Abstract
Adenovirus (Ad), particularly Ad type 7 (Ad7), causes severe lung infection and pneumonia. Initially, Ad causes neutrophilic inflammation of the distal airways and alveoli. Interleukin-8 (IL-8) is the major lung neutrophil chemotaxin, and we have shown that Ad7 induces IL-8 release from the A549 alveolar epithelial cell line. We sought to determine whether ex vivo human and bovine lung tissue containing primary pneumocytes could be used as a more accurate and relevant model to study Ad acute inflammation. We found that cultured lung tissue preserved normal lung architecture for more than 10 days. IL-8 was generated upon exposure of the lung organ culture to Ad7. IL-8 production required activation of the Ras/Erk pathway, since a pharmacological inhibitor blocked the appearance of IL-8 in the medium. Both human and bovine lung explants supported replication of Ad7, and immunohistochemistry experiments demonstrated the presence of the Ad hexon antigen within alveolar epithelial cells. These findings show that our novel human lung organ culture accurately reproduces the in vivo infectious disease process. Thus, this organ culture model represents a valuable tool for studying the acute innate immune response to respiratory infections.
Adenovirus (Ad) infections cause pneumonia and disseminated disease in both immunocompromised and nonimmunocompromised hosts. The human Adenoviridae contain 51 known serotypes defined by immunological methods and are categorized into six subgroups (A to F) based on common hemagglutination patterns and DNA sequences. Human Ad type 7 (Ad7) belongs to subgroup B. In humans, subgroup B Ad, and especially the species B:1, which contains Ad7, causes particularly severe lower-respiratory-tract infections. Ad infections are the most common cause of febrile respiratory tract infection and pneumonia in military recruits and have been responsible for 90% of recruit hospitalizations for pneumonia (2). In young children, mortality rates of Ad7 pneumonia from particularly virulent strains reach 23 to 29% (10, 13). Hence, Ad7 is a significant human pathogen and is especially problematic among children and adults in crowded living conditions.
The initial response to Ad infection of human lung is a neutrophilic infiltration of the lower respiratory tract and alveoli. Neutrophils are prominent in the lungs in animal models of Ad infection, as well as upon exposure of humans and nonhuman primates to genetically modified Ad5 vectors known to induce chemokine production in vitro (4, 6, 9, 11, 45). In severe cases, diffuse alveolar damage occurs during Ad7 infection and is similar to that seen in acute respiratory distress syndrome (ARDS). Chronically, permanent damage to lung tissue can result from Ad7 infection (51).
The CXC chemokine interleukin-8 (IL-8) is an important mediator of the inflammatory response to many stimuli, including viruses (14, 28, 33). Alveolar epithelial cells, which are infected during acute Ad infection, are likely mediators of the inflammatory response to Ad by the production of IL-8 (7, 34). IL-8 may be important in the pathophysiology of asthma and obstructive lung disease, since IL-8 levels are increased in patients with these chronic inflammatory disorders (43, 44, 52). IL-8 is also elevated in bronchoalveolar lavage fluid from patients with ARDS (30). Most importantly, IL-8 is a major neutrophil chemoattractant and activator (reviewed in reference 5).
Our previous studies demonstrated that human pulmonary epithelial cell-like cultured cell lines release IL-8 upon infection with Ad7 (8). Other, similar cell line models of infectious processes caused by agents, including respiratory syncytial virus, lipopolysaccharide, Mycobacterium, and Pseudomonas pyocyanin, have indicated a role for extracellular signal-regulated kinase (Erk) in the induction of IL-8 by host cells (15, 16, 41). We have also demonstrated that Erk activation is important in induction of IL-8 by Ad7 in epithelial cell lines in culture (1).
In addition to detailed mechanistic studies in cultured cell lines, more general studies of the Ad inflammatory response have been conducted with mouse or primate animal models (4, 6, 9, 11, 45). These animal models have significant limitations. There is no single IL-8 homologue in mice, and therefore, two other cytokines, MIP-2 and KC, are measured. More importantly, whereas transformed mouse cell lines support low-level replication of human Ad2 (21), human Ad replication has not been detected in primary mouse tissue (22, 31); thus, studies in rodents do not accurately model the course of the human disease. Primate animal models have also been used, but it is unclear whether there are interspecies differences in the response to primarily human pathogens such as Ad7. Furthermore, primate animal models are difficult to use for detailed mechanistic studies.
These limitations could be overcome by using an ex vivo organ culture system with tissue from closely related animal species or humans. Precision-cut lung slices have frequently been used in toxicology studies and have advantages over the use of isolated, cultured epithelial cells for infectious disease studies. The structural integrity of lung tissue is maintained (48) and this allows cell-cell interaction in a more complex three-dimensional system. Detailed mechanistic studies of intracellular processes such as signal pathway activation and therapeutics can be examined in human tissue without risk to the host. Although the supply of human lung tissue is limited, bovine tissue may be a reasonable substitute for studying cytokine induction since bovine IL-8 is similar to human IL-8 (35, 40) and antibodies to bovine IL-8 cross-react with that of human IL-8 (37). Furthermore, there is evidence that wild-type human Ad can infect bovines in nature (23).
We demonstrate here that induction of IL-8 by Ad7 occurs in a lung slice, organ culture model. We found that Ad7 exposure of lung slices, as for cultured epithelial cells, caused activation of the Ras/Raf-l/MEKI/Erk pathway. Using specific inhibitors of the Erk pathway, we found that Erk activation was essential for IL-8 production stimulated by Ad7 in this human lung tissue model. We also observed that infection of alveolar epithelial cells occurs during Ad exposure, and so these cells probably participate in the innate immune response to Ad. We found similar results with ex vivo bovine or human tissue. Our results demonstrate that the lung slice model is useful for studying the acute tissue response to infectious agents, that the chemokine responses to Ad infection in bovine and human tissue are similar, and that the effect of inhibitors of this response can be assessed in this model.
MATERIALS AND METHODS
Cells and virus.
The human pulmonary epithelial cell line A549 was used to propagate and quantify Ad. This cell line (ATCC CCL-185), derived from a patient with lung carcinoma, has the characteristics of alveolar type II cells and was obtained from the American Type Culture Collection (ATCC), Manassas, Va. (39). Ad7 was also obtained from the ATCC (ATCC VR-7) and was propagated, purified, and quantified as previously described (8). To propagate Ad7, A549 cells were infected and incubated for 3 to 4 days in Dulbecco modified Eagle medium supplemented with 2 mM l-glutamine, 80 μg of gentamicin/ml, and 10% fetal bovine serum. Adenovirus was harvested by three freeze-thaw cycles and purified by ultracentrifugation in CsCl gradients as described previously (32), and the purified viral preparation was dialyzed extensively against phosphate-buffered saline (PBS; pH 7.2), plus 10% glycerol (virus dialysis buffer). The titer of viable virus was quantified by using a cytotoxic plaque assay (36) and subsequently stored at −80°C.
Lung explant culture.
