Abstract
Bed rest induces significant loss of leg lean mass in older adults. Systemic and tissue inflammation also accelerates skeletal muscle loss, but it is unknown whether inflammation is associated to inactivity-induced muscle atrophy in healthy older adults. We determined if short-term bed rest increases toll-like receptor 4 (TLR4) signaling and pro-inflammatory markers in older adult skeletal muscle biopsy samples. Six healthy, older adults underwent seven consecutive days of bed rest. Muscle biopsies (vastus lateralis) were taken after an overnight fast before and at the end of bed rest. Serum cytokine expression was measured before and during bed rest. TLR4 signaling and cytokine mRNAs associated with pro- and anti-inflammation and anabolism were measured in muscle biopsy samples using Western blot analysis and qPCR. Participants lost ∼4% leg lean mass with bed rest. We found that after bed rest, muscle levels of TLR4 protein expression and interleukin-6 (IL-6), nuclear factor-κB1, interleukin-10, and 15 mRNA expression were increased after bed rest (P < 0.05). Additionally, the cytokines interferon-γ, and macrophage inflammatory protein-1β, were elevated in serum samples following bed rest (P < 0.05). We conclude that short-term bed rest in older adults modestly increased some pro- and anti-inflammatory cytokines in muscle samples while systemic changes in pro-inflammatory cytokines were mostly absent. Upregulation of TLR4 protein content suggests that bed rest in older adults increases the capacity to mount an exaggerated, and perhaps unnecessary, inflammatory response in the presence of specific TLR4 ligands, e.g., during acute illness.
Keywords: aging, atrophy, cytokine, hospitalization, physical inactivity
acute hospitalization because of illness or injury can have devastating consequences on the functional capacity and performance of activities of daily living in older adults (3, 25). Profound inactivity characterizes acute hospitalization in older adults and can significantly and independently contribute to muscle and functional loss (11, 12). Several studies have shown that short-term bed rest leads to rapid deterioration of muscle mass and strength of the lower extremity even in healthy older subjects (7, 24). However, the precise mechanisms are still unclear. A better understanding of the cellular mechanisms leading to muscle atrophy with bed rest will allow us to identify specific targets for treatment to preserve physical function and independence in hospitalized older adults.
Muscle loss following bed rest is largely a result of reduced postabsorptive and feeding-induced muscle protein synthesis rates (7, 9, 24). Protein breakdown may also play a role in muscle loss especially in circumstances of muscle wasting such as in critical ill (5, 22) and septic (41) patients and during cast immobilization (4, 16, 23). Pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), have catabolic effects on skeletal muscle (1, 15, 17–19). Moreover, pro-inflammatory cytokines are commonly elevated in the circulation of critical ill patients (5, 22, 41), during prolonged bed rest (2), and with exercise deconditioning (47), all circumstances associated with rapid muscle loss. Conversely, increased levels of physical activity are associated with a reduction in circulating inflammatory levels (10, 20). Moreover, nonimmune cells, such as skeletal muscle, also express pro-inflammatory cytokines (15, 27, 38, 43), suggesting that cytokines may also have local biological effects on the muscle tissue. In support of this notion, Haddad et al. (19) reported decreased myofibrillar protein content in rodent limb muscle following 14 days of local IL-6 infusion. This effect was independent of changes in circulating IL-6 levels.
The biological mechanisms regulating skeletal muscle tissue inflammation are complex but may originate partly through the toll-like receptor 4 (TLR4). TLR4 is most noted for a critical role in innate immunity as a first line of defense against specific pathogens. TLR4 has a number of endogenous ligands, including heat-shock protein 60 (HSP60). Specifically, HSP60 binds TLR4 in monocytes of diabetic patients and under conditions of cellular stress (6, 28, 30, 35). Activated TLR4 in turn initiates a signaling cascade that operates through the inhibitor of κB (IκB)/nuclear factor-κB (NF-κB) pathway. Upon activation, NF-κB increases the expression of several inflammatory cytokines including IL-6, TNF-α, and interleukin-1β (29, 31). TLR4 can be found in various cell types, including human skeletal muscle (14, 42). This raises the possibility that TLR4 may be involved in initiating events associated with the stimulation of pro-inflammatory cytokine transcription within skeletal muscle.
The purpose of the current investigation was to characterize the mechanisms of bed rest-induced muscle wasting by examining the regulatory pathways of muscle inflammation through TLR4 signaling in the postabsorptive state. A secondary aim was to determine the effect of bed rest on muscle anti-inflammatory mediators (suppressor of cytokine signaling-3, interleukin-10) (44, 50) and cytokines associated with anabolism (interleukin-15) (34, 40). We hypothesized that a 7-day bed-rest protocol, mimicking an acute hospitalization, would increase systemic and local levels of pro-inflammatory cytokines, including TLR4 signaling in skeletal muscle biopsy samples of older adults. Furthermore, we hypothesized that anti-inflammatory and anabolic cytokines would be reduced after short-term bed rest in skeletal muscle samples of these older adults.
METHODS
Participants.
Six healthy older adults (5 men, 1 woman; age 67 ± 2 yr; height 174 ± 2 cm; weight 75 ± 4 kg) were recruited from the University of Texas Medical Branch Pepper Center Volunteer Registry. Subjects were community-dwelling, overall healthy, independent older adults as determined by clinical history, mini-mental state exam, physical examination, and laboratory tests. All subjects read and signed a written informed consent before participating in the study. The informed consent and protocol were approved by the Institutional Review Board of the University of Texas Medical Branch. Body composition and protein turnover data from this study have been previously published (7).
Experimental design.
