Abstract
The master transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) regulates the expression of antioxidant and phase II-metabolizing enzymes by activating the antioxidant response element (ARE) and thereby protects cells and tissues from oxidative stress. Pulmonary complications remain the leading cause of death in human immunodeficiency virus (HIV)-1-infected individuals, who display systemic oxidative stress and glutathione deficiency that can be modeled in transgenic rats where HIV-1-related viral proteins decrease glutathione levels and cause epithelial barrier dysfunction within the alveolar space by as yet unknown mechanisms. We hypothesized that HIV-1-related proteins inhibit Nrf2-mediated antioxidant defenses and thereby disrupt the normally tight alveolar epithelial barrier. Nrf2 RNA silencing dampened Nrf2/ARE activity, decreased the expression of the tight junction proteins zonula occludens-1, occludin, and claudin-18, increased paracellular permeability of alveolar epithelial monolayers derived from wild-type rats, and therefore reproduced the effects of HIV-1 transgene expression on the epithelial barrier that we had previously described. In contrast, upregulating Nrf2 activity, either by plasmid-mediated overexpression or treatment with the Nrf2 activator sulforaphane, increased the expression of ARE-dependent antioxidants, including NAD(P)H dehydrogenase, quinone 1 and glutathione, improved the expression of tight junction proteins, and restored the ability to form tight barriers in alveolar epithelial cells from HIV-1 transgenic rats. Taken together, these new findings argue that HIV-1-related proteins downregulate Nrf2 expression and/or activity within the alveolar epithelium, which in turn impairs antioxidant defenses and barrier function, thereby rendering the lung susceptible to oxidative stress and injury. Furthermore, this study suggests that activating the Nrf2/ARE pathway with the dietary supplement sulforaphane could augment antioxidant defenses and lung health in HIV-1-infected individuals.
Keywords: nuclear factor (erythroid-derived 2)-like 2, sulforaphane, epithelial barrier function, tight junction protein, human immunodeficiency virus-1 transgene
nuclear factor (erythroid-derived 2)-like 2 (Nrf2 or NFE2L2) is a transcription factor that regulates various genes, including the enzymes and related molecules such as glutathione that comprise the antioxidant response element (ARE). Nrf2 is constitutively expressed in all tissues but particularly so in the kidney, muscle, lung, heart, and brain (27). The Nrf2-signaling pathway induced by oxidative stress promotes cytoprotection by activating many proteins that are crucial to 1) the metabolism of drugs and toxins, 2) the protection against oxidative stresses and inflammation, and 3) the stability of proteins and the removal of damaged proteins (23). The importance of Nrf2 in cytoprotective roles is illustrated in Nrf2 knockout mice that have lower levels of glutathione reductase, increased levels of oxidized glutathione, and increased cytotoxicity during oxidative stress (18). In the lung, deficiency of Nrf2 augments injury caused by bleomycin, hyperoxia, and cigarette smoke (10). However, direct evidence of epithelial barrier function mediated by Nrf2/ARE activity has yet to be elucidated. Although the incidence and mortality of opportunistic infections in human immunodeficiency virus (HIV)-1-infected individuals has decreased dramatically in the highly active anti-retroviral therapy (HAART) era, “routine” bacterial lung infections and other presumably “nonopportunistic” lung diseases such as chronic obstructive pulmonary disease (COPD) and asthma and bronchitis have become more common (28). The mechanisms by which chronic HIV-1 infection renders individuals susceptible to pneumonia as well as to chronic airway diseases, particularly despite apparently effective viral control with HAART, are unknown. However, studies in HIV-1 transgenic rodents, in which HIV-1-related proteins are expressed and cause a progressive acquired immunodeficiency syndrome (AIDS)-like phenotype, are beginning to provide some intriguing clues. For example, HIV-1 transgene expression in rats significantly impaired alveolar macrophage phagocytic capacity (19). More recently we determined that HIV-1 transgene expression caused significant oxidative stress and glutathione depletion within the alveolar space that was associated with alterations in the expression of tight junction proteins and impaired alveolar epithelial barrier function (22). In addition, we and others have determined that HIV-1-related viral proteins such as gp120 and Tat can directly cause oxidative stress and glutathione depletion in target cells and tissues that cannot be infected with the virus, and thereby impair barrier functions in the brain and lung (22, 33, 34). Tight junction proteins are critical to forming epithelial and endothelial barriers in the brain, lung, and other organs, and experimental evidence supports the interpretation that the HIV-1-related viral proteins gp120 and Tat alter the expression and/or function of tight junction proteins, including members of the occludin and zonula occludens families via redox-dependent mechanisms (22, 33, 34, 41). Therefore, there is now compelling evidence that HIV-1 infection, at least in part through the toxic effects of its related proteins, including gp120 and Tat, causes oxidative stress and impairs alveolar macrophage immune capacity and epithelial barrier function. However, the fundamental mechanism(s) by which HIV-1-related proteins causes oxidative stress, particularly within the alveolar space, remains poorly understood.
A previously unrecognized clue to at least one mechanism by which HIV-1-related proteins induce oxidative stress is our recent finding that Nrf2 expression was decreased in alveolar epithelial cells (AEC) from HIV-1 transgenic rats compared with their wild-type counterparts (15). Because Nrf2 is critically required to activate the ARE and induce the synthesis of glutathione and other components of the antioxidant defenses within the airway, in the present study, we sought to investigate whether or not Nrf2 expression played a role in mediating the alveolar epithelial barrier dysfunction caused by HIV-1 transgene expression. Specifically, we examined the effects of manipulating Nrf2 expression on AEC tight junction proteins and barrier formation. Our results indicate that inhibiting the Nrf2/ARE pathway recapitulates the toxic effects of HIV-1-related proteins on the alveolar epithelium and that activating this pathway restores tight junction protein expression and barrier function in the alveolar epithelia of HIV-1 transgenic rats.
MATERIALS AND METHODS
Cell culture.
Primary AEC were isolated from HIV-1 transgenic (HIV-1 Tg) and wild-type (WT) Fischer 344 rats (Harlan Laboratories, Indianapolis, IN) at 9–12 mo of age and cultured in DMEM/F-12 (Cellgro, Manassas, VA) with 10% FBS (Atlanta Biologicals, Lawrenceville, GA) and an antibiotic-antimycotic reagent (Sigma-Aldrich, St. Louis, MO) at 37°C in 5% CO2 (32). The rat lung epithelial cell line, L2 cells (ATCC CCL-149, Manassas, VA), were cultured in F-12K with 10% FBS and the same antibiotic-antimycotic reagent at 37°C in 5% CO2 but with the FBS concentration decreased to 2% during the treatments. Sulforaphane was from LKT Laboratories (St. Paul, MN), and all other chemicals were obtained from Sigma-Aldrich except where indicated otherwise. All procedures were approved by the Institutional Animal Care and Use Committee at the Atlanta Veterans Affairs and Emory University.
Silence RNA transfection.
Nrf2 Stealth Select RNAi [set of 3 small-interfering RNA (siRNA)] were obtained from Invitrogen (Nfe2l2, oligo ID: RSS343557, RSS343558, RSS343559; Carlsbad, CA). Primary AEC (180,000 cells/cm2) from WT rats or L2 cells (60,000 cells/cm 2) were seeded and then transfected the next day with 6 nM of either stealth RNAi for rat Nrf-2 or stealth RNAi for negative control plus HiPerFect as described in the Qiagen protocol (Valencia, CA). Posttransfection (48–72 h), mRNA and protein expression, monolayer permeability, and immunocytochemistry analyses were performed. In the sulforaphane supplement experiment, 1 μM of sulforaphane was added into cultures for 2 days after 48 h transfection.
