Abstract
In contrast to other vertebrate hemoglobins (Hbs) whose high intrinsic O2 affinities are reduced by red cell allosteric effectors (mainly protons, CO2, organic phosphates, and chloride ions), crocodilian Hbs exhibit low sensitivity to organic phosphates and high sensitivity to bicarbonate (HCO3−), which is believed to augment Hb-O2 unloading during diving and postprandial alkaline tides when blood HCO3− levels and metabolic rates increase. Examination of α- and β-globin amino acid sequences of dwarf caiman (Paleosuchus palpebrosus) revealed a unique combination of substitutions at key effector binding sites compared with other vertebrate and crocodilian Hbs: β82Lys→Gln, β143His→Val, and β146His→Tyr. These substitutions delete positive charges and, along with other distinctive changes in residue charge and polarity, may be expected to disrupt allosteric regulation of Hb-O2 affinity. Strikingly, however, P. palpebrosus Hb shows a strong Bohr effect, and marked deoxygenation-linked binding of organic phosphates (ATP and DPG) and CO2 as carbamate (contrasting with HCO3− binding in other crocodilians). Unlike other Hbs, it polymerizes to large complexes in the oxygenated state. The highly unusual properties of P. palpebrosus Hb align with a high content of His residues (potential sites for oxygenation-linked proton binding) and distinctive surface Cys residues that may form intermolecular disulfide bridges upon polymerization. On the basis of its singular properties, P. palpebrosus Hb provides a unique opportunity for studies on structure-function coupling and the evolution of compensatory mechanisms for maintaining tissue O2 delivery in Hbs that lack conventional effector-binding residues.
Keywords: allosteric interaction, Bohr effect, carbon dioxide, crocodilians, oxygen-binding
the role of hemoglobin (Hb) in transporting O2 to respiring tissues is governed by its intrinsic O2 affinity, its sensitivity to the effectors that modulate Hb-O2 affinity, levels of those effectors in the red blood cells, and the O2 tensions for loading and unloading O2 at the respiratory surfaces and in tissues, respectively (91). Each of these factors may vary among different species and may also undergo reversible changes within the same individual animals in response to changes in environmental conditions, metabolic requirements, and mode of life.
In vertebrate Hbs that comprise two α chains and two β chains, O2 binding at the heme groups triggers a transition of the protein molecules from the low-affinity, tense (T) state to the high-affinity, relaxed (R) state, which is basic to cooperativity in O2 binding. Hb-O2 affinity is modulated by allosteric effectors, chiefly protons (low pH) and CO2 that decrease O2 affinity (increase O2 unloading in the tissues via the Bohr effect), and organic phosphate and chloride ions that commonly reduce Hb-O2 affinity by preferential binding at specific sites of the molecules in the T state (62, 91). Thus, whereas protons mainly bind at β146His (the COOH-terminal histidines of the β chains), the organic phosphates [typically 2,3-diphosphoglycerate (DPG) in mammals, and ATP in ectothermic vertebrates] interact with seven amino acid residues in the cavity between the β chains (β1Val of one chain; and β2His, β82Lys, and β143His of both β chains), CO2 binds at the unprotonated NH2-terminal residues of both chains, and Cl− ions at one α chain site (between α1Val and α131Ser) and one β chain site (between β1Val and β82Lys) (42, 53, 62, 70, 78).
Crocodilian Hbs exhibit striking, distinguishing characteristics. In contrast to the vast majority of vertebrates, they show little or no response to organic phosphates, Cl−, or CO2 (8, 9, 39). The insensitivity to phosphates correlates with the replacement of three phosphate binding residues. Thus, compared to human Hb, β143His is replaced by Ala and β1Val-β2His by acetylated-Ala-Ser in the Nile crocodile (Crocodylus niloticus) and the American alligator (Alligator mississippiensis) and by Ser-Pro in the spectacled caiman (Caiman crocodilus) (51, 75). Perhaps the best known feature of crocodilian Hbs is that O2 affinity is drastically decreased by HCO3− ions (8), which is considered to play an important role in unloading O2 and maintaining aerobic metabolism when crocodilians dive and drown their prey (8, 95), compensating for their low myoglobin O2 stores (46). The HCO3− effect may also play a vital role in unloading O2 from the blood during postprandial alkaline tides (95) when increased blood HCO3− concentrations (resulting from HCl secretion into the stomach to digest bone) coincide neatly with the increased demand for O2 (the specific dynamic action of food) (19). This view aligns with the fact that crocodilians consume large amounts of bone (one alligator stomach contained remnants of up to 12 turtles) (20) and that the postfeeding metabolic peak in C. porosus was 70% higher when fed bone-rich chicken necks than when fed homogenized chicken (30).
The mechanisms basic to allosteric regulation of Hb-O2 affinity in crocodilians remain controversial. Although modeling indicates deoxygenation-linked binding of HCO3− at three residues in the central cavity between the two β chains; viz., β82Lys, β144Glu of one β chain, and the NH2-terminal residue of the partner β chain (Ser in C. crocodilus) (63), mutagenic replacement of β82Lys did not change the HCO3− sensitivity of Nile crocodile (C. niloticus) Hb, and replacements of 12 other amino acid residues clustered at the α1β2 interface were required to transplant the bicarbonate sensitivity into human HbA (16, 46, 47). Also, in contrast to the reported absence of oxygenation-linked binding of CO2 in Cai. crocodilus Hb (8), carbamate (carbamino) formation in crocodilians is reflected by the much (twofold to fivefold) larger CO2 Bohr effect than fixed-acid Bohr effect (measured when pH is changed by adding CO2 and buffers, respectively) in Cai. crocodilus and A. mississippiensis blood (39, 95). Finally, contrary to previous reports [cf. (46)], crocodilian red cells may contain a wide spectrum of organic phosphate effectors, albeit at low concentrations. In fact Cai. crocodilus red cells contain ATP and DPG, as well as inositol pentaphosphate (IPP, found in avian red cells), inositol hexaphosphate (IHP), and guanosine triphosphate (GTP, which is commonly encountered in fish red cells) (86).
Aiming to elucidate molecular adaptations and structure-function relationships in crocodilian Hbs, we determined the amino acid sequences of the α and β chains of the Hb of the dwarf caiman, Paleosuchus palpebrosus. This species differs from larger alligators and crocodiles in that it frequents stony creeks with clean, fast-running and cooler water, and mainly feeds on small vertebrates (e.g., tadpoles, frogs, fish, small mammals) and a variety of insects and snails (18). Finding a unique combination of amino acid exchanges at highly conserved effector binding sites, we investigated the O2 binding properties of the Hb and its sensitivities to chloride ions, ATP, DPG, and IHP, and to CO2 and temperature, over a wide pH range to discern possible alternative allosteric regulatory mechanisms. On the basis of the aggregation of deoxygenated Hbs to octamers and larger polymers (that may occur in vivo) observed in a diverse array of sauropsid taxa (birds and nonavian reptiles) (67, 71, 80), we also assessed polymerization in dwarf caiman Hb, its dependence on oxygenation state, and its possible effects on Hb-O2 affinity.
MATERIALS AND METHODS
Primary Structure
Blood from two young (2–3 yr old) specimens of dwarf caiman, P. palpebrosus, sampled in connection with routine diagnostic controls by the veterinarian at Cologne Zoological Gardens in accordance with existing regulations, was a generous gift from the zoo. Red cells separated by centrifugation were lysed in five times their volumes of 20 mM Tris·HCl, 40 mM DTE, pH 8.5 buffer. The hemolysate resolved into three electrophorectic bands by native alkaline disc electrophoresis, each of which was identified by NH2-terminal sequences as a mixture of the same αA and β chains without any hint of the presence of additional subunits (not shown). This indicates that P. palpebrosus Hb consists of a single component whose αA/β chain subunits show a strong tendency to form aggregates that involve disulfide bonds.
For a better yield of purified peptides we subjected a 1:1 mix (wt/wt) of lyophilized native and oxidized globin to a tryptic digest followed by size-exclusion chromatography (Sephadex G-25 fine) with acidic elution (0.1 N acetic acid), which resolved the digest into 10 fractions of variably sized peptides. Next, we employed reverse-phase HPLC with a LiChrospher 60 RP select B column and different trifluoroacetic acid (TFA)/acetonitrile gradients to separate the 10 fractions into a total of 281 peptide-containing peaks, including all αA and β chain tryptic peptides (Tp). The complete primary sequences of the αA and β subunits were then determined by means of automated Edman degradation in conjunction with amino acid analyses of these tryptic and some additional chymotryptic peptides. NH2-terminal and peptide sequencing schemes of either subunit and tables of the amino acid analyses of αA and β peptides are available upon request.
For proper placement of tryptic peptides within the αA or β chain, comparison to the complete globins from the closely related Cai. crocodilus was crucial. In addition, enriched native subunits and pyridyl-ethylated globin, whose reactive cysteinyl SH-groups could no longer form disulfide-based aggregates, were NH2-terminally sequenced up to αA position 24 and β position 58. This approach allowed the unequivocal identification of alkylated cysteines at αA positions 18/19 and β position 23, respectively (see Fig. 1).
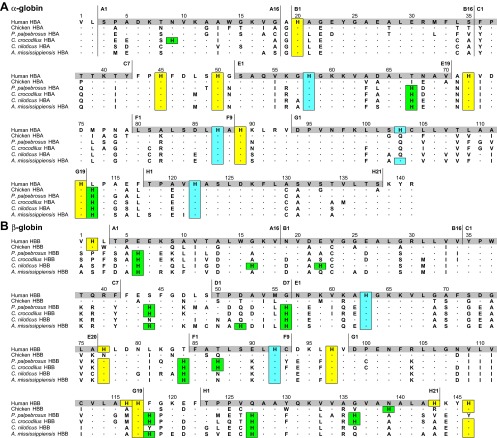
Fig. 1.
Amino acid sequences of the α and β globins of dwarf caiman (Paleosuchus palpebrosus) hemoglobin (Hb) compared with those for spectacled caiman (Caiman crocodilus), Nile crocodile (Crocodylus niloticus), the American alligator (Alligator mississippiensis) (51), chicken, and human. Residues in the other species are shown only where they differ from those in human Hbs. His residues highlighted in yellow and blue are titratable (surface) residues and nontitratable residues, respectively, in human Hb (11, 53); those highlighted in green represent potential gains of titratable His residues compared with human Hb.
Recovery of β Tp9a from the HPLC column occurred in two peaks, 70% of which contained Glu and 30% Gln at position 73. This Glu/Gln ambiguity might be explained either by a true allelic difference (heterozygosity) at site 73 or by the spontaneous deamidation of the original amide (Gln). Proof of the critically important replacement of the COOH-terminal His by Tyr in the β chain (i.e., of β146 Tyr) was obtained by: 1) amino acid analyses of Tp 14b+15I (position 136–141) and Tp 14b+15II (position 142–146), which provided evidence for a single His existing in Tp 14b+15I, whereas two Tyr residues and no His residues were recovered for Tp 14b+15II; and 2) the sequence of the entire Tp 14b+15 that demonstrated a sole His residue in position 1 (β136) along with Tyr phenylthiohydantoin (PTH)-derivative peaks of almost equal yields for β145 and β146.