Human lung tissue was obtained by written consent from patients undergoing lung resection for cancer in accordance with protocols approved by the University of Oklahoma Institutional Review Board. The patients' ages ranged from 45 to 81 years. All volunteers had a significant tobacco smoking history (15 to 100 pack-years). Normal, healthy bovine lung was obtained from a local abattoir (Mikkelson Beef, Inc., Oklahoma City, Okla.) after inspection by a U.S. Department of Agriculture veterinarian. In all cases, the lung tissue was transported on ice in sterile PBS (pH 7.2) containing 200 μg of gentamicin/ml, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2.5 μg of amphotericin B/ml (PBS+Abx). The tissue was subsequently stored at 4°C in PBS+Abx for no longer than 8 h. The subsegmental bronchi of lung tissue with intact outer pleura were isolated and cannulated with a flexible Teflon catheter, and the lung segments were gently inflated with 37°C lung explant medium (LEM) containing 2% low-melting-low-gelling agarose (BioWhittaker, Rockland, Maine). LEM consisted of minimal essential medium (Sigma Chemical Co., St. Louis, Mo.) supplemented with 1.0 μg of bovine insulin/ml, 0.1 μg of hydrocortisone/ml, 0.1 μg of retinyl acetate/ml, 200 μg of gentamicin/ml, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 1.25 μg of amphotericin B/ml (48). The agarose-inflated lung was solidified at 4°C, after which 1-cm-diameter tissue cores were prepared and sliced into 500-μm thick sections by using a tissue slicer (Vibroslice model 752 M; Campden Instruments, Sileby, Loughborough, United Kingdom). During slicing, the cores were submerged in chilled PBS+Abx. Each slice was placed in 0.5 ml of LEM in a single well of a 24-well plate, and the slices were placed in a humidified incubator at 37°C in 5% CO2. The LEM was replaced prior to subjecting the slices to the experimental treatments.
Kinase assay, SDS-PAGE, and immunoblotting.
Lung explant slices maintained in 24-well plates for 24 to 72 h were infected with 2 × 109 PFU of Ad7/ml in 0.5 ml of fresh LEM and incubated at 37°C in 5% CO2 for the indicated times. Two lung slices per condition were used for Erk assays. Phorbol 12-myristate 13-acetate (PMA; Sigma) at 100 ng/ml was used as a positive control, and mock-infected, negative control slices were exposed to a volume of virus-free, virus dialysis buffer (PBS plus 10% glycerol) equal to that of the Ad7. Slices treated with various concentrations of the specific MEK inhibitor PD98059 (Calbiochem) (3, 17) were incubated for 2 h at 37°C in 5% CO2 in 0.5 ml of LEM prior to the addition of Ad7 or PMA. Negative control slices were preincubated with the inhibitor solvent dimethyl sulfoxide. After infection for the specified times, the paired slices were harvested, stored at −80°C, homogenized, and then lysed in 50 μl of cold lysis buffer (150 mM NaCl; 50 mM Tris [pH 8.0]; 10 mM [each] EDTA, NaF, and sodium pyrophosphate; 1% NP-40; 0.5% sodium deoxycholate; 0. 1% SDS; 3 mM sodium vanadate; 10 μg of aprotinin/ml; 10 μg of leupeptin/ml). Lung slice homogenates were clarified by centrifugation at 4°C, and the resultant postnuclear lysates were mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (60 mM Tris [pH 6.8], 10% glycerol, 2.3% SDS) and heated to 95°C for 5 min. The samples were separated by SDS-12% PAGE and electrophoretically transferred to nitrocellulose membranes. To detect activated phosphorylated Erk (25, 46), the membranes were immunoblotted with a mouse monoclonal antibody specific for both the p44/42 diphosphorylated forms of Erk (Cell Signaling Technology, Beverly, Mass.). The filters were developed with horseradish peroxidase-labeled goat anti-mouse immunoglobulin G (IgG; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) and chemiluminescent reagents (Pierce Biotechnology, Rockford, Ill.). The same membrane was stripped for 30 min at 60°C with 2% SDS-100 mM 2-mercaptoethanol in 62.5 mM Tris (pH 6.7) to remove the primary antibody. The membrane was reprobed with polyclonal anti-Erkl/2 antibody that recognizes both phosphorylated and nonphosphorylated Erk (Cell Signaling), and the blots were developed by chemiluminescence with horseradish peroxidase-labeled goat anti-rabbit IgG (Cell Signaling). Blots were quantified by using the LumiImager F1 system and LumiAnalyst software (Boehringer Mannheim GmbH, Mannheim, Germany).
IL-8 ELISA.
After initial maintenance in 24-well plates at 37°C, 5% CO2, lung slices were infected with 2 × 109 PFU of Ad7/ml in 0.5 ml of fresh LEM and incubated for the specified times at 37°C in 5% CO2. PMA (100 ng/ml) served as a positive control, a volume of virus dialysis buffer equal to that of Ad7 was used as a negative control, and all controls were treated similar to Ad-infected slices. Lung slices exposed to various concentrations of PD98059 in fresh LEM were preincubated for 2 h prior to the addition of Ad7, PMA, or virus dialysis buffer, and the indicated final concentration of PD98059 was maintained throughout the experiment. Negative control cells were treated with an equivalent concentration of the inhibitor diluent dimethyl sulfoxide. At the indicated times, the culture supernatants were collected, stored at −80°C, and then analyzed by a sandwich enzyme-linked immunosorbent assay (ELISA) specific for either human or bovine IL-8 protein.
Sandwich capture ELISA systems were developed to quantify both bovine and human IL-8. Both mouse monoclonal antibody (clone 170.13) generated against recombinant bovine IL-8 and recombinant bovine IL-8 protein were generously provided by S. K. Maheswaran (University of Minnesota, St. Paul) (35, 40). Anti-human IL-8 mouse monoclonal antibody and recombinant human IL-8 protein (MAB208 and 208-IL; R&D Systems, Minneapolis, Minn.) were used to quantify human IL-8. Goat polyclonal antibody generated against human IL-8 (AB-208-NA; R&D Systems) was biotinylated as described previously (26) and used in both bovine and human ELISA systems. Microtiter plates (Thermo Labsystems, Franklin, Mass.) were coated overnight at room temperature with 50 μl of either bovine (5 μg/ml) or human (0.5 μg/ml) monoclonal antibody diluted in 50 mM carbonate buffer (pH 9.6) (coating buffer)/well. The plates were washed three times with 20 mM Tris-150 mM NaCl-0.1% Tween 20 (pH 7.5) (wash buffer) and then incubated overnight at 4°C with 200 μl of 20 mM Tris, 150 mM NaCl, and 1% bovine serum albumin (BSA; pH 7.5) (blocking buffer)/well. Samples and standards were diluted in 20 mM Tris-150 mM NaCl-0.1% BSA (pH 7.5; diluting buffer) and were added in triplicate (50 μl/well) to plates that were first washed three times. The plates were incubated for 1.5 h at 37°C and washed three times; 50 μl of biotinylated polyclonal antibody in diluting buffer (1 μg/ml)/well was then added, and the plates were incubated for 1 h at 37°C. After three washes, 50 μl of streptavidin-alkaline phosphatase conjugate (Invitrogen, Carlsbad, Calif.) diluted 1:1,000 in dilution buffer was added/well, and the plate was incubated for 1 h at 37°C. After a thorough washing, 50 μl of NADPH substrate stock solution from the ELISA amplification system kit (Invitrogen) was added/well, and the plate was incubated for ca. 5 min at room temperature. Amplifier solution (50 μl/well) was then added, and incubation continued at room temperature for 5 to 10 min until color developed. The A490 was measured with a V-max Microplate reader, and the data were analyzed by using Softmax Pro software (Molecular Devices, Menlo Park, Calif.).
Lactose dehydrogenase assay.