Nrf2 expression vector and transfection.
We used the mouse Nrf2 vector (pCMV-SPORT6-Nrf2) from Invitrogen (MCG clone ID: 3663276) and created a pCMV-SPORT6 empty control vector by removing the mouse Nrf2 sequence. Rat primary AEC from HIV-1 Tg rats were transfected with either pCMV-SPORT6-Nrf2 or pCMV-SPORT6 using SuperFect (Qiagen). Three to five days after transfection, cells were analyzed for protein expression, immunocytochemistry, and paracellular permeability.
RNA isolation and real-time RT-PCR.
Total RNA was extracted using the Qiagen RNeasy Mini kit. Reverse transcription and real-time PCR (Bio-Rad Lab, Hercules, CA) were performed as described previously (22) using primer pairs shown in Table 1. Each PCR product from the specific gene was normalized to 9S in the same RT sample.
Table 1.
Primer sequences for real-time PCR
| Gene Name | Oligo Sequences |
|---|---|
| Rat Nrf-2 | 5′-CAGTGACTCGGAAATGGAAGAG-3′ |
| 5′-AATGTGTTGGCTGTGCTTTAGG-3′ | |
| Rat NQO-1 | 5′-TGCTTTCAGTTTTCGCCTTT-3′ |
| 5′-GAGGCCCCTAATCTGACCTC-3′ | |
| Rat glutathione transferase | 5′-CACAAGATCACCCAGAGCAA-3′ |
| 5′-CCATAGCCTGGTTCTCCAAA-3′ | |
| Rat glutathione synthetase | 5′-TCACTGGACATGGGTGAAGA-3′ |
| 5′-TCCATGAGGATGTAGGAGGC-3′ | |
| Rat glutathione reductase | 5′-GGGTGGTGTGCCCACGGTTC-3′ |
| 5′-ATAACGTGCGGCTGGGCAA-3′ | |
| Rat glutamate-cysteine ligase, catalytic subunit | 5′-TGGCCAGCCGTACGGAGGAA-3′ |
| 5′-CAGGGCAGCCTAGCCTGGGA-3′ | |
| Rat occludin | 5′-AATTCCCGAATCCCAATTTC-3′ |
| 5′-AAGGGCAGATGGGACTGAGA-3′ | |
| Rat ZO-1 | 5′-AGCGAAGCCACCTGAAGATA-3′ |
| 5′-GATGGCCAGCAGGAATATGT-3′ | |
| Mouse claudin-18 | 5′-TGTGGAGCACTCAAGACCTG-3′ |
| 5′-AGATGGACACGAGGATACCG-3′ | |
| Rat 9S | 5′-ATCCGCCAACGTCACATC-3′ |
| 5′-CCGCCACCATAAGGAGAAC-3′ |
Nrf2, nuclear factor (erythroid-derived 2)-like 2; NQO-1, NAD(P)H dehydrogenase, quinone 1; ZO, zonula occludens.
Nrf2/ARE activity assay.
L2 cells were seeded (10,000/well) in 96-well plates and the next day transfected with the Cignal ARE reporter using HiPerFect transfection reagent. For Nrf2 siRNA experiments, cells were cotransfected with Cignal ARE reporter and 6 nM of either RNAi for Nrf2 or negative control duplexes and lysed for luciferase reporter assay after 48 h. For sulforaphane experiments, transfected cells were cultured ± gp120 (100 ng/ml; ImmunoDiagnostics, Woburn, MA) for 3 days, and sulforaphane (LKT Laboratories) was added 6 h before lysing cells and measuring luciferase activity. A dual-luciferase reporter assay was performed as described by the manufacturer (Promega, Madison, WI). Nrf2/ARE promoter activity values were expressed as ratios of arbitrary units of firefly luciferase/Renilla luciferase activity.
Epithelial monolayer permeability assay.
Barrier function of AEC monolayers was determined by two complementary methods: paracellular permeability to FITC-labeled Dextran (Sigma-Aldrich) and transepithelial electrical resistance (TER), as previously described (15).
Western blotting.
Total proteins were isolated from primary AEC by using RIPA buffer containing 150 mM NaCl, 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1 SDS, 50 mM Tris (pH 8.0), 0.2 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail (Pierce Biotechnology, Rockford, IL), and protein electrophoresis and blotting were performed as described previously (15). The immunoreactive bands were captured with the ChemiDoc XRS system (Bio-Rad). The same membrane was reprobed with anti-actin antibody for protein-loading control.
Immunocytochemistry.
Primary AEC were cultured on glass cover slips, and monolayers were fixed and stained for claudin-18, occludin, and zonula occludens (ZO)-1, as described previously (16). The images were captured using Leica DM4000B microscopy (Leica Mircosystems, Buffalo Grove, IL).
Glutathione measurement.
Epithelial monolayers were washed with PBS and lysed in cold 5-sulfo-salicylic acid dehydrate solution at 5%, and assay was performed as described in the protocol from the glutathione colorimetric detection kit (Arbor Assays, Ann Arbor, MI). All standards and samples were run with duplicates.
Cytotoxicity assays.
After 3-day cultures, primary epithelial cells were treated with or without 50 μM buthionine sulfoximine (BSO) for 48 h. Cell culture media were used for measuring lactate dehydrogenase (LDH) released into medium, and cell monolayers were washed and replaced with fresh culture medium with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent for 2 h. LDH assay (Pierce Biotechnology) and MTT assay (ATCC) were performed per the manufacturers' instructions.
Statistical analyses.
One-way ANOVA with Newman-Keuls posttests were performed for multiple comparisons, and Student's t-tests were used for single comparisons using Prism (GraphPad, San Diego, CA). All data are presented as means ± SE, and significance was accepted at P < 0.05.
RESULTS
Nrf2 RNA interference inhibited Nrf2/ARE-regulated antioxidant gene expressions, decreased intracellular glutathione levels, and increased epithelial barrier permeability.
