Abstract
Shifting the onset of light, acutely or chronically, can profoundly affect responses to infection, tumor progression, development of metabolic disease, and mortality in mammals. To date, the majority of phase-shifting studies have focused on acute exposure to a shift in the timing of the light cycle, whereas the consequences of chronic phase shifts alone on molecular rhythms in peripheral tissues such as skeletal muscle have not been studied. In this study, we tested the effect of chronic phase advance on the molecular clock mechanism in two phenotypically different skeletal muscles. The phase advance protocol (CPA) involved 6-h phase advances (earlier light onset) every 4 days for 8 wk. Analysis of the molecular clock, via bioluminescence recording, in the soleus and flexor digitorum brevis (FDB) muscles and lung demonstrated that CPA advanced the phase of the rhythm when studied immediately after CPA. However, if the mice were placed into free-running conditions (DD) for 2 wk after CPA, the molecular clock was not phase shifted in the two muscles but was still shifted in the lung. Wheel running behavior remained rhythmic in CPA mice; however, the endogenous period length of the free-running rhythm was significantly shorter than that of control mice. Core body temperature, cage activity, and heart rate remained rhythmic throughout the experiment, although the onset of the rhythms was significantly delayed with CPA. These results provide clues that lifestyles associated with chronic environmental desynchrony, such as shift work, can have disruptive effects on the molecular clock mechanism in peripheral tissues, including both types of skeletal muscle. Whether this can contribute, long term, to increased incidence of insulin resistance/metabolic disease requires further study.
Keywords: phase shift, tau, bmal1, period2::luciferase, molecular clock, chronic jet lag, shift work
acute shifts of the light-dark (LD) cycle have been associated with enhanced tumor progression, impaired immune response, cardiovascular pathologies, and disrupted pregnancy in experimental animals (10, 22, 50, 51). In humans, exposure to scheduled shifts in the LD cycle either acutely or chronically can have deleterious effects on health and well being, including elevated insulin and glucose values, increased mean arterial pressure, and risk for cardiovascular disease (20, 35, 48). Most dramatically, repeated exposure to shifting LD cycles can lead to premature death in aged mice (16). Davidson et al. observed that aged mice exposed to repeated advances in the onset of the LD cycle had an increased mortality rate compared with age-matched mice exposed to chronic delays in the LD cycle or control mice not exposed to LD shifts (16). Evidence from this study and others (46) suggests that phase advance (earlier onset of light) is more detrimental than phase delay (later onset of light) and that phase advances require more than 4 days for locomotor behavior (in mice) to adjust to the new lighting schedule (45, 61).
Shifts in the lighting schedule strongly influence the expression of daily rhythms in physiology and behavior, which are regulated by the circadian timing system (1, 41, 42). The circadian timing system includes a master pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus that is endogenously rhythmic under constant conditions but is also sensitive to changes in environmental time cues, especially the LD cycle, and is capable of transmitting rhythmic output signals to many tissues and organs (19, 30, 44). In addition to the SCN, virtually all cells in the body possess intrinsic circadian oscillators (collectively called peripheral oscillators), whose functions and mechanisms of regulation are just beginning to be understood (61, 63). These peripheral oscillators respond to signals from the master pacemaker but have also been shown to respond to other environmental cues, such as time of feeding or time of physical activity, independent of the LD cycle (13, 18, 58). At the core of the central and peripheral oscillators is the ubiquitous molecular clock mechanism that is based on a transcription-translational feedback system consisting of the circadian genes Brain and Muscle Arnt-like 1 (Bmal1), Circadian Locomotor Output Cycles Kaput (Clock), Period 1 and 2 (Per1 and Per2), and Cryptochrome 1 and 2 (Cry1 and Cry2) (3, 5, 8, 32).
Following phase advances of the LD cycle, molecular clocks may require several days to adjust (61). Studies have shown that the central molecular clock, located in the SCN, and peripheral clocks adjust to phase shifts of the LD cycle at different rates (14, 61). Using a firefly luciferase reporter rat, Yamazaki et al. demonstrated that, after an acute 6-h phase advance, the rhythm of expression of a molecular clock gene (Per1) in the rat SCN adjusted rapidly (within 1 day) to the new LD cycle, wheras the Per1 expression rhythms in skeletal muscle, liver, and lung were not fully shifted until day 6 (61). Age also affects the ability of the clocks to adjust to phase shifts; the central and peripheral clocks synchronize to the new LD cycle more quickly in young rats than in old ones (18). It is thought that a primary function of circadian oscillators is to maintain synchronization between the environment and behavioral and physiological rhythms by allowing the organism to anticipate daily and seasonal environmental changes (42). In contrast to gradual, seasonal changes in natural photoperiod, which have persisted throughout the ages, life in the modern era also includes frequent abrupt changes in photoperiod as a consequence of shift work and jet travel. Some evidence suggests that these abrupt changes may be deleterious, but the effect of frequent, repeated shifting of the LD cycle on physiological rhythms or on the molecular clock in peripheral tissues, such as skeletal muscle, has not been much explored (11, 31).
The goal of this study was to investigate the effects of frequent, abrupt photoperiodic changes on physiological and behavioral rhythms and on the molecular rhythms in peripheral tissues as well as the SCN. The hypothesis was that chronic phase advance (CPA) caused by advancing the time of lights-on by 6 h every 4 days for 8 wk would produce disrupted wheel running rhythms and phase shifts in the rhythms of PER2 expression in the SCN as well as peripheral oscillators including two phenotypically different skeletal muscles and the lung. We also hypothesized that CPA would alter circadian rhythms in core body temperature (Tb), heart rate (HR), and locomotor activity, as well as physiological outcomes such as tissue mass, blood glucose levels, and muscle force production. SCN, skeletal muscles, and lung explants from Per2::Luciferase (Per2::Luc) mice (63) were used to measure real-time molecular clock expression in vitro. Transmitter telemetry units were implanted to measure rhythms in locomotor cage activity, body temperature, and HR. Additionally, blood glucose, body/tissue mass, muscle function, and wheel running characteristics were measured to determine whether CPA affects these physiological outcomes. We report that CPA for 8 wk alters locomotor wheel running behavior (free-running period), HR, and body temperature rhythms as well as the phase of Per2::Luc expression in the peripheral clocks, but without detectable changes in the physiological outcomes stated above.
MATERIALS AND METHODS
Animals.
Twelve male and female C57BL/6 and 26 male and female Per2::Luc mice on a C57BL/6 background (63) ∼6 mo of age were housed individually in plastic cages measuring 30.5 × 15.2 × 12.7 cm. Bmal1−/− mice were included in this study as a positive control for arrhythmic locomotor rhythms and attenuated muscle function. Due to the absence of a non-redundant clock gene, Bmal1−/− mice (n = 5) display a loss of locomotor rhythms under an LD cycle and in constant darkness (DD); these mice also have impaired muscle function (2, 8). Male and female mice were equally distributed among the groups in this study. We did not observe any statistical differences between males and females in the circadian variables measured. All mice were housed singly in cages equipped with a running wheel and enclosed in a light-controlled box with constant air exchange and ad libitum access to mouse chow and water. The lighting schedule was 12 h of light and 12 h of dark (12L:12D; lights on at 8AM EST) except as otherwise indicated. The light source was green LEDs that provided ∼200 lux. At the conclusion of the study, mice were anesthetized with isoflurane followed by decapitation. All procedures, which complied with the guidelines of the American Association for Accreditation of Animal Care (AAALAC) were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Transmitter implantation.