Lung slices in 24-well plates in 0.5 ml of fresh LEM were infected with 2 × 109 PFU of Ad7/ml or mock infected with a volume of virus-free virus dialysis buffer equal to that of Ad7 (negative control) and incubated at 37°C in 5% CO2. Because PBS+Abx lacks the nutrients needed to maintain long-term tissue health and viability, lung slices incubated only in 0.5 ml of PBS+Abx served as a positive control for cytotoxicity. At the indicated times, both culture supernatants and lung slices were collected and stored at −80°C. The slices were homogenized with 75 μl of cold lysis buffer (150 mM NaCl; 50 mM Tris [pH 8.0]; 10 mM [each] EDTA, NaF, and sodium pyrophosphate; 1% NP-40; 0.5% sodium deoxycholate; 0. 1% SDS; 3 mM sodium vanadate; 10 μg of aprotinin/ml; 10 μg of leupeptin/ml). Lung slice homogenates were clarified by centrifugation at 4°C, and triplicate 2.5-μl aliquots of the resultant lysates were added to a microtiter plate. Then, 25 μl of a mixture containing 0.75 mM pyruvate and 1.3 mM NADH (Sigma) was added to each sample and, after incubation for 30 min at 37°C, 25 μl of color reagent (2,4-dinitrophenylhydrazine in 1 N HCl at 20 mg/ml (Sigma) was added. After further incubation for 20 min. at room temperature, the reaction was terminated and the final color developed by adding 250 μl of 0.4 N NaOH. The A450 of the samples was measured with a V-max Microplate reader and compared to the absorbance produced from known amounts of pyruvate substrate standards by using Softmax Pro software (Molecular Devices). Cytotoxicity, expressed as the percentage of lactate dehydrogenase (LDH) released, was calculated as follows: [supernatant LDH activity/(supernatant LDH activity + cell extract LDH activity)] × 100.
Immunohistochemistry and microscopy.
To examine the morphology of viable lung, individual slices of both human and bovine lung were placed in a 40-mm culture dish with 1.5 ml of LEM and were maintained in a humidified incubator at 37°C in 5% CO2. At 24-h intervals, photomicrographs were obtained by using a Nikon TS-100 inverted microscope equipped with a Spot Insight QE digital camera controlled with Spot Advanced software (Diagnostic Instruments, Sterling Heights, Mich.).
To confirm Ad7 infection, both bovine and human lung slices were infected with 2 × 109 PFU of Ad7/ml in 0.5 ml of fresh LEM at 37°C in 5% CO2. Uninfected, negative control slices were treated with virus dialysis buffer. After infection for 24 h, the slices were fixed in 10% formaldehyde in PBS, transferred to 70% ethanol, and embedded in paraffin. Sections (5 μm) were immunostained with a mouse monoclonal anti-adenovirus antibody that reacts to all Ad serotypes (MAB8052; Chemicon, Temecula, Calif.). The sections were then probed with a biotinylated, anti-mouse, anti-rabbit, anti-goat IgG polyclonal antibody (K0678; Dako Corp.). Streptavidin-alkaline phosphatase conjugate was added to the sections (Dako Corp.) and, after a rinsing step, fast red color reagent (Biopath Laboratories, Inc., Oklahoma City, Okla.) was used to develop the slides. Photomicrographs were obtained with an Olympus BX 40 microscope equipped with an Olympus DP11 digital camera.
Viral replication assay.
To determine the ability of Ad7 to replicate in bovine and human lung slices, 2 × 107 PFU of Ad7/ml was added to lung slices in 24-well plates in 0.5 ml of LEM and incubated at 37°C, 5% CO2. The slices were harvested at 24-h intervals, rinsed in PBS+Abx, stored at −80°C, and subsequently homogenized in 1 ml of PBS+Abx by using a ground glass pestle and homogenizer. The number of infectious particles present in the clarified slice homogenates was quantified with a cytotoxic plaque assay (36) on A549 cells and the results expressed as PFU of homogenate/ml. Also, a sandwich ELISA system was used to assess the amount of Ad hexon protein present in the homogenates. Serial dilutions of a purified, Ad7 stock were used for the standard curve and thus, the ELISA results were expressed as PFU derived from the titer of the Ad7 preparation per milliliter. For the Ad hexon ELISA, an Ad hexon monoclonal antibody (MAB8015; Chemicon) was used to coat microtiter plates (50 μl/well) at 2 μg/ml in coating buffer. After being washed, the plates were blocked overnight at 4°C with blocking buffer. Triplicate samples and Ad7 standards were prepared in diluting buffer, and 50 μl was added to the washed plates/well. The plates were incubated for 1.5 h at 37°C and then washed three times; biotinylated monoclonal antibody in diluting buffer (1 μg/ml) was then added, and the plates were incubated for 1 h at 37°C. Prior to use, MAB8052 was biotinylated as described previously (26) and stored at 4°C. After a washing step, streptavidin-alkaline phosphatase conjugate (1:1,000) in dilution buffer was added, and the plate was incubated for 1 h at 37°C. After a thorough washing, the plate was developed, and the A490 was measured as described above.
Data analysis.
Results are expressed as means ± the standard errors of the mean (SEM). Statistical difference between groups was determined by using multiple comparisons among treatment means by using the Bonferroni t test. The effect of increasing doses of different treatments was determined by using a repeated-measures analysis of variance (53).
RESULTS
Precision-cut lung preparation maintains morphology and minimizes cytotoxicity.
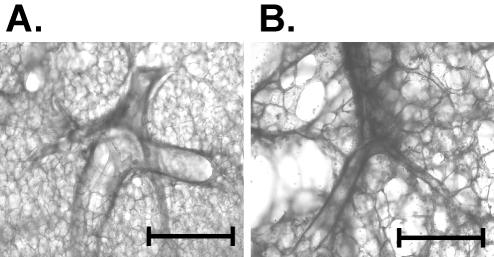
Microscopic examination of the lung slices during long-term culture demonstrated preservation of three-dimensional architecture for up to 10 days in culture in bovine and human tissues (Fig. 1A and B, respectively). Airways were visible, and the pneumocyte appearance did not change over the course of the culture. Furthermore, the tissue showed no evidence of contamination by adventitious agents.
FIG. 1.
Normal morphology of bovine and human lung explant slices is maintained over time. Sections of normal bovine lung tissue or normal human lung tissue were prepared and incubated as described in Materials and Methods. Photomicrographs of bovine (A) and human (B) lung slices show both bronchiolar and alveolar structures after 10 days of incubation. Scale bars, 1 mm.
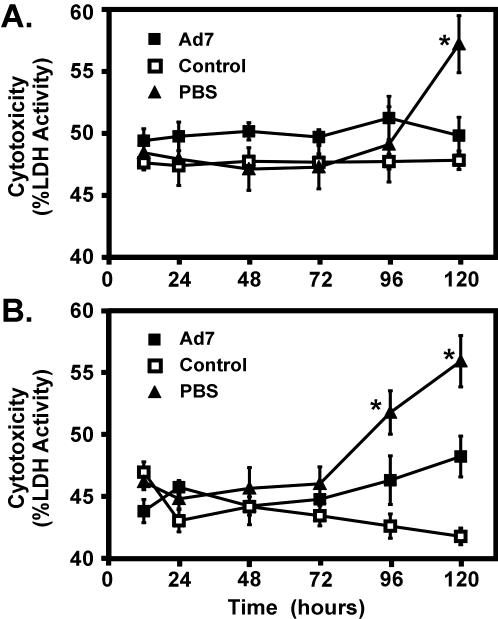
In order to determine whether culture conditions and tissue preparation caused cytotoxicity, LDH levels in supernatants and tissue slices were measured (Fig. 2). Cytotoxicity is determined by the ratio of LDH released in the medium versus the amount in the medium and tissue combined. There was no spontaneous increase in cytotoxicity in either control bovine or human lung tissue cultured in growth medium for 5 days. Upon infection with 2 × 109 PFU of Ad7, we observed a minimal increase in cytotoxicity in infected bovine tissue after introduction of the pathogen (Fig. 2A). Human lung slices displayed a slightly greater increase in cytotoxicity upon Ad7 infection; however, this increase did not reach statistical significance (Fig. 2B). In contrast, the culture of both human and bovine slices in nonnutrient PBS+Abx resulted in significant cytotoxicity by 96 and 120 h, respectively, in the absence of exposure to Ad, as reflected by increased LDH release (P < 0.05; Fig. 2).
