Abstract
Alveolar type II (ATII) epithelial cells play a crucial role in the repair and remodeling of the lung following injury. ATII cells have the capability to proliferate and differentiate into alveolar type I (ATI) cells in vivo and into an ATI-like phenotype in vitro. While previous reports indicate that the differentiation of ATII cells into ATI cells is a complex biological process, the underlying mechanism responsible for differentiation is not fully understood. To investigate factors involved in this differentiation in culture, we used a PCR array and identified several genes that were either up- or downregulated in ATI-like cells (day 6 in culture) compared with day 2 ATII cells. Insulin-like growth factor-I (IGF-I) mRNA was increased nearly eightfold. We found that IGF-I was increased in the culture media of ATI-like cells and demonstrated a significant role in the differentiation process. Treatment of ATII cells with recombinant IGF-I accelerated the differentiation process, and this effect was abrogated by the IGF-I receptor blocker PQ401. We found that Wnt5a, a member of the Wnt-Frizzled pathway, was activated during IGF-I-mediated differentiation. Both protein kinase C and β-catenin were transiently activated during transdifferentiation. Knocking down Wnt5a using small-interfering RNA abrogated the differentiation process as indicated by changes in the expression of an ATII cell marker (prosurfactant protein-C). Treatment of wounded cells with either IGF-I or Wnt5a stimulated wound closure. These results suggest that IGF-I promotes differentiation of ATII to ATI cells through the activation of a noncanonical Wnt pathway.
Keywords: alveolar epithelial cell, prosurfactant protein-C, alveolar type I, alveolar type II
alveolar type ii (ATII) cells are facultative progenitor cells that play a crucial role in the repair of the lung epithelium following injury (9, 32). Following lung injury, ATII cells spread, migrate, proliferate, and differentiate into alveolar type I (ATI) cells to cover the denuded surface. The underlying mechanism of this differentiation process is not fully understood (1, 8, 17, 45), but some aspects of this process are recapitulated in the differentiation of cultured ATII cells to ATI-like cells (3, 4, 10, 15, 20, 21). The microenvironment of the injured alveolus includes many cytokines, chemokines, and growth factors secreted from injured tissues, immune cells, and nonimmune cells (9). One of these components, insulin-like growth factor-I (IGF-I), has previously been shown to be involved in epithelial differentiation in the lungs (31, 35, 37). Alveolar epithelial cells and resident macrophages are the major two cells producing IGF-I in lung (27, 31, 37, 44, 46). Mice deficient in either IGF-I or its receptor exhibited lung hypoplasia and high mortality rates associated with respiratory failure (2, 38). Embryonic lungs of mice with deletion of the IGF-I gene had an increased proportion of ATII cells and less differentiated ATI cells (35). ATII cells isolated from hyperoxia-treated lungs transiently expressed increased IGF-I during transdifferentiation into type I-like cells (37). Although these studies suggest an important role for IGF-I in the differentiation of ATII cells, the underlying mechanism is not fully understood.
In the current study, we investigated the role of IGF-I in the differentiation of cultured rat ATII. Consistent with previous reports, we observed that ATII cells differentiate to ATI-like cells after 6 days of culture, and IGF-I expression was increased in ATI-like cells. We hypothesized that IGF-I-mediated signaling in ATII cell differentiation occurs through the Wnt-Frizzled (Wnt-Fzd) pathway based upon previous reports describing an association of the Wnt-Fzd system with IGF-I during organ development (39, 42). In addition, Wnt/β-catenin signaling has been associated with aberrant epithelial-mesenchymal transition in idiopathic pulmonary fibrosis (6, 32, 34) and an experimental model of emphysema (31). Targeted expression of an activated form of β-catenin caused ectopic differentiation of ATII cells in conducting airways (36). We found that IGF-I activates Wnt5a, which regulates the differentiation of ATII cells into ATI-like cells.
MATERIALS AND METHODS
Reagents.
Dulbecco's modified Eagle's medium (DMEM), penicillin-streptomycin, trypsin-EDTA solution, and PBS were purchased from GIBCO Life Technologies (Grand Island, NY). FBS was obtained from Hyclone (Logan, UT). HEPES, KCl, MgCl2, Triton X-100, sodium vanadate, phenylmethylsulfonyl fluoride, aprotinin, and leupeptin were purchased from Sigma (St. Louis, MO). Tween 20 was purchased from Bio-Rad (Hercules, CA). DRAQ-5 (nuclear stain) was obtained from Molecular Probes (Eugene, OR). Rat IGF-I was purchased from Peprotech (Rocky Hill, NJ). The specific blocker for IGF receptor, PQ401, was purchased from Sigma Chemicals. Gradient gels (4–12%) for Western blot, and 10% gelatin gels, were purchased from Invitrogen (Carlsbad, CA). Antibodies for Wnt5a and Wnt3a were purchased from R & D (Minneapolis, MN). Control small-interfering RNA (siRNA) and Wnt5a siRNA were purchased from Dharmacon (Lafayette, CO). Anti-rat antibodies for prosurfactant protein C (pro-SPC), a marker for ATII cells, were purchased from Millipore (Billerica, MA). Antibodies for rTI40 and rTII70, which are markers for rat ATI and ATII cells, respectively, were generous gifts from Dr. Leland Dobbs, University of California-San Francisco. Antibodies for pan-protein kinase C (PKC) and β-catenin were purchased from abCAM (Cambridge, MA) and Cell Signaling Technology (Denver, CO), respectively.
