Abstract
The C1 neurons reside in the rostral and intermediate portions of the ventrolateral medulla (RVLM, IVLM). They use glutamate as a fast transmitter and synthesize catecholamines plus various neuropeptides. These neurons regulate the hypothalamic pituitary axis via direct projections to the paraventricular nucleus and regulate the autonomic nervous system via projections to sympathetic and parasympathetic preganglionic neurons. The presympathetic C1 cells, located in the RVLM, are probably organized in a roughly viscerotopic manner and most of them regulate the circulation. C1 cells are variously activated by hypoglycemia, infection or inflammation, hypoxia, nociception, and hypotension and contribute to most glucoprivic responses. C1 cells also stimulate breathing and activate brain stem noradrenergic neurons including the locus coeruleus. Based on the various effects attributed to the C1 cells, their axonal projections and what is currently known of their synaptic inputs, subsets of C1 cells appear to be differentially recruited by pain, hypoxia, infection/inflammation, hemorrhage, and hypoglycemia to produce a repertoire of stereotyped autonomic, metabolic, and neuroendocrine responses that help the organism survive physical injury and its associated cohort of acute infection, hypoxia, hypotension, and blood loss. C1 cells may also contribute to glucose and cardiovascular homeostasis in the absence of such physical stresses, and C1 cell hyperactivity may contribute to the increase in sympathetic nerve activity associated with diseases such as hypertension.
Keywords: C1 neurons, blood pressure, brain stem
best known for their contribution to the control of arterial pressure (AP), the C1 neurons have also been implicated in many other physiological processes ranging from neuroendocrine responses to infection and inflammation, glucose homeostasis, reproduction, breathing, thermoregulation, hypothalamo-pituitary axis (HPA)-mediated stress responses, and food consumption. The purpose of this review is to summarize the most salient information concerning the C1 cells, to point out some of the remaining gaps in our current knowledge, and to suggest a few unifying physiological principles that could account for these seemingly disparate observations.
Based on the various effects attributed to the C1 cells and what is currently known of their synaptic inputs, we propose that these neurons are, figuratively speaking, the body's “emergency medical technicians.” By this we imply that these neurons produce stereotyped autonomic, metabolic, and neuroendocrine responses designed to help the organism survive major acute physical stresses such as accidental, pathological, or dive-related hypoxia or physical injury and its associated cohort of acute infection, blood loss, and hypotension. These emergency responses include, in the short term and depending on the stress, vasoconstriction, cardioinhibition, or acceleration, breathing stimulation, antidiuresis, changes in metabolism, and gastrointestinal (GI) functions designed to conserve peripheral glucose supplies for selective use by the brain, adjustment of body temperature via regulation of brown adipose fat, and positive or negative stimulation of food intake.
Identification and Definition of the C1 Neurons
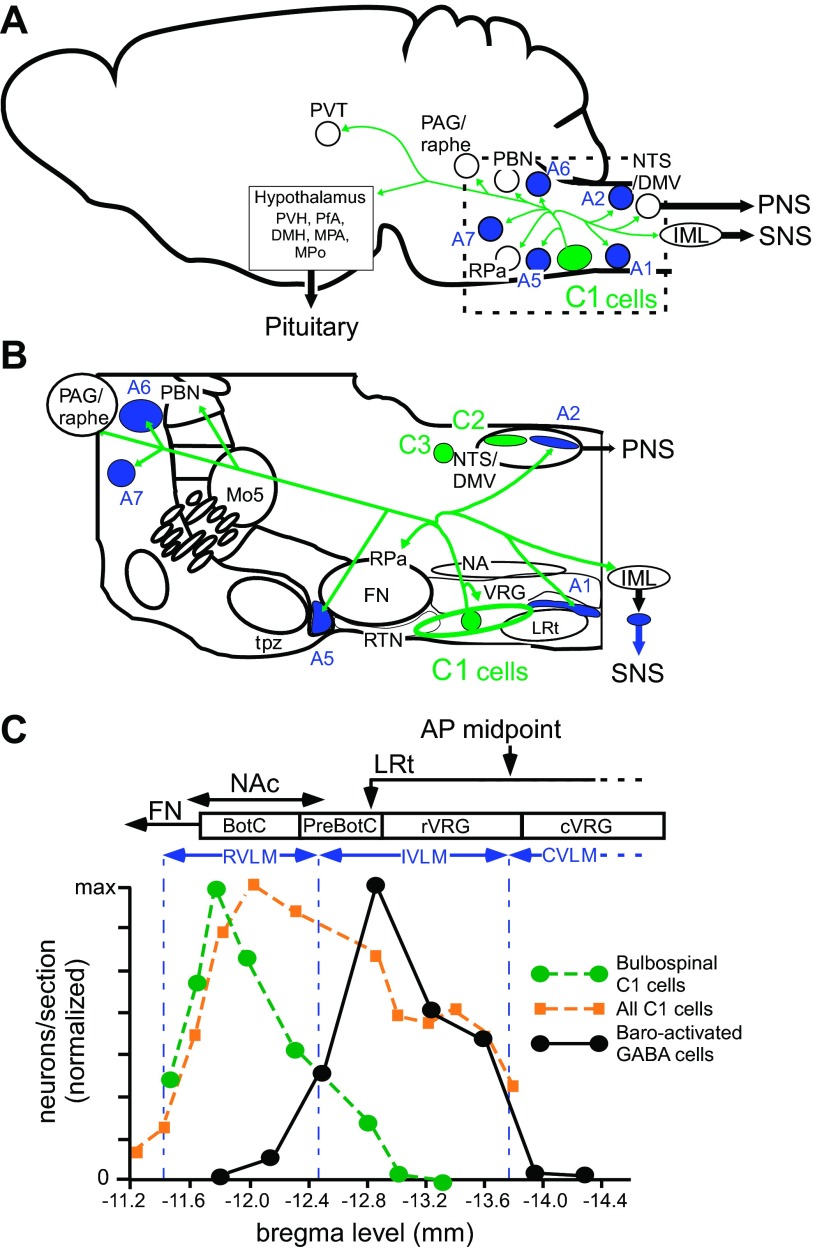
The ability of brain tissue to synthesize epinephrine from norepinephrine enzymatically was demonstrated by Barchas et al. in 1969 (38). The responsible enzyme phenylethanolamine N-methyl transferase (PNMT) was purified by Joh and Goldstein (87) in 1973, and PNMT was soon after detected by immunohistochemistry in the brain of rats (74, 75). The largest cluster of PNMT immunoreactive (ir) neurons, called C1, was found in the ventrolateral aspect of the medulla oblongata (VLM) roughly from the caudal end of the facial motor nucleus to the rostral third of the lateral reticular nucleus corresponding to the level of the area postrema in the transverse plane (Fig. 1, A–C). The C1 cells represent the rostral half of a column of VLM catecholaminergic neurons that extends down to the spinomedullary junction. This cell column was originally identified as catecholaminergic using the Falck-Hillarp fluorescence method and was called the A1 group (45). The term A1 group is now used exclusively in reference to the VLM noradrenergic neurons (catecholaminergic neurons that do not make PNMT). The location of the A1 neurons and of other lower brain stem noradrenergic neurons is also shown in Fig. 1, A and B. The C1 group contains around 72% of the medullary PNMT ir neurons in rats (124). The only other PNMT-ir neurons, the C2 and C3 clusters, are located respectively in the rostral portion of the nucleus of the solitary tract and in the rostral part of the dorsomedial medulla (Fig. 1). They contain 13% (C2) and 15% (C3) of the PNMT-ir neuron population in rats (124). The C2 and C3 clusters are often lumped together in the literature as the C2 group. Almost all C1 neurons also contain tyrosine-hydroxylase (TH) and dopamine-β-hydroxylase (DβH), and a large majority (75%) possess detectable levels of aromatic amino acid decarboxylase (139). Accordingly, the C1 neurons should in theory be able to synthesize both norepinephrine and epinephrine.
Fig. 1.
Location and principal axonal projections of the C1 neurons. A: schematic representation of the location and main axonal projections of the C1 neurons (parasagittal view). B: higher resolution view of the pontomedullary region outlined in A showing the location of the C1 cells in relation to nearby anatomical structures. The location of the other groups of central nervous system (CNS) adrenergic neurons (C2 and C3) is also represented. The C1 neurons (in green) reside in the ventrolateral medulla (green oval in B) from the retrotrapezoid nucleus rostrally (RTN, putative central respiratory chemoreceptors) to the rostral one third of the lateral reticular nucleus (LRt, a precerebellar nucleus). The C1 neurons are located slightly ventral to the ventral respiratory group (VRG). The main projections of the C1 cells include lower brain stem noradrenergic neurons (A1, 2, 5, 6, 7, in blue), selected serotonergic neuron-rich regions (raphe pallidus RPa, dorsal raphe), the dorsal vagal complex (nucleus of the solitary tract and dorsal motor nucleus of the vagus, NTS/DMV), the intermediolateral cell column (IML) and numerous brain nuclei involved in the regulation of the sympathetic nervous system (SNS), parasympathetic nervous system (PNS) and the pituitary. The main rostral targets are the lateral parabrachial nuclei (PBN), the periacqueductal grey matter (PAG), and, in the hypothalamus, the paraventricular nucleus (PVH), the dorsomedial nucleus (DMH) and the perifornical area (PfA; the orexin neuron-rich area) and others (median preoptic area and medial preoptic nucleus, MPA, MPo). The C1 neurons also innervate the paraventricular nucleus of the thalamus (PVT), noted for its role in stress responses. FN, facial motor nucleus; Mo5 trigeminal motor nucleus; NA, nucleus ambiguus; tpz, trapezoid body. The axonal projections of the C1 neurons are based primarily on the result of virus-based tracing methods in rat and mouse (1, 30). Subsets of C1 neurons have more limited axonal projections. In general, most presympathetic C1 neurons do not innervate the hypothalamus. C: rostrocaudal distribution of the C1 cells (bulbospinal and other) and GABAergic interneurons that relay the baroreflex. The ordinate represents the number of neurons per transverse section (normalized to maximum) and the abscissa the Bregma level of the sections as per an atlas (138). The primary data are from Refs. 195 and 157. For comparison, the location of the three overlapping segments of the ventral respiratory column (BotC, Bötzinger complex; PreBotC, pre-Bötzinger complex; rVRG, rostral ventral respiratory group) are also represented (166). AP midpoint, midpoint of area postrema; CVLM, caudal VLM; IVLM, intermediate VLM; RVLM, rostral VLM; NAc, compact portion of nucleus ambiguus. The rostral end of the LRt (arrow) refers to the anterior tip of the bilobated portion of the nucleus according to (138).
In the early 1970s Hökfelt et al. (74, 75) provided a first-pass description of the collective axonal projections of the C1–3 PNMT-ir neurons. The identified targets consisted of the dorsal vagal complex, including the dorsal motor nucleus of the vagus (DMV), the intermediolateral cell column (IML), the locus coeruleus, the periacqueductal gray matter, the hypothalamic paraventricular nucleus (PVH), the perifornical region, and dorsomedial nucleus of the hypothalamus. Based on this projection pattern and the location of the somata, Hökfelt et al. (75) speculated that central nervous system (CNS) adrenergic neurons might control AP and respiration, food and water intake, gonadotrophin and oxytocin secretion, and body temperature. A role of the CNS adrenergic neurons in the control of vigilance was also hypothesized based on the presence of PNMT-ir terminals within the locus coeruleus.
Signaling by the C1 Neurons
Although most C1 neurons contain all the enzymes required to synthesize catecholamines, there is no direct evidence (e.g., electrochemical or electrophysiological) that they actually release such compounds and, if so, which (norepinephrine, epinephrine, or both), where (dendrites, subsets of terminals), and how (action potential-dependent release, receptor-mediated intracellular calcium release). Neurons innervated by the C1 cells (e.g., sympathetic preganglionic neurons, locus coeruleus, and DMV) typically respond to exogenously applied catecholamines via α (1 and/or 2) and/or β adrenergic receptors (6, 82, 116), but these receptors could be activated by norepinephrine released by noradrenergic neurons that also target the regions innervated by the C1 cells (e.g., A5 neurons in the intermediolateral cell column). Most C1 cells (90%) lack a plasmalemmal monoamine transporter suggesting that these cells, unlike their noradrenergic counterparts, lack the means of replenishing their catecholamine stores via reuptake (40, 107).
At present, the best documented aspect of C1 cell communication is their “wiring transmission” (201), which operates via conventional ionotropic glutamatergic synapses and likely accounts for the short-term effects that have been attributed to C1 cell activation in vivo; i.e., short time-scale sympathetic reflexes and acute AP stabilization (66, 128). The axonal varicosities of the C1 cells typically form conventional synapses; e.g., in the IML, locus coeruleus, rostral ventrolateral medulla (RVLM), and DMV (5, 52, 121–123). These synapses are usually (75%) asymmetric and the C1 neurons express vesicular glutamate transporter-2 (VGLUT2) mRNA and lack markers of inhibitory neurons such as glutamic acid decarboxylase (GAD), and glycine transporter 2 (GlyT2) (39, 160, 176, 178). VGLUT2 is a protein whose presence is necessary and sufficient for neurons to release glutamate by calcium-dependent exocytosis (183). For these and other reasons of a pharmacological nature, the C1 neurons have long been suspected to signal via conventional glutamatergic transmission. This hypothesis has now been verified in the case of the C1 projections to the DMV using electron microscopy and optogenetics (52).
The C1 neurons express various combinations of neuropeptides; e.g., neuropeptide Y (NPY), substance P, enkephalins, thyrotropin-releasing hormone (TRH), cocaine- and amphetamine-regulated transcript (CART), and pituitary adenylate cyclase-activating polypeptide (PACAP) (141, 174). TRH and substance P receptors are Gq-linked and typically produce excitatory effects, whereas enkephalin and NPY receptors are Gi/o-linked and cause pre- and postsynaptic inhibition. Therefore, in theory, the C1 neurons should be capable of exerting complex pre- and postsynaptic effects depending on the types of peptides that they release and the composition of the nearby neuropil. These peptides do indeed have distinctive actions when injected intrathecally or into the RVLM (e.g., 27, 57 and other papers from this group), but their cellular mechanisms of action and the physiological context of their release are unknown.
