Abstract
The neuropeptide pigment-dispersing factor (PDF) has been studied extensively in Drosophila, and its role in circadian time-keeping has been firmly established. The role of PDF outside of the clock circuit, however, is poorly understood. A recent study suggested that PDF may act on the ellipsoid body (EB) to link the clock and sleep/activity circuits. We performed whole brain optical imaging with the fluorescence resonance energy transfer (FRET)-based cAMP sensor Epac1-camps expressed under control of the pdfR promoter to address how the clock and sleep deprivation affect the physiology of these cells. Basal cAMP levels in EB were regulated both by PDF and synaptic inputs that are controlled by the circadian clock. Acute application of PDF to the brain caused a significant, and PDF-receptor-dependent, increase in cAMP in EB cells. Application of TTX to block circuit-mediated effects of PDF increased the morning response but not the response at night, implying the existence of a temporally regulated, PDF-stimulated input that blocks cAMP generation. ACh produced both direct (TTX-insensitive) and indirect (TTX-sensitive) increases in cAMP during the day but was totally TTX-insensitive at night, indicating that ACh-stimulated inputs to the EB are suppressed at night. Sleep deprivation did not affect the cAMP responses of these cells to either PDF or ACh. These results suggest a novel role for PDF as a modulator of activity outside of the clock circuit. By elucidating the mechanisms by which the neuropeptide PDF act on its target cells, our work contributes to our understating of how the central clock coordinates activity and sleep.
Keywords: Drosophila, cAMP imaging, sleep, neuropeptide PDF, ellipsoid body, circadian clock
the mechanisms by which daily cycles of activity are regulated have been widely studied, and much is known about the molecular nature of clocks both in mammals as well as in invertebrates. In contrast to the mammalian central clock, the circadian network in Drosophila is relatively simple, being composed of ∼200 neurons (Helfrich-Forster 2003; Kaneko and Hall 2000). These neurons are organized in clusters and are named according to their morphological location in the brain (Helfrich-Forster 2003). One of these clusters, the ventrolateral neurons (LNvs), is critical for controlling sleep and arousal as well as many other aspects of circadian timing (Chung et al. 2009; Helfrich-Forster 1998; Parisky et al. 2008; Renn et al. 1999; Shang et al. 2008; Sheeba et al. 2008b; Yoshii et al. 2009). At the molecular level, one of the most important players in the regulation of circadian function in the Drosophila brain is the neuropeptide pigment-dispersing factor (PDF), which in the adult fly brain is expressed by both the small and large groups of LNvs. This peptide is critical for morning activity and maintenance of daily rhythms in constant conditions (Renn et al. 1999). It has also been shown to regulate the total amount of sleep (Lear et al. 2009; Parisky et al. 2008; Sheeba et al. 2008a; Yoshii et al. 2009). Loss of PDF peptide or its receptor leads to a number of circadian locomotor behavioral problems (Hyun et al. 2005; Lear et al. 2005; Mertens et al. 2005; Renn et al. 1999).
The receptor for PDF is widely expressed in the circadian network, not only in the PDF+ LNvs, but also in cells from other clusters such as the LNd and DN1 groups (Helfrich-Forster 1995; Hyun et al. 2005; Im and Taghert 2010; Lear et al. 2005; Shafer et al. 2008). The PDF receptor is encoded by a single gene (pdfR) and is part of the family of G protein-coupled receptors (Hyun et al. 2005; Lear et al. 2005; Mertens et al. 2005). In cells responsible for morning behavior, PDF signals trough the adenylate cyclase isoform AC3 (Duvall and Taghert 2012). Studies in transfected cells have suggested that this receptor can also cause increases in intracellular calcium as well as cAMP (Hyun et al. 2005; Mertens et al. 2005), although this has not been tested in vivo.
Although the importance of the role of PDF in the synchronization of the different clusters of clock neurons has been established (Lin et al. 2004; Yoshii et al. 2009), very little is known about the role of PDF and its receptor outside of the clock circuit. Parisky et al. (2008) showed that the pdfR promoter was capable of driving expression outside of the clock circuit in a structure called the ellipsoid body (EB), suggesting that this brain area might be responsive to PDF and be part of the output circuitry of the circadian clock. The EB is part of the central complex, a structure in the Drosophila brain that has been suggested to be a locomotor center (Strauss 2002; Strauss and Heisenberg 1993). By studying mutations with altered central complex morphology, it was shown that this structure is involved in the control of many behaviors, including walking activity, walking speed, and leg coordination (Strauss and Heisenberg 1993). With this in mind, we asked what role the PDF receptor has in the EB and how responses to PDF, which synchronizes the most relevant pacemakers in the fly brain, change with circadian time and sleep deprivation (SD). Given the fact that PDF is a wake-promoting peptide, we might expect that altering the behavioral state of the animals by SD would represent a big enough disturbance to modify the responsiveness to this peptide. We addressed these issues using live imaging as our main experimental approach, since it allowed us to record simultaneous responses of a population of neurons. Here, we show for the first time that the PDF receptor is functional outside of the clock circuit, and we demonstrate that the PDF peptide is capable of activating its receptor on the EB neuropil, causing clear increases in levels of cAMP but not calcium. Dissecting the mechanism by which PDF acts on downstream neurons could shed light on the flow of information between circadian and locomotor circuits.
MATERIALS AND METHODS
Fly stocks.
Flies were raised in a 12:12-h light-dark cycle at 25°C. Flies expressing the pdfR-Gal4 (Parisky et al. 2008) and flies expressing the UAS-Epac1-cAMPs (50AII; Nikolaev et al. 2004; Shafer et al. 2008) sensor were crossed to get the experimental lines used (pdfR-GAL4>EPAC). Male flies were collected the day they eclosed, housed individually, and entrained for ∼5 days at 25°C before imaging. At time of imaging, the flies ranged from 5 to 10 days in age. For experiments in Fig. 2, the calcium-sensitive reporter GCaMP3 (Tian et al. 2009) was used in combination with the pdfR-Gal4 line. pdfRhan5304 flies were obtained from Paul H. Taghert (Washington University in St. Louis), UAS-Epac1-cAMPs (50AII) flies were obtained from Orie T. Shafer (University of Michigan), and UAS-GCaMP3 flies were obtained from the Janelia Fly Core Facility (Howard Hughes Medical Institute, Janelia Farm).