For comparisons of Paleosuchus globins with orthologous chains of other species, percentage similarities and identities of amino acid residues (Table 1) were calculated using the EMBOSS tool (http://www.ebi.ac.uk/Tools/psa/emboss_needle/) based on the global alignment Needleman-Wunsch algorithm operating with the Blosum62 matrix.
Table 1.
Identities and similarities in α and β chain sequences of P. palpebrosus hemoglobin compared with those of other crocodilian, chicken and human hemoglobins
| α Chains |
β Chains |
|||
|---|---|---|---|---|
| Identity | Similarity | Identity | Similarity | |
| Cai. crocodilus | 83.7 | 90.1 | 89.0 | 98.6 |
| C. niloticus | 79.4 | 87.2 | 67.8 | 83.6 |
| A. mississippiensis | 79.4 | 87.2 | 75.3 | 87.0 |
| Chicken | 75.2 | 84.4 | 54.1 | 74.7 |
| Human | 67.4 | 75.9 | 48.6 | 68.5 |
All values are %.
O2 Binding Measurements
Hemoglobin preparation.
Hb solutions obtained by lysing washed red blood cells were centrifuged for 10 min at 400 g to remove cellular debris, and were stripped of organic phosphates on a 38 × 2.1 cm (height × diameter) column of Sephadex G25 Fine gel and dialysed for 24 h against 0.01 mol/l HEPES buffer containing 5 × 10−4 mol/l EDTA, as earlier described (92). The Hb showed no oxidation as judged from equal absorption values at 539 and 569 nm after brief equilibration with carbon monoxide, and was frozen at −80°C in 150-μl aliquots that were thawed individually for O2 equilibrium and other measurements.
O2 equilibria.
Hb-O2 equilibria were measured in the presence of 0.05 mol/l HEPES buffer (89) unless otherwise specified, using a modified gas diffusion chamber coupled to two cascaded Wösthoff pumps (Bochum, Germany) for mixing of air and pure (>99.998%) N2 (88, 93). P50 and n50 (respectively, O2 tension and Hill's cooperativity coefficients at half-saturation) values were interpolated from linear regressions (r2 > 0.99) of Hill plots [log Y/(1 − Y) vs. log Po2, where Y is the fractional saturation] of four or more equilibration steps between 30% and 70% O2-Hb saturation. The effects of ATP (disodium salt), DPG (pentacyclohexyl-ammonium salt), IHP (inositol hexaphosphate, sodium salt), and Cl− (KCl) were investigated by adding accurate volumes of standard, ∼100 mM solutions of these effectors to the stripped Hb solutions. ATP was assayed using Sigma test chemicals, and Cl− was assayed using a CMT10 chloride titrator (Radiometer, Copenhagen, Denmark). The pH values were measured in oxygenated Hb samples at the same temperature and CO2 tension as the O2 equilibria using thermostatted pH electrode units (BMS2 Blood Micro System; Radiometer).
The allosteric parameters [the allosteric constant, L, and the association equilibrium constants of the T and R structures (KT and KR, respectively)] were assessed by analyzing precise O2 equilibrium data for a wide range of O2 saturations (extended Hill plots) in terms of the two-state Monod-Wyman-Changeux (MWC) equation (57) that was fit to the data in the form log [Y/(1 − Y)] vs. log Po2 (end weighting) using the curve-fitting procedure previously described (94), and fitting the number of binding sites, q, along with the other parameters to obtain the best possible fit. Additional fits were performed with q fixed at 4 (as applies to tetrameric Hb). Derived parameters, including the median oxygen tension, Pm; the maximum slope of the Hill plot, nmax; and the free energy of heme-heme interaction, ΔG; were evaluated as previously detailed (94). Heats of oxygenation were calculated from the van't Hoff isochore [ΔH = 2.303 · R · Δlog P50/Δ(1/T)], where R is the gas constant and T is the absolute temperature (°K). All quoted ΔH values are exclusive of the heat of solvation of O2 (−12.6 kJ/mol).
Size Exclusion Chromatography
Hb quaternary structure and its oxygenation dependence were investigated by gel filtration of Hb preparations that had not been in contact with CO on a 59.3 × 2.6 cm (height × diameter) column of Sephacryl S-200 HR. The proteins were eluted with 0.025 M Tris buffer (pH 7.40 at 5°C) containing 0.025 M NaCl and 0.003 M NaN3. The partition coefficients, Kav [(= Ve − Vo/Vt − Vo) (cf. 49a)], where Ve is the elution volume, Vt is the total volume of the gel bed, and Vo is the void volume (the elution volume of Blue Dextran that is excluded by the gel)] of Hb fractions were compared with those of horse heart cytochrome c, oval albumin, and aldolase obtained from Boehringer-Mannheim; myoglobin and bovine serum albumin obtained from Sigma (A-0380 and M-4503, respectively); and catalase and ferritin from GE Health Care. Gel filtration of deoxygenated Hb was carried out by adding Na-dithionite (1 mg/ml) to the Hb sample applied to the column and to the elution buffer after both solutions had been equilibrated with gaseous N2. Hb fractions retrieved for O2 binding experiments were concentrated by ultrafiltration in Millipore ultrafree filter units (cutt-off molecular mass 10,000 Daltons). Fractions showing slight oxidation were briefly equilibrated with CO and reduced by dialysis (30 min) against N2-equilibrated 0.01 M HEPES buffer (pH ∼7.6) containing freshly added Na-dithionite (0.1%) followed by extensive dialysis against N2/CO equilibrated HEPES buffer without Na-dithionite.
RESULTS
Structural Analyses
Figure 1 shows the amino acid sequences of P. palpebrosus Hb aligned with those for Cai. crocodilus, C. niloticus, A. mississippiensis, chicken, and human. The entire collection of globin peptides isolated from the 10 size exclusion fractions (see materials and methods) was free of peptides that matched αD subunits of birds and nonavian reptiles, revealing that P. palpebrosus, like other adult crocodilians, does not express αD globin (31). The α and β chain sequences of P. palpebrosus (Table 1) show higher identity with those of the other caimanine member (Cai. crocodilus) than with those of C. niloticus and A. mississippiensis and with chicken than with human α and β chains. Relative to the α chains, the β chains showed higher sequence divergence in comparisons among species, which is consistent with previous reports of unusually high substitution rates in the β chain subunits of crocodilian Hbs (31, 36).
The primary structures of P. palpebrosus Hb chains reveal a unique combination of amino acid substitutions, including β82Lys→Gln, β143His→Val, and β146His→Tyr (Fig. 1) that delete positively charged (anion-binding and proton-dissociating) residues and thus may be expected to drastically impact the sensitivities of the Hb to allosteric effectors. Another impinging substitution of P. palpebrosus Hb is β93Cys→Tyr, which removes a solvent-exposed cysteine residue that is highly conserved among mammalian, avian, and reptilian Hbs.
Charge, Polarity, and Histidine Content
The sequence of P. palpebrosus Hb differs markedly from that of other crocodilian, avian, and human Hbs in charge, polarity, and hydropathy (hydrophobicity) indices of amino acid residues, as well as the number and position of histidine residues that may function as buffer groups and source of Bohr protons and in stabilizing the Hb's T-structure through the formation of internal salt bridges (11). P. palpebrosus Hb contains 24 (11 α chain and 13 β chain) His residues in each dimeric half-molecule (Fig. 1), which is considerably more than in other vertebrates, including humans, who have 19 (10 + 9) residues, but they align with the high numbers of physiological buffer groups in crocodilian Hbs (11).
Remarkably, the α and β chain sequences of P. palpebrosus exhibit distinctive features that are shared with human Hb, but not with other hitherto investigated crocodilian Hbs (Table 2). Thus, both P. palpebrosus and human Hbs have neutral Asn residues at β19 and at β108 compared with negatively charged Asp in other crocodilians. Also both have negatively charged residues (Glu and Asp, respectively) at β52 compared with neutral Gln or weakly positive His in the crocodilians. Also both have residues with opposite side-chain polarities than other crocodilian Hbs at positions α34 and β93. However, P. palpebrosus Hb also shows distinguishing differences in residue polarity compared with human and other crocodilian Hbs (at positions α19, α28, β22, β49, and β143) that may contribute to distinctive functional properties.
Table 2.
Amino acid residues specifically encountered in α and β chains of P. palpebrosus hemoglobin compared with those of other species
| Comparison* |
|||||||
|---|---|---|---|---|---|---|---|
| Human | P. palpebrosus | Cai. crocodilus | C. niloticus | A. mississippiensis | Charge | Polarity | |
| α12 | Ala | Gly | Ala | Ala | Ala | = | = |
| α18 | Gly | Cys | Ala | Ala | Ser | = | np (nnp) |
| α19 | Ala | Cys | Gly | Gly | Gly | = | np (nnn) |
| α23 | Glu− | Asp− | Glu− | Glu− | Glu− | = | = |
| α28 | Ala | Tyr | Ala | Ala | Ala | = | np (nnn) |
| α32 | Met | Leu | Met | Met | Met | = | = |
| α34 | Leu | Phe | Cys | Cys | Cys | = | nn (ppp) |
| α35 | Ser | Val | Ala | Ala | Ala | = | pn (nnn) |
| α49 | Ser | Tyr | Ser | Ser | Ser | = | = |
| α64 | Asp− | Leu | Ala | Ala | Ser | − o (o o o) | pn (nnp) |
| α100 | Leu | Leu | Phe | Phe | Phe | = | = |
| α134 | Tyr | Tyr | Ala | Ser | Ala | = | pp (npn) |
| β12 | Tyr | Leu | Val | Gly | Val | = | pn (nnn) |
| β19 | Asn | Asn | Asp− | Asp− | Asp− | o o (- - -) | = |
| β22 | Asp− | Ala | Ser | His+ | Gln | −o (o + o) | pn (ppp) |
| β31 | Leu | Leu | Met | Met | Met | = | = |
| β49 | Ser | Ala | Ser | Ser | Cys | = | pn (ppp) |
| β52 | Asp− | Glu− | Gln | Gln | His+ | − − (o o +) | = |
| β82 | Lys+ | Gln | Lys+ | Arg+ | Lys+ | +o (+++) | = |
| β93 | Cys | Tyr | Phe | Phe | Phe | = | pp (nnn) |
| β108 | Asn | Asn | Asp− | Asp− | Asp− | o o (- - -) | = |
| β121 | Glu− | Glu− | Asp− | Asp− | Asp− | = | = |
| β124 | Pro | Met | Leu | Leu | Val | = | = |
| β134 | Val | Ala | Val | Val | Val | = | = |
| β143 | His+ | Val | Ala | Ala | Ala | +o (o o o) | pn (nnn) |
| β146 | His+ | Tyr | His+ | His+ | His+ | +o (+ + +) | = |
Underlined residues have polar side chains; + and − denote residues whose side chains are positively and negatively charged, respectively, at pH 7.4.
Comparison between hemoglobins of, respectively, humans, P. palpebrosus, and (in brackets) Cai. crocodilus, C. niloticus,and A. mississipiensis: =, same charge/polarity in all species; +, positively charged side chains; −, negatively charged side chains, o, neutral side-chains. p, Polar side chain; n, nonpolar side chain.
Oxygen Binding
O2 affinity and its pH and chloride sensitivities.