The PVH has been investigated in greater detail and provides a possible model of how catecholamines and NPY, two transmitters presumably released by the C1 cells and other lower brain stem catecholaminergic neurons, operate at the cellular level. The NPY innervation of the PVH originates from three sources, adrenergic neurons (primarily C1 and to a lesser degree C3 for a total of ∼42% of the NPY-positive terminals in the PVH), noradrenergic neurons (primarily A1 and a small fraction of A2 for a total of 21%) while the remaining 36% of PVH NPY terminals originate from agouti-related peptide-(AgRP)-synthesizing arcuate neurons (59). The A1 neurons target primarily the magnocellular component, whereas the C1 and A2 neurons innervate predominantly the parvocellular division of the PVH (59). As a result, the lower brain stem, particularly the C1 group, is the main source of NPY-ir terminals present within the parvocellular division of the PVH. All known NPY receptors are Gi/o coupled and are therefore assumed to cause presynaptic and/or postsynaptic inhibition by activating inwardly rectifying potassium conductances or by inhibiting calcium channels (187). Accordingly, any excitatory effect of NPY ought to be mediated by disinhibition. NPY released along with GABA by the nerve terminals of arcuate neurons in the PVH appears to stimulate food intake by inhibiting a subset of melanocortin-4 receptor (MC4R)-expressing oxytocinergic neurons that innervate the dorsal medulla and the parabrachial region (16, 60, 61). The same mechanism could conceivably contribute to glucoprivic feeding, a response that is mediated by the C1 and or the A1 neurons and is attenuated by silencing NPY expression in these cells, albeit only if DβH is also downregulated (97). The effects of catecholamines on bulbospinal presympathetic PVH neurons have been investigated in rat brain slices. The most conspicuous effect of exogenously applied norepinephrine on these PVH cells is an increased excitatory postsynaptic currents (EPSC) frequency (α1-adrenoceptor mediated) and a reduced inhibitory postsynaptic currents (IPSC) frequency (α2-adrenoceptor mediated) (35, 98). In other words, in vitro, catecholamines exert a globally excitatory effect on the presympathetic PVH neurons, and this effect seems largely mediated by presynaptic modulation of other PVH inputs.
In summary, conventional ionotropic glutamatergic transmission mediates the short-term effects of the C1 cells. C1 cell-derived peptides and catecholamines have detectable effects in the regions innervated by the C1 cells. However, the only physiological deficit that has been associated with the downregulation of a peptide (NPY) made by the C1 cells is a reduction of the glucoprivic response, and even in this case, the deficit was observable only when catecholamine synthesis was simultaneously downregulated (97). The conditions under which the C1 cells release peptides or catecholamines and the effects that these substances produce need clarification.
Anatomical Nomenclature
In simple terms, the ventrolateral medulla (VLM) describes the ventrolateral quadrant of the medullary reticular formation. This region extends from the level of the facial motor nucleus to the spinomedullary junction. The C1 neurons are interspersed with various other neurons within the rostral half of the VLM. Approximately one-third of the C1 cells are presympathetic; i.e., innervate sympathetic preganglionic neurons, and these C1 cells are concentrated at the rostral pole of the VLM in a region described here as the RVLM (Fig. 1C). This region corresponds to the VLM “pressor area” defined by Sapru and colleagues (196), where injection of excitatory amino acids increases AP. As Fig. 1C emphasizes, C1 neurons are not restricted to the RVLM “pressor area.” C1 neurons are found within the VLM down to the level of the area postrema. Thus C1 neurons are also located in the “depressor area” of the VLM where injection of excitatory agents decreases AP in anesthetized rats (62, 196), presumably due to the activation of GABAergic interneurons that relay the baroreflex (see C1 Cells, Hypotension, and Baroreflexes). We define the VLM region that harbors these GABAergic neurons as the IVLM (intermediate VLM) (Fig. 1C) (195), and reserve the term caudal VLM (CVLM) to describe the region of the VLM located between the IVLM and the medullospinal junction (Fig. 1C). With the use of this nomenclature, the CVLM contains the A1 noradrenergic neurons and VLM regions areas where injections of excitatory agents increases AP in anesthetized rats (62). In the aggregate, the RVLM and IVLM are coextensive with the C1 cells. For reference, Fig. 1C also describes the location of four major functional subdivisions of the VLM defined by respiratory physiologists (for review see Ref. 166). The three most rostral regions reside dorsal to the C1 cell column, the fourth is approximately coextensive with the CVLM.
Projections of the C1 Neurons
The global reach of the C1 cells is extensive, well-described using viral vectors, and includes numerous brain stem and diencephalic structures typically associated with autonomic responses and stress behavior (Fig. 1, A and B) (1, 30). The projections and functions of the C2 group are essentially unknown. The C3 group (Fig. 1B) seems to have many of the same connections and properties as the C1 cells and may be an ectopic cluster of C1 cells (164). The axonal projections of individual C1 neurons are not precisely known but there is a strong presumption that these cells, including the presympathetic ones, are highly collateralized. In rats for example, at least 43% of RVLM bulbospinal barosensitive neurons could be antidromically activated from at least one of several structures such as the lateral parabrachial nucleus and the periaqueductal gray matter (70). In agreement with anatomical data (186) at least 10% of physiologically identified RVLM presympathetic neurons also innervate the hypothalamus (70, 175). This population includes NPY-expressing C1 neurons (70, 175). According to Llewellyn-Smith and colleagues, the presympathetic C1 neurons may contribute no more than 2% of the total synaptic input to sympathetic preganglionic neurons (102).
The terminal fields of C1 neurons are found throughout the VLM and innervate both catecholaminergic and noncatecholaminergic neurons. The noncatecholaminergic neurons targeted by the C1 neurons may mediate the stimulation of breathing produced by C1 neuron activation (see later section). Some C1 neurons are interconnected by recurrent collaterals (123). The presence of PNMT-ir synapses within the RVLM/IVLM was first described by Milner et al. (123) and has been recently confirmed (5). These connections may contribute to the tendency of sympathetic vasomotor efferents to discharge synchronously in vivo, even in the absence of baroreceptor input and respiratory network activity (19). Both recurrent excitation and inhibition can theoretically produce network synchronization. The nature of the recurrent interactions between C1 cells is unclear. The recurrent collaterals identified by Agassandian et al. (5) were ultrastructurally asymmetric and filled with small clear vesicles and could represent excitatory glutamatergic synapses. However, ionotropic glutamate transmission does not seem to contribute much to maintaining the on-going activity of the vasomotor C1 cells in vivo (182). An alternative possibility, supported by physiological evidence, is that the recurrent collaterals between C1 cells release catecholamines and are inhibitory. Indeed, the C1 neurons express α2-adrenergic receptors whose activation inhibits these cells in various ways (100) and iontophoretic application of α2-adrenergic receptor antagonists increases the activity of the C1 cells in vivo (10).
In brief, the C1 neurons are highly collateralized and influence many brain regions simultaneously. The C1 cells have recurrent interactions that may synchronize, amplify or restrain the discharge of subsets of these neurons.
C1 Cells, Hypotension, and Baroreflexes
The contribution of the C1 cells to the baroreflexes (the sympathetic, respiratory, and neuroendocrine responses elicited by hypotension via a reduction in the activity of arterial baroreceptors) is summarized in Fig. 2. Seminal experiments carried out in the late 1970s and early 1980s demonstrated that the RVLM is the source of a dense projection to the intermediolateral cell column (IML) and suggested that this region contained neurons whose on-going activity was required for the maintenance of AP and sympathetic vasomotor tone in anesthetized mammals (12, 46, 66, 150). Because the RVLM is approximately coextensive with a subset of C1 neurons that directly innervate sympathetic preganglionic neurons (Fig. 1C), these investigators, chief among them D. J. Reis, proposed that the bulbospinal C1 neurons drive vaso- and cardiomotor sympathetic efferents and stabilize AP under the regulatory influence of the arterial baroreflex (63, 148, 149). In support of this interpretation, the RVLM was found to contain spinally projecting neurons that were active at rest and inhibited by baroreceptor stimulation to a degree roughly commensurate with the change in sympathetic vasoconstrictor or cardiomotor outflow (e.g., 18, 25, 66). A large fraction of these neurons were later identified as C1 cells by intracellular and juxtacellular labeling methods, but these studies also identified a class of bulbospinal baro-inhibited neurons devoid of detectable catecholamine-biosynthetic enzyme (101, 156). These “non-C1” presympathetic neurons were unaffected by injecting the ribosome-inactivating toxin anti-DβH-saporin (anti-DβH-sap) into the spinal cord (161) which gave additional credence to the notion that they were not catecholaminergic. Anti-DβH-sap binds selectively to the DβH enzyme when it is exteriorized on the plasma membranes of catecholaminergic neurons during exocytosis (140). After internalization and retrograde transport to the cell bodies, the toxin kills the DβH-expressing neurons that project to the site of toxin injection (140). Neurons that are resistant to anti-DβH-sap are therefore presumed to be neither noradrenergic nor adrenergic. Based on juxtacellular labeling, 72% of the population of bulbospinal barosensitive (putative presympathetic) neurons of the rat RVLM could be C1 cells (154, 156). This proportion was estimated at only 26% when cell labeling was performed by intracellular recording (101). The higher estimate is in closer agreement with retrograde tracer anatomical experiments (e.g., 57) but each method has biases and the exact proportion of C1 versus non-C1 presympathetic neurons is unknown.
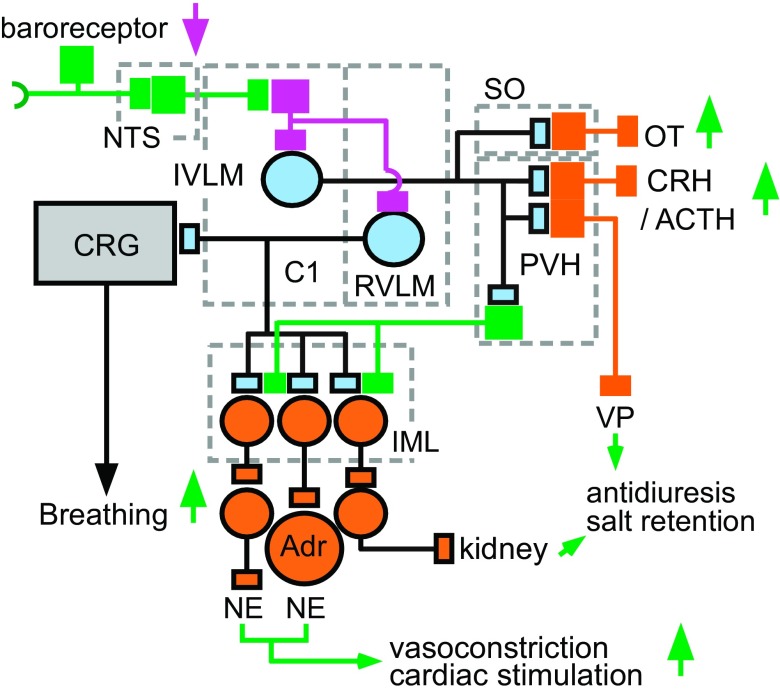
Fig. 2.
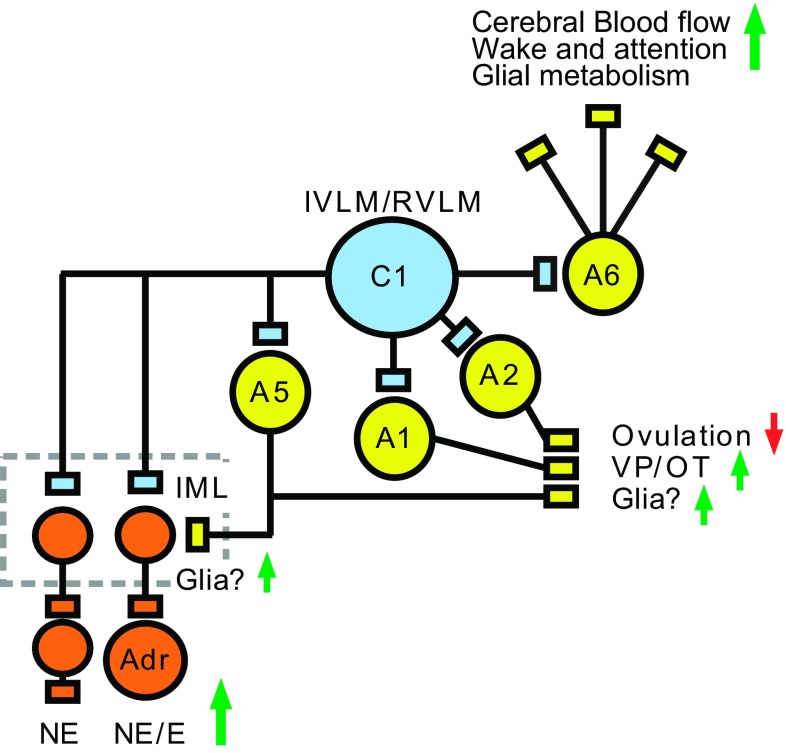
Contribution of the C1 cells to the autonomic responses to hypotension. This and the following three figures are color-coded as follows: C1 neurons in pale blue, autonomic neurons and hormone releasing PVH neurons in orange, excitatory glutamatergic neurons (except C1 cells) in green, inhibitory presumably GABAergic neurons in magenta. Hypotension disinhibits (activates) many C1 cells via a pathway that consists minimally of three neurons: baroreceptor afferent neurons (baroreceptor), glutamatergic neurons located in the NTS and projecting to the ventrolateral medulla and, finally, IVLM GABAergic interneurons innervating the C1 cells (158). Barosensitive C1 neurons located in the rostral ventrolateral medulla (RVLM) are predominantly bulbospinal. Barosensitive C1 neurons located in the IVLM innervate the hypothalamus among other places. The spinally projecting C1 cells innervate preganglionic sympathetic neurons located in the intermediolateral cell column (IML) that control a variety of vascular beds and the heart plus the norepinephrine (NE)-releasing chromaffin cells of the adrenal medulla (Adr). Hypotension also activates breathing by stimulating the central respiratory pattern generator (CRG). There is no direct evidence that this effect is mediated by axonal collaterals of presympathetic barosensitive C1 neurons as depicted. Hypotension also triggers the CRH/ACTH/corticosterone cascade, presumably through direct projections from IVLM C1 neurons to the paraventricular nucleus of the hypothalamus (PVH). Direct projections of barosensitive C1 cells to the PVH may also activate magnocellular vasopressin (VP)-releasing neurons and preautonomic PVH neurons that control renal sodium excretion. C1 cells, directly and/or indirectly (i.e., via A1 noradrenergic neurons) also activate the release of oxytocin (OT) from the supraoptic nucleus (SO). Barosensitive C1 cells also activate subsets of CNS noradrenergic neurons (not illustrated, see Fig. 5).
Both C1 and non-C1 presympathetic neurons express VGLUT2 and are therefore presumably glutamatergic (161). The non-C1 presympathetic neurons and about 40% of the C1 presympathetic neurons have lightly myelinated axons and axonal conduction velocity in the 1–8 m/s range, whereas the rest of the RVLM bulbospinal C1 cells have unmyelinated axons and axonal conduction velocity of less than 1 m/s (130, 156). Morphological differences have been observed between RVLM presympathetic neurons, but the distinguishing features (dendritic complexity, prominent axonal initial segment, abundance of enkephalin-ir synapses, lack of NPY mRNA) seem to correlate best with the degree of axonal myelination of these neurons (i.e., their axonal velocity) rather than with the presence of a catecholaminergic marker (TH or PNMT) (101, 103, 175). The C1 neurons of the IVLM that innervate the hypothalamus do not have myelinated axons (189). A unique diagnostic marker of the noncatecholaminergic RVLM presympathetic neurons has not been found. These neurons may be identified using a combination of markers such as VGLUT2, PACAP-, or preproenkephalin-mRNA (57, 177) but the presence of spinal projections and the absence of TH remain the key to their histological identification. These cells have not been characterized in species other than the rat.
