Abstract
Loading on the limbs has a powerful influence on locomotion. In the present study, we examined whether robotic-enhanced loading during treadmill training improved locomotor recovery in rats that were spinally transected as neonates. A robotic device applied a force on the ankle of the hindlimb while the rats performed bipedal stepping on a treadmill. The robotic force enhanced loading during the stance phase of the step cycle. One group of spinally transected rats received 4 wk of bipedal treadmill training with robotic loading while another group received 4 wk of bipedal treadmill training but without robotic loading. The two groups exhibited similar stepping performance during baseline tests of bipedal treadmill stepping. However, after 4 wk, the spinally transected rats that received bipedal treadmill training with robotic loading performed significantly more weight-bearing steps than the bipedal treadmill training only group. Bipedal treadmill training with robotic loading enhanced the ankle trajectory and ankle velocity during the step cycle. Based on immunohistochemical analyses, the expression of the presynaptic marker, synaptophysin, was significantly greater in the ventral horn of the lumbar spinal cord of the rats that received bipedal treadmill training with robotic loading. These findings suggested that robotic loading during bipedal treadmill training improved the ability of the lumbar spinal cord to generate stepping. The results have implications for the use of robotic-enhanced gait training therapies that encourage motor learning after spinal cord injury.
Keywords: synaptophysin, stepping, spinal cord injury, kinematics
load-related afferent feedback is critical for stepping after a complete spinal cord injury (SCI; Norton and Mushahwar 2010; Rossignol et al. 2008), and increasing load has a positive effect on locomotor output (Dietz et al. 2002). Robotic-enhanced loading of the foot and ankle has previously been shown to influence stepping characteristics in SCI humans. When ankle and foot loading was increased, hip moment and hip extensor EMG activity improved (Gordon et al. 2009). We have found similar results in a rodent model of SCI (Timoszyk et al. 2002). While spinally transected (ST) rats performed bipedal treadmill stepping, a robotic device applied a downward-directed force field at the ankle of the hindlimb during the stance phase (Timoszyk et al. 2002). Increasing loading in this manner improved EMG activity in extensor muscles, ankle trajectory, and dynamics during treadmill stepping. These findings suggested that applying load to the foot and ankle enhanced loading reflexes that improved stepping performance.
New evidence suggests that robotic loading of the foot and ankle could have long-term effects beyond reflexive changes in stepping. Gordon and colleagues (2010) showed that after the removal of the loading forces, aftereffects persisted for several step cycles. The implication was that brief exposure to loading was sufficient to stimulate temporary changes within the nervous system. Similar adaptations have been reported following split-treadmill belt walking (Reisman et al. 2007) and walking against resistive forces (Wu et al. 2012; Yen et al. 2012). Short-term locomotor adaptations such as these may be an essential component for motor learning. Repetitive practice of motor adaptations over time may induce permanent changes that translated into long-term improvements in stepping (Reisman et al. 2010). Therefore, integrating robotic loading into a regimen of treadmill training may improve long-term locomotor recovery. Robotic loading during training on a sliding platform has been shown to be beneficial in ST rats (Nessler et al. 2011). Whether robotic loading during treadmill training can enhance stepping function has not yet been examined.
One effect of treadmill training is to stimulate plasticity within the lumbar spinal circuits. Previous studies have shown that when SCI rats received treadmill training, the levels of the presynaptic marker, synapsin, was increased in the lumbar spinal cord of SCI rats (Beaumont et al. 2008; Hutchinson et al. 2004; Ilha et al. 2011; Ying et al. 2005). Synapses within the ventral horn in particular were enhanced based on immunohistochemical expression of the presynaptic marker, synaptophysin, and the increased expression of synaptophysin around ventral horn motor neurons was associated with improved stepping function (de Leon et al. 2011; Macias et al. 2009). Based on these findings, if robotic loading enhances the effects of treadmill training on locomotor recovery, then it is likely that synapses within the ventral horn will be impacted, but this has not yet been examined.
In the present study, we examined the effects of integrating robotic-enhanced loading during treadmill training in rats that were ST as neonates. A number of studies have already shown that treadmill training by itself improves stepping after spinal cord transection in rats (Cantoria et al. 2011; Cha et al. 2007; de Leon et al. 2011; Ichiyama et al. 2011; Kubasak et al. 2005). Here, we tested the hypothesis that imposing limb loading during treadmill training would enhance the therapeutic effects of treadmill training. ST rats were trained to perform bipedal treadmill stepping with robotic-enhanced loading. We compared locomotor recovery between ST rats that received robotic-enhanced loading and ST rats that received regular treadmill training (i.e., without the enhanced load). In addition, the expression of synaptophysin was examined in the lumbar spinal cord. Synaptophysin is a marker for synaptic plasticity and previously was shown to be increased in the lumbar spinal circuitry of ST rats following several weeks of treadmill training (de Leon et al. 2011; Macias et al. 2009). The findings suggested that bipedal treadmill training with robotic-enhanced loading improved the ability of the lumbar spinal circuitry to generate stepping. The implication is that applying novel robotic forces may facilitate motor learning and enhance the effectiveness of current body weight-supported treadmill training (BWSTT) techniques.
MATERIALS AND METHODS
Research design.
A total of 16 female Sprague-Dawley rats received a complete midthoracic spinal cord transection at 5 days of age. The spinal cords of the rat pups were transected at a midthoracic level as previously described (Cha et al. 2007). Following surgery, the pups were returned to the mothers and weaned at 21 days old. The bladders and colons of the rats were checked daily. After weaning (21 days old), baseline tests of bipedal, hindlimb stepping were performed using a robotic treadmill system (de Leon and Acosta 2006). The ST rats were distributed into two experimental training groups that were balanced according to their locomotor performance during the baseline tests. One group received daily bipedal treadmill training with robotic-enhanced loading (TT+RL; n = 8) while the other group received bipedal treadmill training without robotic-enhanced loading (TT; n = 8). All training was performed 5 days/wk for 4 wk. Stepping was retested 24 h after the last training session. All procedures with rats were carried out in accordance with National Institutes of Health guidelines, and the protocols were approved by the Institutional Animal Care and Use Committee at California State University, Los Angeles.