Cell culture.
Primary rat ATII cells were isolated according to the methods described previously (13, 14). The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center. Briefly, ATII cells were isolated from male Sprague-Dawley rats by elastase digestion and differential adherence on IgG-coated dishes. ATII cells were identified using Nile Red (Sigma-Aldrich) staining of lamellar bodies, and >95% of the cells were Nile Red-positive on day 2. We used plastic six-well plates that were coated with rat lung fibroblast (RLF) matrix deposited by RLF-6 cells (American Type Culture Collection) for culture of ATII cells (13). Freshly isolated cells were seeded to confluence at 3.5 × 106 cells/well in ATII culture medium (DMEM with 10% FBS, 4 mM glutamine, 1% penicillin/streptomycin, and 0.25 μM amphotericin B), and experiments were performed on day 2 after isolation. To obtain ATI-like cells, ATII cells were cultured until day 6 from the day of isolation with changing of the media every day. On day 2 or day 6, cells were harvested, and lysate was made. Lysate was stored at −70°C for future use. Media were collected and stored at −70°C for estimation of IGF-I. A549 cells and MLE-12 cells were cultured in DMEM with 10% FBS and 0.5% penicillin/streptomycin.
Treatment of ATII cell.
Cells were treated with IGF-I at 50 ng/ml as indicated. PQ401 (IGF receptor blocker) was used at a dose of 2 μg/ml. The incubation times for both IGF-I and blocker were 24 h wherever applicable. There was no toxicity or cell death observed by Trypan blue exclusion test after 24 h of incubation for either of those reagents. For combined treatment, cells were treated with PQ401 for 2 h before addition of IGF-I.
PCR array.
A 96-well-based quantitative real-time PCR Array kit that targets rat stem cell-associated genes was purchased from SA-Biosciences (Valencia, CA) (catalog no. PARN-405). Total RNA was isolated from ATII and ATI-like cells followed by conversion of RNA to cDNA using a kit (Bio-Rad). Equal amounts of RNA were used (500 ng) from ATII and ATI-like cells to make cDNA. Real-time PCR array reagents were purchased from SA-Bioscience. Raw data of real-time PCR were analyzed by using software provided by SA-Bioscience.
Luminex assay.
Phosphorylation of insulin receptor substrate-1 was examined by running a Luminex-based assay kit purchased from Millipore (catalog no. 48–61). ATII cells were treated with IGF-I for different time points, and lysate was made using the lysis buffer supplied in the kit. Equal amounts of protein (50 μg) were used for the assay. All the steps were performed according to instructions of the manufacturer.
ELISA of IGF-I.
The amount of secreted IGF-I was measured from media of ATII and ATI-like cultured cells using an ELISA kit purchased from Mediagnost (Reutlingen, Germany) (catalog no. m/rIGF-I ELISA). Media were collected when cells became confluent on the plates. Multiple scratch wounds were made on the confluent layer of cells following the method previously described (12, 19). The amount of IGF-I secreted from the cells after 24 h was determined.
Wound healing assay.
Cell migration was measured according to our previous methods (12, 19). Confluent monolayers of ATII or MLE-12 cells were wounded by scraping a pipette tip across the monolayer to produce initial wounds of 1,000–1,200 μm. Images were collected with a Cool Snap charge-coupled device camera (Roper Scientific, Trenton, NJ) mounted on an Eclipse TE300 inverted microscope with a 4× objective (Nikon, Melville, NY). Images were obtained at the initial time of wounding and then 24 h postwounding. Metamorph software was used to record the coordinates for each wound location using a computer-controlled stage so that the same location was used for postwounding measurements, and data were analyzed by Metamorph imaging software. The mean wound width at 24 h was calculated and normalized to the original wound width. All results reported are based on at least three independent wells of two separate experiments (n = 6).
SDS-PAGE.
Protein concentration was determined by the Bradford (5) method. Equal amounts of protein were electrophoretically separated by 4–12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Membranes were blocked for 1 h in 5% nonfat milk (Bio-Rad) in Tris-buffered saline containing 0.01% Tween 20 (TBST) and incubated overnight at 4°C with the appropriate primary antibody. Membranes were washed with TBST and then incubated with the secondary antibody for 1 h at room temperature. Finally blots were developed on X-ray film using the Luminata method (Millipore).
Transient knockdown of Wnt5a in ATII cells.
Wnt5a was transiently knocked down in ATII cells using siRNA purchased from Dharmacon. Subconfluent (50–60%) ATII cells were transfected with Wnt5a siRNA or control siRNA using the siRNA transfection kit (Santa Cruz, CA). After 48 h of transfection, cells were harvested, and lysate was made for immunoblot.
Confocal microscopy.
Expression of pro-SPC in ATII cells was assessed by confocal microscopy. ATII cells transfected with Wnt5a or control siRNA were fixed with 4% paraformaldehyde. Cells were permeabilized with 0.1% Triton X-100 followed by blocking with 2% BSA. Pro-SPC primary antibody was incubated at 1:100 dilution overnight at 4°C. The secondary antibody labeled with Alexa fluor-488 (Molecular Probes, Eugene, OR) was used at 1:500 dilution at room temperature for 30 min. The nucleus was stained with DRAQ-5 (Molecular Probes) at 1:1,000 dilution for 15 min at room temperature. Images were obtained using a confocal microscope (Olympus) using a ×20 objective.
Densitometry of immunoblot.
