Abstract
Ovarian hormones are thought to modulate sleep and fluctuations in the hormonal milieu are coincident with sleep complaints in women. In female rats, estradiol increases waking and suppresses sleep. In this study, we asked whether this effect is mediated via circadian or homeostatic regulatory mechanisms. Ovariectomized female rats received daily injections of estradiol benzoate (EB) or sesame oil that mimicked the rapid increase and subsequent decline of circulating estradiol at proestrus. In one experiment, animals were sleep deprived for 6 h starting at lights-on, so that recovery began in the mid-light phase; in the second experiment, animals were sleep deprived starting in the mid-light phase, so that recovery began at lights-off. EB suppressed baseline rapid eye movement (REM) and non-REM (NREM) sleep and increased waking in the dark phase. In both experiments, EB enhanced REM recovery in the light phase while suppressing it in the dark compared with oil; this effect was most pronounced in the first 6 h of recovery. By contrast, NREM recovery was largely unaffected by EB. In summary, EB enhanced waking and suppressed sleep, particularly REM sleep, in the dark under baseline and recovery conditions. These strong temporally dependent effects suggest that EB consolidates circadian sleep-wake rhythms in female rats.
Keywords: hormone, paradoxical sleep, sex differences, insomnia, menopause
women have increased risk for insomnia or insufficient sleep compared with men (30, 41, 57, 66). Short- and long-term alterations in the circulating ovarian hormone profile, either at major life events such as adolescence, pregnancy, and at menopause or across the menstrual cycle, are associated with elevated sleep disturbance (3, 23, 27, 33), suggesting that these hormones may play a role in the physiological regulation of sleep. For example, delta sleep and wake after sleep onset are associated with the rate of change in ovarian hormones across the menopausal transition (58), whereas nighttime elevations in luteinizing hormone pulses are associated with increased wakefulness in postmenopausal women (37). In younger women, low circulating estradiol levels across the menstrual cycle reduce subjective variability in sleep (31). Rapid-eye movement (REM) sleep decreases, whereas stage 2 non-REM (NREM) sleep and spindle frequency activity increase in the luteal phase of the menstrual cycle (18, 53). In addition, nighttime alertness and cognitive performance during an all-night sleep deprivation are increased at the luteal phase, consistent with reduced sleep propensity at this time (61). Exogenous ovarian hormones have also been shown to alter subjective and objective sleep measures (4, 21, 47). Thus both endogenous and exogenous ovarian hormones alter sleep in women over much of their lifespan. However, the biological basis for these effects is still unknown.
The regulation of sleep by gonadal steroids can be directly studied in laboratory rodents whose ovulatory cycles are much shorter in length but have a strikingly similar hormonal profile to those of humans. Cycling female rats (12, 22, 25, 50, 63, 67) and mice (26) suppress sleep at proestrus, when circulating estradiol (E2) levels peak. The periovulatory decrease in sleep is abolished by ovariectomy (OVX) (7, 44, 45, 64) and is restored by exogenous E2 treatment in OVX rats (7, 13, 64) and mice (45), demonstrating that E2 powerfully and directly influences spontaneous sleep in both intact and OVX/hormone-replaced rodents. Interestingly, intact rats suppress both REM and NREM sleep in the dark phase (22, 50), particularly around the light-to-dark transition when circadian promotion of wakefulness is strong (12, 22, 51); similarly, OVX rats treated with E2 exhibit more frequent brief awakenings, shorter NREM episodes, and fewer REM episodes in the dark phase (17). Together, these data suggest that E2-mediated sleep suppression is more powerful in the nighttime (i.e., the dark phase of a LD cycle), when rats are normally awake, than in the daytime, when they normally sleep.
We have recently used sleep deprivation paradigms to investigate effects of E2 on sleep. Sleep is regulated by a homeostatic drive that increases sleep propensity in proportion to the duration of prior waking and a circadian drive that alternately promotes sleep and waking across the day (5, 6). Sleep deprivation (SD) induces a compensatory increase or “rebound” that is proportional to the duration of the SD and to the stage of sleep that was lost (e.g., REM vs. NREM) (42, 52). However, rebound may be attenuated when circadian promotion of wakefulness is strong (24, 62). Previously, we and others have shown that spontaneous REM and NREM sleep decrease on the day of proestrus without a subsequent REM recovery (22, 50), whereas NREM sleep (12) and delta power (50) increase on the day of estrus (when E2 levels are declining). Furthermore, OVX rats exposed to low physiological doses of E2 suppress spontaneous REM sleep in the dark phase and significantly attenuate REM recovery in the dark following a 12-h SD in the light phase (49). Based on this, we hypothesize that E2 acts to consolidate or otherwise strengthen the circadian sleep-wake rhythm. If this hypothesis is true, SD ending in the light phase (the rat's normal rest phase) should induce a larger rebound in E2-treated rats than a similar SD ending in the dark phase. Conversely, if E2 acts on homeostatic mechanisms that regulate NREM and/or REM sleep, SD should attenuate sleep rebound in E2-treated rats regardless of what time of day the deprivation ends. We therefore asked whether physiological doses of exogenous E2 would attenuate the recovery from a 6-h SD during the light phase when recovery was initiated in either the light phase or the dark phase.
METHODS
Animals.
Adult female Sprague-Dawley rats (n = 35; Charles River, Kingston, NY) were singly housed in polyethylene cages under a 12:12 light-dark cycle, with lights on = Zeitgeber time (ZT) 0 and lights off = ZT 12 (35). Animals received ad libitum access to food and water for the duration of the experiment. Animals weighed 275–300 g at the start of the study. All surgical procedures took place during the animals' light phase. All animal-related procedures were submitted to and approved by the University of Maryland Institutional Animal Care and Use Committee and were performed in accordance with NIH Guide for the Case and Use of Laboratory Animals.
Surgery.
Animals were ovariectomized and implanted with telemetry units for recording electroencephalogram (EEG) and electromyogram (EMG) activity under isoflurane anesthesia. A single 3- to 4-cm longitudinal incision was made through the skin on the dorsal abdominal surface, and single incisions were made in the muscle wall on each flank. Ovaries were extracted and clamped between the oviduct and uterus and then removed; OVX was confirmed by visual inspection of the excised ovary and the uterine horn. The uterine horn was then replaced into the abdominal cavity and the muscle wall was sutured. A second 3-cm longitudinal skin incision exposed the skull and neck muscle. With the use of a handheld drill, two burr holes were drilled and stainless steel screw electrodes (Plastics One; Roanoke, VA) were implanted at +2.0 mm AP/+1.5 mm LM and −7.0 mm AP/−1.5 mm LM relative to bregma. The telemetry transmitter (TL11M2-F40-EET; Data Sciences International, St. Paul, MN) was inserted subcutaneously into a blunt-dissected pocket to the right or left of the midline body, and electrode leads were threaded subcutaneously to the scalp incision. EEG leads were wrapped around the screw electrodes; screws were tightened flush with the skull and secured with dental cement. EMG leads were inserted directly into the neck muscle just left of the midline, ∼1.5 mm apart. The scalp was sutured and the body incision was closed with wound clips. Animals were treated postoperatively with antibiotic ointment, topical lidocaine, and 0.1 ml buprenorphine and allowed to recover for at least 10 days before the start of experiments.
Sleep EEG data collection and analysis.
EEG and EMG data were continuously collected using a PC running Dataquest ART 4.0 software (DSI). Home cages containing the implanted animals were placed on receiver bases that relayed transmitter data to the PC. Digitized signal data were scored offline with Neuroscore 2.0 (DSI). For each individual, two representative 3-h long blocks of the data record were hand scored in 10-s epochs as wake (low-amplitude, high-frequency EEG combined with high-amplitude EMG), NREM (high-amplitude, low-frequency EEG and low-amplitude EMG), or REM (low-amplitude, high-frequency EEG with very low EMG tone). These hand-scored blocks were used to calibrate the software's autoscoring algorithm to at least 95% agreement between the two blocks; the calibrated autoscoring algorithm was then applied to the entire record. Accuracy of autoscoring was confirmed via visual inspection of the records and corrected by hand as necessary.