The specific contribution of the C1 neurons to AP control in conscious rats was first tested by exploring the consequences of their destruction with anti-DβH-sap (110, 111). Despite very large reductions in C1 cell numbers (>80%) and substantial collateral damage to the A5 noradrenergic neurons, only modest reductions of resting mean AP were observed in conscious rats (<10 mmHg) (111). In these rats, plasma catecholamine levels were only marginally lower than in controls, corresponding to a small drop in resting sympathetic tone. However, there was a substantial reduction in the rise of plasma norepinephrine, vasopressin, and oxytocin following hydralazine-induced hypotension (respectively ∼40%, 73%, and 40%), indicating permanent deficits in the neuroendocrine adjustments to baroreceptor unloading (110). In other experiments, the presympathetic C1 neurons (and A5 neurons) were destroyed by injecting the same toxin into the spinal cord (157, 161). These lesions spared the bulbospinal barosensitive non-C1 RVLM neurons (161) and, as in the above-mentioned studies of Madden and Sved (110, 111), had inconsequential effects on resting AP (157). However, sympathoexcitatory responses evoked by electrical stimulation of the RVLM were severely attenuated as were the baroreflex and the increase in sympathetic nerve activity (SNA) elicited by carotid body stimulation. Together, these studies demonstrate that chronic lesions of the C1 neurons with anti-DβH-sap only modestly decreases resting AP and resting SNA, but permanently and substantially reduces the neuroendocrine response to hypotension, hypoxia, and nociception.
Interpreting the effects of chronic lesions of the C1 neurons is complicated by the potential development of compensatory mechanisms. For example, volume expansion by the kidney, baroreflex compensation, peripheral catecholaminergic receptor supersensitivity, and compensation by non-C1 presympathetic neurons may explain why C1 neuron lesions produce such modest hypotension at rest (110). The slow compensatory mechanisms that may be maintaining AP despite the absence of C1 neurons may be circumvented by applying rapid and selective loss of function approaches. Using the allatostatin receptor (R) method introduced by Callaway (184), Marina et al. (114) showed that acutely inhibiting the C1 cells produced large reductions of SNA, (∼50%) and AP (∼25 mmHg) in anesthetized rats and in an arterially perfused midcollicular transected preparation of the same species. These results, contrary to those of chronic lesions, support the view that the C1 cells make a large contribution to resting SNA and AP as first hypothesized by Reis and colleagues. A few caveats must be evoked however. The rat preparations used by Marina et al. (114) to test the effects of C1 cell inhibition were barodenervated and anesthetized or hypothermic mid-collicular transected arterially perfused preparations. Additionally, the lentiviral vector used in this study to introduce the allatostatin R into RVLM neurons is not entirely selective for the C1 neurons. The selectivity of this vector relies on the artificial promoter PRSx8, which is a binding site for transcription factors Phox2a and b (79). Phox2 is expressed by other RVLM neurons besides the C1 cells, notably by the retrotrapezoid nucleus and by subsets of cholinergic neurons (3). Also, the construct is somewhat leaky, which means that viral titer-dependent transgene expression eventually occurs even in Phox2-negative neurons exposed to PRSx8-lenti- or adeno-virus, albeit at a slower rate (see Ref. 79, Guyenet PG and Stornetta RL, unpublished results). In our experience (1) a more selective way to target the C1 neurons is to use the Cre-Lox technology with rodents transgenically engineered to express Cre under a catecholamine promoter in combination with directed injections of a floxed viral vector (31). Finally, the reduction of SNA observed in the study by Marina et al. (114) was still much smaller than that produced by administration into the RVLM of muscimol, a nonselective inhibitor of neuronal activity. Accordingly, the contribution of the C1 cells versus other RVLM neurons to resting AP in conscious mammals is still uncertain.
RVLM presympathetic neurons are probably organized in a roughly viscerotopic manner (skeletal muscles, viscera, heart, etc.). The principal evidence is that the sympathetic nerves to these organs can be differentially activated by microinjecting glutamate into separate regions of the RVLM of anesthetized cats (118). Similarities between the discharge pattern of individual presympathetic neurons and regional SNA in anesthetized rats also suggest that there exist at least two broad types of RVLM presympathetic neurons that differentially control skeletal muscle versus visceral vascular beds (e.g., 71, 153 and, for review, see Ref. 66). Cutaneous arterioles are preferentially controlled by supraspinal drives that originate from the medullary raphe (131). Although, the viscerotopic organization of the RVLM is an attractive theory, there is also anatomical evidence that supports the existence of C1 neurons with very broad target specificity that could potentially mediate the type of generalized sympathoactivation envisioned by Cannon (85).
RVLM presympathetic neurons are presumably inhibited by arterial baroreceptors via a chain of at least two CNS neurons (Fig. 2) but the monosynaptic nature of the connections between the postulated links has not been established (e.g., 33, 159 and, for reviews see Refs. 66 and 158). The first CNS neuron of the baroreflex is glutamatergic, resides in the dorsolateral aspect of the intermediate nucleus of the solitary tract (NTS), and activates GABAergic interneurons located in the IVLM (Figs. 1C and 2). The projection from NTS to IVLM is largely unilateral. There is almost complete overlap between the location of the baro-activated GABAergic neurons and the caudal portion of the C1 cell column (Figs. 1C and 2). The baro-activated GABA neurons of the IVLM innervate the RVLM bilaterally. Many C1 neurons located in the IVLM are also barosensitive (189) and presumably also receive input from the comingled baroactivated GABAergic interneurons like their presympathetic counterparts (Fig. 2). The baroinhibited C1 neurons located in the IVLM innervate the hypothalamus (189) and presumably regulate the secretion of oxytocin and vasopressin (Fig. 2).
In anesthetized rodents, C1 and other presympathetic neurons are extremely active even in the absence of cardiopulmonary (including baroreceptor) or supracollicular input (up to 35 Hz) (66, 76). This high level of activity is apparently not driven by conventional ionotropic glutamatergic inputs and is absent in dissociated C1 cells, which typically lack dendrites (66). The high activity of C1 cells present in vivo could possibly be partly intrinsic to these neurons, perhaps of dendritic origin, or it could be under the control of unconventional and as yet unidentified transmitters (66, 76). The basal discharge rate of various subsets of C1 cells could be regulated, directly or via the glia, by local factors such as hypoxia, hypoglycemia, inflammatory cytokines (IL1), blood flow restriction, and circulating steroid hormones (32, 146, 163).
Although respiratory control was originally listed as a probable function of the brain adrenergic system (75), the close proximity between the C1 cells and the respiratory column (Fig. 1, B and C) has made it difficult to assess whether these neurons control breathing. In mouse slices, fictive respiration is strongly activated by α1-adrenergic receptor stimulation, but unresolved species differences persist and these experiments have not demonstrated whether the C1 neurons or other catecholaminergic neurons regulate breathing (190, 199). Using optogenetics, we have shown that selective C1 neuron stimulation activates breathing in conscious mice (1). Barosensitive C1 neurons presumably contribute to the ventilatory baroreflex; i.e., the breathing stimulation elicited by hypotension, well described in conscious humans (173).
The parvocellular division of the PVH contains many types of neurons including presympathetic neurons thought to regulate renal function, the heart, and regional blood flows (Fig. 2) (23, 60). C1 neurons located in the IVLM densely innervate the PVN and likely control the circulation albeit via a more circuitous route than their presympathetic (bulbospinal) counterparts (Fig. 2). The evidence is twofold. The C1 cells densely innervate the parvocellular region of the PVH (1, 30) and norepinephrine produces presynaptic facilitation in presympathetic PVH neurons (35, 98). This evidence is still circumstantial because the C1 cells have not been shown to actually release catecholamines in the PVH but the fact that the C1 cells are glutamatergic renders the hypothesis that they activate PVH presympathetic neurons more plausible.
The extent to which the C1 cells contribute to sympathetic tone and AP in conscious mammals is still debatable because the consequences of a complete and instantaneous inhibition of these cells in the conscious state are unknown. The notion that these cells are critically important to AP maintenance in conscious mammals is still an extrapolation of data acquired under anesthesia or in reduced preparations in which resting SNA and the resting discharge of the C1 cells is abnormally high due to surgery and pharmacological issues (anesthetics). C1 lesion experiments (110, 111) suggest the C1 neurons contribute modestly to AP under normal resting conditions and are most important during acute hypotension. Such a drop in blood pressure would likely only occur in a pathological situation (e.g., hemorrhage).
Regulation of the Parasympathetic Outflow by the C1 Neurons: A Gastrointestinal, Cardiovagal, or Immune Connection?
The regulation of the parasympathetic nervous system by the C1 cells is rarely mentioned, perhaps because the bulk of the work dedicated to these cells has been focused on their role in controlling sympathetic vasomotor efferents and AP. Yet, the high density of PNMT-ir terminals within the dorsal vagal complex, especially within the DMV, was identified in the first paper dedicated to the immunohistochemical distribution of this enzyme (74). This observation was replicated by Siaud et al. (165) who suggested a direct PNMT innervation of the DMV neurons that control the stomach and pancreas. Loewy and coworkers used the retrograde migration of pseudorabies virus (PRV) from the pancreas to find that the PNMT innervation comes primarily from C1 cells with lesser contributions from the C2 and C3 neurons (104). This work was performed in rats subjected to a spinal cord transection at the C8 level, therefore, the C1 neurons could not have been labeled retrogradely via the sympathetic nerves. According to these authors, at least 25% of the C1 neurons, located in what appears to be the IVLM, innervate the DMV (104). A projection of the C1 cells to the DMV was also observed by Card and Sved (30) in rats. Using a Cre-dependent viral vector in both TH-Cre transgenic rats and DβH-Cre mice, we confirmed that the C1 cells innervate the DMV massively and we demonstrated that the connection between the C1 cells and parasympathetic preganglionic neurons is monosynaptic and excitatory (52). In this study 70% of the electrophysiologically sampled DMV neurons received input from C1 neurons. This high percentage may not be representative of the entire DMV but it suggests that C1 neurons probably target DMV neurons that innervate multiple organs besides the pancreas. Given the excitatory nature of this connection, C1 neurons have the potential of accelerating GI transit, secretion rate and blood flow within exocrine GI glands by activation of parasympathetic innervation to these targets. The potential role of this connection in glucoprivic responses or hypoxic adjustments is examined later.
The C1 cell projection to the DMV could also produce some form of cardioinhibition (rate and or ventricular contractility) or modulate immune responses via the spleen (36, 134, 170). Under most physiological circumstances cardiovagal tone and vasomotor sympathetic activity vary in opposite directions (e.g., exercise, baroreflex) but there are exceptions. Coactivation of these outflows occurs during diving, a behavior that triggers Fos activation throughout the A1/C1 group (119, 135). The most rostral of these activated neurons are most likely presympathetic neurons that contribute to the muscle and gut vasoconstriction associated with diving (119, 135). However, the rest of the C1 cells activated by diving do not innervate the spinal cord and may contribute to other autonomic responses associated with diving such as hepatic glucose release, epinephrine release favoring anaerobic metabolism and, possibly, bradycardia. Hypoxia is a condition under which coactivation of the cardiovagal and sympathetic outflows have also been observed (91, 92, 137).
C1 Neurons and Responses to Hypoxia
The contribution of the C1 cells to the cardiorespiratory, endocrine and thermoregulatory effects of hypoxia are summarized in Fig. 3. The level of evidence supporting each of the represented mechanisms varies considerably. The primary response of most RVLM presympathetic barosensitive neurons to carotid body stimulation (cyanide or brief hypoxia) in rodents is a vigorous activation (93, 144, 179), although this response can be modified by the baroreflex (93) and central respiratory generator drive (71, 113). Consistent with this observation, a large proportion of C1 neurons express Fos in conscious mammals exposed to hypoxia (54, 73), and sympathetic nerve activation elicited by carotid body stimulation is severely depressed after selective lesions of the C1 neurons (157). The pathway between the carotid bodies and the C1 cells may involve a single interneuron located within the commissural part of the NTS (7, 37, 151). Hypoxia also activates Fos in many IVLM C1 neurons suggesting that the effect of this stimulus is not restricted to the presympathetic neurons.
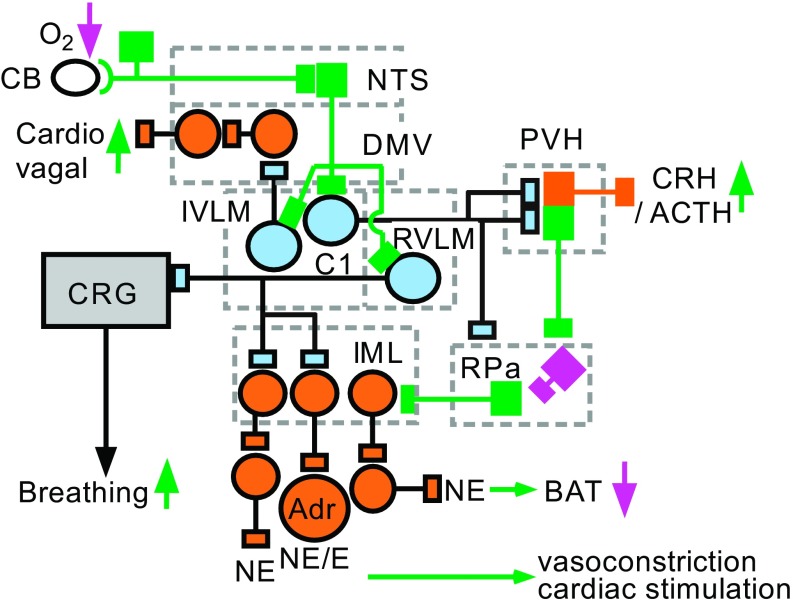
Fig. 3.
Contribution of the C1 cells to the autonomic responses to hypoxia. Hypoxia activates the C1 cells via a minimally disynaptic pathway that consists of carotid body (CB) afferents activated by a reduction in Po2 and a projection from the caudal portion of the NTS to the VLM. CNS hypoxia may also directly or indirectly (via the glia) activate the C1 cells, an effect that may contribute to the Cushing reflex. Three classes of hypoxia-activated C1 cells are represented. RVLM C1 cells innervate preganglionic sympathetic neurons that regulate several vascular beds, the myocardium, and the adrenal medulla, including noepinephrine and epinephrine (E)-releasing cells. This projection likely consists of several subsets of C1 cells (see Fig. 6). Activation of the C1 cells by hypoxia may also increase breathing via stimulation of the CRG. Hypoxia also probably activates the cardiovagal outflow via direct inputs from IVLM C1 neurons to cardiovagal neurons located in the DMV. Activation by hypoxia of IVLM C1 neurons increases CRH release and reduces the activity of presympathetic neurons in the raphe pallidus (RPa) that regulate sympathetic efferent activity to brown adipose tissue (BAT). This inhibition probably operates via projections from IVLM C1 cells to both to the RPa and to the PVH. For color coding, see Fig. 1.
