bold.
Comparison of Features Selected by the Different Methods
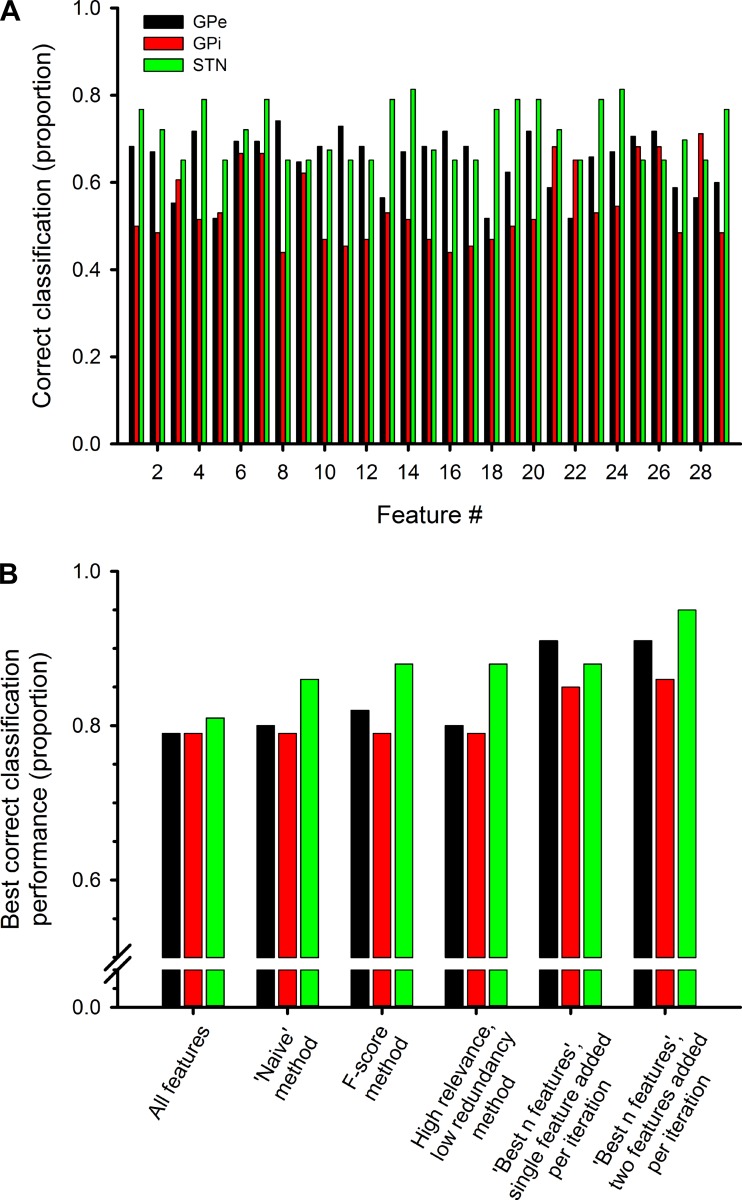
As shown in Fig. 3A, the single feature discrimination performances typically varied substantially from one feature to another. The single feature discrimination performances also varied widely between the different nuclei for some features. Figure 3B shows a comparison of the discrimination performance of combinations of features, added in order of their ranking by the different feature selection algorithms used in this study. For each approach, the results reflect the performance obtained by including the set of features that produced the highest discrimination level in the respective performance graph. When the full set of 29 features was used (“all features” in Fig. 3B), 81% correct discrimination between the normal and parkinsonian states was achieved for the cells in the STN data set and 79% was achieved for both the GPe and GPi data sets. Although each of the methods for feature selection improved on the “all features” approach, Fig. 3B shows that the pairwise “best n features” method generated the rank order of features that resulted in the best discrimination performance.
Fig. 3.
A: RBF SVM discrimination performance using single features (shown for features 1–29). Discrimination performance is shown for features 1–29 for GPe, GPi, and STN. B: summary of RBF SVM discrimination performance for all feature selection methods, applied to data from the GPe, GPi, or STN.
The top features selected with each of the associated methods were not necessarily the same. However, within a particular nucleus, many features were seen to be common across methods. For example, in the GPe data, 3 of the top 11 features (features 4, 7, 8) were common across all methods, with feature 7 (spectral power in the 8–13 Hz band) being the strongest. Four of the remaining eight features were common among three methods (features 10, 11, 25, 26). For the GPi data, 3 of the top 11 features appeared in all four methods (features 21, 22, 28), with feature 28 (proportion of LTS bursts) as the strongest feature. Four of the remaining eight features were common among three methods (features 3, 24, 25, 26). In the STN data, 6 of the top 11 features appeared in all methods (features 4, 7, 14, 20, 23, 24), with feature 24 (median intraburst frequency) being the single strongest feature.