Sleep deprivation.
SD was accomplished via gentle handling (50) in their home cages. Experimenters lightly tapped the cage, introduced novel objects (e.g., toys, paper towels, cotton), and occasionally changed the cage orientation when animals exhibited signs of sleep (eyes closed, adopting a sleep-like posture). In this manner, animals were kept awake using the minimum level of experimenter interaction possible. Aside from hormone injections and regular husbandry during the experiment, we did not physically handle the rats before or during SD. At the conclusion of SD, experimenters removed novel objects and animals were allowed to recover undisturbed.
Experimental schedule.
Hormone treatment consisted of sequential daily injections of 17-β estradiol benzoate in sesame oil (EB; 150 μg/ml), or sesame oil alone (Oil) in the first half of the dark phase (Fig. 1A). The EB treatment consisted of oil on the first day, 5 μg EB on the second day, and 10 μg EB on the third; this ascending dosage paradigm was designed to reflect the natural preovulatory increase in circulating E2. The oil treatment consisted of daily injections of sesame oil alone for 3 days. Within each experiment, hormone conditions and subsequent EEG recording were repeated; thus, animals were sleep deprived once with EB on board and once without. In both experiments animals were given 12–14 days to clear residual hormones between hormone conditions.
Fig. 1.
Schematic showing the experimental schedule. Light-dark (LD) cycle is represented by alternating light and dark bars. Animals received daily injections of oil followed by estradiol benzoate (EB) (black arrows) or oil only (gray arrows) before baseline and sleep deprivation (SD). In the first experiment, SD began at lights on (A), whereas in the second experiment SD began in the midlight phase (B). Recovery thus consisted of 6 h of light followed by 12 h of darkness (Recovery-Light; A) or vice versa (Recovery-Dark; B).
We conducted two separate experiments (n = 5 rats per experiment). In the first experiment (Recovery-Light cohort), all animals were given EB as described above, left undisturbed for a 24-h baseline, sleep deprived from Zeitgeber time 0 (ZT 0, where ZT 0 corresponds to lights on and ZT 12 corresponds to lights off in a 12:12 light-dark cycle) to ZT 6 via gentle handling (50), and allowed to recover undisturbed (Fig. 1A). After a washout period, baseline and recovery sleep was assessed again following 3 days of oil treatment. In the second experiment (Recovery-Dark cohort), EB and oil treatments were fully counterbalanced with some animals receiving EB first while others received oil first. The order in which hormone treatments were given did not influence sleep in this cohort (data not shown). After a 24-h baseline, animals were sleep deprived from ZT 6 to ZT 12 (Fig. 1B). Thus, in experiment 1, recovery began with 6 h of the light phase plus the entire 12-h dark phase (Fig. 1A), whereas in experiment 2 recovery began with the 12-h dark phase and continued into the first 6 h of the next light phase (Fig. 1A).
Serum E2 quantification.
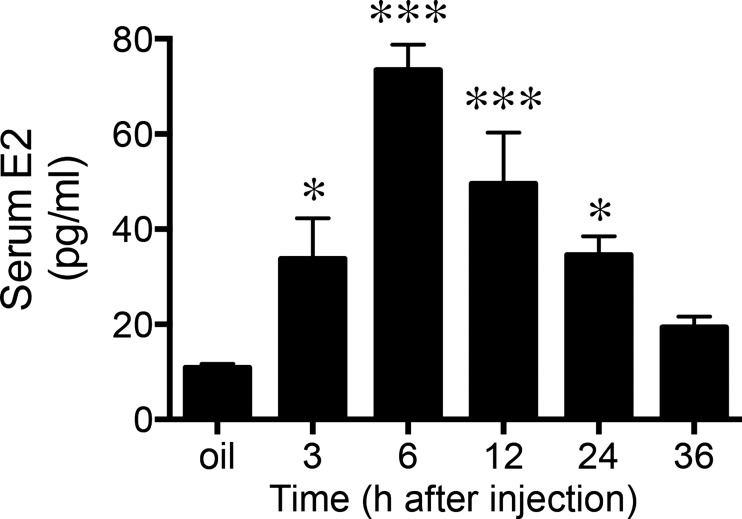
EB is a synthetic ester of estradiol; esterification with benzoate results in prolonged availability. Deesterification of EB by nonspecific steroidal esterases, which have high activity in rats, produces biologically active E2 in vivo (29, 43). To confirm that our exogenous EB treatment mimicked the natural rise and fall of E2, we measured E2 levels via an enzyme immunoassay in a separate cohort of female rats. Females (n = 25) were ovariectomized and treated with either oil (n = 5) or EB (n = 20) as described above. The EB-treated females were divided into the following groups: 3 h (n = 5), 6 h (n = 5), 12 h (n = 4), 24 h (n = 3), and 36 h (n = 3). After the last injection, the animals were decapitated and trunk blood collected according the postinjection times their assigned groups. Serum was separated within 90 min and stored at −20°C. Serum E2 levels were measured using an enzyme-linked immunosorbant assay (ELISA) kit (estradiol ELISA, Calbiotech, Spring Valley, CA) in duplicate and replicated across two separate assay plates according to the stated protocols. Data are presented in picograms per milliliter (pg/ml) as means ± SE.
Data analysis and statistics.
Total sleep duration (min) was summed in 3-h bins for each vigilance state (REM, NREM, Wake). The average number of REM, NREM, and Wake bouts per hour and the average bout length in seconds were calculated for the light and dark phases in baseline and recovery periods. A bout was defined as an uninterrupted episode of a single behavioral state lasting at least one full epoch. NREM delta power was normalized to each individual's 24 h mean baseline delta power. To control for baseline differences in sleep duration between hormone treatments (see results), recovery REM and NREM sleep in each individual were also normalized to their respective baselines using a gain-loss ratio, where [gain-loss ratio = (recovery − corresponding portion of baseline)/(baseline corresponding to SD − SD)] (16).
Baseline vigilance state data (REM, NREM, wake duration) and NREM delta power were analyzed by a repeated-measures ANOVA comparing hormone treatment (EB × oil) by time (ZT 0, 3, 6, 9, 12, 15, 18, 21) in Statistica (Statsoft; Tulsa, OK). Recovery sleep and delta power were analyzed by a repeated-measures ANOVA comparing hormone treatment by time of day (experiment 1, ZT 6, 9, 12, 15, 18, 21; experiment 2, ZT 12, 15, 18, 21, 0, 3). Sleep bout numbers and average bout length were analyzed by a repeated-measures ANOVA comparing hormone treatment by time of day (L vs. D). Gain-loss ratios were analyzed by Student's t-tests. Serum E2 levels were analyzed by a one-way between-subjects ANOVA. Where appropriate, significant effects were followed up with Fisher LSD post hoc analysis (sleep EEG data) or Tukey's multiple comparison test (serum E2). All effects were considered significant at P = 0.05.
RESULTS
Exogenous EB treatment mimics proestrus levels of estradiol.
Exogenous EB treatment significantly elevated serum estradiol concentration compared with oil-treated controls (F2,23 = 15.3; P < 0.001). Tukey's multiple comparisons test revealed estradiol levels measured at 3, 6, 12, and 24 h post-EB treatment were significantly greater than oil-treated animals (P < 0.05), whereas by 36 h post-EB injection estradiol level had returned to baseline (Fig. 2). By 3 h post-EB injection, estradiol concentrations reached ∼30 pg/ml with the peak concentrations reaching ∼75 pg/ml at 6 h postinjection. Additionally, the EB treatment paradigm induced a gradual rise and fall of serum estradiol over a 24-h period. Resulting serum estradiol levels from our exogenous EB treatment paradigm closely resembled the 1) range of values and 2) pattern of rise reported for intact cycling females (9, 56).
Fig. 2.
Mean serum estradiol (E2) concentrations following EB treatment. Values are presented at means ± SE. *P < 0.05 vs. oil; ***P < 0.0005 vs. oil.