Per2::Luc mice (n = 5) were anesthetized using isoflurane, and transmitter units (PhysioTel ETA-F10, Data Sciences) were implanted surgically in the peritoneal cavity under aseptic conditions. The two electrocardiography (ECG) leads were secured near the apex of the heart and the right acromion. Subcutaneous injections of the analgesic carprofen (10 mg/kg) were administered at the end of surgery and every 12 h for the next 48 h. Mice were housed singly to allow recovery for 3 wk under a 12L:12D lighting schedule before beginning the CPA protocol (described below). ECG, body temperature, and locomotor data were collected and analyzed using Dataquest ART4.1 telemetry software (Data Sciences). Onset of physiological measurements from the transmitters was defined as 12 h of increased values preceded by 12 h of lower values and was measured using ClockLab (Actimetrics, Wilmette, IL). ClockLab determines onsets using an activity pattern template. Mean activity is subtracted from each data point over the day, and a template is created where activity below the 20th percentile is given a negative value and above is assigned a positive value. The point that has the highest matching (12 h of negative followed by 12 h of positive) is denoted as the onset. Since physiological data are not “on” or “off,” we used 12-h onsets to find a pattern of lower and higher values because, during rest, there is still a signal. For activity data (described below), we used 6 h on/6 h off because locomotor activity patterns display clear rest and active patterns. Physiological measurements including body temperature (Tb), HR, and locomotor activity were measured before CPA began and at the end of the study to compare longitudinal changes in physiological rhythms. To identify the relationship between the timing of the rhythms studied and the lighting cycle, the phase angle was determined as the difference between the onset of wheel running, locomotor cage activity, Tb, and HR rhythms and the time of lights-off. The phase angles exhibited before CPA and during the last four shifts of the CPA protocol were determined for mice that were not released into DD. Changes in phase angle represent alterations in the adjustment or re-entrainment of a circadian rhythm.
CPA schedule without DD.
After the 2-wk acclimation period, the transmitter-implanted mice (Per2::Luc) were either exposed to a 6-h advance in the times of lights-on and lights-off every 4 days for 8 wk (CPA, n = 5) or exposed to the same 12L:12D schedule for 8 wk (control; n = 5). On the day following the final phase shift, the mice were euthanized 10 h before lights-off (activity onset for nocturnal animals). Physiological data (activity, HR, and Tb) were not included from control mice because two mice died following complications from surgery, and the transmitters from the remaining three mice malfunctioned such that data could not collected. However, the control mice (n = 3) that exhibited wheel running rhythms and tissues from these mice were included in the bioluminescence studies.
CPA schedule with DD.
Mice were acclimated as described above, then randomly assigned to either the control group (C57BL/6, n = 6; Per2::Luc, n = 8) or the CPA group (C57BL/6, n = 6; Per2::Luc, n = 8). The lighting schedule was the same as described above. After the final phase shift, the mice were released into DD for 2 wk. The timing of euthanasia and tissue collection for the mice in DD was determined from activity onset [defined by convention as circadian time 12 (CT12)] on the day before collection. Activity onset was determined for each mouse individually by visual inspection and defined by ClockLab analysis software as a period of 6 h of activity that was preceded by 6 h of inactivity. Further description of ClockLab analysis is provided above. All tissues (from DD mice) were collected under dim red light at CT2 (i.e., 10 h before activity onset). Before euthanasia, body weight for all of the mice was obtained.
Wheel activity monitoring.
Voluntary wheel running was continuously recorded and monitored throughout the experiment using ClockLab hardware and software. Activity was evaluated using voluntary running wheel rotations plotted in 1-min bins. The free-running period (tau) during the 2 wk in DD was calculated using periodogram analysis.
Explant cultures.
Explants were taken from the Per2::Luc reporter mice and placed in culture using methods reported previously (58, 61–63). Briefly, the brain was removed and placed in chilled Hanks' balanced salt solution supplemented with 25 U/ml penicillin, 25 μg/ml streptomycin (Invitrogen), 10 mM HEPES (Sigma), and 4 mM NaHCO3 (Fisher Scientific). The brain was sectioned using a vibrating microtome (Vibratome series 1000 EM, Chestnut Hill, MA). The SCN was dissected and placed on a Millicell insert (Millipore) in a 35-mm tissue culture dish (Sigma) containing 1 ml of DMEM without phenol red (Invitrogen) supplemented with 4 mM NaHCO3, 10 mM HEPES, 25 U/ml penicillin, 25 μg/ml streptomycin, 3.5 g of d-glucose (Sigma), 2% B27 (Gibco), and 0.1 mM d-luciferin firefly, potassium salt (Biosynth). Soleus (oxidative slow-twitch muscle), flexor digitorum brevis (FDB; glycolytic fast-twitch muscle), and one other peripheral tissue (lung) were hand sliced in dissection media and cultured in the same culture media containing 5% FBS (Invitrogen). Tissue bioluminescence was measured using a LumiCycle (Actimetrics, Wilmette, IL) housed in a light-tight, water-jacketed incubator at 36.5°C. LumiCycle software was used to collect raw bioluminescence data in 1.2-min bins every 10 min that was stored on an attached computer. Similar to what others have published (39, 63), the raw data were smoothed by 0.5-h adjacent averaging using LumiCycle analysis software. Baseline-subtracted data were then used to calculate phase of the bioluminescence rhythm, using ClockLab. The phase was measured as the time of the first peak of the PER2::LUC rhythm after 24 h in culture. The period length and phase of the rhythms of luciferase expression over 6 days in vitro were determined.
Physiological outcome measures.
Blood glucose measurements were made with a Freestyle Flash glucometer (Abbott) in mid-light cycle (ZT6) following 8 wk of CPA before entering DD. Similar to what has been previously published (21), ZT 6 was chosen as the sample time because mice usually have lower locomotor and drinking activity during lights-on (34, 36). Specific muscle force was measured as published previously (24, 28). Briefly, extensor digitorum longus muscles (EDL) were excised and placed in Krebs-Ringer solution containing 137 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM NaH2PO4, 24 mM NaHCO3, 2 mM CaCl2 equilibrated with 95% O2-5% CO2 (pH 7.4). The EDL was attached to a force transducer (BG Series 100 g, Kulite, Leonia, NJ), and electrical field stimulation (Grass S48, Quincy, MA) was applied. Maximal force measurements were recorded using an oscilloscope (546601B; Hewlett-Packard, Palo Alto, CA). Muscle length and weight were measured to determine cross-sectional area (CSA) so that force/CSA could be calculated.
Statistics.
To determine the period length of the wheel running rhythm, χ2 periodograms were calculated using ClockLab software. Physiological data (Table 1) from the CPA, control, and Bmal1−/− groups were analyzed using ANOVA. Free-running periods that were calculated using ClockLab were analyzed using Student's t-test between groups within time grouping (Fig. 1G). The phase of the bioluminescence rhythms were analyzed using two-way ANOVA with post hoc Bonferroni for soleus and lung, and Student's t-test was used for FDB and SCN. Phase angle data were analyzed using paired Student's t-test, pre- vs. post-CPA. For all tests and χ2 periodogram analyses, the significance level was defined as α = 0.05.
Table 1.