Densitometry of bands obtained by immunoblot was performed using the software Image-J developed by the National Institutes of Health. Each band was normalized against the corresponding loading control, β-actin, or GAPDH.
Quantification of immunofluorescence.
Immunofluorescence was quantified using the mean fluorescence intensity determined using Image-J software. Mean fluorescence intensity was measured from five different fields that were randomly selected.
Statistical analysis.
All values are presented as means ± SE. Each value represented the mean of at least three independent experiments with SE. A t-test was used when only two groups were compared, and one-way ANOVA with the Holm-Sidak method was performed for comparisons of multiple treatments to determine significant differences between individual conditions. Significant differences were determined based on a threshold of P < 0.05. Statistical comparisons were made using SigmaStat 3.5 (Jandel Scientific, San Rafael, CA).
RESULTS
ATII cells differentiate to ATI-like cells in vitro.
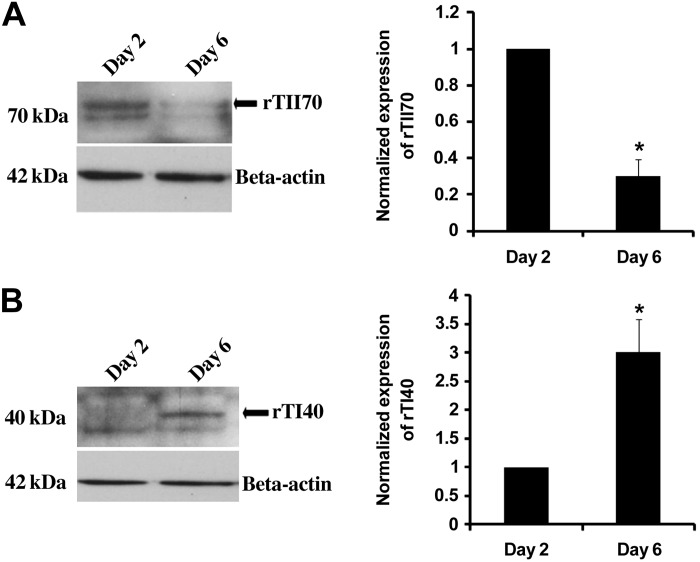
To confirm previous findings that ATII cells differentiate into ATI-like cells in culture, we compared ATII cells isolated from rats after 2 or 6 days of culture. The ATII surface marker rTII70 was significantly decreased after 6 days in culture compared with day 2 cells (Fig. 1A), whereas the ATI surface marker rTI40 was significantly higher (P < 0.01) on the 6th day compared with day 2 ATII cells (Fig. 1B).
Fig. 1.
Alveolar type II (ATII) cells differentiate to alveolar type I (ATI)-like cells in vitro. ATII cells were isolated from rats and cultured for 2 or 6 days. Surface markers of ATII (rTII70) and ATI (rTI40) were immunoblotted from the lysate (A and B, respectively). β-Actin was used as a loading control. Representative immunoblots and densitometry from three different isolations are shown. *Value was significant at P < 0.05 level compared with day 2 (n = 3).
IGF-I was upregulated during differentiation of ATII cells.
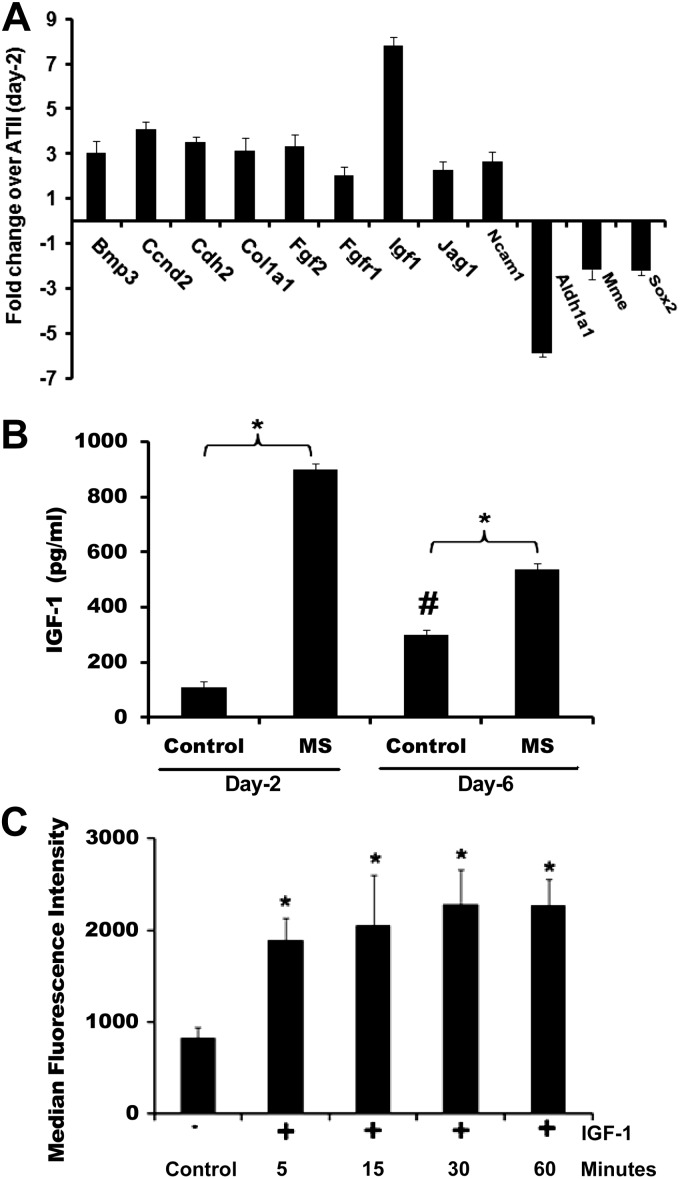
To gain insight into the mechanisms of this differentiation, we used a PCR-based array that targeted the genes of rat stem cell differentiation to compare day 2 ATII cells with day 6 ATI-like cells. As shown in Fig. 2A, at least nine genes were upregulated, and three genes were downregulated more than twofold. Among the upregulated genes, IGF-I was increased approximately eightfold in ATI-like cells compared with ATII cells. To determine whether secretion of IGF-I was increased in ATI-like cells, we measured the IGF-I from conditioned media. Secretion of IGF-I was significantly increased in ATI-like cells compared with ATII cells after 24 h (Fig. 2B). Interestingly, when we applied multiple scratch wounds to cell monolayers, there was a significant increase in IGF-I secretion in both ATII and ATI-like cells (Fig. 2B). To determine the responsiveness of ATII cells to IGF-I in our system, we measured the phosphorylation of insulin receptor substrate-1 (22) in response to exogenous IGF-I using the Luminex system. Treatment with IGF-I (50 ng/ml) caused a significant increase in phosphorylation of insulin receptor substrate-1 in ATII cells within 5 min that was sustained for at least 60 min (Fig. 2C).
