Abstract
During skeletal muscle contractions, the concentration of ATP increases in muscle interstitial fluid as measured by microdialysis probes. This increase is associated with the magnitude of blood flow, suggesting that interstitial ATP may be important for contraction-induced vasodilation. However, interstitial ATP has solely been described to induce vasoconstriction in skeletal muscle. To examine whether interstitial ATP induces vasodilation in skeletal muscle and to what extent this vasoactive effect is mediated by formation of nitric oxide (NO) and prostanoids, three different experimental models were studied. The rat gluteus maximus skeletal muscle model was used to study changes in local skeletal muscle hemodynamics. Superfused ATP at concentrations found during muscle contractions (1–10 μM) increased blood flow by up to 400%. In this model, the underlying mechanism was also examined by inhibition of NO and prostanoid formation. Inhibition of these systems abolished the vasodilator effect of ATP. Cell-culture experiments verified ATP-induced formation of NO and prostacyclin in rat skeletal muscle microvascular endothelial cells, and ATP-induced formation of NO in rat skeletal muscle cells. To confirm these findings in humans, ATP was infused into skeletal muscle interstitium of healthy subjects via microdialysis probes and found to increase muscle interstitial concentrations of NO and prostacyclin by ∼60% and ∼40%, respectively. Collectively, these data suggest that a physiologically relevant elevation in interstitial ATP concentrations increases muscle blood flow, indicating that the contraction-induced increase in skeletal muscle interstitial [ATP] is important for exercise hyperemia. The vasodilator effect of ATP application is mediated by NO and prostanoid formation.
Keywords: nitric oxide, prostanoids, microdialysis, rat gluteus maximus skeletal muscle model
skeletal muscle blood flow is regulated by a complex process involving competing vasodilator and vasoconstrictor influences (13, 16); however, the specific role of different vasodilators and the contribution from intravascular and interstitial vasodilators are not fully understood (27). With regard to the latter, the cellular sources of the vasoactive compound ATP in the blood are likely to be erythrocytes and the luminal surface of endothelial cells, whereas likely sources of interstitial ATP are skeletal muscle cells and abluminal surface of capillary endothelial cells. During muscle contractions, skeletal muscle cells are likely the primary source of ATP (27). The different cellular sources of ATP and the fact that the endothelium is an effective barrier for nucleotides (41, 44) are likely explanations for why the measured concentration of skeletal muscle interstitial ATP is several-fold higher than plasma [ATP] during muscle contractions (26, 47). Furthermore, ATP in the blood vessel lumen has, in general, been described to be a vasodilator, whereas ATP outside of the lumen in close proximity to smooth muscle cells induces vasoconstriction (39). Collectively, these observations accentuate the importance of differentiating between the contributions of intravascular ATP vs. interstitial ATP to vascular tone.
During skeletal muscle contractions, the concentration of ATP increases in the interstitial space in proportion to the intensity of contraction (26, 36). One of the differences between intravascular and interstitial ATP is that interstitial ATP has been shown to contribute to the contraction-induced activation of the sympathetic nervous system, known as the exercise pressor reflex (2, 62), via activation of P2x receptors, which, in turn, stimulate and sensitize thin-fiber muscle afferents (22, 38). The resulting release of norepinephrine from sympathetic nerve endings stimulate α-adrenergic receptors to produce contraction of smooth muscle cells, which leads to vasoconstriction. Furthermore, release of ATP from perivascular nerve terminals and increases in ATP concentrations around vascular smooth muscle cells induces vasoconstriction via activation of P2x receptors (28, 63). In skeletal muscle, ATP released during muscle contractions has been suggested to stimulate presynaptic P2x receptors on sympathetic nerve fibers, which increases a local release of norepinephrine (37), indicating that interstitial ATP acts as a local vasoconstrictor in skeletal muscle.
However, experiments in which ATP was applied to the abluminal surface of isolated rat cerebral arterioles (17, 18) and intact blood perfused arterioles in the cheek pouch of hamsters (19) have both shown brief dose-dependent local vasoconstriction to ATP, which was immediately followed by conducted vasodilation. Overall, the primary response to ATP applied to the outer surface of the arterioles in both cases was vasodilation. ATP is rapidly degraded by ectophosphatases on the sarcolemma and plasma membrane of endothelial cells, which ultimately leads to adenosine formation (24, 25), indicating that the vasodilator effect of ATP applied abluminally could be mediated via adenosine. Although this is in congruence with the findings from the study on arterioles in the cheek pouch of hamsters (19), it is in contrast to observations on rat cerebral arterioles (17, 18). To what extent an increase in interstitial ATP leads to vasodilation of vascular beds within skeletal muscle, a tissue in which interstitial [ATP] increases substantially during contractions (26, 36), remains unclear. However, the potential of interstitial ATP to induce vasodilation and contribute to regulation of skeletal muscle blood flow during contraction appears plausible, considering the association between interstitial [ATP] and the magnitude of exercise hyperemia (26).
Arterial infusion of ATP into the femoral artery can induce vasodilation close to that observed during maximal exercise (20, 58). Although ATP in the blood is rapidly degraded by membrane-bound and soluble nucleotidases to form ADP, AMP, and adenosine (67), the vasodilator effect of intravascular ATP does not appear to be mediated by the actions of adenosine (34, 42, 57). Intravascular ATP has been shown to induce vasodilation by stimulating the formation of the endothelium-derived vasodilators nitric oxide (NO) (4, 11, 21, 42) and prostanoids (42). This vasodilator effect is thought to be mediated via P2y receptors located on the microvascular endothelium and on the assumption that P2y receptors are located also on the abluminal side of capillaries, interstitial ATP may also induce vasodilation by inducing NO and prostanoid formation.
Therefore, our goal was to determine whether interstitial ATP at concentrations found during muscle contractions causes an increase in skeletal muscle blood flow. To address this goal, we used the in vivo rat gluteus maximus skeletal muscle preparation with intravital video microscopy. The intravital approach with rat skeletal muscle allows for detection of changes in local skeletal muscle hemodynamics in response to changes in interstitial ATP concentrations in a locomotor muscle resembling human skeletal muscle. Furthermore, to elucidate whether NO and prostanoids are essential for the vasoactive effect of interstitial ATP, we inhibited these systems in the same model. To establish the cellular sources of ATP-induced formation of NO and prostacyclin, we treated cultured rat microvascular endothelial cells isolated from skeletal muscle and skeletal muscle cells with ATP. To resolve whether an increase in interstitial ATP stimulates the formation of NO and prostacyclin in human skeletal muscle, we infused ATP directly into the muscle interstitium using microdialysis probes and measured changes in the concentration of these substances using the same probes. As infusion of ATP via dialysis probes into human skeletal muscle affects only a small region of muscle, changes in global blood flow are not detectable; thus, this model could not be used for blood flow determination. We hypothesized that raising interstitial [ATP] to levels found during muscle contractions would lead to vasodilation and increases in blood flow in skeletal muscle. Furthermore, the vasodilator effect of interstitial ATP would be mediated by formation of NO and prostanoids.
METHODS
Rat Gluteus Maximus Skeletal Muscle Model
Animal care and use.
The Council on Animal Care at the University of Western Ontario approved the experimental protocol. Experiments were performed using seven male Sprague-Dawley rats (6–7 wk old; mass: 154.4 ± 6 g, mean ± SD), purchased from Charles River Laboratories (Saint-Constant, Quebec, Canada), and housed on site for at least 1 wk prior to the study. Rats were housed in animal care facilities of the University of Western Ontario, at 24°C on a 12-12-h light-dark cycle with access to food and water ad libitum. Upon completion of experimental procedures each day, the anesthetized rat was euthanized with an overdose of α-chloralose and urethane cocktail mix (intraperitoneal injection), and cervical dislocation.