Robotic treadmill training and testing.
A commercially available robotic device (Rodent Robot 3000; Robomedica) was used to train and test bipedal treadmill stepping in the rats as previously described (Cha et al. 2007). Two robotic arms were attached to the ankles via a cuff made of neoprene (Fig. 1A). A third arm raised the rat's body above the treadmill, thereby controlling the amount of weight on the hindlimbs (90–85% body wt support). The robotic device was used to count the number of hindlimb steps during training (Cha et al. 2007). Briefly, a step was detected whenever the robotic arm was displaced by 1 mm in the horizontal direction. A training session was completed when the total number of steps performed by both hindlimbs was 1,000 steps. To enhance loading during stance, a force field was produced by the robotic arms. The robotic arm applied a downward vertical force to the ankle whenever the robotic arm moved backward (Fig. 1B). The magnitude of the force was proportional to the horizontal velocity, i.e., Fy = Δx, x < 0, where Fy is the vertical force and Δx is the horizontal velocity. We have found at a constant treadmill speed (8 cm/s) that the amount of force applied is equivalent to 8% body wt.
Fig. 1.
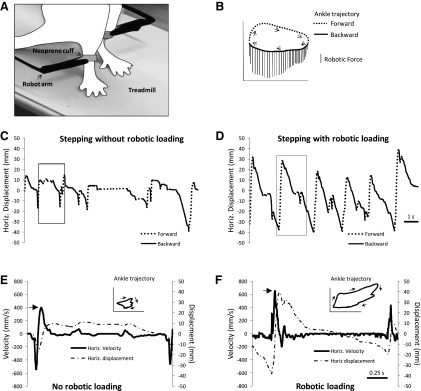
Robot arms were attached to the ankles of rats (A). The diagram of the ankle trajectory (B) shows that a downward force was applied whenever the ankle moved backward. In B, forward and backward movements of the ankle during the step cycle are indicated by the dashed and solid lines, respectively. Arrows show direction of ankle movement. Vertical lines are the downward forces applied by the robot on the ankle. The force calibration bar is 10 g. Examples of horizontal (Horiz.) movement of the ankle are shown for 1 rat stepping without robotic loading (C) and with robotic loading (D). Forward and backward movements are indicated by the dashed and solid lines, respectively. The data shown are from 12 s of training performed at week 4. Horizontal velocity and movement of the ankle during 1 step cycle is shown during stepping without robotic loading (E) and with robotic loading (F). The data shown are from step cycles indicated by the boxes in C and D. The corresponding ankle trajectory with directional arrows is shown in the top right sides of E and F.
During testing, no robotic forces were applied to the ankle. Ankle movements were recorded by the robotic device for 30 s (8 cm/s, 85% weight support). The arms of the robotic device recorded all ankle movements, and all information was stored on a computer for subsequent analysis (Cha et al. 2007). To detect stepping movements, custom-made software was used to identify toe off (TO) and paw contact (PC) based on changes in the horizontal and vertical velocities recorded by the robotic arms. Peaks in velocity that exceeded a criterion threshold value were used to identify TO and PC. The criterion threshold was based on the detection of steps in normal rats (de Leon and Acosta 2006), and the same threshold value was used for the detection analyses in both experimental groups. Using these criteria, weight-bearing steps and nonweight-bearing steps were detected, and this is referred to as the total number of steps. We then differentiated between nonweight-bearing steps and weight-bearing steps. Nonweight-bearing steps occur when the paw drags on the treadmill belt. We have found when the paw drags, small amplitude movements (i.e., <1 mm) occur in the ankle (Heng and de Leon 2009). Successful weight-bearing steps were defined as stepping movements in which the ankle moved ≥1 mm in the horizontal direction. The percentage of weight-bearing steps was calculated by dividing the number of successful weight-bearings steps by the total number of steps (×100). Step height was defined as the distance between the maximum and minimum vertical positions of the ankle during a step. Step length was defined as the horizontal distance between the TO and PC. Step area was the product of step height and length. Cycle period was the time between consecutive TO. Stance duration was the time between PC and TO. Velocity analyses was performed as previously described (Heng and de Leon 2009). Briefly, the step-cycle trajectory was divided into forward and backward phases. Peaks in horizontal and vertical velocities during forward and backward movements were measured. One-way ANOVA was used to determine significant differences in kinematic characteristics between groups, whereas within-group statistical analyses were performed via repeated-measures ANOVA (SPSS 13.0 for Windows).
Immunohistochemical experiments.
One day after the final training session, TT rats (n = 5) and TT+RL rats (n = 5) were anesthetized with isoflurane and then underwent intracardiac perfusion (4% paraformaldehyde), and the spinal cords were dissected, blocked, and cut into 30-μm sections as previously described (Tillakaratne et al. 2002). Tissue sections from all groups were processed simultaneously in a single experiment. The conditions for all procedures were identical for all of the sections in every group. For the synaptophysin labeling, the spinal cord sections (L2–L5; 3–5 sections per rat) were washed and then transferred to a blocking solution of 3% normal donkey serum (NDS). Spinal cord sections were then transferred into 96-well plates and incubated overnight with mouse synaptophysin antibody (1:300). Afterward, the spinal sections were washed, blocked in 3% NDS, and then incubated for 1 h in anti-mouse secondary antibody (FITC 1:200). The spinal cord sections were transferred to glass slides, air-dried, and coverslipped with VECTASHIELD with 4′,6′-diamidino-2-phenylindole (DAPI) staining.