The Cushing response is the neurogenic increase in AP induced by cerebral ischemia (44) and is likely the direct consequence of extreme brain stem hypoxia. This response is independent of the carotid bodies and is largely mediated by the activation of the barosensitive neurons of the RVLM including the C1 cells (47, 48, 67, 180). Their depolarization, like that of the pre-Bötzinger complex, could be partly a cell-autonomous response, for example, an increase in persistent sodium current or calcium permeability (69, 136) or could result from the release of gliotransmitters such as ATP (65, 115, 169, 181, 202). Reis and collaborators championed the idea that the C1 cells are oxygen sensors, implying that these neurons are activated by small “physiologically relevant” reductions in brain Po2 and then trigger countervailing responses including an increase in AP and a neurogenic rise in cerebral blood flow without concomitant change in brain metabolism (180). This appealing theory has been difficult to prove or refute but new evidence detailed later does suggest that the C1 cells could be regulating cortical blood flow via the locus coeruleus and its effect on the glia. In the respiratory field the general consensus is that hypoxia does not activate breathing in the absence of the carotid bodies although, in their presence, CNS hypoxia does stimulate breathing somewhat (42). One could therefore argue that the cardiorespiratory depressant effect of even moderate hypoxia would be more profound were it not for the countervailing influence of CNS oxygen “sensors.” These sensors could be conceivably be the C1 neurons or surrounding glial cells that secondarily activate the C1 cells via ATP or some other gliotransmitter (115).
How the C1 neurons activate breathing has yet to be determined. A direct connection between the C1 cells and elements of the central respiratory generator is plausible (Figs. 2 and 3) because the C1 cells have axonal collaterals within the VLM that contact non-aminergic hence putatively respiratory cells (5, 101). Indirect connections between the C1 cells and the respiratory centers may occur via C1 projections to the retrotrapezoid nucleus, the lateral parabrachial region, the periqueductal gray matter or even via hypothalamic nuclei such as the dorsomedial nucleus/perifornical region (1, 30).
Hypoxia is a condition under which coactivation of the cardiovagal and sympathetic outflows has been observed (91, 92, 137) even though cardiac rate is typically increased by hypoxia in conscious mammals. Cardiovagal activation may improve the efficiency (90) and reduce the metabolic cost (191) of blood pumping during periods of high sympathetic drive to the heart. The projections of the C1 cells to the DMV could in theory underlie this increase in cardiovagal tone. C1 cell projections to the cardiovagal motoneurons located in the VLM are also plausible on anatomical grounds but have not been characterized.
Sustained hypoxia produces hypothermia in rodents. The hypothermia is caused by an active suppression of shivering and brown adipose tissue (BAT) SNA, which eventually leads to a reduced core temperature and metabolic rate. Similar responses are produced by hypoglycemia (Figs. 3 and 4). Hypoxia- and or hypoglycemia-sensitive C1 cells participate in these responses by inhibiting raphe pallidus presympathetic neurons that control BAT SNA. The pathway includes a direct projection to the raphe pallidus and, possibly, an indirect pathway via the PVH (109, 112). The inhibition of the presympathetic neurons that regulate BAT SNA may be mediated by a catecholamine released by the C1 cells (112), although an inhibitory interneuron could be interposed (Fig. 5). Finally, hypoxia is a powerful stimulus for the release of adrenal epinephrine, a response which, in the adult as opposed to the neonate, is neurogenic (24). Hypoxia-activated presympathetic C1 cells are likely to contribute to this response since these cells demonstrably contribute to the activation of other sympathetic efferents (Fig. 3) (157).
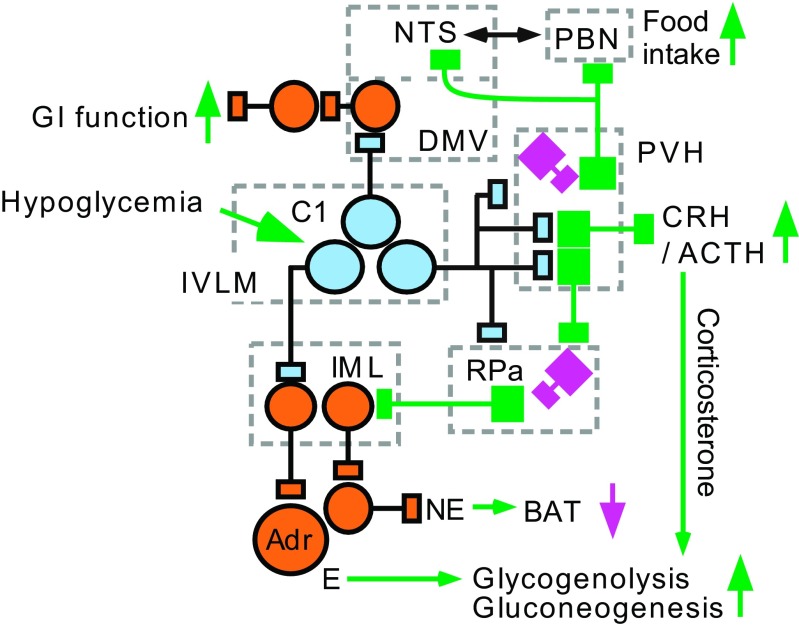
Fig. 4.
Contribution of the C1 cells to the glucoprivic responses. Hypoglycemia activates a subset of presympathetic C1 cells that regulate the epinephrine-releasing chromaffin cells. Hypoglycemia-responsive C1 cells may stimulate gastrointestinal (GI) activity via a direct input to the DMV. C1 cells with projections to the PVH activate the CRH/ACTH/corticosterone cascade and stimulate appetite for carbohydrates. Appetite stimulation by the C1 cells may operate via inhibition of a subset of PVH neurons that target the NTS and the parabrachial region (PBN). The C1 neurons may inhibit these PVH neurons by releasing NPY and catecholamines or, since the C1 neurons are glutamatergic, may recruit unspecified inhibitory interneurons represented in magenta. Hypoglycemia also inhibits BAT activity possibly via activation of IVLM C1 neurons innervating the RPa and the PVH. Three subsets of hypoglycemia-responsive C1 cells are represented but the actual number is unknown.
Fig. 5.
C1 cells activate central and peripheral noradrenergic neurons. C1 cells innervate and activate the locus coeruleus (A6) and other pontine noradrenergic neurons (A5). Evidence that the C1 neurons activate A1 and A2 neurons is based on unpublished evidence from our laboratory and is consistent with the sensitivity of these noradrenergic neurons to hypotension (inhibition) and hypoxia (activation). Given the ubiquitous nature of noradrenergic terminals in the forebrain and the prominent effect of norepinephrine (NE) on glial cells, activation of the LC by the C1 cells may be beneficial to neuronal function in the face of a reduction of AP, partial pressure of oxygen or glucose. Strictly by analogy, one function of the A5 projection to the IML could be to stimulate glial metabolism when the activity of sympathetic preganglionic neurons, hence their metabolic demand, is highest (e.g., hypotension, hypoxia, hemorrhage, and hypoglycemia). A1 and A2 neurons may have more specialized functions (neuroendocrine regulation, control of VP/OT release).
In summary (Fig. 3), a high proportion of C1 neurons are vigorously activated by hypoxia via carotid body stimulation and, possibly via direct effects of hypoxia on the ventrolateral medulla. These C1 neurons contribute to the sympathetic, respiratory, neuroendocrine and thermoregulatory consequences of hypoxia.
Acute hypoxia is often a pathological or accidental situation and brain stem hypoxia of a magnitude that would cause the Cushing response qualifies as a critical situation. Hypoxia only produces hypothermia if severe and sustained. These considerations underlie the present concept that the C1 neurons intervene under extreme (emergency) conditions. This view does not exclude the possibility that the hypoxic sensitivity of these cells also contributes to cardiorespiratory regulation under more physiological circumstances. Strenuous exercise comes to mind since the carotid bodies and SNA are greatly activated under such conditions.
Control of the CRH-ACTH-Corticosterone Cascade by the C1 Neurons
Based on Fos expression and unit recording data, the C1 neurons are activated by the same physical stresses that elicit the CRH-ACTH-corticosterone surge, for example, interleukin-1(IL-1), lipopolysaccharide (LPS), pain, hypotension, hemorrhage, hypoxia, and hypoglycemia (66, 143, 146, 155) (Figs. 3 and 4). In two well-documented cases, IL-1 administration and hypoglycemia, the integrity of the C1 neurons was shown to be required for the CRH-ACTH-corticosterone response to occur (146, 155). The corticosterone surge is attributed to the direct projection of the C1 neurons to the parvocellular division of the PVH (59) but this plausible interpretation has not been strictly demonstrated. To test the contribution of the C1 cells to the release of CRH, the tool of choice has been to lesion the catecholaminergic neurons that innervate the PVH by injecting anti-DβH-sap into this nucleus (146, 155). This procedure also destroys a substantial proportion of A1, A2, A5, and locus coeruleus neurons. Accordingly, the hormonal deficits induced by administering the toxin in the PVH region cannot yet be attributed exclusively to the loss of the C1 neurons (as opposed to other catecholaminergic cells) and cannot yet be exclusively attributed to the direct projections of these catecholaminergic neurons to the PVH as opposed to their projections elsewhere in the brain.
The mechanism by which LPS triggers the CRH-ACTH-corticosterone cascade probably involves the production of IL-1 in blood, the activation of vascular endothelial IL-1 receptors in the brain stem, and the production of prostaglandin E2 (PGE2) by the endothelium and perivascular macrophages. PGE2 presumably reaches the C1 neurons by diffusion and activates these cells via EP3 receptors (155, 163). Lesions of the lateral parabrachial nuclei attenuate the activation of the A1 neurons by systemic administration of IL-1β but, based on Fos expression, these lesions have no effect on the activation of C1 and PVH neurons elicited by the same stimulus (26). This observation reinforces the notion that some of the C1 cells are a primary target of circulating IL-1 and that these cells rather than the A1 neurons mediate the activation of CRH-producing parvovellular PVH neurons by an immune challenge. Hypotension, hypoxia, hypoglycemia, and nociception could conceivably elicit the corticosterone surge by activating a common set of C1 neurons that directly innervate the CRH-releasing neurons of the PVH. Alternately, the CRH neurons may receive input from multiple modality-specific subsets of C1 cells (i.e., glucose sensitive, barosensitive, hypoxia sensitive).
Bacterial infections, hemorrhage, and pain are acute pathophysiological events that are associated with trauma. In keeping with the theme of this review, it appears that the C1 cells are especially implicated in these emergency situations.
Contribution of the C1 Neurons to Glucoprivic Responses
The C1 neurons contribute to nutrient intake in a general sense by regulating sympathetic vasomotor tone to GI organs (152) but C1 cells seem to be more specifically implicated in the homeostatic responses to glucose deficiency (glucoprivic responses, Fig. 4). Glucoprivic responses include the release of epinephrine, glucagon, and corticosterone, increased food consumption and GI activity, and decrease in BAT activity (108, 147, 188). The following evidence implicates the C1 neurons in all of these responses. The strong excitatory input from the C1 cells to DMV neurons suggests that subsets of C1 cells produce GI stimulation (52). In theory, some of these C1 neurons could be hypoglycemia responsive since glucoprivation is a strong signal for the initiation of GI contractions (120). C1 cell lesions reduce the plasma epinephrine increase caused by administration of 2-deoxyglucose by ∼75% (110). A group of C1 neurons located predominantly in the IVLM is activated by 2-deoxyglucose administration and their activation causes glucose release (147, 188). Some of these hypoglycemia-sensitive C1 neurons are bulbospinal and likely to be a source of excitatory drive to the sympathetic efferents that regulate adrenal epinephrine release (147). Like the latter, these C1 neurons respond to hypoglycemia but not to baroreceptor activation (129, 188). Food intake is stimulated by administering 2-deoxyglucose into the IVLM, the hot spots being similar to the region that produced an increase in epinephrine release (145). Administration of TRH into the VLM also produces hyperglycemia and hyperinsulinemia (13). The hyperglycemia is mediated by the sympathetic outflow (epinephrine release) whereas the hyperinsulinemia is mediated via parasympathetic activation of the pancreas, consistent with the direct projection of the C1 cells to the DMV. Last, but not least, feeding behavior is stimulated by injecting norepinephrine or NPY into the PVH, two transmitters presumably released by the C1 cells (172), NPY administration into the PVH elicits a selective increase in carbohydrate consumption with little or no effect on protein or fat consumption (171), and the combined downregulation of NPY and catecholamines in the RVLM reduces glucoprivic feeding (97). Hypoglycemia could conceivably stimulate food intake by inhibiting a subset of PVH MC4R-expressing oxytocinergic neurons that innervate the dorsal medulla (nucleus of the solitary tract) and the parabrachial region (16, 60, 61) (Fig. 4). These PVH neurons are most likely different from the presympathetic ones (23).
There is also evidence that the glucose-activated C1 cells reduce BAT activity (Fig. 4). For example, administration of the glucoprivic agent 5-thioglucose into the IVLM inhibits BAT (108). The inhibitory effect of the C1 cells on BAT activity could be partially mediated via the PVH since activation of this nucleus also suppresses BAT thermogenesis (109) (Fig. 4). C1 neuron-mediated inhibition of BAT activity may also occur via direct projections to the raphe pallidus (1, 30) (Fig. 4). Conceivably, BAT SNA is regulated by a single subset of C1 cells that respond to both hypoglycemia and hypoxia (Figs. 3 and 4).
ACTH release is another consequence of hypoglycemia. Glucose production is increased by corticosterone which, as indicated above, is partly under the control of C1 neurons. Corticosterone promotes gluconeogenesis and shifts the metabolism of non-neural tissues away from glucose utilization to fatty acids and protein utilization. Under hypoxia, glucose release is adaptive by facilitating anaerobic metabolism.
In brief, C1 neurons seem implicated in every aspect of the glucoprivic response including its hormonal and appetitive component (Fig. 4). The C1 cells influence glucoprivic responses at the level of sympathetic and parasympathetic preganglionic autonomic neurons and the PVH but other structures are also implicated. For example, glucoprivation may reduce BAT activity by increasing the activity of C1 cells innervating the raphe pallidus and stimulate appetite by increasing the activity of C1 cells innervating the NTS and parabrachial region (these demonstrated projections are not represented in Fig. 4). Several important knowledge gaps persist. The first question is what degree of hypoglycemia triggers C1 cell-mediated responses. The second question is how to identify the specific subset of C1 cells that mediate the glucoprivic responses. The third is to understand whether and how hypoglycemia is detected by the C1 neurons.