Anesthesia and skeletal muscle preparation.
Using intraperitoneal injection, the rat was anesthetized with a cocktail of α-chloralose (80 mg/kg) and urethane (500 mg/kg). A mid-neck incision was made, and the animal was tracheomotized (PE-205) to facilitate spontaneous respiration. The right jugular vein was cannulated (PE-50 tubing) to maintain a constant infusion of anesthetic to the animal (α-chloralose: 8–16 mg·kg−1·h−1, urethane: 50–100 mg·kg−1·h−1), and a T-connector was used to inject fluorescent red blood cells. The left carotid artery was cannulated (PE-50 tubing) to allow for the recording of arterial blood pressure via the amplified signal of a pressure transducer using a PowerLab system (model ML118 PowerLab Quad Bridge Amplifier; model MLT0699 BP Transducer; AD Instruments, Colorado Springs, CO). The rat was placed prone on a custom-fabricated animal platform using conducted heat with animal temperature feedback to maintain the rectal temperature at 37°C. As described elsewhere (1), the gluteus maximus muscle was carefully exposed by dissecting from its origin along the spine and along its rostral and caudal borders, while maintaining its neurovascular supply. The tissue was pinned out onto a transparent pedestal to approximate in situ dimensions. The exposed tissue was superfused continuously (4–5 ml/min) with bicarbonate-buffered PSS (35°C at tissue, pH 7.4) of the following composition (in mM): 137 NaCl, 4.7 KCl, 1.2 MgSO4, 2 CaCl2, 18 NaHCO3, and equilibrated with 5% CO2-95% N2.
Fluorescent labeling of red blood cells.
Full description of red blood cell labeling is described elsewhere (1). Briefly, red blood cells were isolated and incubated in a freshly prepared FITC dye solution (FITC mixed into dimethyl sulfoxide and Tris-buffered Ringer's albumin solution for 2 h). Cells were washed in Tris-buffered Ringer's albumin solution and stored overnight at 4°C. On the day of experiment, cells were washed, and hematocrit was adjusted to 30–35% with buffer. After checking cell viability, cells were injected into the animal (at 1% of total animal blood volume) via the jugular vein, and the line was flushed with saline.
Intravital video microscopy.
Upon completion of microsurgical procedures, the preparation was transferred to the stage of the intravital microscope (Olympus BX51, Olympus, Tokyo, Japan). Microvessels were observed under Kohler illumination using a long working distance condenser (NA = 0.80) and a long working distance water immersion objective (Olympus LUMPLFL: 10× NA = 0.30) with illumination from a 100-W halogen light source. To assess microvascular red blood cell velocity, fluorescent red blood cells were epi-illuminated using a 120-W Mercury Vapor Short Arc light source (EXFO, X-Cite 120PC Q, Photonic Solutions, Mississauga, ON, Canada) in line with an FITC (excitation: 460–480 nm, emission: 495–540 nm) filter. The optical image was coupled to a front-illuminated interline charge-coupled device camera (Qimaging EXi Blue, Qimaginga, Surrey, BC, Canada) and viewed and stored to a hard drive using a specialized imaging software (MetaMorph 7.6; Molecular Devices, Sunnyvale, CA). Video (.tiff) images were collected (15 fps) under epi-illumination for off-line analysis of red blood cell velocity, and blood flow. Corresponding bright-field video (.tiff) images were collected (15 fps) under Kohler bright-field illumination for off-line analysis of red blood cell (RBC) column (and luminal) diameters.
RBC streaks and flow calculations.
Centerline RBC velocities were acquired by taking multiple centerline streak length (camera exposure: 10–20 ms depending on RBC velocity) measurements using ImageJ software (manual; ImageJ 1.43 u; National Institutes of Health, Bethesda, MD). Mean RBC velocity was calculated by correcting centerline streak lengths with a velocity ratio factor (Vratio) previously determined based on the arteriolar diameter of interest, where Vratio = (0.0071 × diameter) + 1.15 (1). Blood flow was calculated as a product of mean RBC velocity and vessel cross-sectional area.
Experimental procedures.
Second-order (2A) and third-order (3A) branches of the arteriolar network were chosen for the study because they are ideally positioned to control the distribution of blood flow within the muscle (3). Because of the consistent overall architecture of the network among animals, these data were obtained from approximately the same (middle) region of each preparation.
Following initial equilibration and stabilization of the preparation, baseline internal vessel lumen diameters were recorded. As an index of muscle viability (and to ensure responses to a treatment outside of the experiment protocol were similar across animals), the preparation was subjected to an oxygen sensitivity test. This was done by elevating superfusate O2 from 0% to 21% (5% CO2, balance N2) for 5–10 min, and recording arteriolar diameter [mean arteriolar changes from baseline were −11 ± 2 μm (for 2A), and −7 ± 2 μm (for 3A)]. Equilibration with 5% CO2-95% N2 was restored for the duration of experimental procedures. The vasoactive effects of ATP (Sigma-Aldrich, St. Louis, MO) were evaluated by cumulative addition (10−8, 10−6, 5 × 10−6, and 10−5 M) to the superfusion solution to mimic increases in interstitial ATP concentrations measured in contracting skeletal muscle. The ATP concentrations were chosen on the basis of the observations that in rat (37, 65, 66), canine (41), and cat (36) skeletal muscle, interstitial ATP levels at rest are comparable to those obtained from humans (26, 44), and the concentration increases in a tension-dependent manner with mechanical stretch and electrical stimulation up to ∼3 μM in animal models (36, 37, 41, 66) and up to 8 μM during light- to moderate-intensity exercise in humans (26, 44). At each ATP concentration, arteriolar diameter was allowed to stabilize for at least 5 min and recorded afterward. Resting diameter was then allowed to recover (typically within 30 min of restoring control superfusate), and ATP (10−5 M) was then added to the superfusion solution. When arteriolar diameter had stabilized (∼3–5 min), a solution with ATP (10−5 M) and either l-NA (100 μM; Sigma-Aldrich) or indomethacin (10 μM; Sigma-Aldrich) was superfused over the muscle surface. When a new stable arteriolar diameter was observed and had been recorded, resting diameter was allowed to recover (typically within 60 min of restoring control superfusate). To investigate the isolated effect of both drugs, ATP (10−5 M) was added to the superfusion solution and when arteriolar diameter had stabilized (∼3–5 min), a solution with ATP (10−5 M) and either l-NA (100 μM) or indomethacin (10 μM) was used to superfuse the muscle until a new stable arteriolar diameter was observed. The solution was then switched to a solution with ATP (10−5 M) and both l-NA (100 μM) and indomethacin (10 μM) to evaluate the effect of double blockade. To test the effect of l-NA on baseline hemodynamics, a solution containing only l-NA (100 μM) was used to bathe the muscle (n = 4). Previous studies have shown that indomethacin does not affect baseline hemodynamics in either animals or human subjects (29, 43). To confirm this in the present preparation, the effect of indomethacin was assessed in one rat and found not to change blood flow (2A: 18 vs. 16 nl/s and 3A: 9 vs. 8 nl/s for control and indomethacin, respectively). At the end of experimentation, maximal arteriolar diameters to sodium nitroprusside (SNP; 10−5 M) were evaluated [mean arteriolar changes from baseline were 35 ± 8 μm (for 2A), and 28 ± 5 μm (for 3A)]. Working concentrations were prepared fresh daily.
Cell Culture
Materials.