Synaptophysin labeling in the ventral horn was measured as previously described. Microscopic images were acquired using an Olympus FluoView FV500 confocal laser-scanning microscope (×100 magnification). Using the Simple PCI 6 (Compix) software, an elliptical object was generated around lamina IX. The area of synaptophysin expression was measured within the object. The same object size was maintained for all rats in all groups. Images from at least five spinal cord sections per rat were analyzed. Differences in synaptophysin expression between groups were analyzed using one-way ANOVA (SPSS 13.0 for Windows).
RESULTS
Stepping characteristics during robotic loading.
We used a robotic device to apply a force field that enhanced loading during bipedal treadmill stepping. Detailed analyses of the effect of the loading on stepping in ST rats have been previously published (Timoszyk et al. 2002), and the overall effects are summarized here. The robotic device applied a downward force whenever the ankles moved backward (Fig. 1, A and B), and the magnitude of the force was proportional to horizontal ankle velocity. We previously reported that the average amount of force applied to the ankle was equivalent to 8% body wt (Timoszyk et al. 2002). Figure 1 illustrates these effects in a representative ST rat. The ankle trajectory during stepping changed when the force field was applied. For example, the ankle moved further backward during stance (compare solid lines in Fig. 1, C and D). In addition, forward movement during swing was also increased even though no force was applied to the forward-moving limb (compare dashed lines in Fig. 1, C and D). The overall effect was a larger ankle trajectory when the force field was active (see Ankle trajectory in Fig. 1, E and F). The ankle also moved faster when the force field was applied. For example, Fig. 1, E and F, shows the horizontal velocities corresponding to step cycles in Fig. 1, C and D (see boxes in Fig. 1, C and D). The peak forward velocity was greater with the force field than without the force field (see large arrows in Fig. 1, E and F).
Bipedal treadmill training with robotic loading enhanced stepping recovery.
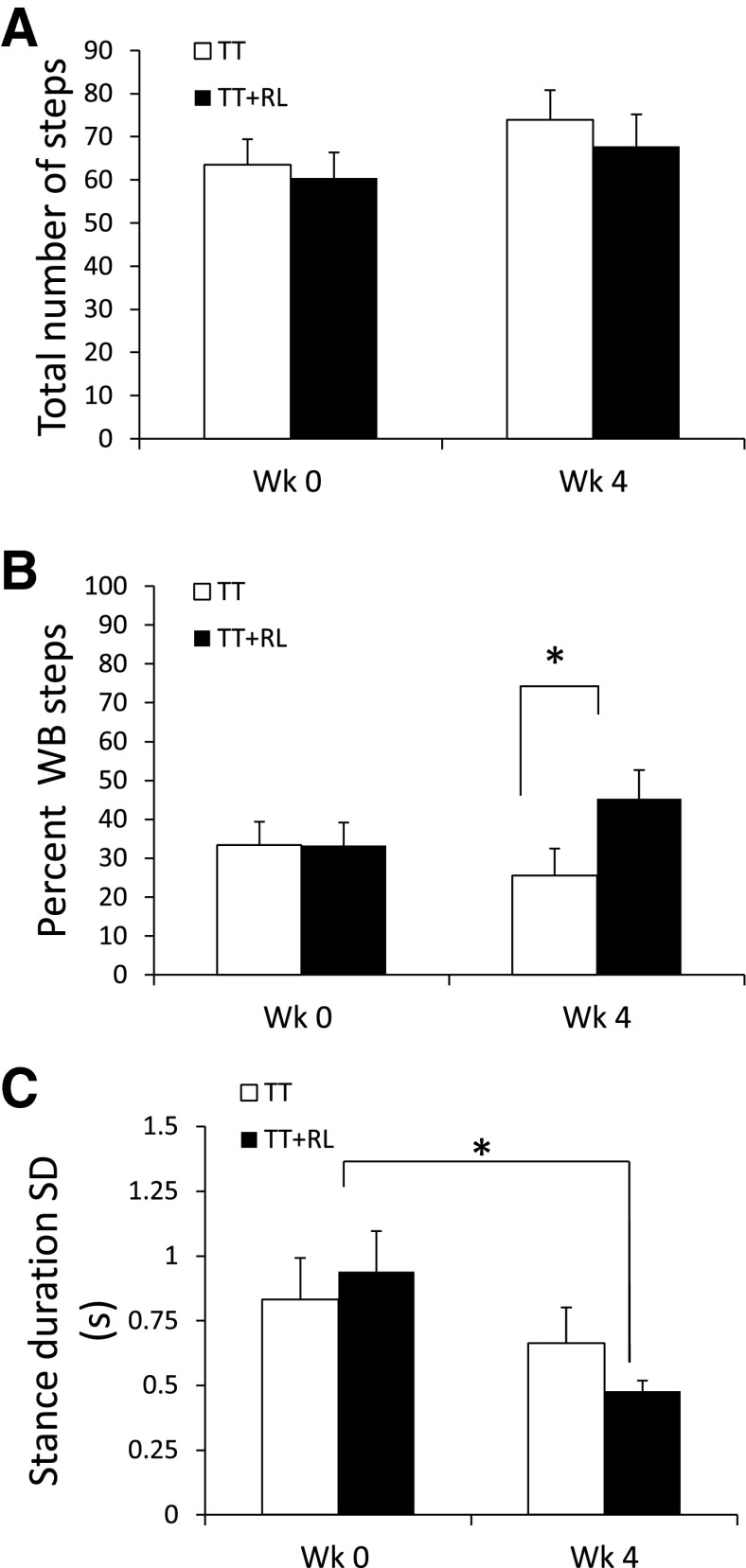
Tests of bipedal treadmill stepping were performed to compare the effect of bipedal treadmill training (1,000 steps per training session) with robotic loading (TT+RL) vs. bipedal treadmill training alone (TT). The ability to perform independent hindlimb stepping was assessed, and thus no robotic forces were applied during testing. During testing, ST rats performed stepping movements that consisted of both weight-bearing and nonweight-bearing steps (Cha et al. 2007). At week 0, the total number of stepping movements (weight-bearing + nonweight-bearing steps) and the percentage of stepping movements that were weight-bearing were similar between the groups (compare TT and TT+RL at week 0, Fig. 2, A and B). By week 4, the total number of hindlimb stepping movements was still similar between the groups (compare TT and TT+RL at week 4, Fig. 2A); however, the TT+RL group performed a greater percentage of weight-bearing steps than the TT group (compare TT and TT+RL at week 4, Fig. 2B; 1-way ANOVA, P < 0.05). No significant differences in stepping number between weeks 0 and 4 were found in either group (compare weeks 0 and 4, Fig. 2, A and B). The consistency of stepping was examined by measuring the variability in the duration of stance. Stance duration standard deviation was similar between the two groups at week 0 (compare TT and TT+RL at week 0, Fig. 2C). By week 4, stance duration standard deviation significantly decreased in the TT+RL group but not in the TT group (compare weeks 0 and 4, Fig. 2C; repeated-measures ANOVA, P < 0.05).
