Abstract
Purpose
Neuroblastoma is one of the commonest extra-cranial tumors of childhood. The majority of patients present with metastatic disease for which outcome remains poor. Immunotherapy is an attractive therapeutic approach for this disease, and a number of neuroblastoma tumor antigens have been identified. Here we examine the therapeutic potential of combining immunomodulatory monoclonal antibodies (mAb) with peptide vaccination in murine neuroblastoma models.
Experimental design
Neuroblastoma bearing mice were treated with mAb targeting 4-1BB, CD40 and CTLA-4 alone, or in combination with a peptide derived from the tumor antigen survivin (GWEDPPNDI). Survivin-specific immune response and therapeutic efficacy was assessed.
Results
In the Neuro2a model, treatment of established tumor with either anti-4-1BB, anti-CD40 or anti-CTLA-4 mAb results in tumor regression and long-term survival in 40-60% of mice. This is dependent on NK and CD8+ T cells and is associated with tumor CD8+ lymphocyte infiltrate. Successful therapy is achieved only if mAb is given to mice once tumors are established, suggesting dependence on sufficient tumor to provide antigen. In the more aggressive AgN2a and NXS2 models, single agent mAb therapy provides ineffective therapy. However if mAb (anti-CTLA-4) is given in conjunction with survivin peptide vaccination then 60% long term survival is achieved. This is associated with the generation of survivin-specific T cell immunity, which again is only demonstrated in the presence of tumor antigen.
Conclusions
These data suggest the combination of antigen and co-stimulatory mAb may provide effective immunotherapy against neuroblastoma and may be of particular use in the minimal-residual disease setting.
Keywords: neuroblastoma, immunotherapy, survivin, immunostimulatory mAb
Introduction
Neuroblastoma, an embryonal tumour originating from sympathetic neural crest cells, is one of the commonest extra-cranial malignancies of childhood, accounting for 6-8% of all childhood cancers and over 15% of pediatric cancer deaths [1]. Over 50% of children have metastatic disease at presentation and long term survival is seen in only around 30-40 % of these patients despite intensive multi-modal therapies (1, 2). Treatment associated mortality is significant (5-8%), so there is little room to further intensify therapies (3). Immunotherapy is an attractive alternative therapeutic option for these children as it potentially offers a more specific and less toxic treatment than conventional therapies.
Endogenous cellular and humoral anti-tumor immune responses in children with neuroblastoma are well established, and it is the most frequent human tumor to spontaneously undergo regression (4). Lymphoid infiltration is found in approximately 25% of tumors, and has been correlated favorably with outcome (5, 6) . A number of tumor antigens have been identified, including survivin, tyrosine hydroxylase, MYC-N, GD2 and a number of cancer testis antigens, and endogenous immune responses to some of these have been identified (4). Coughlin et al (2006) demonstrated survivin-specific CD8+ T-cells in the peripheral blood of 8 out of 9 children with high risk neuroblastoma, not seen in healthy controls, accounting for up to 0.64 % of the circulating CD8+ lymphocytes (7). Although tumor growth was not controlled in vivo, the majority of these T-cells demonstrated cytotoxicity against human neuroblastoma following in vitro re-stimulation. Such T-cells may be inadequate to eradicate the tumors in patients due to limited tumor antigen, lack of co-stimulation and/or host immunoregulation. Immunomodulatory monoclonal antibodies (mAb) potentially offer a means of overcoming immune escape mechanisms to generate effective anti-tumor immunity (8) by enhancing endogenous anti-tumor T-cell responses through targeting key receptors in the immune system. Antibodies targeting co-stimulatory molecules expressed on T-cells (e.g. CD28, 4-1BB and OX40) may act agonistically, functioning as surrogate ligands and augmenting T-cell proliferation and survival (9). Alternatively, agonistic mAbs recognizing molecules expressed on dendritic cells (DC) (e.g. CD40) may mature and activate DC, increasing the expression of both co-stimulatory ligands and MHC molecules, which will in turn promote T-cell responses (10). In addition, blocking mAbs have been employed to counteract the inhibitory, immune-regulating check-point blocker molecules such as CTLA-4 and PD-1 (9, 11). Importantly, immune responses generated in this way do not require target antigen identification and may be directed against multiple epitopes, reducing the chances of tumor escape variants. Each of these approaches has been shown to not only augment antigen-specific T-cell responses, but also to provide therapeutic benefit in murine syngeneic tumor models (12-15).
Monoclonal antibodies targeting a number of these receptors (CTLA-4, CD40, 4-1BB, OX40, PD-1) have entered clinical trial in adult oncology patients, showing considerable promise. Ipilimumab, a human anti-CTLA-4 mAb, has been recently granted FDA approval for first line treatment of metastatic melanoma, having been shown to offer survival advantage in Phase III trial in this population (16). Smaller, early phase studies of anti-CTLA-4 mAb have suggested potential benefit in a number of other adult malignancies (17, 18). Although earlier in clinical development, a number of other agents (e.g. anti-CD40, anti-PD-1 and anti-4-1BB) are also showing promise in adult oncology patients (19). However despite these encouraging results, there is, as yet, no reported pediatric experience of this class of agents.
Here we demonstrate that immunostimulatory mAb, either alone or in combination with peptide vaccine, can be used to generate potent anti-tumor immunity in murine neuroblastoma models.
Materials and methods
Animals and cells
A/J mice were supplied by Harlan, UK. Animal experiments were cleared through local ethical committee and performed under Home Office licenses PPL30/2450 and 30/2451. Neuro2a (ECACC), AgN2a (Dr Rimas Orentas, Medical College of Wisconsin) and NXS2 (Dr Holger Lode, Charité Children’s Hospital, Berlin) cell lines were maintained in DMEM supplemented with 2 mM glutamine, 0.1 mM non essential amino acids and 10 % FCS or with addition of 0.55 mM Arginine 0.014 mM Folic Acid, 0.27 mM asparagine and 50 μM β-mercaptoethanol (AgN2a). Splenocytes were maintained in RPMI containing 2 mM glutamine, 1 mM pyruvate, 100 IU/ml penicillin and streptomycin, 50 μM β-mercaptoethanol and 10 % FCS (Invitrogen).
Antibodies
Hybridomas for LOB12.3 (anti-4-1BB) and Mc106A5 (anti-BCL1 Id, irrelevant control) were generated in house (20, 21). The 3/23 (anti-CD40) hybridoma was originally provided by G. Klaus, NIMR, London. (22). The UC10 4F10-11 (anti-CTLA-4) hybridoma was obtained from the ATCC. Antibodies for flow cytometry were obtained from BD Biosciences unless otherwise stated.
Murine neuroblastoma therapy models
Age matched 8-12 week old A/J mice were injected subcutaneously (s.c.) with 2 × 106 freshly prepared tumor cells (> 90 % viability) in 100 μl PBS on day 0 and received antibody/peptide vaccine as specified in individual experiments. All antibodies were given by intra-peritoneal (i.p.) injection diluted in PBS. Survivin (GWEPDDNPI) and control (SIINFEKL or FEANGNLI) peptides were in PBS were emulsified in equal volumes of incomplete Freund’s adjuvant (IFA) prior to intra-dermal (i.d.) injection. Tumor diameter was measured regularly and mice culled when cross-sectional area exceeded 225 mm2.
Where indicated CD8+/CD4+/NK cells were removed by administration of depleting antibodies as previously described (23). 500 μg anti-CD8 mAb (YTS169), 1 mg anti-CD4 mAb (YTA 3.1.2) or 200 μl of a 1 in 10 dilution of anti-asialo GM1 (polyclonal rabbit anti-asialo GM1, Wako Chemicals) were injected i.p. every 4-5 days.