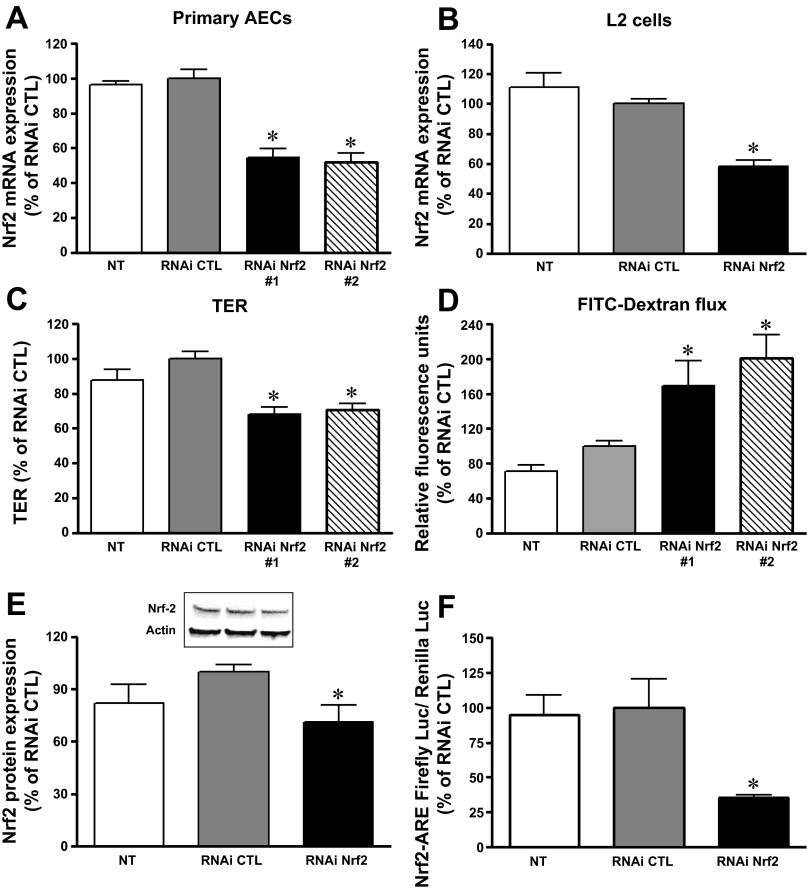
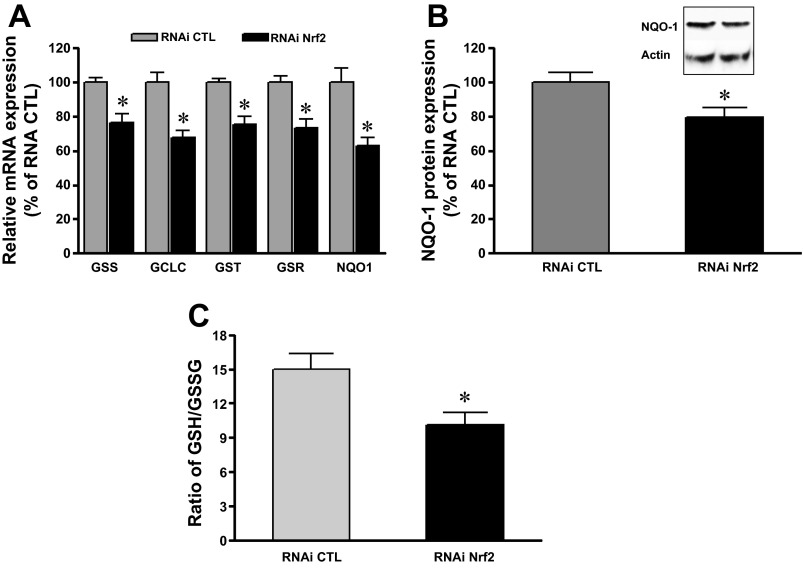
We previously reported that chronic alcohol ingestion and/or chronic HIV-1-related viral protein expression attenuated Nrf2 expression and caused barrier dysfunction in the rat alveolar epithelium (15). To test whether a selective decrease in Nrf2 expression alone could impair lung epithelial barrier function, Nrf2 RNAi was introduced into AEC derived from WT rats or into L2 cells. The concentration of Nrf2 RNAi used was targeted to reproduce a similar inhibition in Nrf2 expression that we had previously identified in primary AEC derived from HIV-1 Tg rats, in which Nrf2 expression was decreased 20–30% compared with cells from littermate WT rats (12). As shown in Fig. 1, A and B, Nrf2 RNAi (6 nM) inhibited Nrf2 mRNA expression by 30–40% (P < 0.05) in both primary AEC and L2 cells and decreased Nrf2 protein levels as well (Fig. 1E). The MTT assay (ATCC) revealed no differences in viability between the cells treated with Nrf2 RNAi or the negative control RNAi (104 ± 3 vs. 100 ± 2%). Because interfering with Nrf2 expression altered the redox balance in lung epithelial cells, we next examined whether it also directly affected epithelial permeability and therefore could recapitulate the oxidative stress and the barrier dysfunction we had previously identified in the HIV-1 Tg rat model. As shown in Fig. 1, C and D, TER dramatically decreased (P < 0.0001) while the paracellular flux of FITC-labeled Dextran (4 kDa) significantly increased (P < 0.02) in AEC monolayers treated with RNAi Nrf2. These distinct but complementary indicators of epithelial barrier function indicate that inhibiting Nrf2 expression compromised the AEC ability to form a tight monolayer. Three RNAi Nrf2 were used in primary AEC cultures. Two of three Nrf2 RNAi at 6 nM effectively inhibited Nrf2 expressions and compromised epithelial monolayer barrier function as seen in Fig. 1, A, C, and D. Therefore, we used Nrf2 RNAi #1 for further studies. In parallel, Nrf2 RNA silencing significantly attenuated the Nrf2/ARE activity by ∼60% (P < 0.05) (Fig. 1F). Consistent with inhibiting Nrf2 gene expression, there was decreased expression of the downstream Nrf2/ARE-mediated enzymes glutathione synthetase, glutathione transferase (GST), glutathione reductase, glutamate-cysteine ligase catalytic subunit, and NAD(P)H dehydrogenase, quinone 1 (NQO-1) (Fig. 2, A and B). Furthermore, the intracellular reduced glutathione (GSH)-to-glutathione disulfide (GSSG) ratio, reflecting the levels of GSH and its primary oxidized form, GSSG, was significantly decreased in cells transfected with RNAi Nrf2 (P < 0.05) (Fig. 2C). Therefore, Nrf2 RNAi treatment significantly decreased Nrf2-ARE function and altered the intracellular redox balance in lung epithelial cells.
Fig. 1.
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) RNA interference decreased Nrf2 expression and impaired barrier function in alveolar epithelial cells (AEC). Three RNAi Nrf2 were added to cultures (n = 8–16) of primary AEC from wild-type (WT) rats. Two RNAi Nrf2 significantly inhibited Nrf2 mRNA expression in primary AEC (A) and L2 cells (B). Three days posttransfection, Nrf2 interference significantly decreased transepithelial electrical resistance (TER, C) and increased FITC-Dextran flux (D) in primary AEC with two RNAi Nrf2 (n = 4–14). The baseline TER values in untreated (NT) monolayers were 700–1,200 ohms·cm2. E: RNAi Nrf2 decreased Nrf2 protein expression in primary AEC (n = 5). Representative Western blot shown in inset. F: RNAi Nrf2 decreased Nrf2/antioxidant response element (ARE) activity (as reflected by a dual-luciferase reporter assay) in L2 cells (n = 6–15). *P < 0.05 compared with treatment with RNAi control (RNAi CTL).
Fig. 2.
Nrf2 RNA interference decreased Nrf2/ARE-regulated antioxidants and intracellular glutathione levels in primary AEC. A: Nrf2 RNAi decreased mRNA expression for glutathione synthetase (GSS), glutathione transferase (GST), glutamate-cysteine ligase catalytic subunit (GCLC), glutathione reductase (GSR), and NAD(P)H dehydrogenase, quinone 1 (NQO-1) (n = 8–15). B: Western analysis showing that silence Nrf2 gene decreased NQO-1 protein level (n = 5). Representative Western blot shown in inset. C: Nrf2 RNAi decreased the intracellular reduced glutathione (GSH)-to-glutathione disulfide (GSSG) ratios (n = 3). *P < 0.05 compared with treatment with RNAi CTL.
Selective interference of Nrf2 expression altered tight junction protein expression and localization.