Characteristics of mice on CPA protocol compared with control and Bmal1−/− mice
| Control | CPA | Bmal1−/− | |
|---|---|---|---|
| Tissue mass | |||
| Body mass, g | 29.6 ± 6.3 | 29.1 ± 4.1 | 20.1 ± 2.2* |
| Heart mass, g | 0.148 ± 0.017 | 0.139 ± 0.015 | 0.119 ± 0.014* |
| Heart mass/body mass | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.006 ± 0.001* |
| Running measures | |||
| Average time run, h/day | 10.39 ± 2.71 | 9.74 ± 2.01 | 1.31 ± 0.65* |
| Average distance, km/day | 10.31 ± 3.10 | 11.92 ± 4.00 | 0.25 ± 0.15* |
| Physiological measures | |||
| Force/CSA, N/cm2 | 24.95 ± 2.69 | 23.65 ± 3.44 | 20.01 ± 3.20* |
| Blood glucose, mg/dl | 138.1 ± 30.1 | 140.9 ± 35.3 | N/A |
Values are means ± SD. Chronic phase advance (CPA) did not alter basic characteristics or physiological outcomes. Bmal1−/− mice have significantly lower body and tissues mass as well as decreased activity compared with control. Muscle function in CPA mice measured as maximal force/cross-sectional area (CSA) in the extensor digitorum longus muscle was also similar to control, whereas Bmal1−/− mice produced significantly less force/CSA than control. Blood glucose levels were also similar between control and CPA.
Statistically different from control (P < 0.05; ANOVA, post hoc Tukey's test).
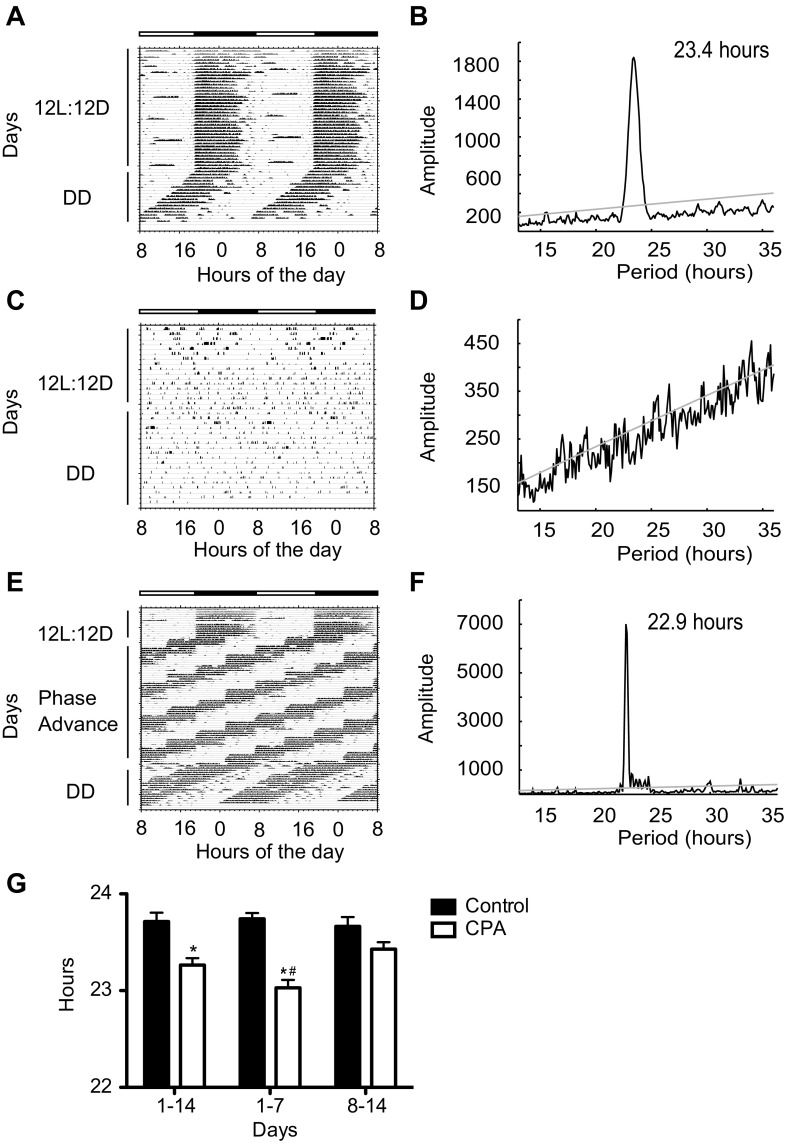
Fig. 1.
Wheel running rhythms in chronic phase advance (CPA) and control mice. Representative double-plotted actograms of voluntary wheel running behavior for control, Bmal1−/−, and CPA. Control mice (A) and Bmal−/− mice (C) were exposed to 12-h light/12-h dark (12L:12D) for 8 wk and then to complete darkness (DD) for 2 wk. The CPA group (E) was placed on a phase-shifting schedule consisting of a 6-h advance in time of lights-on every 4 days for 6 wk (phase advance) then released into DD for 2 wk. Dark horizontal bars indicate time of lights-off for control and Bmal1−/− for the entire study and the first 2 wk for the CPA group. Representative χ2 periodograms generated with ClockLab are shown for control (B), Bmal1−/− (D), and CPA (F), with significance level (α = 0.05) represented by the gray line. The prominent peaks in B and F demonstrate the presence of circadian rhythms with the period lengths indicated. In D, the absence of a single prominent peak shows that the activity does not represent a circadian rhythm (G). The average free-running period (tau) is shown for all control and CPA mice. Values are means ± SE (control, n = 14; CPA, n = 14; Bmal1−/−, n = 5). *Significant difference by Student's t-test between groups within day-group (P < 0.05). #Significant difference by Student's t-test between CPA 1–7 and CPA 8–14 (P < 0.05).
RESULTS
Effect of CPA on basic physiological characteristics and outcomes.
Table 1 provides a summary of body mass, tissue mass, blood glucose, muscle function, and wheel running characteristics for 6-mo-old control, CPA, and Bmal1−/− mice. Mice exposed to CPA were similar to control mice of the same age for all variables measured. Bmal1−/− mice were used as an established model of arrhythmic behavior to provide reference for the physiological and behavioral data in this study. Bmal1−/− mice, 6 mo of age, had significantly lower body and tissue mass and ran minimally compared with control mice, as previously reported (2, 8). Muscle function was not different in the CPA mice compared with controls; however, the EDL muscles of Bmal1−/− mice displayed deficits in force production as previously reported (2). The data in Table 1 demonstrate that 8 wk of CPA did not alter any general physiological outcomes, including body mass, tissue mass, blood glucose, muscle function, and running characteristics.
Effects of CPA on the wheel-running rhythm.
Figure 1, A–F, contains representative wheel-running actograms and χ2 periodograms for control, CPA, and Bmal1−/− mice. Control mice maintained a running pattern in phase with the 12L:12D schedule, remaining active almost exclusively in the dark, as is expected for nocturnal rodents, and once released into darkness (DD) had an earlier onset of wheel-running activity each day and a free-running period (tau) <24 h (Fig. 1, A and B) consistent with the C57BL6 strain (49, 54). In contrast to the control mice, the Bmal1−/− mice ran for less time and shorter distances, and displayed scattered running behavior (Fig. 1C), and although several peaks crossed the gray significance line, no high-amplitude signal or discernable rhythm under 12L:12D or DD conditions could be found (Fig. 1D). All CPA mice showed robust wheel-running rhythms with a daily onset of activity at the time of lights-off on the day of phase advance under 12L:12D conditions (labeled “phase advance”; Fig. 1E) and remained rhythmic in DD (labeled “DD”; Fig. 1, E and F).