Research participants underwent a 7-day bed-rest experiment in the University of Texas Medical Branch Institute for Translational Sciences Clinical Research Center. Bed rest was preceded by a 3-day in-hospital run in (maintaining the subject's habitual diet and physical activity) and followed by a 3-day in-hospital rehabilitation period (7). Over the bed-rest period, nonpharmacological deep venous thrombosis prevention was performed, which consisted of intermittent lower leg compression devices, compression stockings, and daily passive range of motion by a physical therapist. Bathing and hygiene activities were performed in the bed with nursing help, while toilet privileges were limited to a bedside commode. Adherence to bed rest was continuously monitored via closed-circuit cameras and reinforced daily by nursing staff and study personnel. A dual-energy X-ray absorptiometry (DXA) scan to measure body tissue composition was conducted before and after bed rest as reported previously (7). DXA scan measurements were carefully controlled between measurements, which entailed measurements taken at the same time of day (8:00 AM) after an overnight fast, participants wearing the same clothing and controlling for fluid shifts with participants laying supine before scans.
After an inpatient stay and an overnight fast, percutaneous muscle biopsies were collected in the morning of the initiation of bed-rest day 1 (pre-bed rest) and at the same time in the morning of bed-rest day 7 (post-bed rest). Muscle biopsies were taken from the vastus lateralis muscle of one leg (pre-bed rest) and the opposite leg (post-bed rest) using aseptic technique, local anesthesia (1% lidocaine), and a 5-mm Bergström biopsy needle with suction. All muscle tissue was immediately blotted and dissected free of visible nonmuscle tissue, flash-frozen in liquid nitrogen, and stored at −80°C for protein and mRNA analysis.
Serum cytokines.
Venous blood samples from participants were collected before bed rest and on the second, third, fourth, and seventh day of bed rest. Samples were collected at the same time in the morning, after an overnight fast. Blood samples were allowed to clot at room temperature for 20 min in a serum separator tube, of which the tubes were then centrifuged. Supernatant serum was collected and stored at −80°C until analysis. Cytokines [IL-1β, -2, -4, -5, -6, -7, -8, -10, -12, -13, -17, G-CSF, granulocyte macrophage colony stimulating factor (GM-CSF), interferon-γ (INF-γ), monocyte chemoattractant protein-1 (MCP-1), MIP-1β, and TNF-α] were measured in serum samples using a Bio-Plex Pro Human Cytokine 17-plex Assay (M50-00031YV; Bio-Rad, Hercules, CA) on a Bio-Plex 200 Instrument (Bio-Rad). For each analyte, a standard curve was generated using recombinant proteins to estimate protein concentration in the unknown sample (BioPlex Array Manager).
SDS-PAGE and immunoblotting.
Muscle tissue was homogenized using a glass pestle and prechilled tube in a buffer cocktail with protease and phosphatase inhibitors (50 mM Tris·HCl, 250 mM mannitol, 50 mM sodium flouride, 5 mM sodium pyrophophate, 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 1 mM benzamidine, 0.1 mM phenylmethylsulfonyl fluoride, and 5 μg/ml soybean trypsin inhibitor). Whole muscle homogenates were centrifuged and the supernatant was collected. Total protein concentration for each sample was determined on a SmartSpec (Bio-Rad) using a colorimetric protein assay (Bio-Rad; Bradford) and an albumin standard curve. Whole muscle homogenates were diluted 1:1 in a 2× sample buffer. Homogenates (50 μg of total protein) were loaded on a 7.5% and 15% polyacrylamide gel (Criterion; Bio-Rad), depending on the molecular weight of the protein, and subjected to SDS-PAGE (150 V) for 1 h in running buffer. Each gel contained alternating pre- and post-bed rest samples loaded in duplicate and a molecular weight ladder. An internal control (rodent muscle homogenate) was loaded in duplicate on each gel for band normalization and comparisons across blots. Protein was transferred (50 V; 1 h) to a polyvinylidene fluoride membrane in transfer buffer and then blocked for 1 h at room temperature with 5% non-fat dry milk (NFDM) in Tris-buffered saline in 0.1% Tween-20 (TBST). Membranes were incubated overnight in primary antibody diluted in 5% NFDM or bovine serum albumin in TBST. The next morning, blots were rinsed in TBST for 5 min, rocked in secondary antibody for 1 h at room temperature in 5% NFDM in TBST, and then serially washed (15 min, 3 × 5 min) in TBST. Chemiluminescence reagent (ECL Plus, GE Healthcare) was applied to each blot for 5 min. Optical density measurements were obtained with a digital imager (ChemiDoc XRS, Bio-Rad). Membranes containing phospho-specific proteins were stripped (25 mM glycine, pH 2.0, and 1% SDS) of primary and secondary antibodies then reprobed for the total protein of specific target. Densitometric analysis was performed using Quantity One 4.5.2 software (Bio-Rad). After the background was subtracted out, all Western blot data were normalized to the internal control and replicate samples were averaged. α-Tubulin was used to verify equal loading across lanes.
Antibodies.
The following antibodies were used in this experiment: TLR4 (cat. no. sc-10741) from Santa Cruz Biotechnology (Santa Cruz, CA), whereas the following antibodies were purchased from Cell Signaling Technology (Boston, MA): HSP60 (cat. no. 4870), phosphorylated IκBα (S32; cat. no. 2859) and total IκBα (cat. no. 9242), phosphorylated NF-κB p65 (S536; cat. no. 3033) and total NF-κB p65 (cat. no. 8242). α-Tubulin was purchased from Sigma-Aldrich (cat. no. F2168). Donkey anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from GE Healthcare Amersham, whereas a mouse secondary antibody was purchased from Santa Cruz Biotechnology.