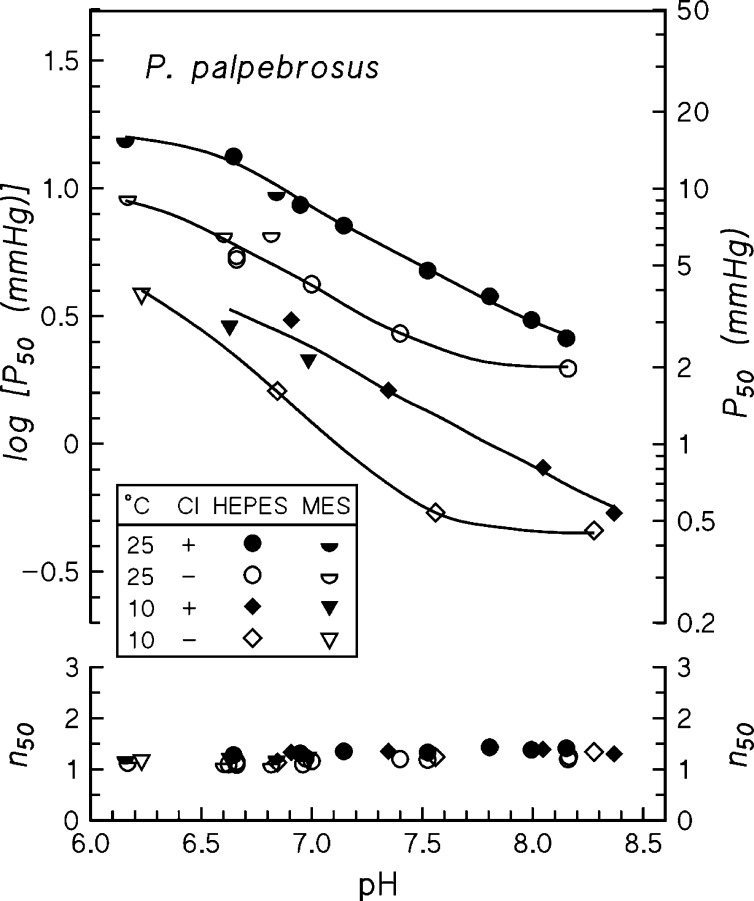
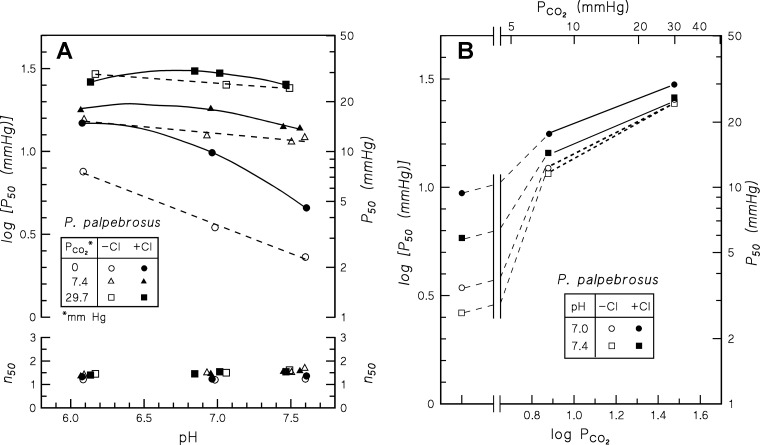
Stripped P. palpebrosus Hb exhibits a high O2 affinity that is substantially reduced by Cl− ions, decreased pH, and increased temperature (Fig. 2; Table 3); at pH 7.4, P50 values at 10° and 25°C are 0.65 and 2.69 mmHg, respectively, in the stripped Hb; and 1.53 and 5.43 mmHg, respectively, in the presence of 0.1 M Cl−. Expressed as log P50,Cl − log P50,str, the chloride effect decreases markedly at high pH where cationic binding sites are neutralized (Table 3). Cooperativity of O2 binding was low (n50 = 1.2–1.5) under all conditions investigated (Fig. 2).
Fig. 2.
Oxygen tension (P50) and Hill's cooperativity coefficients (n50) at half O2 saturation of P. palpebrosus Hb measured at 10°C (triangles and diamonds) and 25°C (full and half circles), in 0.05 M HEPES buffer (circles and diamonds) or 0.05 M MES buffer (half-circles and triangles) and in the absence (open symbols) and presence (closed symbols) of 0.1 M Cl−. Heme concentration, 0.37 mM.
Table 3.
P50 values and Bohr factors of stripped P. palpebrosus hemoglobin and their dependence on pH, [Cl−] and temperature
| °C | Property | pH 8.0 | pH 7.4 | pH 7.0 | φ (7.0–7.4) |
|---|---|---|---|---|---|
| 10 | P50, str. (mmHg) | 0.45 | 0.65 | 1.20 | −0.67 |
| P50, str. + 0.1 M Cl (mmHg) | 0.78 | 1.53 | 2.66 | −0.61 | |
| log P50,Cl − log P50,st. | 0.23 | 0.37 | 0.35 | ||
| 25 | P50, str. (mmHg) | 2.02 | 2.69 | 4.26 | −0.45 |
| P50, str. + 0.1 M Cl (mmHg) | 3.09 | 5.43 | 8.61 | −0.50 | |
| log P50,Cl − log P50,str | 0.18 | 0.31 | 0.32 |
φ, Bohr factor; str., stripped.
Significantly, P. palpebrosus Hb exhibits a pronounced Bohr effect. At pH 7.0–7.4, which characterizes physiological values in crocodilians (77), the Bohr factors (φ = ΔlogP50/ΔpH) in the absence and presence of 0.1 M chloride were −0.67 and −0.61, respectively, at 10°C; and −0.45 and −0.50, respectively, at 25°C (Fig. 2; Table 3). The decrease in Bohr effects with increasing temperature accords with the temperature dependence of ionization of Bohr groups. Unlike in human and most vertebrate Hbs (37, 73, 92) the Bohr effect is not increased by 0.1 M chloride, suggesting that allosteric proton binding to this Hb is not enhanced by chloride. O2 equilibria of stripped P. palpebrosus Hb in MES buffer (Fig. 2) reveal the absence of a reverse (acid) Bohr effect (increasing O2 affinity with decreasing pH) as found in human Hb below ∼6.5.
Phosphate sensitivities.
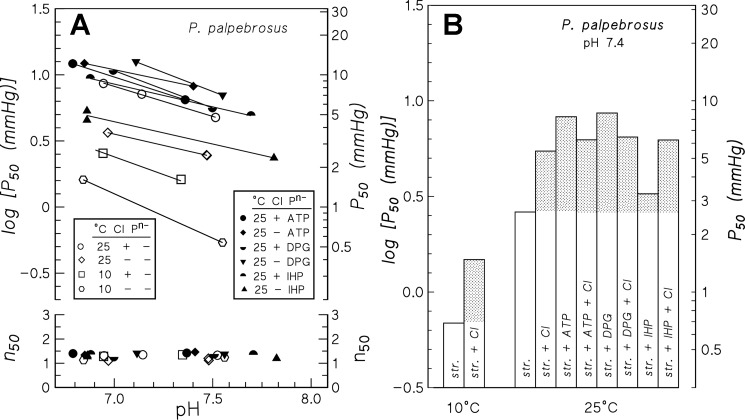
Measurements of the interactive effects of organic phosphates and chloride at physiological pH (Fig. 3) show insensitivity of P. palpebrosus Hb to organic phosphates in the presence of chloride ions, as observed in other crocodilians (8, 9, 63). In contrast, pronounced sensitivities to ATP and DPG are unmasked in the absence of chloride, as is also observed in A. mississippiensis and Cai. crocodilus Hbs (96). Strikingly, however, the Hb-O2 affinity is virtually insensitive to polyanionic IHP. Thus in the absence of chloride, addition of ATP, DPG, and IHP increase log P50 by 0.49, 0.52, and 0.089, respectively, at 25°C and pH 7.4 (Fig. 3B). Also, whereas the effects of IHP and chloride are additive (i.e., chloride plus IHP depress O2 affinity more than IHP alone), addition of chloride increases Hb-O2 affinity in the presence of ATP or DPG (Fig. 3B). Similar relative sensitivities to these effectors were observed in duplicate measurements at pH 7.0 (data not shown).
Fig. 3.
A: P50 and n50 values of P. palpebrosus Hb measured in 50 mM HEPES buffer at 10° and 25°C in the absence (−) and presence (+) of 0.1 M Cl−, and the absence (open symbols) and presence (closed symbols) of saturating levels [ATP/Hb and 2,3-diphosphoglycerate (DPG)/Hb ratio 6.2; inositol hexaphosphate (IHP)/Hb ratio ∼28] of the polyanionic phosphate effectors (Pn−) ATP, DPG, and IHP. B: histograms showing the P50 values of the stripped Hb (str., open columns) and the log P50 shifts (shaded columns) induced by chloride, ATP, DPG, and IHP at pH 7.4 and 10° and 25° C. Other conditions as in Fig. 2.
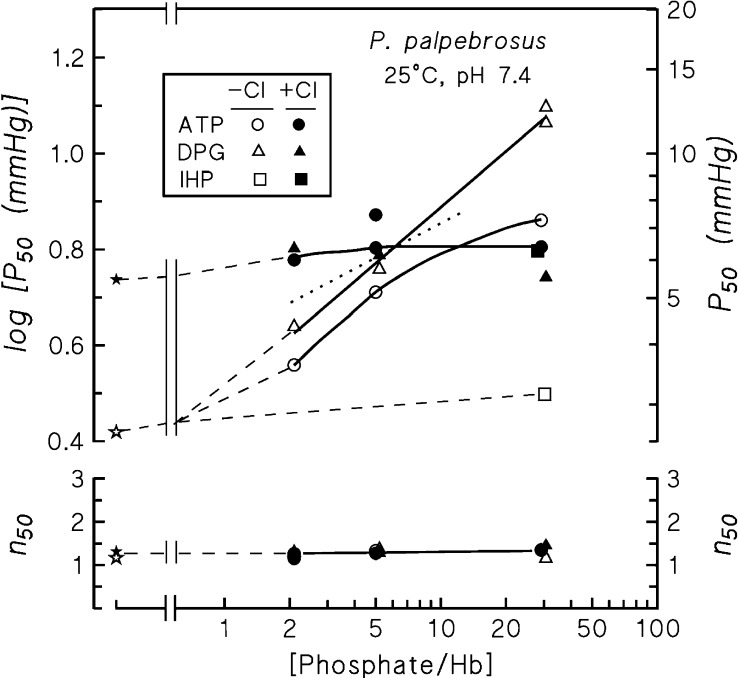
Dose-response curves (Fig. 4) for the effects of increasing concentrations of free phosphates calculated as [total phosphate] − 0.5[Hb4], on the assumption that at P50 half of the tetrameric Hb molecules are phosphate-liganded (97), demonstrate virtual annihilation of the phosphate effects in the presence of 0.1 M chloride. Strikingly, the double logarithmic plots reveal slopes that markedly exceed 0.25 (i.e., the value expected for one-to-one stoichiometry between phosphate and tetrameric Hb molecules if phosphate binding is limited to the T state). For DPG binding at pH 7.4 the slope of 0.37 (Fig. 4) indicates a stoichiometry of ∼1.5 DPG molecules bound per Hb tetramer.
Fig. 4.