In vitro proliferation and viability assays
0.5 μCi [3H]-thymidine / well was added to cultures for the last 16 hr, after which cells were harvested onto glass fibres with an automated harvester. [3H]-thymidine incorporation was subsequently determined via liquid scintillation counting.
Cells were assessed for viability by flow cytometry after staining with Fluorescein isothiocyanate (FITC)-labeled annexin V and propidium iodide (AnV/PI) as detailed previously (24).
Immunofluorescence
OCT (RA Lamb) frozen tumor sections were fixed in 100 % acetone for 10 min at 4 °C and non-specific binding was blocked by 30 min pre-incubation with PBS containing 5 % normal goat serum and 2 % BSA (pH 7.4). Sections were incubated with rat anti-mouse CD8α, CD4 (both BD Pharmingen) or NK2A/C/E (eBiosciences) diluted in 2 % BSA/PBS, followed by Alexa Fluor 488-conjugated goat anti-rat IgG (Life Technologies). Nuclei were counterstained with DAPI (Sigma) and sections mounted in Vectashield (Vector Laboratories). Images were collected using a CKX41 inverted microscope reflected fluorescence system equipped with a CC12 colour camera running under Cell B software (Olympus), using a Plan Achromat 10X/0.25 objective lens (Olympus, UK).
Flow cytometry
Flow cytometry was performed as described previously (25) with samples assessed on a FACSCanto II™ and data analyzed using FACSDiva™ software (all BD Biosciences). To determine surface expression of 4-1BB, CD40 or CTLA-4, cells were labeled with 10 μg/ml unlabelled mAb (in-house) before incubation with R-Phycoerythrin (PE)-conjugated anti-mouse Fc. T cells were identified with allophycocyanin (APC)-labeled anti-mouse CD3ε (clone 145-2c11).
Intracellular IFN-γ
Mice were immunized with mAb and/or peptide as specified. Splenocytes were cultured for 6 days with control (FEANGNLI) or GWEPDDNPI-pulsed, irradiated splenocytes in the presence of 20 IU/ml IL-2. T cells secreting IFN-γ were then detected by direct immunofluorescence staining using FITC-conjugated CD3ε, and APC-Cyano dye Cy7 (APC-Cy7)–conjugated CD8α (clone 53-6.7). Cells were fixed for 20 min at 4°C with 1% paraformaldehyde, washed once with PBS and permeabilized using 0.5% saponin before staining with IFNγ-APC for 20 min at 4°C, washed once with 0.1% saponin, before samples were analyzed on the FACSCanto™.
Survivin Real-Time Quantitative PCR
Forward (AGCCTGATTTGGCCCAGTGT) and reverse (TCTTCCATCTGCTTCTTGACAGTGA) primers (Sigma-Aldrich) and a splice-junction spanning TaqMan probe (AACCCGATGACAACCCGATAGAGGAGC) were designed from the murine survivin-121 and survivin-140 gene transcripts (ENSMUSG00000017716). cDNA was produced from 1μg of total RNA with the Invitrogen Superscript® III First-Strand Synthesis System. Quantitative PCR reactions were performed in triplicate using Invitrogen Platinum® qPCR SuperMix-UDG on the Bio-Rad CFX96™ real time system. The fold expression of survivin was calculated using the 2−ΔΔCT method with respect to hypoxanthine-guanine phosphoribosyltransferase (TaqMan assay Mm00446968_m1, Applied Biosystems, Warrington, UK).
Chromium-51 (51Cr) cytotoxic T-lymphocyte killing assays
Splenocytes were re-stimulated with GWEPDDNPI or control peptide-pulsed, irradiated splenocytes in the presence of 20 IU/ml rHu IL-2 for 6 days. A standard 51Cr release assay was then used to assess cytotoxic activity of splenic effectors, as previously described (13). 51Cr labeled target-cells were incubated with splenocytes for 5 hr under tissue culture conditions (5% CO2, 37°C), centrifuged at 500 × g for 5 min before estimation of 51Cr release from supernatant using a gamma-counter (Wallace 1470 WIZARD, PerkinElmer). Maximum release of radioactivity was calculated from target cells using 150 μl of 1% Nonidet P-40. % specific release was calculated as ((sample release – spontaneous release)/(maximum release – spontaneous release)) x 100.
Adoptive transfer assay
Splenocytes from naïve A/J mice were pulsed with 25 μg/ml GWEPDDNPI or control (FEANGNLI) peptide for 1 hr at 37°C, washed once with FCS free RPMI and then stained with high (5 μM) and low (0.5 μM) concentrations of Carboxyfluorescein succinimidyl ester (CFSE) respectively. A 1:1 ratio of target (T; GWEPDDNPI-pulsed) to non-target (NT; FEANGNLI-pulsed) cells. were injected i.v. into recipient mice. After 24 hr mice were culled and the splenic T:TN ratio remaining assessed by flow cytometry (FACSCanto II™).
Statistics
All statistical analysis was performed using GraphPad Prism version 5 for Windows (GraphPad software, San Diego, California). The statistical significance between treatment groups in the tumor models was analyzed using the log-rank test comparing survival curves. The statistical significance between IFN-γ responses in different groups of treated mice was calculated using Student’s t-test. The statistical significance between CTL killing was compared using a one-way ANOVA.
Results
Anti-4-1BB treatment of established, weakly immunogenic tumors resulted in resolution of tumor, long-term survival and protection from tumor re-challenge
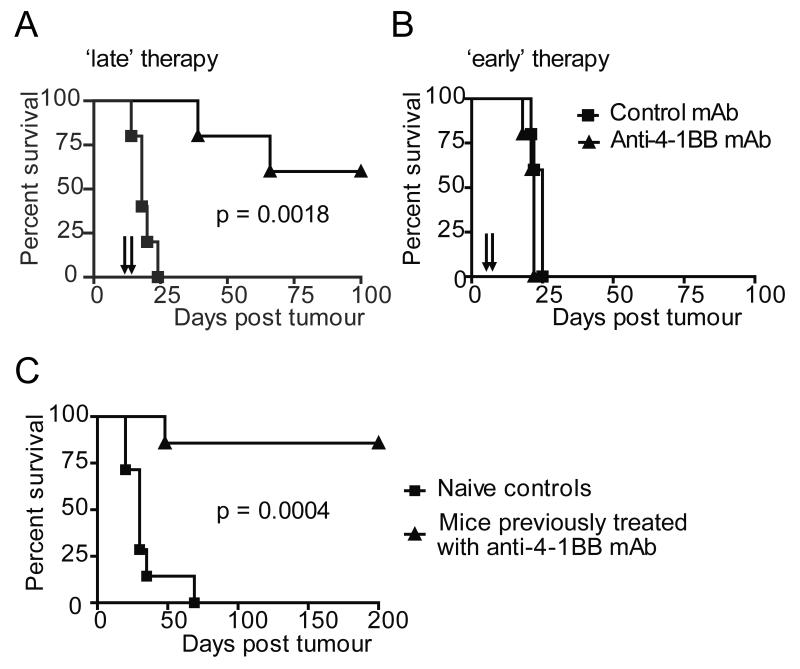
Initially, therapeutic activity of the immunomodulatory mAbs were explored in the Neuro2a neuroblastoma model. This cell line expresses relatively low levels of MHC Class I but has been shown to be susceptible to T-cell mediated immunotherapies (26). Anti-4-1BB mAb efficacy was investigated first, as it has been shown to directly promote T-cell development of CTL effector function and is also known to have agonistic effects on NK cells, making it an attractive agent to use in neuroblastoma, where MHC down regulation may make NK cell responses important in generating effective immunity (12, 27-30). Mice with small, palpable (1-2 mm diameter) Neuro2a tumors were treated with systemic anti-4-1BB mAb, resulting in complete tumor regression and long term survival(> 180 days) in approximately 60% of the mice treated (Figure 1A). Interestingly, effective therapy was only achieved when mice received mAb therapy after tumors were well-established, and not if therapy was given soon after tumor inoculation, prior to development of palpable tumors (Figure 1B). Furthermore, mice cured of Neuro2a by anti-4-1BB mAb treatment acquire long-term immunity against re-challenge. The majority of mice (~ 80%) receiving 2 × 106 of the same tumour after a period of > 120 days were protected from tumor re-growth, suggesting long term immunity had been achieved (Figure 1C).
