Abstract
Metabolic syndrome patients have insulin resistance, which causes hyperinsulinemia, which in turn causes aberrant increased renal sodium reabsorption. The precise mechanisms underlying this greater salt-sensitivity of hyperinsulinemic patients remain unclear. Abnormal activation of the recently-identified WNK kinase-OSR1/SPAK kinases-NCC transporter phosphorylation cascade results in the salt-sensitive hypertension of pseudohypoaldosteronism type II. Here, we report a study of renal WNK-OSR1/SPAK-NCC cascade activation in the db/db mouse model of hyperinsulinemic metabolic syndrome. Thiazide sensitivity was increased, suggesting greater activity of NCC in db/db mice. In fact, increased phosphorylation of OSR1/SPAK and NCC was observed. In both SpakT243A/+ and Osr1T185A/+ knock-in db/db mice, which carry mutations that disrupt the signal from WNK kinases, increased phosphorylation of NCC and elevated blood pressure were completely corrected, indicating that phosphorylation of SPAK and OSR1 by WNK kinases is required for the increased activation and phosphorylation of NCC in this model. Renal phosphorylated Akt was increased in db/db mice, suggesting that increased NCC phosphorylation is regulated by the PI3K/Akt signaling cascade in the kidney in response to hyperinsulinemia. A PI3K inhibitor (NVP-BEZ235) corrected the increased OSR1/SPAK-NCC phosphorylation. Another more specific PI3K inhibitor (GDC-0941) and an Akt inhibitor (MK-2206) also inhibited increased NCC phosphorylation. These results indicate that the PI3K/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in db/db mice. This mechanism may play a role in the pathogenesis of salt-sensitive hypertension in human hyperinsulinemic conditions such as the metabolic syndrome.
Keywords: WNK, PI3K, Akt, NaCl cotransporter, insulin, obesity, sodium dependent hypertension
Introduction
Pseudohypoaldosteronism type II (PHAII) is an autosomal dominant disease characterized by salt-sensitive hypertension due to increased renal salt reabsorption.1-3 Mutations in with-no-lysine kinases 1 and 4 (WNK1 and WNK4) have been reported to cause PHAII.4 Previously WNK4D561A/+ knock-in mice, an ideal mouse model of PHAII, were analyzed; the pathogenesis of PHAII was shown to involve abnormal constitutive activation of the WNK kinase-oxidative stress-responsive kinase-1 (OSR1), STE20/SPS1-related proline/alanine-rich kinase (SPAK)-NaCl cotransporter (NCC) phosphorylation cascade, resulting in increased NCC function.5
Recently, several physiological regulators of NCC phosphorylation have been reported. We have reported that NCC phosphorylation was increased by a low-salt diet and decreased by a high-salt diet through aldosterone, which is a strong regulator of NCC phosphorylation.6 Angiotensin II was also found to regulate NCC phosphorylation.7-9 In addition, extracellular potassium ions are reported to regulate the WNK-OSR1/SPAK-NCC phosphorylation cascade.10 Moreover, Vallon et al. reported that serum and glucocorticoid inducible kinase 1 (SGK1) is involved in the regulation of NCC phosphorylation by potassium intake.11 These findings indicate that the WNK-OSR1/SPAK-NCC phosphorylation cascade is important for NaCl homeostasis and blood pressure regulation under physiological conditions, as well as in PHAII.
There has been a striking worldwide increase in prevalence of the metabolic syndrome, which is characterized by hypertension, glucose intolerance, obesity and dyslipidemia.12 It has been reported that the metabolic syndrome enhances salt-sensitivity, leading to salt-sensitive hypertension.13-15 The metabolic syndrome causes hyperinsulinemia as a result of insulin resistance,12 and hyperinsulinemia causes an aberrant increase in sodium reabsorption by the kidney.16-19 However, the precise mechanisms responsible for the increased salt-sensitivity of hyperinsulinemic patients have not been clarified. Recently, it was demonstrated that acute insulin stimulation increases OSR1/SPAK and NCC phosphorylation in vivo.20 However, it remains unclear whether insulin increases NCC phosphorylation under physiological and chronic hyperinsulinemic conditions. It is also uncertain what type of signaling pathway links NCC phosphorylation with insulin.