Top Features for Each Nucleus
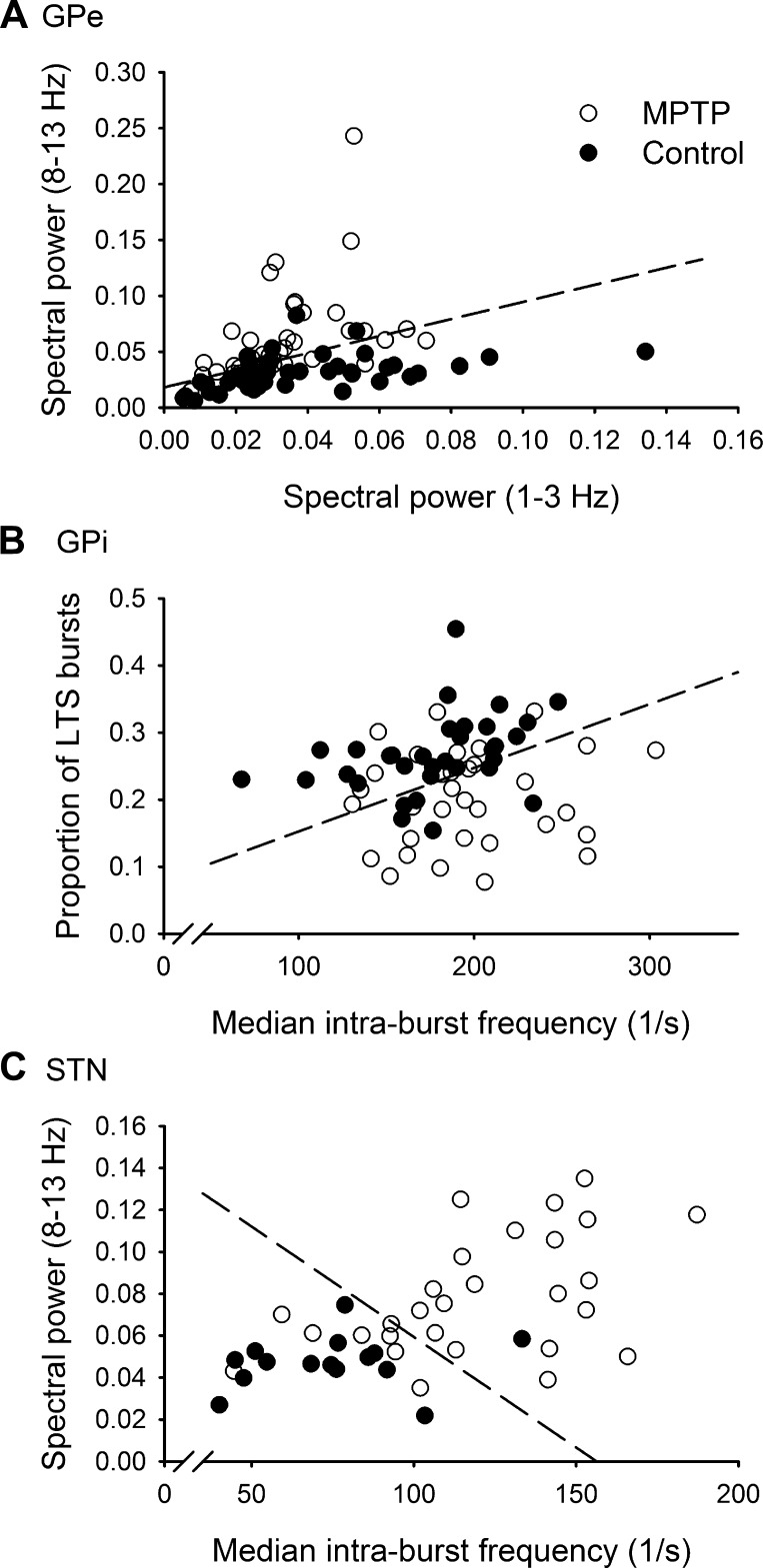
Figure 4 shows scatterplots of the two top-ranked features for discrimination for each of the three nuclei, as selected by the “best n features” method. Although more than two features were necessary for peak discrimination, the two-dimensional scatterplots provide a visual indication of how the neuronal discharge patterns changed in the parkinsonian state and how much discrimination is achievable using only the two features that were most changed. Standard linear discrimination analysis (LDA) of the two-dimensional data yielded 76% discrimination for the GPe, 71% for the GPi, and 82% for the STN data (dashed lines in Fig. 4 show LDA discrimination). As can be seen, the top features in each nucleus were related to either bursting or fractional spectral power. For GPe, the top two features were the fractional spectral power in the 8–13 Hz (feature 7) and 1–3 Hz (feature 5) bands. Under baseline conditions, the mean 8–13 Hz fractional power was 0.03 ± 0.016 (i.e., ∼3% of the entire power in the 1–100 Hz range was in this band; mean ± SD). Under parkinsonian conditions, the mean increased to 0.06 ± 0.041. The mean 1–3 Hz fractional power decreased from 0.04 ± 0.026 (baseline) to 0.03 ± 0.016 (parkinsonism). For the GPi data, the two highest-ranking features were the fraction of LTS bursts (feature 28) and the mean intraburst frequency (feature 23). Under baseline conditions, the fraction of LTS bursts was 0.27 ± 0.059 (i.e., 27% of all bursts were designated as LTS bursts). In the parkinsonian state, the mean decreased to 0.20 ± 0.070. The mean intraburst frequency increased from 185.06 ± 41.147 spikes/s at baseline to 204.03 ± 43.881 spikes/s in the parkinsonian state. For the STN data, the two top features were the median intraburst frequency (feature 24) and the spectral power in the 8–13 Hz band (feature 7). Under baseline conditions, the median intraburst frequency was 74.37 ± 24.934 spikes/s. Under parkinsonian conditions, the mean increased to 119.15 ± 33.599 spikes/s. The mean fractional power in the 8–13 Hz range increased from 0.05 ± 0.012 (baseline) to 0.08 ± 0.028 (parkinsonism). The minimum and maximum values and the data distributions for the top two features from each nucleus can be seen in the scatterplots in Fig. 4. The individual (and 2-dimensional) feature distributions for the baseline and parkinsonian conditions overlapped. However, as seen in Fig. 2D, the ability to discriminate between the data increased as more features were included.