Fig. 2.
One group received daily bipedal treadmill training with robotic-enhanced loading (TT+RL) while the other group received bipedal treadmill training without robotic-enhanced loading (TT). The total number of stepping movements (A), the percentage of weight-bearing (WB) steps (B), and the stance duration standard deviation (C) are shown for the TT and TT+RL groups. The data are from tests of stepping performed at weeks 0 and 4. The total number of steps is all of the weight-bearing and nonweight-bearing stepping movements in the hindlimbs that were performed during 30 s of testing. Weight-bearing steps were defined as steps that were >1 mm in length. The percentage of weight-bearing steps is the number of weight-bearing steps/total number of steps. White and black bars indicate the average data for the TT and TT+RL groups, respectively. Standard errors of the average are shown (n = 8 in each group). *Significant difference between the TT and TT+RL groups (P < 0.05).
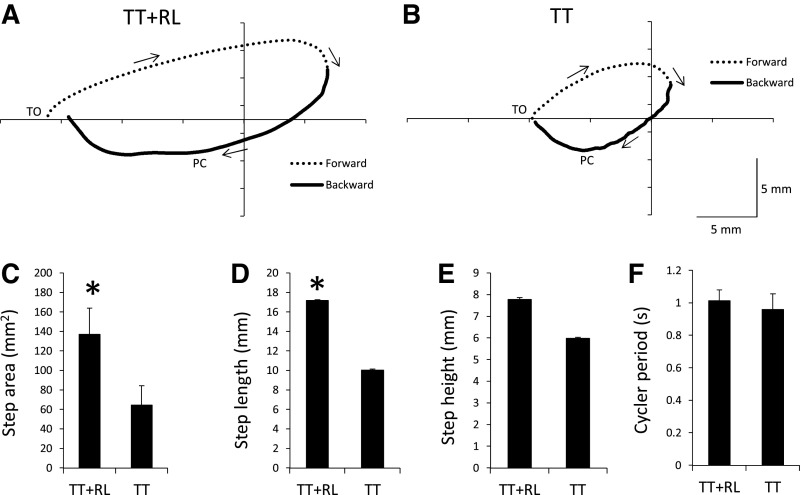
We next analyzed the step cycle characteristics in the TT+RL and TT groups from the tests performed at week 4. Figure 3 shows the average ankle trajectory during stepping in the TT+RL and TT groups. The ankle movements during the step-cycle trajectory generally followed a similar trajectory pattern in both groups (see dashed and thick lines in Fig. 3, A and B). During forward movement, the ankle was lifted and swung forward (see dashed lines in Fig. 3, A and B). The ankle then moved backward and downward until PC (see solid lines in Fig. 3, A and B). After PC, the ankle continued to move backward and was lifted toward the end of the stance phase (see solid lines in Fig. 3, A and B).
Fig. 3.
The ankle trajectory during stepping is shown for the TT+RL (A) and TT (B) groups. Data from tests performed on week 4 were used to generate the average trajectory for each group (n = 8 in each group). Forward and backward movements of the ankle during the step cycle are indicated by the dashed and solid lines, respectively. Arrows show direction of ankle movement. TO and PC indicate approximate occurrences of toe off and paw contact, respectively. Plots of step area (C), step length (D), step height (E), and cycle period (F) are shown for the TT+RL and TT groups. Mean + standard errors are shown (n = 8 in each group) and were generated from data collected during week 4 tests. *Significant difference between the TT and TT+RL groups (P < 0.05).
The average trajectory was larger in the TT+RL group than in the TT group (compare Fig. 3, A and B). For example, the area of the step cycle (i.e., length × height) was significantly greater in the TT+RL group (Fig. 3C; 1-way ANOVA, P < 0.05). The difference in area was due to a significantly longer step in the TT+RL group than in the TT group (Fig. 3D). No significant difference in step height (Fig. 3E) or in cycle period (Fig. 3F) was found between the groups. Together, these data indicated that the ankle in the TT+RL group moved through a larger trajectory than the TT group.