Fig. 2.
Insulin-like growth factor-I (IGF-I) was upregulated during differentiation of ATII to ATI-like cells. A: mRNA was collected from ATII (day 2) and ATI-like (day 6) cells and converted to cDNA using an RT-PCR kit. PCR Array was run according to the procedure described in materials and methods. Values indicate the fold change relative to day 2 ATII cells (n = 3). B: IGF-I was secreted from wounded and unwounded ATII and ATI-like cells. Multiple scratch wounds were used to increase the percentage of cells near a wound edge. IGF-I was determined by ELISA. *Significant difference from unwounded cells; #significant difference from day 2 ATII cells (P < 0.05, n = 3–9). C: phosphorylation of insulin receptor substrate-1 was examined using the Luminex system. ATII cells (day 2) were treated with IGF-I (50 ng/ml) for the indicated times, and lysate was collected. *Significant difference from untreated cells (P < 0.05; n = 6).
Activation of Wnt5a and Wnt3a during transdifferentiation.
Because the Wnt-Fzd system has previously been shown to be involved in epithelial cell differentiation, we investigated the expression of Wnt5a and Wnt3a in ATII cells as a function of time in culture. Wnt5a is part of the noncanonical Wnt pathway, whereas Wnt3a is part of the canonical pathway. Both Wnt5a and Wnt3a were upregulated during the course of differentiation in culture, but, while Wnt5a expression peaked by day 4 and remained elevated, Wnt3a expression peaked by day 3 and then declined (Fig. 3). In parallel, we examined the level of pro-SPC, an ATII cell marker during the course of differentiation. We observed that the amount of pro-SPC was high in the first 2 days in culture but decreased significantly by day 3 (Fig. 3, C and D). For comparison, we measured the expression of Wnt5a and pro-SPC in A549 cells following treatment with IGF-I for 72 h. Although the magnitude of the changes was not as high as in primary ATII cells, we found that treatment with IGF-I did cause a significant decrease in expression of pro-SPC and a significant increase in expression of Wnt5a (Fig. 3, E and F).
Fig. 3.
Activation of Wnt3a and Wnt5a during transdifferentiation. Representative immunoblots of Wnt3a, Wnt5a, and prosurfactant protein-C (pro-SPC) from lysate from ATII cells cultured from 0 to 6 days are shown (A, B, and C, respectively). β-Actin was immunoblotted as a loading control. D: densitometry for Wnt3a, Wnt5a, and Pro-SPC (n = 4). *Significant difference from day 0 (P < 0.05). E and F: representative immunoblots and densitometry of Wnt5a (n = 5) and pro-SPC (n = 3) in A549 cells treated with IGF-I for 72 h. *Significant difference from untreated control (P < 0.05).
Upregulation of pan-PKC and β-catenin during transdifferentiation of ATII cells.
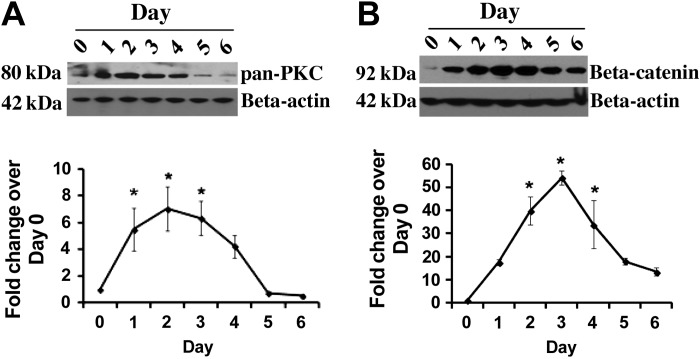
Activation of Wnt5a and Wnt3a is associated with upregulation of PKC and β-catenin, respectively. Therefore, we investigated the status of these two proteins during differentiation of ATII cells to ATI cells. Immunoblots demonstrated that upregulation of both pan-PKC and β-catenin occurred during differentiation of ATII cells (Fig. 4). The maximal level was observed at day 2 for pan-PKC and day 3 for β-catenin followed by gradual decline in the expression of each.