Fig. 4.
Scatterplot of the 2 best features for all cells, GPe (A), GPi (B), and STN (C). Linear discrimination analysis (LDA) of the 2-dimensional data yielded 76% for GPe, 71% for GPi, and 82% for the STN data (dashed lines show LDA discrimination boundaries). MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Although increased bursting occurred in all three nuclei, the observed changes differed between the STN and the two pallidal segments. The GPe and GPi data showed a large increase in the average proportion of spikes in bursts (feature 26), while in STN the major effect was the increased intraburst frequency. The median intraburst frequency (feature 24) increased by ∼60% in the STN in the parkinsonian state compared with the normal condition, although the average proportion of spikes in bursts only increased by ∼7%. In contrast, the median intraburst frequency in the GPe decreased by 16% while the average proportion of spikes in bursts increased by 80% in the parkinsonian state. In the GPi, the median (and mean) intraburst frequency increased by 10% while the average proportion of spikes in bursts increased by 50%.
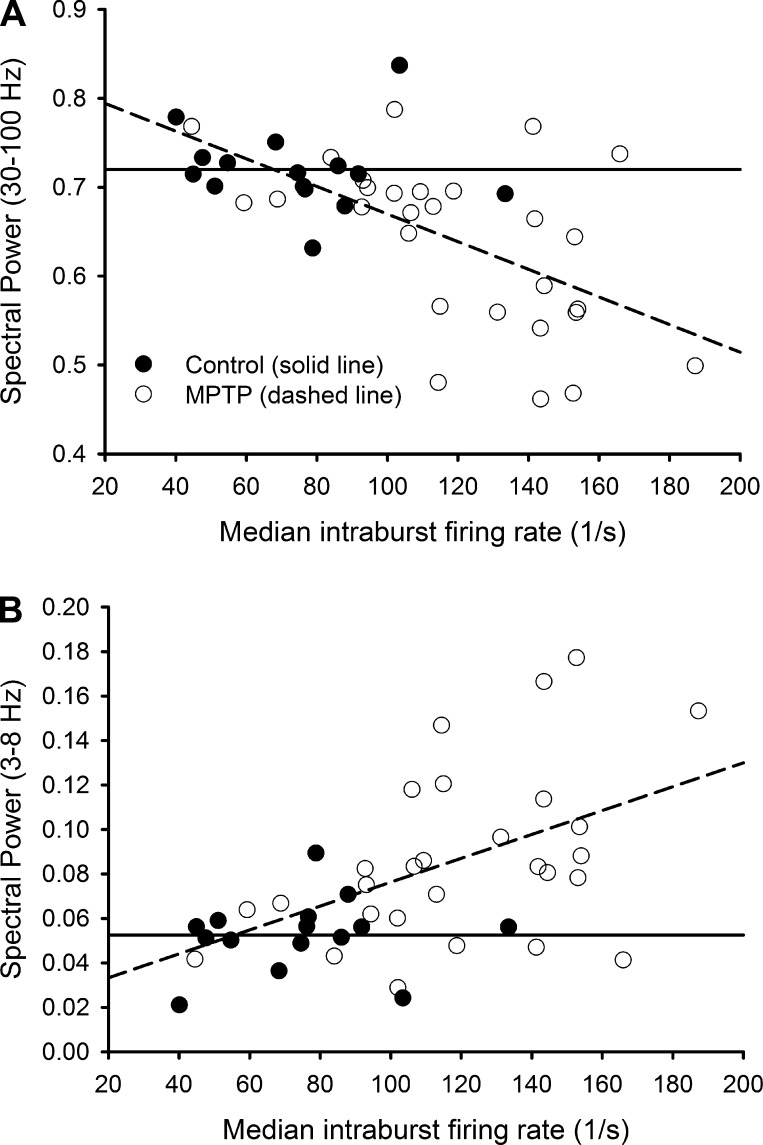
Some spectral characteristics were shared between nuclei, while other spectral features showed significant differences. For example, the 30–100 Hz fractional spectral power in each band for GPe, GPi, and STN was reduced while the fractional power in the 3–30 Hz frequency bands was higher in the parkinsonian state. In the GPi, characteristics of burst discharges (features 23–26) and fractional spectral power in the 3–100 Hz frequency bands (features 6–9) were not significantly correlated in either the normal or the parkinsonian state (P ≥ 0.1). In the GPe, these sets of features were related, but the significance (and direction) of correlation between them was similar for both states [P ≤ 0.008; 30–100 Hz (negatively correlated with bursting), 3–8 Hz and 8–13 Hz (positively correlated with bursting)]. Thus there was no parkinsonism-related change in the relationship between burst features and spectral power in either pallidal segment. This was different for the STN, however. In this nucleus, the intraburst frequency (features 23 and 24) and the power in the 3–100 Hz bands were not correlated under normal conditions (P > 0.3; see flat solid least-square fit lines in Fig. 5). In contrast, in the parkinsonian state the intraburst frequency correlated negatively with the power in the 30–100 Hz band (P = 0.002, Fig. 5A, dashed line) and positively with the power in the bands between 3 and 30 Hz [P = 0.01 for 3–8 Hz (Fig. 5B), P = 0.009 for 8–13 Hz, and P = 0.04 for 13–30 Hz].