DMEM, FCS, horse serum (HS), Dulbecco's phosphate-buffered saline (DPBS), Pen Strep [penicillin (10,000 U/ml), streptomycin (10,000 U/ml)], and trypsin were all obtained from Life Technologies. Serum growth supplement containing FBS, fibroblast growth factor, heparin, and epidermal growth factor were obtained from Cascade Biologics (Portland, OR), while DNAse, trypsin/EDTA solution, glucose, l-arginine, ATP, and S-nitroso-N-acetyl-penicillamine (SNAP) were all products from Sigma-Aldrich. Collagenase (type II) was obtained from Worthington Biochemicals (Lakewood Township, NJ). TriReagent was obtained from the Molecular Research Center (Cincinnati, OH) and biotinylated Griffonia simplicifolia lectin and Ulex europaeus agglutinin I from Vector Laboratories (Burlingame, CA). Fluorochrome 4-amino-5-methylamino-2′,7-difluorescein (DAF-FM) and Dynabeads were obtained from Invitrogen (Carlsbad, CA), and medium 131, microvascular growth supplement, and attachment factor were obtained from Cascade Biologics.
Cell culture and analyses.
Skeletal muscle and microvascular endothelial cell cultures were prepared from male Wistar rats (Taconic M&B A/S, Denmark). Rats weighing 100 g were killed by cervical dislocation. Carefully, the muscle fascia was removed, and soleus, gastrocnemius, and quadriceps femoris muscles were removed and placed on ice in DPBS with 1% glucose + 1% Pen Strep. The muscle tissue was minced into small pieces with scissors and then digested with 0.2% collagenase II in DMEM containing 1% penicillin-streptomycin (Pen Strep) for 1.5 h at 37°C with rotation. After centrifugation at 200 g for 15 min, the pellet was incubated with rotation in a solution of 0.2% collagenase, 0.01% DNase, and 0.25% trypsin in DMEM containing 1% Pen Strep for 30 min at 37°C.
The skeletal muscle cells were suspended in primary growth medium (PGM) [DMEM supplemented with 1% Pen Strep, HS (10%), and FCS (10%)], counted, seeded out on 35-mm dishes (∼30 × 104 cells/dish), coated with 1% Matrigel, and incubated at 8% CO2 and 37°C. The cells were not passaged, and, after 2 days, PGM was changed to primary fusion medium [DMEM supplemented with l-glutamine (2 mM) and HS (10%)], and, after seven additional days, the primary skeletal muscle cells were ready for experiments.
To isolate skeletal muscle microvascular endothelial cells, 10 ml of the cell suspension was extracted, and 50 μl of Dynabeads coated with lectin I was added. After 25 min of incubation, a magnet was applied to separate Dynabeads and bead-bound microvascular endothelial cells. Cells were then resuspended in medium 131 containing microvascular growth supplement and counted and seeded onto 35-mm dishes coated with attachment factor. After 4 or 5 days, medium 131 and growth supplement were changed and after 1–3 additional days, cells were passaged with trypsin/EDTA solution. After being seeded onto 35-mm dishes coated with attachment factor cells were ready for experiments 3–5 days later.
All treatment of animals complied with the European Convention for the protection of Vertebrate Animal Used for Experimental or other Scientific Purposes (Council of Europe No. 123; Strasbourg, France, 1985).
Before experiments were performed, the cells were washed once with PBS containing 5 mM glucose, and then PBS containing 100 μM l-arginine and 5 mM glucose was added. After 30 min of incubation, the cells were washed once with PBS containing 5 mM glucose and then PBS containing 10 μM DAF-FM, and 5 mM glucose was added. To investigate the role of ATP for microvascular endothelial cell and skeletal muscle cell NO and prostacyclin formation, either ATP (50, 250, or 750 μM in PBS containing 5 mM glucose) or PBS containing 5 mM glucose (control) was added to culture medium. Medium for determination of DAF-FM fluorescence was collected after 5 min of incubation and transferred to microplates, and fluorescence was measured immediately with a fluorescence microplate reader (Fluoroskan Ascent, Thermo Labsystems) calibrated for excitation at 485 nm and emission at 520 nm. Medium for determination of 6-keto PGF1α was collected after 10 min of incubation and immediately stored in a freezer (−80°C) for later analysis. The reported values in Figs. 3 and 4 are, therefore, the difference between the ATP-treated dishes and control dishes. This approach was chosen because of the low volume of culture medium required for the detection of changes in the respective substances, making repeated measurements from each dish impossible. Total protein concentrations of microvascular endothelial and skeletal muscle cells were determined by BCA protein assay using BSA as the standard (Pierce Reagents, Rockford, IL).
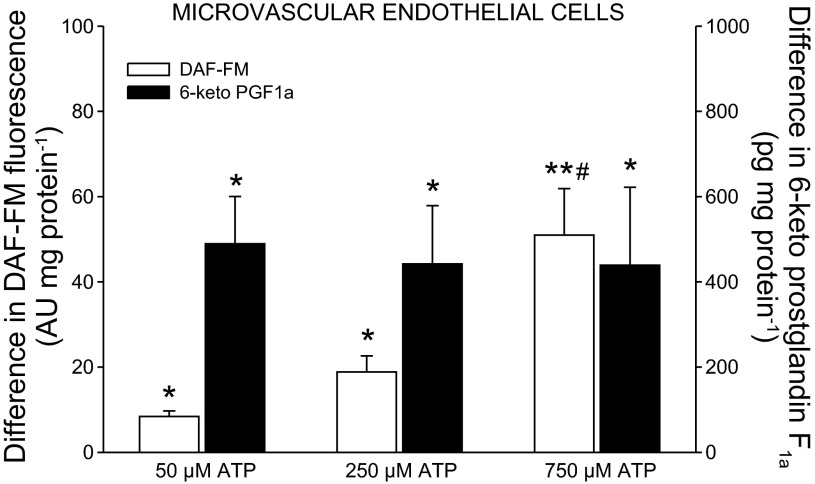
Fig. 3.
Effect of ATP on release of NO and 6-keto PGF1α from microvascular endothelial cells. n = 7–17; AU, arbitrary units. *P < 0.05, **P < 0.001, significant formation. #P < 0.05 significantly different from 50 and 250 μM ATP.
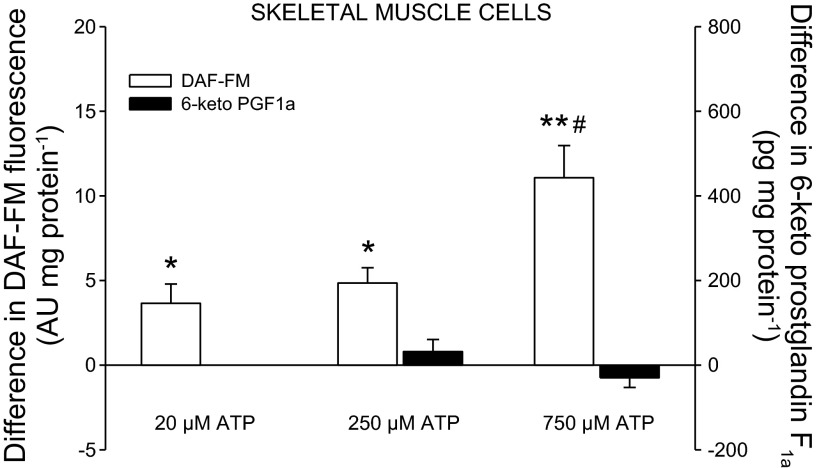
Fig. 4.
Effect of ATP on release of NO and 6-keto PGF1α from skeletal muscle cells. *P < 0.05, **P < 0.001, significant formation. #P < 0.05 significantly different from 50 and 250 μM ATP.