Fig. 4.
Activation of protein kinase C (PKC) and β-catenin during transdifferentiation. Representative immunoblots of pan-PKC and β-catenin from lysate from ATII cells cultured from 0 to 6 days are shown (A and B). β-Actin was immunoblotted as a loading control. Bottom: densitometry for pan-PKC (n = 4) and β-catenin (n = 3). *Significant difference from day 0 (P < 0.05).
Wnt5a upregulation was stimulated by IGF-I.
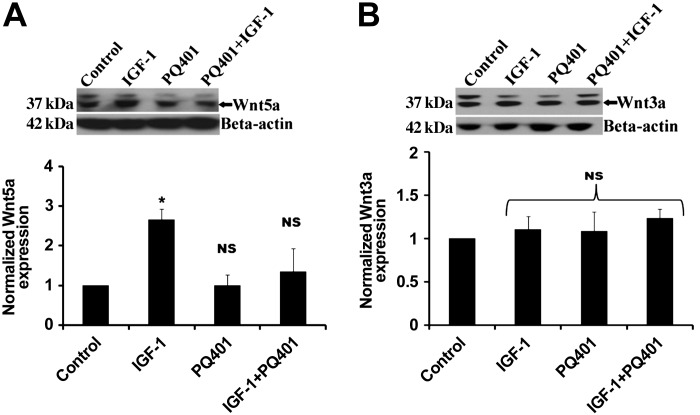
To determine whether Wnt5a or Wnt3a induction was stimulated by IGF-I, we treated ATII cells from day 2 with IGF-I (50 ng/ml) for 24 h and immunoblotted for Wnt5a and Wnt3a. As shown in Fig. 4A, IGF-I treatment stimulated increased expression of Wnt5a (Fig. 5A) but not Wnt3a (Fig. 5B). When cells were pretreated with the IGF-I receptor blocker PQ401 before IGF-I, there was no increase in Wnt5a expression.
Fig. 5.
Wnt5a activation was stimulated by IGF-I. ATII cells (day 2) were treated with IGF-I (50 ng/ml), PQ401 (500 ng/ml), or the combination for 24 h, and lysates were collected. Representative immunoblots of Wnt5a and Wnt3a are shown (A and B) along with densitometry for Wnt5a (n = 4) and Wnt3a (n = 4). *Significant difference from untreated controls (P < 0.05). NS, values were not significant compared with control.
Downregulation of Wnt5a delays transdifferentiation.
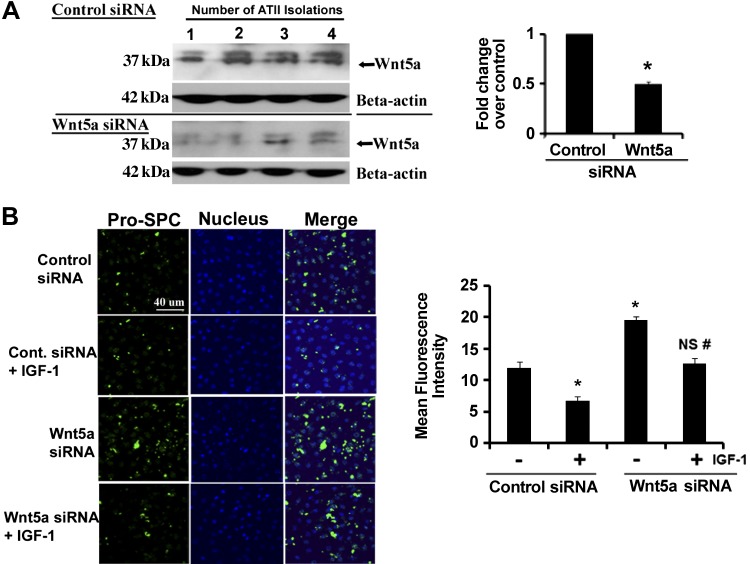
To examine the role of Wnt5a in the differentiation of ATII cells, we knocked down Wnt5a using siRNA. Figure 6A shows that siRNA directed against Wnt5a caused a 50% reduction in expression compared with cells transfected with a control siRNA. To determine the effect of Wnt5a siRNA on transdifferentiation, we measured the expression of pro-SPC as a phenotypic marker of ATII cells. When ATII cells were transfected with control siRNA on day 1 and treated with IGF-I for 24 h beginning on day 2, there was a loss of pro-SPC expression (Fig. 6B). Cells with knockdown of Wnt5a showed markedly increased pro-SPC expression in the absence of IGF-I, but treatment with IGF-I reduced pro-SPC expression in these cells (Fig. 6B).
Fig. 6.
Knockdown of Wnt5a by small-interfering RNA (siRNA) increased the expression of pro-SPC. Wnt5a was knocked down by siRNA in ATII cells according to the procedure described in materials and methods. A: immunoblot of Wnt5a from lysates from 4 different isolations with corresponding densitometry. β-Actin was used as a loading control. *Significant difference from cells transfected with control siRNA (P < 0.05). B: expression of pro-SPC was examined by immunofluorescence in control and Wnt5a siRNA-transfected cells. Mean fluorescence intensity was measured in 5 different fields from 5 different experiments. *Significant difference from cells transfected with control siRNA (P < 0.05; n = 5); NS, values were not significantly different from control siRNA; #significant difference from cells transfected with Wnt5a siRNA.