Figure 1. Anti-4-1BB mAb provides effective therapy in Neuro2a neuroblastoma model.
A/J mice inoculated s.c. with 2 × 106 Neuro2a cells on day 0 received 0.5 mg anti-4-1BB mAb or 200 μl PBS i.p on A, days 9 and 12 or B, days 3 and 6. Anti-4-1BB treatment increased survival in mice treated on days 9 and 12 (p = 0.0018), but not with treated on days 3 and 6. C, Long term survivors from (A) re-challenged with tumor (2 × 106 cells s.c) 180 days after initial inoculation showed increased survival compared with naïve age-matched controls (p = 0.0004). Data represent examples of at least two experiments, where n = 5 mice/group.
Neuro2a tumor resolution after anti-4-1BB treatment requires CD8+ T-cells and NK-cells, but not CD4+ cells
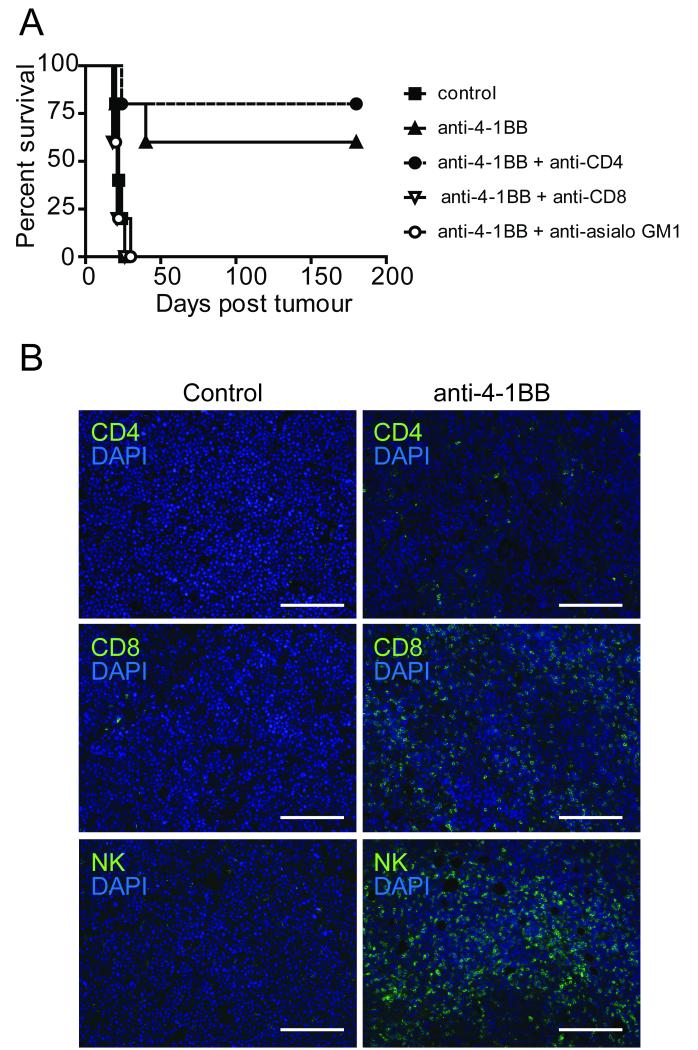
In vivo depletion of CD8+ T cell and NK cell populations had minimal effect on the rate of Neuro2a tumor growth (data not shown), but completely abrogated the therapeutic effects of anti-4-1BB (Figure 2A). In contrast, mice lacking CD4+ T cells remained completely sensitive to anti-4-1BB therapy. To further explore the effector population mediating immunotherapy, treatment was delayed, thus avoiding complete resolution of tumor and allowing tumors excision for immunohistochemical staining. A significant CD8+ and NK lymphocyte infiltrate was observed in the majority of tumors taken from treated mice, as was scanty CD4+ infiltration in treated but not untreated mice (Figure 2B).
Figure 2. Anti-4-1-BB immunotherapy is dependent on CD8+ T and NK cells.
A, Neuro2a inoculated A/J mice received anti-4-1BB or 200 μl PBS i.p. on days 9 and 12. Mice also received anti-CD4, anti-CD8 or anti-asialo GM1 antibodies. Anti-4-1-BB immunotherapy was abrogated after CD8+ T-cell (p = 0.0215) or NK-cell depletion (p = 0.0228), but was retained after CD4+ depletion (p = 0.5203). B, Neuro2a tumors were excised from mice 8 days after mAb treatment. Tumors from anti-4-1BB mAb treated mice showed evidence of CD8+ and NK cell infiltrates. X10 magnification, scale bar is equal to 200 μm. Data represents examples of at least three experiments, where n = 5 mice/group.
mAb against CD40 and CTLA-4 are also effective in the Neuro2a model
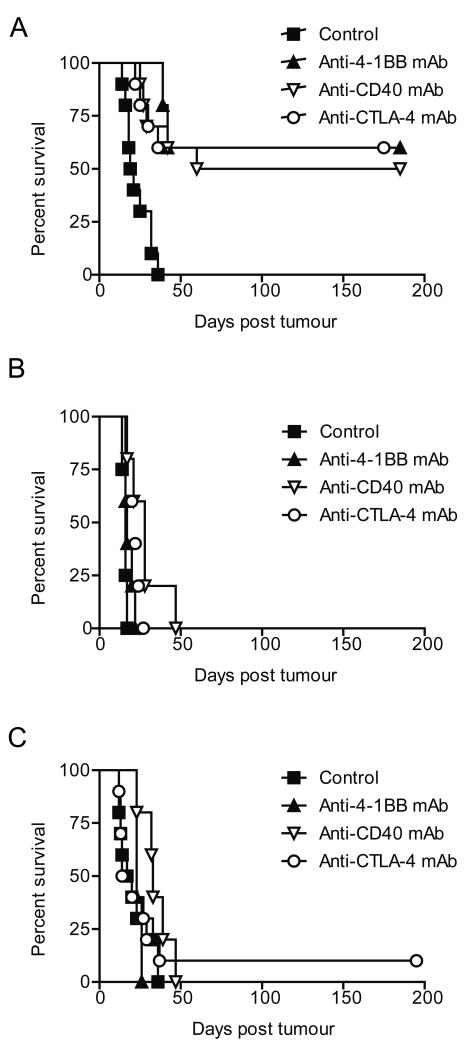
Antibodies targeting both CD40 and CTLA-4 were also demonstrated to be therapeutic in the Neuro2a model (p < 0.0001 for both) (Figure 3A); with efficacy comparable to that observed with anti-4-1BB mAb. None of the neuroblastoma cell lines investigated showed significant expression of 4-1BB, CD40 or CTLA-4 (Figure S1), and there was no in vitro evidence of either direct-cell death (as determined by AnV/PI staining) or inhibition of cell proliferation (as determined by 3H thymidine incorporation) by mAb targeting any of these receptors (Figure S2). By contrast proliferation of the B cell lymphoma cell line, A20, which expresses CD40, was significantly inhibited by anti-CD40 mAb (Figure S2). These data demonstrate that although immunomodulatory mAb may have a direct effect on cells which express their target molecule, these do not include the neuroblastoma cell lines.