Daily EB injections suppress baseline sleep and increase wakefulness at night.
Both cohorts exhibited the expected estradiol-mediated effects that we and others have observed (16, 17, 49): specifically, estradiol suppressed spontaneous REM and NREM sleep in the dark, with no effects on either sleep stage in the light. Therefore, baseline data from the two cohorts (Recovery-Light and -Dark) were pooled for analysis. At baseline, EB suppressed REM sleep compared with oil (hormone, F1,9 = 25.978, P < 0.001; Fig. 3A). This effect was strongest in the dark, when EB reduced total REM duration to 16.4 ± 3.8 min over the 12-h dark phase compared with 43.4 ± 3.4 min for oil-treated rats. EB-treated rats had fewer REM bouts per hour (bouts/h) in the dark phase compared with oil (hormone × time, F1,9 = 13.563, P = 0.005), as well as shorter average REM bout duration (hormone × time, F1,9 = 12.731, P = 0.006; Table 1).
Fig. 3.
Total minutes spent in rapid eye movement (REM) (A), non-REM (NREM) (B), and wake (C) during baseline in ovariectomized (OVX) rats after EB (black lines) and oil (gray lines) injections. Time of day is expressed as Zeitgeber time (ZT), where ZT 0 = lights on, indicated below LD bar on X-axis. *P < 0.05 vs. oil.
Table 1.
Baseline sleep architecture
| Baseline |
||||
|---|---|---|---|---|
| Light |
Dark |
|||
| EB | Oil | EB | Oil | |
| Bouts per Hour | ||||
| REM | 12.41 ± 1.68 | 12.50 ± 1.89 | 2.54 ± 1.13* | 5.50 ± 1.83 |
| NREM | 19.76 ± 2.06 | 20.23 ± 2.29 | 8.33 ± 2.01 | 10.62 ± 2.46 |
| Wake | 10.34 ± 2.11 | 10.69 ± 1.79 | 8.92 ± 1.68 | 8.65 ± 1.42 |
| Average Bout Duration, s | ||||
| REM | 39.49 ± 4.57 | 42.72 ± 5.14 | 19.58 ± 8.29* | 28.67 ± 8.71 |
| NREM | 120.33 ± 17.65 | 113.91 ± 21.32 | 59.02 ± 13.11 | 62.45 ± 14.28 |
| Wake | 118.86 ± 52.15 | 123.83 ± 61.89 | 614.67 ± 233.89 | 566.38 ± 230.42 |
Average sleep bouts per hour and bout duration (in seconds) for the baseline light and dark phase. Values are means ± SE.
P < 0.05 vs. oil.
EB also decreased NREM sleep duration compared with oil at ZT 15 and ZT 18 (hormone × time; F7,63 = 2.475, P = 0.026; Fig. 3B). EB did not influence NREM sleep in the light phase and did not affect either NREM bout number or bout length (Table 1). Complementing the REM and NREM result, EB increased waking in the dark phase at ZT 15 and ZT 18 (hormone × time; F7,63 = 2.418, P = 0.029; Fig. 3C). As was the case for NREM sleep, there were no effects of EB on waking in the light phase and no effects on wake bout number or bout length. As we previously reported (49), EB injections thus suppressed REM and NREM sleep and increased wake in the dark phase but not the light.
When recovery begins in the light phase, EB concentrates REM rebound in the light.
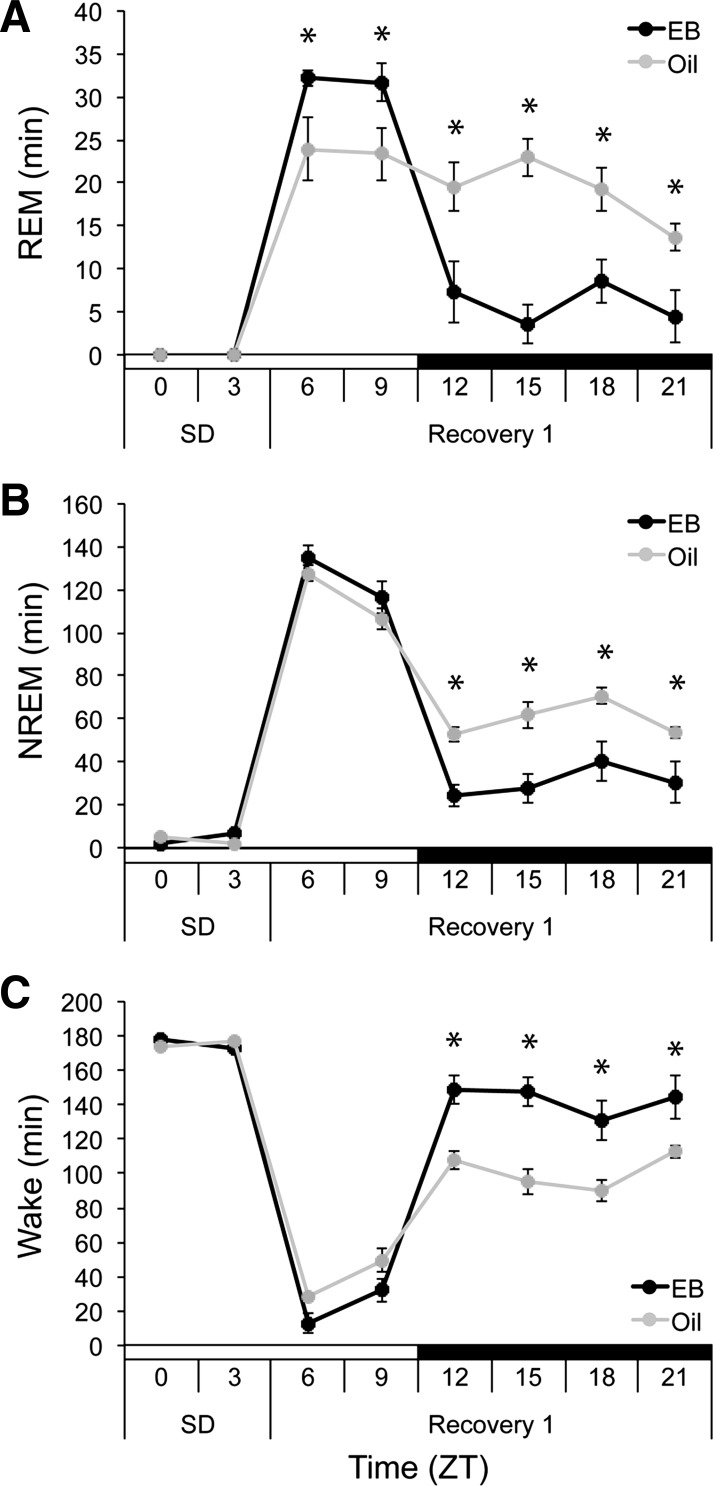
The Recovery-Light cohort was sleep deprived in the first half of the light phase (n = 5), so that sleep recovery began in the middle of the day (ZT 6). SD eliminated 100% and 99.87% of baseline REM sleep in the EB- and oil-treatment conditions, respectively. Upon release from SD, EB increased REM duration in the light phase compared with oil (ZT 6 and ZT 9) and decreased REM duration in the dark phase compared with oil (ZT 12-ZT 21; F5,20 = 10.990, P < 0.001; Fig. 4A). EB-mediated changes in REM duration were driven by increases and decreases in REM bout number, respectively (hormone × time, F1,4 = 44.160, P = 0.003; Table 2). This differential timing of REM recovery preserved the normal day-night REM sleep distribution when rats received EB, whereas this pattern was flattened when the same rats received oil (Fig. 4A).
Fig. 4.
Total minutes spent in REM (A), NREM (B), and wake (C) during SD from ZT 0-ZT 6 and subsequent 18 h recovery in OVX rats treated with EB (black) or oil (gray). *P < 0.05 vs. oil.
Table 2.