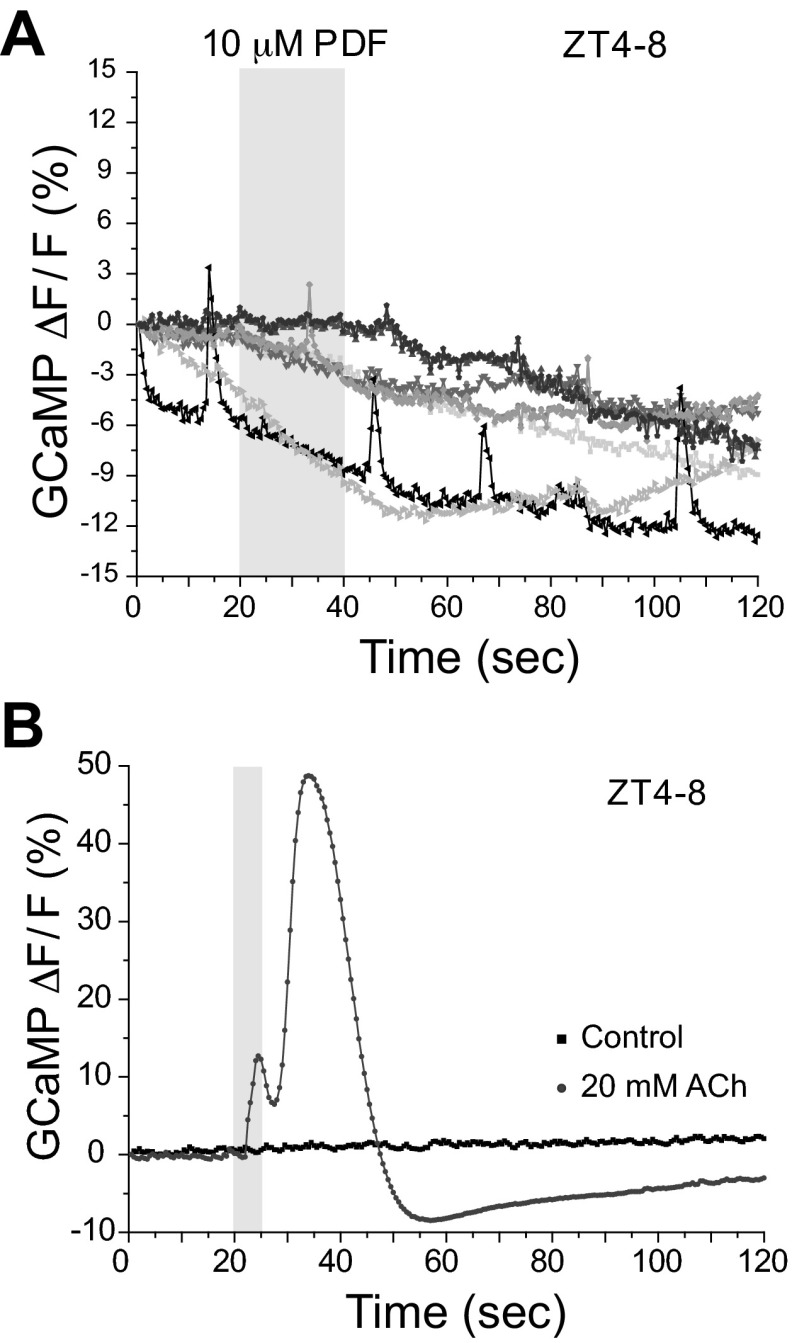
Fig. 2.
PDF stimulation does not elicit changes in calcium levels. A: relative calcium change produced by 10 μM PDF as assessed by GCaMP3 expressed under control of pdfR-GAL4. Each line represents a different animal. No significant increase in calcium was seen, although occasional spontaneous synaptic transients can be seen. Light gray box on figure represents the duration of stimulation. F, fluorescence. B: relative calcium change produced by a short pulse of 20 mM ACh (light gray trace) or vehicle control (black trace). Light gray box on figure represents the duration of stimulation.
Brain imaging and data analysis.
Imaging experiments were performed using a naked brain preparation. Briefly, whole brains were dissected in ice-cold artificial hemolymph-like (AHL) as previously described (Shang et al. 2011; Wang et al. 2003) and placed on a perfusion chamber (Harvard Apparatus) immediately before the experiments. Brains were allowed to recover in the perfusion chamber for a few minutes before the recording started while the preparation was under constant perfusion with AHL Ringers flowing by gravity feed at approximately 3–4 ml/min. AHL contained 5 mM HEPES, 4 mM NaHCO3, 108 mM NaCl, 5 mM KCl, 2 mM CaCl2, 8.2 mM MgCl2, 1 mM NaH2PO4, 5 mM trehalose, and 10 mM sucrose (based on Wang et al. 2003). All experiments were performed using an Olympus BX51WI microscope and a ×40 water immersion lens (Olympus LUMPlanFl), and all recordings were done using a charge-coupled device camera (Hamamatsu ORCA C472-80-12AG). The exchange protein directly activated by cAMP (EPAC) sensor expressed in the EB neuropil was excited with 45-ms pulses of light and emitted light from both channels [cyan and yellow fluorescent proteins (CFP and YFP)] was concomitantly collected by means of a splitter (Optical Insights). The filters used were the following: excitation, 86002v1 JP4 filter (436; Chroma Technology); and emission, D480/30m and D535/40m (Optical Insights). To avoid light-induced effects, a 25% Neutral Density Filter (Chroma Technology) was used to reduce light intensity. Fluorescent signals were imaged at a 1-Hz frequency for 240 s. Volocity software (PerkinElmer) was used for acquisition. Under these conditions, we determined that the baseline fluorescent signal in these cells stabilized after preexposing the brains to light for a period of 5 min. Imaging was performed at different times of the day in animals entrained to a 12:12-h light-dark cycle; throughout the paper, those times are expressed using their zeitgeber time (ZT) with lights on at ZT0. Drugs were added to the bath by means of a three-way valve solenoid (Cole Parmer) that was manually controlled. The baseline images were collected for 30 s before applying any drug to the brain. The fluorescence resonance energy transfer (FRET) signal (YFP/CFP ratio) for each time point was calculated and normalized to the ratio of the first time point before drug application. The relative cAMP changes were determined by plotting the normalized CFP/YFP ratio (percentage) over time. We also determined the average fluorescence change (area under the relative cAMP change curve) by calculating an average CFP/YFP ratio increase from 30 to 110 s. This calculation was done by first adding all of the time points within the defined time interval, dividing by the number of time points, and then subtracting from 100 (adapted from Shang et al. 2011).
For experiments in Fig. 2, we used the calcium-sensitive reporter GCaMP3 (Tian et al. 2009), and for these experiments different filter sets and recording conditions were used. We used a ×60 (0.9-numerical aperture) water-immersion lens (Olympus LUMPlanFl) and the following filter set (Chroma Technology): exciter, HQ470/×40; dichroic, Q495LP; emitter, HQ525/50m; and data were acquired using μManager software (Edelstein et al. 2010) at 2 Hz with 50-ms exposure and 4× binning. For the calcium-imaging experiments, we calculated the change in fluorescence using the following formula: ΔF/F = (Fn − F0)/F0 × 100%, where Fn is the fluorescence at time point n, and F0 is the fluorescence at time 0.
For all experiments, data were analyzed offline using custom software written in ImageJ (National Institutes of Health), MATLAB (The MathWorks), and Excel (Microsoft). All statistical analyses were performed using JMP (SAS Software) and InfoStat [Grupo InfoStat, Facultad de Ciencias, Universidad Nacional de Córdoba (FCA, UNC)]. In the cases in which there was significance in the ANOVA, a post hoc Tukey honestly significant difference test was used to determine the significance among the different means. Results are expressed as means ± SE unless otherwise stated. Numbers in parentheses represent the number of preparations for each condition, and different letters represent different significance groups.
SD experiments.