CPA did affect the endogenous period length (tau) assessed during DD (Fig. 1G). The average free-running period was significantly shorter for CPA mice compared with controls after the first week (23.03 ± 0.08 vs. 23.74 ± 0.06 h) and when analyzed over both weeks (23.26 ± 0.07 vs. 23.71 ± 0.09 h) (CPA vs. control, respectively; P < 0.05; means ± SE). However, during the second week of DD, there was no significant difference in the period lengths exhibited by the CPA mice (23.43 ± 0.07 h) and control mice (23.66 ± 0.09 h) in DD. (Because Bmal1−/− mice have no measurable behavioral rhythm, tau cannot be calculated.) The data presented in Fig. 1 provide evidence that the CPA imposed did not cause the mice to behave arrhythmically but did shorten tau under free-running conditions, suggesting a change at the level of the molecular clock in the SCN.
Effect of CPA on HR and body temperature rhythms.
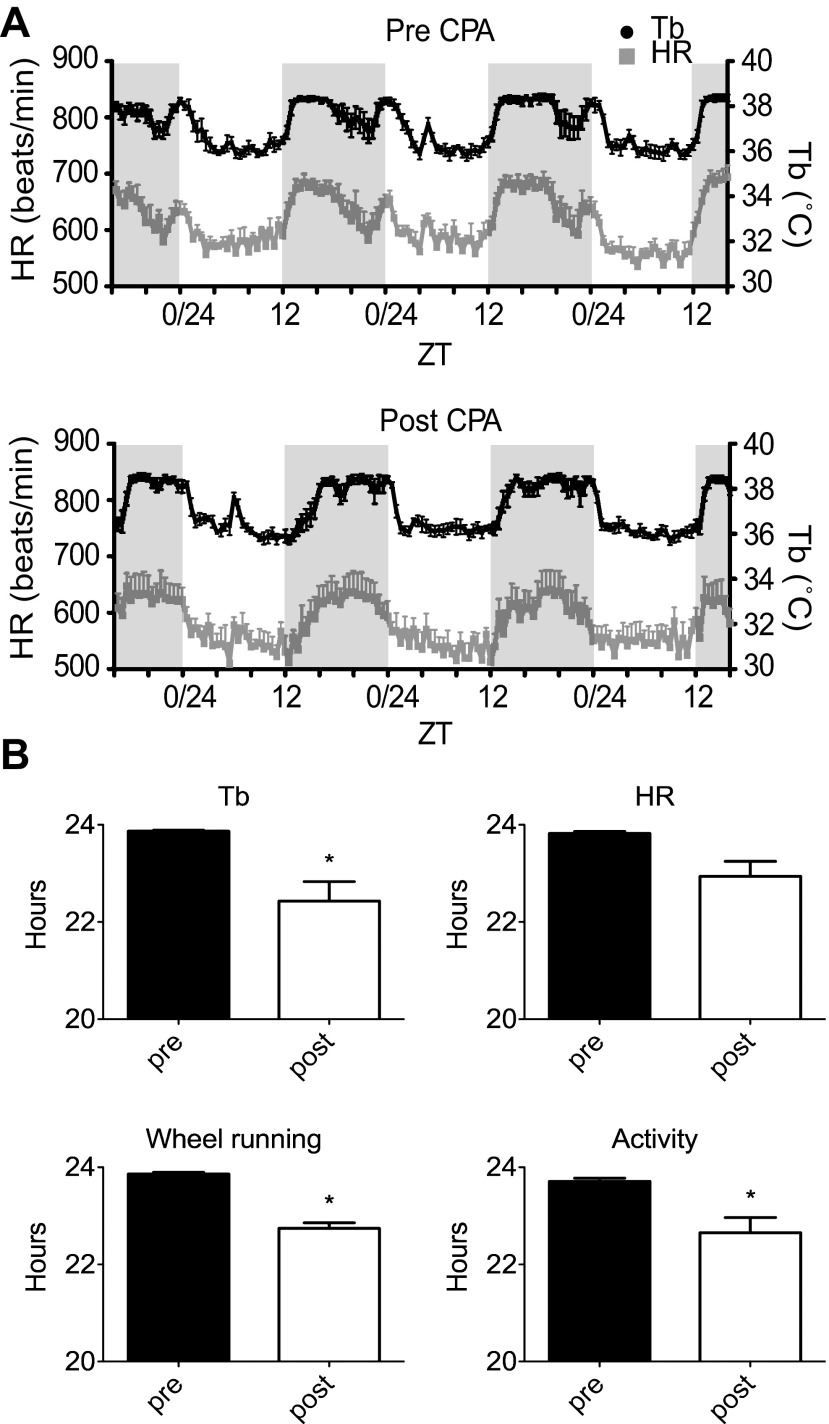
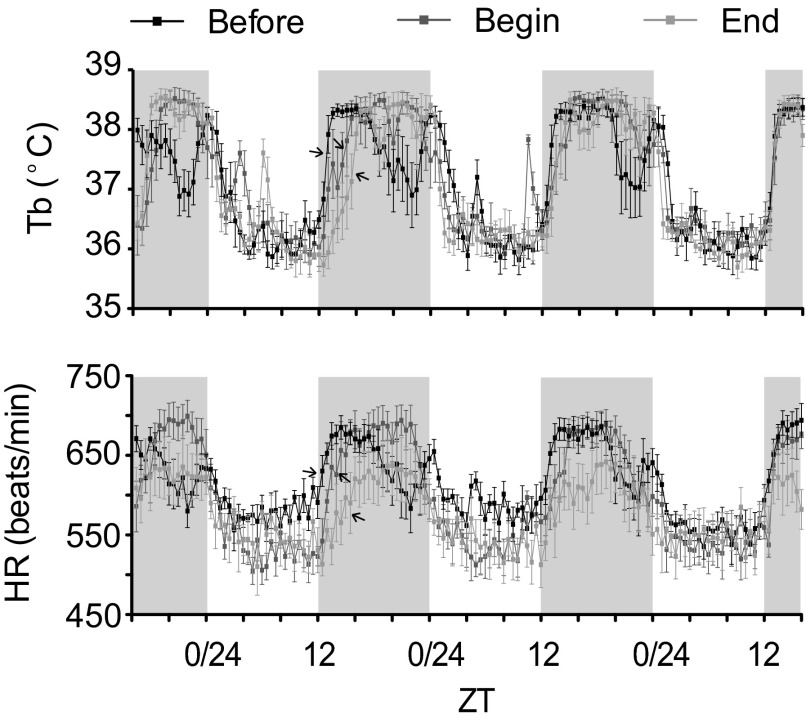
Tb and HR are physiological parameters that are largely regulated through central autonomic mechanisms and are well known to exhibit circadian oscillations (6, 27, 57). This series of experiments used in vivo telemetry to monitor the effect of CPA on the pattern of Tb and HR responses. We examined the data before CPA (pre-CPA, Before), following the first 6-h phase advance at the beginning (acute measurement) of the phase advancing protocol (Begin), and at the conclusion of CPA (post-CPA, End). In Fig. 2, we measured phase angle, which was calculated on day 2 of the shift at the conclusion of CPA relative to the time of lights-off, for locomotor activity (wheel and cage), Tb, and HR. As seen in Fig. 2, all measures showed a delay of ∼2 h on the day after the phase advance compared with preshift responses. It was interesting to note that these rhythms also showed other changes at this time, including a slowing, more gradual rising phase and, in the case of the body temperature rhythm, a faster, more abrupt falling phase. By days 3 and 4 post-CPA, the phase angles of the body temperature and HR rhythms were not different from pre-CPA (data not shown), suggesting that the rhythms had adjusted to the new LD cycle within 72 h. We also found that acute phase advance (Begin), determined after the first phase advance, produced a phase delay in the onset of Tb and HR rhythms that was between the Before measurement, before phase advancing began, and the End measurement, which was measured at the end of CPA (see Fig. 3). These observations suggest that, in young adult mice, physiological rhythms were affected similarly after a single shift compared with chronic phase shifting. Mean HR or Tb were not different between groups (data not shown).