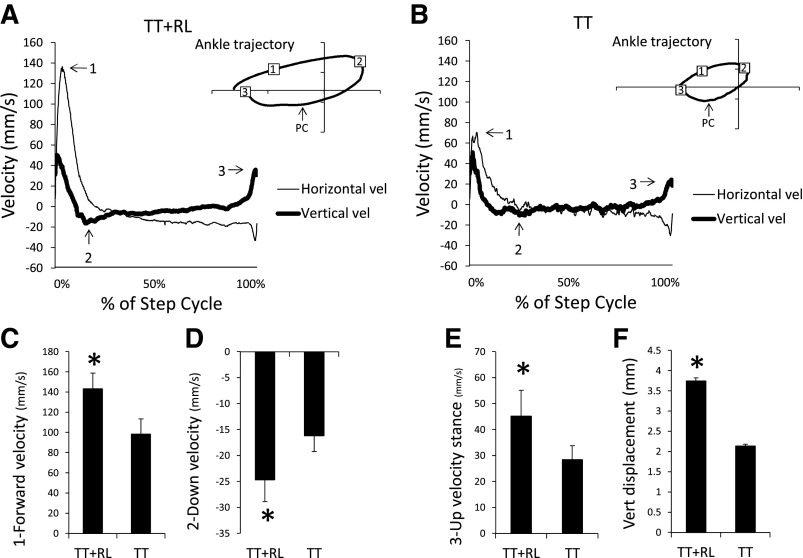
Figure 4 shows the average ankle horizontal and vertical velocities during stepping in the TT+RL and TT groups. These are normalized with respect to time and expressed as percentage of the step cycle. The ankle in the TT+RL group moved faster during swing than in the TT group. For example, there was a peak in horizontal velocity (see arrow 1 in Fig. 4, A and B) that occurred during early swing in both groups (see 1 in Ankle trajectory in Fig. 4, A and B). This peak was significantly greater in the TT+RL group than in the TT group (Fig. 4C; 1-way ANOVA, P < 0.05). A minimum in the vertical velocity (see arrow 2 in Fig. 4, A and B) occurred at the end of forward swing (see 2 in Ankle trajectory in Fig. 4, A and B). The vertical velocity during downward movement was significantly faster in the TT+RL group than in the TT group (Fig. 4D; 1-way ANOVA, P < 0.01).
Fig. 4.
The velocity of the ankle is shown for the TT+RL (A) and TT (B) groups. Horizontal and vertical velocities (vel) are indicated by the thin and thick lines, respectively. Data from tests performed on week 4 were used to generate the average trajectory for each group (n = 8 in each group). 1, 2, And 3 indicate maximum forward velocity, maximum downward velocity, and maximum upward velocity and correspond to the numbers shown in ankle trajectory. In the ankle trajectory, PC indicates approximate occurrence of PC. Plots of maximum forward velocity (C), maximum downward velocity (D), maximum upward velocity (E), and vertical (Vert) displacement during stance (F) are shown for the TT+RL and TT groups. Mean + standard errors are shown (n = 8 in each group) and were generated from data collected during week 4 tests. *Significant difference between the TT and TT+RL groups (P < 0.05).
After PC, the ankle moved backward and then rose quickly at the end of stance (see PC to 3 in Ankle trajectory in Fig. 4, A and B). At the end of stance, it reached a peak vertical velocity (see arrow 3 in Fig. 4, A and B). The peak vertical velocity was significantly greater in the TT+RL group than in the TT group (Fig. 4E; 1-way ANOVA, P < 0.05). This difference was accompanied by a significantly greater vertical displacement of the ankle in the TT+RL group from PC to the end of stance (Fig. 4F; 1-way ANOVA, P < 0.05).
Synaptophysin label in ventral horn was increased by treadmill training with robotic loading.
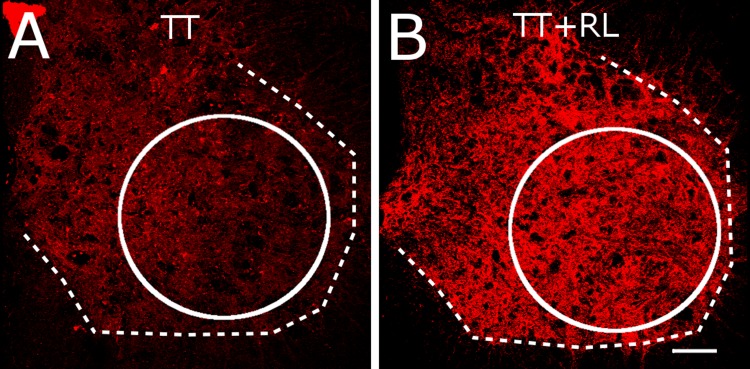
Treadmill training has been shown to increase synaptophysin expression in the ventral horn, and this is associated with improved stepping (de Leon et al. 2011; Macias et al. 2009). Figure 5 shows synaptophysin labeling in the ventral horn of the lumbar spinal cord of representative TT and TT+RL rats (Fig. 5). Greater synaptophysin labeling was found in the TT+RL rats than in the TT rats (compare Fig. 5, A and B). We performed semiquantitative analyses of the mean intensity of synaptophysin labeling in the ventral horn (see circles in Fig. 5, A and B). The mean intensity in the TT+RL rats and TT rats was 88.6 ± 2.6 and 75.6 ± 2.3 intensity units, respectively. One-way ANOVA revealed that the intensity of synaptophysin labeling was significantly different between the TT+RL and TT groups (P < 0.05).
Fig. 5.
Synaptophysin label in the ventral horns of the lumbar spinal cord in representative TT (A) and TT+RL (B) rats are shown. The dashed lines indicates approximate boundary between gray and white matter. The circle indicates the area of the ventral horn that was used in analyses. Calibration bar is 150 μm.
DISCUSSION
The influence of loading during locomotion is well-established (Duysens et al. 2000). Recent evidence indicates that load-related afferent feedback was essential for spinally generated locomotion, and when load-related afferents were eliminated, locomotion was not possible (Norton and Mushahwar 2010). The goal of the present study was to enhance load-related afferent signals during bipedal treadmill training and thereby improve the effectiveness of the training. We used robotic technology to enhance loading. We found that bipedal treadmill training with robotic loading improved stepping in ST rats relative to bipedal treadmill training alone. The TT+RL group performed more hindlimb weight-bearing steps than the TT group. Stepping was also more consistent in the TT+RL group. Moreover, based on step-cycle trajectory and dynamics, the quality of the stepping was better in the TT+RL group. Given that ∼20% of rats that are transected as neonates recover weight-bearing stepping (Giszter et al. 2008; Miya et al. 1997; Stelzner et al. 1975), we were careful to balance the TT and TT+RL groups according to baseline stepping characteristics. Finally, there was greater expression of the synaptic plasticity marker, synaptophysin, in the ventral horns of the TT+RL group. These results suggested that the therapeutic effects of bipedal treadmill training were improved by robotic-enhanced loading.