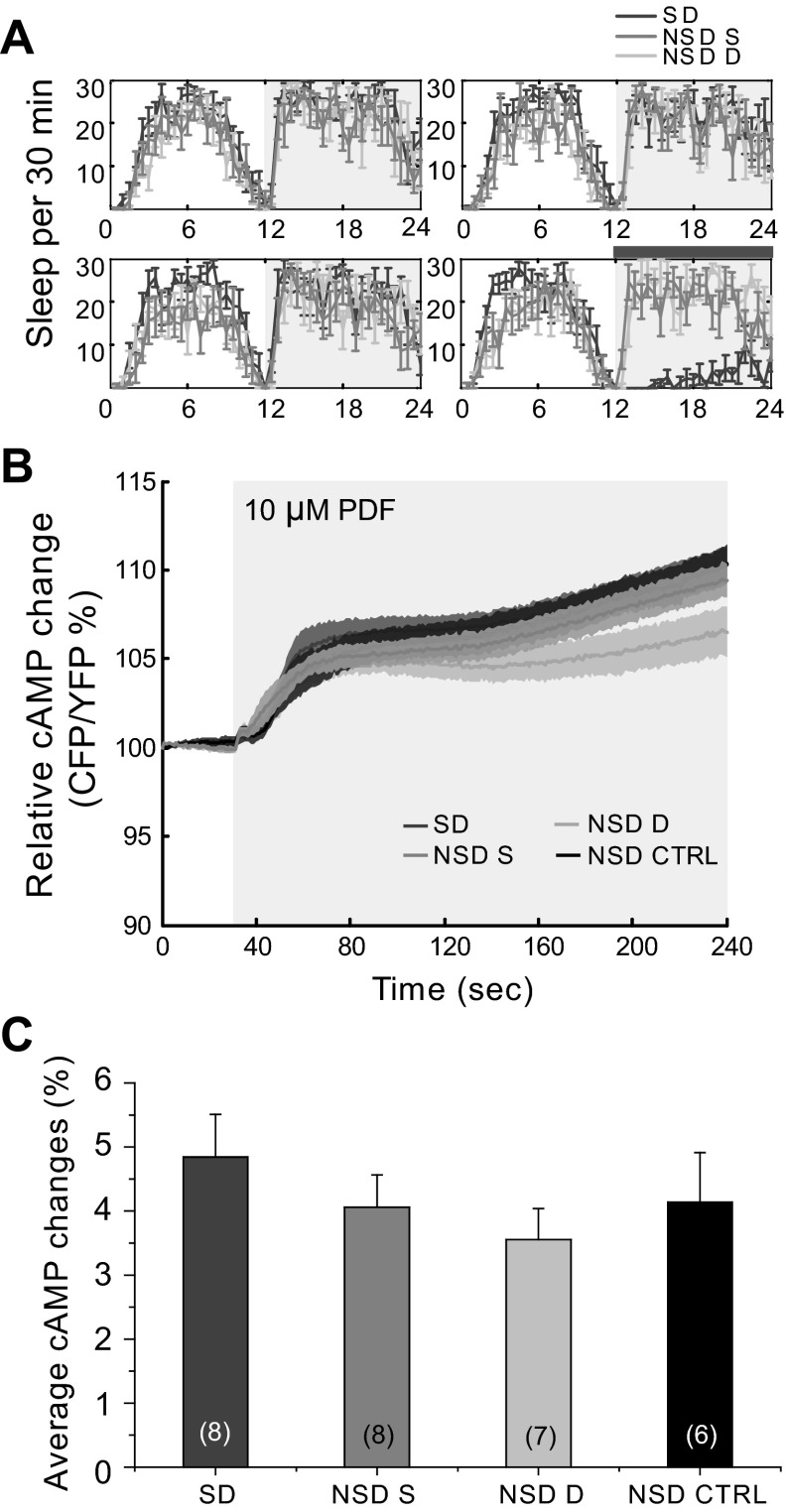
For the SD experiments, 2- to 4-day-old flies were housed separately in 65- × 5-mm glass behavior tubes (TriKinetics) containing 5% agarose with 2% sucrose. Flies were acclimated in the tubes for at least 24 h at 25°C in 12:12-h light-dark conditions before data collection. Flies were then entrained for 3 days in 12:12-h light-dark before performing the SD during the last 12-h dark period. Locomotor activity was collected with Drosophila Activity Monitoring System monitors (TriKinetics) in 1-min bins as previously described (Agosto et al. 2008). Sleep was defined as at least 5 continuous minutes of inactivity. SD was achieved by using a modified shaker (VWR; modified by TriKinetics) that shakes the flies for 2 s of every 10 s for 12 h. Using custom-written software (Donelson et al. 2012), we verified the sleep/wake state of individual flies used in the imaging studies. Groups were: SD; NSD same, control flies that were not in the SD machine but were in the same shelf of the incubator; NSD different, a second control group of flies that were placed on a different shelf within the same incubator; and NSD control, flies that were not in the same incubator as the previous three groups and were imaged in an independent session. Immediately after the end of the SD period, flies were removed from the incubator, dissected, and imaged as described above. In a separate experiment, flies were kept for 4 h in the incubator after the end of the SD to test for the effect of sleep recovery on the physiological responses of these cells.
Pharmacology.
To stimulate the preparations, we used commercially synthesized PDF peptide (H-NSELINSLLSLPKNMNDA-NH2; PolyPeptide Group). PDF was stored as powder at room temperature and then dissolved in DMSO at 20 mM. Aliquots were desiccated using a SpeedVac (Savant) and stored at −20°C. Other pharmacological agents, such as ACh, forskolin, and TTX (all from Sigma-Aldrich), were used for different experiments. In all cases, solutions were made fresh the day of the experiment. When TTX was added to the bath, it was added to the perfusion chamber as soon as the brains were placed there. This means that brains were preincubated in TTX for a period of 5 min while the brain was preexposed to light to control bleaching. A relevant side note is that we experienced an important quantitative (but not qualitative) difference in the biological activity of different PDF batches (data not shown). We believe this is important enough to be pointed out, mainly due to the fact that this difference in biological activity was such that prevented us from comparing data collected with different batches. For all of the experiments shown here, we used only one batch of PDF peptide, which was not the one that gave the highest biological activity but the one that allowed us to perform all of the experiments we had planned. When we stimulated wild-type fly brains with 10 μM PDF of the batch with the highest biological activity, the averaged cAMP change was 8.43 ± 0.96 (n = 15, data not shown). Doing the same experiment with the PDF batch that was used for all of the experiments presented here, we obtained an averaged cAMP change as follows: 3.03 ± 0.41 (n = 8; see Fig. 1B). Reversal of PDF responses with washout was not seen in the time window of our experiments, and further investigation of washout suggested that this peptide is very difficult to remove from tissue, being incomplete even after 10 min (N. Pírez and C. G. Vecsey, unpublished observations).