FIG. 2.
Cytotoxicity in bovine and human lung explant culture. Bovine (A) and human (B) lung slices were infected with 2 × 109 PFU of Ad7/ml or treated with of PBS plus 10% glycerol (control) in 0.5 ml of fresh LEM. Slices grown in 0.5 ml of PBS plus antibiotics were a positive control. At various times the lactose dehydrogenase activities in both culture supernatants and lung slices were assessed in triplicate (see Materials and Methods). Cytotoxicity is expressed as the percentage of LDH released. The data are the means ± the SEM for three experiments. An asterisk indicates a significant difference (P < 0.05) from the control at that incubation time.
Lung slice preparations support Ad7 replication.
A limitation of rodent models is that they are nonpermissive for human Ad replication. We measured Ad replication in the tissue slice model by using a conventional plaque assay and an ELISA for Ad hexon protein. This was done by harvesting lung slices and supernatants after various times after Ad7 exposure.
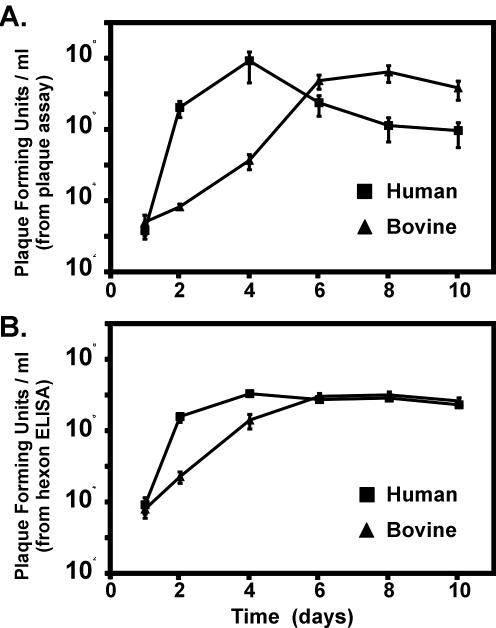
Plaque assays of both bovine and human lung tissue slices revealed significant production of viral infectious particles. In human slices, this was apparent by 2 days of culture and peaked at 4 days (Fig. 3A). Although the total quantity of infectious virus produced was similar in bovine tissue, replication was delayed, peaking after 6 days of culture (Fig. 3A). Replication in the human and bovine tissue represented an increase of 4 orders of magnitude in the amount of infectious particles present. There was no increase in the infectious particle concentration from either human or bovine medium supernatants (not shown).
FIG. 3.
Replication of Ad7 in bovine and human lung slices. Lung slices were exposed to wild-type Ad7 and cultured for various times and viral replication in the resultant lung slice homogenates was assessed in triplicate by both a plaque assay and by an Ad hexon ELISA (see Materials and Methods). (A) Virus titer, expressed as PFU per milliliter, in human and bovine lung slices as determined by cytotoxic plaque assay; (B) Ad hexon quantitation in human and bovine lung slices as determined by ELISA and expressed as the PFU per milliliter derived from the amount of Ad hexon present in the purified, titered Ad7 standards.
Quantification of total Ad hexon present in these tissue homogenates by ELISA also demonstrated viral replication (Fig. 3B). The amount of Ad hexon detected was expressed in terms of the number of purified infectious particles that contain an equal amount of Ad hexon. Because purified virus was used as the standard, the plotted values are proportional to the amount of virus present in the tissue. Ad hexon increases significantly in both human and bovine tissue. As in the plaque assays, Ad hexon levels peak earlier in human tissue than in bovine tissue (4 days versus 6 days, Fig. 3B). This increase is equal to the amount expected for an increase in infectious particles of 4 orders of magnitude. There was no increase in the amount of Ad hexon present in either human or bovine supernatants (not shown).
These data show that the lung slice culture model supports Ad replication and therefore offers an advantage to lower animal models.
IL-8 protein production is induced by Ad7 in bovine and human lung tissue slices.
The ability of precision-cut lung slices to respond to Ad7 infection by the production of IL-8 protein was investigated. Lung slices were stimulated by either Ad7 or PMA or were mock infected with viral diluent. After various incubation times from 12 to 120 h, supernatants were harvested and assessed for IL-8 levels by using a sandwich ELISA.
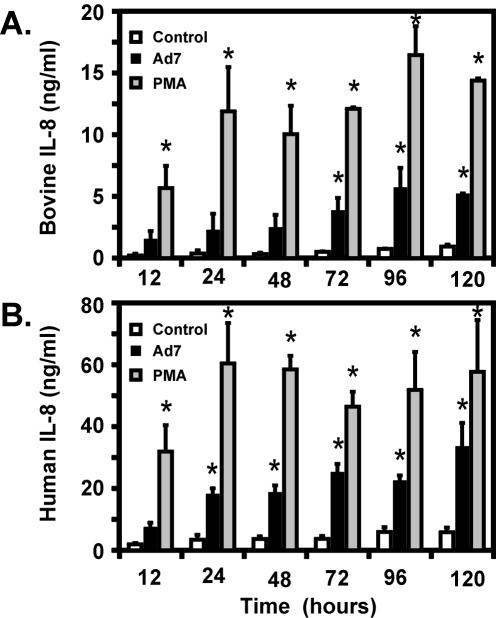
In bovine lung tissue, Ad7 infection of lung slices caused a six- to eightfold increase in IL-8 production above that seen in mock-infected cells (Fig. 4A). Increased IL-8 from Ad7-infected tissue was detectable by 12 h and was significantly higher than in the control by 72 h and remained so for the duration of the experiment. IL-8 levels from Ad7-infected tissue reached a maximum at 96 h and persisted for 120 h. IL-8 production from PMA-stimulated tissue was significantly higher than that of controls at all time points. The amounts of IL-8 induced by Ad7 were 19 to 36% of the amounts induced by PMA.
FIG. 4.
Ad7 induces IL-8 protein in both bovine and human lung slice models. Bovine (A) and human (B) lung slices were prepared as described in Materials and Methods and infected with 2 × 109 PFU of Ad7/ml, mock infected with virus-free buffer (control), or stimulated with 100 ng of PMA/ml. The medium supernatants were harvested at the times indicated, and the IL-8 levels in the supernatants were measured by ELISA. All measurements were performed in triplicate and are expressed as the means ± the SEM from three separate experiments. An asterisk indicates a significant difference (P < 0.05) from the control at that incubation time.
Similar results were obtained with human lung tissue. Slices were mock infected or were exposed to Ad7 or PMA as described above. We found that Ad7 infection caused a five- to sevenfold increase in IL-8 induction. Increased IL-8 from Ad7-infected tissue was detectable by 12 h and was significantly higher than the controls at all subsequent time points, peaking at 120 h (Fig. 4B). PMA significantly increased IL-8 levels at all time points. The amounts of IL-8 induced by Ad7 in human lung tissue were 23 to 58% of the amounts induced by PMA.