Fig. 2.
Body temperature (Tb) and heart rate (HR) rhythms before and after CPA. A: mean Tb and HR measured over 3 days before CPA (pre-CPA) and at the end of the protocol (post-CPA). B: phase angles measured for wheel running onset, locomotor cage activity onset, HR onset, and Tb onset relative to lights-off. The pre values (black bars; n = 5) were calculated from the mean onset before CPA, and the day 2 post values (open bars; n = 5) were calculated from the mean onset on day 2 of the shift using the last four shifts of the protocol. Values are means ± SE. *Significant difference (paired Student's t-test, pre- and post-CPA; P < 0.05).
Fig. 3.
Body temperature and heart rate (HR) rhythms acute vs. chronic phase shifting. Mean body temperature (Tb) and HR measured over 3 days before CPA (Before), after the first phase advance (Begin), and at the end of the protocol (End) (n = 5). The arrows point to the areas of the curves where HR and Tb are rising following lights-off. Values are means ± SE.
Effects of CPA on molecular rhythms.
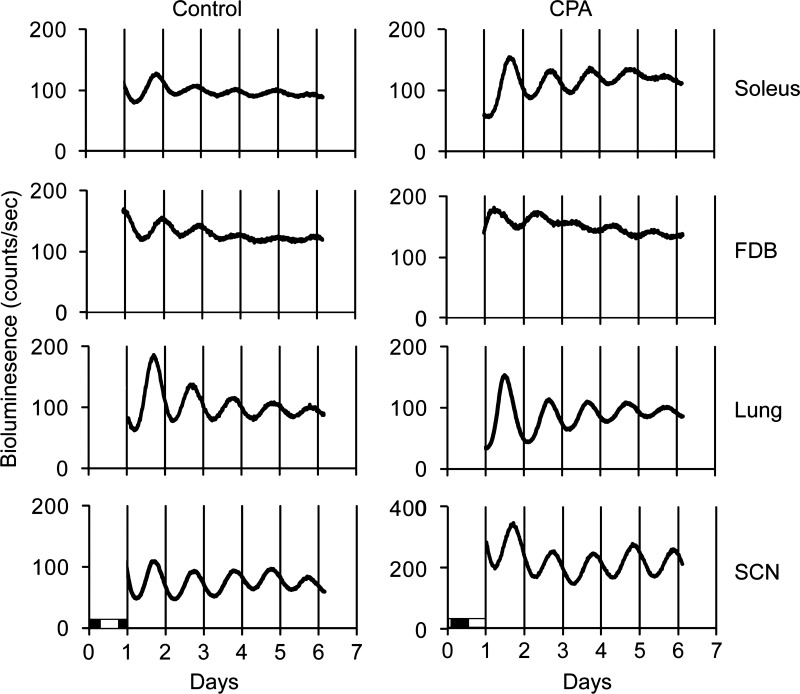
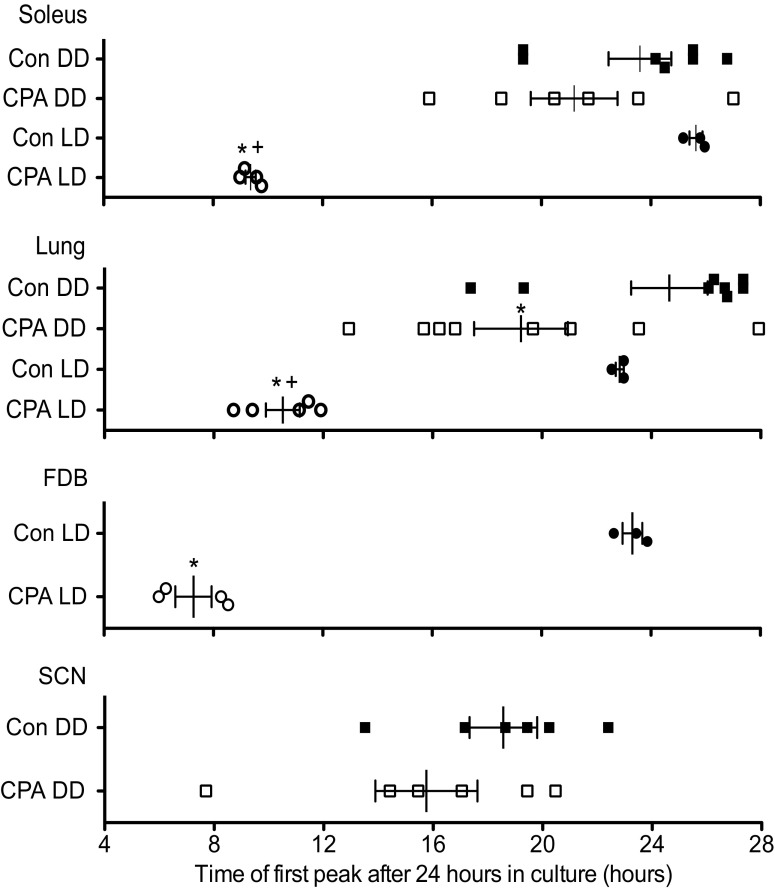
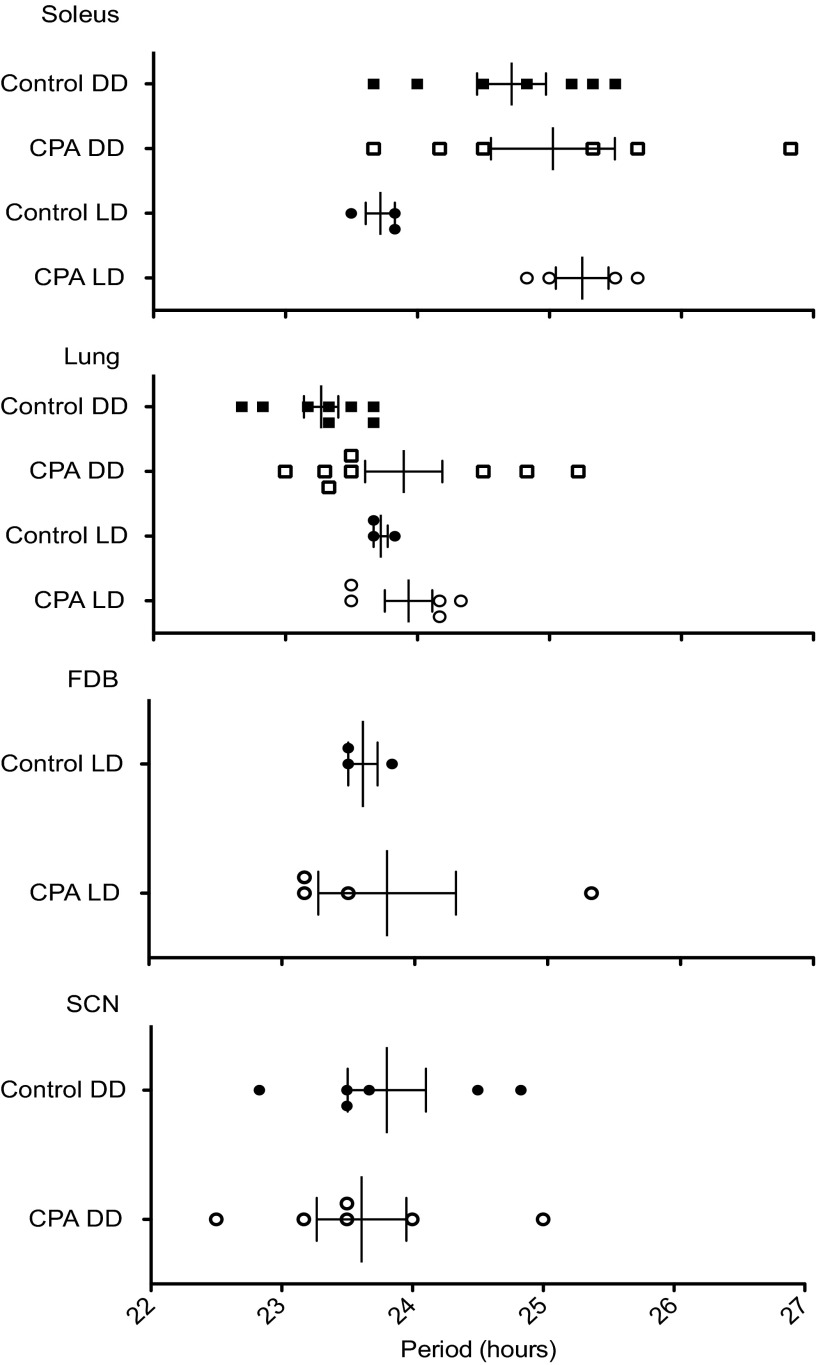
Robust circadian oscillations of PER2::LUC bioluminescence were exhibited by all tissues for at least 5 days in culture, with the phases varying among the tissues and previous lighting conditions (Figs. 4 and 5). SCN explants were cultured only from the DD group (control DD and CPA DD), whereas FDB cultures were only done immediately after CPA (control LD and CPA LD). For cultures prepared after 2 wk of DD, prior exposure to CPA advanced the phase of the PER2::LUC bioluminescence rhythm in lung by ∼5 h but did not affect the phase of this rhythm exhibited by the soleus muscle or SCN. In the case of cultures prepared from peripheral tissues explanted from mice without prior DD exposure, CPA advanced the PER2::LUC bioluminescence rhythm in all tissues studied by as much as 16 h in soleus (oxidative slow twitch muscle used for stabilization) and FDB (glycolytic fast twitch used to flex the toes) muscles and by ∼12.5 h in the lung. Additionally, the phase of PER2::LUC bioluminescence in CPA LD was significantly advanced compared with CPA DD in both soleus (∼12 h) and lung (∼8.5 h). These data show that the phase changes to the peripheral molecular clock were more severe immediately after CPA and suggest that the molecular clock had been free running during DD and reached a phase approaching that occurring during the original 12L:12D conditions. All peripheral tissues advanced robustly after CPA and showed a similar progression toward original conditions following DD; however, the lung remained significantly shifted. Also, skeletal muscles with two distinct uses advanced to a similar extent in contrast to another peripheral tissue, the lung. This suggests that peripheral tissues may respond differently to the same cue or use additional signals to synchronize. In contrast to the phase changes, the period length (peak-to-peak measurement) of PER2::LUC protein bioluminescence rhythms in the tissue explants (for all tissues) was not altered in either the LD or DD groups (Fig. 6), suggesting that the behavioral rhythm does not necessarily predict what the molecular oscillators in the SCN and peripheral clocks are doing. Because the phase of the PER2::LUC rhythm in the peripheral tissues is significantly advanced even after DD (Figs. 4 and 5), chronically advancing the LD cycle has persistent effects on the timing of the circadian clock in these tissues.