The current study was an investigation of whether and how NCC phosphorylation is increased in the db/db mouse model of the metabolic syndrome; results demonstrated that the PI3K/Akt signaling pathway plays a key role in this process, thereby revealing a mechanism that links hyperinsulinemia with salt-sensitive hypertension.
Materials and Methods
See the online-only Data Supplement.
Results
Hyperinsulinemic db/db Mice Have Elevated Systolic Blood Pressure and Increased Phosphorylation of OSR1/SPAK and NCC
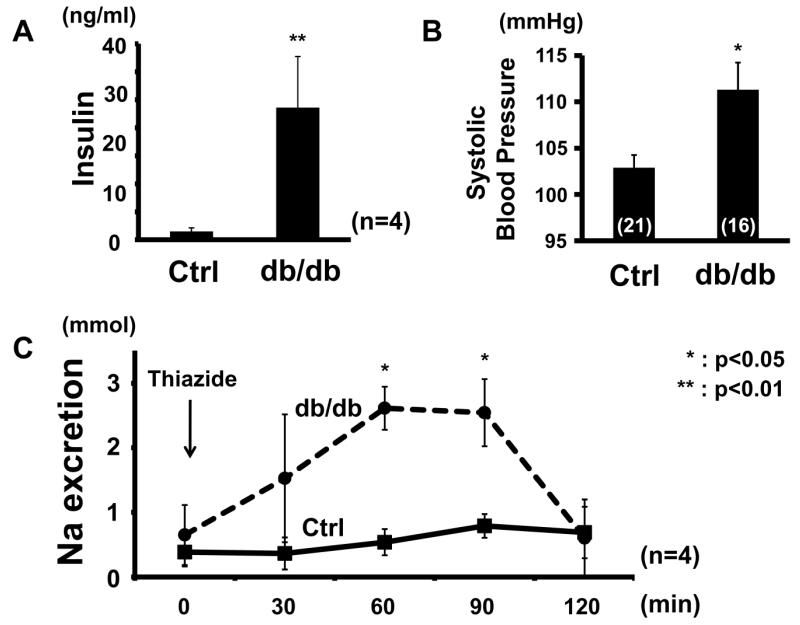
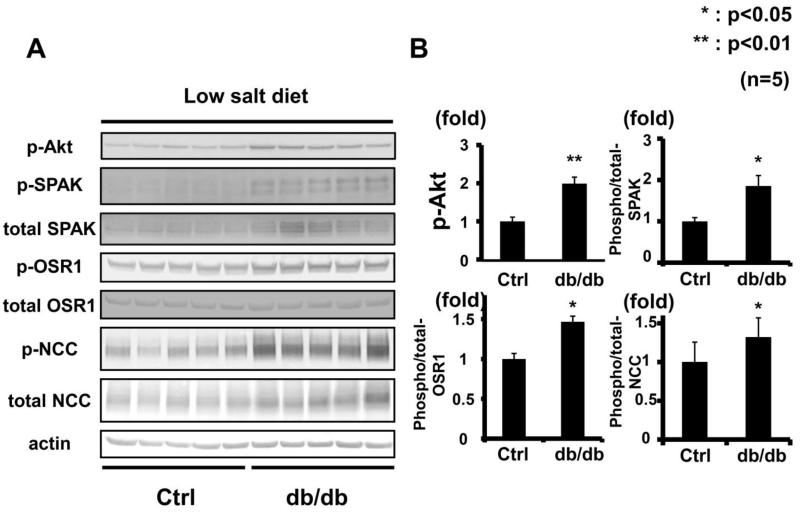
To investigate whether chronic hyperinsulinemia can activate the WNK-OSR1/SPAK-NCC signaling cascade (as can acute insulin administration20), hyperinsulinemic db/db mice were used (Figure 1A). Db/db mice lack leptin receptors, and therefore develop features of metabolic syndrome.21 It has been previously reported that db/db mice have salt-sensitive hypertension.22-26 This study confirmed presence of higher blood pressure in these animals, as compared to control mice (db/db, 111.3 ± 2.93 vs. control, 102.9 ± 1.36 mmHg, p<0.05) (Figure 1B). The blood pressure of db/db mice at the same age was comparable with the previous report.24 To confirm the contribution of NCC to the enhanced salt sensitivity, responsiveness to thiazide, an inhibitor of NCC, was investigated. For 7 days before the thiazide infusion test, mice were fed a low-salt diet to allow the salt intake of db/db and control mice to equilibrate. As a result, although plasma aldosterone levels in the db/db mice were not significantly lower compared to controls (db/db 632 ± 461.7 vs. control 822.4 ± 309.6 pg/ml, p=0.11), thiazide sensitivity was higher in the hyperinsulinemic mice (Figure 1C), suggesting greater NCC activity. Therefore, whether renal phosphorylation of OSR1/SPAK and NCC was increased in the db/db mice was ascertained, and as expected from the results of thiazide infusion test, increased phosphorylation of OSR1/SPAK and NCC was observed in db/db mice fed a low-salt diet (Figure 2).