IGF-I and Wnt5a augment wound healing in MLE-12 cells.
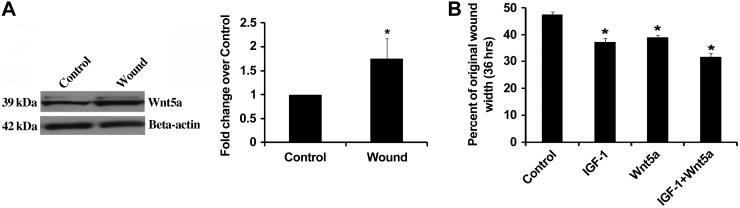
To determine whether Wnt5a expression was changed following injury, we measured the expression of Wnt5a in a cell line of mouse lung epithelial cells (MLE-12) exposed to multiple scratch wounds. Compared with unwounded cells, cells exposed to multiple scratch wounds had significantly increased Wnt5a expression after 36 h (Fig. 7A). We then investigated whether treatment with either IGF-I or Wnt5a would affect wound healing following a scratch wound. Figure 7B shows that wound closure was stimulated by treatment with either IGF-I, Wnt5a, or the combination of the two.
Fig. 7.
Wounding stimulated Wnt5a expression, and IGF-I and Wnt5a augmented wound healing in MLE-12 cells. A: MLE-12 cells were either unwounded (control) or wounded with multiple scratches, and Wnt5a expression was determined from cell lysates after 36 h. A representative immunoblot is shown, and densitometry is summarized. *Significant difference from unwounded control (P < 0.05, n = 3). B: MLE-12 cells were wounded and then treated with either murine IGF-I (50 ng/ml), murine recombinant Wnt5a (100 ng/ml), or the combination of the two. IGF-I and Wnt5a were added at time 0 and at 24 h, and wound width measurements were made at the initial time and after 36 h. *Significant difference compared with control (P < 0.05; n = 6).
DISCUSSION
Epithelial repair following lung injury involves differentiation of ATII cells, but the mechanisms involved in ATII cell differentiation into ATI cells is not fully understood. Consistent with previous reports (3, 15, 20, 21), our data showed that ATII cells in culture differentiated into ATI-like cells over 2–6 days as indicated by specific markers rTII70 and rTI40 (Fig. 1). To investigate factors involved in this differentiation in culture, we used a PCR array targeted at stem cell differentiation and identified several genes that were either up- or downregulated in ATI-like cells (day 6 in culture) compared with day 2 ATII cells. IGF-I mRNA was increased nearly eightfold (Fig. 2A). We then measured IGF-I protein levels in the conditioned media and found a significant increase in IGF-I in ATI-like cells compared with ATII cells (Fig. 2B). Gonzalez et al. (20) had previously performed a comparison of freshly isolated ATII cells (day 0) and freshly isolated ATI cells (day 0) from rats. Using microarray analysis, they found a 5.5-fold increase in gene expression of IGF-binding protein 6 (IGFBP6) in ATI cells compared with ATII cells. IGFBP6 is expressed in the lung and regulates the bioavailability of IGF-I by binding with its active conformation (41). While our data and the microarray analysis of Gonzalez et al. (20) suggested differential expression of numerous other genes between ATII and ATI cells (and between ATII cells cultured for 6 days), these results support a potential role for IGF-I in the differentiation of ATII cells to ATI cells.
Previous studies have suggested that IGF-I is elevated in the bronchoalvoelar lavage fluid in patients with early acute respiratory distress syndrome (ARDS) (40), and patients with fibroproliferative ARDS exhibited increased IGF-I immunostaining in biopsy tissue sections (27). IGF-I mRNA was also elevated in a mouse model of bleomycin-induced pulmonary fibrosis (30), and the use of IGF-I receptor antibodies improved survival of mice with bleomycin-induced lung injury (7). Based upon these findings, it has been proposed that IGF-I may promote the survival of lung fibroblasts and subsequent fibrosis. However, an additional role of IGF-I may be the promotion of differentiation of ATII to ATI cells following injury. We found that IGF-I secretion was significantly increased in both ATII and ATI-like cells following a scratch wound injury (Fig. 2B). A previous report demonstrated that treatment of ATII cells with antibodies against IGF-I or the IGF I receptor inhibited transdifferentiation (as indicated by expression of ATII and ATI markers) when the cells were isolated from rats following exposure to hyperoxia in vivo (37). This study also found that mRNA expression for IGF-I, IGF-II, and the IGF-I receptor increased during exposure to hyperoxia in vivo, and protein levels remained elevated during 3 days of recovery and then decreased. Ionescu et al. (24) demonstrated that treatment with recombinant IGF-I reduced LPS-induced lung injury in mice and suggested that IGF-I may be secreted by mesenchymal stem cells during repair following injury. To investigate the signaling pathways initiated by IGF-I, we first examined the phosphorylation of insulin receptor substrate-1, which binds with the IGF-I receptor and becomes phosphorylated (22). Luminex data demonstrated the rapid phosphorylation of the insulin receptor substrate-1 upon treatment with IGF-I, indicating activation of the IGF-I receptor (Fig. 2C).