P50 and n50 of P. palpebrosus Hb at pH 7.4 and 25°C in the absence (open symbols) and presence (solid symbols) of 0.1 M Cl−; the absence of organic phosphates (stars); and the presence of ATP (circles), DPG (triangles), and IHP (squares) at different phosphate/tetrameric Hb ratios, measured in 0.05 M HEPES buffer. Fine dotted line shows a slope of 0.25 expected if deoxygenation of the Hb molecules were linked to binding of one phosphate molecule. Heme concentration, 0.36 mM. (This figure replots earlier published P50 values (96) against the [free phosphate]/[Hb] ratios).
CO2 sensitivity.
Admixture of CO2 in the equilibration gases conspicuously lowers O2 affinity of the stripped P. palpebrosus Hb at constant pH. This specific CO2 effect increases with increasing pH and is markedly reduced by Cl− (Fig. 5). Thus at pH 7.0 and 7.4, addition of 29.7 mmHg CO2 raises log P50 by 0.85 and 0.98 units, respectively, in the absence of chloride, compared with 0.49 and 0.64, respectively, in 0.1 M chloride. This CO2 effect and its pH and Cl− dependencies are consistent with carbamate formation at the (NH2-terminal) α-amino residues of the chains. Slopes of log P50 vs. log Pco2 plots (in the 7–30 mmHg Pco2 range) of ∼0.5 in the absence, and ∼0.4 in the presence of Cl, indicate binding of one CO2 molecule per two O2 molecules released.
Fig. 5.
A: pH dependence of P50 and n50 values of P. palpebrosus Hb in the presence of 0, 1, and 4% CO2 (Pco2 = 0, 7.4, and 29.4 mm, respectively), measured at 25°C in 50 mM HEPES buffer. B: the specific (pH-independent) effect of CO2 on Hb-O2 affinity at pH 7.0 and 7.4. Heme concentration 0.37 mM.
Allosteric control.
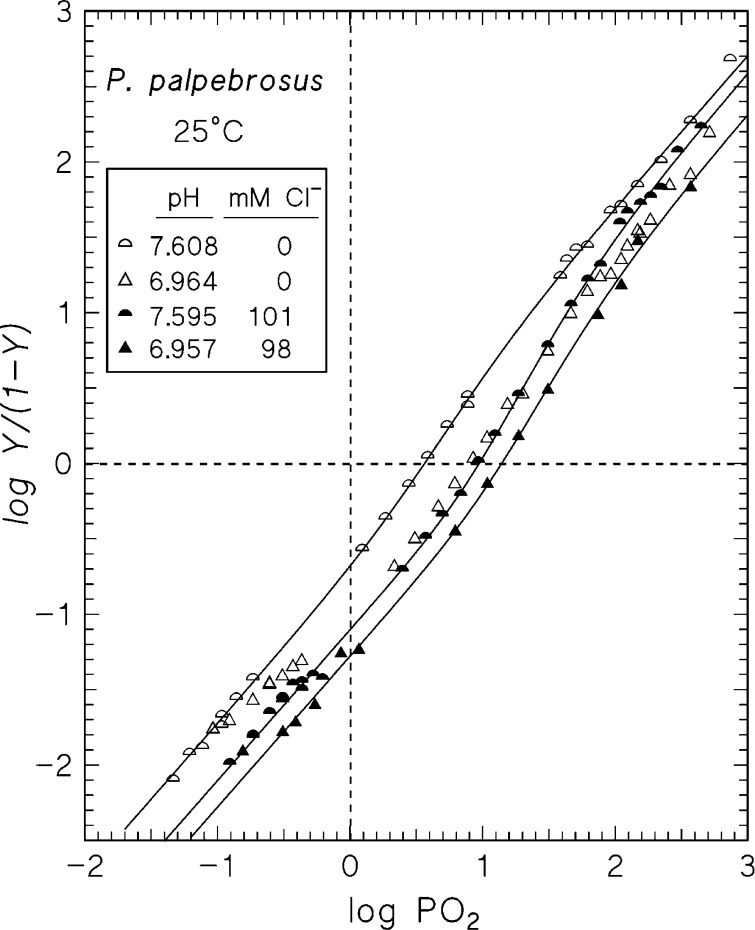
Extended Hill plots (Fig. 6) reveal slopes of unity at extreme low and high O2 saturations consistent with noncooperativity in binding the first and last O2 molecules to the Hb, and good correspondence between Pm and P50 values (O2 tensions at, respectively, median and half-saturations), permitting assessment of allosteric effects from alterations in P50 values. As shown (Table 4), the n50 values are low, which hampered fits of the MWC two-state model to the data. Thus, at high pH and in the presence of chloride, it was not possible to fit the model to the data with q (the number of interacting binding sites) floating and with the constraint that the allosteric constant (L) > 1. For the other curves, the fitted values of q were close to 4, indicating that the functional units are tetramers, despite Hb polymerization (see below). As illustrated (Fig. 6), lowered pH decreases O2 affinity by reducing KT and KR, whereas chloride ions decrease O2 affinity mainly by lowering KT. Fits with q fixed at 4 (Table 4) show that the interaction energy is increased in the presence of 0.1 M chloride and that this was due in part to a stabilizing effect of chloride on the T state (lowered KT). At pH 7.6 this was augmented by a destabilizing effect of chloride on the R state (increased KR), whereas this was not the case at pH 6.9. As evident from the standard error on the fits, however, it is not safe to draw firm conclusions about KR at the low pH.
Fig. 6.
Extended Hill plots of P. palpebrosus Hb at pH ∼7.60 (half circles) and ∼6.96 (triangles) in the absence (open symbols) and presence (closed symbols) of 0.10 M Cl−. Heme concentration, 0.37 mM.
Table 4.
MWC and derived parameters obtained by fitting the MWC two-state model to the data with four fixed interacting O2-binding sites
| pH | Cl mM | P50 mmHg | Pm mmHg | n50 | log L | SE* | log KT | SE* | log KR | SE* | ΔG kJ·(M heme)−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7.608 | 0 | 3.58 | 3.53 | 1.29 | 1.66 | 0.333 | −0.758 | 0.040 | −0.146 | 0.075 | 2.44 |
| 6.964 | 0 | 8.02 | 7.62 | 1.21 | 3.49 | 1.593 | −0.993 | 0.025 | −0.0562 | 0.386 | 2.07 |
| 7.595 | 0.10 | 9.38 | 8.31 | 1.43 | 65.61 | 0.0026 | −1.102 | 0.019 | 15.46 | 0.011 | 4.16 |
| 6.961 | 0.10 | 14.00 | 12.84 | 1.37 | 4.07 | 4.085 | −1.279 | 0.063 | −0.117 | 1.012 | 3.31 |
P50, O2 tension at half-saturation; Pm, median O2 tension; n50, Hill's cooperativity coefficient at half-saturation; L, allosteric constant; KT and KR, association equilibrium constants of the T and R structures, respectively; ΔG, free energy of heme-heme interaction.
Standard errors as estimated from the covariance matrix associated with the fit.
Temperature sensitivity and enthalpic effects.
At pH 7.5–8.0, at which stripped P. palpebrosus Hb virtually lacks a Bohr effect (cf. Fig. 2), the overall oxygenation enthalpy (−56.3 kJ/mol) corresponds closely with the intrinsic heat of oxygenation of human Hb (−59 kJ/mol) (4). The numerically lower value (−48.8 kJ/mol) found at pH 7.0 where the Bohr effect is operative, is consistent with endothermic contributions from Bohr proton dissociation [∼26 kJ/mol for imidazole groups of histidines (3, 4)]. Analogously, the reduced enthalpy values observed in the presence of 0.1 M Cl− (−47.7, −46.6, and −42.3 kJ/mol at pH 8.0, 7.5, and 7.0, respectively) reflect endothermic contributions (+8.6, +7.7, and +6.5 kJ/mol, respectively) from oxygenation-linked chloride ion dissociation. The semblance of these enthalpic effects with those in other (nonheterothermic) vertebrates (90) indicates the absence of distinguishing adaptive traits related to body temperature variation in P. palpebrosus Hb.
Quaternary Structure and Effects
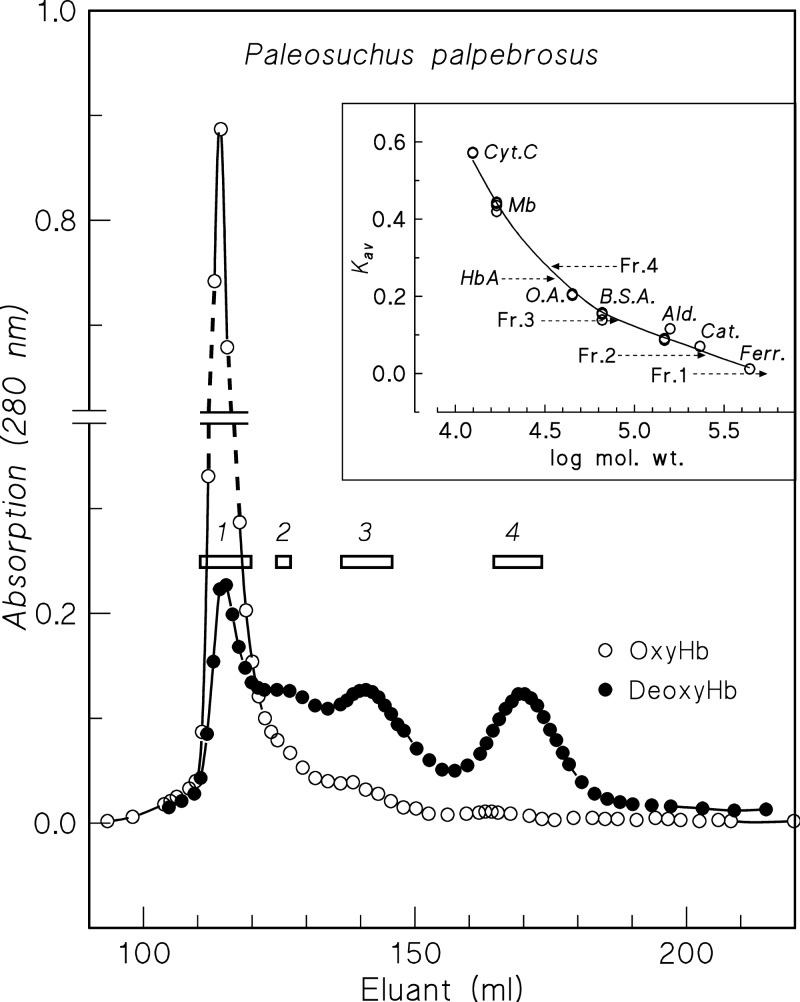
Unexpectedly, the size exclusion gel-filtration chromatography experiments showed that oxygenated P. palpebrosus Hb elutes with the void volume of the Sephacryl S-200 HR medium, revealing an exceptionally high degree of polymerization (Fig. 7). In contrast, the elution pattern of Hb deoxygenated with dithionite reveals the presence of three fractions of smaller molecules, whose partition coefficients (Kav) indicate molecular masses of 31.6, 81.3, and 263 kDa. The value of 38 kDa obtained for human Hb (Fig. 7, inset) is consistent with observations that molecular mass estimates for tetrameric vertebrate Hbs on the basis of gel filtration are lower than established values (64–68 kDa) due to reversible dissociation to dimers (17) and to elution volumes being proportional to molecular Stokes radii of proteins rather than their molecular weights (1). In this light, the three fractions observed in deoxygenated Hb (labeled 4, 3, and 2 in Fig. 7) likely comprise tetramers, octamers (dimers of tetramers), and high-order polymers, respectively.