The concentrations of ATP used to stimulate NO and prostacyclin formation in the cell cultures were higher than the endogenous concentrations measured using microdialysis probes in vivo during muscle contractions. However, ATP is very rapidly degraded both in vivo and in cell culture. In addition, local ATP concentrations near the cell membrane have been found to be much higher than that observed in the bulk medium (35, 64). The concentrations used in the current study are comparable to those used in previous studies (9, 10, 12).
Analysis of 6-keto prostaglandin F1α in culture medium.
The stable metabolite of prostacyclin, 6-keto prostaglandin F1α, was measured with an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI).
Human Study
Ethical approval.
Three male and three female subjects with a mean (± SE) age of 46 ± 1 yr, body weight of 78 ± 4 kg, height of 176 ± 3 cm, and V̇o2max relative to body mass of 35.7 ± 3.0 ml·min−1·kg−1 participated in the study. The purpose, nature, and potential risks were explained to the subjects before they gave their informed, written consent to participate in the study. The study was approved by the Ethics Committee of Copenhagen and Frederiksberg communities (H-2-2009-096) and conducted in accordance with the guidelines of the Declaration of Helsinki. All subjects were nonsmokers, and none of the subjects had been diagnosed with cardiovascular disease, renal dysfunction, insulin resistance, diabetes, or hypercholesterolemia.
Microdialysis.
Subjects refrained from caffeine, alcohol, and exercise for 24 h before the experimental day. After local anesthesia (lidocaine), two microdialysis probes (CMA 63, CMA microdialysis, Stockholm) with a 30-mm membrane (20-kDa cut-off) were inserted into the thigh muscle (∼70 mm; m. vastus lateralis) of the experimental leg. Thirty minutes after insertion of the probes, the subjects performed 10 min of knee-extensor exercise (12 W) with the purpose of minimizing the tissue response to insertion trauma (49). To re-establish resting conditions, the subjects rested for another 30 min, and dialysate was then collected for 10 min during resting conditions and for 10 min during infusion of ATP (0.09 μmol/min) through the probes (interstitial ATP infusion). The infusion rate of ATP was based on observations from a pilot study demonstrating that the infusion rate needed to be in this range to detect changes in NOx and 6-keto prostaglandin F1α. The microdialysis probes were perfused at a rate of 5 μl/min with Ringer acetate and to determine the relative exchange of substances across the membrane, a small amount (2.7 nM) of [2-3H]ATP (<0.1 μCi/ml) was added to the perfusate for calculation of probe recovery. The molecular probe recovery (PR) was calculated as [PR = (dpminfusate − dpmdialysate)/dpminfusate], where dpm denotes disintegrations per minute (31, 60). The 3H activity (in dpm) was measured on a liquid scintillation counter (Tri-Carb 2910 TR; Perkin Elmer, Waltham, MA) after the addition of the perfusate to 3 ml of Ultima Gold scintillation liquid (Perkin Elmer). After collection of microdialysate, the sample was weighed, and the flow rate was calculated to estimate any loss of fluid or abnormal decrease in perfusion rate.
Analysis of nitrate and nitrite and 6-keto prostaglandin F1α in microdialysate.
The stable metabolites of NO, nitrite and nitrate (NOx), were measured using fluorometric assay kit (Cayman Chemical). The stable metabolite of prostacyclin, 6-keto prostaglandin F1α, was measured with an enzyme immunoassay kit (Cayman Chemical).
Statistical Analysis
A one-way repeated-measures ANOVA was used to test the effect of ATP and inhibitors in the rat gluteus maximus muscle superfusion preparation. A one-way ANOVA was used to test the effect of ATP on microvascular endothelial cells and skeletal muscle cells, and the effect of interstitial ATP infusion was determined by a paired t-test. Following a significant F-test, pair-wise differences were identified using Student-Newman-Keuls post hoc procedure. The effect of 10−8 and 10−6 M ATP was assessed with a paired t-test. SigmaPlot 11.0 (Systat Software, San Jose, CA) was used for all analyses. The significance level was set at P < 0.05, and data are expressed as means ± SE unless otherwise indicated.
RESULTS
Control Responses in Rat Gluteus Maximus Muscle
Resting diameters after equilibration averaged 68 ± 1 (n = 7) and 53 ± 3 μm (n = 12) for 2A and 3A, respectively. Elevating superfusate O2 constricted arterioles by 9 ± 2 and 7 ± 1 μm (P < 0.05). Maximal diameters of arterioles during SNP were 92 ± 5 and 72 ± 4 μm. l-NA reduced (P < 0.05) resting diameter by 18 ± 4 and 17 ± 3 μm, respectively (n = 4).
Hemodynamic Responses to Changes in Interstitial ATP in Rat Gluteus Maximus Muscle
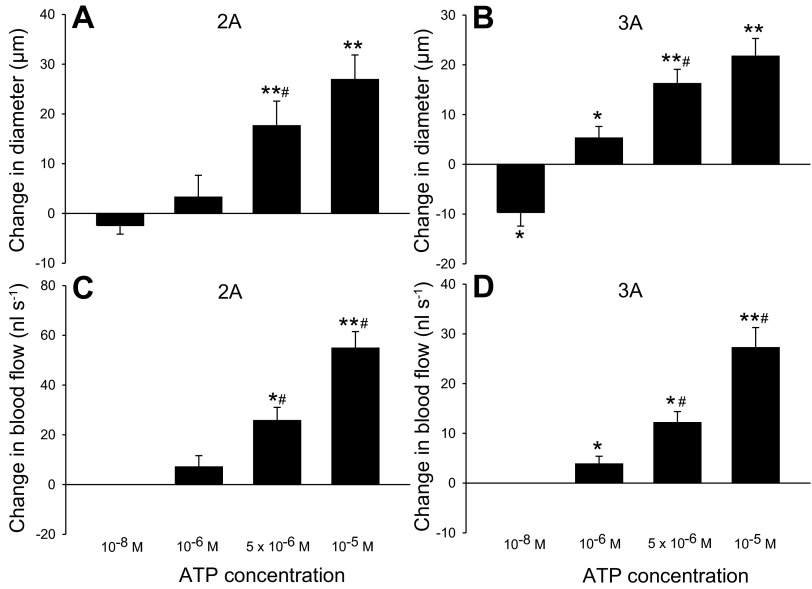
The addition of 10−8 M ATP induced a 7 ± 3 (5 ± 2 μm) and 7 ± 3% (4 ± 2 μm) reduction (P < 0.05) in diameter of 2A and 3A, respectively (Fig. 1). The addition of 5 × 10−6 and 10−5 M ATP caused a 26 ± 7 (18 ± 5 μm) and 39 ± 7% (27 ± 5 μm) increase in diameter of 2A (P < 0.001), and the addition of 10−6, 5 × 10−6, and 10−5 M ATP caused a 10 ± 5 (5 ± 2 μm) (P < 0.05), 36 ± 11 (16 ± 3 μm), and 47 ± 12% (22 ± 4 μm) (both P < 0.001) increase in diameter of 3A, respectively. The addition of 5 × 10−6 and 10−5 M ATP induced a 127 ± 12 (26 ± 2 nl/s) (P < 0.05) and 274 ± 32% (55 ± 7 nl/s) (P < 0.001) increase in blood flow in 2A and the addition of 10−6, 5 × 10−6, and 10−5 M ATP induced a 37 ± 10 (4 ± 2 nl/s), 194 ± 76 (12 ± 2 nl/s) (both P < 0.05) and 392 ± 105% (27 ± 4 nl/s) (P < 0.001) increase in blood flow in 3A, respectively. There was no difference in baseline diameter (2A: 68 ± 1 vs. 65 ± 3 μm; 3A: 53 ± 3 vs. 55 ± 3 μm) and blood flow (2A: 20 ± 2 vs. 18 ± 2 nl/s; 3A: 9 ± 2 vs. 9 ± 2 nl/s) before and after the addition of ATP. Mean arterial pressure (MAP) was 87 ± 2 mmHg during baseline conditions and did not change with the addition of 10−6 (88 ± 3 mmHg), 5 × 10−6 (84 ± 6 mmHg), or 10−5 M (87 ± 5 mmHg) ATP.