Figure 3. Single agent mAbs are not therapeutic in aggressive neuroblastoma models.
A/J mice inoculated s.c. with 2 × 106 A, Neuro2a, B, AgN2a or C, NXS2 cells received 0.5 mg of anti-4-1BB, anti-CD40 or anti-CTLA-4 or 200 μl isotype control i.p. on days 9 and 12. These mAb were therapeutic in the Neuro2a model (p = 0.0006, 0.0012 & 0.0006, respectively), but not in the AgN2a (p = 0.1258, 0.8361 & 0.1034, respectively) or NXS2 (p = 7726, 0.0635 & 0.6108, respectively) models. Data represent examples of at least three experiments, where n = 5 mice/group.
Immunomodulatory mAb alone are not effective against more aggressive neuroblastoma tumors
Next, the in vivo activity of these three immunomodulatory mAb was investigated in the more aggressive, AgN2a and NXS2 neuroblastoma models. Here, single agent immunostimulatory mAb therapy with anti-CD40, anti-CTLA4 or anti-4-1BB resulted in only marginal slowing of tumor growth (Figure 3B & C). Although occasionally long term survival was observed, there was no significant overall survival advantage in these models.
Combination of immunomodulatory mAb with peptide vaccination provides effective therapy against aggressive neuroblastoma tumors
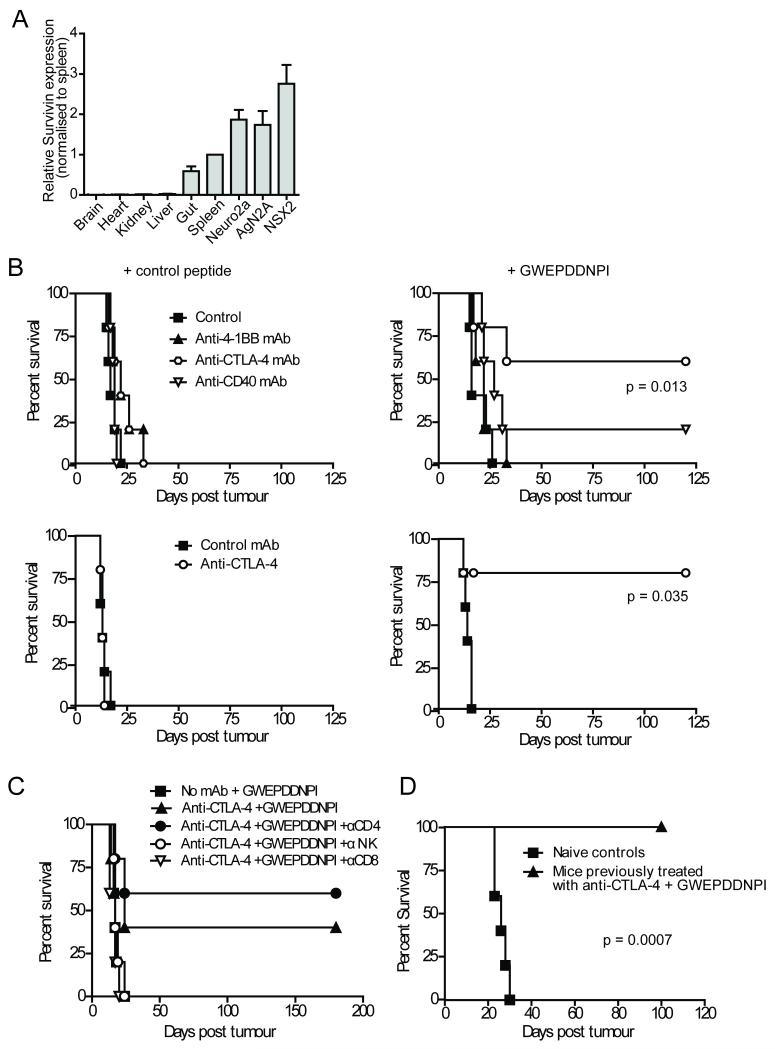
It was postulated that provision of extra tumor antigen in the form of peptide vaccination, may enhance the therapeutic effects of the immunomodulatory mAb. The survivin peptide GWEPDDNPI is a nonamer with predicted high affinity binding to murine Class I H2-Kk (31) and has been shown by others to be biologically active, where T cell responses targeting this epitope have contributed to therapeutic immunity in mice treated with a cytokine-transfected Neuro2a cellular vaccine therapy (26). Quantative PCR confirmed survivin expression in the three neuroblastoma cell lines with little expression observed in normal murine tissues other than the spleen and gut as previously reported (Figure 4A).
Figure 4. Combining anti-CTLA-4 mAb with survivin peptide vaccination provides effective therapy in the aggressive AgN2a and NXS2 models.
A, PCR determined that survivin expression (normalized to expression in brain) in normal A/J mouse tissue was low and in the malignant neuroblastoma cell lines was high. Data represents average of three experiments, with error expressed as SEM. B, A/J mice inoculated s.c. with 2 × 106 AGN2a (top panels) or NXS2 (bottom panels) on day 0 received 50 μg IFA emulsified survivin (GWEPDDNPI) or control (SIINFEKL) peptide vaccine i.d. day 3 and 0.5 mg of mAb or 200 μl isotype control i.p. on day 3 and day 6. In the AgN2a and NXS2 models combining GWEPDDNPI-peptide with anti-CTLA-4 mAb resulted in increased survival (p < 0.05). C, Anti-CTLA-4/GWEPDDNPI-peptide immunotherapy was abrogated after CD8+ T-cell (p = 0.0110) or NK-cell depletion (p = 0.0307) but was retained after CD4+ depletion (p = 0.4405). D, Long term survivors from (B) re-challenged with tumor (2 × 106 cells s.c) 120 days after initial inoculation showed increased survival compared with naïve age-matched controls (p = 0.0007). Data represent examples of three experiments, where n = 5 mice/group.
Anti-CTLA-4 mAb given with IFA emulsified GWEPDDNPI peptide (but not control peptide) resulted in effective therapy in both the AgN2a and NXS2 models (Figure 4B). The combination therapy significantly prolonged survival compared to either GWEPDDNPI peptide (p = 0.013 & p = 0.0353 for AgN2a & NXS2, respectively) or anti-CTLA-4 mAb alone (p = 0.047 & p = 0.0346 for AgN2a & NXS2, respectively) by abrogating tumor growth (Figure S3 and data not shown). Again, therapy was dependent on the presence of CD8+ and NK cells (Figure 4C) and long term survivors from the anti-CTLA-4/GWEPDDNPI peptide treatment groups were protected against NXS2 tumor re-challenge (p < 0.0001) (Figure 4D). Interestingly, despite similar efficacy to anti-CTLA-4 mAb in the Neuro2a model, neither anti-CD40 nor anti-4-1BB mAbs were found to provide effective therapy when combined with the GWEPDDNPI peptide in the more aggressive tumor models (Figure 4B). These data suggest that the GWEPDDNPI peptide in combination with certain immunomodulatory mAb is able to generate effective anti-tumor immunity in aggressive neuroblastoma models.