Fig. 7.
Elution profile of oxygenated (○) and deoxygenated (●) P. palpebrosus Hb on a column Sephacryl S-200 HR gel showing fractions pooled for analyses (open horizontal bars 1–4). Inset: Kav values of these fractions compared with those of human Hb A and proteins of known molecular mass, viz., cyt.C (cytochrome c), Mb (myoglobin), oval albumin (O.A.), bovine serum albumin (B.S.A.), aldolase (Ald.), catalase (Cat.), and ferritin (Ferr.).
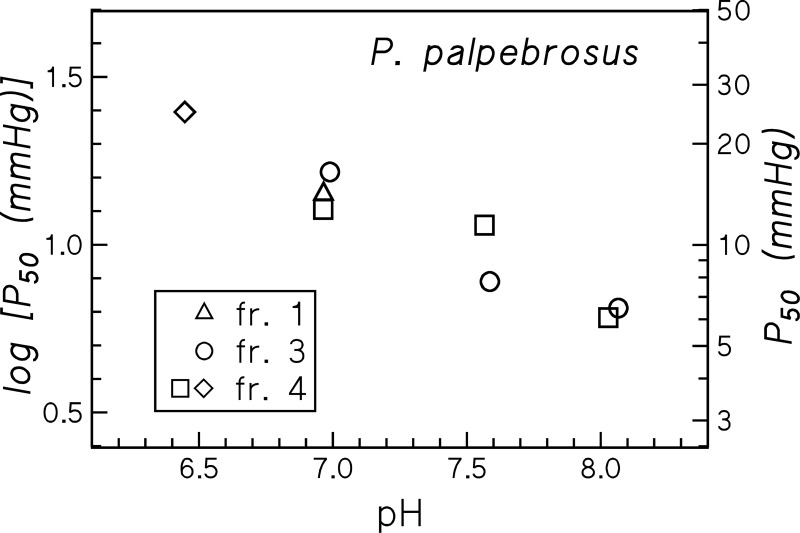
O2 equilibrium measurements of three major molecular mass fractions obtained from gel filtration of deoxygenated Hb show similar O2 affinities and Bohr effects (Fig. 8) indicating that aggregation state does not affect oxygenation properties of P. palpebrosus Hb.
Fig. 8.
P50 values and pH dependence of three molecular mass fractions (fr.) of P. palpebrosus Hb isolated by gel filtration (cf. Fig. 7), measured at 25°C in HEPES (▵, ○, and ▫) and MES (◇) buffers.
DISCUSSION
O2 Affinity and its Sensitivity to Allosteric Effectors
P. palpebrosus Hb exhibits a high intrinsic O2 affinity combined with low cooperativity, suggesting that the allosteric T-R equilibrium in this Hb is markedly shifted to the high-affinity R state. Its O2 affinity is reduced by allosteric effectors (protons and CO2, and organic phosphate and chloride anions) despite replacements of functionally important amino acid residues compared with other Hbs. P. palpebrosus Hb provides a unique opportunity to reevaluate the roles of specific amino acid residues implicated in the allosteric regulation of O2 affinity, and the possible compensatory mechanisms in naturally occurring Hbs that lack specific effector binding sites.
CO2 Effects
P. palpebrosus Hb binds molecular CO2. The distinct, specific effect of CO2 on O2 affinity (Fig. 5) is at variance with the reported absence of carbamate formation in crocodilian Hbs (63). The reduction in the CO2 effect observed in the presence of chloride and increased proton activity (pH decrease) (cf. Fig. 5) is consistent with competition between these effectors for binding at the NH2-terminal amino acid residues, and thus is analogous to the antagonism between binding of CO2 on the one hand, and of lactate (58) and DPG (7) on the other, in human HbA.
The slope of ∼0.5 for the log P50/log Pco2 relationship (Fig. 5) signifies a stoichiometry of two CO2 molecules bound per Hb tetramer under physiological conditions, which indicates that CO2 binds to either the α or the β chains of P. palpebrosus Hb as in human Hb, where O2-linked carbamate formation is confined to the β chains (10). The identical NH2-terminal amino acid sequences (Fig. 1) of P. palpebrosus and Cai. crocodilus Hbs predict similar CO2 binding capacities in these species. In C. niloticus and A. mississippiensis, however, the β chain NH2-terminal residues are acetylated (75) and are therefore not available for carbamate formation.
In A. mississippiensis Hb carbamate, free HCO3− and Hb-bound HCO3− contribute approximately equally to the high CO2 carrying capacity of deoxygenated red cells (41). Two observations indicate that HCO3− binding to P. palpebrosus Hb is markedly reduced compared with that of other crocodilians. First, the CO2 effect increases strongly with increasing pH (Fig. 5A), whereas the association constants for the reaction of Cai. crocodilus Hb with HCO3− are essentially pH independent below pH 7.3 (8). Second, compared with other crocodilian Hbs, P. palpebrosus Hb exhibits substitutions (viz., α34Cys→Phe, α35Ala→Val, α100Phe→Leu, and β31Met→Leu) (Fig. 1) that replace 4 of the 12 amino acid residues (viz., 34Cys, 35Ala, 36Tyr, 38Gln, 41Ile, 100Phe, and 103Gln of the α chain; and 29Ser, 31Met, 38Lys, 39Arg, and 41Tyr of the β chain) that are present in other crocodilian Hbs, and confer a HCO3− effect when engineered into human Hb (47). Among these the loss of polar α34Cys in P. palpebrosus Hb may be significant. If β82-Lys is a bicarbonate-binding site as proposed by Perutz and his colleagues (63), its substitution by Gln would similarly contribute to loss of bicarbonate binding in P. palpebrosus Hb, but not in Cai. crocodilus and A. mississippiensis Hbs that have retained this residue, nor in C. niloticus Hb that shows a polar- and charge-neutral substitution (β82Lys→Arg).1
The Bohr Effect
β146His→Tyr substitution.
The pronounced Bohr effect in stripped Hb (φ = −0.50 at 25°C) (Fig. 2, Table 3) is remarkable given the substitution of β146His by neutral Tyr in P. palpebrosus, and the prodigious evidence for the importance of β146His for expression of the Bohr effect in vertebrate Hbs. In humans where His residues account for almost 90% of the total alkaline Bohr effect (11, 27, 53), the majority of this effect (63% at pH 7.4) (27) originates from proton release upon oxygenation-linked breakage of the T-state salt bridge between β146His and β94-Asp (61). The overriding role of β146His in expression of the alkaline Bohr effect is supported by a host of investigations. Enzymatic removal of the β chain COOH-terminal residues (145-Tyr and 146-His) from human HbA drastically reduces the Bohr effect and cooperativity but increases O2 affinity, confirming its role in stabilizing the T state relative to the R state (12, 43, 49, 56). Moreover, abnormal human Hb with β146His replaced by a wide range of other residues [viz., by Asp in Hb Hiroshima2 (38, 64), Pro in Hb York (48, 55), Tyr in Hb Bologna-St. Orsola (also called Hb Halamshire) (50, 52), Gln in Hb Kodaira (33), Leu in Hb Cowtown (76), and Arg in Hb Cochin-Port Royal (87)] show a similar reduction in the Bohr effect, and generally decreased heme-heme cooperativity and increased O2 affinities.
Available data indicate a similarly important role for β146His in ectothermic vertebrates. The Bohr effect of carp Hb is halved following enzymatic cleavage of the β chain COOH-terminal His residues (60), and that of Aethotaxis mitopteryx Hb (β146His→Val) is about half of that in other Antarctic fishes (21). In conjunction with the pervasive correlation between β146His and Bohr effects in widely different vertebrates, the strong Bohr effect in P. palpebrosus Hb indicates that other titratable residues take over the role of β146His. Which residues might these be?
The high His content of P. palpebrosus Hb (24 per αβ dimer; Fig. 1) confirms the generality of this trait in crocodilians (11, 40), predicting high nonbicarbonate buffer values in P. palpebrosus blood that reduces arteriovenous pH changes and thus the in vivo contribution of the fixed-acid Bohr effect to tissue O2 release. In bicarbonate-sensitive Hbs, pH changes are further dampened because proton binding by Hb is balanced by protons liberated upon O2-linked HCO3− binding (41).
Apart from increasing the buffer capacity, the additional His residues in crocodilian Hbs may take over the role of β146His in accounting for a strong fixed-acid Bohr effect expressed in P. palpebrosus Hb (Fig. 2). Although four of the His residues found in human Hb are substituted for other residues (i.e., β2Pro, β116Met, β143Val, and β146Tyr), P. palpebrosus has 10 His residues (at positions α67, α113, β6, β44, β56, β84, β87, β118, β127, and β136), which are not found in human Hb and may be sources of Bohr protons (Fig. 1).
β143His→Val substitution.
Because β143His makes the largest contribution to this reverse (acid) Bohr effect (71% at pH 5.1 in human Hb) (27), its replacement by neutral Val (Fig. 1) correlates deftly with the lack of a reverse Bohr effect in P. palpebrosus at low pH (Fig. 2). The β143His→Val substitution, however, also deletes a phosphate binding site that would decrease phosphate binding. Indeed in humans, γ143His→Ser substitution in the fetal β type chains increases the O2 affinity of fetal blood via lesser DPG interaction. Analogously, abnormal human Hbs in which β143His is substituted by neutral residues, including Hb Little Rock (β143His→Gln), Hb Syracuse (β143His→Pro), and Hb Old Dominion (β143His→Tyr) all exhibit increased affinities in the presence of DPG [cf. (22)].
Phosphate Effects
Contrary to the reported insensitivity of crocodilian Hbs to organic phosphates (8, 46, 63), ATP and DPG decrease O2 affinities of crocodilian Hb at low chloride levels (96) as may occur during the postprandial alkaline tide (20).
The observation that slopes of double logarithmic plots of P50 against the free DPG and ATP concentrations (Fig. 4) clearly exceed the maximum value (0.25) expected for one-to-one stoichiometry between bound phosphate and Hb molecules provides evidence for an additional phosphate binding site (compared with that between the β chains), as observed for Hbs of several other vertebrates including eel, billfish, and dromedary (2, 59, 97). Additional evidence for a second binding site derives from association/dissociation kinetics of the reaction of IHP with deoxygenated and carboxy human Hb (99) and molecular dynamic simulations of skua (seabird Catharacta maccormicki) and pheasant Hbs (32, 68, 84). This second site that comprises a cluster of positive charges between the α chains may serve as an entry-leaving site, implying the existence of a migration pathway for phosphates along the central cavity between the two phosphate binding sites (68). In conjunction with the studies of fish, bird, and mammalian Hbs, our findings in P. palpebrosus provide further evidence of a wide occurrence of an α chain phosphate binding site among vertebrates.
Given the distinct effects of ATP and DPG, the insensitivity of P. palpebrosus Hb to IHP molecules (which carry six negative charges compared with four for ATP at physiological pH) indicates that binding of IHP molecules in the cavity between the β chains is impeded by steric hindrance or stereo-chemical mismatch with the positive charges lining the cavity. The observation that 0.1 M chloride decreases Hb-O2 affinity in the presence of IHP, but increases it in the presence of DPG and ATP indicates that chloride is a less potent effector than ATP or DPG but obstructs binding of these phosphates at shared cationic binding sites.