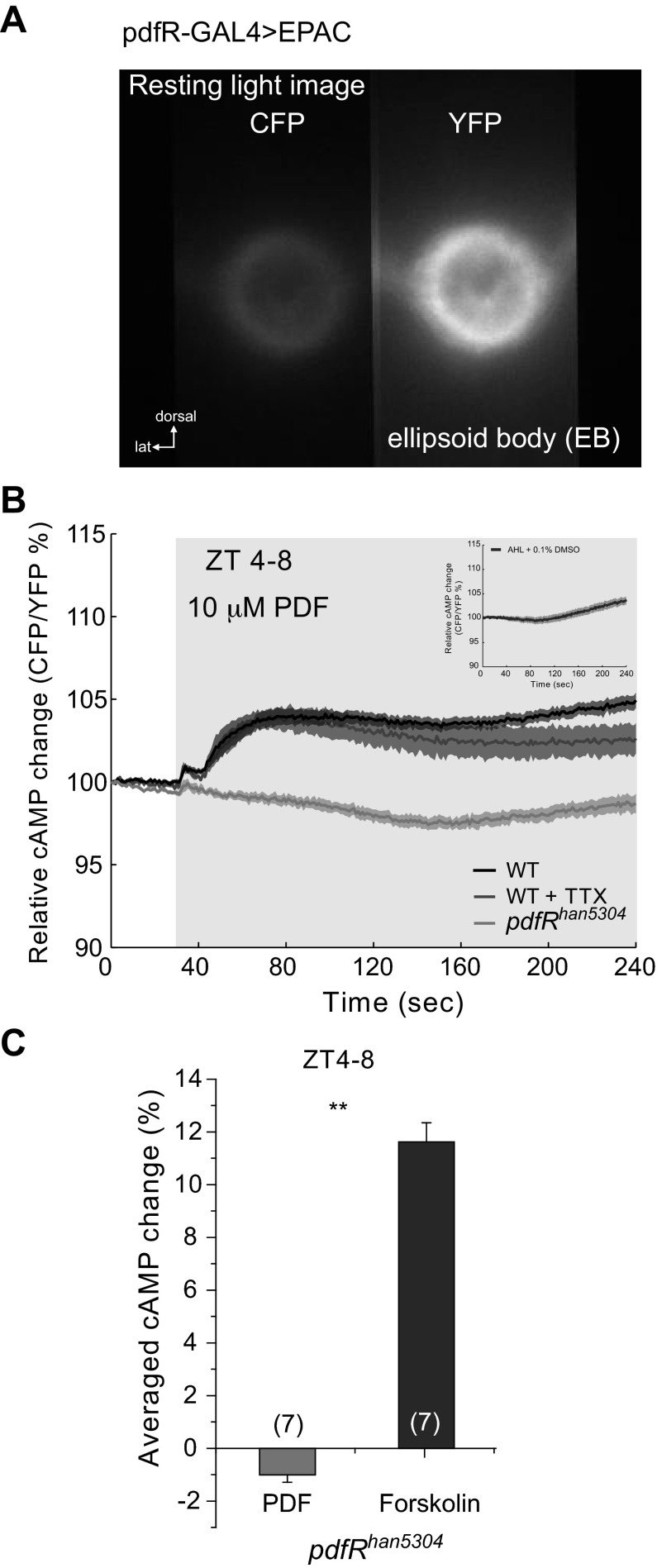
Fig. 1.
Pigment-dispersing factor (PDF) increases cAMP levels in ellipsoid body (EB). A: resting light image showing the expression of the UAS-Epac1-cAMPs (50AII) driven by pdfR-Gal4 in the EB. Left channel shows the cyan fluorescent protein (CFP) emission, and right channel shows the yellow fluorescent protein (YFP) emission, as is seen by using a splitter. EPAC, exchange protein directly activated by cAMP; lat, lateral. B: relative cAMP change, measured as the CFP/YFP percentage change. Average responses of EB neuropil of wild-type (WT) flies to the perfusion of 10 μM PDF (black trace) and 10 μM PDF + 1 μM TTX (dark gray trace). When pdfRhan5304 mutant flies were perfused with 10 μM PDF, no response was observed (light gray trace). Light gray box on figure represents the duration of stimulation. See text for statistical analysis. Inset: figure shows the response to the vehicle [artificial hemolymph-like (AHL) + 0.1% DMSO]. ZT, zeitgeber time. C: average data using the area under the curve of response of pdfRhan5304 mutants to 10 μM PDF as shown in B. As a control, 10 μM forskolin was perfused onto pdfRhan5304 brains. Numbers in parentheses represent the number of preparations for each condition (1-way ANOVA, **P < 0.0001). Data are expressed as means ± SE.
RESULTS
EB neurons express a functional PDF receptor.
It is unknown how information from the circadian clock regulates locomotor circuits. It has been recently shown that a pdfR-Gal4 line, made using sequences upstream of the pdfR gene, drives expression in cells that form the EB (Parisky et al. 2008). Figure 1A shows the expression of UAS-Epac1-camps (50AII) under the control of pdfR-Gal4. This FRET sensor was previously shown to report changes in cAMP concentration efficiently following stimulation of clock cells in Drosophila (Lelito and Shafer 2012; Shafer et al. 2008; Shang et al. 2011; Yao et al. 2012). Here, we used this tool to study the role of PDF receptor in the EB. When 10 μM PDF is perfused onto the brains of flies at midday (ZT4–8), we observed a significant increase in cAMP levels (measured as CFP/YFP; 1-way ANOVA, P < 0.0001; Fig. 1B). Adding 1 μM TTX to the bath did not significantly alter the response to PDF (Tukey test: α = 0.05, Q = 2.58033), suggesting that the effect seen on cAMP levels is mainly due to direct stimulation of the PDF receptor in these cells and not from activation of other neurons in the circuit that signal to EB. Additionally, this indicates that the PDF receptor expressed by these EB cells is not only functional, but also responds with high affinity to its known ligand, the neuropeptide PDF.
To test whether this response is truly mediated by the PDF receptor, we tested PDF responses in the pdfRhan5304 mutant. These flies have a large deletion that removes all of the transmembrane domains and the COOH terminus of the PDFR protein, rendering the receptor inactive (Hyun et al. 2005; Im and Taghert 2010; Mertens et al. 2005). When PDF was perfused onto mutant brains, we found that it elicited no response (Fig. 1, B and C). To determine whether the lack of response in these mutant flies is due to the absence of functional PDF receptors or the lack of a functional second messenger cascade, we perfused the pdfRhan5304 mutant brains with 10 μM forskolin, an adenylyl cyclase activator that is commonly used to raise intracellular levels of cAMP. As expected, these brains showed a significant FRET response to this drug (Fig. 1C; 1-way ANOVA, P < 0.0001), confirming that the lack of response to PDF in the mutants is due not to dysfunctional second messenger machinery but the lack of PDF receptor. As a control for these experiments, we perfused vehicle control (AHL + 0.1% DMSO) onto wild-type brains and found that this stimulation elicited no significant changes in the cAMP levels (Fig. 1B, inset). When we compared this response to the response elicited by other stimulation paradigms, we found that the amplitude of the signal elicited by vehicle control was significantly smaller than the amplitude of the response elicited by PDF stimulation onto wild-type brains but not different from the response observed in Han5304 mutants to a PDF stimulation (Tukey test: α = 0.05, Q = 2.88432).