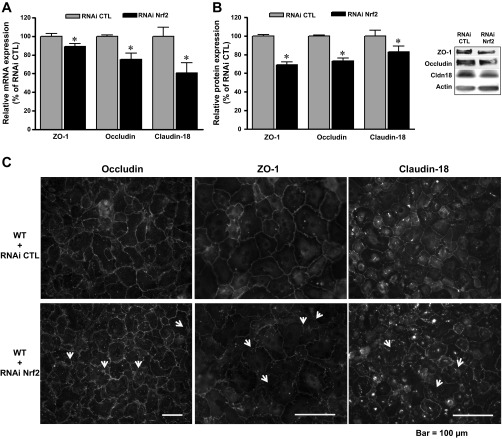
Because tight junction proteins are critical to the formation of a normal alveolar epithelial barrier, we next examined the expression of several key tight junction proteins. As shown in Fig. 3, A and B, the expression of occludin, zonula occludens-1 (ZO-1), and claudin-18 was decreased in cells transfected with RNAi Nrf2 compared with cells transfected with the RNAi negative control. These decreases in occludin and ZO-1 expression were comparable to those in AEC derived from HIV-1 Tg in which we previously reported that there were 10–20% decreases compared with cells from littermate WT rats (12). In parallel, inspection of these monolayers by immunocytochemistry (Fig. 3C) showed weak and nonuniform staining of occludin, ZO-1, and claudin-18 in cell-cell borders of primary AEC transfected with RNAi Nrf2. Overall, these alterations in tight junction protein expression and localization into the cell membrane are consistent with the barrier dysfunction shown in Fig. 1, C and D.
Fig. 3.
Nrf2 RNA interference altered the expression of tight junction proteins in primary AEC. Relative mRNA expression (A) and relative protein expression (B) (summary data and representative Western blot; normalized to actin) for occludin, zonula occludens (ZO)-1, and claudin-18 (n = 7–20). C: representative immunocytochemistry images showing that Nrf2 RNAi impaired the assembly of occludin, ZO-1, and claudin-18 protein into intercellular junctions (see arrows).
Nrf2 overexpression improved epithelial barrier function as well as tight junction expression and localization in AEC from HIV-1 tg rats.
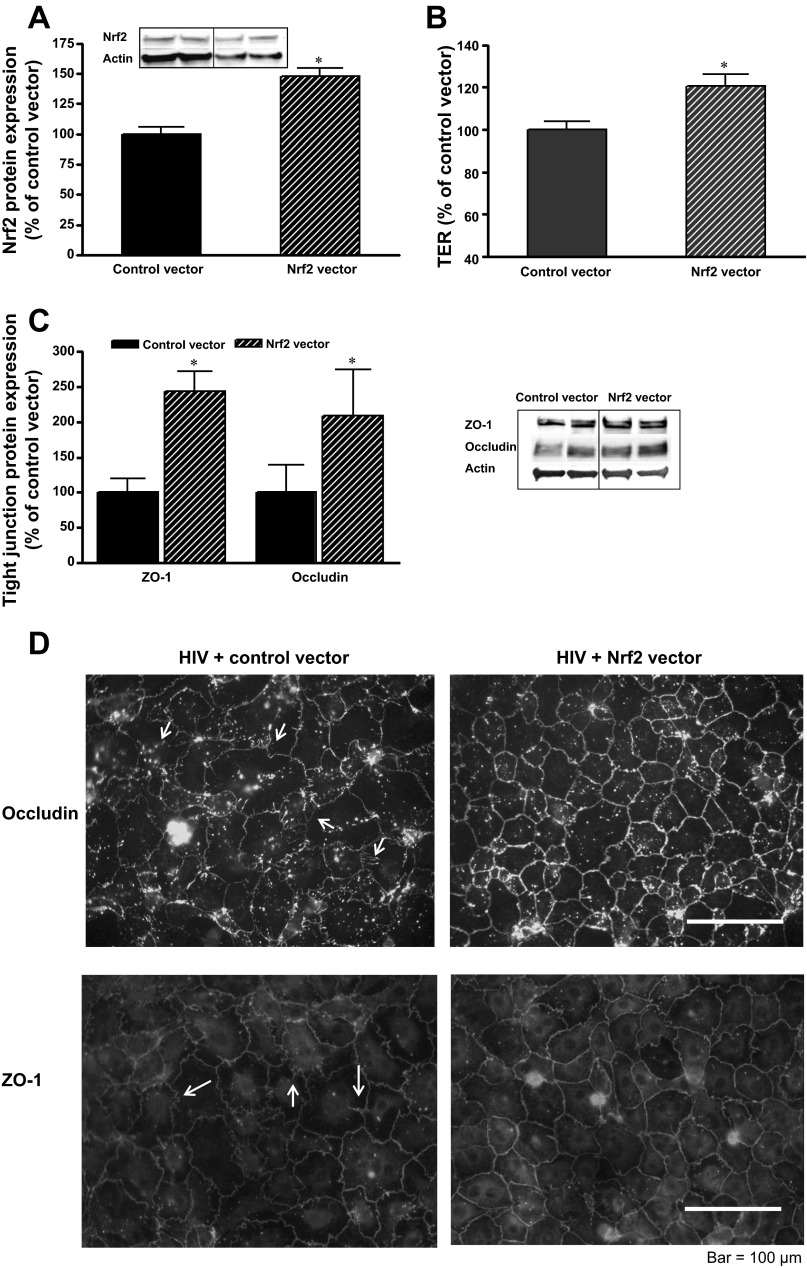
To this point, we had determined that targeted inhibition of Nrf2 expression recapitulated the effects of HIV-1 transgene expression on the alveolar epithelium, as reflected by redox imbalance, aberrant expression and localization of tight junction proteins, and impaired barrier function. These findings are consistent with our previously published studies showing that the epithelial barrier dysfunction in the HIV-1 Tg rats was associated with decreased Nrf2 expression in the alveolar epithelium and evidence of redox imbalance within the alveolar space (15, 22). However, these similarities alone do not provide sufficient evidence that HIV-1-related proteins impair alveolar epithelial barrier function by inhibiting Nrf2 expression. Therefore, we next asked if augmenting the expression and/or the activity of Nrf2 in AEC derived from HIV-1 Tg rats could improve their tight junction protein expression and barrier function. Because the rat and mouse Nrf2 protein sequences are 96% identical, we used a mouse Nrf2 overexpression vector from Invitrogen and delivered this into primary AEC derived from HIV-1 Tg rats. We first determined that the epithelial cells treated with the mouse Nrf2 expression vector had increased Nrf2 protein levels compared with cells treated with a control vector (Fig. 4A). In these Western analyses, both endogenous rat Nrf2 protein expression and mouse Nrf2 protein expression driven by the vector were detected by the same antibody. Therefore, the Nrf2 protein expression represents the sum of mouse and rat Nrf2. In parallel to this increase in Nrf2 protein expression, epithelial monolayer barrier function, as determined by TER, was increased (P < 0.05) by increasing Nrf2 expression (Fig. 4B). Furthermore, augmenting Nrf2 expression increased (P < 0.05) the expression of the tight junction proteins occludin and ZO-1 (Fig. 4C) and improved their localization into the cellular membranes as reflected by immunocytochemistry (Fig. 4D). Taken together, the results thus far provide compelling evidence that the chronic expression of HIV-1-related proteins causes redox imbalance and barrier dysfunction within the alveolar epithelium by inhibiting the expression of Nrf2.
Fig. 4.