Recovery sleep architecture
| Recovery-Light |
Recovery-Dark |
|||||||
|---|---|---|---|---|---|---|---|---|
| Light |
Dark |
Dark |
Light |
|||||
| EB | Oil | EB | Oil | EB | Oil | EB | Oil | |
| Bouts per Hour | ||||||||
| REM | 12.06 ± 1.98* | 8.83 ± 1.63 | 3.43 ± 1.82* | 8.02 ± 2.58 | 6.12 ± 2.45* | 8.10 ± 2.49 | 12.23 ± 2.3* | 10.72 ± 1.82 |
| NREM | 15.50 ± 2.13 | 13.83 ± 2.12 | 8.83 ± 2.67* | 14.58 ± 2.99 | 12.85 ± 3.53 | 11.57 ± 3.15 | 19.62 ± 2.69 | 19.87 ± 3.31 |
| Wake | 4.93 ± 1.34 | 6.27 ± 1.79 | 8.30 ± 1.85 | 9.62 ± 2.03 | 11.27 ± 3.02 | 8.33 ± 1.87 | 10.23 ± 2.56 | 12.82 ± 3.27 |
| Average Bout Duration, s | ||||||||
| REM | 57.35 ± 13.59 | 58.09 ± 10.04 | 21.62 ± 10.14 | 42.18 ± 13.52 | 35.78 ± 12.96 | 44.53 ± 12.34 | 49.05 ± 7.85 | 50.79 ± 11.19 |
| NREM | 182.34 ± 33.44 | 185.60 ± 32.86 | 69.91 ± 18.84 | 80.37 ± 18.02 | 110.39 ± 50.49 | 77.62 ± 20.11 | 120.53 ± 24.28 | 113.37 ± 26.28 |
| Wake | 85.26 ± 32.34 | 149.66 ± 63.74 | 494.14 ± 165.15* | 293.57 ± 142.55 | 427.71 ± 274.87 | 555.57 ± 394.69 | 93.05 ± 40.29 | 109.41 ± 68.61 |
Average sleep bouts per hour and bout duration (in seconds) for the Recovery-Light and Recovery-Dark light and dark phase. Values are means ± SE.
P < 0.05 vs. oil.
SD eliminated 96.43% and 97.25% of baseline NREM sleep in the EB- and oil-treatment conditions, respectively. On release, rats showed large acute increases in NREM duration in the light phase in both hormone conditions, but EB decreased NREM duration compared with oil in the dark phase (ZT 12-ZT 21; F5,20 = 5.667, P = 0.002; Fig. 4B). Similarly, EB had no effect on wake duration in the light phase of the recovery while increasing it in the dark phase compared with oil (ZT 12-ZT 21; F5,20 = 9.309, P < 0.001; Fig. 4C). Both the decrease in NREM time and the increase in wake time in the dark were associated with decreased NREM bout number (hormone × time, F1,4 = 36.791, P = 0.004) and increased wake bout number (hormone × time, F1,4 = 47.947, P = 0.002; Table 2), respectively. EB thus appeared not to influence the acute SD-induced increase in NREM sleep time in the second half of the light phase; however, it did continue to suppress NREM sleep and promote wakefulness compared with oil in the dark phase.
When recovery begins in the dark phase, EB suppresses REM sleep until the next light phase.
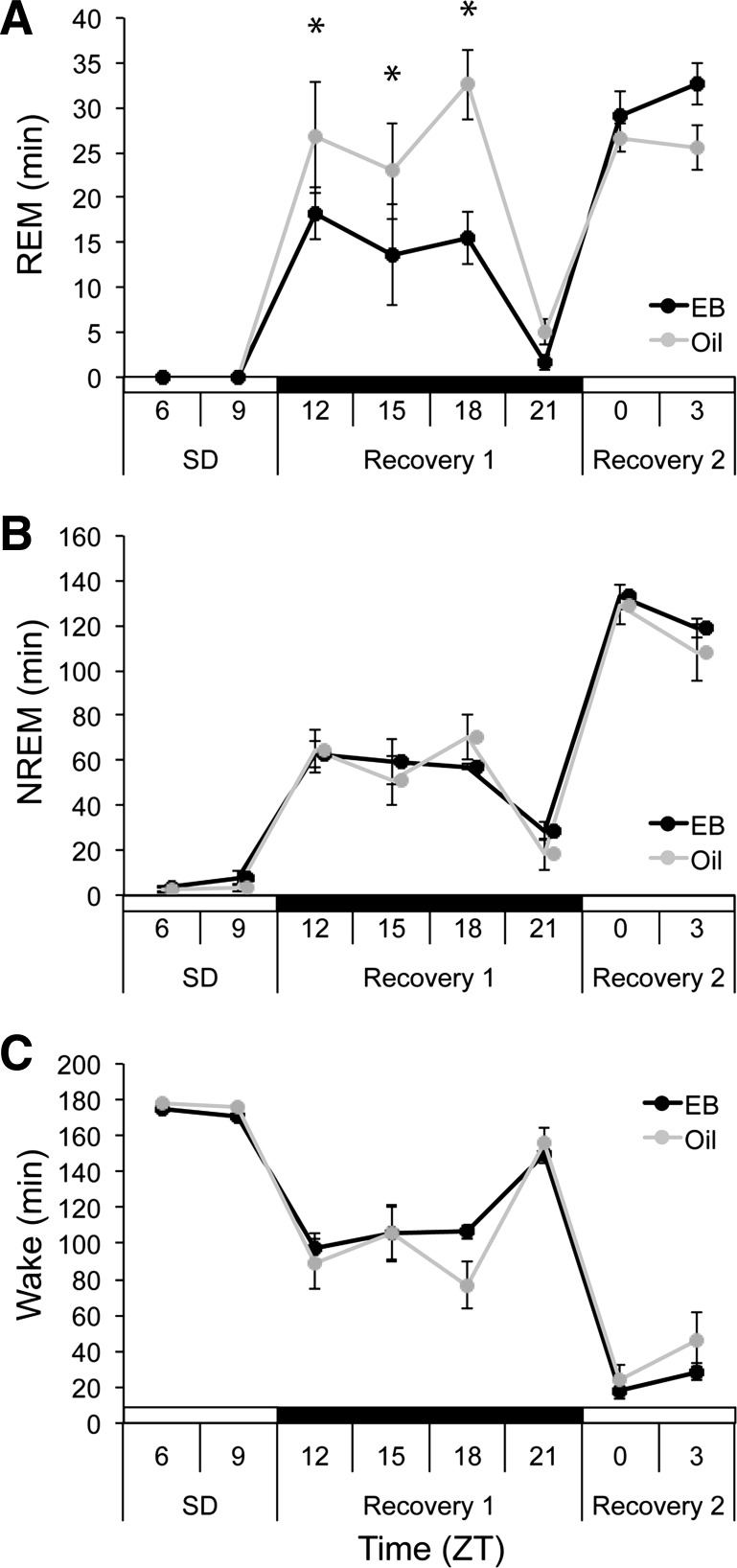
The Recovery-Dark cohort was sleep deprived in the second half of the light phase (ZT 6-ZT 12; n = 5), so that sleep recovery began at lights out (ZT 12). In these animals, SD eliminated 99.89% and 100% of baseline REM sleep in the EB- and oil-treatment conditions, respectively. During recovery from SD, EB decreased REM duration in the dark phase compared with oil (ZT 12-ZT 18; F5,20 = 4.272, P = 0.008; Fig. 5A) but had no effect on REM sleep in the light phase. EB also modulated REM bouts per hour (hormone × time, F1,4 = 7.785, P = 0.049; Table 2). While post hoc comparisons between EB and oil conditions were not significant, EB increased the difference in REM bouts per hour between light and dark phases compared with oil, consistent with the general trend for EB to decrease nighttime REM bout number. EB thus promoted a persistent weighting of REM recovery sleep to the daytime, despite the elevated need for REM sleep in the dark phase induced by the SD; by contrast, this rhythm was apparently blunted out when the same animals received oil, as was observed in the first cohort.
Fig. 5.