Infection with Ad7 induces Erk phosphorylation.
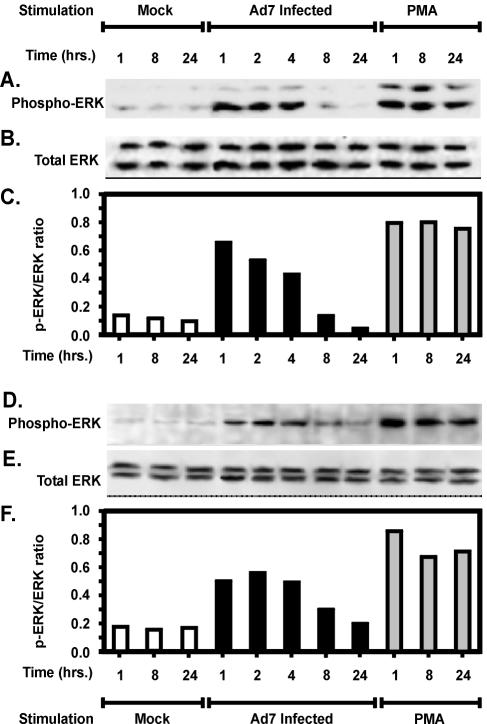
To determine whether Ad7-induced production of IL-8 was accompanied by Erk activation, we first assessed Erk phosphorylation in bovine lung tissue slices infected with Ad7. At various time intervals after infection, tissue lysates were prepared, and Erk 1/2 phosphorylation was determined as described in Materials and Methods (25, 46). Samples, including PMA-stimulated positive controls and virus-free buffer negative controls, were obtained at 1, 8, and 24 h. The results in bovine lung slices are shown in Fig. 5A to C. Figure 5A shows the phosphorylation of Erk in unstimulated control lung slices or those after stimulation by Ad7 of PMA. Figure 5B shows the same membrane reprobed for total Erk protein, and Fig. 5C shows the ratio of phospho-Erk to total Erk. Erk phosphorylation and activation were evident by 1 h after Ad7 exposure to the tissue (Fig. 5). Erk phosphorylation peaked at 1 h of exposure and returned to background over a 24-h period. These results indicate that Ad7 infection elicits Erk activation, as we have previously shown for A549 cultured pneumocytes (1). Peak Erk activation by Ad7 was 82% of that seen with PMA and precedes the maximal induction of IL-8 by Ad7 in bovine lung tissue.
FIG. 5.
Kinetics of Erk phosphorylation by Ad7 in bovine and human lung slice models. Normal bovine (A to C) or human (D to F) lung slices were stimulated with either 2 × 109 PFU of Ad7/ml, virus-free buffer (Mock) or 100 ng of PMA/ml for various times. Tissue lysates were analyzed for Erk activation by immunoblotting for both phosphorylated (active) and total Erk. Panels A and D show Western blots performed with antibody specific for phosphorylated Erk 1/2. Panels B and E are the same blots stripped and reprobed with an antibody specific for both phosphorylated and nonphosphorylated forms of Erk 1/2. Panels C and F show Erk activation graphed as the ratio of active, phosphorylated Erk to total Erk.
In human lung slices, Erk activation was evident by 1 h of Ad7 exposure to the tissue (Fig. 5D to F). Erk phosphorylation peaked at 2 h of exposure, and as for bovine tissue, Erk activity returned to background level by 24 h. Peak Erk activation was 63% of that seen with PMA. As in the results in bovine tissue, maximal Erk induction preceded maximal IL-8 induction by Ad7. The kinetics of Erk and IL-8 production are consistent with our previous findings that Erk activation is causally related to IL-8 production (1).
MEK inhibitor prevents Erk activation by Ad.
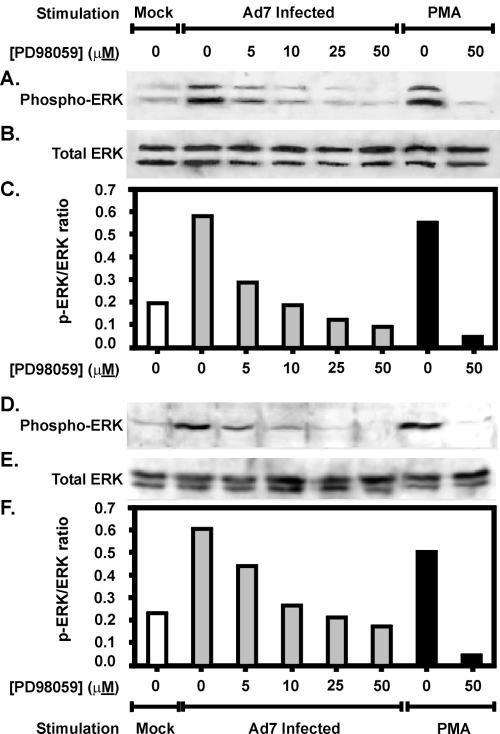
Erk is activated through a series of serine-threonine kinases. Activation of the immediate upstream kinase of Erk, MEK-1, is blocked by a specific inhibitor: PD98059 (3, 17). We used PD98059 to determine whether Ad7-triggered Erk activation is important for IL-8 production in the lung tissue slice model, as we have earlier shown in a cultured lung epithelial cell line. First, to determine whether activation of Erk by Ad7 occurred through the expected Raf-I/MEK-1 pathway, we treated lung tissue with increasing doses of PD98059 prior to infection with Ad7 or stimulation with PMA. Mock-infected tissue was exposed to an equal volume of virus-free buffer containing the same concentration of inhibitor solvent (dimethyl sulfoxide) as that used at the highest dose of inhibitor. The results in bovine lung tissue are shown in Fig. 6A to C. Ad7 inducible Erk l/2 phosphorylation was reduced in a dose-dependent fashion by the MEK inhibitor PD98059. Inhibitor doses above 10 μM reduced Erk phosphorylation in Ad7-exposed cells to below background levels. These amounts of PD98059 are slightly smaller than but comparable to those found in similar studies using the A549 cell line as a model to study Erk activation by Ad7 (1).
FIG. 6.
The MEK inhibitor PD98059 prevents Erk 1/2 activation by Ad7 in bovine and human lung slice models. Normal bovine (A to C) or human (D to F) lung slices were preincubated with various doses of the MEK inhibitor PD98059 and then stimulated with either 2 × 109 PFU of Ad7/ml, virus-free buffer (Mock) or 100 ng of PMA/ml. The indicated final concentration of PD98059 was maintained throughout the experiment. After a 1-h incubation at 37°C, tissue lysates were prepared and analyzed for Erk activation by immunoblotting for both phosphorylated (active) and total Erk. Panels A and D show Western blots performed with antibody specific for phosphorylated Erk 1/2. Panels B and E are the same blots stripped and reprobed with an antibody specific for both phosphorylated and nonphosphorylated forms of Erk 1/2. Panels C and F are graphs of Erk activation expressed as the ratio of phosphorylated Erk to total Erk.
The results in human lung tissue are shown in Fig. 6D to F. Ad7-inducible Erk 1/2 phosphorylation was reduced in a dose-dependent fashion by PD98059, similar to the bovine experiment. Treatment of the human tissue with doses at or above 25 μM PD98059 showed reduced Erk phosphorylation in Ad7-exposed tissue to below background levels. These amounts are similar to the amounts necessary to inhibit Erk activation by Ad7 in A549 pulmonary epithelial cells (1).