Enhanced locomotor recovery with robotic loading.
Previous studies have reported beneficial effects of robotic loading during the performance of stepping. For example, EMG activity patterns and leg movements were improved during robotic ankle-foot loading in SCI human subjects (Gordon et al. 2009). Similarly, robotic loading in ST rats improved stepping consistency and enhanced step-cycle trajectory and EMG activity (Timoszyk et al. 2002). Stepping characteristics returned to baseline levels when the robotic forces were removed, confirming that these changes were mostly reflexive responses to loading (Gordon et al. 2010; Timoszyk et al. 2002). In the present study, similar locomotor adaptations were observed when the robotic forces were applied during daily training. However, the finding that, after 4 wk of training, the adaptations were maintained during testing (i.e., when no robotic forces were applied) suggested that these beneficial effects were not merely reflex responses. These results provide the first evidence that robotic foot-ankle loading during treadmill training induced long-term changes in stepping function following SCI.
Proper control of loading on the limbs is essential for treadmill training after SCI. Specifically, there is an optimal range of weight-bearing that facilitates the generation of stepping (Harkema et al. 1997). When weight-bearing is raised beyond or falls below this range, the generation of stepping ceases. Previously, we used a robotic-controlled weight support system that gradually lowered the position of the rat (i.e., increased hindlimb loading; Timoszyk et al. 2005). Enhancing load during training in this manner did not improve stepping recovery in the ST rats. One possibility was that loading the entire hindlimb via weight support control alters leg kinematics, and this may interfere with the generation of stepping. Loading of the ankle and foot may be effective because it provides more precise control over load-related afferent stimuli without producing large changes in the kinematic characteristics of the entire hindlimb (Timoszyk et al. 2002). Our findings suggested that an additional increase of loading equivalent to 8% body wt was sufficient to produce significant gains. The effect of robotic loading at the ankle may extend to more proximal joints in the hindlimb. This possibility is supported by our previous findings that robotic ankle foot loading increased EMG activity in hip and knee extensor muscles in ST rats (Timoszyk et al. 2002). Although further studies are necessary, these findings suggest that robotic loading applied locally to the foot and ankle elicited a loading response across multiple hindlimb joints.
Another finding was that training with robotic loading had a beneficial effect on the swing phase of stepping even though robotic forces were applied only during the stance phase. We have speculated that when the robot pushed down on the ankle, the ankle plantarflexor muscles were stretched, and this may boost push-off of the paw at the end of stance (Timoszyk et al. 2002). However, the mechanical effect of loading cannot explain the observed effects on locomotor recovery since there were no robotic forces applied during the tests of stepping. Rather, the improved hindlimb movements during swing likely reflected an improved activation of the spinal networks controlling hindlimb flexion. How robotic loading could influence activity in flexor pathways is not clear. Applying a downward force caused the ankle to move further backward (Timoszyk et al. 2002 and see Fig. 1) such that swing was initiated when the hindlimb was more extended. This finding suggested training with robotic loading may alter the timing of ipsilateral swing. Other findings suggested there may be a contralateral effect. When one limb was unilaterally loaded during stance, there were adaptations in the swing phase in the contralateral limb (Timoszyk et al. 2002). Likewise, in SCI humans, loading one leg induced muscle activity in the contralateral leg (Ferris et al. 2004), and a similar effect was observed in able-bodied humans (Dietz et al. 2002). Although further studies are necessary, these findings suggested that load-related afferent signals generated in one hindlimb may be transmitted to pathways that control movement in the contralateral limb.
Motor learning and robotic forces during treadmill training.
The effect of robotic-assisted treadmill training has previously been examined in a number of studies (Swinnen et al. 2010). Although there was some evidence that stepping improved, it did not appear that robotic-assisted treadmill training was as good as therapist-assisted treadmill training. One problem was that constant robotic guidance failed to encourage motor-learning processes (Israel et al. 2006). Indeed, the nervous system appears to adapt to robotically generated movement, making it less capable of generating independent movement (Reinkensmeyer et al. 2009). The lack of variability in practicing stepping movements was also cited as a factor that may have interfered with motor learning (Ziegler et al. 2010). More recent studies have experimented with applying resistive instead of assistive forces to the legs during treadmill training (Wu et al. 2012; Yen et al. 2012). Initial findings have been promising and suggested that robotic forces may best be used in conjunction with intrinsically generated movements rather than using robots to drive leg movements.
The present study used a novel robotic algorithm to facilitate learning to step. This strategy differs from the robotic assistance that has been previously used with treadmill training. First, instead of enforcing a movement pattern in the limbs, the robotic forces were only used to stimulate loading reflexes. Given loading reflexes contribute to locomotor control, the effect of robotic forces was to enhance afferent feedback naturally generated during stance. Second, robotic forces were applied only during a portion (i.e., stance phase) of the step cycle. Thus, while one hindlimb was engaged, the contralateral hindlimb was allowed to move freely during swing. Third, a relatively simple algorithm was used. Only a downward-directed force was applied at a single point on the limb. In short, robotic-enhanced loading in the present study was a simpler way of using robotic forces to augment bipedal treadmill training. Robotic ankle-foot loading interfered less with stepping movements and thus allowed more independent control over stepping.