Nrf2 overexpression increased Nrf2 protein expression, barrier function, and tight junction protein expression in primary AEC from human immunodeficiency virus (HIV)-1 transgenic (Tg) rats. A: protein expression shown as densitometry data (n = 3) and representative Western blot showing increased Nrf2 protein expression. B: improved barrier function as reflected by increased TER by ∼20%. C: representative Western blot and summary analyses of 3 determinations of relative protein expression of ZO-1 and occludin. D: representative immunocytochemistry images showing that Nrf2 overexpression improved the assembly of occludin and ZO-1 into intercellular junctions; for comparison, note areas of strand breaks observed in untreated monolayers (see arrows). *P < 0.05 compared with treatment with control vector.
Sulforaphane, an Nrf2 activator, increased Nrf2/ARE-regulated cellular antioxidants and protected AEC against HIV-1 viral protein-induced barrier dysfunction.
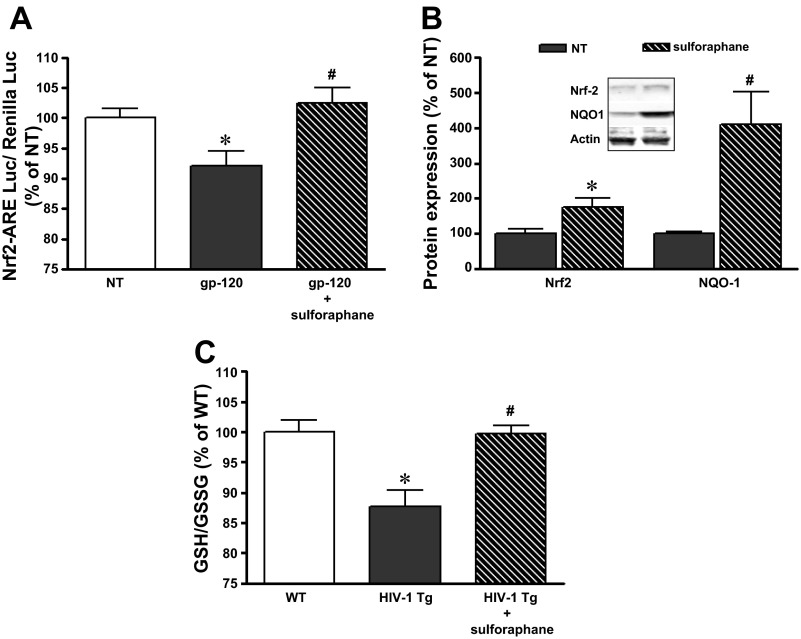
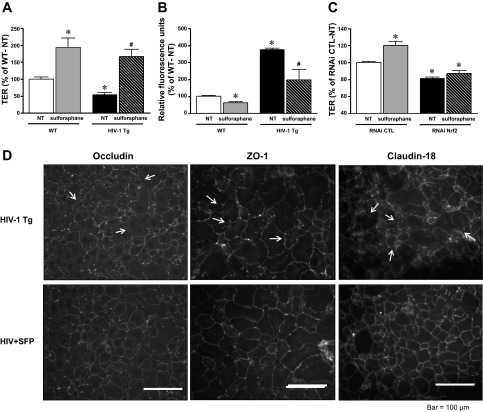
To provide further evidence of the role of decreased Nrf2 activity in mediating the pathological effects of HIV-1-related proteins on the alveolar epithelium, we next tested the effects of a Nrf2 activator, sulforaphane, in our model. We first examined L2 cells treated directly with the HIV-1-related protein gp120. As shown in Fig. 5A, treating L2 cells with gp120 significantly decreased Nrf2/ARE activity (P < 0.05) (as determined by the dual-luciferase activity assay). In contrast, concomitant treatment with sulforaphane (1 μM) restored Nrf2/ARE activity to the same levels (P > 0.05) as untreated cells (Fig. 5A). In parallel, primary AEC derived from HIV-1 Tg rats increased their Nrf2 expressions as well as NQO-1 levels in response to sulforaphane (Fig. 5B). Treatment with sulforaphane at a concentration as low as 1 μM significantly increased the intracellular GSH-to-GSSG ratio (P < 0.05), which reflected an increase in ARE-dependent triol-redox biosynthesis/recycling enzyme activities (Fig. 5C). Cell viability assay showed no toxic effects of 1 μM sulforaphane (untreated: 100 ± 1.7%, 1 μM sulforaphane: 106 ± 1.3%). As shown in Fig. 6, A and B, treatment with sulforaphane significantly (P < 0.05) improved barrier function (as reflected by increased TER and decreased paracellular flux of FITC-labeled Dextran) in alveolar epithelial monolayers derived from HIV-1 Tg rats. In fact, somewhat surprising, treatment with sulforaphane also significantly increased barrier function in alveolar epithelial monolayers derived from WT rats (P < 0.05). Furthermore, shown in Fig. 6C, selective Nrf2 RNA interference in primary AEC blocked the effect of sulforaphane (as reflected by no changes in TER with or without sulforaphane treatment) compared with AEC transfected by control RNAi with significant increase in TER. Consistent with the salutary effects of sulforaphane treatment on epithelial barrier function, immunocytochemistry images from these monolayers shown in Fig. 6D indicated that sulforaphane enhanced the localization of the tight junction proteins occludin, ZO-1, and claudin-18 in cells from HIV-1 Tg rats that, in the absence of treatment, demonstrated areas of strand breaks and weak staining in the intercellular junctions.
Fig. 5.
Sulforaphane restored Nrf2/ARE activity in L2 cells treated with the HIV-1-related protein gp120 and increased Nrf2 and NQO-1 protein expression as well as the GSH-to-GSSG ratios in primary AEC from HIV-1 Tg rats. A: sulforaphane treatment (1 μM) restored the relative Nrf2/ARE activity (by a dual-luciferase reporter assay) in L2 cells treated with gp120 (n = 11). B: representative Western blot and summary of densitometry data (n = 6) showed relative Nrf2 and NQO1 protein expression in primary AEC from HIV-1 Tg rats untreated or treated with sulforaphane (1 μM) for 4 days. C: relative intracellular GSH-to-GSSG ratios were measured in primary AEC from WT and HIV-1 Tg rats cultured for 4 days ± sulforaphane (1 μM) (n = 6). *P < 0.05 compared with untreated L2 cells (A) or cells from WT rats (C). #P < 0.05 compared with L2 cells treated with gp120 (A) or with cells from HIV Tg rats (C).
Fig. 6.
Nrf2 activation with sulforaphane improved barrier function and tight junction protein assembly in AEC monolayers from HIV-1 Tg rats. Relative TER (n = 13–16) (A) and relative FITC-Dextran flux (B) (n = 3–12) in primary AEC monolayers derived from WT and HIV-1 Tg rats were measured in culture for 5 days ± sulforaphane (1 μM). C: RNAi Nrf2 blocked the effect of sulforaphane on improvement of epithelial monolayer function as reflected by no changes in TER with sulforaphane treatment compared with an increase in TER in AEC treated with control RNAi (n = 3). D: representative immunocytochemistry images showed that sulforaphane (SFP) treatment (1 μM) improved the assembly of occludin, ZO-1, and claudin-18 into intercellular junctions of monolayers derived from HIV-1 Tg rats; for comparison, note areas of strand breaks observed in untreated monolayers (see arrows). *P < 0.05, different (either increased or decreased) compared with untreated monolayers derived from WT rats. #P < 0.05, improved (either increased TER or decreased FITC-Dextran flux) compared with untreated monolayers derived from HIV-1 Tg rats.