RNA extraction, cDNA synthesis, and semiquantitative qPCR.
Total RNA, cDNA synthesis, and real-time qPCR were conducted as previously reported (8). Total RNA was isolated by homogenizing 15–20 mg tissue with a hand-held homogenizing dispenser (T10 Basic Ultra Turrax, IKA, Wilmington, NC) in a solution containing 1 ml of Tri reagent. The RNA was separated into an aqueous phase using 0.2 ml of chloroform and precipitated from the aqueous phase using 0.5 ml of isopropanol. Extracted RNA was washed with 1 ml of 75% ethanol, dried, and then suspended in a known amount of nuclease-free water. RNA integrity for these samples (RIN: ∼9) have been published previously (7). RNA was DNase-treated using a commercially available kit (DNA-free, Ambion, Austin, TX). Afterwards, 1 μg of total RNA was reverse transcribed into cDNA (iScript, Bio-Rad). All isolated RNA and cDNA samples were stored at −80°C until analyzed with qPCR. Real-time qPCR was carried out with an iQ5 Multicolor Real Time PCR cycler (Bio-Rad). Taqman predesigned primers were purchased from Applied Biosystems (Carlsbad, CA). The thermal cycling conditions were the following: a 10-min initial denaturing step at 95°C followed by 40 cycles consisting of denaturation for 15 s at 95°C and anneal and elongation for 1 min at 60°C. Values were normalized to β2-microglobulin (since Ct values remained stable following bed rest) and then fold change from pre-bed rest values was calculated (32).
Statistical analysis.
A linear model (SAS v9.2 Cary, NC) with subject as a blocking factor was used to test differences across time (day 2, 3, 4, 7) for serum cytokine samples. Cytokine measurements were corrected for multiple hypotheses testing using the Tukey-Kramer method. For ease of readership, actual means are reported in Table 1, but data were adjusted for statistical analysis. Correlations were determined between TLR4 and IL-6 and changes in leg lean mass following bed rest using a linear regression model. Analysis of baseline protein content and mRNA expression between pre- and post-bed rest was conducted using a paired t-test (SigmaPlot; Version 12.0). Significance was set at P < 0.05. All values are presented as means ± SE.
Table 1.
Serum cytokines before and during 7 days of bed rest in healthy older adults
| Bed Rest (Days) |
||||||
|---|---|---|---|---|---|---|
| Cytokine | Baseline | Day 2 | Day 3 | Day 4 | Day 7 | Main Effect-Time (P Value) |
| IL-1β | 1.4 ± 0.4 | 1.6 ± 0.3 | 1.7 ± 0.5 | 1.4 ± 0.3 | 1.4 ± 0.2 | 0.68 |
| IL-4 | 2.0 ± 0.4 | 2.1 ± 0.2 | 2.6 ± 0.7 | 2.0 ± 0.5 | 2.4 ± 0.4 | 0.41 |
| IL-5 | 2.4 ± 0.7 | 2.6 ± 0.6 | 3.0 ± 0.8 | 2.6 ± 0.6 | 2.5 ± 0.5 | 0.94 |
| IL-6 | 3.5 ± 0.4 | 3.8 ± 0.4 | 4.4 ± 0.7 | 3.5 ± 0.5 | 3.8 ± 0.4 | 0.77 |
| IL-7 | 5.1 ± 1.0 | 5.9 ± 0.5 | 6.8 ± 1.2 | 5.9 ± 0.8 | 6.2 ± 0.8 | 0.80 |
| IL-8 | 7.0 ± 1.5 | 7.6 ± 0.9 | 8.4 ± 1.8 | 7.5 ± 1.2 | 8.5 ± 1.3 | 0.19 |
| IL-10 | 2.5 ± 0.6 | 3.1 ± 0.8 | 3.0 ± 0.8 | 2.3 ± 0.4 | 2.5 ± 0.3 | 0.39 |
| IL-12 | 9.6 ± 1.8 | 10.2 ± 1.1 | 12.3 ± 2.5 | 10.0 ± 1.7 | 11.0 ± 1.8 | 0.78 |
| IL-13 | 2.5 ± 0.8 | 2.7 ± 0.7 | 3.3 ± 1.1 | 2.7 ± 0.9 | 2.4 ± 0.5 | 0.84 |
| IL-17 | 4.5 ± 0.9 | 5.5 ± 2.4 | 6.4 ± 2.2 | 3.6 ± 1.8 | 6.4 ± 2.1 | 0.30 |
| G-CSF | 9.0 ± 3.1 | 12.6 ± 1.5 | 10.2 ± 1.3 | 8.2 ± 2.2 | 11.1 ± 2.6 | 0.57 |
| GM-CSF | 7.4 ± 1.1 | 5.6 ± 0.9 | 4.9 ± 1.2 | 4.2 ± 1.2 | 6.7 ± 1.5 | 0.29 |
| IFN-γ | 58.4 ± 10.0 | 60.4 ± 4.6 | 76.2 ± 18.2 | 61.8 ± 12.2 | 72.9 ± 16.7 | 0.05 |
| MCP-1 | 19.7 ± 3.9 | 17.2 ± 4.3 | 17.9 ± 4.7 | 16.4 ± 3.1 | 21.9 ± 5.9 | 0.50 |
| MIP-1β | 49.5 ± 25.2 | 52.1 ± 24.8 | 51.8 ± 23.1 | 52.9 ± 24.0 | 62.0 ± 30.2 | 0.02 |
| TNF-α | 14.0 ± 3.5 | 15.8 ± 2.7 | 18.1 ± 4.4 | 15.1 ± 3.0 | 15.1 ± 2.0 | 0.93 |
Values are pg/ml and are means ± SE (n = 6). P value signifies main effect for time (days of bed rest). Values for IL-17, G-CSF, and GM-CSF are n = 4. IL-1β, interleukin-1β; IL-4, interleukin-4; IL-5, interleukin-5; IL-6, interleukin-6; IL-7, interleukin-7; IL-8, interleukin-8; IL-10, interleukin-10; IL-12, interleukin-12; IL-13, interleukin-13; IL-17, interleukin-17; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte macrophage-colony stimulating factor; IFN-γ, interferon-γ; MCP-1, monocyte chemotactic protein-1; MIP-1β, macrophage inflammatory protein-1β; TNF-α, tumor necrosis factor-α.