It has been suggested that the PDF receptor may signal both through calcium and cAMP pathways, although the specific components of this calcium signaling cascade are not known (Hyun et al. 2005; Mertens et al. 2005), and this has not been tested in vivo. To determine whether PDF could cause changes in calcium levels in the EB, we performed experiments similar to those in Fig. 1B but using flies expressing the calcium sensor UAS-GCaMP3 under the control of pdfR-Gal4. PDF application did not elicit any significant changes in calcium levels (Fig. 2A), although we observed spontaneous synaptic responses, confirming the health of the preparations and our capability to record calcium transients with our imaging setup. The lack of change in calcium levels following PDF stimulation implies that, in the EB, the PDF receptor signals preferentially through the cAMP pathway. As a positive control to show that EB could generate calcium responses following stimulation, we perfused brains with 20 mM ACh to stimulate the EB. Figure 2B shows an example recording. Here, we can clearly see that a brief application of ACh (5 s) is capable of eliciting significant calcium transients in the EB neuropil significantly greater than in the vehicle control.
Responses to PDF early in the morning are different from late at night.
To determine whether there is a daily regulation of the response to PDF in the EB, we also dissected brains early in the morning (ZT0–3) and in the middle of the night (ZT16–20) with flies under the same circadian entrainment as described above in addition to the ZT4–8 time point. We found that the response dynamics to a 10 μM PDF stimulation were similar across time points (Fig. 3, A and B). Nevertheless, when we compared the amplitude of the responses measured by the area under the curve, we found that responses were significantly higher in the early morning time points when compared with the rest of the time points during the day (2-way ANOVA, P = 0.0029; ZT effect, P = 0.0015; letters in Fig. 3C refer to time-of-day statistical groups).
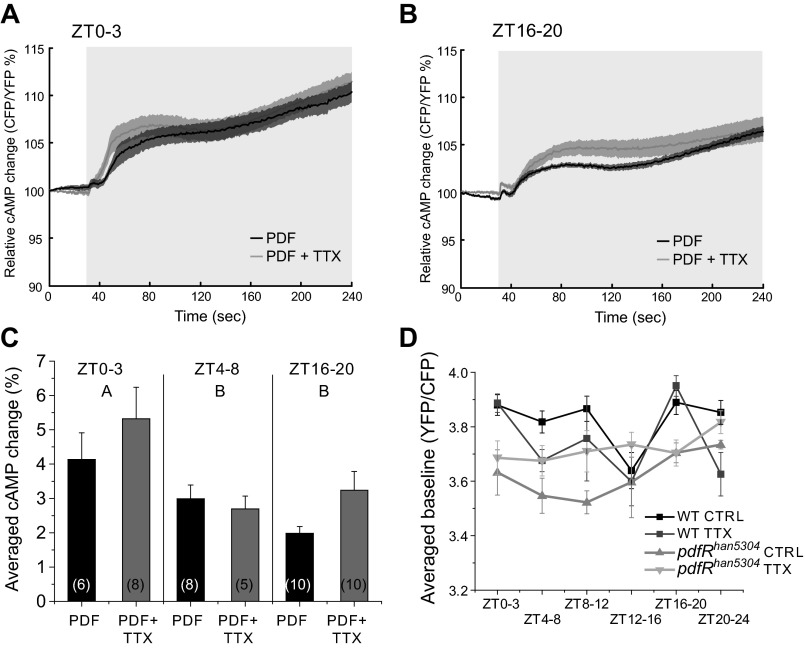
Fig. 3.
Responses to PDF stimulation are higher early in the day. A and B: PDF perfusion elicited clear cAMP responses with and without 1 μM TTX early in the morning (ZT0–3; A) and late at night (ZT16–20; B). Light gray box on figure represents the duration of stimulation. C: summary bar graph from data of all of the conditions on A as well as WT data from Fig. 1B (2-way ANOVA: P = 0.0029, ZT effect: P = 0.0015, treatment effect: P = 0.1492; ZT × treatment effect: P = 0.3763). Responses early in the morning were significantly different from the other 2 time points. Different letters represent different significance groups. Numbers in parentheses represent the number of preparations for each condition. Data are expressed as means ± SE. D: baseline cAMP levels of WT and pdfRhan5304 mutant flies with and without 1 μM TTX. Levels were significantly different between genotypes (3-way ANOVA, P = 0.0003) and time of day (3-way ANOVA, P = 0.0117). We also found significant interactions between genotype and TTX treatment (3-way ANOVA, P = 0.0055) and genotype and time of day (3-way ANOVA, P = 0.0383). CTRL, control.
Similar to what we found with the ZT4–8 recordings (Fig. 1), adding 1 μM TTX at these times did not cause a significant change in the response to PDF (2-way ANOVA, P = 0.0029; treatment effect, P = 0.1492; ZT × treatment effect, P = 0.3763), although there was a trend toward an increase in response that could reflect some inhibition by TTX of an inhibitory input. These results support the hypothesis that the observed response following PDF stimulation across the day is due to direct stimulation of the receptor, although they also suggest that the effect of this neuropeptide is different early in the morning and imply that there may be network inputs that modulate EB cAMP levels at particular times of the day. It is important to point out here that in these experiments we are not able to claim that these are circadian changes in the responses of the EB to this neuropeptide. Although we believe that at least some of the EB responses are circadianly regulated, experiments using circadian mutants, such as per01, would be necessary to determine the relative contributions of the clock and light/dark cycling.
This apparently circadian effect of TTX on the responses elicited by PDF and the fact that in all of the animals we had imaged there was potentially some level of endogenous PDF led us to ask whether we could detect rhythms in circuit activity and/or PDF effects on basal cAMP levels in EB. We measured basal levels of cAMP in AHL alone and compared wild-type and pdfRhan5304 mutants with and without TTX (Fig. 3D). We found significant differences between genotypes (3-way ANOVA, P = 0.0003) and time of day (3-way ANOVA, P = 0.0117) as well as significant interactions between genotype and TTX treatment (3-way ANOVA, P = 0.0055) and genotype and time of day (3-way ANOVA, P = 0.0383).
Comparison of basal cAMP levels across the day (Fig. 3D) indicates that the major daily variation is accounted for by a combination of the actions of PDF and TTX-sensitive circuit effects since cAMP levels in the pdfRhan5304 mutant in the presence of TTX (which should eliminate both types of EB input) are essentially flat across the day. Allowing either just PDF action (wild-type + TTX) or just non-PDF-mediated circuit effects (pdfRhan5304) allowed us to dissect their relative contributions. ANOVA of data from each time bin in Fig. 3D indicates that at ZT0–3 and 16–20 the EB is primarily responding to PDF and not to other inputs (P = 0.0002 and 0.0036, respectively), whereas at ZT12–16 neither PDF nor other circuit inputs are modulating the EB. At other time points (ZT4–8, 8–12, and 20–24), there appears to be a complex interaction of circuit inputs and PDF, which in part could reflect actions of PDF on the clock or other EB inputs. These data support the idea that the EB is part of the output of the circadian clock and that it can be modulated both by PDF and by synaptic inputs that are regulated by the clock. It is, however, formally possible that the observed changes in basal cAMP in the pdfRhan5304 mutant might be developmental in origin due to some indirect effect of loss of PDF signaling.
EB cholinergic inputs are under circadian regulation.