Total minutes spent in REM (A), NREM (B), and wake (C) during SD from ZT 6-ZT 12 and subsequent 18 h recovery in OVX rats treated with EB (black) or oil (gray). *P < 0.05 vs. oil.
SD eliminated 94.23% and 97.41% of baseline NREM sleep in the EB- and oil-treatment conditions, respectively. EB did not influence NREM recovery duration [hormone, F1,4 = 0.750, not significant (ns); hormone × time, F5,20 = 0.383, ns; Fig. 5B] nor did it affect wake duration (hormone, F1,4 = 0.907, ns; hormone × time, F5,20 = 0.122, ns; Fig. 5C). However, NREM duration during the dark phase was elevated in both groups relative to baseline values, consistent with a homeostatically driven increase in NREM sleep in both groups, independent of hormone treatment.
EB strengthens daily rhythms of REM sleep before and after SD.
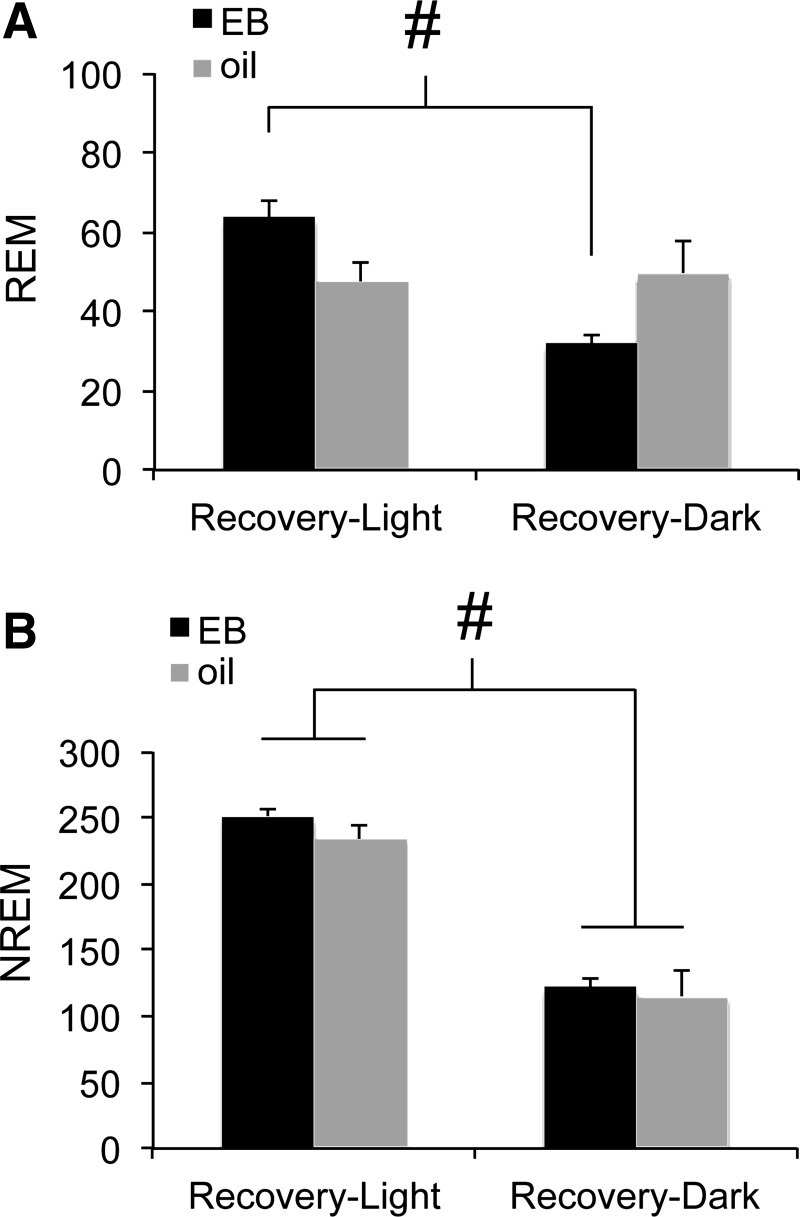
EB consistently promoted wakefulness and suppressed sleep in the dark (active) phase as previously reported (49), suggesting that daily sleep-wake rhythms were stronger in the presence of EB. If this is the case, then EB should suppress sleep in the dark compared with the light when homeostatic pressure is held constant. Figure 6 shows total REM (Fig. 6A) and NREM (Fig. 6B) sleep time for the first 6 h following SD for each cohort (ZT 6–12 for Recovery-Light, ZT 12–18 for Recovery-Dark). EB decreased REM sleep following SD in the Recovery-Dark animals compared with Recovery-Light (hormone × time; F1,8 = 10.35, P = 0.012), whereas oil-treated rats had similar REM sleep duration in both light and dark phases. Interestingly, REM duration in oil-treated rats was approximately midway between the high daytime and low nighttime values for EB rats, suggesting that EB acts to both promote REM sleep in the light and suppress it in the dark. By contrast, NREM recovery sleep time was always shorter in the dark than the light and was unaffected by hormone treatment (time F1,8 = 2351.755, P < 0.001; hormone F1,8 = 0.684, ns; hormone × time, F1,8 = 0.121, ns; Fig. 6B). Together with the consistent EB-mediated suppression of baseline and recovery sleep in the dark (Figs. 3–5), these results show that EB acts to promote the normal circadian expression of REM sleep across the day.
Fig. 6.
Total minutes spent in REM (A) and NREM (B) sleep during the first 6 h of recovery from each of the two sleep deprivation conditions in OVX rats treated with EB (black) or oil (gray). #P < 0.05 vs. Recovery-Dark.
Net gain in recovery sleep is not altered by EB.
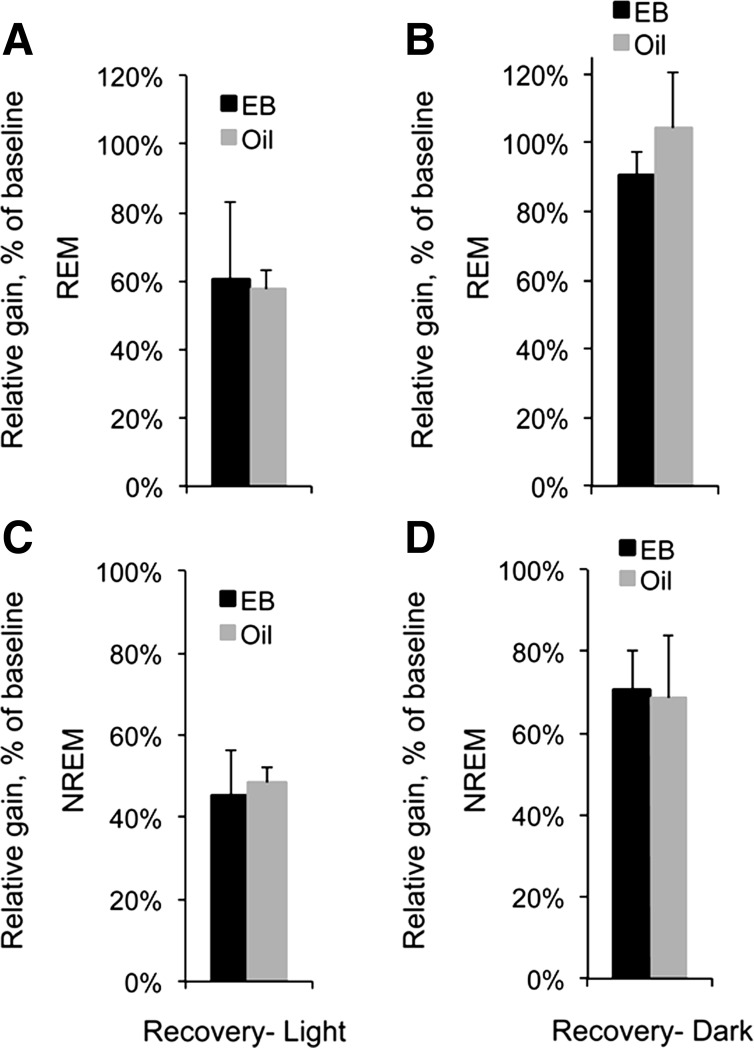
EB altered REM and NREM sleep duration at both baseline and recovery compared with oil, making it difficult to directly compare the effects of EB treatment on recovery sleep using absolute duration alone. Therefore, to determine whether total amounts of sleep recovered were different after EB and oil treatment, REM and NREM rebounds over the 18-h recovery period were assessed as a gain-loss ratio, where the net gain in sleep time over baseline in the first 18 h after SD was divided by the amount lost during SD (Fig. 7, A–D). SD elicited moderate to large gains in REM sleep and NREM sleep relative to baseline in both recovery cohorts and both hormone conditions. However, neither REM nor NREM gain-loss ratios for the 18-h recovery period were influenced by hormone condition when recovery began in the light phase (REM, t9 = 0.15, ns; NREM, t9 = 0.39, ns; Fig. 7, A and C) or the dark phase (REM, t9 = 1.14, ns; NREM, t9 = 0.10, ns; Fig. 7, B and D). Differences in the amounts of REM and NREM recovery sleep were therefore specific to the temporal profile of the recovery (Fig. 6), not the total amount of sleep recovered, suggesting that EB treatment did not influence the homeostatic need for REM or NREM sleep.