Fig. 1.
Hemodynamic responses to changes in superfusate ATP in rat gluteus maximus muscle. Change in diameter [A, n = 6 or 7; B, n = 10 or 11 (10−8 ATP; n = 7)] and blood flow [C, n = 3–4; D, n = 5 or 6] of second-order and third-order branches of the arteriolar network in rat gluteus maximus muscle with cumulative addition of ATP to the superfusate. *P < 0.05, **P < 0.001, significant change from baseline. #P < 0.05 significantly different from previous ATP concentration.
Role of NO and Prostanoids for the Vasoactive Effect of Interstitial ATP in Rat Gluteus Maximus Muscle
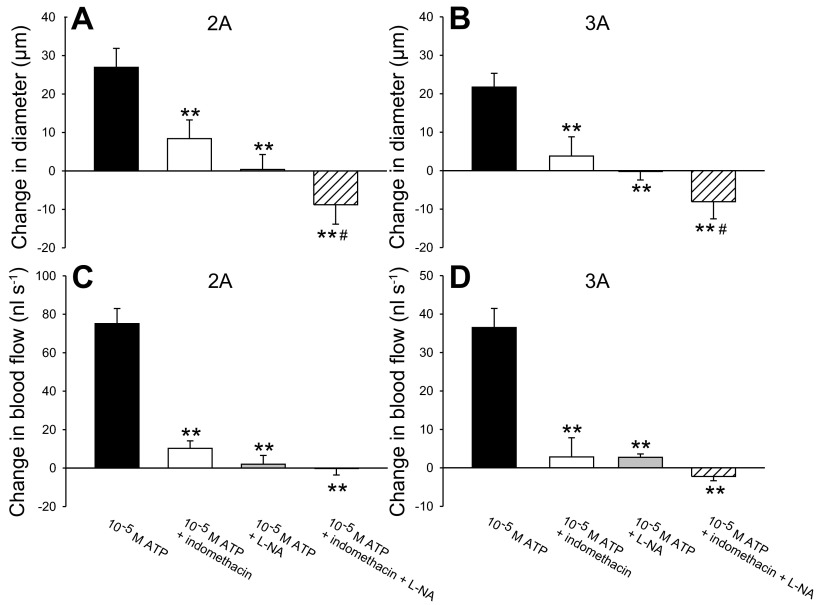
The addition of 10−5 M ATP followed by 10−5 M ATP + 10 μM indomethacin abolished the ATP-mediated vasodilation as evidenced by a 95 ± 33 (19 ± 4 μm) and 99 ± 21% (19 ± 4 μm) reduction (P < 0.001) in the ATP-induced change in diameter of 2A and 3A, respectively (Fig. 2). Similarly, the addition of 10−5 M ATP + 100 μM l-NA caused a 120 ± 23 (27 ± 4 μm) and 122 ± 18% (23 ± 3 μm) reduction (P < 0.001) in the ATP-induced change in diameter of 2A and 3A, respectively, as the diameters were smaller than at baseline. The addition of 10−5 M ATP + 10 μM indomethacin and 100 μM l-NA reduced (P < 0.001) the ATP-induced increase in diameter by 208 ± 81 (36 ± 2 μm) and 194 ± 42% (31 ± 5 μm) in 2A and 3A, respectively. The effect of 10−5 M ATP + 10 μM indomethacin and 100 μM l-NA was more pronounced (P < 0.05) than that of 10−5 M ATP + 10 μM indomethacin alone. The addition of 10−5 M ATP + 10 μM indomethacin caused an 88 ± 5 (73 ± 5 nl/s) and 93 ± 7% (40 ± 4 nl/s) reduction (P < 0.001) in the ATP-induced change in blood flow in 2A and 3A, respectively, whereas the addition of 10−5 M ATP + 100 μM l-NA caused a 98 ± 4 (70 ± 10 nl/s) and 92 ± 2% (33 ± 6 nl/s) reduction (P < 0.001). The addition of both inhibitors reduced (P < 0.001) the ATP-induced increase in blood flow by 101 ± 4 (72 ± 10 nl/s) and 105 ± 4% (38 ± 8 nl/s) in 2A and 3A, respectively. There was no difference in baseline diameter before and after 10−5 M ATP + 10 μM indomethacin in 2A (63 ± 3 vs. 60 ± 3 μm) and 3A (52 ± 4 vs. 49 ± 4 μm). Baseline diameter was lower (P < 0.05) after the addition of 10−5 M ATP + 100 μM l-NA in 2A (68 ± 5 vs. 51 ± 9 μm) and 3A (56 ± 4 vs. 40 ± 6 μm), but this did not affect ATP-mediated vasodilation (2A: 91 ± 12 vs. 88 ± 11 μm; 3A: 71 ± 7 vs. 66 ± 7 μm). The effect of l-NA and/or indomethacin was typically evident after ∼15 min. MAP was 89 ± 7 mmHg during baseline conditions and did not change with the addition of 10−5 M ATP + 10 μM indomethacin (88 ± 5 mmHg), 10−5 M ATP + 100 μM l-NA (93 ± 3 mmHg) or 10−5 M ATP + 10 μM indomethacin and 100 μM l-NA (90 ± 2 mmHg).
Fig. 2.
Role of nitric oxide (NO) and prostanoids for the vasoactive effect of ATP in the superfusate in rat gluteus maximus muscle. Effect of 10 μM indomethacin and/or 100 μM l-NA on arteriolar dilation (A; n = 7; B, n = 11 or 12) and blood flow (C, n = 3 or 4; D, n = 4 or 5) of second-order and third-order branches in rat gluteus maximus muscle with the addition of 10−5 M ATP to the superfusate. **P < 0.001, significantly different from 10−5 M ATP. #P < 0.05, significantly different from 10−5 M ATP + indomethacin.
Effect of ATP on DAF-FM Fluorescence and 6-keto PGF1α Levels in Cultured Microvascular Endothelial and Skeletal Muscle Cells
The addition of 50, 250 (P < 0.05), and 750 μM (P < 0.001) of ATP to microvascular endothelial cells induced an increase in DAF-FM fluorescence (Fig. 3). The increase in DAF-FM fluorescence was more pronounced with 750 μM ATP than with 50 and 250 μM ATP (P < 0.05). Similarly, the level of 6-keto PGF1α was increased (P < 0.05) with the addition of 50, 250, and 750 μM of ATP.
Addition of 50, 250 (P < 0.05), and 750 μM (P < 0.001) of ATP to skeletal muscle cells increased DAF-FM fluorescence (Fig. 4). The increase in DAF-FM fluorescence was more pronounced with 750 μM ATP than with 50 and 250 μM ATP (P < 0.05). The levels of 6-keto PGF1α remained unchanged with ATP application.