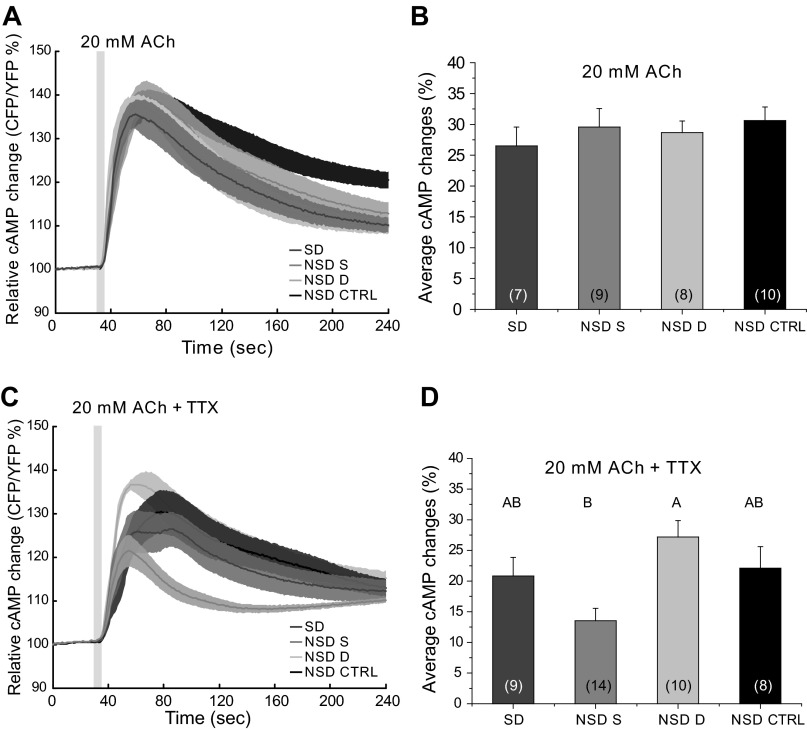
What is the nature of the TTX-sensitive synaptic input to the EB? We first tested ACh because it is the major excitatory transmitter in the Drosophila brain (Breer and Sattelle 1987) and because muscarinic ACh receptors can regulate cAMP levels (Knipper and Breer 1989). To determine whether the EB was responsive to ACh, we applied short pulses (5 s) of 20 mM ACh and measured the cAMP response. We found that ACh caused a significant increase in cAMP levels measured in the EB neuropil at all time points tested, although when the interaction between time of day and treatment is analyzed, we found that there is no significant interaction effect (Fig. 4, A–C; 2-way ANOVA, P < 0.0001; ZT effect, P = 0.0051; ZT × treatment effect, P = 0.2081; treatment effect, P = 0.0001). The response in the middle of the night (ZT16–20), however, was smaller than during the day (significantly different from ZT0 to 3 with α = 0.05 and from ZT4 to 8, α = 0.1; Fig. 4D, letters refer to statistical groups for time of day). When 1 μM TTX was added to the bath, the responses during the day were significantly decreased (2-way ANOVA, P < 0.0001; treatment effect, P = 0.0001) to the level seen in the middle of the night, i.e., there was no effect of time of day on the stimulated cAMP levels. This indicates that the intrinsic responsiveness of the EB (in terms of cAMP levels) to ACh is not regulated by the clock. These data also suggest the existence of daytime, but not nighttime, circuit level inputs to the EB that are activated by ACh.
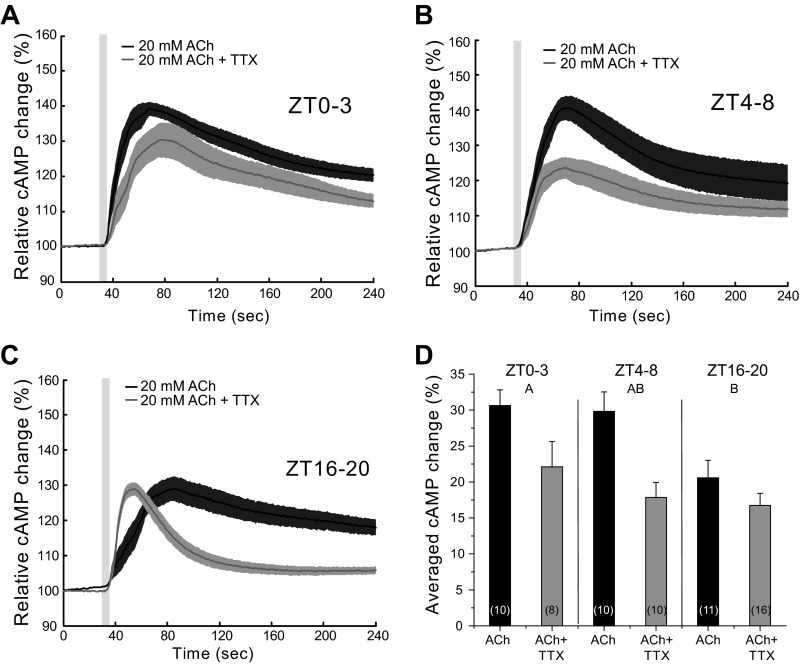
Fig. 4.
ACh causes a transient increase in cAMP in EB neurons. A–C: responses of WT flies to 5-s pulses of 20 mM ACh (black trace) and with 1 μM TTX added to the bath (gray trace) at different time points during the day (A: ZT0–3; B: ZT4–8; C: ZT16–20). Light gray box on figure represents the duration of stimulation. D: bar graph showing the averaged responses of the different preparations (2-way ANOVA, P < 0.0001; ZT effect, P = 0.0051; ZT × treatment effect, P = 0.2081; treatment effect, P = 0.0001). Numbers in parentheses represent the number of preparations for each condition. Data are expressed as means ± SE. Letters represent different significance groups for time of day effect.
The time course of the ACh responses suggests that at ZT16–20 they are qualitatively different from the other time points tested. The time course of the ZT16–20 response to ACh in the presence of TTX showed kinetics very different from those seen at the same time point with ACh alone or with ACh + TTX at other times of day (Fig. 4). When the slope of the onset of these responses was measured, we observed a striking difference between ACh and ACh + TTX (1-way ANOVA, P < 0.0001), the addition of TTX to the bath caused a faster response (Fig. 4C). Decay time in the presence of TTX was also significantly faster (1-way ANOVA, P = 0.0002). This difference in kinetics was observed in an independent set of experiments done several months later, and we combined both data sets before doing the statistical analysis since were no significant differences among the data. These observations argue for a nighttime input to the EB that has properties distinct from that of the daytime inputs, which act to increase the magnitude of the daytime cAMP response.
SD does not alter the response to PDF.
The role of the wake-promoting PDF and the circadian clock in the regulation of sleep suggested that the EB might also be involved and have a relevant role in its regulation (Lear et al. 2009; Parisky et al. 2008; Sheeba et al. 2008b). To address this, we assessed the effects of SD on the ability of the EB to respond to PDF. The sleep of wild-type flies was monitored for 4 days in a 12:12-h light-dark cycle (Fig. 5A). During the last night, flies were mechanically SD for 12 h (gray horizontal bar in Fig. 5A). The behavioral analysis of these experiments showed that only the group of flies that were mechanically SD (dark gray trace in Fig. 5A) showed any loss of sleep during the stimulation period. Both control groups (NSD same and NSD different, see below) showed normal levels of sleep. We imaged four experimental groups: 1) flies that were SD (SD); 2) flies that were not SD but located in the same shelf in the incubator as the SD flies (NSD same); 3) flies that were not SD and were placed on a different shelf in the same incubator (NSD different); and finally 4) flies that were not SD and kept in a different incubator, which was on the same light schedule (NSD control; data from Fig. 3A, black trace). We removed the flies from the incubator immediately after the end of the SD period (gray horizontal bar in Fig. 5A, ZT0), so these experiments were conducted in the ZT0–3 time frame. We observed no significant differences when we compared the responses to PDF stimulation for the 4 different conditions (Fig. 5, B and C; 1-way ANOVA, P = 0.5102); nevertheless, we observed a small trend where the response of the SD flies was slightly increased, although this difference was not significant (Fig. 5C).