Fig. 4.
Representative graphs of raw bioluminescence data from PER2::LUC tissue explants monitored in culture for 6 days. The light and dark bars shown at bottom left indicate the final light/dark (LD) cycle before culture. FDB, flexor digitorum brevis; SCN, suprachiasmatic nucleus.
Fig. 5.
Effects of CPA and DD on the phases of the peripheral and central molecular clocks. Phase plot of first peak of PER2::LUC bioluminescence from cultured explants after 24 h in culture. The phases of PER2::LUC bioluminescence rhythms in the soleus, FDB, and lung were significantly shifted following exposure to CPA alone (compare control LD and CPA LD). When mice were exposed to 2 wk in DD following CPA, only the molecular clock in the lung remained significantly shifted (compare control DD and CPA DD). The phase of PER2::LUC bioluminescence rhythm in the lung was significantly shifted from controls in both the CPA DD and CPA LD groups, and there was a significant difference between the CPA group that was in DD from that cultured immediately following CPA (CPA LD). There was no significant interaction between group (CPA or control) and lighting condition (LD or DD), except in the rhythm expressed by the soleus. In the soleus muscle, there was no significant difference between CPA DD and control DD; only the CPA LD group was significantly different from control LD; however, CPA LD was still significantly shifted from CPA DD. Values are means ± SE (control DD, n = 8; CPA DD, n = 8; control LD, n = 3; CPA LD, n = 5). Not all tissues from each mouse could be used for analysis due to tissue culture survival. *Significant difference compared with control within lighting condition (P < 0.05). +Significant difference between CPA DD and CPA LD (P < 0.05; Student's t-test for FDB and SCN, two-way ANOVA with post hoc Bonferroni for soleus and lung).
Fig. 6.
Effects of CPA and DD on the period length of the peripheral and central molecular clocks. Plot of period length between the first and second peaks of PER2::LUC bioluminescence from cultured explants following 24 h in culture. The period length was not significantly shifted in the LD or DD groups in central (SCN) or peripheral clocks. See Fig. 5 legend for n values. Values are means ± SE.
DISCUSSION
Because several studies have demonstrated that phase advancing the LD cycle can have a variety of negative consequences on health when combined with pathology (e.g., infection, carcinogenesis, and metabolic disorders) (10, 22, 23, 48, 52) and can decrease the lifespan in mice (16), we tested the effects of CPA alone in mice. We hypothesized that behavioral and molecular circadian rhythms would be unable to re-synchronize to phase shifts of 6 h every 4 days for 8 wk and that such an aggressive phase-advancing protocol would produce arrhythmic locomotor behavior, altered clock gene rhythms, and changes in physiological outcomes. Surprisingly, the results showed that young adult mice, 6 mo of age, were able to adjust to the rapid CPA changes in the LD cycle by continuously and rapidly shifting their behavioral rhythms. Mice exposed to CPA also showed robust circadian rhythms in HR and Tb, and did not exhibit detectable impairments in body weight, tissue weight, circulating glucose levels, or muscle force. However, we found that the phase of the PER2::LUC bioluminescence rhythms of the peripheral tissues, i.e., soleus, FDB, and lung, were significantly phase shifted after CPA.