Collectively the β143His→Val and β82Lys→Gln substitutions in P. palpebrosus Hb delete four of seven phosphate binding sites commonly found in vertebrates, which accords with the loss of phosphate sensitivity in the presence of 0.1 M Cl− (Fig. 4). Given that β82-Lys also is a Cl− binding site, its replacement by neutral Gln predictably reduces the chloride effect in P. palpebrosus, but not in C. niloticus, where it is replaced by another positively charged residue (Arg) (Fig. 1).
Polymerization and Its Effect on Hb-O2 Binding
Polymerization of Hbs may begin at hemolysis (69), but may also occur in vivo, as observed in the turtle Pseudemys scripta (81) and witnessed by inclusions resembling Hb crystals in erythrocytes of healthy iguanas (79). In vivo polymerization moreover may be associated with red cell sickling found in iguanas and several teleosts (45). In polymerizing to large aggregates (Fig. 7), oxygenated P. palpebrosus Hb differs from Hbs of other reptiles, including Cai. crocodilus (9) and a large majority of turtles (69, 82) in which polymerization is deoxygenation-linked and does not proceed beyond octamers (dimers of tetramers).
Hb polymerization commonly involves Cys residues, as evident from the correlation between disulfide bridge formation and polymerization in ectotherm Hbs (13, 25, 67, 69, 85). In human Hb mutants Mississippi (β9Ser→Cys) and Hb Ta-li (β83Gly→Cys), polymerization is induced by the incorporation of a single Cys residue (6).
The dissociation of the large complexes of oxygenated P. palpebrosus Hb into smaller molecules upon deoxygenation contrasts starkly with the deoxygenation-linked self-association encountered in Hbs of other ectothermic vertebrates (65), including hagfish and lampreys (monomer to dimer) (14, 26), snakes (dimer to tetramer) (29, 54), frogs Rana esculenta and R. temporaria (tetramer to dimer of tetramers) (5, 23), and bullfrog R. catesbiana (tetramer to heterotrimer of tetramers) (83).
Given that dithionite (used to deoxygenate Hb) may reduce disulfide bonds (44), the possibility cannot be excluded that such interaction contributed to the dissociation of the large Hb complexes upon deoxygenation. Nevertheless P. palpebrosus Hb appears to be unique in polymerizing to giant complexes in the oxygenated state (in the absence of dithionite).
The residues mediating the distinctive self-association remain unknown. P. palpebrosus Hb lacks the only surface Cys residue known in humans (β93Cys) (98), and β49Cys, which causes polymerization through intermolecular disulfide bridges in a teleost fish (25), but has six Cys residues per dimer (at α18, α19, α104, α130, β23, and β100). Available data (36) show that apart from the highly conserved α104Cys, all crocodilian Hbs (as with chicken Hb) have Cys at α130 and β23 (Table 5). Strikingly, P. palpebrosus differs from other crocodilians in lacking α34Cys and α81Cys and uniquely possessing two adjacent Cys residues at α18 and α19 (surface helical positions A16 and AB1) (28) (Fig. 1), implying that these residues may be involved in its unusual, oxygenation-linked polymerization.
Table 5.
Positions of cysteine residues in the α and β chains of crocodilian, human, and chicken hemoglobins
| α Chain | β Chain | |
|---|---|---|
| Human HbA | 104 | 93, 112 |
| Chicken HbA | 104, 130 | 23, 93, 126 |
| P. palpebrosus | 18, 19, 104, 130 | 23, 100 |
| Cai. crocodilus | 34, 81, 104, 115, 130 | 23, 100 |
| C. niloticus | 8, 34, 81, 104, 130 | 23, 76, 93, 126 |
| A. mississippiensis | 34, 81, 104, 130 | 23, 49, 93, 126 |
The similar O2 affinities and Bohr effects in the three major molecular mass fractions isolated in the deoxygenation state (cf. Figs. 7 and 8) indicates that polymerization does not affect the O2 binding properties of Hb. This inference is supported by q values (the number of interacting O2 binding sites) near 4 obtained in fitting the two-state allosteric model to the data. It also aligns with observations that different quaternary assemblies of C. porosus Hb exhibit the same CO2 effect (9) and that the O2 binding properties of turtle (Dermatemys mawi) Hb are not altered by mercaptoethanol-induced dissociation (80). Similarly, polymerization of teleost fish (25) and mouse (72) Hbs has no significant effect on O2 affinity, and recombinant human Hb β83Gly→Cys, which oligomerizes through disulfide bonds, has similar CO binding properties as native human HbA (24).
The lack of polymerization effects on O2 binding properties indicates that the highly variable number of Cys residues in vertebrate Hbs has no consequence for O2 transport. Given that Cys (-SH) residues contribute to redox buffering during oxidative stress in periodic hypoxia and reoxygenation (66), it would seem appropriate to compare Hb polymerization and red cell antioxidant defenses in crocodilians (in which Hbs have high Cys contents) and sauropsids such as the side-neck turtle, Podocnemis unifilis, whose Hbs completely lack Cys residues (34).
Physiological and Evolutionary Implications
The singular structural and functional properties of P. palpebrosus Hb compared with other crocodilian (and vertebrate) Hbs may be either conserved ancestral traits or more recent adaptations related to specific differences in its habit and habitat. In contrast to other large and predominantly aquatic crocodilians in which HCO3−-induced O2 unloading may support aerobic metabolism during prolonged submergence and activity (drowning of prey) and fuel the postprandial rise in metabolic rate, this property is unlikely to retain utility in the small, semiterrestrial dwarf caiman that frequents running streams of clear and cool waters, feeding on snails, insects, and small vertebrates (18). It follows that P. palpebrosus may not have been subjected to selection pressure that favored evolution of the HCO3− effect or that this trait may have been secondarily lost to cope with changes in habitat and/or diet. To place these evolutionary events in perspective, the divergence between the alligator-caiman and the Paleosuchus-Caiman lineages, inferred from the genetic distances between the mitochondrial genome sequences of these taxa, occurred between approximately 65–70 and 37–41 million years ago, respectively (74). Framed by these boundaries, recent fossil-based studies (15, 35) support the view of Paleosuchus evolving as the most basal (plesiotypic) lineage among extant caimanines, indicating that it well may represent the ancestral trait rather than some specialized advanced condition. Either way, P. palpebrosus Hb exhibits allosteric control of O2 affinity on the basis of reciprocating interaction between O2 binding and nonbicarbonate end products of oxidative metabolism (viz., a substantial fixed-acid Bohr effect and the carbamino-mediated CO2 effect) adopted by apparently novel compensatory mechanisms. Accordingly, some key amino acid exchanges observed in dwarf caiman Hb (compared with other crocodilians) closely resemble those in human Hb, whereas other exchanges are distinct compared with human and other crocodilian Hbs. As with other crocodilians, dwarf caiman Hb is rich in titratable His residues that could serve as Bohr groups and compensate for replacement of β146His (the major Bohr group in other vertebrate Hbs). Similarly, a large number of reactive sulphhydryl groups would increase the tendency to form disulfide bridges. The biological significance, if any, of the unique oxygenated-linked polymerization observed in P. palpebrosus is not clear.
Perspectives and Significance
P. palpebrosus Hb exhibits a unique combination of distinctive amino acid exchanges compared with other vertebrate and crocodilian Hbs that could potentially disrupt the allosteric regulation of Hb function. The finding that Hb sustains marked sensitivities to allosteric effectors demonstrates the existence of alternative, compensatory molecular mechanisms to maintain O2 delivery to the tissues in this species. The functional properties of P. palpebrosus Hb disprove several published assumptions and tenets; notably: 1) that β146His is essential for expression of pronounced Bohr effects (P. palpebrosus Hb lacks this residue but expresses a strong Bohr effect); 2) that crocodilian Hbs lack phosphate effects, that β82Lys is essential for phosphate binding, and that IHP invariably is a more potent effector than ATP and DPG (P. palpebrosus Hb lacks β82Lys and is sensitive to ATP and DPG but not to IHP); 3) that crocodilian Hbs bind bicarbonate and do not form carbamino compounds (P. palpebrosus Hb binds CO2); and 4) that oxygenated reptilian Hbs do not polymerize (the oxygenated Hb aggregates to large complexes). P. palpebrosus Hb thus provides unique opportunity for further studies on structure-function coupling and the evolution of effector sensitivities in vertebrate Hbs.
GRANTS
Support for this study was provided by a Faculty of Science and Technology, Aarhus University grant to R. E. Weber, by Danish Council for Independent Research Natural Sciences Grant 10-084565 to A. Fago, by National Heart, Lung, and Blood Institute Grants R01 HL087216 and HL087216-S1 and National Science Foundation Grant IOS-0949931 to J.F. Storz, and by personal fellowships from the Studienstiftung des Deutschen Volkes and Max Planck Society to T.A. Gorr.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.E.W. and T.A.G. conception and design of research; R.E.W. and T.A.G. performed experiments; R.E.W., H.M., J.F.S., and T.A.G. analyzed data; R.E.W., A.F., J.F.S., and T.A.G. interpreted results of experiments; R.E.W., H.M., J.F.S., and T.A.G. prepared figures; R.E.W. and T.A.G. drafted manuscript; R.E.W., A.F., H.M., J.F.S., and T.A.G. approved final version of manuscript; A.F., H.M., J.F.S. and T.A.G. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Anny Bang (Aarhus) for technical assistance, and Prof. W. Böhme, Dr. O. Behlert, Dr. T. Ziegler, and U. Bott (Zoological Gardens of Cologne, Germany) for the blood samples. Primary structure determinations were carried out by T.A. Gorr under expert supervision of the late Prof. G. Braunitzer and Dr. T. Kleinschmidt, Max Planck Institute of Biochemistry, Munich-Martinsried, Germany.
Footnotes
The β82Arg residue in C. niloticus is incorrectly listed as β82Lys in Perutz et al. 1981 (63).
The His→Asp replacement in Hb Hiroshima originally reported at β143 was later shown to be at β146.