Fig. 5.
Sleep deprivation (SD) does not alter the response of EB to PDF. A: flies were placed in an incubator and entrained in a 12:12-h light-dark cycle at 25°C for 4 days. The last night flies were mechanically SD. There were 3 different groups together in the incubator: 1) SD; 2) NSD S, non-SD animals placed in the same shelf as the SD flies; and 3) NSD D, non-SD animals placed in a different shelf as the SD flies. The gray horizontal bar represents the SD period. Only the SD group suffered sleep loss. B: immediately after the end of the SD period, flies from different groups were dissected and imaged. SD caused no difference in the response of these cells to the PDF perfusion nor did stress from proximity to the shaker in non-SD flies. We added a NSD CTRL group with flies that were housed in a different incubator on the same cycle. Light gray horizontal box on figure represents the duration of stimulation. C: bar graph showing the summary data from all of the conditions. No statistical differences were found (1-way ANOVA, P = 0.5102). Numbers in parentheses represent the number of preparations for each condition. Data are expressed as means ± SE.
SD does not alter the response to ACh.
To determine whether SD had effects on the ability of EB to respond to ACh, we performed a similar SD experiment and stimulated the brains with ACh. When 20 mM ACh was used to stimulate the brains following 12 h of SD (ZT0–3), we found that these responses were not different from any of the controls (Fig. 6, A and B; 1-way ANOVA, P = 0.7255). When TTX was added to the bath, SD flies were not significantly different from any of the non-SD control groups, but we observed a difference between NSD same and NSD different flies indicating a potential effect of stress due to proximity to the shaker (1-way ANOVA, P = 0.0044), although we were not able to see any indication of stress in the sleep behavior of any of the groups (data not shown). Previous work by Hendricks et al. (2000) and Shaw et al. (2000) showed that flies recover most of their sleep during the first few hours following SD. To see whether responses were altered in that time window, we also kept flies in the incubator for a recovery period of 4 h before imaging between ZT4 and 8. By doing this, we allowed our flies to have substantial sleep rebound. In this experiment, we observed no differences in the responses to ACh for the four tested conditions, although we did observe a clear behavioral effect of the SD in terms of sleep rebound (data not shown).
Fig. 6.
The response of EB neurons to ACh stimulation is not affected by SD. A: responses of EB neurons to ACh following SD were not different from responses of non-SD flies. Light gray box on figure represents the duration of stimulation. B: bar graph shows the average response for all conditions tested (1-way ANOVA, P = 0.7255). C: adding 1 μM TTX to the bath did not alter the response to ACh following SD. Light gray box on figure represents the duration of stimulation. D: bar graph shows the average response for all conditions tested (1-way ANOVA, P = 0.0044). Numbers in parentheses represent the number of preparations for each condition. Data are expressed as means ± SE. Different letters represent different significance groups.
DISCUSSION
Both sleep and locomotion are highly complex behaviors, and for them to be executed properly there must be a significant flow of information between the neuronal networks that control them. PDF is a wake-promoting peptide and considered to be the molecule in charge of the synchronization of the circadian network in Drosophila (Lear et al. 2009; Parisky et al. 2008; Renn et al. 1999; Yoshii et al. 2009). As such, it seemed a good candidate for a role in linking circadian and locomotor networks. Here, we show that the pdfR-GAL4 used by Parisky et al. (2008) captures a subset of EB cells that are directly responsive to the neuropeptide PDF. By using a combination of imaging, pharmacology, and behavioral manipulation (SD), we demonstrate for the first time that the neuropeptide PDF directly elicits an increase in cAMP levels in cells outside of the clock circuit. This result provides evidence for a novel role for this peptide as a circadian neuromodulator of locomotion, linking clock and motor networks in the Drosophila brain.
The cAMP responses to PDF observed in the EB are comparable with those observed by others using similar stimulation and recording paradigms in different structures in the adult Drosophila central nervous system (Duvall and Taghert 2012; Shafer et al. 2008; Shang et al. 2011). It has been suggested that the PDF receptor may also signal through calcium (Hyun et al. 2005; Mertens et al. 2005). Our in vivo experiments showed that, at least in the EB, the main second messenger for PDF is cAMP. Importantly, we found that the EB response to PDF is dependent on having the PDF receptor, as shown by the fact that the response is absent in flies carrying a mutation of the receptor gene (pdfRhan5304). The fact that concurrent incubation with TTX did not block the actions of PDF implies that they are direct (i.e., due to expression of PDF receptor in the EB and not simply actions of PDF on cells that are synaptically connected to EB).
The modulatory effects of neuropeptide signaling are often apparent only in the context of synaptic signaling (cf. Shang et al. 2011), so we were interested in the interplay between PDF signaling and other EB inputs and how they might be modulated by time of day. To test the effect that circuit activity has on the response of the EB cells to the neuropeptide PDF, we performed experiments in the presence of the Na+ channel blocker TTX. If this structure receives either excitatory or inhibitory input from other areas of the brain that are either PDF-regulated or could regulate the EB response to PDF, we would expect to find that the response of the EB to the peptide would change. If the input is inhibitory toward the EB, we would expect the response to increase; on the other hand, if the input is excitatory, we would expect the response to decrease. When TTX was added to the bath, it did not decrease the amplitude of the EPAC response to PDF. There was a slight trend toward increased response in early morning and nighttime in the presence of TTX, which might be due to loss of some minor inhibitory input, but from our present results the major conclusion is that PDF perfusion seems to activate cAMP production in EB directly without causing much indirect effect via input circuitry.
PDF is central to the working of the circadian clock and is expressed in the LNvs of adult brain. It is known that the levels of PDF (Park et al. 2000) and the morphology of the dorsal processes of the small LNvs (Fernandez et al. 2008) change across the day and that this is important for the circadian control by the LNvs. To test whether there was circadian control of the response of the EB neuropil to PDF, we compared the responses at multiple time points. We found that the responses to exogenous PDF were higher early in the morning. This result demonstrates that PDF responsiveness is regulated in a circadian fashion in these EB cells. The timing is consistent with the known morning role of PDF and suggests that upregulation of PDF responsiveness may accompany the higher PDF immunoreactivity seen early in the morning (Park et al. 2000). The regulation of both the signal and the activity of the receptor by time of day suggests that there are multiple levels of regulation of the action of this neuropeptide that allow signaling to accommodate to a changing environment.
ACh is the major excitatory neurotransmitter in the Drosophila brain (Breer and Sattelle 1987) and therefore is likely to participate in the regulation of EB at the circuit level. Application of ACh to the brain increased cAMP in the EB neuropil at all time points tested. Adding TTX to the bath reduced the amplitude of the response in all cases, although the magnitude of the TTX-sensitive cAMP signal was very small at night. These data imply that in addition to activating cyclase in EB directly, ACh can stimulate inputs during the day that act to increase cAMP.