Fig. 7.
Gain-loss ratios for REM (A and B) and NREM sleep (C and D) in the 18-h recovery starting in the midlight phase (A, C) or at lights off (B, D) in OVX rats treated with EB (dark bars) or oil (light bars). Gain-loss ratios were calculated according to the formula (sleep gained over baseline/sleep lost during recovery).
EB modulates NREM delta power in a time-dependent manner.
Baseline and recovery NREM delta power was expressed as a percentage of the 24-h average NREM delta power on the baseline day. At baseline, EB significantly modulated NREM delta power compared with oil (hormone × time, F7,63= 4.197, P < 0.001; Fig. 8A). Specifically, EB suppressed NREM delta power at ZT 15 and ZT 18 and increased delta power at ZT 0 and ZT 6.
Fig. 8.
Normalized NREM delta power in OVX rats treated with EB (black) or oil (gray). For each individual under each hormone condition, total delta power per 3-h bin was normalized to the 24-h mean baseline delta power. Data are shown for baseline day (A), recovery starting in the light phase (B), and recovery starting in the dark phase (C). LD cycle is indicated on X-axis. *P < 0.05 vs. oil.
The phase-specific modulation of NREM delta power persisted in recovery sleep. In the Recovery-Light cohort EB increased NREM delta power at ZT 6 and suppressed it at ZT 12 and ZT 15 (hormone × time, F5,20 = 6.519, P = 0.001; Fig. 8B). In the Recovery-Dark cohort, there was a near-significant trend toward an effect of EB (hormone × time, F5,20= 2.329, P = 0.08; Fig. 8C), such that NREM delta power was slightly elevated in the light phase (ZT 0-ZT 6) compared with oil. While not statistically significant, this trend is consistent with the phase-specific effects seen at baseline and in the Recovery-Light cohort; namely that EB tended to increase NREM delta power in the light while decreasing it in the dark.
DISCUSSION
In the present study, we challenged OVX female rats treated with EB or oil with 6 h SD ending either in the midlight phase or at lights off and asked whether the sleep-suppressing effects of EB would depend on time of day. EB consistently facilitated REM recovery sleep in the light phase and suppressed REM recovery sleep in the dark phase compared with oil, regardless of whether that recovery began in the light or the dark. EB also increased NREM delta power during the light phase and decreased it in the dark compared with oil, both before and after SD. Together, these data strongly suggest that EB acts to consolidate the circadian sleep-wake rhythm.
Intact female rats suppress spontaneous REM and NREM sleep at proestrus, when circulating E2 levels increase up to fivefold over 24 h (12, 22, 50). Previously, we showed that capsule implants delivering low physiological doses of E2 were sufficient to suppress spontaneous REM sleep within 3 days of capsule implantation (49); however, spontaneous NREM sleep was not suppressed for another 24–48 h, raising the possibility that a higher E2 dose would have stronger baseline effects, or alternatively that additional hormones such as progesterone (7, 16) might be necessary to achieve the degree of suppression seen in intact proestrous rats (12, 22, 64). In the present study, sequential daily injections of 5 and 10 μg EB, administered without progesterone, closely approximated the rapid E2 increase at proestrus (56) and its subsequent decline ∼24 h later. This injection protocol also suppressed spontaneous REM and NREM sleep 24 h after the last injection, demonstrating that our acute EB protocol is sufficient to mimic the proestrous suppression of REM and NREM sleep in intact female rats (22, 50), and supporting the hypothesis that in intact rats this suppression is mediated directly by elevated E2 at proestrus. These data do not preclude the possibility that other ovarian steroids including progesterone, LH, and FSH may also act to regulate sleep. Nevertheless, the present data point clearly to estrogen signaling as a mechanism for sleep regulation.
Exogenous E2 began to suppress sleep ∼12–24 h after administration and continued to do so well into recovery, more than 36 h after the last injection. This time course is consistent with alterations in gene expression that are most likely mediated by activated estrogen receptors. The precise targets where E2 may influence sleep are still unknown and are likely to be diverse. However, several candidates have been documented. In the preoptic area, E2 downregulates the enzyme lipocalin-type prostaglandin D synthase (34); its product prostaglandin D2 promotes sleep (46, 48). The wake-promoting hypocretin system has recently been shown to be highly sensitive to fluctuations in endogenous and exogenous ovarian steroids (54, 55). E2 also increases wake-associated Fos expression in hypocretin neurons and in the histaminergic tuberomammillary nuclei (15), while decreasing Fos expression in the sleep-active ventrolateral preoptic area (15, 22). Thus exogenous E2 may influence sleep-wake state via coordinated action in multiple cell groups that modulate sleep-wake state in distinct ways.
As we and others have previously shown (7, 16, 49, 64), EB decreased spontaneous REM sleep during the dark phase while leaving it unaffected in the light phase. After 6 h SD, EB substantially altered the distribution of REM within the recovery so that it was increased in the light and decreased in the dark compared with oil (Fig. 6). This pattern persisted independently of the start time of the recovery when rats were given EB but was almost completely abolished when the same rats received oil. We therefore conclude that E2 potentiates and/or inhibits REM sleep in a time-dependent manner, leading to consolidation of the circadian rhythm in REM sleep.
Indeed, ovarian hormones, including E2, are known to influence circadian rhythms. Women have a significantly shorter circadian period than men (19) and exhibit phase-advanced endogenous temperature and melatonin rhythms (10). Women also exhibit a circadian rhythm in endothelial function that disappears at menopause (60). Similarly, in rodents, E2 shortens free-running period length and advances the daily activity onset (2, 14, 20, 36). This response appears to depend on exposure to the appropriate gonadal hormones in development (1, 8, 28). Ovarian steroids including E2 also modulate circadian gene expression in both central nervous system and the periphery (38, 39, 65). While E2 may not act directly on the circadian pacemaker itself in adult rats (40), our findings suggest that E2 contributes to circadian organization at the behavioral and physiological level.
At proestrus, sleep is strongly suppressed at the light-dark transition (22, 50). Accordingly, we selected the timing of the two SD and recovery conditions so that 1) rats would always be sleep deprived during the light phase, when sleep pressure is normally greatest, while 2) allowing observation of recovery starting before and after this transition. One potential problem with this design is that the Recovery-Light cohort was sleep deprived immediately after their 12-h long active phase, whereas the Recovery-Dark cohort was able to sleep for 6 h in the light phase before SD. The Recovery-Light rats thus may have begun SD with a larger sleep debt than the Recovery-Dark rats, which could account for the large REM rebound seen in the first 6 h of light phase recovery. Indeed, prior sleep-wake history can account for at least some day-night variations in recovery sleep parameters (59). However, EB and oil treatment resulted in similar net gains in REM sleep over the 18-h recovery period in both SD/recovery conditions (Fig. 7), and EB also suppressed REM recovery in the dark following 12 h SD starting at lights on (49). It is thus unlikely that EB attenuated REM homeostasis.