Muscle Interstitial NOx and 6-keto PGF1α Levels with Interstitial ATP Infusion in Humans
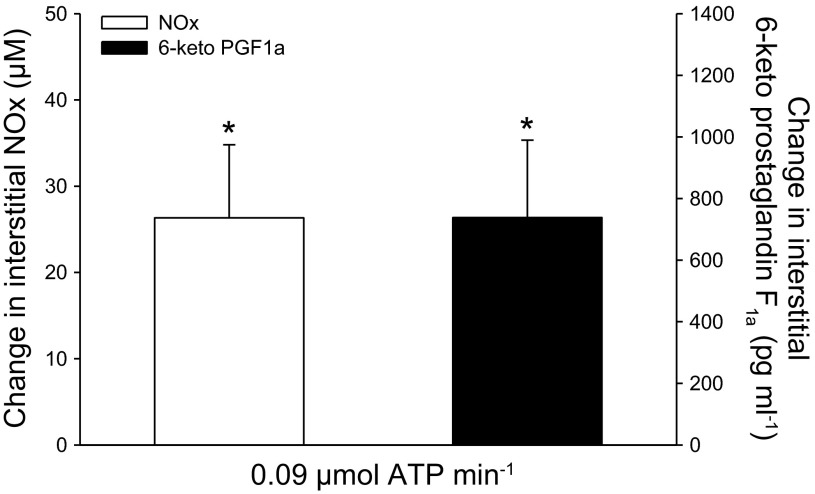
Interstitial NOx was 44 ± 6 μmol/l at baseline and the level increased (P < 0.05) to 71 ± 13 μmol/l during ATP infusion (Fig. 5). Similarly, interstitial 6-keto PGF1α was 1,885 ± 752 pg/ml at baseline and increased (P < 0.05) to 2,624 ± 864 pg/ml during ATP infusion. The levels of interstitial NOx (49 ± 9 vs. 44 ± 6 μmol/l) and PGF1α (2,127 ± 813 vs. 1,885 ± 752 pg/ml) were unchanged during 1 h of rest (infusion of Ringer acetate only). Heart rate (62 ± 3 vs. 63 ± 5 bpm) and MAP (96 ± 5 vs. 97 ± 4 mmHg) were unchanged in response to interstitial ATP infusion.
Fig. 5.
Changes in human skeletal muscle interstitial NOx and 6-keto PGF1α concentrations with interstitial ATP infusion. *Significant change from baseline, P < 0.05; n = 6.
DISCUSSION
The results from the present investigation demonstrate for the first time that raising the concentration of ATP in rat skeletal muscle interstitial fluid to concentrations measured in human and animal skeletal muscle interstitium during contractions leads to vasodilation and increases in blood flow in skeletal muscle. This finding suggests that the contraction-induced increase in skeletal muscle interstitial [ATP] is important for exercise hyperemia. Furthermore, the vasodilator effect induced by interstitial ATP application is dependent on formation of NO and prostanoids by endothelial and skeletal muscle cells. Future studies should aim at elucidating the extent by which P2 vs. P1 receptors are involved.
Increasing the Concentration of ATP in the Interstitial Fluid Induces Vasodilation and Increases Blood Flow in Rat Skeletal Muscle
The vasoactive effect of ATP when applied on the outside of arterioles has only been addressed in a few studies (17–19), and the studies examining skeletal muscle tissue have all proposed that interstitial ATP induces vasoconstriction via P2x receptors (22, 37, 38). This is in contrast to evidence from isolated rat cerebral arterioles and hamster cheek pouch, demonstrating that extraluminal application of ATP causes a biphasic response with an initial transient constriction followed by dilation (17–19). In congruence with the observation of an initial constriction, adding ATP to the superfusate in the present rat gluteus maximus preparation evoked vasoconstriction when the lowest concentration of ATP of 10 nM was applied. Furthermore, vasodilation and increases in blood flow were observed when the concentration of ATP was increased to 1–10 μM in the superfusion. In human skeletal muscle, the interstitial concentration of ATP is ∼150 nM at rest and increases to values of ∼1–8 μM during light- to moderate-intensity exercise (26, 44). In rat (37, 65, 66), canine (41), and cat (36) skeletal muscle, interstitial ATP levels at rest are comparable to those obtained from humans, and the concentration increases in a tension-dependent manner with mechanical stretch and electrical stimulation and concentrations up to ∼3 μM have been reported (36, 37, 41, 66). Importantly, because of release of ATP from endothelial cells and skeletal muscle cells in response to skeletal muscle contractions, the in vivo ATP concentration at the cell surface is likely to be several-fold higher than that reported for the bulk solution (35, 64). This indicates that, to mimic the in vivo situation, a higher concentration of ATP in the superfusion in the current experimental setup was needed to mirror the actual concentration at the cell surface.
The increase in skeletal muscle blood flow in response to contraction is very rapid, increasing within the first second following release of a brief contraction in both human and animal models (13). During repeated contractions, once the effects of muscle mechanical factors stabilize, additional factors contribute to increase exercise hyperemia with an onset latency of two to four contractions (54). In the present in vivo rat gluteus maximus skeletal muscle preparation, vasodilation was evident within the first few seconds after the superfusion with ATP reached the skeletal muscle, suggesting that interstitial ATP could be important for the increase in vasodilation during the initial phase of exercise. Collectively, the findings from the current study demonstrating that elevating the concentration of interstitial ATP to 1–10 μM leads to (rapid) vasodilation and increases in blood flow provides evidence that interstitial ATP is important for the regulation of exercise hyperemia.
It has been proposed that skeletal muscle fibers release ATP when intracellular H+ is increased (65) and by membrane depolarization, intracellular Ca2+ or mechanical stress (9). Once released, ATP is rapidly degraded by ectophosphatases on the sarcolemma and plasma membrane of endothelial cells where ecto AMP 5′ nucleotidase catalyzes the conversion of AMP to adenosine (24, 25). Adenosine has been shown to be essential for exercise hyperemia (45, 52, 53, 56), and the concentration of interstitial adenosine is, similar to ATP, associated with the magnitude of exercise hyperemia (26). These observations suggest that the adenosine that contributes to skeletal muscle blood flow regulation in contracting muscle originates from ATP released from skeletal muscle fibers, which is supported by the observation that skeletal muscle cells take up rather than release adenosine (25). This suggestion implies that the vasodilator effect of interstitial ATP is, at least in part, mediated via the formation of adenosine, as previously suggested in the study by Duza and Sarelius (19), focusing on the arterioles in the hamster cheek pouch. This potential mechanism of action of interstitial ATP would then be in contrast to that of intravascular ATP as the vasodilator effect of ATP within the blood vessels does not appear to be dependent on the action of adenosine (34, 42, 57). However, the role of adenosine as a mediator of interstitial ATP-induced vasodilation is in congruence with the finding that NO and prostacyclin also mediate the vasodilator effect of adenosine (45, 51). On the basis of the putative role of adenosine in ATP-induced vasodilation, it could be speculated that a vasodilator effect of increasing interstitial ATP would be expected; however, a strong vasoconstrictor action of ATP could have overruled such a vasodilator effect of adenosine. Further studies should aim at determining the specific role of adenosine in interstitial ATP-induced vasodilation.
Role of NO and Prostanoids in Interstitial ATP-Induced Vasodilation in Rat Skeletal Muscle
Intravascular ATP has been shown to induce vasodilation in various vascular beds by stimulating the formation of NO (4, 11, 14, 21, 40, 42) and prostanoids (42), although this is not a universal finding (57). To examine to what extent the synthesis of NO and/or prostanoids is an inherent component of the vasodilator effect of increasing the concentration of skeletal muscle interstitial ATP, responses to ATP were monitored under control conditions and in the presence of inhibitors of prostanoid and/or NO formation in the rat gluteus maximus skeletal muscle preparation. Inhibition of either prostanoid or NO formation caused a large attenuation of the ATP-induced increase in vasodilation and blood flow. The finding that NO is important for the dilator effect of increasing the level of interstitial ATP is in congruence with observations in isolated rat cerebral arterioles (17). However, the vasodilation induced by ATP is not dependent on prostanoid formation in these vessels (17), suggesting that the mediation by prostanoids observed in the present study may be specific for skeletal muscle. Notably, no additive effect of combining l-NA and indomethacin was detected, which is in agreement with NO mediating a part of the prostacyclin-induced vasodilation (33, 48). We have previously shown by immunohistochemistry that P2y2 receptors are present on capillary endothelial cells (42), whereas other P2y isoforms, including P2y11, appear to be present on skeletal muscle cells (5). The specific role of these receptors in the ATP-induced formation of NO and prostanoids remains to be resolved.