RESULTS
Lean mass.
After 7-days of bed rest, older adults lost ∼1.6 kg of total body lean mass (Pre: 50.4 ± 2.9; Post: 48.8 ± 2.5 kg; P = 0.03), whereas ∼50% (0.8 kg) of this loss came from leg (right + left) lean mass (Pre: 18.3 ± 1.1; Post: 17.5 ± 1.0 kg; P = 0.01).
Serum cytokines.
We found no changes across time in 14 of the 17 cytokines. INF-γ (P = 0.05) and MIP-1β (P = 0.02) increased over time. Serum IL-2 was undetectable throughout the experimental period, and IL-17, G-CSF, and GM-CSF were detectable only in n = 4. All detectable serum cytokines were within range of the standard curve.
Skeletal muscle TLR4 and NF-κβ signaling.
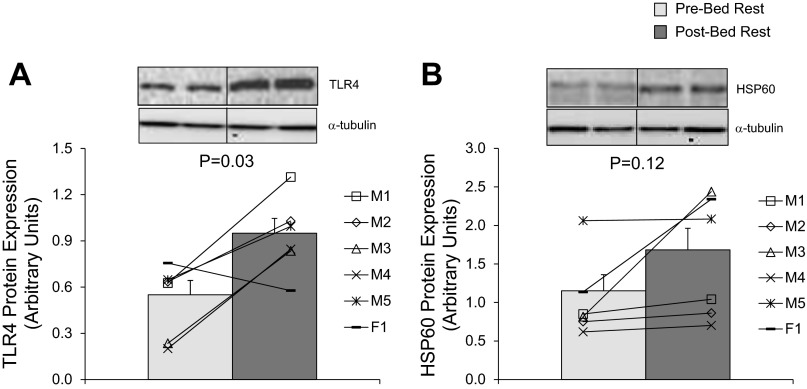
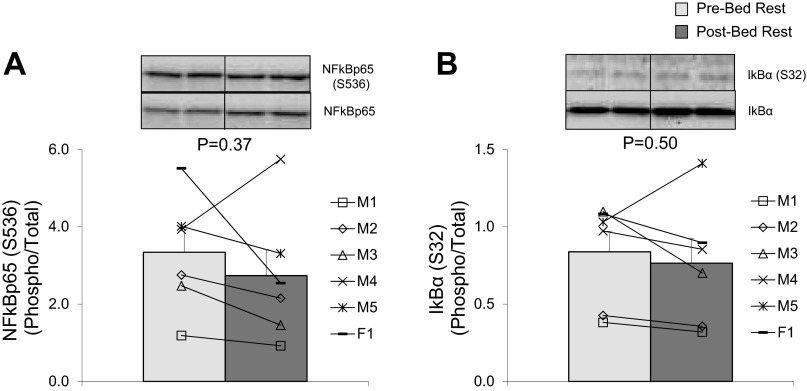
We found that the 7-day bed rest increased the abundance of TLR4 in skeletal muscle by ∼70% compared with pre-bed rest values (Figure 1A; P = 0.03). However, heat shock protein 60 (HSP60), a ligand for TLR4, was unaltered following bed rest (Fig. 1B; P = 0.12). To evaluate the effect of increased TLR4 protein expression, we measured NF-κB signaling. We found no differences when NF-κB phosphorylation (S536) was expressed relative to total NF-κB p65 protein abundance (Fig. 2A; P = 0.37) following 7-days of bed rest in skeletal muscle of healthy older subjects. Similarly, bed rest did not affect the phosphorylation of NF-κB at S536 (Pre: 3.53 ± 0.58; Post: 2.77 ± 0.31 AU; P = 0.26) or NF-κB p65 protein levels (Pre: 1.18 ± 0.21; Post: 1.27 ± 0.25 AU; P = 0.42). Furthermore, there were no changes in IκBα phosphorylation (S32) relative to its total protein levels (Fig. 2B; P = 0.50) or the independent phosphorylation of IκBα at S32 (Pre: 0.99 ± 0.26; Post: 1.03 ± 0.25 AU; P = 0.50) and total IκBα protein levels (Pre: 1.78 ± 0.77; Post: 2.14 ± 0.93 AU; P = 0.13).
Fig. 1.
Effect of bed rest on toll-like receptor 4 (TLR4) and heat shock protein 60 (HSP60) protein expression. Data represent protein expression for TLR4 (A) and HSP60 (B) before (light gray bar) and after (dark gray bar) 7 days of bed rest in vastus lateralis skeletal muscle biopsy samples of healthy older adults (n = 6). Insets are representative immunoblot images. Equal protein loading was confirmed with α-tubulin. Line plots within figures represent individual means for males (M1–5) and female (F1). P values are indicated on figures. Values are in arbitrary units and presented as means ± SE.
Fig. 2.