Fig. 5.
Scatterplots with lines showing least-squares fits to baseline (Control) and parkinsonian state (MPTP) data from the STN for median intraburst frequency vs. 30–100 Hz (A) and median intraburst frequency vs. 3–8 Hz (B). Note that the slopes of the least-squares fit lines for the data from the parkinsonian state show a negative correlation between intraburst frequency and the 30–100 Hz power in A and a positive correlation between intraburst frequency and the 3–8 Hz power in B.
Reproducibility of Results
Recognizing the importance of reproducibility in generating a set of best features for each nucleus, we reran the “best n features” algorithm using half of the data, randomly selected, for training and optimizing parameters and then used SVM prediction to classify the other half of the data. We then repeated the process, swapping the training and testing data sets. As can be expected, the performance results were slightly lower (81–83% for the GPe data set, 68–75% for the GPi data set, and 77–86% for the STN data set), but the list of best features did not change significantly. In particular, the top features for each nucleus remained the same.
Discrimination Between Nuclei
We also explored whether the “best n features” method could be used to discriminate single-cell spiking recorded in the different nuclei. Under baseline conditions, the discrimination between GPe and GPi was 83.1%, between STN and GPe was 98.3%, and between STN and GPi was 100%. The top features for discrimination were for GPe/GPi zc3, CV of ISIs, H3, SD of ISIs, and mean spikes per burst; for STN/GPe median intraburst frequency, 13–30 Hz power, and firing rate; and for STN/GPi H1, zc5, and firing rate. In the parkinsonian condition, the corresponding accuracies were 89.1%, 97.0%, and 96.8%, respectively. Concerning the GPe/GPi discrimination, the best features were mean intraburst frequency, 13–30 Hz power, and mean ISI; for STN/GPe discrimination, the best features were 13–30 Hz power, H1, and zc1; and for STN/GPi discrimination, the best features were 13–30 Hz power, median intraburst frequency, and zc2.