The mechanism by which robotic loading enhances stepping function cannot be determined by the present study. Recent evidence indicates that locomotor adaptations to perturbations may play a role in motor learning that is involved in gait rehabilitation (Reisman et al. 2010). Repetitive exposure to the perturbations may trigger long-term changes in stepping. For example, walking speed in SCI subjects was improved by applying robotic forces that resisted the forward movement of the leg during treadmill training (Wu et al. 2012; Yen et al. 2012). Ankle-foot loading perturbs stepping and elicits short-term adaptations in locomotion (Gordon et al. 2009, 2010). Thus it is feasible that repetitive exposure to ankle-foot loading during treadmill training may trigger long-term plasticity within locomotor-generating circuits.
Alternatively, the improved stepping may be a reflection of short-term adaptation to robotic loading. A persistence of the adaptation to loading could account for the enhanced stepping observed during testing in the TT+RL rats. One characteristic of locomotor adaptations is that when stepping continues without the perturbation, deadaptation occurs and baseline stepping characteristics return (Reisman et al. 2010). Previously, we found that deadaptation to a single bout of robotic loading was quite rapid and occurred within 3–7 step cycles after loading forces were removed (Timoszyk et al. 2002). In the present study, the TT+RL rats produced 60 steps on average during tests (Fig. 2A), but we cannot be certain whether this amount of steps was sufficient to “wash out” adaptation effects. It is possible that if the TT+RL rats subsequently underwent a period of training without the robotic loading, deadaptation may have been observed. Clearly, more extensive tests of deadaptation to robotic loading are necessary. In particular, it will be important to understand how the time course of deadaptation was influenced by the amount of training with robotic loading. The presence or absence of deadaptation then can help distinguish between short-term responses vs. long-term plasticity.
Loading enhances treadmill training-induced synaptic plasticity.
Based on a number of findings, synaptic plasticity in the lumbar spinal cord of ST animals is modified by treadmill training (Beaumont et al. 2008; Hutchinson et al. 2004; Ilha et al. 2011; Ying et al. 2005). Other studies have shown that synapses in the ventral horn region in particular are affected, and these changes are associated with improved stepping function (de Leon et al. 2011; Macias et al. 2009). The present findings are consistent with these results and suggest that the effect of treadmill training on ventral horn synapses was further enhanced when robotic loading was added.
The specific synapses in the lumbar spinal cord that were influenced by robotic loading cannot be determined from the present study. There is evidence that treadmill training strengthened synaptic connections onto motor neurons. For example, the number of synapses onto motor neurons in ST rats was increased by treadmill training (de Leon et al. 2011; Macias et al. 2009). Treadmill training also improved the ratio of excitatory to inhibitory inputs to motor neurons (Ichiyama et al. 2011), and this likely involved a modulation of glutamatergic and glycinergic terminals (Cantoria et al. 2011). It is well-known that load-related afferent signals influence extensor motor neuron activity during locomotion (Donelan and Pearson 2004). By applying a downward force at the ankle, robotic loading may have enhanced afferent feedback from cutaneous receptors on the paw or proprioceptors in ankle extensor muscles (Cote and Gossard 2004). Other data suggested that treadmill training enhanced activity in load-related polysynaptic pathways to extensor motor neurons (Cote et al. 2003). Thus one effect of robotic loading during training may be to increase the sensitivity of extensor motor neurons to load-related afferents.
In addition to spinal adaptations, it is possible that skeletal muscle adaptations were induced by robotic loading. Training with robotic loading has recently been shown to increase the mass of ankle extensor muscles in ST rats (Nessler et al. 2011). No effects on the mass of ankle flexor muscles were found. These findings suggested that extensor muscles may be selectively strengthened in response to training with loading.
Clinical implications.
BWSTT is a gait-training therapy that has been used to improve locomotor function after SCI (Harkema et al. 2012). Recent findings from randomized clinical trial studies have raised questions regarding the effectiveness and value of BWSTT (Dobkin and Duncan 2012). It is therefore important to continue exploring ways to augment BWSTT so as to enhance the impact on plasticity. Current robotic devices have succeeded in reducing the labor involved in treadmill training, but robots that fully assist stepping may not be optimal for the recovery of independent stepping function. The present findings suggest that using robotic devices to alter loading increases the effectiveness of treadmill training. This result is consistent with recent findings that show resistive rather than assistive forces may be a useful component in the overall rehabilitation strategy (Wu et al. 2011). Discovering the different types of robotic forces that facilitate motor learning is essential and may increase the overall value of BWSTT as a rehabilitation tool.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grant 1R01-NS-055911.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.A.S. and R.D.d.L. conception and design of research; P.A.S. and R.D.d.L. performed experiments; P.A.S. and R.D.d.L. analyzed data; P.A.S. and R.D.d.L. interpreted results of experiments; P.A.S. and R.D.d.L. prepared figures; P.A.S. and R.D.d.L. drafted manuscript; P.A.S. and R.D.d.L. edited and revised manuscript; P.A.S. and R.D.d.L. approved final version of manuscript.