C1 and Female Reproductive Function
The C1 neurons may contribute to reproductive function, perhaps via A1/A2/A6 regulation of gonadotropin-releasing hormone (GnRH). While there is evidence of projections from A1, A2, and A6 neurons to the region of the GnRH neurons (29, 198), a specific projection of the C1 neurons to this area has not been reported. A proportion of C1 cells (∼20%) express the estrogen receptor-α (95, 193) that may be involved in estrogen's feedback on GnRH. During most of the reproductive cycle estrogen receptor-α signaling inhibits GnRH, but in the late follicular stage it switches to positive feedback and causes a surge in GnRH and luteinizing hormone (LH) release. C1 cells may participate in the GnRH/LH surge but the evidence for this is limited (41, 95, 167). Alternatively, C1 neurons may inhibit GnRH, in particular, in response to physical stressors to conserve energy. Indeed, combined lesions of both C1 and A1 neurons with hypothalamic projections prevented the inhibition of reproductive function caused by chronic glucose deficit in rats (80). Thus a disruption of energy homeostasis may activate a subset of A1 or C1 neurons and result in the inhibition of estrous cycles.
Activation of CNS Noradrenergic Neurons by the C1 Neurons
Noradrenergic terminals are ubiquitous in the brain. In the neocortex and hippocampus these varicosities originate exclusively from the locus coeruleus and number about 1 to 2 million/mm3 of tissue (1–2 varicosities for each cube of tissue with 10 μm side) with relatively small (threefold) variations between regions and layers (133, 162). Thus, by simple diffusion, norepinephrine probably has access to every CNS glial cell and neuron. Norepinephrine promotes both glycogenolysis and glycogen resynthesis in glia and activates sodium-potassium-ATPase, which facilitates glutamate reuptake, lactate production, and glucose uptake from blood by these cells (168). Glial cell activation increases cerebral blood flow (17) as does the activation of the locus coeruleus (185).
The input from the C1 cells to the locus coeruleus (A6 cell group, Fig. 5) was originally thought to be inhibitory and mediated by catecholamines, but a reinvestigation of this issue with optogenetic techniques in vivo has suggested that the C1 cells actually excite the LC by releasing glutamate (2, 14). We recently confirmed the monosynaptic and glutamatergic nature of this input using optogenetics in mouse brain slices (B. B. Holloway and P. G. Guyenet, unpublished results). The C1 cells also activate the A5 noradrenergic neurons in vivo and make glutamatergic close appositions with the A1 noradrenergic neurons (2, 30) and the A2 neurons (Holloway BB, Stornetta RL, and Guyenet PG, unpublished results) (Fig. 5).
The discharge rate of locus coeruleus neurons is increased by hypoglycemia, hypotension, and hypoxia in conscious mammals (43, 54, 127). These effects are likely mediated via the C1 cells because subsets of these neurons are vigorously activated by these stimuli and the C1 cell input to locus coeruleus neurons is dense and excitatory (2, 11). As mentioned previously, the C1 cells probably also target additional groups of lower brain stem noradrenergic neurons (2, 23). These neurons (A1, A2, A5, for anatomical location see Fig. 1) are weakly inhibited by arterial baroreceptor stimulation, consistent with a reduction in excitatory drive from a barosensitive input such as the C1 neurons (77, 126). Hypoxia causes varying degrees of Fos expression in brain stem noradrenergic cell groups and activates locus coeruleus unit activity in vivo, an effect that is also consistent with an excitatory drive from the C1 cells (53, 54, 68). Thus the (or subsets of) C1 cells increase the release of norepinephrine throughout the CNS under conditions such as hypoxia, hypoglycemia, and hypotension (Fig. 5). One interpretation is that, via a brain-wide upregulation of norepinephrine release, the C1 cells stimulate glial metabolism (increased glycogenolysis, glucose uptake from blood, glucose-lactate shuttle) (168) and cerebral blood flow (185) and thus help preserve brain function during systemic challenges such as hypotension, hypoxia, and hypoglycemia or any form of severe somatic stress. Such a regulation must be somehow superimposed and integrated with the local reactive hyperemia and glial metabolic adjustments elicited by increases in neuronal activity (17, 64). The A5 neurons whose function is still elusive but are activated by hypoxia and hypotension may exert similar types of “housekeeping” effects at the level of the IML under conditions when sympathetic efferents are strongly activated.
The potential regulation of the A1 neurons by the C1 cells can also be viewed in the light of an integrated physiological response to acute injury. The A1 cells preferentially innervate the magnocellular cells of the PVH and supraoptic nuclei, whereas the C1 cells preferentially target the parvocellular division of the PVH (59). These anatomical observations and much physiological evidence indicate that the A1 neurons provide an important excitatory drive to hypothalamic magnocellular neurons, which is mediated by NPY, catecholamines, and perhaps ATP (e.g., 22, 50, 197). As previously mentioned, C1 cell lesions that spare the A1 neurons severely attenuate the release of vasopressin and oxytocin elicited by hypotension (110). These deficits could be partly explained by the loss of the excitatory input from the C1 cells to the A1 neurons (Fig. 5). This pathway is poised to help preserve blood volume in case of traumatic injury involving hypoxia, hypotension, and blood loss because each of these stimuli vigorously activates the C1 cells.
C1 Cells and Disease
C1 cells degenerate in multiple system atrophy (MSA) and advanced Parkinson's disease (PD), two conditions associated with postural hypotension (20, 21). Congenital central hypoventilation syndrome (CCHS) is a developmental disease caused by mutations of the transcription factor Phox2b and characterized by sleep apnea and the loss of the respiratory chemoreflexes (194). A defect in the number or connectivity of the C1 neurons may contribute to the dysautonomias also experienced by CCHS patients because the development of the C1 neurons is Phox2b dependent (49).
Resting SNA is mildly elevated in human diseases associated with hypertension such as essential hypertension, obesity, and obstructive sleep apnea (51, 56) and severely elevated in congestive heart failure (55, 96). An increase in the resting activity of the C1 cells and other presympathetic neurons of the RVLM could plausibly contribute to the increased SNA in such diseases (9, 78). Chronic baroreceptor stimulation, which presumably decreases the activity of the C1 cells in the conscious state as under anesthesia, produces a sustained AP reduction in experimental animals and in humans with drug-refractory essential hypertension (106). In human heart failure, the overflow of catecholamine metabolites from the brain is enhanced suggesting that the release of CNS norepinephrine, and possibly epinephrine, is elevated (94).
In theory, a pathological increase in C1 neuron activity could have three causes. The most plausible is a change in the activity of the neurons that regulate the C1 cells. Indeed, abnormal activity within the PVH, the NTS, and the carotid bodies contributes to various forms of hypertension (4, 8, 192) and heart failure is associated with excessive carotid body activity and changes in the activity of cardiopulmonary and muscle afferents (e.g., 142). A second possible cause of C1 cell hyperactivity, also supported by experimental evidence, may be some change in the local environment of these neurons, perhaps because of microvascular inflammation or glial activation (81, 83, 89, 117, 200). For example, endothelial nitric oxide (NO) synthase (eNOS) overexpression in RVLM, which plausibly increases local blood flow causes an espcially large hypotension in spontaneously hypertensive rats of the stroke prone variety (SHRSP) (88), whereas excessive levels of radical oxygen species in the RVLM seem to contribute to hypertension in this strain (89). In these models, there is little evidence that the changes in NO or reactive oxygen species (ROS) are specifically associated with the C1 cells and, in heart failure, signs of CNS inflammation are widespread and involve many regions that control the RVLM, e.g., the PVH (58). The “selfish brain hypothesis” of hypertension is a variation on the theme of the Cushing response (32). The idea is that flow restriction within the brain stem vasculature, including the VLM and NTS, caused by compression of hypertrophied vessels or by an inflammatory process that reduces the arteriolar lumen, produces local hypoxia or some other biochemical change leading to neuronal activation and ultimately activation of the sympathetic outflow, in other words a graded Cushing response that restores brain blood flow (32). Finally, the activity of the C1 neurons could theoretically be upregulated by a change in their intrinsic properties. Consistent with this possibility the RVLM seems hyperreactive to angiotensin II in several hypertension models (9, 83). This last evidence implicates the C1 cells perhaps more directly in the pathological process because, in the RVLM, angiotensin-1A receptors (AT1R) are predominantly expressed by these neurons (34).
In brief, C1 cell damage may contribute to the dysautonomias present in several degenerative diseases (MSA and PD) and, possibly, to one developmental disease (CCHS). C1 cell hyperactivity is a plausible albeit not directly demonstrated factor contributing to the sympathetic hyperactivity associated with hypertension and heart failure. The postulated hyperactivity of the C1 neurons could be caused by an altered balance of synaptic inputs. Local factors such as vascular inflammation and increased local ROS production and changes in the intrinsic properties of these cells (e.g., increased AT1R expression) may also contribute to their hyperactivity in hypertension.
In conclusion, the C1 neurons regulate the HPA axis via direct projections to the PVH and possibly by more circuitous routes. The C1 neurons also regulate both the sympathetic and parasympathetic nervous systems via direct projections to the preganglionic neurons. Only a third of the C1 cells are presympathetic and a large proportion of these presumably regulate the circulation. The presympathetic C1 neurons target several pontomedullary and midbrain regions in addition to the IML. Therefore, in the conscious state, their effects on the circulation and other physiological functions probably result from their action on a network of interconnected structures. Finally, the C1 cells that do not innervate the spinal cord likely also regulate AP and SNA via their projections to the dorsomedial nucleus of the hypothalamus, the midbrain central gray area, the parabrachial nuclei, and the PVH. The presympathetic C1 cells that regulate epinephrine release and glucoprivic responses presumably also regulate networks rather than just preganglionic autonomic or PVH neurons.
The control of the parasympathetic system is the least understood aspect of the function of the C1 cells. The C1 projection to the DMV is dense and presumably originates from the IVLM. These C1 neurons likely regulate GI motility and GI hormonal secretions and could conceivably also influence cardiac performance and the immune system.
The predominant short-term synaptic effect of the C1 cells is an excitation that is mediated by conventional ionotropic glutamatergic transmission. The C1 cells synthesize catecholamines and inhibitory as well as excitatory neuropeptides but the circumstances of their release and the resulting cellular effects need clarification.
Based on neuroanatomical evidence, Loewy and colleagues (85) proposed that many C1 cells have widely divergent projections and are wired to produce generalized increases in SNA that are not limited to any particular vascular bed or even peripheral organ. Remarkably, the C1 cells are also wired to produce a generalized increase in CNS noradrenergic release. CNS noradrenergic neurons have both a support role (astrocyte stimulation, blood flow control, microglia stimulation) and a neuroeffector role (modulation of neuronal activity) (132). The same duality (metabolic role and circulatory control) applies to catecholamines released by the peripheral sympathetic system, for example, in skeletal muscles (72, 84). Activation of the locus coeruleus by the C1 cells may therefore help preserve forebrain function during hypotension, hypoxia, and hypoglycemia by stimulating glial metabolism and cerebral blood flow above and beyond the level that would be required by local neuronal activity in the absence of such systemic perturbation (185). This level of regulation could potentially complicate the interpretation of functional magnetic resonance imaging. Forebrain neuron activation, the other expected consequence of activating the locus coeruleus by the C1 cells, may help heighten arousal or attention in situations associated with injury or other bodily harm (15). Additionally, activation of the C1 cells, e.g., by LPS or circulating interleukin-1 as would happen during injury and sepsis presumably has adaptive immunological consequences that are mediated by the central noradrenergic system (microglia stimulation) (132), by the release of ACTH and, possibly, by activation of the parasympathetic system. Finally, the activation of selected lower brain stem noradrenergic neurons by the C1 cells may have more specialized effects such as to enhance the release of oxytocin and vasopressin from magnocellular neurons under conditions such as hypotension and hemorrhage.
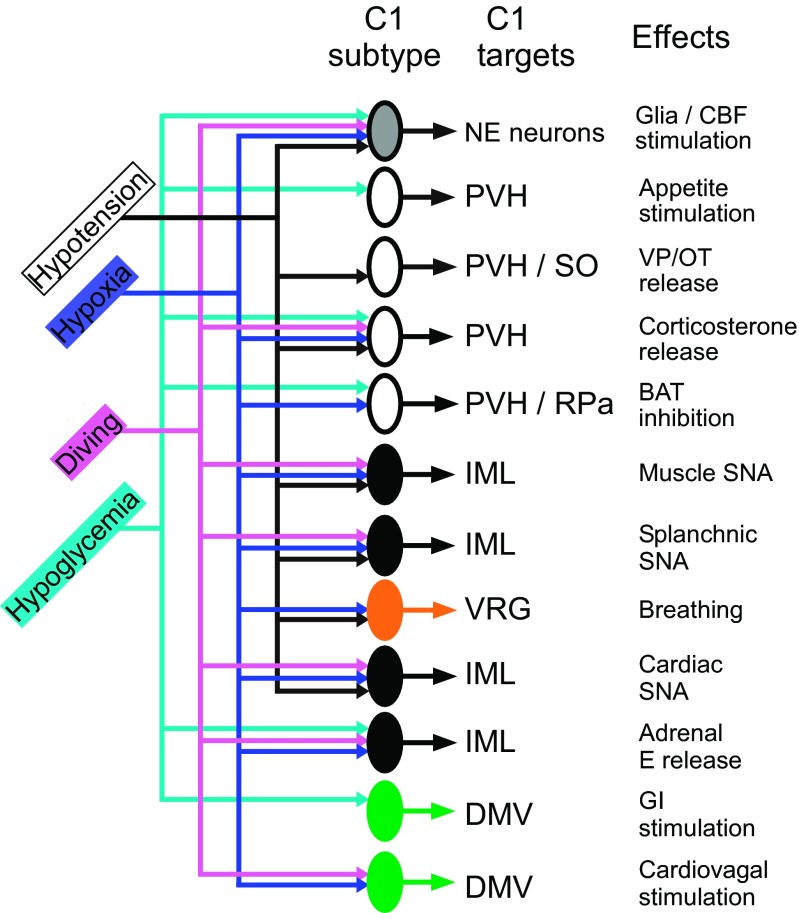
The number of functional subtypes of C1 cells is not known. Figure 6 is a highly hypothetical attempt to illustrate how the differential recruitment of 12 subsets of C1 cells by hypotension, hypoxia, diving, or hypoglycemia might produce a response pattern adapted to each situation. The number of C1 cell groups is arbitrary. There could be fewer subsets than represented. For example, the CNS noradrenergic neurons may receive collaterals from glucosensitive and or barosensitive C1 cells and breathing could also be activated by collaterals of baro- and hypoxia-sensitive presympathetic neurons. On the other hand Fig. 6 does not consider the pattern elicited by infection and inflammation, which may engage additional cell types. From a neurophysiological standpoint, the presympathetic C1 neurons include at least four types of neurons that have variable influence over the heart, skeletal muscle arterioles, gut arterioles, the kidney, and the adrenal medulla. Three of them (muscle, gut, and cardiac types, Fig. 6) are barosensitive and are differentially activated or inhibited by such factors as GI hormones (e.g., CCK) and the respiratory pattern generator (66). These “cardiovascular” neurons are activated by carotid body stimulation but are presumably not directly influenced by hypoglycemia. A fourth type of presympathetic C1 neurons may be dedicated to the control of epinephrine-releasing chromaffin cells. These neurons are probably less numerous than those that control the circulation. They respond to hypoglycemia and likely to hypoxia but not to hypotension (Fig. 6). With exceptions (at least 10% of the total), the C1 neurons that innervate the hypothalamus are different from the presympathetic ones and one would think that they are as functionally diverse as the presympathetic C1 neurons but there is little evidence of this yet. Figure 6 depicts ACTH release as being triggered by a single class of C1 neurons that respond indifferently to hypotension, hypoxia, hypoglycemia but this assumption has not been tested. The number of C1 cell subtypes that innervate the DMV (two are represented in Fig. 6) is equally uncertain.