Changes in intracellular GSH levels altered epithelial barrier function, which was associated with alterations of tight junction protein expression.
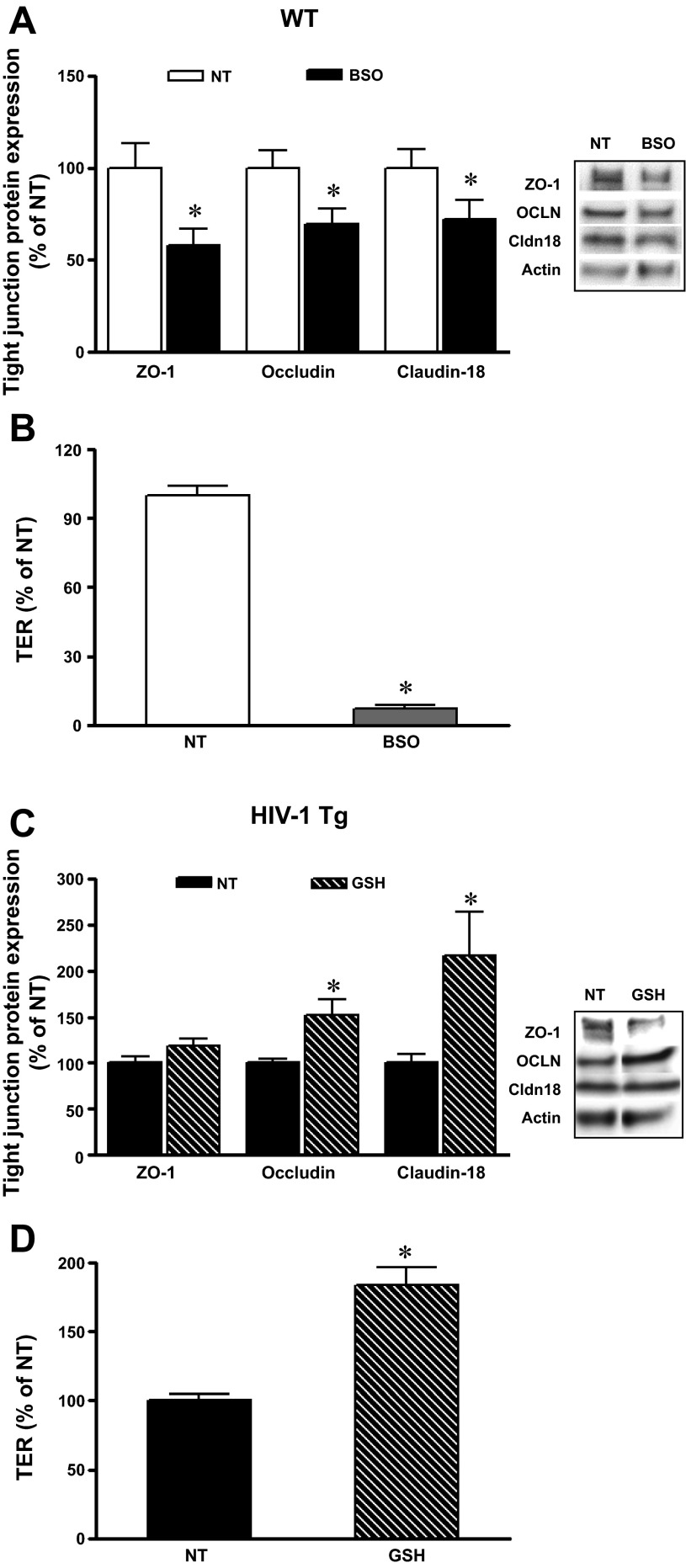
Oxidative stress induced by various reactive oxygen species disrupts barrier function by altering tight junctions (35), and treatment with triol-redox antioxidants protects barrier integrity in the lung and intestine (15, 29, 31). Baneriee et al. (5) reported that the thiol antioxidant N-acetylcysteine amide could protect the blood-brain barrier from oxidative damage in HIV-1 Tg mice by increasing tight junction proteins and restoring blood-brain barrier function. To further investigate whether oxidative stress induced by decreasing antioxidants such as GSH can directly cause the observed changes in epithelial tight junction expression and the consequent impaired epithelial barrier function, we manipulated intracellular GSH levels in primary AEC by either decreasing intracellular GSH with the glutathione synthetase inhibitor BSO (Sigma-Aldrich) (2) or supplementation with exogenous GSH (Sigma-Aldrich). BSO treatment had no detectable effect on cell viability, as reflected by the LDH release assay (100.0 ± 2.4 vs. 86.0 ± 1.2%) or MTT (100.0 ± 3.0 vs. 109.0 ± 3%) in untreated primary AEC compared with primary AEC with 50 μM BSO. The intracellular GSH levels in BSO-treated cells decreased to below the GSH kit detection level compared with untreated cells, whereas the intracellular GSH levels significantly increased with 500 μM of exogenous GSH treatment (100.0 ± 15% in untreated cells vs. 378 ± 41% in GSH-treated cells). As shown in Fig. 7A, BSO at 50 μM in primary AEC culture for 2 days significantly reduced the expression of the tight junction proteins ZO-1, occludin, and claudin-18 compared with untreated AEC derived from WT rats. In parallel, BSO treatment increased epithelial monolayer permeability as reflected by significantly decreased TER (Fig. 7B). The monolayer with BSO treatment appeared more “leaky” than cells transfected with Nrf2 RNAi or treated with gp120. The significantly lower level of intracellular GSH in BSO-treated cells could contribute to this relative increase in the epithelial monolayer permeability. In contrast, expression of the tight junction proteins occludin and claudin-18 significantly increased when primary AEC were treated with 500 μM exogenous GSH for 2 days (Fig. 7C). In parallel, epithelial monolayer barrier function was significantly improved by GSH treatment, as reflected by an increase in the TER (Fig. 7D). Thus, changes in intracellular levels of the thiol antioxidant GSH directly alter tight junction protein expression and modulate epithelial barrier function.
Fig. 7.
Changes in intracellular GSH levels altered tight junction protein expression and AEC monolayer permeability. AEC from WT rats treated with 50 μM buthionine sulfoximine (BSO) significantly decreased tight junction protein ZO-1, occludin, and claudin-18 expressions (A) as well as increased epithelial monolayer permeability (B). Addition of GSH (500 μM) in culture media increased tight junction protein occludin and claudin-18 expression (C) and enhanced the epithelial monolayer barrier function (D) by increasing TER compared with untreated cells from HIV-1 Tg rats. *P < 0.05, different compared with untreated AEC derived from WT (A and B) or HIV-1 Tg (C and D) rats.
DISCUSSION
Previously we had determined that chronic expression of HIV-1-related proteins in vivo caused oxidative stress and epithelial barrier dysfunction within the alveolar space in a rat model (22) and that these HIV-1-related proteins inhibited the expression of Nrf2, induced oxidative stress, and disrupted tight junction protein expression and assembly in AEC in vitro. In this current study, we provide additional and novel evidence that implicates Nrf2 expression as a pathophysiological target of HIV-1-related proteins and we thereby elucidate a previously unrecognized mechanism by which chronic HIV-1 infection renders the lung susceptible to injury. Specifically, we determined that selective inhibition of Nrf2 expression by RNA interference in lung epithelial cells recapitulated the redox imbalance, barrier disruption, and aberrant expression of tight junction proteins that we had previously identified in the HIV-1 Tg rat model (15, 22). This circumstantial evidence suggested a central role for Nrf2 in these conditions, but a direct causal role could not be inferred. Therefore, we determined that selectively increasing the expression of Nrf2 with an expression vector or activating existing Nrf2 with sulforaphane restored tight junction protein expression and membrane assembly in AEC from HIV-1 Tg rats and, as a consequence, normalized the ability of these cells to form a tight epithelial barrier. Taken together, these results provide novel evidence that HIV-1-related proteins inhibit Nrf2 expression in the alveolar space and thereby further induce oxidative stress and barrier dysfunction.