It is well accepted that oscillations in both Tb and HR coincide with the locomotor activity of a mammal, increasing during active wakeful hours and decreasing during rest (53, 55). The phase angle relationship between the activity onset of nocturnal rodents and onset of darkness is very stable, such that when a wheel is present most mice begin running almost as soon as the lights go off. Consistent with previous studies, the phase angle of circadian rhythms to the ambient photoperiod was very small before CPA such that, within minutes of lights-off, Tb and HR rose as the mice became active (4, 26). During CPA, the phase angle of entrainment, for all measures, was delayed on day 2 of the shift relative to time of lights-off, but on days 3 and 4 there was no difference, demonstrating that >1 day was required for physiological and locomotor rhythms to “catch up” to the shifting LD cycle. Because the rhythms appeared to adjust within ∼3 days after an abrupt advance of the LD cycle, it is not surprising that similar changes were seen after short-term as well as after chronic CPA.
Wheel-running behavior, like the other physiological measures, adjusted rapidly to the shifting LD cycle. However, once released into darkness, the period length of free-running rhythm was shorter in CPA mice than in control mice, suggesting that the endogenous central clock (SCN) that controls this rhythm was affected by CPA. Similar alterations of free-running period have been observed in mice exposed to non-24-h LD cycles (so called “T cycles”). Wild-type mice exposed to shorter T cycles (21–22 h) display a free-running period that is shorter than that observed after exposure to a 24-h LD cycle (9, 40, 43). In the present study with a 6-h phase advance of the LD cycle every 4 days, the mice experienced a compression of 4 days into 90 h (24 h/day × 4 days − 6 h = 90 h). Thus, on average, each “day” was ∼22.5 h, resembling a short T cycle. This compression of days may have affected the endogenous period length that was observed. Alternatively, the shortened free-running period exhibited by the CPA mice might have been caused by lack of stable or complete adjustment after each shift. The presence of a running wheel may have potentiated the rate at which the mice were able to adjust to the 6-h shifts. There is evidence that when mice are given a running wheel and a shifted LD cycle together, the mice adjust more quickly to the new LD cycle (60). In addition, scheduled access to a running wheel can produce phase shifts in the molecular clock, suggesting that circadian rhythms are sensitive to scheduled bouts of activity (58). The current observations describe how physiological as well as behavioral rhythms change in response to chronic shifting of the LD cycle.
Others have shown that the SCN and peripheral oscillators respond differently to acute phase shifts of the LD cycle (56). In previous studies, the SCN was able to adjust most rapidly, whereas the peripheral tissues took 3–6 days to fully shift following a single 6-h advance in the LD cycle (14, 18, 61). In the present study, the phase of the molecular clock, as determined from PER2::LUC bioluminescence rhythms, of the peripheral tissues, i.e., soleus, FDB, and lung, was significantly altered after CPA. It is interesting to note that, although the PER2::LUC rhythm was significantly shifted in both skeletal muscle and lung tissue taken immediately after CPA, after 2 wk in DD, only the lung, although trending toward the same phase as control, was still significantly advanced, indicating perhaps that the molecular clock in the lung lagged behind the skeletal muscle clock and had not yet recovered from the chronically shifting LD cycle. There is also evidence that peripheral oscillators and the SCN respond differently to nonphotic cues presented in the presence of a normal LD cycle. For example, restricting the time of food presentation shifts the phase of the molecular clock in the liver, lung, and other tissues (13, 15, 17), whereas the SCN remains synchronized with light (29, 37). Also, exercise scheduled at specific times of the day phase shifts the skeletal muscle and lung clocks (58). We demonstrated that with restricted feeding (RF) the molecular clock in the lung phase shifted by ∼12 h, whereas the skeletal muscle only shifted by ∼3 h (58). Scheduled activity had a similar effect on the lung and skeletal muscle clocks, ∼2- to 3-h phase shifts (58). These data suggest that the lung is more sensitive to time of food presentation, whereas the skeletal muscle clock responds similarly to either RF or scheduled exercise. This difference, as well as the present finding of different rates of adjustment to CPA, provides additional evidence suggesting that multiple mechanisms and cues mediate the temporal adjustment of the oscillators in these tissues.
There is some evidence that various peripheral tissues may be synchronized to the LD cycle via different physiological pathways. Guo et al. reported that the molecular clocks in some peripheral tissues can be synchronized by circulating factors (or behavior), whereas others cannot (25). Using a parabiosis technique, SCN-lesioned mice, with arrhythmic behavior and altered circadian clock gene expression, were surgically joined to SCN-intact mice. Expression of core clock genes in the liver and kidneys exhibited time of day variations (suggesting rhythms) similar to control mice, whereas clock gene expression in the heart, spleen, and skeletal muscle did not show time of day variations (25). This finding suggests that peripheral clocks in various tissues are synchronized by the SCN using different pathways, humoral, neural, behavior, or possibly others. Another study demonstrated that body temperature rhythms constitute a potent entraining signal for the pituitary and lungs (7). Whether temperature rhythms can also entrain circadian oscillations in skeletal muscle has not yet been tested. The findings that the PER2::LUC rhythms in the lung and soleus muscle adjusted to CPA at different rates suggest that different entraining mechanisms may be employed by these two tissues. In addition to the variation reported between tissue-specific oscillators, the pathways whereby physiological rhythms, which employ multiple cell types and organs, are synchronized by environmental cues and groups of cells within a single tissue could be quite different. At the conclusion of CPA in the present study, physiological rhythms, HR, Tb, and locomotor activity all showed a delay on day 2 of the phase shift while molecular rhythms advanced. Studies have shown that, with acute phase advances, circadian rhythms in Tb advance relatively quickly to the new LD cycle (38, 47), and they advance more rapidly with exercise (59). After 8 wk of chronic phase shifting, we observed a delay, suggesting that physiological rhythms and molecular rhythms may be uncoupled or that, after such an aggressive phase advancing protocol, the resetting signals or sensitivity to them may have changed.
In summary, this is the first study to assess behavior and physiological rhythms, physiological end points, and molecular oscillators within the same cohort of mice, during and after exposure to CPAs of the LD cycle. No detrimental effects of 8 wk of CPA were observed in this study of young adult mice, consistent with a previous report that chronic exposure (8 wk) to shifting LD cycles profoundly decreases survival in aged (27- to 31-mo-old) but not younger (8- to 12-mo-old) mice (16). Although the CPA mice in this study did not exhibit illness or functional impairments, they did show alterations in molecular oscillations and desynchrony of rhythms among tissues, including skeletal muscle, which has not been studied before. In the present study, CPA had the most profound effect on the lung. Recently, it was demonstrated that repeated phase shifts promoted cancer growth in the lung (33). Since persistent circadian misalignment is associated with pathology and increased risk for disease in rodents and humans (12, 22, 23, 48, 50), it is interesting to speculate that CPA, which occurs during chronic shift work or extensive travel across time zones, may be a risk factor for respiratory disorders or lung cancer. Because the data presented here demonstrate that CPA disrupts the synchrony of peripheral clocks, further work should be done to understand this fundamental connection between chronic exposure to shifting LD cycles and molecular clocks in both peripheral and central tissues to counteract potential consequences of CPA on human health and well-being.
GRANTS
Support for this study was provided by the National Institutes of Health Grants AR-055246 and ES-018636 to K. A. Esser and T32 fellowship grant no. HL-086341-02 to G. Wolff.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.W. and K.A.E. conception and design of research; G.W. performed experiments; G.W. analyzed data; G.W., M.J.D., and K.A.E. interpreted results of experiments; G.W. prepared figures; G.W. drafted manuscript; M.J.D. and K.A.E. edited and revised manuscript; K.A.E. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Dr. John J. McCarthy for assistance with tissue collection, Tanya Seward for implanting the transmitters, and Dr. Elizabeth Schroder for assisting with the Dataquest ART4.1 telemetry software. The authors also thank the laboratory of Dr. Shin Yamazaki for valuable insight.