Fig. 6.
C1 cells as “emergency medical technicians.” Differential recruitment of subsets of C1 cells for different emergency responses. Hypothetical scheme that illustrates how the differential recruitment of 12 subsets of C1 cells by hypotension, hypoxia, diving, or hypoglycemia might produce a response pattern adapted to each situation. The anatomical target of each type of C1 cells and the postulated physiological effect produced by their activation are also indicated. Green: responses mediated by C1 cells that innervate parasympathetic preganglionic neurons. Black: responses mediated by C1 cells that innervate sympathetic preganglionic neurons. White: responses mediated by C1 cells that innervate the PVH and other forebrain regions. Gray: responses mediated via activation of the CNS noradrenergic system (locus coeruleus, etc.). Orange: response mediated by activation of the respiratory pattern generator. The number of C1 cell subsets could be smaller if some of the responses listed are produced by axonal collaterals. For example the CNS noradrenergic neurons could be activated by collaterals of the presympathetic neurons that regulate the circulation and by collaterals of glucose-sensitive cells (presympathetic or others).
The A1 cells are likely postsynaptic to the C1 neurons and these interactions are not reciprocal because the A1 cells do not seem to innervate the C1 region (111). Also, VGLUT2 is not detectable in the terminals of the A1 neurons (52), whereas the norepinephrine transporter is generally absent from the C1 cells (11).
The SNA hyperactivity observed in hypertension, heart failure, and other diseases is of CNS origin and plausibly partly mediated by an increase in the rate of discharge of the C1 cells but the evidence is still circumstantial. In human heart failure, CNS catecholaminergic systems do seem hyperactive (94), although the measured metabolites probably originate from the quantitatively dominant noradrenergic terminals rather than from the C1 cells. A comparable increase in CNS catecholaminergic turnover is produced in humans by ganglionic blockade (125) a stimulus that almost certainly activates the C1 cells via baroreceptor unloading. These observations are consistent with our hypothesis that the C1 cells upregulate the activity of the CNS noradrenergic neurons.
Perspectives and Significance
The C1 cells are activated in response to physical stresses such as pain, hypoxia, hemorrhage, infection, inflammation, hypotension, and hypoglycemia and many C1 cells are also activated by psychological stress (99). C1 cell activation increases the release of norepinephrine both peripherally and centrally with broad and presumably adaptive consequences on the metabolism of various tissues and on the membrane polarization of excitable cells (neurons, vascular smooth muscle and glands). How severe these stresses need to be for the C1 cells to produce such responses is uncertain. The physical stresses to which experimental animals are typically subjected to reveal changes in C1 cell activity, for example, with the Fos expression method, are severe by human standards. Tissue injury, hemorrhage, pain, severe hypoxia, and bacterial infection (LPS) are clearly accidental or pathological events. The physiological responses elicited by C1 cell activation facilitate survival by preventing cardiovascular collapse, maintaining oxygenation of essential organs, and providing tissues with a readily usable energy substrate, glucose, hence the concept of C1 neurons as EMTs proposed in this review. The postulated “emergency” nature of their physiological role is also in keeping with the fact that very extensive lesions of the C1 cells produce only minor effects on AP but impair bodily responses to severe acute hypotension.
Many fundamental questions regarding the biology of the C1 neurons remain unanswered. The developmental lineage of these neurons is unexplored. By analogy with on-going work on the serotonergic system (86), specific combinations of transcription factors presumably govern the differentiation of subsets of medullary catecholaminergic neurons. Such studies may clarify whether the RVLM presympathetic C1 and non-C1 neurons, which appear to have many common structural and physiological features, are developmentally related. The catecholaminergic phenotype of the presympathetic C1 neurons could conceivably be a vestige of evolution that may have disappeared altogether from the presympathetic neurons referred to as the non-C1 cells. Indeed the latter neurons have the same location and transmitters (glutamate, enkephalin, PACAP, lack of NPY), similar projections and inputs as a subset of C1 presympathetic cells (174). The only notable difference is that they lack (or express undetectably low levels of) catecholamine biosynthetic enzymes.
The functional differences between A1 and C1 neurons, especially between the A1 and the caudal C1 cells, are still incompletely delineated. There appear to be fundamental differences between the biology of these neurons (e.g., A1 cells lack VGLUT2), but the work of Lipski and others (40) suggests that transitional phenotypes between A1 and C1 neurons exist (e.g., neurons that express both norepinephrine transporter, NET, and PNMT). Also there is very little information concerning the role of the C2 and C3 adrenergic neurons besides evidence that they target some of the same CNS neurons as the C1 cells (e.g., 105).
RVLM presympathetic neurons can discharge up to 35 Hz under anesthesia if AP is low. A reduction in GABAergic inhibition contributes to this activation and presumably underlies the tremendous increase in SNA that is unleashed by hypotension in conscious mammals, humans included. However, disinhibition does not explain why neurons become active and the source of the excitatory inputs to RVLM presympathetic neurons is an enduring mystery. The use of retrograde transsynaptic vectors (rabies, pseudorabies) may help identify these excitatory synaptic inputs (28). Further understanding of the local factors that regulate the activity of the C1 neurons (gliotransmitters, oxygen, blood vessel-derived factors) will probably also be critical.
One would also want to find out how many subtypes of C1 neurons there are and which brain neurons are targeted by each C1 cell subtype. However, this information must be complemented by a thorough understanding of the inputs of each subtype of C1 cell. Based on Fos studies, a very large fraction of the C1 cells can be activated by a single stimulus such as hypoxia, pain, LPS, and psychological stress suggesting that each of these stimuli may cause a broad activation of the sympathetic system and of the brain's noradrenergic system. Other stimuli may selectively activate much smaller subsets of C1 cells thereby enabling organ-specific changes in SNA.
In this review we proposed that the C1 cells intervene mostly under dire “emergency” situations. This view largely derives from the nature of the stimuli known to increase their activity (pain, hemorrhage, bacterial infection, hypoxia, hypoglycemia). However, subsets of C1 cells may also contribute to homeostasis under more physiological conditions. This could be the case of the highly barosensitive RVLM presympathetic C1 cells that control the vasoconstrictor tone, the heart, and ultimately AP. Opto/pharmacogenetic experiments in conscious animals may provide answers to these questions. Finally, the increased SNA associated with hypertension and heart failure (94) could be caused in part by an increase in the discharge rate of RVLM presympathetic C1 cells. This also remains to be demonstrated in conscious animals.
GRANTS
This work was supported by the grants from the National Institutes of Health (HL-28785 and HL-74011 to P. G. G, 1F32HL-096280-01 to S. D. D) and by the American Heart Association (11post7170001 to S. B. G. A).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.G.G., R.L.S., G.B., S.D.D., and S.B.A. conception and design of research; P.G.G., R.L.S., G.B., S.D.D., and S.B.A. performed experiments; P.G.G., R.L.S., G.B., S.D.D., and S.B.A. analyzed data; P.G.G., R.L.S., G.B., S.D.D., and S.B.A. interpreted results of experiments; P.G.G. and S.B.A. prepared figures; P.G.G. and P.G.B. drafted manuscript; P.G.G., R.L.S., G.B., and S.B.A. edited and revised manuscript; P.G.G., R.L.S., G.B., S.D.D., P.G.B., and S.B.A. approved final version of manuscript.
REFERENCES
- 1.Abbott SB, Depuy SD, Nguyen T, Coates MB, Stornetta RL, Guyenet PG. Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice. J Neurosci 33: 3164–3177, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott SB, Kanbar R, Bochorishvili G, Coates MB, Stornetta RL, Guyenet PG. C1 neurons excite locus coeruleus and A5 noradrenergic neurons along with sympathetic outflow in rats. J Physiol 590: 2897–2915, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci 29: 5806–5819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol 590: 4269–4277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agassandian K, Shan Z, Raizada M, Sved AF, Card JP. C1 catecholamine neurons form local circuit synaptic connections within the rostroventrolateral medulla of rat. Neurosci 227: 247–259, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aghajanian GK, Wang YY. Common alpha 2- and opiate effector mechanisms in the locus coeruleus: intracellular studies in brain slices. Neuropharmacology 26: 793–799, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: Comparison with input from the caudal ventrolateral medulla. J Comp Neurol 373: 62–75, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39: 275–280, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Allen AM. Role of angiotensin in the rostral ventrolateral medulla in the development and maintenance of hypertension. Curr Opin Pharmacol 11: 117–123, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Allen AM, Guyenet PG. α2-Adrenoceptor-mediated inhibition of bulbospinal barosensitive cells of rat rostral medulla. Am J Physiol Regul Integr Comp Physiol 265: R1065–R1075, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Amara SG, Simerly RB. Cell-type-specific expression of catecholamine transporters in the rat brain. J Neurosci 14: 4903–4914, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amendt K, Czachurski J, Dembowsky K, Seller H. Bulbospinal projections to the intermediolateral cell column: a neuroanatomical study. J Auton Nerv Syst 1: 103–107, 1979 [DOI] [PubMed] [Google Scholar]
- 13.Ao Y, Ko M, Chen A, Marvizon JC, Adelson D, Song MK, Go VL, Liu YY, Yang H. Potent hyperglycemic and hyperinsulinemic effects of thyrotropin-releasing hormone microinjected into the rostroventrolateral medulla and abnormal responses in type 2 diabetic rats. Neurosci 169: 706–719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aston-Jones G, Astier B, Ennis M. Inhibition of noradrenergic locus coeruleus neurons by C1 adrenergic cells in the rostral ventral medulla. J Neurosci 273: 1991 [DOI] [PubMed] [Google Scholar]
- 15.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28: 403–450, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature 488: 172–177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barman SM, Gebber GL. Axonal projection patterns of ventrolateral medullospinal sympathoexcitatory neurons. J Neurophys 53: 1551–1566, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Barman SM, Gebber GL, Calaresu FR. Differential control of sympathetic nerve discharge by the brain stem. Am J Physiol Regul Integr Comp Physiol 247: R513–R519, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Benarroch EE, Schmeichel AM, Parisi JE. Involvement of the ventrolateral medulla in parkinsonism with autonomic failure. Neurology 54: 963–968, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Benarroch EE, Smithson IL, Low PA, Parisi JE. Depletion of catecholaminergic neurons of the rostral ventrolateral medulla in multiple systems atrophy with autonomic failure. Ann Neurol 43: 156–163, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Beroukas D, Willoughby JO, Blessing WW. Neuropeptide Y-like immunoreactivity is present in boutons synapsing on vasopressin-containing neurons in rabbit supraoptic nucleus. Neuroendocrinology 50: 222–228, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong HW. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: A study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol 520: 6–33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biesold D, Kurosawa M, Sato A, Trzebski A. Hypoxia and hypercapnia increase the sympathoadrenal medullary functions in anesthetized, artificially ventilated rats. Jap J Physiol 39: 511–522, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res 56: 359–369, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Buller KM, Allen T, Wilson LD, Munro F, Day TA. A critical role for the parabrachial nucleus in generating central nervous system responses elicited by a systemic immune challenge. J Neuroimmunol 152: 20–32, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Burke PG, Abbott SB, McMullan S, Goodchild AK, Pilowsky PM. Somatostatin selectively ablates post-inspiratory activity after injection into the Botzinger complex. Neurosci 167: 528–539, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol 18: 617–623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell RE, Herbison AE. Definition of brainstem afferents to gonadotropin-releasing hormone neurons in the mouse using conditional viral tract tracing. Endocrinology 148: 5884–5890, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol 499: 840–859, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cates MJ, Dickinson CJ, Hart EC, Paton JF. Neurogenic hypertension and elevated vertebrobasilar arterial resistance: is there a causative link? Curr Hypertens Rep 14: 261–269, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Chan RKW, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in the brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol 348: 433–460, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Chen D, Jancovski N, Bassi JK, Nguyen-Huu TP, Choong YT, Palma-Rigo K, Davern PJ, Gurley SB, Thomas WG, Head GA, Allen AM. Angiotensin type 1A receptors in C1 neurons of the rostral ventrolateral medulla modulate the pressor response to aversive stress. J Neurosci 32: 2051–2061, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Q, Li DP, Pan HL. Presynaptic alpha1 adrenergic receptors differentially regulate synaptic glutamate and GABA release to hypothalamic presympathetic neurons. J Pharmocol Exp Ther 316: 733–742, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Cheng ZX, Powley TL, Schwaber JS, Doyle FJIII. Projections of the dorsal motor nucleus of the vagus to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol 410: 320–341, 1999 [PubMed] [Google Scholar]
- 37.Chitravanshi VC, Kachroo A, Sapru HN. A midline area in the nucleus commisuralis of NTS mediates the phrenic nerve responses to carotid chemoreceptor stimulation. Brain Res 662: 127–133, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Ciaranello RD, Barchas RE, Byers GS, Stemmle DW, Barchas JD. Enzymatic synthesis of adrenaline in mammalian brain. Nature 221: 368–369, 1969 [DOI] [PubMed] [Google Scholar]