DISCUSSION
We found that SVM discrimination between ISI features from extracellular recordings in the STN, GPe, and GPi allowed successful discrimination between the normal and parkinsonian states and between the three nuclei in monkeys.
Combining the SVM discrimination techniques with optimal feature selection methods enabled quantitative analysis of the most changed features, and the relationships between them, in the three basal ganglia regions under dopamine depletion conditions. As seen in Fig. 3B, the “best n features” method provided the highest discrimination percentages of the feature selection approaches tested. The top features identified by that method were also highly ranked by most of the other methods, adding to our confidence that the features identified and discussed below are important for distinguishing between the normal and parkinsonian conditions.
Best Feature Sets for Discriminating Between Normal and Parkinsonian States in Different Basal Ganglia Nuclei
As shown in Table 3, the set of best features included multiple categories of features for all three basal ganglia nuclei, reinforcing the idea that it is relevant to examine different aspects of cellular activity to gain optimal information. As suggested by the summary results, the best number of features for this type of discrimination may be ∼6–10. The optimal feature sets did not include all features in any one category, indicating that some of the features in each category were redundant (and were thus removed). For example, optimal feature lists included the ISI CV (feature 3) but not the ISI mean and standard deviation (features 1 and 2), likely because the former is a linear function of the latter two.
The feature categories most relevant for discrimination were the bursting statistics and fractional power of oscillations (spectral power), although firing rate/ISI statistics and entropy measures were also useful. Interestingly, the relationship between oscillatory activities and bursting may be different in the STN and the GPe/GPi. The burst statistics indicated that, although increased bursting occurs in all three nuclei in the parkinsonian state, the effect in the STN was an increase in intraburst frequency while the GPi and GPe data showed larger increases in the proportion of spikes in bursts.
Our analysis showed that the fractional spectral power in the 8–13 Hz and 13–30 Hz bands was higher on average in the parkinsonian condition for all three nuclei, agreeing with previous findings of prominent oscillations in the beta band in the parkinsonian state (see, for instance, Bergman et al. 1994; Hammond et al. 2007). The STN intraburst frequency feature was not correlated to the oscillatory power in these frequency bands at baseline (normal) but under parkinsonian conditions became positively correlated for bands between 3 and 30 Hz and negatively correlated for the 30–100 Hz band. This significant change in the correlation between bursting and spectral power is likely the reason that better discrimination was possible for the STN compared with GPe and GPi data. It also suggests a link between the increased burst intensity of STN neurons and the parkinsonian profile of increased beta and reduced gamma oscillations.
Although increased fractional spectral power in the 3–30 Hz frequency bands was also seen in GPe and GPi, the magnitude and direction of correlation between burst features and fractional spectral power in these structures did not change significantly in the parkinsonian state compared with the normal state.
We found that the proportion of LTS bursts (feature 28), the top-ranked feature for GPi discrimination, was smaller in the parkinsonian state than in the baseline condition in the GPi data, indicating that, at least for GPi cells, the increased bursting is substantially different from typical LTS bursting activity. This result may be understandable by the fact that LTS bursts tend to be associated with increased membrane hyperpolarization. While several structures within the basal ganglia-thalamocortical network of connections are hypothesized to be subject to greater phasic or tonic GABAergic inhibition and may thus be prone to develop LTS bursts, this is not the case for GPi, which, according to the classic models of basal ganglia activity changes in Parkinson's disease, is instead subject to an increased excitatory drive from the STN (see, e.g., Galvan and Wichmann 2008).
As seen in Table 3, three of the most relevant features from the GPe and GPi data sets were based on measures of entropy (features 10, 11, and 15). H1 (feature 10) is the entropy calculated considering only single ISIs, while H2 (feature 11) takes all sets of two consecutive ISIs into account. zc1 (feature 15) is calculated by extrapolating the entropy to infinite signal length through regression of H1 (see Dorval et al. 2010). The entropy features indicate the general level of variability in the cell firing (Brown et al. 2004). It is interesting to note that entropy features did not appear in the STN “best” feature set even though the STN data allowed the best overall discrimination. Even for GPi and GPe, entropy measures did not appear in the top two features. Thus, while measures of entropy may be helpful when used in conjunction with other features, they do not perform as well as other features for the type of discrimination performed in this study and, if the ease of discrimination is considered a proxy for importance, may be less important than other features.
Although basic descriptors of firing were not top-ranked features for any nucleus, the firing rate and mean, standard deviation, and CV of the ISIs ranked fairly high in the single feature discrimination performance for all nuclei (see Fig. 3A, features 1–4). In our study, no firing rate-related features were necessary for optimal discrimination of the STN data; however, the CV of the ISIs (feature 3) was included in the optimal feature lists for both the GPe and GPi data sets (see Table 3). These features continue to be widely used in models of the pathophysiology of parkinsonism, perhaps in part because they are easily quantifiable smoothed statistics that reflect the underlying bursting activity that this study found most relevant for distinguishing between the normal and parkinsonian states.