Splenocytes from mice treated with anti-CTLA-4 and GWEPDDNPI demonstrate tumor directed killing
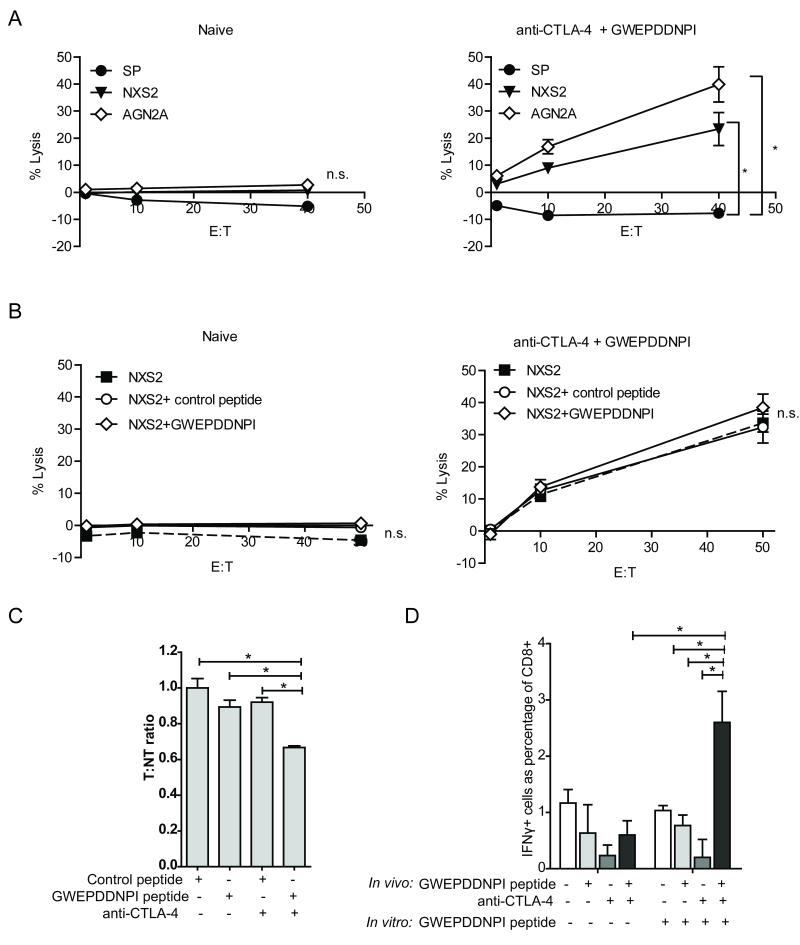
Splenocytes from tumor inoculated mice receiving anti-CTLA-4/GWEPDDNPI peptide treatment in vivo demonstrated effective lysis of tumor cells in CTL killing assays ex vivo (Figure 5A). In contrast, splenocytes from naïve mice similarly stimulated in vitro were inefficient at killing tumor cells (Figure 5A). Lysis was observed against the cell line that the mice had been inoculated with (NXS2) and additionally the AgN2a neuroblastoma cell line (Figure 5A). This may be because both cell lines express survivin, or because immunity has been generated against other shared antigens. Lytic activity was not significantly increased when tumor cells or splenocytes, were pulsed with the GWEPDDNPI peptide during the in vitro assay (Figure 5B and Figure S4). Initially it was hypothesized that the relative high expression of survivin on these tumors cells may mean that survivin-specific lysis was already maximal. However, as splenocytes pulsed with GWEPDDNPI peptide also did not show enhanced lysis, it is more likely that epitope spreading to other target antigens is involved in the anti-tumour response observed.
Figure 5. Anti-CTLA4 and GWEPDDNPI peptide immunotherapy generates tumor directed cytotoxicity.
Splenocytes from NXS2 inoculated mice receiving anti-CTLA-4/GWEPDDNPI-peptide immunotherapy as previously described were harvested after 35 days and re-stimulated alongside splenocytes from naïve mice with GWEPDDNPI-pulsed, irradiated splenocytes for 6 days. Target-cells labeled with 51Cr were incubated with splenocytes (effectors) at 50:1, 10:1 and 1:1 effector:target (E:T) ratio for 5 hr under tissue culture condition. A, Splenocytes from mice treated with anti-CTLA-4/GWEPDDNPI peptide but not from naïve mice demonstrated lytic activity against NXS2 and AgN2a tumors (p < 0.05). B, NXS2 cell lysis was not enhanced when the NXS2 cells were pulsed with a control or GWEPDDNPI peptide. C. Fourteen days after therapy, CSFE-labeled peptide-pulsed splenocytes were adoptively transferred into NXS2 inoculated mice treated with control peptide, GWEPDDNPI, anti-CTLA-4 alone or anti-CTLA-4/GWEPDDNPI-peptide. After 24 hrs peptide-specific killing was observed only with anti-CTLA-4/GWEPDDNPI treatment, as determined by the T:NT ratio. D. Splenocytes from (C) re-stimulated as described above, were incubated with unlabeled NXS2 target cells at a 50:1 E:T ratio for 5 hr in the presence of brefeldin A under tissue culture conditions. Using intracellular flow cytometry, IFN-γ production was detected in CD8+ T-cells in splenocytes from anti-CTLA-4/GWEPDDNPI treated mice only following in vitro re-stimulation with GWEPDDNPI. Data represent examples of at least three experiments, where n = 3 mice/group. Error is expressed as SEM. * p < 0.05, ** p < 0.01, n.s. not significant.
Combined therapy with anti-CTLA-4 mAb and GWEPDDNPI peptide generates anti-survivin immunity
Having established that splenocytes from mice treated with anti-CTLA-4/GWEPDDNPI-peptide had anti-tumor lytic activity ex vivo we next wished to determine whether these mice had developed survivin specific immune responses. Fourteen days after therapy with control peptide, GWEPDDNPI peptide, anti-CTLA-4 or anti-CTLA-4/GWEPDDNPI peptide, CFSE-labeled, GWEPDDNPI-pulsed target and control peptide-pulsed non-target splenocytes were adoptively transferred into treated mice. Specific depletion of target-cells was observed 24 hr later only in mice treated with the combined anti-CTLA-4/GWEPDDNPI peptide therapy and not in mice that received the control or single agent therapy (p = 0.05) (Figure 5C). This demonstrates that anti-CTLA-4 combined with GWEPDDNPI peptide vaccine was able to generate anti-survivin immunity. Furthermore, following 6 day ex vivo stimulation with GWEPDDNPI-pulsed irradiated splenocytes, CD8+ T-cells from mice treated with anti-CTLA-4 and GWEPDDNPI demonstrated IFN-γ production after 5 hr incubation with NXS2 cells. This was in comparison to mice receiving control peptide, GWEPDDNPI or anti-CTLA-4 alone (p = 0.0138, 0.0017 & 0.0172, respectively) (Figure 5D).
Effective anti-CTLA-4 and GWEPDDNPI therapy was not observed in the absence of tumor
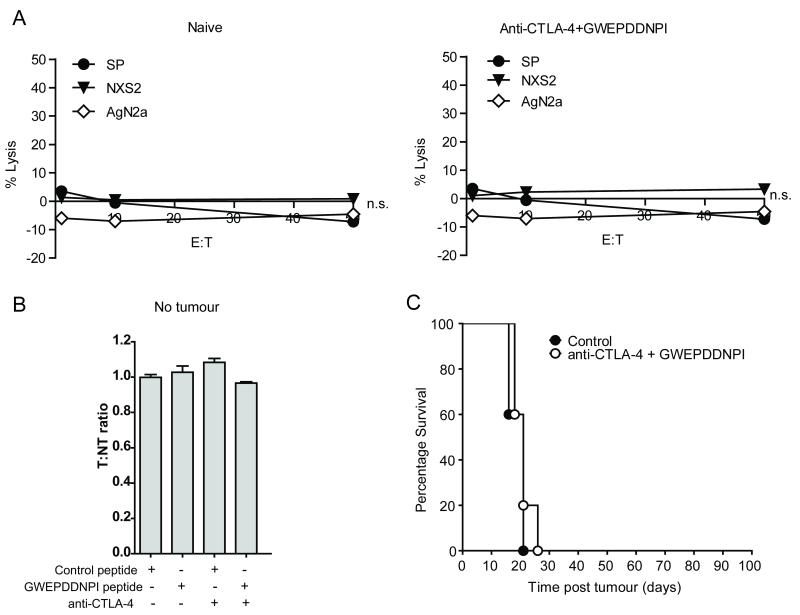
In the absence of tumor, splenocytes from mice that had received anti-CTLA-4/GWEPDDNPI therapy for 35 days in vivo were unable to generate anti-tumor cell lysis in CTL killing assays against either NXS2 or AgN2a neuroblastoma cells (Figure 6A). Alongside this, in vivo depletion of the target GWEPDDNPI-pulsed population was absent when labeled splenocytes were adoptively transferred into mice treated as previously described, but without prior tumor inoculation (Figure 6B). Lastly, prophylactic administration of anti-CTLA-4/GWEPDDNPI peptide (prior to tumor inoculation) was not protective against tumor growth, and did not achieve long term survival (Figure 6C). These data therefore suggest that the presence of tumor is required to generate an effective anti-tumor response in vivo.