We also previously determined that chronic alcohol ingestion, which similarly causes profound oxidative stress and epithelial barrier dysfunction within the alveolar space, and/or chronic HIV-1-related protein expression, decrease Nrf2 expression in the alveolar epithelium (15), suggesting that decreased Nrf2 expression may be an important mechanism underlying seemingly diverse lung pathologies. Nrf2 activation is critical for protection in pulmonary diseases such as asthma, acute lung injury, hyperoxia, and pulmonary fibrosis (9). Interestingly, Nrf2-regulated antioxidant gene expression declines in COPD (24), and functional polymorphisms in the Nrf2 promoter, which significantly affect Nrf2 basal expression, are associated with increased risk for developing acute lung injury after major trauma and sepsis (26). Experimentally, Nrf2-deficient mice have decreased GSH synthesis that is associated with aberrant tissue repair and persistent inflammation following hyperoxia-induced acute lung injury (36).
Nrf2-deficient mice also have an exaggerated response to Staphylococcus aureus-induced pneumonia and demonstrate increased acute lung injury as assessed by neutrophil infiltration and overt tissue damage compared with WT controls (3). Athale et al. (3) found that upregulation of claudin-4 by Nrf2-deficient mice in response to pneumonia was blunted, which is consistent with an exacerbated injury response (42). Recently, Nrf2 was shown to interact with the claudin-4 promoter, but not the claudin-1 promoter (8). Reddy et al. (37) showed that glutathione supplementation in a Nrf2-deficient mouse upregulated gene expressions of the tight junction proteins occludin, ZO-2, claudin-18, claudin-6–7, and claudin-3–4 and rescued cells from the defects associated with Nrf2 deficiency, consistent with the ability of Nrf2 to directly and specifically regulate tight junction protein expression. Here, we found that changes to Nrf2 expression correlated with changes in the expression of claudin-18, ZO-1, and occludin, three key alveolar tight junction proteins (21). Whether Nrf2 directly regulates the expression of these proteins is not known at present. In silico analysis of the promoter regions of rat ZO-1, occludin, or claudin-18 did not reveal consensus Nrf2/ARE binding motifs (data not shown), suggesting an indirect effect of Nrf2 in regulating claudin-18, ZO-1, and occludin. Nonetheless, direct experimental analysis is needed to determine whether Nrf2 can bind to the promoters of these and other tight junction proteins.
We found siRNA depletion of Nrf2 inhibited TER by ∼30% and caused a twofold increase in permeability of FITC-Dextran (4 kDa). These changes are comparable to previous barrier function measurements of primary AEC from HIV-1 Tg rats and from rats fed an alcohol-containing diet (15, 22). While these changes do not lead to overt pulmonary edema, both HIV Tg rats and rats fed an alcohol-containing diet have decreased lung liquid clearance in vivo (22, 32). Clinically, we have identified that alcohol abuse increases the risk of acute respiratory distress syndrome approximately fourfold and that otherwise healthy alcoholics have profound oxidative stress and increased alveolar leak (17). Therefore, small changes to alveolar permeability have significant pathological consequences, particularly when the lung faces an acute inflammatory insult such as sepsis or pneumonia.
Nrf2/ARE activators such as sulforaphane have been identified and tested in experimental models and in clinical studies. Sulforaphane is an organosulfur compound found in cruciferous vegetables such as broccoli. Sulforaphane ingested orally has been shown to induce phase II enzymes such as glutathione-S-transferase, NQO1, and hemoxygenase-1 (HO-1) in the human upper airway (39). Other studies have shown that, following traumatic brain injury, early sulforaphane treatment upregulates Nrf2-dependent genes for GST and HO-1, attenuates the loss of expression of the tight junction proteins occludin and claudin-5, reduces endothelial cell death, and preserves blood-brain barrier function (12, 43, 44). In the blood-brain barrier, enhancing the expression of Nrf2-driven genes with a specific peptide that binds to Keap1 and releases Nrf2 into the nuclear compartment significantly reduced endothelial barrier compromise after traumatic brain injury (45). Furthermore, sulforaphane treatment of human epithelial cells in culture inhibits cigarette smoke-induced production of IL-8 and MCP-1 production (40) as well as the associated endoplasmic reticulum stress and cell death (25). In this context, it is exciting that there is evidence that targeting the Nrf2/ARE pathway could improve innate immune response, protect against oxidative stress, and prevent bacterial exacerbations in subjects with COPD (7).
These results could have important implications for the care of HIV-1-infected individuals. Although the mortality for individuals with HIV-1 infection/AIDS has declined significantly since the introduction of HAART, infectious and noninfectious pulmonary diseases remain a major cause of morbidity and the most common cause of death in this vulnerable population (1, 13). Consistent with our experimental findings, HIV-1-infected individuals have evidence of systemic oxidative stress and decreased levels of antioxidants, such as GSH and superoxide dismutase compared with uninfected individuals, even if they have adequate viral control with HAART (4). Importantly, these effects impair diverse physiological barriers in addition to the lung, including the blood-brain barrier and the intestinal mucosa barrier (6, 14, 22). Our previously reported observation that administering the glutathione precursor procysteine restores glutathione levels and reverses HIV-1 viral protein-induced superoxide anion production and restores nitric oxide-mediated vasorelaxation provides further evidence that oxidative stress and glutathione depletion is part of the systemic manifestation of AIDS (20). These and other experimental observations suggest that enhancing antioxidant defenses could be a therapeutic strategy to protect cells and tissues against oxidative stress during HIV-1 infection. Importantly, targeted activation of Nrf2 activity with agents such as sulforaphane, which is already being widely tested in diverse clinical trials, has the advantage of restoring the cells' ability to turn on the expression of an entire program of antioxidants and therefore is likely to be much more effective than monotherapy with specific antioxidants.
A concern inherent in our studies is that we used a noninfectious HIV-1 Tg rat model to test our hypothesis that HIV-1-related viral proteins induce oxidative stress and alveolar epithelial barrier dysfunction by inhibiting Nrf2/ARE activity. However, there is now overwhelming evidence that many of the manifestations of AIDS are not due to direct viral infection per se, but rather to the toxic effects of HIV-1-related proteins such as Tat and gp120 that circulate systemically even when viral loads are suppressed by HAART. In this context, the HIV-1 Tg rodent models, including the rat model we have used, was developed specifically to study these “noninfectious” aspects of AIDS. This HIV-1 Tg rat develops a progressive AIDS-like phenotype as it ages and begins to display systemic findings that mimic clinical AIDS by the age of 5–7 mo (38). This is consistent with studies showing that the HIV-1 viral proteins gp120 and Tat directly affect blood-brain barrier and epithelial barrier function (11, 22, 30). Importantly, this animal model has provided an opportunity to examine the fundamental mechanisms by which these proteins cause oxidative stress and cellular dysfunction within the alveolar space, including these new observations regarding a potential role for Nrf2 expression that can now be translated to the clinical setting to determine if this mechanism is involved in human HIV-1 infection.