Effect of bed rest on nuclear factor-κB (NF-κB) signaling. Data represent NF-κB phosphorylation (S536) relative to NF-κB total protein expression (A) and IκBα phosphorylation (S32) relative to IκBα total protein expression (B) before (light gray bars) and after (dark gray bars) 7 days of bed rest in vastus lateralis skeletal muscle of healthy older adults (n = 6). Insets are representative immunoblot images. Line plots below figures represent individual means for males (M1–5) and female (F1). P values are indicated on figures. Values are presented as means ± SE.
Pro-inflammatory mRNA expression.
We found that NF-κB1 mRNA expression in skeletal muscle biopsy samples was increased ∼40% compared with pre-bed rest values (Fig. 3A, P = 0.03) but independent of changes in total protein (Fig. 3B). Additionally, IL-6 mRNA expression increased ∼130% after 7-days of bed rest in older adult skeletal muscle (Fig. 3B; P = 0.04). Bed rest did not affect TNF-α (Fig. 3C; P = 0.64), TNFR1SA1 (Fig. 3D; P = 0.42), or IL1β mRNA expression (Fig. 3E; P = 0.89). CD45 mRNA expression, a cell surface marker of immune cells, did not change as a result of bed rest (Pre: 1.11 ± 0.20; Post: 1.29 ± 0.19 fold change; P = 0.56).
Fig. 3.
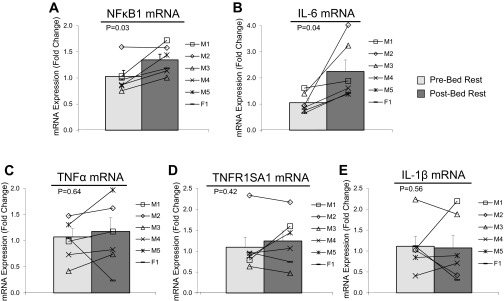
Effect of bed rest on pro-inflammatory muscle mRNA markers. Data represent NF-κB1 (A), IL-6 (B), tumor necrosis factor-α (TNF-α) (C), TNFR1SA1 (D), and IL-1β mRNA expression (E) before (light gray bars) and after (dark gray bars) 7 days of bed rest in vastus lateralis skeletal muscle of healthy older adults (n = 6). Line plots below figures represent individual means for males (M1–5) and female (F1). Values are reported as fold change from baseline. P values are indicated on figures. Values are presented as means ± SE.
Cytokines associated with anti-inflammation and anabolism.
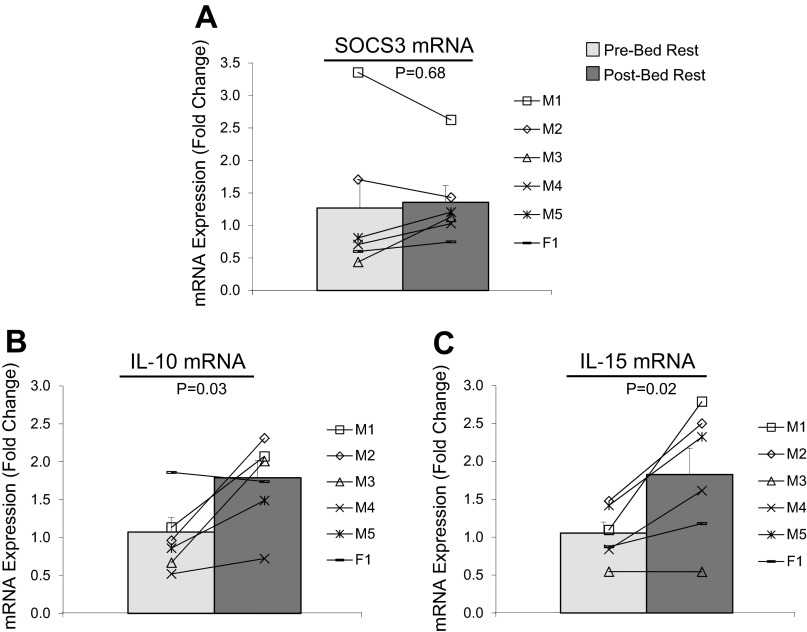
There were no differences in SOCS3 mRNA expression after bed rest in older adult skeletal muscle samples (Fig. 4A; P = 0.68). However, we found that IL-10 (Fig. 4B; P = 0.03) and IL-15 (Fig. 4C; P = 0.02) mRNA expression increased as a result of bed rest.
Fig. 4.
Effect of bed rest on the mRNA expression of anti-inflammatory and anabolic cytokines. Data represent SOCS3 (A), IL-10 (B), and IL-15 mRNA expression (C) before (light gray bars) and after (dark gray bars) 7 days of bed rest in vastus lateralis skeletal muscle of healthy older adults (n = 6). Line plots below figures represent individual means for males (M1–5) and female (F1). Values are reported as fold change from baseline. P values are indicated on figures. Values are presented as means ± SE.