The finding that the vasodilator effect of increasing interstitial [ATP] in the rat gluteus maximus model was almost abolished during inhibition of NO, and prostanoid formation suggests that the overall contribution of interstitial ATP to exercise hyperemia is highly dependent on these vasodilator systems. Previous studies have shown that combined inhibition of NO and prostanoid formation reduces blood flow to the exercising leg by ∼30% (43, 50). The limited reduction in blood flow during such inhibition is thought to be due to a highly complex and redundant system of blood flow regulation, in which several vasodilator compounds are involved and interact to produce the highly precise level of blood flow needed in the muscle and in which vasodilator systems can compensate when the formation of a vasodilator is impaired. This proposition may also explain why simultaneous inhibition of NO and prostanoid formation only has a transient effect on leg hemodynamics during prolonged low-intensity exercise (61).
It cannot be excluded that ATP from the superfusate crossed the endothelial layer and interacted with P2y and/or P1 receptors on the luminal side of endothelial cells to induce vasodilation via the formation of NO and prostanoids. However, it has been shown in skeletal muscle that the concentration difference of ATP over the capillary wall has to be 50-fold in order for ATP to cross the endothelium (41). Moreover, ATP infused into the femoral artery of humans is not detectable in the muscle interstitium (44). In the current study, pronounced vasodilation was evident at a superfusate concentration of 1 μM, and arterial and venous concentrations of ATP in Sprague-Dawley rats are ∼3 and 5 μM (30), respectively, suggesting that the concentration gradient was toward the interstitium. Nevertheless, the fact that the concentrations of ATP used in the present investigation were comparable to those observed during muscle contraction, underscores that such a mechanism of action would be physiologically relevant.
Sources of ATP-Induced Formation of NO and Prostacyclin in Rat Skeletal Muscle
To determine which cells are responsible for the increase in NO and prostacyclin in vivo, experiments were performed on cell cultures of rat skeletal muscle myotubes and microvascular endothelial cells isolated from skeletal muscle tissue. Previous observations have demonstrated that ATP stimulates the release of NO (55) and prostacyclin (10, 12) from human umbilical vein endothelial cells. The current study extends these observations to microvascular endothelial cells in skeletal muscle, thereby confirming that these cells may be a source of NO and prostacyclin formed during ATP stimulation in skeletal muscle. Furthermore, ATP stimulated the release of NO from skeletal muscle cells, whereas no changes in prostacyclin levels were detected. The lack of release of prostacyclin from myotubes is in agreement with the observation that prostacyclin levels remain unaltered when myotubes are treated with adenosine (51) and when electrostimulated (Nyberg M and Hellsten Y, unpublished results). These observations suggest that the increase in interstitial prostacyclin in response to interstitial ATP primarily originates from microvascular endothelial cells in the muscle tissue.
Skeletal muscle cells and endothelial cells were obtained from m. gastrocnemius, m. soleus and m. quadriceps, whereas the skeletal muscle of interest in the intravital microscopy model was m. gluteus maximus. Although the response to ATP could be different in various skeletal muscles, the finding that the skeletal muscle cell preparation and the gluteus maximus model responded to ATP suggests a similar signaling pathway. Taken together, we conclude from our data that skeletal muscle cells and endothelial cells contribute to skeletal muscle interstitial NO and prostacyclin levels during ATP stimulation in vivo.
Interstitial ATP Stimulates the Formation of NO and Prostacyclin in Human Skeletal Muscle
To confirm the observations in rat skeletal muscle in humans, ATP was infused through microdialysis probes directly into the muscle interstitium of human subjects. When ATP was administered, the concentration of NO and prostacyclin was found to increase in the dialysate. This finding confirmed that increasing the level of interstitial ATP stimulates the formation of these vasoactive substances in human skeletal muscle, supporting the suggestion that the vasodilator effect of increasing the concentration of interstitial ATP is dependent on formation of NO and prostanoids. In addition, this finding in humans supports a role of interstitial ATP in the regulation of blood flow to contracting muscle, as inhibition of these systems lowers exercise hyperemia in humans (6, 23, 43, 45).
Vasoconstrictor Effect of Interstitial ATP
There is growing evidence that P2x receptors on vascular smooth muscle play an important role in vasoconstriction at rest and during exercise (7, 8, 15). This effect of ATP on hemodynamics is in congruence with the current finding that ATP evoked vasoconstriction at the lowest concentration of 10 nM. The potent vasodilator effect of ATP also found in the current study with the higher concentrations is likely to mask the underlying vasoconstrictor effect. In this setting, the increase in interstitial ATP during muscle contraction would induce vasodilation, although the increase in blood flow would be a result of a concomitant vasodilator and vasoconstrictor stimuli. This suggestion is in agreement with the observation that P2X blockade increases vascular conductance in exercising muscle (8). The dual role of ATP remains speculative, but it may serve to optimize blood flow within the contracting muscle and/or to increase peripheral resistance to ensure adequate mean arterial pressure and perfusion pressure at lower exercise intensities.
Perspectives and Significance
To complement the current finding that infusion of ATP via microdialysis probes leads to formation of potent vasodilators, future studies should aim at developing methods that reliably allow for determination of the microvascular response to interstitial ATP infusion in humans. In addition, the interstitial ATP concentration in contracting skeletal muscle has been found to be higher in endurance-trained subjects (46). This observation, combined with the present finding of a vasodilator effect of interstitial ATP, suggests that interstitial ATP could be important for blood flow distribution and improved conditions for O2 diffusion associated with exercise training (32, 59). This aspect will be important to focus on in future investigations.
GRANTS
This work was supported by a grant from the Danish Medical Research Council and the Lundbeck Foundation. All animal research was conducted in Canada and was supported by a Natural Sciences and Engineering Research Council (NSERC) grant (R4218A03) awarded to D.N.J. M.N. was supported by a grant from the Lundbeck foundation. S.P.M. was supported by a grant from the Danish Council for Independent Research - Medical Sciences. B.K.A.-K. was supported by OGS and NSERC CGS-D scholarships.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.N., B.K.A.-K., S.P.M., D.N.J., C.G.E., and Y.H. conception and design of research; M.N., B.K.A.-K., and S.P.M. performed experiments; M.N. and B.K.A.-K. analyzed data; M.N., B.K.A.-K., S.P.M., D.N.J., C.G.E., and Y.H. interpreted results of experiments; M.N. prepared figures; M.N., B.K.A.-K., S.P.M., D.N.J., C.G.E., and Y.H. drafted manuscript; M.N., B.K.A.-K., S.P.M., D.N.J., C.G.E., and Y.H. edited and revised manuscript; M.N., B.K.A.-K., S.P.M., D.N.J., C.G.E., and Y.H. approved final version of manuscript.
ACKNOWLEDGMENTS
Karina Olsen is gratefully acknowledged for her isolation of microvascular endothelial cells and other excellent technical assistance.