- 39.Comer AM, Gibbons HM, Qi J, Kawai Y, Win J, Lipski J. Detection of mRNA species in bulbospinal neurons isolated from the rostral ventrolateral medulla using single-cell RT-PCR. Brain Res Brain Res Protoc 4: 367–377, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Comer AM, Qi J, Christie DL, Gibbons HM, Lipski J. Noradrenaline transporter expression in the pons and medulla oblongata of the rat: localisation to noradreneric and some C1 adrenergic neurones. Mol Brain Res 62: 65–76, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Conde GL, Bicknell RJ, Herbison AE. Changing patterns of Fos expression in brainstem catecholaminergic neurons during the rat oestrous cycle. Brain Res 672: 68–76, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Curran AK, Rodman JR, Eastwood PR, Henderson KS, Dempsey JA, Smith CA. Ventilatory responses to specific CNS hypoxia in sleeping dogs. J Appl Physiol 88: 1840–1852, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Curtis AL, Drolet G, Valentino RJ. Hemodynamic stress activates locus coeruleus neurons of unanesthetized rats. Brain Res Bull 31: 737–744, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Cushing H. Concerning a definitive regulatory mechanism of the vaso-motor centre which controls blood pressure during cerebral compression. B Johns Hopkins Hosp 12: 290–292, 1901 [Google Scholar]
- 45.Dahlstrom A, Fuxe K. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 62, Suppl 232: 1–55, 1964 [PubMed] [Google Scholar]
- 46.Dampney RA, Goodchild AK, Robertson LG, Montgomery W. Role of ventrolateral medulla in vasomotor regulation: a correlative anatomical and physiological study. Brain Res 249: 223–235, 1982 [DOI] [PubMed] [Google Scholar]
- 47.Dampney RA, Kumada M, Reis DJ. Central neural mechanisms of the cerebral ischemic response. Characterization, effect of brainstem and cranial nerve transections, and simulation by electrical stimulation of restricted regions of medulla oblongata in rabbit. Circ Res 45: 48–62, 1979 [DOI] [PubMed] [Google Scholar]
- 48.Dampney RA, Moon EA. Role of ventrolateral medulla in vasomotor response to cerebral ischemia. Am J Physiol Heart Circ Physiol 239: H349–H358, 1980 [DOI] [PubMed] [Google Scholar]
- 49.Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development 130: 6635–6642, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Day TA. Control of neurosecretory vasopressin cells by noradrenergic projections of the caudal ventrolateral medulla. In: The Central Neural Organization of Cardiovascular Control, Progress in Brain Research, edited by Ciriello J, Caverson MM, Polosa C. Amsterdam: Elsevier, 1989, p. 303–317 [DOI] [PubMed] [Google Scholar]
- 51.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Depuy SD, Stornetta RL, Bochorishvili G, Deisseroth K, Witten I, Coates MB, Guyenet PG. Glutamatergic neurotransmission between the C1 neurons and the parasympathetic preganglionic neurons of the dorsal motor nucleus of the vagus. J Neurosci 33: 1486–1497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and Hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic sympathetic nerve. Brain Res 222: 373–381, 1981 [DOI] [PubMed] [Google Scholar]
- 54.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce c-Fos-like immunoreactivity within catecholaminergic and serotinergic neurons of the rat brainstem. J Comp Neurol 348: 161–182, 1994 [DOI] [PubMed] [Google Scholar]
- 55.Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J Cardiovasc Pharmacol 35: S1–S7, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Esler M, Rumantir M, Kaye D, Lambert G. The sympathetic neurobiology of essential hypertension: Disparate influences of obesity, stress, and noradrenaline transporter dysfunction? Am J Hypertens 14: 139S–146S, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Farnham MM, Li Q, Goodchild AK, Pilowsky PM. PACAP is expressed in sympathoexcitatory bulbospinal C1 neurons of the brain stem and increases sympathetic nerve activity in vivo. Am J Physiol Regul Integr Comp Physiol 294: R1304–R1311, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. Am J Physiol Regul Integr Comp Physiol 284: R259–R276, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Fuzesi T, Wittmann G, Liposits Z, Lechan RM, Fekete C. Contribution of noradrenergic and adrenergic cell groups of the brainstem and agouti-related protein-synthesizing neurons of the arcuate nucleus to neuropeptide-y innervation of corticotropin-releasing hormone neurons in hypothalamic paraventricular nucleus of the rat. Endocrinology 148: 5442–5450, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol 518: 1460–1499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghamari-Langroudi M, Srisai D, Cone RD. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proc Natl Acad Sci USA 108: 355–360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodchild AK, Moon EA. Maps of cardiovascular and respiratory regions of rat ventral medulla: focus on the caudal medulla. J Chem Neuroanat 38: 209–221, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Goodchild AK, Moon EA, Dampney RA, Howe PR. Evidence that adrenaline neurons in the rostral ventrolateral medulla have a vasopressor function. Neurosci Lett 45: 267–272, 1984 [DOI] [PubMed] [Google Scholar]
- 64.Gordon GR, Howarth C, MacVicar BA. Bidirectional control of arteriole diameter by astrocytes. Exp Physiol 96: 393–399, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci 25: 1211–1218, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Guyenet PG, Brown DL. Unit activity in nucleus paragigantocellularis lateralis during cerebral ischemia in the rat. Brain Res 364: 301–314, 1986 [DOI] [PubMed] [Google Scholar]
- 68.Guyenet PG, Koshiya N, Huangfu D, Verberne AJM, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Physiol Regul Integr Comp Physiol 264: R1035–R1044, 1993 [DOI] [PubMed] [Google Scholar]
- 69.Hammarstrom AK, Gage PW. Hypoxia and persistent sodium current. Eur Biophys J 31: 323–330, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Haselton JR, Guyenet PG. Ascending collaterals of medullary barosensitive neurons and C1 cells in rats. Am J Physiol Regul Integr Comp Physiol 258: R1051–R1063, 1990 [DOI] [PubMed] [Google Scholar]
- 71.Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol 256: R739–R750, 1989 [DOI] [PubMed] [Google Scholar]
- 72.Hertz L, Lovatt D, Goldman SA, Nedergaard M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int 57: 411–420, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirooka Y, Polson JW, Potts PD, Dampney RAL. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neurosci 80: 1209–1224, 1997 [DOI] [PubMed] [Google Scholar]
- 74.Hokfelt T, Fuxe K, Goldstein M, Johansson O. Evidence for adrenaline neurons in the rat brain. Acta Physiol Scand 89: 286–288, 1973 [DOI] [PubMed] [Google Scholar]
- 75.Hokfelt T, Fuxe K, Goldstein M, Johansson O. Immunohistochemical evidence for the existence of adrenaline neurons in the rat brain. Brain Res 66: 235–251, 1974 [Google Scholar]
- 76.Horiuchi J, Killinger S, Dampney RA. Contribution to sympathetic vasomotor tone of tonic glutamatergic inputs to neurons in the RVLM. Am J Physiol Regul Integr Comp Physiol 287: R1335–R1343, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Huangfu D, Koshiya N, Guyenet PG. A5 noradrenergic unit activity and sympathetic nerve discharge in rats. Am J Physiol Regul Integr Comp Physiol 261: R393–R402, 1991 [DOI] [PubMed] [Google Scholar]
- 78.Huber DA, Schreihofer AM. Altered regulation of the rostral ventrolateral medulla in hypertensive obese Zucker rats. Am J Physiol Heart Circ Physiol 301: H230–H240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hwang DY, Carlezon WA, Jr, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther 12: 1731–1740, 2001 [DOI] [PubMed] [Google Scholar]
- 80.I'Anson H, Sundling LA, Roland SM, Ritter S. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology 144: 4325–4331, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Iigaya K, Kumagai H, Nabika T, Harada Y, Onimaru H, Oshima N, Takimoto C, Kamayachi T, Saruta T, Itoh H. Relation of blood pressure quantitative trait locus on rat chromosome 1 to hyperactivity of rostral ventrolateral medulla. Hypertension 53: 42–48, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Inokuchi H, Yoshimura M, Polosa C, Nishi S. Adrenergic receptors (alpha 1 and alpha 2) modulate different potassium conductances in sympathetic preganglionic neurons. Can J Physiol Pharmacol 70, Suppl:S92–S97, 1992 [DOI] [PubMed] [Google Scholar]
- 83.Ito S, Hiratsuka M, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension 41: 744–750, 2003 [DOI] [PubMed] [Google Scholar]
- 84.James JH, Wagner KR, King JK, Leffler RE, Upputuri RK, Balasubramaniam A, Friend LA, Shelly DA, Paul RJ, Fischer JE. Stimulation of both aerobic glycolysis and Na+-K+-ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol Endocrinol Metab 277: E176–E186, 1999 [DOI] [PubMed] [Google Scholar]
- 85.Jansen ASP, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system:basis of the fight-or flight response. Science 270: 644–646, 1995 [DOI] [PubMed] [Google Scholar]
- 86.Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci 11: 417–419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joh TH, Goldstein M. Isolation and characterization of multiple forms of phenylethanolamine N-methyltransferase. Mol Pharmacol 9: 117–129, 1973 [PubMed] [Google Scholar]
- 88.Kishi T, Hirooka Y, Ito K, Sakai K, Shimokawa H, Takeshita A. Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone spontaneously hypertensive rats. Hypertension 39: 264–268, 2002 [DOI] [PubMed] [Google Scholar]
- 89.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 109: 2357–2362, 2004 [DOI] [PubMed] [Google Scholar]
- 90.Koizumi K, Terui N, Kollai M. Neural control of the heart: significance of double innervation re-examined. J Auton Nerv Syst 7: 279–294, 1983 [DOI] [PubMed] [Google Scholar]
- 91.Koizumi K, Terui N, Kollai M, Brooks CM. Functional significance of coactivation of vagal and sympathetic cardiac nerves. Proc Natl Acad Sci USA 79: 2116–2120, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kollai M, Koizumi K, Brooks CM. Nature of differential sympathetic discharges in chemoreceptor reflexes. Proc Natl Acad Sci USA 75: 5239–5243, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Res 609: 174–184, 1993 [DOI] [PubMed] [Google Scholar]
- 94.Lambert GW, Kaye DM, Lefkovits J, Jennings GL, Turner AG, Cox HS, Esler MD. Increased central nervous system monoamine neurotransmitter turnover and its association with sympathetic nervous activity in treated heart failure patients. Circulation 92: 1813–1818, 1995 [DOI] [PubMed] [Google Scholar]
- 95.Lee EJ, Moore CT, Hosny S, Centers A, Jennes L. Expression of estrogen receptor-alpha and c-Fos in adrenergic neurons of the female rat during the steroid-induced LH surge. Brain Res 875: 56–65, 2000 [DOI] [PubMed] [Google Scholar]
- 96.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986 [DOI] [PubMed] [Google Scholar]
- 97.Li AJ, Wang Q, Dinh TT, Ritter S. Simultaneous silencing of Npy and Dbh expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding. J Neurosci 29: 280–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li DP, Atnip LM, Chen SR, Pan HL. Regulation of synaptic inputs to paraventricular-spinal output neurons by alpha2 adrenergic receptors. J Neurophysiol 93: 393–402, 2005 [DOI] [PubMed] [Google Scholar]
- 99.Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci USA 93: 2359–2364, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li YW, Bayliss DA, Guyenet PG. C1 neurons of neonatal rats: Intrinsic beating properties and α2-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol 269: R1356–R1369, 1995 [DOI] [PubMed] [Google Scholar]
- 101.Lipski J, Kanjhan R, Kruszewska B, Smith M. Barosensitive neurons in the rostral ventrolateral medulla of the rat in vivo: Morphological properties and relationship to C1 adrenergic neurons. Neurosci 69: 601–618, 1995 [DOI] [PubMed] [Google Scholar]
- 102.Llewellyn-Smith IJ, Minson JB, Pilowsky PM, Chalmers JP. There are few catecholamine- or neuropeptide Y-containing synapses in the intermediolateral cell column of rat thoracic spinal cord. Clin Exp Pharmacol Physiol 18: 111–115, 1991 [DOI] [PubMed] [Google Scholar]
- 103.Llewellyn-Smith IJ, Schreihofer AM, Guyenet PG. Distribution and amino acid content of enkephalin-immunoreactive inputs onto juxtacellularly-labeled bulbospinal barosensitive neurons in rat rostral ventrolateral medulla. Neurosci 108: 307–322, 2001 [DOI] [PubMed] [Google Scholar]
- 104.Loewy AD, Franklin MF, Haxhiu MA. CNS monoamine cell groups projecting to pancreatic vagal motor neurons: a transneuronal labeling study using pseudorabies virus. Brain Res 638: 248–260, 1994 [DOI] [PubMed] [Google Scholar]
- 105.Loewy AD, Haxhiu MA. CNS cell groups projecting to pancreatic parasympathetic preganglionic neurons. Brain Res 620: 323–330, 1993 [DOI] [PubMed] [Google Scholar]
- 106.Lohmeier TE, Iliescu R, Dwyer TM, Irwin E, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol 299: H402–H409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lorang D, Amara SG, Simerly RB. Cell-type-specific expression of catecholamine transporters in the rat brain. J Neurosci 14: 4903–4914, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Madden CJ. Glucoprivation in the ventrolateral medulla decreases brown adipose tissue sympathetic nerve activity by decreasing the activity of neurons in raphe pallidus. Am J Physiol Regul Integr Comp Physiol 302: R224–R232, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R831–R843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Madden CJ, Stocker SD, Sved AF. Attenuation of homeostatic responses to hypotension and glucoprivation after destruction of catecholaminergic rostral ventrolateral medulla (RVLM) neurons. Am J Physiol Regul Integr Comp Physiol 291: R751–R759, 2006 [DOI] [PubMed] [Google Scholar]
- 111.Madden CJ, Sved AF. Cardiovascular regulation after destruction of the C1 cell group of the rostral ventrolateral medulla in rats. Am J Physiol Heart Circ Physiol 285: H2734–H2748, 2003 [DOI] [PubMed] [Google Scholar]
- 112.Madden CJ, Tupone D, Cano G, Morrison SF. alpha2 Adrenergic receptor-mediated inhibition of thermogenesis. J Neurosci 33: 2017–2028, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol 572: 881–896, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marina N, Abdala AP, Korsak A, Simms AE, Allen AM, Paton JF, Gourine AV. Control of sympathetic vasomotor tone by catecholaminergic C1 neurones of the rostral ventrolateral medulla oblongata. Cardiovasc Res 91: 703–710, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marina N, Tang F, Figueiredo M, Mastitskaya S, Kasimov V, Mohamed-Ali V, Roloff E, Teschemacher AG, Gourine AV, Kasparov S. Purinergic signalling in the rostral ventro-lateral medulla controls sympathetic drive and contributes to the progression of heart failure following myocardial infarction in rats. Basic Res Cardiol 108: 317, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martinez-Pena y Valenzuela I, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 286: G333–G339, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsuura T, Kumagai H, Kawai A, Onimaru H, Imai M, Oshima N, Sakata K, Saruta T. Rostral ventrolateral medulla neurons of neonatal Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 40: 560–565, 2002 [DOI] [PubMed] [Google Scholar]
- 118.McAllen RM, May CN, Shafton AD. Functional anatomy of sympathetic premotor cell groups in the medulla. Clin Exp Hypertens 17: 209–221, 1995 [DOI] [PubMed] [Google Scholar]
- 119.McCulloch PF, Panneton WM. Activation of brainstem catecholaminergic neurons during voluntary diving in rats. Brain Res 984: 42–53, 2003 [DOI] [PubMed] [Google Scholar]