We then investigated the activation of the Wnt-Fzd system since previous studies have suggested that IGF-I can activate this system during organ development (39, 42). In addition, mircroarray comparison between freshly isolated ATII and ATI cells demonstrated a fourfold increase in expression of Fzd-2 in ATI cells relative to ATII cells (20). Surprisingly, we found that activation of both canonical (Wnt3a) and noncanonical (Wnt5a) Wnt pathways occurred in ATII cells with time in culture (Fig. 3). Wnt5a has previously been shown to antagonize canonical Wnt signaling in esophageal carcinoma cells and other cell lines (28, 34, 43), but, to our knowledge, this is the first report in primary ATII cells. We found that expression of both Wnt3a and Wnt5a increased until day 4 in culture at which time Wnt3a expression decreased and Wnt5a remained stable. Wnt3a signaling involves stabilization and activation of β-catenin, whereas Wnt5a signaling involves stimulation of intracellular Ca2+ release and activation of PKC (34). To provide additional support for the simultaneous activation of Wnt5a and Wnt3a, we examined the expression PKC and β-catenin (23, 29, 33, 47). We found that both were transiently upregulated during the differentiation of ATII to ATI-like cells (Fig. 4). We also found that treatment of A549 cells with IGF-I stimulated a decrease in pro-SPC expression and an increase in Wnt5a expression (Fig. 3).
Our results showing increased Wnt3a expression are consistent with the previous finding that β-catenin signaling was not present in freshly isolated ATII cells but increased by day 2 in culture (18). Forced inhibition of β-catenin signaling caused increased cell death and reduced expression of ATI cell markers by day 3 in culture. Flozak et al. (18) also demonstrated that adult ATII cells did not exhibit constitutive β-catenin signaling in vivo using the AXIN2+/LacZ reporter mouse, but β-catenin was activated in ATII cells following bleomycin injury. β-Catenin pathways are reported to be induced in idiopathic pulmonary fibrosis or bleomycin-induced lung fibrosis, which are associated with epithelial-to-mesenchymal transition of alveolar epithelial cells (6, 26). We found for the first time that the activation of Wnt5a was dependent upon IGF-I signaling, but activation of Wnt3a was not affected by IGF-I (Fig. 5). To further confirm the importance of Wnt5a in ATII cell transdifferentiation, we showed that siRNA knockdown of Wnt5a decreased expression of pro-SPC in control and IGF-I-treated ATII cells (Fig. 6).
Previous studies have suggested that IGF-I can stimulate wound repair in other organs (11, 16, 25). In preliminary studies, we found that treatment of rat ATII cells with IGF-I did not stimulate wound closure over 16 h in a scratch wound model (data not shown). It is possible that a longer exposure time is necessary to observe effects on wound closure, but we were concerned that our studies might be complicated by the changes taking place in the cells during transdifferentiation. Therefore, we examined the response in MLE-12 cells over 36 h. We found that both IGF-I and Wnt5a treatment stimulated wound closure in a scratch wound assay (Fig. 7).
Taken together, our results and the previous studies suggest that both Wnt3a and Wnt5a activation occurs in response to injury and upon culture of freshly isolated ATII cells to initiate differentiation into ATI cells. However, activation of Wnt5a may ultimately antagonize Wnt3a signaling while further promoting the transition. Our results suggest that secretion of IGF-I from ATII cells results in autocrine activation of Wnt5a signaling that promotes both wound repair and differentiation.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.C.G. and C.M.W. conception and design of research; M.C.G., V.K.G., P.S.M., and C.L.L. performed experiments; M.C.G. and C.M.W. analyzed data; M.C.G., P.S.M., S.E.S., A.S., and C.M.W. interpreted results of experiments; M.C.G. and C.M.W. prepared figures; M.C.G. and C.M.W. drafted manuscript; M.C.G., P.S.M., S.E.S., A.S., and C.M.W. edited and revised manuscript; M.C.G., P.S.M., S.E.S., A.S., and C.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-094366.
REFERENCES
- 1. Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Tnvest 30: 35–42, 1974 [PubMed] [Google Scholar]
- 2. Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75: 73–82, 1993 [PubMed] [Google Scholar]
- 3. Borok Z, Danto SI, Lubman RL, Cao Y, Williams MC, Crandall ED. Modulation of t1alpha expression with alveolar epithelial cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 275: L155–L164, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, Lee DM, Agre P, Crandall ED. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol 18: 554–561, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 6. Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162: 1495–1502, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi JE, Lee SS, Sunde DA, Huizar I, Haugk KL, Thannickal VJ, Vittal R, Plymate SR, Schnapp LM. Insulin-like growth factor-I receptor blockade improves outcome in mouse model of lung injury. Am J Respir Crit Care Med 179: 212–219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clegg GR, Tyrrell C, McKechnie SR, Beers MF, Harrison D, McElroy MC. Coexpression of RTI40 with alveolar epithelial type II cell proteins in lungs following injury: identification of alveolar intermediate cell types. Am J Physiol Lung Cell Mol Physiol 289: L382–L390, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298: L715–L731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol 12: 497–502, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Delafontaine P. Insulin-like growth factor I and its binding proteins in the cardiovascular system. Cardiovasc Res 30: 825–834, 1995 [PubMed] [Google Scholar]
- 12. Desai LP, Aryal AM, Ceacareanu B, Hassid A, Waters CM. RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L1134–L1144, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol 295: L958–L965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 258: L134–L147, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Dobbs LG, Williams MC, Brandt AE. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta 846: 155–166, 1985 [DOI] [PubMed] [Google Scholar]