Figure 6. Combined therapy with anti-CTLA4 mAb and GWEPDDNPI peptide requires tumor to generate anti-survivin T-cell immunity.
A, Assessment using CTL killing assays demonstrated that splenocytes from naïve mice or mice treated with anti-CTLA-4/GWEPDDNPI-peptide without tumor for 35 days showed negligible lytic activity against splenocytes or neuroblastoma cells. Data represents an example of 2 experiments with 4 mice / group, error is expressed as SEM. B, A/J mice treated for 14 days with control peptide, GWEPDDNPI-peptide or anti-CTLA-4 alone or anti-CTLA-4/GWEPDDNPI-peptide in the absence of NXS2 cells did not show in vivo peptide-specific killing after 24 hr following transfer of CFSE-labeled, peptide-pulsed splenocytes. C, A/J mice received GWEPDDNPI or control peptide i.d. emulsified in IFA on day -7 and 0.5 mg of anti-CTLA-4 or 200 μl PBS i.p. on day -7 and -3. Mice were then inoculated on day 0 with 2 × 106 NXS2 cells s.c. Anti-CTLA-4/GWEPDDNPI peptide prophylactic treatment did not result in significant long term survival. Data in (B) and (C) represents examples of three experiments, where n = 5 mice/group. Error is expressed as SEM.
Taken together these data suggest that anti-CTLA-4 and survivin peptide vaccination can be used to generate potent anti-tumor immunity in these aggressive neuroblastoma models. Despite the fact that this therapy provides tumor antigen, generation of effective anti-tumor immunity appears dependent on the presence of tumor per se. Whether tumor is required to provide other tumor antigens, or to provide some form of ‘danger’ signal is unclear, but is the subject of ongoing investigation.
Finally, it should be noted that in all of the above experiments, in the absence of tumor progression, treated mice remained well with no overt clinical or histological signs of toxicity from the immunotherapy. In particular, there was no obvious weight loss, gut toxicity or histological evidence of colitis (data not shown).
Discussion
Successfully treating metastatic neuroblastoma remains a major challenge of pediatric oncology. Using the immune system to target tumor is an attractive treatment option for these children. Although many immunotherapeutic strategies have proved effective in pre-clinical neuroblastoma models, relatively few have entered clinical trials. To date, most of these have been ‘passive’ immunotherapies, such as anti-GD2 mAb therapy (32). In 2010 the US Children’s oncology Group reported a large randomized control trial demonstrated a significant 2 year event free survival benefit (66 vs. 46%) in children who had received anti-GD2 based immunotherapy in addition to standard high risk neuroblastoma therapy (33). Whilst clearly encouraging, data from this study is still relatively immature and a large proportion of children still die from their disease.
The advantage of ‘active’ immunotherapies is that they potentially achieve long-term immunity and tumor protection. Immunomodulatory mAb offer a potential mechanism of enhancing circulating tumor specific T cells in patients to achieve protective anti-tumor immunity. For effective activation T cells must not only engage their specific antigen but also a number of co-stimulatory molecules (34). Such molecules are usually absent on the surface of neuroblastoma cells themselves (35, 36) and although tumor antigens may be cross-presented by professional antigen presenting cells, expression of co-stimulatory molecules by these cells is likely to be low in the relatively non-inflammatory tumor environment (37). Furthermore, neuroblastoma cells themselves may abrogate expression of co-stimulatory molecules (e.g CD40) on dendritic cells (38). Therefore, despite the presence of potentially immunogenic tumor antigens, anti-tumor T cell responses may be sub-optimal or even rendered tolerogenic (39). Transfection of co-stimulatory ligands (CD80, CD86, 4-1BBL, CD40L) into murine neuroblastoma cell lines may overcome this defect, providing effective prophylactic tumor vaccination (40). Immunomodulatory mAb are a more practical and effective way of achieving this, either by acting agonistically, functioning as surrogate ligands or by blocking immune-regulating, molecules such as CTLA-4. Here we demonstrate that a range of such mAb provide very effective therapy and may be successfully combined with other immunotherapies such as peptide vaccines. Unlike many other immunotherapies, such as cellular vaccines, these mAb do not need to be tailored to individual tumors or patient HLA-types. This makes them a more attractive therapy to take forward to the clinic.
In the Neuro2a model, antibodies targeting CTLA-4, 4-1BB or CD40 all provide effective therapy. The timing of mAb delivery is crucial and is only efficacious if given in a therapeutic rather than prophylactic setting. Although perhaps counterintuitive, this phenomenon has been noted previously when immunostimulatory mAb therapies are used in other tumor models, suggesting that the anti-tumor immune response generated is dependent on the presence of tumor antigen, and therapy is limited when mice are treated with a low tumor burden (13). In these models, rapid in vivo tumor growth kinetics results in there being a relatively narrow therapeutic window, between giving the mAb too early (when there may be insufficient tumor antigen) and too late (when there is insufficient time to mount an effective immune response before the animal succumbs to disease). In more aggressive neuroblastoma models (NXS2 and AgN2a) we were unable to achieve effective therapy with mAb alone. It was unclear whether this was simply due to the faster in vivo growth of these tumors, or because these tumors were less intrinsically immunogenic. We postulated that, in either instance, therapy may be enhanced by combining the immunostimulatory mAb with a peptide vaccine targeting the tumor antigen survivin. Survivin is a member of the inhibitor of apoptosis family of proteins and is attractive as a target antigen for a number of reasons. Not only is it almost universally expressed in human high risk neuroblastoma, it is also expressed in a number of other pediatric malignancies and many adult tumors, but with little expression in normal tissues (7). Expression of survivin appears to confer pro-angiogenic and anti-apoptotic properties giving survival advantage to the tumor. These properties would be lost if the antigen were to be down regulated as a means of immune escape (41). Additionally, as previously mentioned, spontaneous T cell responses to survivin have been recognized in children with neuroblastoma, suggesting the clinical relevance of the antigen (7). Therefore, there is already interest in developing clinical vaccines and immunotherapies targeting this antigen, with a number of adult phase I studies of survivin vaccine based therapies have been reported (42, 43). In general, minimal epitope peptide vaccines given alone have not proved successful as cancer immunotherapeutics (44). In accordance with this we did not observe any survival advantage in mice treated with survivin peptide alone. However, we found that combining survivin peptide with immunomodulatory mAb provided highly effective therapy. In addition broader immune responses were potentially generated, targeting other antigens released when tumor cells were killed by anti-survivin CTLs, and boosted by circulating immunomodulatory mAb. Although initial peptide-specific responses were observed, later on T cell responses did not appear to be predominantly directly against survivin, suggesting epitope spreading. The generation of a broader immune response, directed against non-vaccine antigens is potentially clinically advantageous, and is the focus of ongoing investigation within our laboratory.