MEK inhibitor prevents induction of IL-8 by adenovirus.
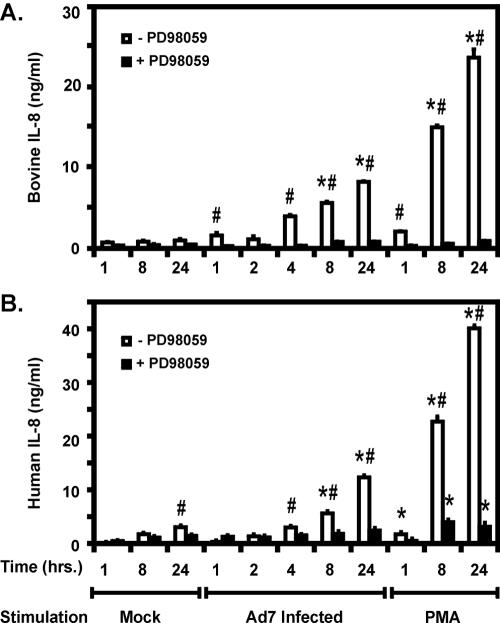
The findings thus far demonstrate that pharmacological inhibition of the immediate upstream kinase activator of Erk with PD98059 prevents Ad7-induced activation of Erk in bovine and human lung slices. We next sought to determine whether Erk activation was necessary for Ad7 induction of IL-8 secretion. Lung slices were preincubated in medium with PD98059 (50 μM) for 2 h prior to stimulation. PD98059 remained in the medium for the duration of the experiments, and slices not treated with PD98059 were incubated in medium containing an equivalent amount of inhibitor solvent (dimethyl sulfoxide). Cells were incubated with Ad7 or PMA for various times, and IL-8 protein levels in the medium supernatants were measured by ELISA. In bovine lung slices, Ad7 induction of IL-8 was detectable by 1 h postinfection and was significantly induced seven- and ninefold over that seen in mock-infected tissues at 8, and 24 h, respectively (Fig. 7A). Similar to the induction of Erk, Ad7-stimulated IL-8 secretion was blocked by 50 μM PD98059 to levels similar to those seen in uninfected control tissue at all times. IL-8 induction by PMA was also blocked to control levels by 50 μM PD98059 at all times tested (Fig. 7A).
FIG. 7.
The MEK inhibitor PD98059 prevents Ad7-induced IL-8 production in both bovine and human lung slice models. Bovine (A) and human (B) tissue slices were preincubated for 2 h in the presence or absence of 50 μM PD98059 prior to exposure to 2 × 109 PFU of Ad7/ml, virus-free buffer (Mock), or 100 ng of PMA/ml. The indicated final concentration of PD98059 was maintained throughout the experiment. After incubation for 24 h at 37°C, the medium supernatants were harvested and the IL-8 protein content was measured in triplicate by ELISA. The data are expressed as the means ± the SEM from three separate experiments. Asterisks indicate a significant difference (P < 0.05) from mock-infected slices at that incubation time; “#” symbols indicate a significant difference (P < 0.05) between −PD and +PD slices at that incubation time and stimulation condition.
In human lung slices, Ad7 induction of IL-8 was detectable by 1 h and was significantly induced by three- and fourfold over that seen in mock-infected tissue at 8 and 24 h, respectively (Fig. 7B). As seen in bovine tissue, Ad7-stimulated IL-8 secretion was blocked by 50 μM PD98059 at all times to levels in uninfected tissue. IL-8 was induced by PMA and was significantly decreased by 50 μM PD98059 at 8 and 24 h, though not to background levels (Fig. 7B). These experiments establish a causal relationship between Ad7-induced Erk activation and subsequent IL-8 production in lung tissue.
Alveolar epithelial cells are infected by Ad in the human lung slice model.
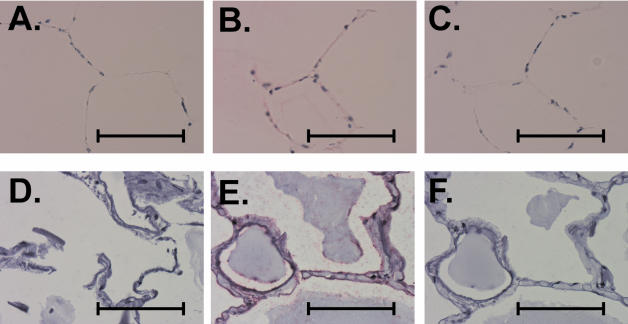
We next sought to determine which cells were infected during exposure of the tissue to Ad. Both bovine (Fig. 8A to C) and human (Fig. 8D to F) lung slices from the same experiment were processed for immunohistochemistry for detection of Ad with a mouse monoclonal antibody to Ad hexon as described in Materials and Methods. Tissue slices unexposed to Ad were used as a negative control for Ad detection. An additional negative control was performed for antigen detection by the same staining protocol but with the Ad hexon antibody omitted. We found no detectable Ad antigen in tissue treated with virus-free buffer (Fig. 8A and D). However, exposure of the tissue to Ad for 24 h resulted in the detection of Ad hexon in multiple cells, which appear morphologically to be alveolar epithelial cells (Fig. 8B and E). Omission of the primary Ad detection antibody resulted in the loss of signal (Fig. 8C and F). This control demonstrates that the staining pattern observed in panels B and E is due to Ad hexon. These findings show that epithelial cells are productively infected during exposure of the lung tissue to Ad7 and probably participate in the inflammatory response.
FIG. 8.
Exposure to Ad7 results in infection of alveolar epithelial cells in both bovine and human lung slice models. Bovine (A to C) and human (D to F) lung tissue slices were infected with 2 × 109 PFU of Ad7/ml or mock infected with virus-free buffer (negative control), followed by incubation for 24 h. Tissue sections were prepared and immunostained for the presence of Ad7 hexon as described in Materials and Methods. Panels A and D are uninfected, negative control bovine and human lung slices, respectively, and demonstrate that Ad hexon is not detected. Panels B and E (bovine and human, respectively) show that exposure of the tissue to Ad7 results in detection of Ad hexon in multiple cells that appear morphologically to be alveolar epithelial cells. Panels C and F (bovine and human, respectively) demonstrate that omission of the anti-Ad antibody from Ad7-infected lung slices results in a loss of signal. Scale bars, 50 μm.
DISCUSSION
The innate defense response to lung pathogens involves elaboration of cytokines from resident cells. This response is important in recruiting and activating inflammatory cells and is also important in the transition to adaptive immunity. The purpose of this response is to clear the pathogen, but the response may result in severe organ damage as occurs with ARDS. IL-8 contributes to recruitment and sequestration of neutrophils, which are the predominant cell type in early Ad infection, and IL-8 is elevated in ARDS (4, 6, 9, 11, 30, 45). Activation of intracellular signaling pathways, specifically the Erk pathway, is important in the induction of IL-8 by Ad in pulmonary epithelial cell lines (1).
These notions are based on studies with cultured cell lines. Although such models are useful and informative, they suffer from a number of deficiencies. First, the normal lung architecture is not reproduced in cultured epithelial cells. Second, other lung cell types are not represented in cultured cell models, which are genetically identical cloned cells. Normal lung contains many cell types, including type I and type II alveolar epithelial cells and hematopoietic cells that serve to respond to and clear infectious agents. Thus, the complex kinetics and course of an infectious disease are less accurately modeled in cultured cell lines.