- 16. el Nahas AM. The role of growth hormone and insulin-like growth factor-I in experimental renal growth and scarring. Am J Kidney Dis 17: 677–679, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22: 142–150, 1975 [DOI] [PubMed] [Google Scholar]
- 18. Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA, Beri R, Mutlu GM, Budinger GR, Gottardi CJ. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem 285: 3157–3167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghosh MC, Makena PS, Gorantla V, Sinclair SE, Waters CM. CXCR4 regulates migration of lung alveolar epithelial cells through activation of Rac1 and matrix metalloproteinase-2. Am J Physiol Lung Cell Mol Physiol 302: L846–L856, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 288: L179–L189, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez RF, Allen L, Dobbs LG. Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol 297: L1045–L1055, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest 95: 2195–2204, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernandez AR, Klein AM, Kirschner MW. Kinetic responses of beta-catenin specify the sites of Wnt control. Science 338: 1337–1340, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thebaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 303: L967–L977, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones JI, Doerr ME, Clemmons DR. Cell migration: interactions among integrins, IGFs and IGFBPs. Prog Growth Factor Res 6: 319–327, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ, Lee SK, Yoon HJ, Shin DH, Park SS, Sohn JW. Blockade of the Wnt/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med 223: 45–54, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Krein PM, Sabatini PJ, Tinmouth W, Green FH, Winston BW. Localization of insulin-like growth factor-I in lung tissues of patients with fibroproliferative acute respiratory distress syndrome. Am J Respir Crit Care Med 167: 83–90, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Li J, Ying J, Fan Y, Wu L, Ying Y, Chan AT, Srivastava G, Tao Q. WNT5A antagonizes WNT/beta-catenin signaling and is frequently silenced by promoter CpG methylation in esophageal squamous cell carcinoma. Cancer Biol Ther 10: 617–624, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Liu AR, Liu L, Chen S, Yang Y, Zhao HJ, Guo FM, Lu XM, Qiu HB. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol 228: 1270–1283, 2013 [DOI] [PubMed] [Google Scholar]
- 30. Maeda A, Hiyama K, Yamakido H, Ishioka S, Yamakido M. Increased expression of platelet-derived growth factor A and insulin-like growth factor-I in BAL cells during the development of bleomycin-induced pulmonary fibrosis in mice. Chest 109: 780–786, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Maitre B, Clement A, Williams MC, Brody JS. Expression of insulin-like growth factor receptors 1 and 2 in the developing lung and their relation to epithelial cell differentiation. Am J Respir Cell Mol Biol 13: 262–270, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2: 206–213, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Medrano EE. Wnt5a and PKC, a deadly partnership involved in melanoma invasion. Pigment Cell Res 20: 258–259, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene 18: 7860–7872, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Moreno-Barriuso N, Lopez-Malpartida AV, de Pablo F, Pichel JG. Alterations in alveolar epithelium differentiation and vasculogenesis in lungs of LIF/IGF-I double deficient embryos. Dev Dyn 235: 2040–2050, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Mucenski ML, Nation JM, Thitoff AR, Besnard V, Xu Y, Wert SE, Harada N, Taketo MM, Stahlman MT, Whitsett JA. Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol 289: L971–L979, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Narasaraju TA, Chen H, Weng T, Bhaskaran M, Jin N, Chen J, Chen Z, Chinoy MR, Liu L. Expression profile of IGF system during lung injury and recovery in rats exposed to hyperoxia: a possible role of IGF-1 in alveolar epithelial cell proliferation and differentiation. J Cell Biochem 97: 984–998, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev 7: 2609–2617, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Richard-Parpaillon L, Heligon C, Chesnel F, Boujard D, Philpott A. The IGF pathway regulates head formation by inhibiting Wnt signaling in Xenopus. Dev Biol 244: 407–417, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Schnapp LM, Donohoe S, Chen J, Sunde DA, Kelly PM, Ruzinski J, Martin T, Goodlett DR. Mining the acute respiratory distress syndrome proteome: identification of the insulin-like growth factor (IGF)/IGF-binding protein-3 pathway in acute lung injury. Am J Pathol 169: 86–95, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sueoka N, Lee HY, Walsh GL, Fang B, Ji L, Roth JA, LaPushin R, Hong WK, Cohen P, Kurie JM. Insulin-like growth factor binding protein-6 inhibits the growth of human bronchial epithelial cells and increases in abundance with all-trans-retinoic acid treatment. Am J Respir Cell Mol Biol 23: 297–303, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Thieme R, Ramin N, Fischer S, Puschel B, Fischer B, Santos AN. Gastrulation in rabbit blastocysts depends on insulin and insulin-like-growth-factor 1. Mol Cell Endocrinol 348: 112–119, 2012 [DOI] [PubMed] [Google Scholar]
- 43. Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol 162: 899–908, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uh ST, Inoue Y, King TE, Jr, Chan ED, Newman LS, Riches DW. Morphometric analysis of insulin-like growth factor-I localization in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 158: 1626–1635, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Uhal BD. Cell cycle kinetics in the alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 272: L1031–L1045, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Wallen LD, Han VK. Spatial and temporal distribution of insulin-like growth factors I and II during development of rat lung. Am J Physiol Lung Cell Mol Physiol 267: L531–L542, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Whang YM, Jo U, Sung JS, Ju HJ, Kim HK, Park KH, Lee JW, Koh IS, Kim YH. Wnt5a is associated with cigarette smoke-related lung carcinogenesis via protein kinase C. PLoS One 8: e53012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]