Our analysis showed that the optimal feature sets differed substantially between neurons recorded in the different structures. Discriminating neurons recorded in the normal and parkinsonian states was easiest for the STN data, possibly because of the previously discussed significant correlation between the median intraburst frequency and spectral features that occurs under parkinsonism. Records from GPe and GPi were consistently more difficult to categorize, resulting in lower rates of correct discrimination. These differences are likely due to the different membrane properties, afferent synaptic connections, and receptor and neurotransmitter distributions found in the GPe, GPi, and STN nuclei. The different optimal feature sets hint at the general response properties of the cell types under parkinsonian conditions. Whereas the STN cells have a clearly discernible pattern of increased intraburst frequency and spectral changes, the pallidal cell responses appear to be more varied and complex.
Discrimination Between Nuclei
Trained investigators can easily distinguish between STN, GPi, and GPe activities, using online audio representations or oscilloscope displays of single-cell activities recorded from these nuclei. The analysis of the internuclear discrimination of the chosen analysis method was therefore included as a “positive control” experiment, demonstrating that the analytical methods are capable of discriminating GPe, GPi, and STN activities. Not surprisingly, the discrimination between the relatively slow-firing STN cells and the high-frequency pallidal cells was found to be excellent, while distinction between the two (relatively similar) pallidal segments was less accurate. In all cases, three to five features were adequate for maximum discrimination. However, the best features for discriminating between nuclei differed between the control and parkinsonian states, supporting the notion of significant parkinsonism-related changes of single-cell activities in these nuclei. Note that, in the parkinsonian state, discrimination between the STN and GPi became less accurate while discrimination between the GPe and GPi became more accurate.
Conclusions
Using multiple feature selection methods tested against a set of neuronal discharge features from three basal ganglia regions, we found that the features that most effectively discriminate the neuron activity in the parkinsonian and normal states differ between the STN and the two segments of the globus pallidus. Although the reasons for these differences remain speculative, it is possible that the cellular responses are strongly influenced by local factors, such as the membrane properties of specific groups of cells, or by the synaptic connections between the recorded neurons and their respective afferents. Concentration on any one feature of single-cell firing (such as firing rates or oscillatory properties) to characterize the parkinsonian state in all nodes of the basal ganglia-thalamocortical network is less accurate than an analysis of multiple nucleus-specific descriptors to predict changes of activity patterns in the basal ganglia-thalamocortical network.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke grants P01 NS-038399, P50 NS-071669, and R01 NS-042250 (T. Wichmann) as well as National Center for Research Resources P51 RR-000165 (currently the Office of Research Infrastructure Programs P51OD011132) to the Yerkes National Primate Research Center. T. H. Sanders was supported through funding from Texas Instruments and the Texas Instruments Leadership University (TILU).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.H.S. analyzed data; T.H.S. and T.W. prepared figures; T.H.S. drafted manuscript; T.H.S., M.A.C., and T.W. edited and revised manuscript; M.A.C. and T.W. approved final version of manuscript; T.W. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Dr. Jesus Soares for help with some of the primate recordings and Dr. Michele Kliem for her expert technical assistance.