Although E2 reliably suppresses spontaneous NREM sleep in the dark phase, it has to date shown little effect on NREM recovery sleep. For example, NREM recovery duration did not differ between rats in estrus and proestrus (50), nor was it influenced by exogenous E2 (16). In the present study, EB suppressed NREM recovery sleep in the dark only after 6 h of recovery sleep in the light phase (Fig. 4B); when recovery started at lights off, there was no suppression of NREM sleep (Fig. 5B). Furthermore, neither the increase in NREM sleep in the first 6 h after SD, nor the net gain in NREM sleep over baseline, were influenced by EB in either SD condition. These data suggest two things. First, the acute homeostatic increase in NREM sleep in response to 6 h SD is preserved in OVX-E2-treated and intact proestrus rats. Second, under both baseline and recovery conditions, the opportunity to sleep in the light appears to be permissive for E2 to suppress NREM sleep in a later dark period; when this opportunity is blocked by SD, the suppressive effect of E2 is canceled by the expected NREM rebound. Consistent with this, NREM sleep is reduced in the dark phase in OVX-E2-treated mice (45) and rats (present study) following SD in the early light phase and recovery in the late light phase, whereas SD in the late light phase blocks the E2-induced suppression of NREM sleep (16) (present study). Together, these data suggest that E2 alters the temporal expression of NREM sleep, rather than altering its homeostatic regulation, yielding a larger impact on baseline NREM sleep than on its recovery.
Paradoxically, E2 previously suppressed NREM recovery sleep in the dark phase following 12 h SD ending at lights off (49). However, in that study both hormone treatment groups retained as much as 20% of their baseline NREM sleep during the 12 h SD; this residual sleep during the light could have relieved some of the accumulated homeostatic pressure and thereby reduced the magnitude of the rebound. An alternative explanation is that E2 may limit the magnitude of a rebound but not suppress it entirely. If this were the case, the magnitude of a NREM rebound would be predicted to increase in proportion to the duration of SD in oil-treated rats, whereas it would plateau in E2-treated rats resulting in suppression at longer durations of SD (e.g., 12 h) compared with oil but not at shorter durations such as 6 h. To our knowledge, no other studies have assessed the effects of exogenous E2 on recovery sleep following SD longer than 6 h, but our data (present study and Ref. 49) support this interpretation.
At baseline, EB increased NREM delta power in the light phase and decreased it in the dark, effectively amplifying the daily rhythm in delta power. This amplification was also seen in recovery sleep beginning in the light but not when recovery began in the dark (Fig. 8, B and C). Notably, EB neither increased nor attenuated the NREM delta rebound in the dark phase in the Recovery-Dark cohort, suggesting that the amplification seen at baseline and in the Recovery-Light condition was probably secondary to other effects on sleep (e.g., continuity). By contrast, E2 replacement was previously shown to attenuate the SD-induced increase in NREM delta power following 6 h of SD ending at lights off (16). Specifically, E2 treatment resulted in a more rapid return to baseline delta power, consistent with the possibility that E2 might limit recovery in the dark phase. If this were true, a longer SD should “unmask” the effect of E2 as described previously; indeed, following 12 h SD, E2 prevented a decline in NREM delta power at the end of a dark phase recovery period compared with oil (49). These studies, despite some differences in the specific effects, do consistently show two things: first, E2 tends to suppress basal NREM delta power in the dark; second, E2 does not block the response to strong homeostatic manipulations such as SD. Together, these findings suggest that EB may facilitate the normal increase in delta power in the light phase caused by buildup of sleep pressure in the dark phase, by consolidating waking in the dark and/or by facilitating sleep in the light.
Perspectives and Significance
Sleep is tightly regulated by the circadian clock in nearly all species studied to date, including humans (11, 32). Indeed, the dysregulation of internal circadian phase with respect to the external temporal environment (e.g., jet lag, shift work) is itself a circadian sleep disorder. While it is increasingly clear that gonadal hormones interact with each other to regulate sleep, it has proven difficult to tease apart exactly how and where in the central nervous system these effects are mediated. One obstacle is that the highly dynamic nature of the ovulatory cycle itself makes standardization of experimental designs, treatments, and outcomes difficult. In humans, moreover, sleep is highly consolidated, making it difficult to study circadian versus homeostatic effects without the aid of constant routine or forced desynchrony protocols. Here, using a rodent model of hormone removal and replacement, we have for the first time shown strong time-of-day modulation of both baseline (spontaneous) and recovery sleep, in the absence of strong modulation of homeostatic sleep regulation. These data offer a novel perspective on understanding and eventually treating sleep problems in women, as well as on the neural mechanisms that regulate sleep in general.
GRANTS
This research was supported by National Institutes of Health Grants 1RO1HL-85037 (J. A. Mong) and T32NS-007375 (to M. D. Schwartz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.S. conception and design of research; M.D.S. performed experiments; M.D.S. analyzed data; M.D.S. interpreted results of experiments; M.D.S. prepared figures; M.D.S. drafted manuscript; M.D.S. and J.A.M. edited and revised manuscript; M.D.S. and J.A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Michael Castello, Danielle Cusmano, Mary Holder, Joseph Sand, and Shaun Viechweg for technical assistance.
REFERENCES
- 1. Albers H. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol Regul Integr Comp Physiol 241: R62–R66, 1981 [DOI] [PubMed] [Google Scholar]
- 2. Albers HE, Gerall AA, Axelson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav 26: 21–25, 1981 [DOI] [PubMed] [Google Scholar]
- 3. Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med 8: 613–622, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflügers Arch 442: 729–737, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Borbely AA, Achermann P. Concepts and models of sleep regulation: an overview. J Sleep Res 1: 63–79, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Borbély AA. A two process model of sleep regulation. Hum Neurobiol 1: 195–204, 1982 [PubMed] [Google Scholar]
- 7. Branchey M, Branchey L, Nadler RD. Effects of estrogen and progesterone on sleep patterns of female rats. Physiol Behav 6: 743–746, 1971 [DOI] [PubMed] [Google Scholar]
- 8. Brockman R, Bunick D, Mahoney MM. Estradiol deficiency during development modulates the expression of circadian and daily rhythms in male and female aromatase knockout mice. Horm Behav 60: 439–447, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 94: 1704–1708, 1974 [DOI] [PubMed] [Google Scholar]
- 10. Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SBS, Santhi N, Schoen MW, Czeisler CA, Duffy JF. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms 25: 288–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev 8: 269–300, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Colvin GB, Whitmoyer DI, Lisk RD, Walter DO, Sawyer CH. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res 7: 173–181, 1968 [DOI] [PubMed] [Google Scholar]
- 13. Colvin GB, Whitmoyer DI, Sawyer CH. Circadian sleep-wakefulness patterns in rats after ovariectomy and treatment with estrogen. Exp Neurol 25: 616–625, 1969 [DOI] [PubMed] [Google Scholar]
- 14. Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol Regul Integr Comp Physiol 244: R93–R105, 1983 [DOI] [PubMed] [Google Scholar]
- 15. Deurveilher S, Cumyn EM, Peers T, Rusak B, Semba K. Estradiol replacement enhances sleep deprivation-induced c-Fos immunoreactivity in forebrain arousal regions of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 295: R1328–R1340, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Deurveilher S, Rusak B, Semba K. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep 32: 865–877, 2009 [PMC free article] [PubMed] [Google Scholar]