One very surprising effect of time of day was observed at the ZT16–20 time point when circuit effects were removed by TTX application. Whereas blocking synaptic activity caused the response to be slightly reduced as during the day, there was an obvious additional effect on the temporal dynamics of the response. TTX application caused a significant increase in the rise time as well as in the decay time of the response. This result has two implications. First, it suggests that the kinetics of the direct response to ACh changes at night. A second conclusion is that there are inputs to the EB present during the night that cause the response to ACh to be kinetically slowed down. This effect was not present in any of the other time points, supporting the idea of a circadianly controlled change in the interaction of EB with brain circuitry. The mechanisms and neuromodulators underlying these changes are unknown but may represent important features of the linkage of the locomotor and circadian circuits.
The presence of both circuit and direct effects of externally applied substances prompted us to look at the endogenous situation. EB cells have a measurable basal level of cAMP, and by looking at basal cAMP in PDF receptor mutants and in the presence of TTX we could characterize the role of endogenous PDF and synaptic inputs to EB. When the baseline cyclic nucleotide levels were measured, we found that cAMP levels during the day were decreased by mutation of the PDF receptor gene (pdfRhan5304), indicating that endogenous PDF contributes to basal daytime levels of cAMP. Additionally, we found an interaction effect of adding TTX to the bath, suggesting that the absence of the PDF receptor may cause a change in the inputs to the EB. These results support the idea that the EB is part of the output of the circadian clock and that this structure is under circadian modulation by the neuropeptide PDF and synaptic inputs, which are themselves subject to regulation by the clock. We believe that the effects seen in our experiments are likely due to circadian regulation of synaptic inputs to the EB; nevertheless, without performing additional experiments where the effect of light is removed (i.e., constant-dark conditions) and where the clock is eliminated (e.g., using the circadian mutant per01), we cannot discriminate between light and clock effects.
It has been suggested that the EB may play a role in sleep regulation (Parisky et al. 2008). With that in mind, we looked at the ability of SD to alter EB responses to PDF and ACh. One might expect responses to PDF to be higher in the early morning due to the fact that PDF is a wake-promoting peptide and animals will have a lower sleep drive, and that is indeed what we see (Fig. 3C). However, when we alter sleep drive by SD before imaging, we did not see a significant change in the response elicited by either 10 μM PDF or 20 mM ACh application. It has been suggested that flies recover most of their sleep following a 12-h SD during the 1st 4 h after SD (Hendricks et al. 2000; Shaw et al. 2000), with full recovery taking up to 8 h. To see whether there was a role for EB in this sleep recovery window, we deprived flies of sleep and left the flies in the incubator to recover for 4 h (i.e., allowed them to initiate rebound sleep) but found no effect of the SD or the recovery on the response of these cells to stimulation with ACh. Taken together, our data suggest that the behavioral effect seen in these flies following SD does not alter the cAMP regulation of EB. If EB is involved in this aspect of sleep regulation, it is likely that some other cellular parameter is being affected.
In summary, PDF and ACh have clear effects on EB (as measured by cAMP level changes). Our results suggest that the activity of these nonclock cells is regulated by an interplay between PDF and synaptic inputs under circadian regulation. It is unlikely that PDF is the only player in this type of linkage, and it will be interesting to look for other neuropeptides that can mediate communication between the clock and output neuronal networks. Nevertheless, we show here for the first time a novel role for the neuropeptide PDF as a potential link between the central clock and the locomotor circuit.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01-MH-067284 to L. C. Griffith. B. L. Christmann was supported by NIH Grant T32-GM-007122.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.P. and L.C.G. conception and design of research; N.P. and B.L.C. performed experiments; N.P. analyzed data; N.P. and L.C.G. interpreted results of experiments; N.P. prepared figures; N.P. and L.C.G. drafted manuscript; N.P. and L.C.G. edited and revised manuscript; N.P. and L.C.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank N. C. Donelson, C. G. Vecsey, and K. Parisky for comments on the manuscript.
Present address of N. Pírez: Laboratorio de Genética del Comportamiento, Fundación Instituto Leloir and Instituto de Investigaciones Bioquímicas-Buenos Aires (IIB-BA, CONICET), Av. Patricias Argentinas 435, 1405-BWE Buenos Aires, Argentina.
REFERENCES
- Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci 11: 354–359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H, Sattelle DB. Molecular properties and functions of insect acetylcholine receptors. J Insect Physiol 33: 771–790, 1987 [Google Scholar]
- Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol 19: 386–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelson N, Kim EZ, Slawson JB, Vecsey CG, Huber R, Griffith LC. High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the “tracker” program. PLoS One 7: e37250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall LB, Taghert PH. The circadian neuropeptide PDF signals preferentially through a specific adenylate cyclase isoform AC3 in M pacemakers of Drosophila. PLoS Biol 10: e1001337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. Computer control of microscopes using microManager. Curr Protoc Mol Biol chapt. 14: Unit 14.20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MP, Berni J, Ceriani MF. Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol 6: e69, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Forster C. The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microsc Res Tech 62: 94–102, 2003 [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci USA 92: 612–616, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Forster C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol A 182: 435–453, 1998 [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron 25: 129–138, 2000 [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, Bae E, Kim J. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron 48: 267–278, 2005 [DOI] [PubMed] [Google Scholar]
- Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J Comp Neurol 518: 1925–1945, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 422: 66–94, 2000 [DOI] [PubMed] [Google Scholar]
- Knipper M, Breer H. Muscarinic receptors modulating acetylcholine release from insect synaptosomes. Comp Biochem Physiol C 93: 287–292, 1989 [DOI] [PubMed] [Google Scholar]
- Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron 48: 221–227, 2005 [DOI] [PubMed] [Google Scholar]
- Lear BC, Zhang L, Allada R. The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS Biol 7: e1000154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelito KR, Shafer OT. Reciprocal cholinergic and GABAergic modulation of the small ventrolateral pacemaker neurons of Drosophila's circadian clock neuron network. J Neurophysiol 107: 2096–2108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci 24: 7951–7957, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 48: 213–219, 2005 [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279: 37215–37218, 2004 [DOI] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60: 672–682, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Helfrich-Forster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci USA 97: 3608–3613, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99: 791–802, 1999 [DOI] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 58: 223–237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci USA 105: 19587–19594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Haynes P, Pírez N, Harrington KI, Guo F, Pollack J, Hong P, Griffith LC, Rosbash M. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci 14: 889–895, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science 287: 1834–1837, 2000 [DOI] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol 18: 1537–1545, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Sharma VK, Gu H, Chou YT, O'Dowd DK, Holmes TC. Pigment dispersing factor-dependent and -independent circadian locomotor behavioral rhythms. J Neurosci 28: 217–227, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol 12: 633–638, 2002 [DOI] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci 13: 1852–1861, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 6: 875–881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112: 271–282, 2003 [DOI] [PubMed] [Google Scholar]
- Yao Z, Macara AM, Lelito KR, Minosyan TY, Shafer OT. Analysis of functional neuronal connectivity in the Drosophila brain. J Neurophysiol 108: 684–696, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T, Wulbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Forster C. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock. J Neurosci 29: 2597–2610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]