There has been recent concern that survivin expression by activated CTLs may actually render them targets, and subject to fratricide, limiting the potential of any survivin-directed immunotherapy (45). The potent therapeutic effects we observed would argue against this being a major problem in vivo and the fact that mice successfully treated with mAb are left protected from re-challenge with the same tumor, suggests that sustained, functional, protective immunity is generated by this combination therapy.
In our experiments, the most promising mAb appeared to be anti-CTLA-4, and it is mAb targeting this molecule that have so far shown most promise in adult clinical trials (16). To date, the majority of clinical trials have been in patients with melanoma. Like melanoma, neuroblastoma is derived from neuroectodermal tissue, and immunologically there are many parallels and common tumor antigens. Although many of the melanoma studies have included peptide vaccination in addition to anti-CTLA-4, this did not confer additional benefit in the phase III study (16). This contrasts with our experience in murine models, and may be because there was pre-existing tolerance to the epitopes of the chosen vaccine, or because in these patients there was sufficient endogenous tumor antigen.
Despite the fact that our experiments demonstrated superior efficacy in the context of increasing tumor burden, it is unlikely that immunotherapy alone will provide effective therapy in children with bulky disease. For most immunotherapies, there is increased likelihood of success in the context of low tumor burden, or minimal residual disease (MRD). Children that initially respond well to chemotherapy often harbor microscopic minimal residual disease (MRD) resulting in eventual tumor relapse. Prior to relapse children are relatively well and can potentially receive a MRD-directed regime such as immunotherapy. In this context, there would be rationale for delivering the immunomodulatory mAb with tumor antigen in the form of peptide vaccine.
There is reservation about the usefulness of T cell mediated immunotherapies in children with neuroblastoma, given that these tumors frequently exhibit low or absent levels of MHC class I (46). However, there is clinical evidence from other experimental T cell mediated therapies that this may not be a barrier for effective therapy. Russell et al (2007) reported a phase I study of a vaccine consisting of an allogeneic human neuroblastoma transfected with IL-2 and lymphotactin, a chemokine with T cell attractant properties. Evidence of tumor-specific T cell responses were reported. Of 28 children treated, there were 4 complete tumor responses to the vaccine (2 sustained for more than 4 years), 2 partial responses and 5 children with stable disease (47). This suggests that, providing CTL are adequately primed, only very low levels of surface MHC/antigen are needed to render tumor cells targets for killing. In addition, surface expression of MHC class I on neuroblastoma is up-regulated in the context of IFN-γ (48), which is potentially released by tumor infiltrating lymphocytes as part of the anti-tumor immune response. This has been found to be the case with the B16-F10 metastatic melanoma model, where expression of both MHC class I and class II is increased on tumor cells following anti-4-1BB therapy (49).
Although we saw no signs of obvious toxicity in mice treated with any of the mAb or peptide therapies, anti-CTLA-4 mAb have been associated with significant toxicity in patients, with severe (grade 3 or 4) immune related adverse events (e.g. colitis, dermatitis, hypophysitis) in up to 10-15 % of patients (16). Remarkably, immune related adverse events have tended to correlate with therapeutic benefit (50). The vast majority resolved with either withdrawal of mAb therapy, or with corticosteroid treatment. The latter of which was shown not to abrogate therapeutic benefit. There is no reason why toxicity should be worse in children than adults, but clearly introduction of these agents into pediatric clinical trials should proceed cautiously, and should build on the adult experience in terms of prevention and management of this spectrum of toxicities, which would be unfamiliar to most pediatric oncologists. There would also be potential concern about targeting survivin therapeutically in children as it expressed during cell division and there may be long term effects on growing and developing tissues that are not seen in adult patients. The combination of peptide vaccines targeting other neuroblastoma antigens (e.g. tyrosine hydroxylase) should therefore also be explored. All of these risks and potential toxicities have to be balanced against the significant toxicity of current neuroblastoma therapies, and the high mortality of the disease.
Finally, although we did not find any evidence of a expression of co-stimulatory molecules, or direct effects of the immunostimulatory mAbs, on these neuroblastoma cell lines, there is some published data suggesting that CD40 is expressed by human neuroblastoma (35). In other tumors in which CD40 is expressed by the tumor, there does seem to be a direct killing effect, in addition to indirect effects on immune system (13). It is therefore possible that additional therapeutic mechanisms may be seen in patients that we have not observed in our murine models.
Supplementary Material
Translational relevance.
The majority of children with neuroblastoma present with metastatic disease for which long-term survival remains poor, despite intensive multi-modal therapies. Immunotherapy is an attractive therapeutic approach for these children, potentially being more specific and less toxic than conventional therapies. Survivin is a particularly promising immunotherapy target as it is expressed in 80-100% of high-risk tumors and only minimally in normal tissue. Spontaneous anti-survivin T-cell responses have also been reported in children with neuroblastoma. Immunomodulatory monoclonal antibodies targeting co-stimulatory molecules (4-1BB, CD40) or checkpoint-blockers (CTLA-4, PD-1) offer a practical and potent means of boosting these weak endogenous responses to achieve therapeutic immunity and have shown promise in adult clinical trials in a number of malignancies. There is, as yet, no clinical experience in pediatric patients. Here, we demonstrate that anti-CTLA-4 combined with survivin-peptide vaccination provides effective therapy and anti-tumor immunity and is a promising immunotherapeutic for the treatment of high-risk neuroblastoma.
Acknowledgements
We would like to thank Dr Rimas Ornetas and Dr Holger Lode for the gift of cell lines and all members of the Antibody and Vaccine Group for their help and assistance with this project
Grant support Funding was provided by Cancer Research UK grant C8574/A11781, Wessex Cancer Trust and Wessex Medical Research.
This work was supported by Cancer Research UK, Wessex Cancer Trust and Wessex Medical Research.