In summary, we provide evidence that Nrf2 activity is necessary to maintain redox balance, tight junction protein expression and membrane assembly, and barrier function in the alveolar epithelium and that its expression and ability to activate the ARE is significantly decreased by exposure to HIV-1-related proteins. In contradistinction, augmenting Nrf2 expression and/or activity mitigated the oxidative stress and barrier dysfunction in the alveolar epithelia of HIV-1 Tg rats. Although these experimental findings need to be translated to the clinical setting, this study raises the intriguing possibility that enhancing global antioxidant defenses by directly activating the Nrf2/ARE pathway could improve lung health in susceptible individuals with HIV-1 infection, chronic alcohol abuse, and/or other chronic oxidative stresses that render them susceptible to both infectious and noninfectious forms of lung injury.
GRANTS
This work was supported by National Institute on Alcoholism and Alcohol Abuse Grants R01-AA-017627 and P50-AA-013757 to P. C. Joshi, M. Koval, and D. M. Guidot and a Veterans Affairs Merit Review to D. M. Guidot.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.F. and D.M.G. conception and design of research; X.F., B.S.S., J.S.J., K.J.M., and J.A.G. performed experiments; X.F. analyzed data; X.F., P.C.J., M.K., and D.M.G. interpreted results of experiments; X.F. prepared figures; X.F. and D.M.G. drafted manuscript; X.F., M.K., and D.M.G. edited and revised manuscript; X.F. and D.M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Robert Raynor and S. Todd Mills for invaluable technical assistance with this project.
REFERENCES
- 1. Afessa B, Green W, Chiao J, Frederick W. Pulmonary complications of HIV infection: autopsy findings. Chest 113: 1225–1229, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Anderson CL, Iyer SS, Ziegler TR, Jones DP. Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione. Am J Physiol Regul Integr Comp Physiol 293: R1069–R1075, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Athale J, Ulrich A, Chou Macgarvey N, Bartz RR, Welty-Wolf KE, Suliman HB, Piantadosi CA. Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic Biol Med 53: 1584–1594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Awodele O, Olayemi SO, Nwite JA, Adeyemo TA. Investigation of the levels of oxidative stress parameters in HIV and HIV-TB co-infected patients. J Infect Dev Ctries 6: 79–85, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free Radic Biol Med 48: 1388–1398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banks WA, Ercal N, Price TO. The blood-brain barrier in neuroAIDS. Curr HIV Res 4: 259–266, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Biswal S, Thimmulappa RK, Harvey CJ. Experimental Therapeutics of Nrf2 as a Target for Prevention of Bacterial Exacerbations in COPD. Proc Am Thorac Soc 9: 47–51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen H, Hu Y, Fang Y, Djukic Z, Tamamoto M, Shaheen NJ, Orlando RC, Chen X. Nrf2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut. Doi: 10.1136/gutjnl-2012-3-3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol 244: 43–56, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 8: 76–87, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Cioni C, Annunziata P. Circulating gp120 alters the blood-brain barrier permeability in HIV-1 gp120 transgenic mice. Neurosci Lett 330: 299–301, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Dash PK, Zhao J, Orsi SA, Zhang M, Moore AN. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci Lett 460: 103–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davaro RE, Thirumalai A. Life-threatening complications of HIV infection. J Intensive Care Med 22: 73–81, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, Amasheh M, Loddenkemper C, Fromm M, Zeitz M, Schulzke JD. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 58: 220–227, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Fan X, Joshi PC, Koval M, Guidot DM. Chronic alcohol ingestion exacerbates lung epithelial barrier dysfunction in HIV-1 transgenic rats. Alcohol Clin Exp Res 35: 1866–1875, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandez AL, Koval M, Fan X, Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol 41: 371–379, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guidot DM, Hart CM. Alcohol abuse and acute lung injury: epidemiology and pathophysiology of a recently recognized association. J Investig Med 53: 235–245, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46: 443–453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joshi PC, Raynor R, Fan X, Guidot DM. HIV-1 transgenic expression in rats decreases alveolar macrophage zinc levels and phagocytosis. Am J Respir Cell Mol Biol 39: 218–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kline ER, Kleinhenz DJ, Liang B, Dikalov S, Guidot DM, Hart CM, Jones DP, Sutliff RL. Vascular oxidative stress and nitric oxide depletion in HIV-1 transgenic rats are reversed by glutathione restoration. Am J Physiol Heart Circ Physiol 294: H2792–H2804, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koval M. Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol 75: 551–567, 2013 [DOI] [PubMed] [Google Scholar]
- 22. Lassiter C, Fan X, Joshi PC, Jacob BA, Sutliff RL, Jones DP, Koval M, Guidot DM. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction (Abstract). AIDS Res Ther 6: 1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr Comp Biol 50: 829–843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med 178: 592–604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, Wise R, Tuder R, Biswal S. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med 180: 1196–1207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, Kleeberger SR. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J 21: 2237–2246, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA 91: 9926–9930, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris A, George MP, Crothers K, Huang L, Lucht L, Kessinger C, Kleerup EC. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc 8: 320–325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Napolitano M, Rainaldi G, Bravo E, Rivabene R. Influence of thiol balance on micellar cholesterol handling by polarized Caco-2 intestinal cells. FEBS Lett 551: 165–170, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 6: e1000852, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel RB, Kotha SR, Sauers LA, Malireddy S, Gurney TO, Gupta NN, Elton TS, Magalang UJ, Marsh CB, Haley BE, Parinandi NL. Thiol-redox antioxidants protect against lung vascular endothelial cytoskeletal alterations caused by pulmonary fibrosis inducer, bleomycin: comparison between classical thiol-protectant, N-acetyl-l-cysteine, and novel thiol antioxidant, N,N′-bis-2-mercaptoethyl isophthalamide. Toxicol Mech Methods 22: 383–396, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pelaez A, Bechara RI, Joshi PC, Brown LA, Guidot DM. Granulocyte/macrophage colony-stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol 286: L106–L111, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res 1045: 57–63, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Pu H, Tian J, Andras IE, Hayashi K, Flora G, Hennig B, Toborek M. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J Cereb Blood Flow Metab 25: 1325–1335, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci 13: 7210–7226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol 182: 7264–7271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reddy NM, Kleeberger SR, Yamamoto M, Kensler TW, Scollick C, Biswal S, Reddy SP. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol Genomics 32: 74–81, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA 98: 9271–9276, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol 130: 244–251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Starrett W, Blake DJ. Sulforaphane inhibits de novo synthesis of IL-8 and MCP-1 in human epithelial cells generated by cigarette smoke extract. J Immunotoxicol 8: 150–158, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Toborek M, Lee YW, Flora G, Pu H, Andras IE, Wylegala E, Hennig B, Nath A. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol 25: 181–199, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol 297: L219–L227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiang J, Alesi GN, Zhou N, Keep RF. Protective effects of isothiocyanates on blood-CSF barrier disruption induced by oxidative stress. Am J Physiol Regul Integr Comp Physiol 303: R1–R7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci 27: 10240–10248, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao J, Redell JB, Moore AN, Dash PK. A novel strategy to activate cytoprotective genes in the injured brain. Biochem Biophys Res Commun 407: 501–506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]