REFERENCES
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989 [DOI] [PubMed] [Google Scholar]
- Baron MS, Wichmann T, Ma D, DeLong MR. Effects of transient focal inactivation of the basal ganglia in parkinsonian primates. J Neurosci 22: 592–599, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, Balzamo E, Bezard E, Tison F, Ghorayeb I. Sleep disorders in Parkinson's disease: the contribution of the MPTP non-human primate model. Exp Neurol 219: 574–582, 2009 [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72: 507–520, 1994 [DOI] [PubMed] [Google Scholar]
- Breit S, Bouali-Benazzouz R, Popa RC, Gasser T, Benabid AL, Benazzouz A. Effects of 6-hydroxydopamine-induced severe or partial lesion of the nigrostriatal pathway on the neuronal activity of pallido-subthalamic network in the rat. Exp Neurol 205: 36–47, 2007 [DOI] [PubMed] [Google Scholar]
- Brotchie JM, Mitchell IJ, Sambrook MA, Crossman AR. Alleviation of parkinsonism by antagonism of excitatory amino acid transmission in the medial segment of the globus pallidus in rat and primate. Mov Disord 6: 133–138, 1991 [DOI] [PubMed] [Google Scholar]
- Brown EN, Kass RE, Mitra PP. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci 7: 456–461, 2004 [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci 21: 1033–1038, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Lin CJ. LIBSVM: a Library for Support Vector Machines. http://www.csie.ntu.edu.tw/∼cjlin/libsvm 2001
- Chen H, Zhuang P, Miao SH, Yuan G, Zhang YQ, Li JY, Li YJ. Neuronal firing in the ventrolateral thalamus of patients with Parkinson's disease differs from that with essential tremor. Chin Med J (Engl) 123: 695–701, 2010 [PubMed] [Google Scholar]
- Chen YW, Lin CJ. Combining SVMs with various feature selection strategies. In: Feature Extraction: Foundations and Applications. Berlin: Springer, 2006, p. 315–324 [Google Scholar]
- Darbin O, Soares J, Wichmann T. Nonlinear analysis of discharge patterns in monkey basal ganglia. Brain Res 1118: 84–93, 2006 [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol 34: 414–427, 1971 [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990 [DOI] [PubMed] [Google Scholar]
- Dorval AD, Kuncel AM, Birdno MJ, Turner DA, Grill WM. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol 104: 911–921, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Panjwani N, Qi RY, Grill WM. Deep brain stimulation that abolishes Parkinsonian activity in basal ganglia improves thalamic relay fidelity in a computational circuit. Conf Proc IEEE Eng Med Biol Soc 2009: 4230–4233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Russo GS, Hashimoto T, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson's disease. J Neurophysiol 100: 2807–2818, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder CM, Vitek JL. The motor thalamus: alteration of neuronal activity in the parkinsonian state. In: Basal Ganglia and Thalamus in Health and Movement Disorders, edited by Kultas-Ilinsky K, Ilinsky IA. New York: Kluwer Academic, 2001 [Google Scholar]
- Elias S, Joshua M, Goldberg JA, Heimer G, Arkadir D, Morris G, Bergman H. Statistical properties of pauses of the high-frequency discharge neurons in the external segment of the globus pallidus. J Neurosci 27: 2525–2538, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 547: 142–151, 1991 [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol 119: 1459–1474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D, Peterson DA, Anderson CW, Thaut MH. Comparison of linear, nonlinear, and feature selection methods for EEG signal classification. IEEE Trans Neural Syst Rehabil Eng 11: 141–144, 2003 [DOI] [PubMed] [Google Scholar]
- Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord 21: 1566–1577, 2006 [DOI] [PubMed] [Google Scholar]
- Gatev PG, Wichmann T. Changes in arousal alter neuronal activity in primate basal ganglia (Abstract). Soc Neurosci Abstr 29: 390.9, 2003 [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease. J Neurosci 22: 4639–4653, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guehl D, Pessiglione M, Francois C, Yelnik J, Hirsch EC, Feger J, Tremblay L. Tremor-related activity of neurons in the “motor” thalamus: changes in firing rate and pattern in the MPTP vervet model of parkinsonism. Eur J Neurosci 17: 2388–2400, 2003 [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci 30: 357–364, 2007 [DOI] [PubMed] [Google Scholar]
- Holgado AJ, Terry JR, Bogacz R. Conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. J Neurosci 30: 12340–12352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CW, Chang CC, Lin CJ.A Practical Guide to Support Vector Classification. Technical report, Department of Computer Science, National Taiwan University, 2003 [Google Scholar]
- Hutchison WD, Lozano AM, Davis K, Saint-Cyr JA, Lang AE, Dostrovsky JO. Differential neuronal activity in segments of globus pallidus in Parkinson's disease patients. Neuroreport 5: 1533–1537, 1994 [DOI] [PubMed] [Google Scholar]