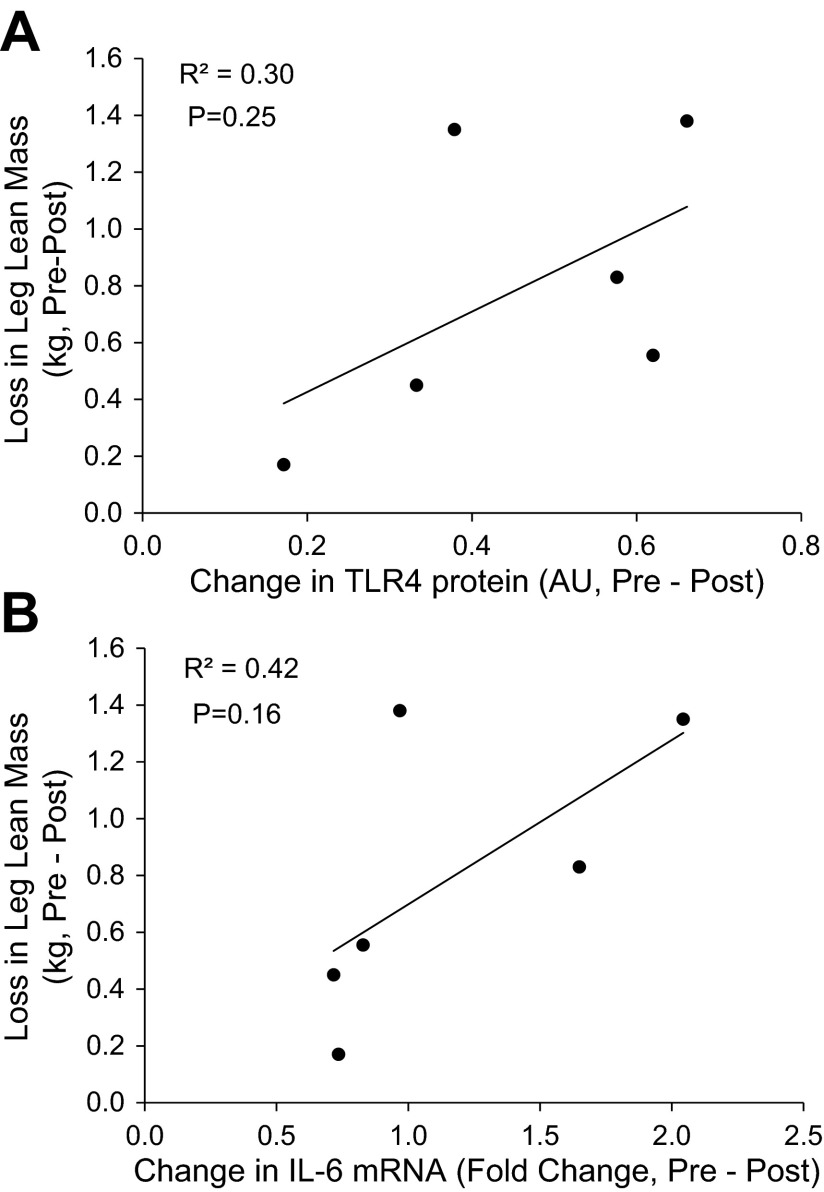
Relationship between bed rest-induced changes in leg lean mass and TLR4 and IL-6 expression.
There was not a statistically significant correlation between changes in TLR4 protein and IL-6 mRNA expression and leg lean mass: TLR4 protein expression and leg (right + left) lean mass (Fig. 5A; R2 = 0.31, P = 0.25) and IL-6 mRNA expression and leg lean mass (Fig. 5B; R2 = 0.42, P = 0.16).
Fig. 5.
Relationship between the changes in leg lean mass and TLR4 and IL-6 expression. Data represent the delta change (pre- and post-bed rest) correlation for the following dependent variables in skeletal muscle of healthy older adults (n = 6): TLR4 protein expression vs. leg (right + left) lean mass loss (A) and IL-6 mRNA expression vs. leg (right + left) lean mass loss (B).
DISCUSSION
The primary finding of this study was that 7 days of controlled bed rest, mimicking the inactivity of an acute hospital stay, markedly increased TLR4 protein abundance and IL-6 mRNA expression in the skeletal muscle biopsy samples of healthy older adults. Conversely, we did not find significant changes in muscle NF-κB signaling while changes in circulating inflammatory levels were modest and mostly absent. We also found that some, but not all, pro-inflammatory and anabolic cytokine mRNA expression in muscle biopsy samples was increased after bed rest. These data provide preliminary evidence that short-term physical inactivity can locally induce a small, but likely important, pro-inflammatory response in skeletal muscle biopsy samples of healthy older adults in the absence of an obvious increase in systemic inflammation. The elevation in TLR4 with bed rest may suggest an increased muscle capacity to mount a rapid and possibly uncontrolled inflammatory response in the presence of TLR4 ligands, such as during an acute illness or infection, in older adults.
This study is the first demonstrating an elevation in TLR4 expression in skeletal muscle biopsy samples following 7 days of controlled bed rest in healthy older subjects. Previous work has shown that increased physical activity and exercise training can reduce TLR4 mRNA in skeletal muscle of healthy (51) and diabetic rats (36) and in frail obese older adults (26). When interpreted from those results, our novel data support the notion that TLR4 expression is highly sensitive and inversely correlated to the level physical activity in human skeletal muscle.
A signature response of increased TLR4 activity is an upregulation of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β (27). We found that bed rest increased the skeletal muscle levels of IL-6 mRNA, but TNF-α, TNF-α receptor (TNFR1SA1), and IL-1β mRNA were unchanged.Our findings are consistent with those reported by Reyna and colleagues (42) who found elevated skeletal muscle levels of TLR4 mRNA and protein and IL-6 mRNA in obese adults with Type 2 diabetes compared with lean nondiabetic controls. Other human models of muscle wasting (critically ill) have also been associated with elevated muscle IL-6 mRNA levels, whereas changes in TNF-α have been inconsistent (5, 22). To the best to our knowledge, until now no one had examined muscle tissue levels of inflammatory cytokines as a result of controlled bed rest. While we cannot exclude that macrophages and other immune cells could have contributed to the increased inflammatory state as noted by detectable levels of CD-45 mRNA levels in our muscle homogenate samples, the finding that 15 of the 17 serum cytokines were unaltered following bed rest strongly suggests the lack of a systemic pro-inflammatory response with bed rest. In addition, even a systemic pro-inflammatory condition, such as Type 2 diabetes in obese subjects, is not necessarily associated with immune cell infiltration of the skeletal muscle tissue, as reported by Reyna et al. (42). Thus the likelihood that our findings were confounded by activated immune cells migrating into the muscle tissue is low. Regardless, an increase in cytokine secreting immune cells in skeletal muscle would have a significant impact on muscle metabolism and homeostasis. Collectively, these data support that as little as 7 days of physical inactivity in healthy older adults increases some markers of local inflammation, including TLR4 protein and IL-6 mRNA expression.