REFERENCES
- 1. Al-Khazraji BK, Novielli NM, Goldman D, Medeiros PJ, Jackson DN. A simple “streak length method” for quantifying and characterizing red blood cell velocity profiles and blood flow in rat skeletal muscle arterioles. Microcirculation 19: 327–335, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol 561: 535–545, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bender SB, Berwick ZC, Laughlin MH, Tune JD. Functional contribution of P2Y1 receptors to the control of coronary blood flow. J Appl Physiol 111: 1744–1750, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borno A, Ploug T, Bune LT, Rosenmeier JB, Thaning P. Purinergic receptors expressed in human skeletal muscle fibres. Purinergic Signal 8: 255–264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543: 691–698, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buckwalter JB, Hamann JJ, Clifford PS. Vasoconstriction in active skeletal muscles: a potential role for P2x purinergic receptors? J Appl Physiol 95: 953–959, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Buckwalter JB, Taylor JC, Hamann JJ, Clifford PS. Do P2x purinergic receptors regulate skeletal muscle blood flow during exercise? Am J Physiol Heart Circ Physiol 286: H633–H639, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Buvinic S, Almarza G, Bustamante M, Casas M, Lopez J, Riquelme M, Saez JC, Huidobro-Toro JP, Jaimovich E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem 284: 34490–34505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carter TD, Hallam TJ, Cusack NJ, Pearson JD. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol 95: 1181–1190, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casey DP, Joyner MJ. Local control of skeletal muscle blood flow during exercise: influence of available oxygen. J Appl Physiol 111: 1527–1538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi J, Hammer LW, Hester RL. Calcium-dependent synthesis of prostacyclin in ATP-stimulated venous endothelial cells. Hypertension 39: 581–585, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res 56: 43–53, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Delorey DS, Buckwalter JB, Mittelstadt SW, Anton MM, Kluess HA, Clifford PS. Is tonic sympathetic vasoconstriction increased in the skeletal muscle vasculature of aged canines? Am J Physiol Regul Integr Comp Physiol 299: R1342–R1349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand 162: 411–419, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Dietrich HH, Horiuchi T, Xiang C, Hongo K, Falck JR, Dacey RG., Jr Mechanism of ATP-induced local and conducted vasomotor responses in isolated rat cerebral penetrating arterioles. J Vasc Res 46: 253–264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dietrich HH, Kajita Y, Dacey RG., Jr Local and conducted vasomotor responses in isolated rat cerebral arterioles. Am J Physiol Heart Circ Physiol 271: H1109–H1116, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Duza T, Sarelius IH. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+. Am J Physiol Heart Circ Physiol 285: H26–H37, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Gorman MW, Rooke GA, Savage MV, Jayasekara MP, Jacobson KA, Feigl EO. Adenine nucleotide control of coronary blood flow during exercise. Am J Physiol Heart Circ Physiol 299: H1981–H1989, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2x agonist in cats. J Appl Physiol 96: 1166–1169, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Heinonen I, Saltin B, Kemppainen J, Sipila HT, Oikonen V, Nuutila P, Knuuti J, Kalliokoski K, Hellsten Y. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. Am J Physiol Heart Circ Physiol 300: H1510–H1517, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Hellsten Y. The effect of muscle contraction on the regulation of adenosine formation in rat skeletal muscle cells. J Physiol 518: 761–768, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hellsten Y, Frandsen U. Adenosine formation in contracting primary rat skeletal muscle cells and endothelial cells in culture. J Physiol 504: 695–704, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation 98: 6–8, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Hellsten Y, Nyberg M, Mortensen SP. Contribution of intravascular versus interstitial purines and nitric oxide in the regulation of exercise hyperaemia in humans. J Physiol 590: 5015–5023, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hopwood AM, Burnstock G. ATP mediates coronary vasoconstriction via P2x-purinoceptors and coronary vasodilatation via P2y-purinoceptors in the isolated perfused rat heart. Eur J Pharmacol 136: 49–54, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Hungerford JE, Sessa WC, Segal SS. Vasomotor control in arterioles of the mouse cremaster muscle. FASEB J 14: 197–207, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Jansson PA, Veneman T, Nurjhan N, Gerich J. An improved method to calculate adipose tissue interstitial substrate recovery for microdialysis studies. Life Sci 54: 1621–1624, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Kalliokoski KK, Oikonen V, Takala TO, Sipila H, Knuuti J, Nuutila P. Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance-trained men. Am J Physiol Endocrinol Metab 280: E1015–E1021, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Kamper AM, Paul LC, Blauw GJ. Prostaglandins are involved in acetylcholine- and 5-hydroxytryptamine-induced, nitric oxide-mediated vasodilatation in human forearm. J Cardiovasc Pharmacol 40: 922–929, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Vasodilatory responsiveness to adenosine triphosphate in ageing humans. J Physiol 588: 4017–4027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785–795, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 95: 577–583, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Li J, King NC, Sinoway LI. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation 111: 2748–2751, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol 283: H2636–H2643, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res 95: 269–280, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Circ Physiol 272: H1886–H1891, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP and ATP in dog skeletal muscle. J Physiol 536: 593–603, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol 296: R1140–R1148, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mortensen SP, Gonzalez-Alonso J, Nielsen JJ, Saltin B, Hellsten Y. Muscle interstitial ATP and norepinephrine concentrations in the human leg during exercise and ATP infusion. J Appl Physiol 107: 1757–1762, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signaling in the human leg. J Physiol 590: 6227–6236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mortensen SP, Thaning P, Nyberg M, Saltin B, Hellsten Y. Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol 589: 1847–1857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension 53: 973–978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol 285: R143–R148, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Nyberg M, Mortensen SP, Saltin B, Hellsten Y, Bangsbo J. Low blood flow at onset of moderate-intensity exercise does not limit muscle oxygen uptake. Am J Physiol Regul Integr Comp Physiol 298: R843–R848, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Nyberg M, Mortensen SP, Thaning P, Saltin B, Hellsten Y. Interstitial and plasma adenosine stimulate nitric oxide and prostacyclin formation in human skeletal muscle. Hypertension 56: 1102–1108, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Poucher SM. The role of the A2A adenosine receptor subtype in functional hyperaemia in the hindlimb of anaesthetized cats. J Physiol 492: 495–503, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Radegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand 171: 177–185, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Radegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol Heart Circ Physiol 274: H314–H322, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic P2y2 receptors mediate rapid Ca2+ mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium 49: 240–248, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Ray CJ, Marshall JM. Elucidation in the rat of the role of adenosine and A2A-receptors in the hyperaemia of twitch and tetanic contractions. J Physiol 587: 1565–1578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rongen GA, Smits P, Thien T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation 90: 1891–1898, 1994 [DOI] [PubMed] [Google Scholar]
- 58. Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558: 351–365, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation 38: 1–78, 1968 [PubMed] [Google Scholar]
- 60. Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods 40: 31–38, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, Curry TB, Joyner MJ. Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol 109: 768–777, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev 19: 313–349, 1991 [PubMed] [Google Scholar]
- 63. Sneddon P, Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol 106: 149–152, 1984 [DOI] [PubMed] [Google Scholar]
- 64. Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem 276: 32925–32932, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Tu J, Le G, Ballard HJ. Involvement of the cystic fibrosis transmembrane conductance regulator in the acidosis-induced efflux of ATP from rat skeletal muscle. J Physiol 588: 4563–4578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tu J, Lu L, Cai W, Ballard HJ. cAMP/protein kinase A activates cystic fibrosis transmembrane conductance regulator for ATP release from rat skeletal muscle during low pH or contractions. PLos One 7: e50157, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673–694, 2008 [DOI] [PubMed] [Google Scholar]