- Kliem MA, Pare JF, Khan ZU, Wichmann T, Smith Y. Ultrastructural localization and function of dopamine D1-like receptors in the substantia nigra pars reticulata and the internal segment of the globus pallidus of parkinsonian monkeys. Eur J Neurosci 31: 836–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere-Roula M, Darbin O, Hutchison WD, Wichmann T, Lozano AM, Dostrovsky JO. Apomorphine reduces subthalamic neuronal entropy in parkinsonian patients. Exp Neurol 225: 455–458, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen LV. Pallidotomy for Parkinson's disease. Neurosurg Clin N Am 6: 105–112, 1995 [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 53: 926–939, 1985 [DOI] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain 125: 1196–1209, 2002a [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci 20: 7766–7775, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. Synchronized neuronal discharge in the basal ganglia of parkinsonian patients is limited to oscillatory activity. J Neurosci 22: 2855–2861, 2002b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DM, Corthesy ME, Cummins A, Oldfield EH. Reversal of experimental parkinsonism by using selective chemical ablation of the medial globus pallidus. J Neurosurg 90: 928–934, 1999 [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lang AE, Galvez-Jimenez N, Miyasaki J, Duff J, Hutchinson WD, Dostrovsky JO. Effect of GPi pallidotomy on motor function in Parkinson's disease. Lancet 346: 1383–1387, 1995 [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience 106: 313–330, 2001 [DOI] [PubMed] [Google Scholar]
- Magill PJ, Sharott A, Bevan MD, Brown P, Bolam JP. Synchronous unit activity and local field potentials evoked in the subthalamic nucleus by cortical stimulation. J Neurophysiol 92: 700–714, 2004a [DOI] [PubMed] [Google Scholar]
- Magill PJ, Sharott A, Bolam JP, Brown P. Brain state-dependency of coherent oscillatory activity in the cerebral cortex and basal ganglia of the rat. J Neurophysiol 92: 2122–2136, 2004b [DOI] [PubMed] [Google Scholar]
- Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience 96: 549–564, 2000 [DOI] [PubMed] [Google Scholar]
- Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In: The Basal Ganglia II, edited by Carpenter MB, Jayaraman A. New York: Plenum, 1987, p. 415–427 [Google Scholar]
- Miller WC, DeLong MR. Parkinsonian symptomatology. An anatomical and physiological analysis. Ann NY Acad Sci 515: 287–302, 1988 [DOI] [PubMed] [Google Scholar]
- Molnar GF, Pilliar A, Lozano AM, Dostrovsky JO. Differences in neuronal firing rates in pallidal and cerebellar receiving areas of thalamus in patients with Parkinson's disease, essential tremor, and pain. J Neurophysiol 93: 3094–3101, 2005 [DOI] [PubMed] [Google Scholar]
- Ni ZG, Gao DM, Benabid AL, Benazzouz A. Unilateral lesion of the nigrostriatal pathway induces a transient decrease of firing rate with no change in the firing pattern of neurons of the parafascicular nucleus in the rat. Neuroscience 101: 993–999, 2000 [DOI] [PubMed] [Google Scholar]
- Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J Neurophysiol 74: 1800–1805, 1995 [DOI] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS. Primary motor cortex of the parkinsonian monkey: differential effects on the spontaneous activity of pyramidal tract-type neurons. Cereb Cortex 21: 1362–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci 25: 1523–1531, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D, Kitai S. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 400: 677–682, 1999 [DOI] [PubMed] [Google Scholar]
- Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J Neurophysiol 76: 2083–2088, 1996 [DOI] [PubMed] [Google Scholar]
- Rye DB, Bliwise DL, Dihenia B, Gurecki P. FAST TRACK: daytime sleepiness in Parkinson's disease. J Sleep Res 9: 63–69, 2000 [DOI] [PubMed] [Google Scholar]
- Schneider JS, Rothblat DS. Alterations in intralaminar and motor thalamic physiology following nigrostriatal dopamine depletion. Brain Res 742: 25–33, 1996 [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of MPTP-induced parkinsonism and lesions of the external pallidal segment. J Neurosci 24: 6417–6426, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 68: 649–742, 1988 [DOI] [PubMed] [Google Scholar]
- Theodoridis S, Koutroumbas K. Pattern Recognition. New York: Academic, 2008 [Google Scholar]
- Vila M, Perier C, Feger J, Yelnik J, Faucheux B, Ruberg M, Raisman-Vozari R, Agid Y, Hirsch EC. Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed by metabolic and electrophysiological measurements. Eur J Neurosci 12: 337–344, 2000 [DOI] [PubMed] [Google Scholar]
- Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, McDonald W, Haber M, Barnhart H, Wahlay N, Triche S, Mewes K, Chockkan V, Zhang JY, DeLong MR. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol 53: 558–569, 2003 [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. I. Functional properties in intact animals. J Neurophysiol 72: 494–506, 1994 [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res 125: 397–409, 1999 [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA, DeLong MR. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp Neurol 167: 410–424, 2001 [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol 95: 2120–2133, 2006 [DOI] [PubMed] [Google Scholar]
- Yu L, Liu H. Efficient feature selection via analysis of relevance and redundancy. J Machine Learn Res 5: 1205–1224, 2004 [Google Scholar]
- Zirh TA, Lenz FA, Reich SG, Dougherty PM. Patterns of bursting occurring in thalamic cells during parkinsonian tremor. Neuroscience 83: 107–121, 1998 [DOI] [PubMed] [Google Scholar]