We did not detect an increase in the TLR4 ligand HSP60 within skeletal muscle homogenate samples after bed rest other than a trend (P = 0.12) suggesting that HSP60 may not be involved during the early stages of bed rest. More importantly, other than a subtle increase in NF-κB1 mRNA, we did not find any significant changes in downstream TLR4 signaling (IκBα/NF-κB) in skeletal muscle biopsy samples after bed rest. It is possible that, because of the limited amount of human muscle sample available, the use of whole muscle homogenates instead of isolated nuclear and cytoplasmic fractions might have reduced our sensitivity to detect changes. Alternatively, TLR4 signaling may occur through another pathway (i.e., p38, JNK) (13), or increased expression of IL-6 mRNA may be mediated by an alternate (37) or unknown mechanism independent of TLR4 and NF-κB. A more likely explanation is that bed rest, in the absence of disease, increased TLR4 protein levels without activating NF-κB because of lack of TLR4 ligands (i.e., HSP60). If this is the case, then inactivity per se in older adults increases the capacity to mount an elevated, yet abnormal, muscle inflammatory response to acute noxious stimuli (e.g., an infection or a cardiovascular event requiring hospitalization). At best, changes in TLR4 and IL-6 showed a tendency to be related to muscle loss following bed rest, but interpretation must be taken with caution with a small sample size and making correlations with DXA measurements. Perhaps, with longer periods of bed rest-induced inactivity, an oversensitization to infection (i.e., TLR4) may lead to a much larger and potentially catastrophic loss of skeletal muscle mass.
We also found that skeletal muscle IL-10 mRNA expression was upregulated with bed rest in older adults, whereas there were no observed changes in SOCS3 mRNA. IL-10 is well known for its function to suppress inflammatory immune responses by targeting the transcription of pro-inflammatory mediators (33). Since IL-10 mRNA was elevated with bed rest, it is possible that this might be a mechanism to counteract a pro-inflammatory response (21). In fact, IL-6 is known to stimulate IL-10 production, thereby providing a physiological brake on the overall pro-inflammatory status (45). We also found that the anabolic cytokine IL-15 mRNA (34, 40) was increased after bed rest as previously reported in animals (39). Taken together, the increased expression of local anti-inflammatory and anabolic cytokines following acute bed rest in older adults may be a mechanism to counter heightened levels of IL-6.
An interesting observation was that bed rest largely did not affect serum inflammatory markers even in the early period of bed rest (days 2–4). In a previous report, 14 days of bed rest in young volunteers resulted in increased circulating IL-6 levels (2). Perhaps we did not observe changes in serum inflammatory cytokines because cellular sources of inflammatory mediators require a longer period of bed rest to be distributed into the circulation. Although a majority of circulating cytokines were unaltered during (and after) 7 days of bed rest even with a comprehensive cytokine profiling, we did find elevated serum levels of MIP-1β and a tendency for INF-γ to be increased. An increase in MIP-1β may represent an immune response to regenerate new tissue during bed rest (46, 49), whereas elevated INF-γ is consistent with an increased pro-inflammatory response as observed following muscle injury in old rodents (48).
We conclude that short-term bed rest in healthy older adults increased some, but not all, local pro-inflammatory mediators, namely TLR4 protein and IL-6 mRNA expression, as well as anti-inflammatory (IL-10) and anabolic (IL-15) cytokine mRNA expression. These changes were largely independent of alterations in local NF-κB signaling and serum markers of inflammation. Increased expression of TLR4 may be an important predisposing factor for catastrophic inflammatory and muscle catabolic responses triggered by a concomitant acute disease and bed rest inactivity in older adults (e.g., inactivity during hospitalization for an acute illness).
GRANTS
This study was supported by National Institutes of Health Grants UL1 TR000071, R01 AG018311, P30 AG024832, R01 AR04987, K01 AG038556.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.D., B.B.R., and E.V. conception and design of research; M.J.D., K.L.T., M.M.M., D.K.W., and J.M.D. performed experiments; M.J.D., M.M.M., and M.J. analyzed data; M.J.D., K.L.T., J.M.D., A.R.B., B.B.R., and E.V. interpreted results of experiments; M.J.D. prepared figures; M.J.D. drafted manuscript; M.J.D., K.L.T., M.M.M., D.K.W., J.M.D., M.J., A.R.B., B.B.R., and E.V. edited and revised manuscript; M.J.D., K.L.T., M.M.M., D.K.W., J.M.D., M.J., A.R.B., B.B.R., and E.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the research volunteers for their time and effort completing this study. Additionally, we are grateful to the nursing staff of the Institute for Translational Sciences Clinical Research Center and the Claude D. Pepper Older Americans Independence Center Volunteer Registry study coordinators. We also thank Junfang Hao for technical assistance.