REFERENCES
- Beaumont E, Kaloustian S, Rousseau G, Cormery B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci Res 62: 147–154, 2008 [DOI] [PubMed] [Google Scholar]
- Cantoria MJ, See PA, Singh H, de Leon RD. Adaptations in glutamate and glycine content within the lumbar spinal cord are associated with the generation of novel gait patterns in rats following neonatal spinal cord transection. J Neurosci 31: 18598–18605, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, de Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J Neurotrauma 24: 1000–1012, 2007 [DOI] [PubMed] [Google Scholar]
- Cote MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci 24: 11317–11327, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Menard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J Neurosci 23: 2789–2796, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Acosta CA. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J Neurotrauma 23: 1147–1163, 2006 [DOI] [PubMed] [Google Scholar]
- de Leon RD, See PA, Chow CH. Differential effects of low versus high amounts of weight supported treadmill training in spinally transected rats. J Neurotrauma 28: 1021–1033, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain 125: 2626–2634, 2002 [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil Neural Repair 26: 308–317, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of sensory feedback to ongoing ankle extensor activity during the stance phase of walking. Can J Physiol Pharmacol 82: 589–598, 2004 [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133, 2000 [DOI] [PubMed] [Google Scholar]
- Ferris DP, Gordon KE, Beres-Jones JA, Harkema SJ. Muscle activation during unilateral stepping occurs in the nonstepping limb of humans with clinically complete spinal cord injury. Spinal Cord 42: 14–23, 2004 [DOI] [PubMed] [Google Scholar]
- Giszter S, Davies MR, Ramakrishnan A, Udoekwere UI, Kargo WJ. Trunk sensorimotor cortex is essential for autonomous weight-supported locomotion in adult rats spinalized as P1/P2 neonates. J Neurophysiol 100: 839–851, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KE, Wu M, Kahn JH, Dhaher YY, Schmit BD. Ankle load modulates hip kinetics and EMG during human locomotion. J Neurophysiol 101: 2062–2076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KE, Wu M, Kahn JH, Schmit BD. Feedback and feedforward locomotor adaptations to ankle-foot load in people with incomplete spinal cord injury. J Neurophysiol 104: 1325–1338, 2010 [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 77: 797–811, 1997 [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil 93: 1508–1517, 2012 [DOI] [PubMed] [Google Scholar]
- Heng C, de Leon RD. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp Neurol 216: 139–147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 127: 1403–1414, 2004 [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Broman J, Roy RR, Zhong H, Edgerton VR, Havton LA. Locomotor training maintains normal inhibitory influence on both alpha- and gamma-motoneurons after neonatal spinal cord transection. J Neurosci 31: 26–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilha J, Centenaro LA, Broetto Cunha N, de Souza DF, Jaeger M, do Nascimento PS, Kolling J, Ben J, Marcuzzo S, Wyse AT, Gottfried C, Achaval M. The beneficial effects of treadmill step training on activity-dependent synaptic and cellular plasticity markers after complete spinal cord injury. Neurochem Res 36: 1046–1055, 2011 [DOI] [PubMed] [Google Scholar]
- Israel JF, Campbell DD, Kahn JH, Hornby TG. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys Ther 86: 1466–1478, 2006 [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Hedlund E, Roy RR, Carpenter EM, Edgerton VR, Phelps PE. L1 CAM expression is increased surrounding the lesion site in rats with complete spinal cord transection as neonates. Exp Neurol 194: 363–375, 2005 [DOI] [PubMed] [Google Scholar]
- Macias M, Nowicka D, Czupryn A, Sulejczak D, Skup M, Skangiel-Kramska J, Czarkowska-Bauch J. Exercise-induced motor improvement after complete spinal cord transection and its relation to expression of brain-derived neurotrophic factor and presynaptic markers. BMC Neurosci 10: 144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya D, Giszter S, Mori F, Adipudi V, Tessler A, Murray M. Fetal transplants alter the development of function after spinal cord transection in newborn rats. J Neurosci 17: 4856–4872, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessler JA, Moustafa-Bayoumi M, Soto D, Duhon JE, Schmitt R. Robot applied stance loading increases hindlimb muscle mass and stepping kinetics in a rat model of spinal cord injury. Conf Proc IEEE Eng Med Biol Soc 2011: 4145–4148, 2011 [DOI] [PubMed] [Google Scholar]
- Norton JA, Mushahwar VK. Afferent inputs to mid- and lower-lumbar spinal segments are necessary for stepping in spinal cats. Ann NY Acad Sci 1198: 10–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Akoner O, Ferris DP, Gordon KE. Slacking by the human motor system: computational models and implications for robotic orthoses. Conf Proc IEEE Eng Med Biol Soc 2009: 2129–2132, 2009 [DOI] [PubMed] [Google Scholar]
- Reisman DS, Bastian AJ, Morton SM. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Phys Ther 90: 187–195, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130: 1861–1872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Barriere G, Frigon A, Barthelemy D, Bouyer L, Provencher J, Leblond H, Bernard G. Plasticity of locomotor sensorimotor interactions after peripheral and/or spinal lesions. Brain Res Rev 57: 228–240, 2008 [DOI] [PubMed] [Google Scholar]
- Stelzner DJ, Ershler WB, Weber ED. Effects of spinal transection in neonatal and weanling rats: survival of function. Exp Neurol 46: 156–177, 1975 [DOI] [PubMed] [Google Scholar]
- Swinnen E, Duerinck S, Baeyens JP, Meeusen R, Kerckhofs E. Effectiveness of robot-assisted gait training in persons with spinal cord injury: a systematic review. J Rehabil Med 42: 520–526, 2010 [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci 22: 3130–3143, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoszyk WK, De Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J Neurophysiol 88: 3108–3117, 2002 [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkensmeyer DJ, de Leon R. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res 1050: 180–189, 2005 [DOI] [PubMed] [Google Scholar]
- Wu M, Hornby TG, Landry JM, Roth H, Schmit BD. A cable-driven locomotor training system for restoration of gait in human SCI. Gait Posture 33: 256–260, 2011 [DOI] [PubMed] [Google Scholar]
- Wu M, Landry JM, Schmit BD, Hornby TG, Yen SC. Robotic resistance treadmill training improves locomotor function in human spinal cord injury: a pilot study. Arch Phys Med Rehabil 93: 782–789, 2012 [DOI] [PubMed] [Google Scholar]
- Yen SC, Schmit BD, Landry JM, Roth H, Wu M. Locomotor adaptation to resistance during treadmill training transfers to overground walking in human SCI. Exp Brain Res 216: 473–482, 2012 [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol 193: 411–419, 2005 [DOI] [PubMed] [Google Scholar]
- Ziegler MD, Zhong H, Roy RR, Edgerton VR. Why variability facilitates spinal learning. J Neurosci 30: 10720–10726, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]