Mouse models have been used but suffer from other disadvantages, including the lack of a single human IL-8 homologue, the lack of Ad7 proliferation in whole mice (31), and the lack of an appropriate host receptor for Ad7. Although CD46 has been identified as a receptor for the Ad of subgroup B:2, the receptor for subgroup B:1, which contains Ad7, is unknown (20, 50).
To overcome these difficulties, we sought to develop a novel organ culture model with human and bovine lung slices. The findings presented here demonstrate that such cultures maintain the appropriate lung architecture and contain the appropriate complex mixture of various cell lineages present in lung. These cultures, unlike rodent models, also permit viral replication. We found, as in cultured pneumocytes, that IL-8 is induced and requires activation of Erk in the lung tissue. Ad7 infection of both bovine and human lung tissue stimulated Erk in a time-dependent manner. Inhibition of Ad7-induced Erk activation by the MEKI inhibitor PD98059 correlated with inhibition of Ad7-induced IL-8.
These findings, which were obtained with a complex tissue model that closely mimics infection in vivo, validate previous results in lung cell lines. These findings are also consistent with a mechanism of IL-8 induction by Ad7 in which the Erk pathway is activated, stimulates the IL-8 promoter, increases IL-8 mRNA, and induces IL-8 protein in virus-infected cells. The final result of IL-8 induction is the recruitment of neutrophils and the initial inflammation seen during Ad infection.
Our findings do not eliminate the possibility that activation of other signaling pathways is important in IL-8 induction. Activation of p38 and c-Jun kinases is important in induction of IL-8 by other stimuli, including other viruses (18, 19, 27, 38, 47). The data presented show that Erk activation is necessary for IL-8 induction and that inhibition of Erk is sufficient to block IL-8 induction by Ad in tissue.
The finding that alveolar epithelial cells are infected in this model is potentially important. First, it confirms that the tissue is healthy enough to support viral infection. Second, since viral antigen is present in alveolar cells during infection of natural hosts, the model replicates this aspect of normal infection. Finally, this finding is also consistent with data in cell culture showing that alveolar epithelial cell lines in culture induce IL-8 in response to Ad7 infection. Taken together, these findings suggest that the alveolar epithelium participates in the inflammatory response through release of cytokines during infection in vivo.
Bovine lung tissue demonstrated responses to Ad infection similar to those seen in human tissue. There is evidence that bovines are infected in nature by wild-type Ad and can be artificially transduced by Ad vectors, and our data demonstrate that bovine tissue supports wild-type Ad replication (12, 23, 24, 29, 42, 49). This may explain why the response is similar to that seen in human tissue. Other studies have shown that bovine cells are suitable for studying other aspects of lung physiology and host-pathogen interactions (12, 29, 42, 49). Our findings show that bovine lung tissue may also be appropriate to study the innate immune response to human lung pathogens.
This organ culture model has potential advantages over other systems. First, it is capable of revealing complex mechanistic information regarding the inflammatory process during infectious disease. Second, it shows actual human cytokine responses to human pathogens in living human tissue. Therefore, the organ culture system described here may provide a safe intermediate model for examining the innate immune response in detail and the effect of treatments on this response. As such, it provides a novel approach to testing new but potentially harmful therapies directed against the host inflammatory response to viral infections and viral vectors.
Acknowledgments
J.P.M. is supported by an American Lung Association of Oklahoma Affiliate Grant and by a grant from the Oklahoma Center for the Advancement of Science and Technology. K.M.C. is a Scholar of the Leukemia and Lymphoma Society (formerly Leukemia Society of America).
We thank Jenny Oblander for excellent technical assistance. We acknowledge the assistance and cooperation of the Departments of Surgery and Pathology, University of Oklahoma Health Sciences Center, and the Pathology and Laboratory Medicine Service, Veterans Affairs Medical Center, Oklahoma City, Okla.
REFERENCES
- 1.Alcorn, M. J., J. L. Booth, K. M. Coggeshall, and J. P. Metcalf. 2001. Adenovirus type 7 induces interleukin-8 production via activation of extracellular regulated kinase 1/2. J. Virol. 75:6450-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2000. Adenovirus rates remain high. US Med. 36:1-22. [Google Scholar]
- 3.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 4.Amin, R., R. Wilmott, Y. Schwarz, B. Trapnell, and J. Stark. 1995. Replication-deficient adenovirus induces expression of interleukin-8 by airway epithelial cells in vitro. Hum. Gene Ther. 6:145-153. [DOI] [PubMed] [Google Scholar]
- 5.Baggiolini, M., B. Dewal, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines: CXC and CC chemokines. Adv. Immunol. 33:97-179. [PubMed] [Google Scholar]
- 6.Batshaw, M., S. Raper, and J. M. Wilson. 1999. Study of adenoviral vector mediated gene transfer in liver in adults with partial ornithine transcarbamylase deficiency (IND 6624): review of data, p. 158-178. In Proceedings of the National Institutes of Health, Recombinant DNA Advisory Committee, December 9-10, 1999. National Institutes of Health, Bethesda, Md.
- 7.Becroft, D. M. 1967. Histopathology of fatal adenovirus infection of the respiratory tract in young children. J. Clin. Pathol. 20:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth, J. L., and J. P. Metcalf. 1999. Type-specific induction of interleukin-8 by adenovirus. Am. J. Respir. Cell Mol. Biol. 21:521-527. [DOI] [PubMed] [Google Scholar]
- 9.Brody, S. L., M. Metzger, C. Danel, M. A. Rosenfeld, and R. G. Crystal. 1994. Acute responses of non-human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum. Gene Ther. 5:821-836. [DOI] [PubMed] [Google Scholar]
- 10.Brown, R. S., M. B. Nogrady, L. Spence, and F. W. Wiglesworth. 1973. An outbreak of adenovirus type 7 infection in children in Montreal. Can. Med. Assoc. J. 108:434-439. [PMC free article] [PubMed] [Google Scholar]
- 11.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campo, M. S. 2002. Animal models of papillomavirus pathogenesis. Virus Res. 89:249-261. [DOI] [PubMed] [Google Scholar]
- 13.Carballal, G., C. Videla, A. Misirlian, P. V. Requeijo, and M. Aguilar. 2002. Adenovirus type 7 associated with severe and fatal acute lower respiratory infections in Argentine children. BMC Pediatr. 2:6-13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter, L. R., J. N. Moy, and K. A. Roebuck. 2002. Respiratory syncytial virus and TNF alpha induction of chemokine gene expression involves differential activation of RelA and NF-κB1. BMC Infect. Dis. 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, W., M. M. Monick, A. B. Carter, and G. W. Hunninghake. 2000. Activation of ERK2 by respiratory syncytial virus in A549 cells is linked to the production of interleukin 8. Exp. Lung Res. 26:13-26. [DOI] [PubMed] [Google Scholar]
- 16.Denning, G. M., L. A. Wollenweber, M. A. Railsback, C. D. Cox, L. L. Stoll, and B. E. Britigan. 1998. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect. Immun. 66:5777-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92:7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eliopoulos, A. G., N. J. Gallagher, S. M. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 274:16085-16096. [DOI] [PubMed] [Google Scholar]