- 120.McDougal DH, Viard E, Hermann GE, Rogers RC. Astrocytes in the hindbrain detect glucoprivation and regulate gastric motility. Auton Neurosci Basic 175: 61–69, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Milner TA, Abate C, Reis DJ, Pickel VM. Ultrastructural localization of phenylethanolamine N- methyltransferase-like immunoreactivity in the rat locus coeruleus. Brain Res 478: 1–15, 1989 [DOI] [PubMed] [Google Scholar]
- 122.Milner TA, Morrison SF, Abate C, Reis DJ. Phenylethanolamine N-methyltransferase-containing terminals synapse directly on sympathetic preganglionic neurons in the rat. Brain Res 448: 205–222, 1988 [DOI] [PubMed] [Google Scholar]
- 123.Milner TA, Pickel VM, Park DH, Joh TH, Reis DJ. Phenylethanolamine N-methyltransferase-containing neurons in the rostral ventrolateral medulla of the rat. I. Normal Ultrastructure. Brain Res 411: 28–45, 1987 [DOI] [PubMed] [Google Scholar]
- 124.Minson J, Llewellyn-Smith I, Neville A, Somogyi P, Chalmers J. Quantitative analysis of spinally projecting adrenaline- synthesising neurons of C1, C2 and C3 groups in rat medulla oblongata. J Auton Nerv Syst 30: 209–220, 1990 [DOI] [PubMed] [Google Scholar]
- 125.Mitchell DA, Lambert G, Secher NH, Raven PB, van LJ, Esler MD. Jugular venous overflow of noradrenaline from the brain: a neurochemical indicator of cerebrovascular sympathetic nerve activity in humans. J Physiol 587: 2589–2597, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moore SD, Guyenet PG. Effect of blood pressure on A2 noradrenergic neurons. Brain Res 338: 169–172, 1985 [DOI] [PubMed] [Google Scholar]
- 127.Morilak DA, Fornal CA, Jacobs BL. Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. III. Glucoregulatory challenge. Brain Res 422: 32–39, 1987 [DOI] [PubMed] [Google Scholar]
- 128.Morrison SF, Callaway J, Milner TA, Reis DJ. Glutamate in the spinal sympathetic intermediolateral nucleus: localization by light and electron microscopy. Brain Res 503: 5–15, 1989 [DOI] [PubMed] [Google Scholar]
- 129.Morrison SF, Cao WH. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am J Physiol Regul Integr Comp Physiol 279: R1763–R1775, 2000 [DOI] [PubMed] [Google Scholar]
- 130.Morrison SF, Milner TA, Reis DJ. Reticulospinal vasomotor neurons of the rat rostral ventrolateral medulla: relationship to sympathetic nerve activity and the C1 adrenergic cell group. J Neurosci 8: 1286–1301, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.O'Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res 37: 2496–2512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oleskevich S, Descarries L, Lacaille JC. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci 9: 3803–3815, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 248: 188–204, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Panneton WM, Gan Q, Le J, Livergood RS, Clerc P, Juric R. Activation of brainstem neurons by underwater diving in the rat. Front Physiol 3: 111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci 9: 311–313, 2006 [DOI] [PubMed] [Google Scholar]
- 137.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Rev 49: 555–565, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Paxinos G, Carrive P, Wang HQ, Wang PY. Chemoarchitectonic Atlas of the Rat Brainstem. Academic Press, 1999 [Google Scholar]
- 139.Phillips JK, Goodchild AK, Dubey R, Sesiashvili E, Takeda M, Chalmers J, Pilowsky PM, Lipski J. Differential expression of catecholamine biosynthetic enzymes in the rat ventrolateral medulla. J Comp Neurol 432: 20–34, 2001 [DOI] [PubMed] [Google Scholar]
- 140.Picklo MJ, Wiley RG, Lappi DA, Robertson D. Noradrenergic lesioning with an anti-dopamine β-hydroxylase immunotoxin. Brain Res 666: 195–200, 1994 [DOI] [PubMed] [Google Scholar]
- 141.Pilowsky PM, Abbott SB, Burke PG, Farnham MM, Hildreth CM, Kumar NN, Li Q, Lonergan T, McMullan S, Spirovski D, Goodchild AK. Metabotropic neurotransmission and integration of sympathetic nerve activity by the rostral ventrolateral medulla in the rat. Clin Exp Pharmacol Physiol 35: 508–511, 2008 [DOI] [PubMed] [Google Scholar]
- 142.Prabhakar NR, Peng YJ. Peripheral chemoreceptors in health and disease. J Appl Physiol 96: 359–366, 2004 [DOI] [PubMed] [Google Scholar]
- 143.Raff H, Fagin KD. Measurement of hormones and blood gases during hypoxia in conscious cannulated rats. J Appl Physiol 56: 1426–1430, 1984 [DOI] [PubMed] [Google Scholar]
- 144.Reis DJ, Golanov EV, Ruggiero DA, Sun MK. Sympatho-excitatory neurons of the rostral ventrolateral medulla are oxygen sensors and essential elements in the tonic and reflex control of the systemic and cerebral circulations. J Hypertens 12, Suppl 10: S159–S180, 1994 [PubMed] [Google Scholar]
- 145.Ritter S, Dinh TT, Zhang YB. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res 856: 37–47, 2000 [DOI] [PubMed] [Google Scholar]
- 146.Ritter S, Li AJ, Wang Q, Dinh TT. Minireview: The value of looking backward: the essential role of the hindbrain in counterregulatory responses to glucose deficit. Endocrinology 152: 4019–4032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-d-glucose induced metabolic challenge. Brain Res 805: 41–54, 1998 [DOI] [PubMed] [Google Scholar]
- 148.Ross CA, Armstrong DM, Ruggiero DA, Pickel VM, Joh TH, Reis DJ. Adrenaline neurons in the rostral ventrolateral medulla innervate thoracic spinal cord: a combined immunocytochemical and retrograde transport demonstration. Neurosci Lett 25: 257–262, 1981 [DOI] [PubMed] [Google Scholar]
- 149.Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Adrenaline synthesizing neurons in the rostral ventrolateral medulla: a possible role in tonic vasomotor control. Brain Res 273: 356–361, 1983 [DOI] [PubMed] [Google Scholar]
- 150.Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci 4: 474–494, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sapru Carotid chemoreflex HN. Neural pathways and transmitters. Adv Exp Med Biol 410: 357–364, 1996 [PubMed] [Google Scholar]
- 152.Sartor DM, Shulkes A, Verberne AJ. An enteric signal regulates putative gastrointestinal presympathetic vasomotor neurons in rats. Am J Physiol Regul Integr Comp Physiol 290: R625–R633, 2006 [DOI] [PubMed] [Google Scholar]
- 153.Sartor DM, Verberne AJ. Cholecystokinin selectively affects presympathetic vasomotor neurons and sympathetic vasomotor outflow. Am J Physiol Regul Integr Comp Physiol 282: R1174–R1184, 2002 [DOI] [PubMed] [Google Scholar]
- 154.Sartor DM, Verberne AJ. Phenotypic identification of rat rostroventrolateral medullary presympathetic vasomotor neurons inhibited by exogenous cholecystokinin. J Comp Neurol 465: 467–479, 2003 [DOI] [PubMed] [Google Scholar]
- 155.Schiltz JC, Sawchenko PE. Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. J Comp Neurol 502: 455–467, 2007 [DOI] [PubMed] [Google Scholar]
- 156.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol 387: 524–536, 1997 [DOI] [PubMed] [Google Scholar]
- 157.Schreihofer AM, Guyenet PG. Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-DβH-saporin. Am J Physiol Regul Integr Comp Physiol 279: R729–R742, 2000 [DOI] [PubMed] [Google Scholar]
- 158.Schreihofer AM, Guyenet PG. The baroreflex and beyond: Control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharm Phys 29: 514–521, 2002 [DOI] [PubMed] [Google Scholar]
- 159.Schreihofer AM, Guyenet PG. Baroactivated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J Neurophysiol 89: 1265–1277, 2003 [DOI] [PubMed] [Google Scholar]
- 160.Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Bötzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol 407: 583–597, 1999 [DOI] [PubMed] [Google Scholar]
- 161.Schreihofer AM, Stornetta RL, Guyenet PG. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol 221–236, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seguela P, Watkins KC, Geffard M, Descarries L. Noradrenaline axon terminals in adult rat neocortex: an immunocytochemical analysis in serial thin sections. Neurosci 35: 249–264, 1990 [DOI] [PubMed] [Google Scholar]
- 163.Serrats J, Schiltz JC, Garcia-Bueno B, van RN, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron 65: 94–106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sevigny CP, Bassi J, Williams DA, Anderson CR, Thomas WG, Allen AM. Efferent projections of C3 adrenergic neurons in the rat central nervous system. J Comp Neurol 520: 2352–2368, 2012 [DOI] [PubMed] [Google Scholar]
- 165.Siaud P, Puech R, Assenmacher I, Alonso G. Adrenergic innervation of the dorsal vagal motor nucleus: possible involvement in inhibitory control of gastric acid and pancreatic insulin secretion. Cell Tissue Res 259: 535–542, 1990 [DOI] [PubMed] [Google Scholar]
- 166.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci 36: 152–162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smith MJ, Jennes L. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction 122: 1–10, 2001 [DOI] [PubMed] [Google Scholar]
- 168.Sorg O, Magistretti PJ. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res 563: 227–233, 1991 [DOI] [PubMed] [Google Scholar]
- 169.St-John WM, Stornetta RL, Guyenet PG, Paton JF. Location and properties of respiratory neurones with putative intrinsic bursting properties in the rat in situ. J Physiol 587: 3175–3188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Standish A, Enquist LW, Escardo JA, Schwaber JS. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci 15: 1998–2012, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides 6: 1205–1211, 1985 [DOI] [PubMed] [Google Scholar]
- 172.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci USA 82: 3940–3943, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stewart JM, Rivera E, Clarke DA, Baugham IL, Ocon AJ, Taneja I, Terilli C, Medow MS. Ventilatory baroreflex sensitivity in humans is not modulated by chemoreflex activation. Am J Physiol Heart Circ Physiol 300: H1492–H1500, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stornetta RL. Neurochemistry of bulbospinal presympathetic neurons of the medulla oblongata. J Chem Neuroanat 38: 222–230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stornetta RL, Akey PJ, Guyenet PG. Location and electrophysiological characterization of rostral medullary adrenergic neurons that contain neuropeptide Y mRNA in rat. J Comp Neurol 415: 482–500, 1999 [DOI] [PubMed] [Google Scholar]
- 176.Stornetta RL, Guyenet PG. Distribution of glutamic acid decarboxylase mRNA-containing neurons in rat medulla projecting to thoracic spinal cord in relation to monoaminergic brainstem neurons. J Comp Neurol 407: 367–380, 1999 [PubMed] [Google Scholar]
- 177.Stornetta RL, Schreihofer AM, Pelaez NM, Sevigny CP, Guyenet PG. Preproenkephalin mRNA is expressed by C1 and non-C1 barosensitive bulbospinal neurons in the rostral ventrolateral medulla of the rat. J Comp Neurol 435: 111–126, 2001 [DOI] [PubMed] [Google Scholar]
- 178.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/GLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol 444: 207–220, 2002 [DOI] [PubMed] [Google Scholar]
- 179.Sun MK. Pharmacology of reticulospinal vasomotor neurons in cardiovascular regulation. Pharm Rev 48: 465–494, 1996 [PubMed] [Google Scholar]
- 180.Sun MK, Reis DJ. Central neural mechanisms mediating excitation of sympathetic neurons by hypoxia. Prog Neurobiol 44: 197–219, 1994 [DOI] [PubMed] [Google Scholar]
- 181.Sun MK, Wahlestedt C, Reis DJ. Action of externally applied ATP on rat reticulospinal vasomotor neurons. Eur J Pharmacol 224: 93–96, 1992 [DOI] [PubMed] [Google Scholar]
- 182.Sun MK, Hackett JT, Guyenet PG. Sympathoexcitatory neurons of rostral ventrolateral medulla exhibit pacemaker properties in the presence of a glutamate-receptor antagonist. Brain Res 438: 23–40, 1988 [DOI] [PubMed] [Google Scholar]
- 183.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2). J Neurosci 21: NIL7–NIL12, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tan EM, Yamaguchi Y, Horwitz GD, Gosgnach S, Lein ES, Goulding M, Albright TD, Callaway EM. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron 51: 157–170, 2006 [DOI] [PubMed] [Google Scholar]
- 185.Toussay X, Basu K, Lacoste B, Hamel E. Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J Neurosci 33: 3390–3401, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tucker DC, Saper CB, Ruggiero DA, Reis DJ. Organization of central adrenergic pathways: I. Relationships of ventrolateral medullary projections to the hypothalamus and spinal cord. J Comp Neurol 259: 591–603, 1987 [DOI] [PubMed] [Google Scholar]
- 187.Van den Pol AN. Neuropeptide transmission in brain circuits. Neuron 76: 98–115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Verberne AJ, Sartor DM. Rostroventrolateral medullary neurons modulate glucose homeostasis in the rat. Am J Physiol Endocrinol Metab 299: E802–E807, 2010 [DOI] [PubMed] [Google Scholar]
- 189.Verberne AJM, Stornetta RL, Guyenet PG. Properties of C1 and other ventrolateral medullary neurones with hypothalamic projections in the rat. J Physiol 517: 477–494, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol 95: 2070–2082, 2006 [DOI] [PubMed] [Google Scholar]
- 191.Vimercati C, Qanud K, Ilsar I, Mitacchione G, Sarnari R, Mania D, Ryan F, Stanley WC, Sabbah HN, Recchia FA. Acute vagal stimulation attenuates cardiac metabolic response to beta-adrenergic stress. J Physiol 590: 6065–6074, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Waki H, Hendy EB, Hindmarch CC, Gouraud S, Toward M, Kasparov S, Murphy D, Paton JF. Excessive leukotriene B4 in nucleus tractus solitarii is prohypertensive in spontaneously hypertensive rats. Hypertension 61: 194–201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res 1094: 163–178, 2006 [DOI] [PubMed] [Google Scholar]
- 194.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H. An official ATS clinical policy statement: Congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med 181: 626–644, 2010 [DOI] [PubMed] [Google Scholar]
- 195.Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J Comp Neurol 460: 525–541, 2003 [DOI] [PubMed] [Google Scholar]
- 196.Willette RN, Barcas PP, Krieger AJ, Sapru HN. Vasopressor and depressor areas in the rat medulla. Identification by microinjection of l-glutamate. Neuropharmacology 22: 1071–1079, 1983 [DOI] [PubMed] [Google Scholar]
- 197.Willoughby JO, Jervois PM, Menadue MF, Blessing WW. Noradrenaline, by activation of alpha-1-adrenoceptors in the region of the supraoptic nucleus, causes secretion of vasopressin in the unanesthetized rat. Neuroendocrinology 45: 219–226, 1987 [DOI] [PubMed] [Google Scholar]
- 198.Wright DE, Jennes L. Origin of noradrenergic projections to GnRH perikarya-containing areas in the medial septum-diagonal band and preoptic area. Brain Res 621: 272–278, 1993 [DOI] [PubMed] [Google Scholar]
- 199.Zanella S, Roux JC, Viemari JC, Hilaire G. Possible modulation of the mouse respiratory rhythm generator by A1/C1 neurones. Respir Physiol Neurobiol 153: 126–138, 2006 [DOI] [PubMed] [Google Scholar]
- 200.Zhang Q, Yao F, Raizada MK, O'Rourke ST, Sun C. îApelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ Res 104: 1421–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, Agnati LF. The emergence of the volume transmission concept. Brain Res Rev 26: 136–147, 1998 [DOI] [PubMed] [Google Scholar]
- 202.Zwicker JD, Rajani V, Hahn LB, Funk GD. Purinergic modulation of preBotzinger complex inspiratory rhythm in rodents: the interaction between ATP and adenosine. J Physiol 589: 4583–4600, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]