REFERENCES
- 1. Ackers GK. Molecular exclusion and restricted diffusion processes in molecular-sieve chromatography. Biochemistry 3: 723–730, 1964 [DOI] [PubMed] [Google Scholar]
- 2. Amiconi G, Bertollini A, Bellelli A, Coletta M, Condò SG, Brunori M. Evidence for two oxygen-linked binding sites for polyanions in dromedary hemoglobin. Eur J Biochem 150: 387–393, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Antonini E, Wyman J, Brunori M, Fronticelli C, Bucci E, Rossi-Fanelli A. Studies on the relations between molecular and functional properties of hemoglobin. V. The influence of temperature on the Bohr effect in human and in horse hemoglobin. J Biol Chem 240: 1096–1103, 1965 [PubMed] [Google Scholar]
- 4. Atha DH, Ackers GK. Calorimetric determination of the heat of oxygenation of human hemoglobin as a function of pH and the extent of reaction. Biochemistry 13: 2376–2382, 1974 [DOI] [PubMed] [Google Scholar]
- 5. Bårdgard A, Fago A, Malte H, Weber RE. Oxygen binding and aggregation of hemoglobin from the common European frog, Rana temporaria. Comp Biochem Physiol B Biochem Mol Biol 117: 225–231, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Baudin-Creuza V, Fablet C, Zal F, Green BN, Prome D, Marden MC, Pagnier J, Wajcman H. Hemoglobin Porto Alegre forms a tetramer of tetramers superstructure. Protein Sci 11: 129–136, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauer C. Antagonistic influence of CO2 and 2,3 diphosphoglycerate on the Bohr effect of human haemoglobin. Life Sci 8: 1041–1046, 1969 [DOI] [PubMed] [Google Scholar]
- 8. Bauer C, Forster M, Gros G, Mosca A, Perrella M, Rollema HS, Vogel D. Analysis of bicarbonate binding to crocodilian hemoglobin. J Biol Chem 256: 8429–8435, 1981 [PubMed] [Google Scholar]
- 9. Bauer C, Jelkmann W. Carbon dioxide governs the oxygen affinity of crocodile blood. Nature 269: 825–827, 1977 [DOI] [PubMed] [Google Scholar]
- 10. Bauer C, Kurtz A. Oxygen-linked CO2 binding to isolated β subunits of human hemoglobin. J Biol Chem 252: 2952–2955, 1977 [PubMed] [Google Scholar]
- 11. Berenbrink M. Evolution of vertebrate haemoglobins: histidine side chains, specific buffer value and Bohr effect. Respir Physiol Neurobiol 154: 165–184, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Bonaventura J, Bonaventura C, Brunori M, Giardina B, Antonini E, Bossa F, Wyman J. Functional properties of carboxypeptidase-digested hemoglobins. J Mol Biol 82: 499–511, 1974 [DOI] [PubMed] [Google Scholar]
- 13. Borgese TA, Harrington JP, Ganjian I, Duran C. Haemoglobin properties and polymerization in the marine teleost Lophius americanus (Goosefish). Comp Biochem Physiol 91: 663–670, 1988 [Google Scholar]
- 14. Briehl RW. The relation between the oxygen equilibrium and aggregation of subunits in lamprey hemoglobin. J Biol Chem 238: 2361–2366, 1963 [PubMed] [Google Scholar]
- 15. Brochu CA. Phylogenetic relationships of Necrosuchus ionensis Simpson, 1937 and the early history of caimanines. Zool J Linnean Soc 163: S228–S256, 2011 [Google Scholar]
- 16. Brunori M, Cutruzzolà F, Vallone B. Haemoglobin engineering: for fun and money. Curr Biol 5: 462–465, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Chiancone E. Dissociation of hemoglobin into subunits. II. Human oxyhemoglobin: gel filtration studies. J Biol Chem 243: 1212–1219, 1968 [PubMed] [Google Scholar]
- 18. Choi H. Paleosuchus palpebrosus (online) Animal Diversity Web. Accessed February 22, 2013 at http://animaldiversity.ummz.umich.edu/accounts/Paleosuchus_palpebrosus/
- 19. Coulson RA, Hernandez T. Increase in metabolic rate of the alligator fed proteins or amino acids. J Nutr 109: 538–550, 1979 [DOI] [PubMed] [Google Scholar]
- 20. Coulson RA, Hernandez T. Alligator metabolism: studies on chemical reactions in vivo. Comp Biochem Physiol B 74: 1–175, 1983 [DOI] [PubMed] [Google Scholar]
- 21. D'Avino R, Fago A, Kunzmann A, Prisco G. The primary structure and oxygen-binding properties of the single haemoglobin of the high-Antarctic fish Aethotaxis mitopteryx DeWitt. Polar Biol 12: 135–140, 1992 [Google Scholar]
- 22. Elder GE, Lappin TR, Horne AB, Fairbanks VF, Jones RT, Winter PC, Green BN, Hoyer JD, Reynolds TM, Shih DT, Mccormick DJ, Kubik KS, Madden BJ, Head CG, Harvey D, Roberts NB. Hemoglobin Old Dominion/Burton-upon-Trent, beta 143 (H21) His→Tyr, codon 143 CAC→TAC—a variant with altered oxygen affinity that compromises measurement of glycated hemoglobin in diabetes mellitus: structure, function, and DNA sequence. Mayo Clin Proc 73: 321–328, 1998 [PubMed] [Google Scholar]
- 23. Elli R, Giuliani A, Tentori L, Chiancone E, Antonini E. The hemoglobin of amphibia. X. Sedimentation behaviour of frog, Triton and Axolotl hemoglobins. Comp Biochem Physiol 36: 163–171, 1970 [DOI] [PubMed] [Google Scholar]
- 24. Fablet C, Marden MC, Green BN, Ho C, Pagnier J, Baudin-Creuza V. Stable octameric structure of recombinant hemoglobin alpha(2)beta(2)83 Gly→Cys. Protein Sci 12: 690–695, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fago A, Romano M, Tamburrini M, Coletta M, D'Avino R, Di Prisco G. A polymerising Root-effect fish hemoglobin with high subunit heterogeneity. Correlation with primary structure. Eur J Biochem 218: 829–835, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Fago A, Weber RE. Hagfish hemoglobins. In: The Biology of Hagfishes, edited by Jørgensen JM, Lomholt JP, Malte H, Weber RE. London: Chapman & Hall, 1996 [Google Scholar]
- 27. Fang TY, Zou M, Simplaceanu V, Ho NT, Ho C. Assessment of roles of surface histidyl residues in the molecular basis of the Bohr effect and of β143 histidine in the binding of 2,3-bisphosphoglycerate in human normal adult hemoglobin. Biochemistry 38: 13423–13432, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Fermi G, Perutz MF. Atlas of Molecular Structures in Biology. 2. Haemoglobin and Myoglobin. Oxford, UK: Clarendon Press, 1981 [Google Scholar]
- 29. Focesi A, Ogo SH, Matsuura MS, Say JC. Further evidence of dimer-tetramer transition in hemoglobin from Liophis miliaris. Braz J Med Biol Res 20: 861–864, 1987 [PubMed] [Google Scholar]
- 30. Gienger CM, Tracy CR, Brien ML, Manolis SC, Webb GJ, Seymour RS, Christian KA. Energetic costs of digestion in Australian crocodiles. Aust J Zool 59: 416–421, 2012 [Google Scholar]
- 31. Gorr TA, Mable BK, Kleinschmidt T. Phylogenetic analysis of reptilian hemoglobins: trees, rates, and divergences. J Mol Evol 47: 471–485, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Grispo MT, Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Storz JF. Gene duplication and the evolution of hemoglobin isoform differentiation in birds. J Biol Chem 287: 37647–37658, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harano T, Harano K, Kushida Y, Imai K, Nishinakamura R, Matsunaga T. Hb Kodaira [beta 146(HC3)His—-Gln]: a new beta chain variant with an amino acid substitution at the C-terminus. Hemoglobin 16: 85–91, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Hasegawa T, Shishikura F, Kuwada T. Side-necked turtle (Pleurodira, Chelonia, reptilia) hemoglobin: cDNA-derived primary structures and X-ray crystal structures of Hb A. IUBMB Life 63: 188–196, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Hastings AK, Bloch JI, Jaramillo CA, Rincon AF, MacFadden BJ. Systematics and biogeography of crocodylians from the Miocene of Panama. J Vert Paleontol 33: 239–263, 2013 [Google Scholar]
- 36. Hoffmann FG, Storz JF, Gorr TA, Opazo JC. Lineage-specific patterns of functional diversification in the alpha- and beta-globin gene families of tetrapod vertebrates. Mol Biol Evol 27: 1126–1138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hofmann O, Carrucan G, Robson N, Brittain T. The chloride effect in the human embryonic haemoglobins. Biochem J 309: 959–962, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Imai K. Oxygen-equilibrium characteristics of abnormal hemoglobin Hiroshima (alpha-2 beta-2 143 Asp). Arch Biochem Biophys 127: 543–547, 1968 [DOI] [PubMed] [Google Scholar]
- 39. Jelkmann W, Bauer C. Oxygen binding properties of caiman blood in the absence and presence of carbon dioxide. Comp Biochem Physiol A 65: 331–336, 1980 [Google Scholar]
- 40. Jensen FB. Hydrogen ion equilibria in fish haemoglobins. J Exp Biol 143: 225–234, 1989 [DOI] [PubMed] [Google Scholar]
- 41. Jensen FB, Wang T, Jones DR, Brahm J. Carbon dioxide transport in alligator blood and its erythrocyte permeability to anions and water. Am J Physiol Regul Integr Comp Physiol 274: R661–R671, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Kilmartin JV. Interaction of haemoglobin with protons, CO2 and 2,3-diphosphoglycerate. Br Med Bull 32: 209–213, 1976 [DOI] [PubMed] [Google Scholar]
- 43. Kilmartin JV, Wootton JF. Inhibition of Bohr effect after removal of C-terminal histidines from haemoglobin β-chains. Nature 228: 766–767, 1970 [DOI] [PubMed] [Google Scholar]
- 44. Knipp M, Taing JJ, He C. Reduction of the lipocalin type heme containing protein nitrophorin – sensitivity of the fold-stabilizing cysteine disulfides toward routine heme-iron reduction. J Inorg Biochem 105: 1405–1412, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Koldkjaer P, Berenbrink M. In vivo red blood cell sickling and mechanism of recovery in whiting, Merlangius merlangus. J Exp Biol 210: 3451–3460, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Komiyama N, Tame J, Nagai K. A hemoglobin-based blood substitute: transplanting a novel allosteric effect of crocodile Hb. Biol Chem 377: 543–548, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Komiyama NH, Miyazaki G, Tame J, Nagai K. Transplanting a unique allosteric effect from crocodile into human haemoglobin. Nature 373: 244–246, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Kosugi H, Weinstein AS, Kikugawa K, Asakura T, Schroeder WA. Characterization and properties of Hb York (beta 146 His leads to Pro). Hemoglobin 7: 205–226, 1983 [DOI] [PubMed] [Google Scholar]
- 49. Kwiatkowski LD, Noble RW. The contribution of histidine (HC3) (146 beta) to the R state Bohr effect of human hemoglobin. J Biol Chem 257: 8891–8895, 1982 [PubMed] [Google Scholar]
- 49a. Laurent TC, Killander J. A theory of gel filtration and its experimental verification. J Chromatogr 14: 317–330, 1964 [Google Scholar]
- 50. Leach M, Greaves M, Porter N, Williamson D, Brown K. Haemoglobin Hallamshire (b146 HIS→TYR): a new high oxygen affinity haemoglobin responsible for familial erythrocytosis. Clin Lab Haematol 18: 237–239, 1996 [PubMed] [Google Scholar]