- 19.Feoktistov, I., A. E. Goldstein, and I. Biaggioni. 1999. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol. Pharmacol. 55:726-734. [PubMed] [Google Scholar]
- 20.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 21.Ganly, I., V. Mautner, and A. Balmain. 2000. Productive replication of human adenoviruses in mouse epidermal cells. J. Virol. 74:2895-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginsberg, H. S., L. L. Moldawer, P. B. Sehgal, M. Redington, P. L. Kilian, R. M. Chanock, and G. A. Prince. 1991. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc. Natl. Acad. Sci. USA 88:1651-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gogev, S., M. Lemaire, and E. Thiry. 2001. Prevalence of antibodies to human adenovirus type 5 in Belgian cattle. Vet. Rec. 148:752-754. [DOI] [PubMed] [Google Scholar]
- 24.Gogev, S., N. Vanderheijden, M. Lemaire, F. Schynts, J. D'Offay, I. Deprez, M. Adam, M. Eloit, and E. Thiry. 2002. Induction of protective immunity to bovine herpesvirus type 1 in cattle by intranasal administration of replication-defective human adenovirus type 5 expressing glycoprotein gC or gD. Vaccine 20:1451-1465. [DOI] [PubMed] [Google Scholar]
- 25.Grammer, T. C., and J. Blenis. 1997. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene 14:1635-1642. [DOI] [PubMed] [Google Scholar]
- 26.Gretch, D. R., M. Suter, and M. F. Stinski. 1987. The use of biotinylated monoclonal antibodies and streptavidin affinity chromatography to isolate herpesvirus hydrophobic proteins or glycoproteins. Anal. Biochem. 163:270-277. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto, S., K. Matsumoto, Y. Gon, T. Nakayama, I. Takeshita, and T. Horie. 1999. Hyperosmolarity-induced interleukin-8 expression in human bronchial epithelial cells through p38 mitogen-activated protein kinase. Am. J. Respir. Crit. Care Med. 159:634-640. [DOI] [PubMed] [Google Scholar]
- 28.Johnston, S. L., A. Papi, P. J. Bates, J. G. Mastronarde, M. M. Monick, and G. W. Hunninghake. 1998. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J. Immunol. 160:6172-6181. [PubMed] [Google Scholar]
- 29.Jones, C. 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin. Microbiol. Rev. 16:79-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorens, P. G., J. Van Damme, W. De Backer, L. Bossaert, R. F. De Jongh, A. G. Herman, and M. Rampart. 1992. Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokine 4:592-597. [DOI] [PubMed] [Google Scholar]
- 31.Kajon, A. E., A. P. Gigliotti, and K. S. Harrod. 2003. Acute inflammatory response and remodeling of airway epithelium after subspecies B1 human adenovirus infection of the mouse lower respiratory tract. J. Med. Virol. 71:233-244. [DOI] [PubMed] [Google Scholar]
- 32.Kanegae, Y., M. Makimura, and I. Saito. 1994. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn. J. Med. Sci. Biol. 47:157-166. [DOI] [PubMed] [Google Scholar]
- 33.Knobil, K., A. M. Choi, G. W. Weigand, and D. B. Jacoby. 1998. Role of oxidants in influenza virus-induced gene expression. Am. J. Physiol. 274:L134-L142. [DOI] [PubMed] [Google Scholar]
- 34.Ladisch, S., F. H. Lovejoy, J. C. Hierholzer, M. N. Oxman, D. Strieder, G. F. Vawter, N. Finer, and M. Moore. 1979. Extrapulmonary manifestations of adenovirus type 7 pneumonia simulating Reye syndrome and the possible role of an adenovirus toxin. J. Pediatr. 95:348-355. [DOI] [PubMed] [Google Scholar]
- 35.Lafleur, R. L., M. S. Abrahamsen, and S. K. Maheswaran. 1998. The biphasic mRNA expression pattern of bovine interleukin-8 in Pasteurella haemolytica lipopolysaccharide-stimulated alveolar macrophages is primarily due to tumor necrosis factor alpha. Infect. Immun. 66:4087-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence, W., and H. Ginsberg. 1967. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J. Virol. 1:851-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, J., and X. Zhao. 2000. Recombinant human interleukin-8, but not human interleukin-1β, induces bovine neutrophil migration in an in vitro coculture system. Cell Biol. Int. 24:889-895. [DOI] [PubMed] [Google Scholar]
- 38.Li, J., S. Kartha, S. Iasvovskaia, A. Tan, R. K. Bhat, J. M. Manaligod, K. Page, A. R. Brasier, and M. B. Hershenson. 2002. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am. J. Physiol. Lung Cell Mol. Physiol. 283:L690-L969. [DOI] [PubMed] [Google Scholar]
- 39.Lieber, M., B. Smith, A. Szakal, W. Nelson-Rees, and G. Todaro. 1976. A continuous tumor cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 17:62-70. [DOI] [PubMed] [Google Scholar]
- 40.Malazdrewich, C., T. R. Ames, M. S. Abrahamsen, and S. K. Maheswaran. 2001. Pulmonary expression of tumor necrosis factor alpha, interleukin-1β, and interleukin-8 in the acute phase of bovine pneumonic pasteurellosis. Vet. Pathol. 38:297-310. [DOI] [PubMed] [Google Scholar]
- 41.Marie, C., S. Roman-Roman, and G. Rawadi. 1999. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or Mycoplasma fermentans membrane lipoproteins. Infect. Immun. 67:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell, G. B., B. N. Albright, and J. L. Caswell. 2003. Effect of interleukin-8 and granulocyte colony-stimulating factor on priming and activation of bovine neutrophils. Infect. Immun. 71:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nocker, R. E., D. F. Schoonbrood, E. A. van de Graaf, C. E. Hack, R. Lutter, H. M. Jansen, and T. A. Out. 1996. Interleukin-8 in airway inflammation in patients with asthma and chronic obstructive pulmonary disease. Int. Arch. Allergy Immunol. 109:183-191. [DOI] [PubMed] [Google Scholar]
- 44.Ordonez, C. L., T. E. Shaughnessy, M. A. Matthay, and J. V. Fahy. 2000. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am. J. Respir. Crit. Care Med. 161:1185-1190. [DOI] [PubMed] [Google Scholar]
- 45.Otake, K., D. L. Ennist, K. Harrod, and B. C. Trapnell. 1998. Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses. Hum. Gene Ther. 9:2207-2222. [DOI] [PubMed] [Google Scholar]
- 46.Pang, L., C. F. Zheng, K. L. Guan, and A. R. Saltiel. 1995. Nerve growth factor stimulates a novel protein kinase in PC-12 cells that phosphorylates and activates mitogen-activated protein kinase kinase (MEK). Biochem. J. 307:513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pati, S., M. Cavrois, H. G. Guo, J. S. Foulke, Jr., J. Kim, R. A. Feldman, and M. Reitz. 2001. Activation of NF-κB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Placke, M. E., and G. L. Fisher. 1987. Adult peripheral lung organ culture-a model for respiratory tract toxicology. Toxicol. Appl. Pharmacol. 90:284-298. [DOI] [PubMed] [Google Scholar]
- 49.Schlender, J., G. Zimmer, G. Herrler, and K. K. Conzelmann. 2003. Respiratory syncytial virus (RSV) fusion protein subunit F2, not attachment protein G, determines the specificity of RSV infection. J. Virol. 77:4609-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sly, P. D., M. E. Soto-Quiros, L. I. Landau, I. Hudson, and H. Newton-John. 1984. Factors predisposing to abnormal pulmonary function after adenovirus type 7 pneumonia. Arch. Dis. Child. 59:935-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto, C., T. Yoneda, M. Yoshikawa, A. Fu, T. Tokuyama, K. Tsukaguchi, and N. Narita. 1997. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest 112:505-510. [DOI] [PubMed] [Google Scholar]
- 53.Zar, J. H. 1996. Biostatistical analysis. Prentice-Hall, Inc., Englewood Cliffs, N.J.