REFERENCES
- 1. Albrecht U, Oster H. The circadian clock and behavior. Behav Brain Res 125: 89–91, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA 107: 19090–19095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell 89: 655–667, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arraj M, Lemmer B. Circadian rhythms in heart rate, motility, and body temperature of wild-type C57 and eNOS knock-out mice under light-dark, free-run, and after time zone transition. Chronobiol Int 23: 795–812, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30: 525–536, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Blazquez A, Martinez-Nicolas A, Salazar FJ, Rol MA, Madrid JA. Wrist skin temperature, motor activity, and body position as determinants of the circadian pattern of blood pressure. Chronobiol Int 29: 747–756, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330: 379–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cambras T, Chiesa J, Araujo J, Diez-Noguera A. Effects of photoperiod on rat motor activity rhythm at the lower limit of entrainment. J Biol Rhythms 19: 216–225, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 185: 5796–5805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci 20: RC66, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clancy J, McVicar A. Circadian rhythms 2: shift work and health. Br J Nurs 3: 712–717, 1994 [PubMed] [Google Scholar]
- 13. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci 29: 171–180, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav 2: 32–39, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol 16: 914–916, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davidson AJ, Stokkan KA, Yamazaki S, Menaker M. Food-anticipatory activity and liver per1-luc activity in diabetic transgenic rats. Physiol Behav 76: 21–26, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Davidson AJ, Yamazaki S, Arble DM, Menaker M, Block GD. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging 29: 471–477, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeCoursey PJ, Buggy J. Circadian rhythmicity after neural transplant to hamster third ventricle: specificity of suprachiasmatic nuclei. Brain Res 500: 263–275, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ. Melatonin and circadian biology in human cardiovascular disease. J Pineal Res 49: 14–22, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM, Young ME. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int 28: 187–203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res 64: 7879–7885, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Filipski E, Subramanian P, Carriere J, Guettier C, Barbason H, Levi F. Circadian disruption accelerates liver carcinogenesis in mice. Mutat Res 680: 95–105, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Gilliam LA, Ferreira LF, Bruton JD, Moylan JS, Westerblad H, St Clair DK, Reid MB. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol 107: 1935–1942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA 102: 3111–3116, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hannibal J, Hsiung HM, Fahrenkrug J. Temporal phasing of locomotor activity, heart rate rhythmicity, and core body temperature is disrupted in VIP receptor 2-deficient mice. Am J Physiol Regul Integr Comp Physiol 300: R519–R530, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Hannibal J, Hsiung HM, Fahrenkrug J. Temporal phasing of locomotor activity, heart rate rhythmicity, and core body temperature is disrupted in VIP receptor 2-deficient mice. Am J Physiol Regul Integr Comp Physiol 300: R519–R530, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, Reid MB. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol 104: 694–699, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Hiroshige T, Honma K, Honma S. SCN-independent circadian oscillators in the rat. Brain Res Bull 27: 441–445, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Kaufman CM, Menaker M. Effect of transplanting suprachiasmatic nuclei from donors of different ages into completely SCN lesioned hamsters. J Neural Transplant Plast 4: 257–265, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuhn G. Circadian rhythm, shift work, and emergency medicine. Ann Emerg Med 37: 88–98, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193–205, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Logan RW, Zhang C, Murugan S, O'Connell S, Levitt D, Rosenwasser AM, Sarkar DK. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol 188: 2583–2591, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marchant EG, Mistlberger RE. Entrainment and phase shifting of circadian rhythms in mice by forced treadmill running. Physiol Behav 60: 657–663, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res 106: 447–462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mistlberger RE, Kent BA, Landry GJ. Phenotyping food entrainment: motion sensors and telemetry are equivalent. J Biol Rhythms 24: 95–98, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc 71: 343–372, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Murphy BA, Elliott JA, Sessions DR, Vick MM, Kennedy EL, Fitzgerald BP. Rapid phase adjustment of melatonin and core body temperature rhythms following a 6-h advance of the light/dark cycle in the horse. J Circ Rhythm 5: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pendergast JS, Friday RC, Yamazaki S. Endogenous rhythms in Period1 mutant suprachiasmatic nuclei in vitro do not represent circadian behavior. J Neurosci 29: 14681–14686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pendergast JS, Friday RC, Yamazaki S. Photic entrainment of period mutant mice is predicted from their phase response curves. J Neurosci 30: 12179–12184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol 25: 159–184, 1960 [DOI] [PubMed] [Google Scholar]
- 42. Pittendrigh CS. On temporal organization in living systems. Harvey Lect 56: 93–125, 1960 [PubMed] [Google Scholar]
- 43. Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. J Comp Physiol A 106: 223–252, 1976 [Google Scholar]
- 44. Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science 247: 975–978, 1990 [DOI] [PubMed] [Google Scholar]
- 45. Reddy AB, Field MD, Maywood ES, Hastings MH. Differential resynchronisation of circadian clock gene expression within the suprachiasmatic nuclei of mice subjected to experimental jet lag. J Neurosci 22: 7326–7330, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sack RL. Clinical practice. Jet lag. N Engl J Med 362: 440–447, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Satoh Y, Kawai H, Kudo N, Kawashima Y, Mitsumoto A. Temperature rhythm reentrains faster than locomotor rhythm after a light phase shift. Physiol Behav 88: 404–410, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106: 4453–4458, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci 10: 3685–3694, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suarez-Barrientos A, Lopez-Romero P, Vivas D, Castro-Ferreira F, Nunez-Gil I, Franco E, Ruiz-Mateos B, Garcia-Rubira JC, Fernandez-Ortiz A, Macaya C, Ibanez B. Circadian variations of infarct size in acute myocardial infarction. Heart 97: 970–976, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Summa KC, Vitaterna MH, Turek FW. Environmental perturbation of the circadian clock disrupts pregnancy in the mouse. PLos One 7: e37668, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9: 764–775, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tankersley CG, Irizarry R, Flanders S, Rabold R. Circadian rhythm variation in activity, body temperature, and heart rate between C3H/HeJ and C57BL/6J inbred strains. J Appl Physiol 92: 870–877, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol Regul Integr Comp Physiol 273: R1957–R1964, 1997 [DOI] [PubMed] [Google Scholar]
- 55. van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav 5: 139–149, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Vansteensel MJ, Yamazaki S, Albus H, Deboer T, Block GD, Meijer JH. Dissociation between circadian Per1 and neuronal and behavioral rhythms following a shifted environmental cycle. Curr Biol 13: 1538–1542, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Winget CM, Card DH, Hetherington NW. Circadian oscillations of deep-body temperature and heart rate in a primate (Cebus albafrons). Aerospace Med 39: 350–353, 1968 [PubMed] [Google Scholar]
- 58. Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exercise 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamanaka Y, Hashimoto S, Tanahashi Y, Nishide SY, Honma S, Honma K. Physical exercise accelerates reentrainment of human sleep-wake cycle but not of plasma melatonin rhythm to 8-h phase-advanced sleep schedule. Am J Physiol Regul Integr Comp Physiol 298: R681–R691, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Yamanaka Y, Honma S, Honma K. Scheduled exposures to a novel environment with a running-wheel differentially accelerate re-entrainment of mice peripheral clocks to new light-dark cycles. Genes Cells 13: 497–507, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol 393: 288–301, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]