REFERENCES
- 1.Argiles JM, Busquets S, Lopez-Soriano FJ. The pivotal role of cytokines in muscle wasting during cancer. Int J Biochem Cell Biol 37: 2036–2046, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bosutti A, Malaponte G, Zanetti M, Castellino P, Heer M, Guarnieri G, Biolo G. Calorie restriction modulates inactivity-induced changes in the inflammatory markers C-reactive protein and pentraxin-3. J Clin Endocrinol Metab 93: 3226–3229, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, Burant C, Covinsky KE. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc 56: 2171–2179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YW, Gregory CM, Scarborough MT, Shi R, Walter GA, Vandenborne K. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol Genomics 31: 510–520, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Constantin D, McCullough J, Mahajan RP, Greenhaff PL. Novel events in the molecular regulation of muscle mass in critically ill patients. J Physiol 589: 3883–3895, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33: 861–868, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, Volpi E. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 302: E1113–E1122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298: E1011–E1018, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Fischer CP, Berntsen A, Perstrup LB, Eskildsen P, Pedersen BK. Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scandi J Med Sci Sports 17: 580–587, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fisher SR, Galloway RV, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV, Goodwin JS. Pilot study examining the association between ambulatory activity and falls among hospitalized older adults. Arch Phys Med Rehabil 92: 2090–2092, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher SR, Goodwin JS, Protas EJ, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc 59: 91–95, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foncea HZ, Raymackers JM, Deldicque L, Renard P, Francaux M. TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Med Sci Sports Exercise 44: 1463–1472, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Frisard MI, McMillan RP, Marchand J, Wahlberg KA, Wu Y, Voelker KA, Heilbronn L, Haynie K, Muoio B, Li L, Hulver MW. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am J Physiol Endocrinol Metab 298: E988–E998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care 8: 255–263, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Glover EI, Yasuda N, Tarnopolsky MA, Abadi A, Phillips SM. Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Appl Physiol Nutr Metab 35: 125–133, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med 205: 182–185, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Goodman MN. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol Endocrinol Metab 260: E727–E730, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Hjelstuen A, Anderssen SA, Holme I, Seljeflot I, Klemsdal TO. Markers of inflammation are inversely related to physical activity and fitness in sedentary men with treated hypertension. Am J Hypertens 19: 669–675; discussion 676–667, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Huey KA, McCusker RH, Kelley KW. Exaggerated expression of skeletal muscle-derived interleukin-6, but not TNFalpha, in mice lacking interleukin-10. J Neuroimmunol 199: 56–62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jespersen JG, Nedergaard A, Reitelseder S, Mikkelsen UR, Dideriksen KJ, Agergaard J, Kreiner F, Pott FC, Schjerling P, Kjaer M. Activated protein synthesis and suppressed protein breakdown signaling in skeletal muscle of critically ill patients. PLos One 6: e18090, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones SW, Hill RJ, Krasney PA, O'Conner B, Peirce N, Greenhaff PL. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J 18: 1025–1027, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. J Am Med Assoc 297: 1772–1774, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R, Evans WJ. Functional impact of 10 days of bed rest in healthy older adults. Biol Sci Med Sci 63: 1076–1081, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol 105: 473–478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle. Shock 19: 538–546, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci 28: 2320–2331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med 86: 1113–1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, Zhang M, Warren HS, Sosnovik DE, Chao W. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem 286: 31308–31319, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10: 2327–2334, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA 102: 8686–8691, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol 584: 305–312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164: 558–561, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Oliveira AG, Carvalho BM, Tobar N, Ropelle ER, Pauli JR, Bagarolli RA, Guadagnini D, Carvalheira JB, Saad MJ. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes 60: 784–796, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Pedersen BK. Muscles and their myokines. J Exp Biol 214: 337–346, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol 536: 329–337, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pistilli EE, Siu PM, Alway SE. Interleukin-15 responses to aging and unloading-induced skeletal muscle atrophy. Am J Physiol Cell Physiol 292: C1298–C1304, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res 280: 55–63, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Rabuel C, Renaud E, Brealey D, Ratajczak P, Damy T, Alves A, Habib A, Singer M, Payen D, Mebazaa A. Human septic myopathy: induction of cyclooxygenase, heme oxygenase and activation of the ubiquitin proteolytic pathway. Anesthesiology 101: 583–590, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57: 2595–2602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest 97: 1111–1116, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem 274: 31868–31874, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285: E433–E437, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol 117: 1027–1035, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Thompson D, Markovitch D, Betts JA, Mazzatti D, Turner J, Tyrrell RM. Time course of changes in inflammatory markers during a 6-mo exercise intervention in sedentary middle-aged men: a randomized-controlled trial. J Appl Physiol 108: 769–779, 2010 [DOI] [PubMed] [Google Scholar]
- 48.van der Poel C, Gosselin LE, Schertzer JD, Ryall JG, Swiderski K, Wondemaghen M, Lynch GS. Ageing prolongs inflammatory marker expression in regenerating rat skeletal muscles after injury. J Inflamm 8: 41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yahiaoui L, Gvozdic D, Danialou G, Mack M, Petrof BJ. CC family chemokines directly regulate myoblast responses to skeletal muscle injury. J Physiol 586: 3991–4004, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa HSOCS. Inflammation, Autoimmunity. Frontiers Immunol 3: 20, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zanchi NE, Lira FS, de Siqueira Filho MA, Rosa JC, de Oliveira Carvalho CR, Seelaender M, Santos RV, Lancha AH., Jr Chronic low frequency/low volume resistance training reduces pro-inflammatory cytokine protein levels and TLR4 mRNA in rat skeletal muscle. Eur J Appl Physiol 109: 1095–1102, 2010 [DOI] [PubMed] [Google Scholar]