- 51. Leclercq F, Schnek AG, Braunitzer G, Stangl A, Schrank B. Direct reciprocal allosteric interaction of oxygen and hydrogen carbonate. Sequence of the haemoglobins of the caiman (Caiman crocodilus), the Nile crocodile (Crocodylus niloticus) and the Mississippi crocodile (Alligator mississippiensis). Hoppe-Seylers Z Physiol Chem 362: 1151–1158, 1981 [PubMed] [Google Scholar]
- 52. Lee YW, Ki CS, Kim HJ, Lee ST, Kim CK, Shin HB, Hong DS, Lee YK. High oxygen-affinity hemoglobin variant associated with high-level venous oxygen saturation. Clin Chem Lab Med 46: 417–418, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Lukin JA, Ho C. The structure–function relationship of hemoglobin in solution at atomic resolution. Chem Rev 104: 1219–1230, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Matsuura MS, Ogo SH, Focesi A., Jr Dimer-tetramer transition in hemoglobins from Liophis miliaris–I. Effect of organic polyphosphates. Comp Biochem Physiol A Comp Physiol 86: 683–687, 1987 [DOI] [PubMed] [Google Scholar]
- 55. Misgeld E, Gattermann N, Wehmeier A, Weiland C, Peters U, Kohne E. Hemoglobinopathy York [beta146 (HC3) His→Pro]: first report of a family history. Ann Hematol 80: 365–367, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Moffat K, Olson JS, Gibson QH, Kilmartin JV. The ligand-binding properties of desHis (146beta) hemoglobin. J Biol Chem 248: 6387–6393, 1973 [PubMed] [Google Scholar]
- 57. Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol 12: 88–118, 1965 [DOI] [PubMed] [Google Scholar]
- 58. Nielsen MS, Weber RE. Antagonistic interaction between oxygenation-linked lactate and CO2 binding to human hemoglobin. Comp Biochem Physiol A Mol Integr Physiol 146: 429–434, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Olianas A, Messana I, Sanna MT, Castagnola M, Manconi B, Masia D, Coluccia E, Giardina B, Pellegrini M. Two sites for GTP binding in cathodic haemoglobins from Anguilliformes. Comp Biochem Physiol B Biochem Mol Biol 141: 400–407, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Parkhurst LJ, Goss DJ, Perutz MF. Kinetic and equilibrium studies on the role of the β-147 histidine in the Root effect and cooperativity in carp hemoglobin. Biochemistry 22: 5401–5409, 1983 [Google Scholar]
- 61. Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Haem-haem interaction and the problem of allostery. Nature 228: 726–734, 1970 [DOI] [PubMed] [Google Scholar]
- 62. Perutz MF. Species adaptation in a protein molecule. Mol Biol Evol 1: 1–28, 1983 [DOI] [PubMed] [Google Scholar]
- 63. Perutz MF, Bauer C, Gros G, Leclercq F, Vandecasserie C, Schnek AG, Braunitzer G, Friday AE, Joysey KA. Allosteric regulation of crocodilian haemoglobin. Nature 291: 682–684, 1981 [DOI] [PubMed] [Google Scholar]
- 64. Perutz MF, Pulsinelli P, Eyck LT, Kilmartin JV, Shibata S, Iuchi I, Miyaji T, Hamilton HB. Haemoglobin Hiroshima and the mechanism of the alkaline Bohr effect. Nat New Biol 232: 147–149, 1971 [DOI] [PubMed] [Google Scholar]
- 65. Rana MS, Knapp JE, Holland RA, Riggs AF. Component D of chicken hemoglobin and the hemoglobin of the embryonic Tammar wallaby (Macropus eugenii) self-associate upon deoxygenation: effect on oxygen binding. Proteins 70: 553–561, 2008 [DOI] [PubMed] [Google Scholar]
- 66. Reischl E. High sulfhydryl content in turtle erythrocytes: is there a relation with resistance to hypoxia? Comp Biochem Physiol B 85: 723–726, 1986 [DOI] [PubMed] [Google Scholar]
- 67. Reischl E, da Diefenbach CO. Heterogeneity and polymerization of hemoglobins of Caiman latirostris (Crocodylia: Reptilia). Comp Biochem Physiol B 54: 543–545, 1976 [DOI] [PubMed] [Google Scholar]
- 68. Riccio A, Tamburrini M, Giardina B, Di Prisco G. Molecular dynamics analysis of a second phosphate site in the hemoglobins of the seabird, south polar skua. Is there a site-site migratory mechanism along the central cavity? Biophys J 81: 1938–1946, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Riggs A, Sullivan B, Agee JR. Polymerization of frog and turtle hemoglobins. Proc Natl Acad Sci USA 51: 1127–1134, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Riggs AF. The Bohr effect. Annu Rev Physiol 50: 181–204, 1988 [DOI] [PubMed] [Google Scholar]
- 71. Riggs AF. Self-association, cooperativity and supercooperativity of oxygen binding by hemoglobins. J Exp Biol 201: 1073–1084, 1998 [DOI] [PubMed] [Google Scholar]
- 72. Riggs A, Rona M. The oxygen equilibria and aggregation behavior of polymerizing mouse hemoglobins. Biochim Biophys Acta 175: 248–259, 1969 [DOI] [PubMed] [Google Scholar]
- 73. Rollema HS, De Bruin SH, Janssen LH, Van Os GA. The effect of potassium chloride on the Bohr effect of human hemoglobin. J Biol Chem 250: 1333–1339, 1975 [PubMed] [Google Scholar]
- 74. Roos J, Aggarwal RK, Janke A. Extended mitogenomic phylogenetic analyses yield new insight into crocodylian evolution and their survival of the Cretaceous-Tertiary boundary. Mol Phylogenet Evol 45: 663–673, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Schäfer W, Braunitzer G, Stangl A. Direct allosteric interaction of oxygen and bicarbonate: N-acetyl-alanyl-seryl-phenylalanine, N-terminal sequence of the b-chains of the haemoglobins of Nile crocodile (Crocodylus niloticus) and Mississippi crocodile (Alligator mississippiensis). Z Naturforsch 36: 902–903, 1981 [PubMed] [Google Scholar]
- 76. Schneider RG, Bremner JE, Brimhall B, Jones RT, Shih TB. Hemoglobin Cowtown (beta 146 HC3 His-Leu): a mutant with high oxygen affinity and erythrocytosis. Am J Clin Pathol 72: 1028–1032, 1979 [DOI] [PubMed] [Google Scholar]
- 77. Seymour RS, Bennett AF, Bradford DF. Blood gas tensions and acid-base balance in the salt-water crocodile, Crocodylus porosus, at rest and after exhaustive exercise. J Exp Biol 118: 143–159, 1985 [Google Scholar]
- 78. Shih T, Jones RT, Bonaventura J, Bonaventura C, Schneider RG. Involvement of His HC3 (146) beta in the Bohr effect of human hemoglobin. Studies of native and N-ethylmaleimide-treated hemoglobin A and hemoglobin Cowtown (beta 146 His replaced by Leu). J Biol Chem 259: 967–974, 1984 [PubMed] [Google Scholar]
- 79. Stacy NI, Alleman AR, Sayler KA. Diagnostic hematology of reptiles. Clin Lab Med 31: 87–108, 2011 [DOI] [PubMed] [Google Scholar]
- 80. Sullivan B. Reptilian hemoglobins. In: Chemical Zoology, Vol. IX. Amphibia and Reptilia, edited by Florkin M, Scheer BT. New York: Academic Press, 1974, p. 377–398 [Google Scholar]
- 81. Sullivan B, Riggs A. Hæmoglobin: reversal of oxidation and polymerization in turtle red cells. Nature 204: 1098–1099, 1964 [DOI] [PubMed] [Google Scholar]
- 82. Sullivan B, Riggs A. Structure, function and evolution of turtle hemoglobins. I. Distribution of heavy hemoglobins. Comp Biochem Physiol 23: 437–447, 1967 [DOI] [PubMed] [Google Scholar]
- 83. Tam LT, Riggs AF. Oxygen binding and aggregation of bullfrog hemoglobin. J Biol Chem 259: 2610–2616, 1984 [PubMed] [Google Scholar]
- 84. Tamburrini M, Riccio A, Romano M, Giardina B, Di Prisco G. Structural and functional analysis of the two haemoglobins of the Antarctic seabird Catharacta maccormicki characterization of an additional phosphate binding site by molecular modelling. Eur J Biochem 267: 6089–6098, 2000 [DOI] [PubMed] [Google Scholar]
- 85. Torsoni MA, Souza-Torsoni A, Ogo SH, Souza Torsoni A. Involvement of available SH groups in the heterogeneity of hemoglobin from the tortoise Geochelone carbonaria. Biochem Mol Biol Int 44: 851–860, 1998 [DOI] [PubMed] [Google Scholar]
- 86. Villar JL, Puigbo P, Riera-Codina M. Analysis of highly phosphorylated inositols in avian and crocodilian erythrocytes. Comp Biochem Physiol B Biochem Mol Biol 135: 169–175, 2003 [DOI] [PubMed] [Google Scholar]
- 87. Wajcman H, Kilmartin JV, Najman A, Labie D. Hemoglobin Cochin-Port-Royal: consequences of the replacement of the β-chain C-terminal by an arginine. Biochim Biophys Acta 400: 354–364, 1975 [DOI] [PubMed] [Google Scholar]
- 88. Weber RE. Cationic control of O2 affinity in lugworm erythrocruorin. Nature 292: 386–387, 1981 [Google Scholar]
- 89. Weber RE. Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J Appl Physiol 72: 1611–1615, 1992 [DOI] [PubMed] [Google Scholar]
- 90. Weber RE, Campbell KL. Temperature dependence of haemoglobin-oxygen affinity in heterothermic vertebrates: mechanisms and biological significance. Acta Physiol (Oxf) 202: 549–562, 2011 [DOI] [PubMed] [Google Scholar]
- 91. Weber RE, Fago A. Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir Physiol Neurobiol 144: 141–159, 2004 [DOI] [PubMed] [Google Scholar]
- 92. Weber RE, Fago A, Val AL, Bang A, Van Hauwaert ML, Dewilde S, Zal F, Moens L. Isohemoglobin differentiation in the bimodal-breathing Amazon catfish Hoplosternum littorale. J Biol Chem 275: 17297–17305, 2000 [DOI] [PubMed] [Google Scholar]
- 93. Weber RE, Jensen FB, Cox RP. Analysis of teleost hemoglobin by Adair and Monod-Wyman-Changeux models. Effects of nucleoside triphosphates and pH on oxygenation of tench hemoglobin. J Comp Physiol B 157: 145–152, 1987 [DOI] [PubMed] [Google Scholar]
- 94. Weber RE, Malte H, Braswell EH, Oliver RW, Green BN, Sharma PK, Kuchumov A, Vinogradov SN. Mass spectrometric composition, molecular mass and oxygen binding of Macrobdella decora hemoglobin and its tetramer and monomer subunits. J Mol Biol 251: 703–720, 1995 [DOI] [PubMed] [Google Scholar]
- 95. Weber RE, White FN. Oxygen binding in alligator blood related to temperature, diving and “alkaline tide”. Am J Physiol 20: R901–R908, 1986 [DOI] [PubMed] [Google Scholar]
- 96. Weber RE, White FN. Chloride-dependent organic phosphate sensitivity of the oxygenation reaction in crocodilian hemoglobins. J Exp Biol 192: 1–11, 1994 [DOI] [PubMed] [Google Scholar]
- 97. Weber RE, Campbell KL, Fago A, Malte H, Jensen FB. ATP-induced temperature independence of hemoglobin-O2 affinity in heterothermic billfish. J Exp Biol 213: 1579–1585, 2010 [DOI] [PubMed] [Google Scholar]
- 98. Zhang N, Palmer AF. Polymerization of human hemoglobin using the crosslinker 1,11-bis(maleimido)triethylene glycol for use as an oxygen carrier. Biotechnol Prog 26: 1481–1485, 2010 [DOI] [PubMed] [Google Scholar]
- 99. Zuiderweg ER, Hamers LF, Rollema HS, De Bruin SH, Hilbers CW. 31P NMR study of the kinetics of binding of myo-inositol hexakisphosphate to human hemoglobin. Observation of fast-exchange kinetics in high-affinity systems. Eur J Biochem 118: 95–104, 1981 [DOI] [PubMed] [Google Scholar]