Footnotes
Conflict of interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 2008;9:247–56. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]
- 2.Zage PE, Kletzel M, Murray K, Marcus R, Castleberry R, Zhang Y, et al. Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51:747–53. doi: 10.1002/pbc.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–58. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 4.Gray JC, Kohler JA. Immunotherapy for neuroblastoma: turning promise into reality. Pediatr Blood Cancer. 2009;53:931–40. doi: 10.1002/pbc.22153. [DOI] [PubMed] [Google Scholar]
- 5.Martin RF, Beckwith JB. Lymphoid infiltrates in neuroblastomas: their occurrence and prognostic significance. J Pediatr Surg. 1968;3:161–4. doi: 10.1016/0022-3468(68)91005-1. [DOI] [PubMed] [Google Scholar]
- 6.Lauder I, Aherne W. The significance of lymphocytic infiltration in neuroblastoma. Br J Cancer. 1972;26:321–30. doi: 10.1038/bjc.1972.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coughlin CM, Fleming MD, Carroll RG, Pawel BR, Hogarty MD, Shan X, et al. Immunosurveillance and survivin-specific T-cell immunity in children with high-risk neuroblastoma. J Clin Oncol. 2006;24:5725–34. doi: 10.1200/JCO.2005.05.3314. [DOI] [PubMed] [Google Scholar]
- 8.Gray JC, Johnson PW. Glennie MJ Therapeutic potential of immunostimulatory monoclonal antibodies. Clin Sci (Lond) 2006;111:93–106. doi: 10.1042/CS20060024. [DOI] [PubMed] [Google Scholar]
- 9.Peggs KS, Quezada SA, Allison JP. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clin Exp Immunol. 2009;157:9–19. doi: 10.1111/j.1365-2249.2009.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalil M, Vonderheide RH. Anti-CD40 agonist antibodies: preclinical and clinical experience. Update Cancer Ther. 2007;2:61–65. doi: 10.1016/j.uct.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–9. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 13.Tutt AL, O’Brien L, Hussain A, Crowther GR, French RR, Glennie MJ. T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J Immunol. 2002;168:2720–8. doi: 10.4049/jimmunol.168.6.2720. [DOI] [PubMed] [Google Scholar]
- 14.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 15.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–96. [PubMed] [Google Scholar]
- 16.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446–53. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calabro L, Danielli R, Sigalotti L, Maio M. Clinical studies with anti-CTLA-4 antibodies in non-melanoma indications. Semin Oncol. 2010;37:460–7. doi: 10.1053/j.seminoncol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Advani R, Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Ren H, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4371–7. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 20.Taraban VY, Rowley TF, O’Brien L, Chan HT, Haswell LE, Green MH, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32:3617–27. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.George AJ, McBride HM, Glennie MJ, Smith LJ, Stevenson FK. Monoclonal antibodies raised against the idiotype of the murine B cell lymphoma, BCL1 act primarily with heavy chain determinants. Hybridoma. 1991;10:219–27. doi: 10.1089/hyb.1991.10.219. [DOI] [PubMed] [Google Scholar]
- 22.Hasbold J, Johnson-Leger C, Atkins CJ, Clark EA, Klaus GG. Properties of mouse CD40: cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur J Immunol. 1994;24:1835–42. doi: 10.1002/eji.1830240817. [DOI] [PubMed] [Google Scholar]
- 23.Cobbold SP, Martin G, Waldmann H. The induction of skin graft tolerance in major histocompatibility complex-mismatched or primed recipients: primed T cells can be tolerized in the periphery with anti-CD4 and anti-CD8 antibodies. Eur J Immunol. 1990;20:2747–55. doi: 10.1002/eji.1830201232. [DOI] [PubMed] [Google Scholar]
- 24.Chan HT, Hughes D, French RR, Tutt AL, Walshe CA, Teeling JL, et al. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane rafts. Cancer Res. 2003;63:5480–9. [PubMed] [Google Scholar]
- 25.Tutt AL, French RR, Illidge TM, Honeychurch J, McBride HM, Penfold CA, et al. Monoclonal antibody therapy of B cell lymphoma: signaling activity on tumor cells appears more important than recruitment of effectors. J Immunol. 1998;161:3176–85. [PubMed] [Google Scholar]
- 26.Croce M, Meazza R, Orengo AM, Fabbi M, Borghi M, Ribatti D, et al. Immunotherapy of neuroblastoma by an Interleukin-21-secreting cell vaccine involves survivin as antigen. Cancer Immunol Immunother. 2008;57:1625–34. doi: 10.1007/s00262-008-0496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–9. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 29.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–15. [PubMed] [Google Scholar]
- 30.Castriconi R, Dondero A, Cilli M, Ognio E, Pezzolo A, De Giovanni B, et al. Human NK cell infusions prolong survival of metastatic human neuroblastoma-bearing NOD/scid mice. Cancer Immunol Immunother. 2007;56:1733–42. doi: 10.1007/s00262-007-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fest S, Huebener N, Bleeke M, Durmus T, Stermann A, Woehler A, et al. Survivin minigene DNA vaccination is effective against neuroblastoma. Int J Cancer. 2009;125:104–14. doi: 10.1002/ijc.24291. [DOI] [PubMed] [Google Scholar]
- 32.Modak S, Cheung NK. Disialoganglioside directed immunotherapy of neuroblastoma. Cancer Invest. 2007;25:67–77. doi: 10.1080/07357900601130763. [DOI] [PubMed] [Google Scholar]
- 33.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16:321–7. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Prigione I, Corrias MV, Airoldi I, Raffaghello L, Morandi F, Bocca P, et al. Immunogenicity of human neuroblastoma. Ann N Y Acad Sci. 2004;1028:69–80. doi: 10.1196/annals.1322.008. [DOI] [PubMed] [Google Scholar]
- 36.Airoldi I, Lualdi S, Bruno S, Raffaghello L, Occhino M, Gambini C, et al. Expression of costimulatory molecules in human neuroblastoma. Evidence that CD40+ neuroblastoma cells undergo apoptosis following interaction with CD40L. Br J Cancer. 2003;88:1527–36. doi: 10.1038/sj.bjc.6600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennaceur K, Chapman J, Brikci-Nigassa L, Sanhadji K, Touraine JL, Portoukalian J. Dendritic cells dysfunction in tumour environment. Cancer Lett. 2008;272:186–96. doi: 10.1016/j.canlet.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 38.Walker SR, Redlinger RE, Jr., Barksdale EM., Jr. Neuroblastoma-induced inhibition of dendritic cell IL-12 production via abrogation of CD40 expression. J Pediatr Surg. 2005;40:244–9. doi: 10.1016/j.jpedsurg.2004.09.050. discussion 249-50. [DOI] [PubMed] [Google Scholar]
- 39.Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003;192:161–80. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 40.Yan X, Johnson BD, Orentas RJ. Induction of a VLA-2 (CD49b)-expressing effector T cell population by a cell-based neuroblastoma vaccine expressing CD137L. J Immunol. 2008;181:4621–31. doi: 10.4049/jimmunol.181.7.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J. 2010;430:199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyazaki A, Kobayashi J, Torigoe T, Hirohashi Y, Yamamoto T, Yamaguchi A, et al. Phase I clinical trial of survivin-derived peptide vaccine therapy for patients with advanced or recurrent oral cancer. Cancer Sci. 2011;102:324–9. doi: 10.1111/j.1349-7006.2010.01789.x. [DOI] [PubMed] [Google Scholar]
- 43.Honma I, Kitamura H, Torigoe T, Takahashi A, Tanaka T, Sato E, et al. Phase I clinical study of anti-apoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother. 2009;58:1801–7. doi: 10.1007/s00262-009-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voskens CJ, Strome SE, Sewell DA. Synthetic peptide-based cancer vaccines: lessons learned and hurdles to overcome. Curr Mol Med. 2009;9:683–93. doi: 10.2174/156652409788970724. [DOI] [PubMed] [Google Scholar]
- 45.Leisegang M, Wilde S, Spranger S, Milosevic S, Frankenberger B, Uckert W, et al. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J Clin Invest. 2010;120:3869–77. doi: 10.1172/JCI43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfl M, Jungbluth AA, Garrido F, Cabrera T, Meyen-Southard S, Spitz R, et al. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother. 2005;54:400–6. doi: 10.1007/s00262-004-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell HV, Strother D, Mei Z, Rill D, Popek E, Biagi E, et al. Phase I trial of vaccination with autologous neuroblastoma tumor cells genetically modified to secrete IL-2 and lymphotactin. J Immunother. 2007;30:227–33. doi: 10.1097/01.cji.0000211335.14385.57. [DOI] [PubMed] [Google Scholar]
- 48.Ponzoni M, Guarnaccia F, Corrias MV, Cornaglia-Ferraris P. Uncoordinate induction and differential regulation of HLA class-I and class-II expression by gamma-interferon in differentiating human neuroblastoma cells. Int J Cancer. 1993;55:817–23. doi: 10.1002/ijc.2910550521. [DOI] [PubMed] [Google Scholar]
- 49.Ju SA, Lee SC, Kwon TH, Heo SK, Park SM, Paek HN, et al. Immunity to melanoma mediated by 4-1BB is associated with enhanced activity of tumour-infiltrating lymphocytes. Immunol Cell Biol. 2005;83:344–51. doi: 10.1111/j.1440-1711.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 50.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.