- 17. Deurveilher S, Rusak B, Semba K. Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep 34: 519–530, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Driver HS, Dijk DJ, Werth E, Biedermann K, Borbély AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab 81: 728–735, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Duffy JF, Cain SW, Chang AM, Phillips AJK, Münch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP, Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA 108, Suppl 3: 15602–15608, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fitzgerald K, Zucker I. Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci USA 73: 2923–2927, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gambacciani M, Ciaponi M, Cappagli B, Monteleone P, Benussi C, Bevilacqua G, Genazzani AR. Effects of low-dose, continuous combined estradiol and noretisterone acetate on menopausal quality of life in early postmenopausal women. Maturitas 44: 157–163, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Hadjimarkou M, Benham R, Schwarz J, Holder MK, Mong J. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci 27: 1780–1792, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci 31: 276–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kas MJ, Edgar DM. Circadian timed wakefulness at dawn opposes compensatory sleep responses after sleep deprivation in Octodon degus. Sleep 22: 1045–1053, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kleinlogel H. The female rat's sleep during oestrous cycle. Neuropsychobiology 10: 228–237, 1983 [DOI] [PubMed] [Google Scholar]
- 26. Koehl M, Battle SE, Turek FW. Sleep in female mice: a strain comparison across the estrous cycle. Sleep 26: 267–272, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, Sowers MR. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 31: 979–990, 2008 [PMC free article] [PubMed] [Google Scholar]
- 28. Lee TM, Hummer D, Jechura T, Mahoney MM. Pubertal development of sex differences in circadian function: an animal model. Ann NY Acad Sci 1021: 262–275, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Lund-Pero M, Jeppson B, Arneklo-Nobin B, Sjögren HO, Holmgren K, Pero RW. Non-specific steroidal esterase activity and distribution in human and other mammalian tissues. Clin Chim Acta 224: 9–20, 1994 [DOI] [PubMed] [Google Scholar]
- 30. McKnight-Eily LR, Liu Y, Perry GS, Presley-Cantrell LR, Strive TW, Lu H, Croft JB. Perceived Insufficient Rest or Sleep Among Adults - United States, 2008. Morbidity Mortality Weekly Rep 58: 1175–1179, 2009 [PubMed] [Google Scholar]
- 31. Merklinger-Gruchala A, Ellison PT, Lipson SF, Thune I, Jasienska G. Low estradiol levels in women of reproductive age having low sleep variation. Eur J Cancer Prev 17: 467–472, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Mistlberger R. Circadian regulation of sleep in mammals: Role of the suprachiasmatic nucleus. Brain Res Rev 49: 429–454, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Moline M, Broch L, Zak R, Gross V. Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev 7: 155–177, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Mong J, Devidze N, Frail D, O'Connor L, Samuel M, Choleris E, Ogawa S, Pfaff DW. Estradiol differentially regulates lipocalin-type prostaglandin D synthase transcript levels in the rodent brain: Evidence from high-density oligonucleotide arrays and in situ hybridization. Proc Natl Acad Sci USA 100: 318–323, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moore-Ede M, Sulzman F, Fuller C. The Clocks That Time Us: Physiology Of The Circadian Timing System. Cambridge MA: Harvard University Press, 1982 [Google Scholar]
- 36. Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science 196: 305–307, 1977 [DOI] [PubMed] [Google Scholar]
- 37. Murphy PJ, Campbell SS. Sex hormones, sleep, and core body temperature in older postmenopausal women. Sleep 30: 1788–1794, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakamura T, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res 82: 622–630, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Nakamura TJ, Sellix MT, Kudo T, Nakao N, Yoshimura T, Ebihara S, Colwell CS, Block GD. Influence of the estrous cycle on clock gene expression in reproductive tissues: effects of fluctuating ovarian steroid hormone levels. Steroids 75: 203–212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakamura TJ, Sellix MT, Menaker M, Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab 295: E1025–E1031, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. National Sleep Foundation 2007 Women and Sleep | National Sleep Foundation-Information on Sleep Health and Safety. 2008 [Google Scholar]
- 42. Ocampo-Garcés A, Molina E, Rodríguez A, Vivaldi EA. Homeostasis of REM sleep after total and selective sleep deprivation in the rat. J Neurophysiol 84: 2699–2702, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Oriowo MA, Landgren BM, Stenström B, Diczfalusy E. A comparison of the pharmacokinetic properties of three estradiol esters. Contraception 21: 415–424, 1980 [DOI] [PubMed] [Google Scholar]
- 44. Paul KN, Dugovic C, Turek FW, Laposky AD. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep 29: 1211–1223, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Paul KN, Laposky AD, Turek FW. Reproductive hormone replacement alters sleep in mice. Neurosci Lett 463: 239–243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qu WM, Huang ZL, Xu XH, Aritake K, Eguchi N, Nambu F, Narumiya S, Urade Y, Hayaishi O. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci USA 103: 17949–17954, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saletu B, Anderer P, Gruber D, Metka M, Huber J, Saletu-Zyhlarz GM. Hormone replacement therapy and vigilance: double-blind, placebo-controlled EEG-mapping studies with an estrogen-progestogen combination (Climodien, Lafamme) versus estrogen alone in menopausal syndrome patients. Maturitas 43: 165–181, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci USA 95: 7754–7759, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwartz MD, Mong JA. Estradiol suppresses recovery of REM sleep following sleep deprivation in ovariectomized female rats. Physiol Behav 104: 962–971, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schwierin B, Borbely AA, Tobler I. Sleep homeostasis in the female rat during the estrous cycle. Brain Res 811: 96–104, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Schwierin B, Borbely AA, Tobler I. Prolonged effects of 24-h total sleep deprivation on sleep and sleep EEG in the rat. Neurosci Lett 261: 61–64, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Shea JL, Mochizuki T, Sagvaag V, Aspevik T, Bjorkum AA, Datta S. Rapid eye movement (REM) sleep homeostatic regulatory processes in the rat: changes in the sleep-wake stages and electroencephalographic power spectra. Brain Res 1213: 48–56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep 33: 647–656, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Silveyra P, Catalano PN, Lux-Lantos V, Libertun C. Impact of proestrous milieu on expression of orexin receptors and prepro-orexin in rat hypothalamus and hypophysis: actions of Cetrorelix and Nembutal. Am J Physiol Endocrinol Metab 292: E820–E828, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Silveyra P, Cataldi NI, Lux-Lantos V, Libertun C. Gonadal steroids modulated hypocretin/orexin type-1 receptor expression in a brain region, sex and daytime specific manner. Regul Pept 158: 121–126, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96: 219–226, 1975 [DOI] [PubMed] [Google Scholar]
- 57. Soares CN. Insomnia in women: an overlooked epidemic? Arch Womens Ment Health 8: 205–213, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Sowers MR, Zheng H, Kravitz HM, Matthews K, Bromberger JT, Gold EB, Owens J, Consens F, Hall MH. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh sleep quality index. Sleep 31: 1339, 2008 [PMC free article] [PubMed] [Google Scholar]
- 59. Vyazovskiy VV, Achermann P, Tobler I. Sleep homeostasis in the rat in the light and dark period. Brain Res Bull 74: 37–44, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Walters JF, Hampton SM, Deanfield JE, Donald AE, Skene DJ, Ferns GAA. Circadian variation in endothelial function is attenuated in postmenopausal women. Maturitas 54: 294–303, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Wright K, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res 103: 185–194, 1999 [DOI] [PubMed] [Google Scholar]
- 62. Wurts SW, Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci 20: 4300–4310, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yamaoka S. Participation of limbic-hypothalamic structures in circadian rhythm of slow wave sleep and paradoxical sleep in the rat. Brain Res 151: 255–268, 1978 [DOI] [PubMed] [Google Scholar]
- 64. Yamaoka S. Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res 185: 385–398, 1980 [DOI] [PubMed] [Google Scholar]
- 65. Yoshikawa T, Sellix M, Pezuk P, Menaker M. Timing of the ovarian circadian clock is regulated by gonadotropins. Endocrinology 150: 4338–4347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep 29: 85–93, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Zhang JH, Sampogna S, Morales FR, Chase MH. Distribution of hypocretin (orexin) immunoreactivity in the feline pons and medulla. Brain Res 995: 205–217, 2004 [DOI] [PubMed] [Google Scholar]