Figure 1. Increased thiazide sensitivity and high blood pressure in db/db mice.
A. Hyperinsulinemia in db/db mice. Plasma insulin was increased in db/db mice, compared to control db/m mice. Mean ± SEM. (n=5). **p<0.01.
B. Systolic blood pressure is higher in db/db mice compared to control db/m mice. Mean ± SEM. (n as indicated). *p<0.05.
C. Thiazide infusion test. Response to hydrochlorothiazide (25 mg/kg ip), an inhibitor of NCC, was significantly greater in db/db mice fed a low-salt diet, compared to control db/m mice. Mean ± SEM. (n=4). *p<0.05.
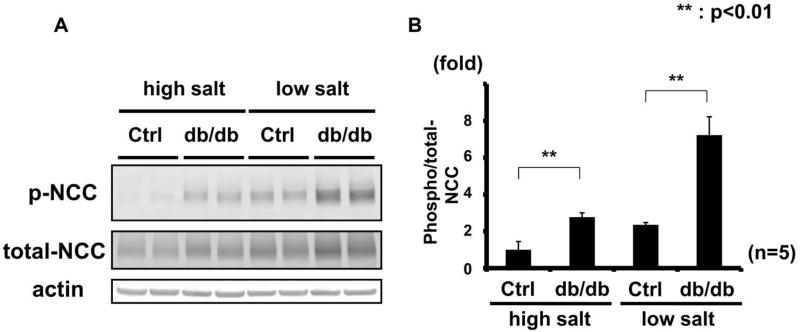
Figure 2. Increased OSR1/SPAK and NCC phosphorylation in kidneys of db/db mice fed a low-salt diet.
A. Immunoblots of OSR1/SPAK and NCC phosphorylation in kidneys of hyperinsulinemic db/db mice fed a low-salt diet. OSR1/SPAK and NCC phosphorylation increased in db/db mice compared to controls. Renal Akt phosphorylation was increased.
B. Densitometry analyses of phosphorylation of Akt, OSR1/SPAK and NCC in the kidney. Values expressed as the ratio to the average of signals in the vehicle group. **p<0.01.
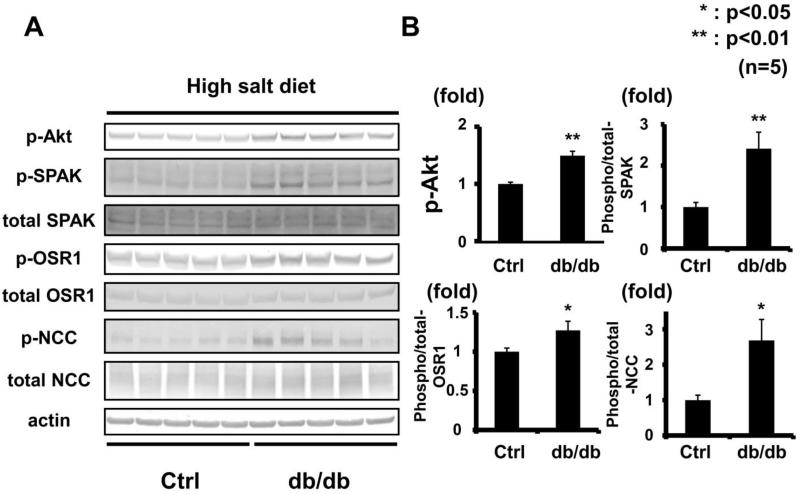
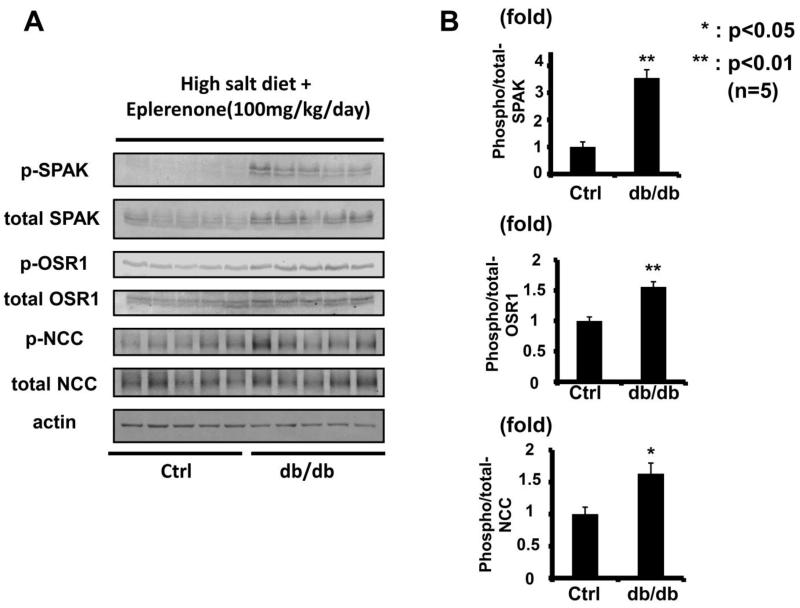
To minimize the effect of aldosterone, after a week of a high-salt diet, the OSR1/SPAK-NCC phosphorylation cascade was assayed in the db/db mouse kidney. Plasma aldosterone levels in db/db mice were significantly lower than in control mice on a high-salt diet (db/db 15.2 ± 14.8 vs. control 58.6 ± 32.5 pg/ml, p<0.05). As previously reported, a low plasma aldosterone level down-regulates the WNK-OSR1/SPAK-NCC phosphorylation cascade in the kidney.6 However, despite lower plasma aldosterone levels, db/db mice on a high-salt diet had increased phosphorylation of OSR1/SPAK and NCC (Figure 3). We also used eplerenone to investigate further the contribution of aldosterone to the enhanced WNK-OSR1/SPAK-NCC phosphorylation cascade in db/db mice. Eplerenone (100 mg/kg/day) suppressed WNK-OSR1/SPAK-NCC phosphorylation cascade in both db/db and control mice (please see http://hyper.ahajournals.org Figures S1-2). However, as shown in Figure 4, even after administration of eplerenone, the phosphorylation of OSR1/SPAK and NCC was still increased in db/db mice, compared to control mice. These results indicated that aldosterone-independent mechanism(s) might be involoved in the increased NCC phosphorylation in db/db mice.
Figure 3. Increased OSR1/SPAK and NCC phosphorylation in kidneys of db/db mice fed a high-salt diet.
A. Immunoblots of OSR1/SPAK and NCC phosphorylation in kidneys of hyperinsulinemic db/db mice fed a high-salt diet. Despite lower plasma aldosterone, OSR1/SPAK and NCC phosphorylation increased in db/db mice fed high- and low-salt diets. Akt phosphorylation increased.
B. Densitometry analyses of phosphorylation of Akt, OSR1/SPAK and NCC in the kidney. Values expressed as the ratio to the average of signals in the vehicle group. *p<0.05, **p<0.01.
Figure 4. Increased OSR1/SPAK and NCC phosphorylation in kidneys of db/db mice fed a high-salt diet with eplerenone.
A. Immunoblots of OSR1/SPAK and NCC phosphorylation in kidneys of hyperinsulinemic db/db mice fed a high-salt diet with eplerenone (100 mg/kg/day). Phosphorylation of OSR1/SPAK and NCC was still increased in db/db mice, even with eplerenone treatment.
B. Densitometry analyses of phosphorylation of OSR1/SPAK and NCC in the kidney. Values expressed as the ratio to the average of signals in the vehicle group. *p<0.05, **p<0.01.
Moreover, as shown in Figure 5, NCC phosphorylation in db/db mice on a high-salt diet was similar to the increase seen in control mice on a low-salt diet, suggesting that the magnitude of increased NCC phosphorylation in db/db mice reached a level sufficient to explain their increased salt-sensitivity.
Figure 5. Increased NCC phosphorylation in db/db mice is independent of aldosterone.
A. Representative immunoblot of the renal crude membrane fraction of db/db mice fed high- and low-salt diets, probed with phosphorylated NCC antibody.
B. Densitometry analysis; values expressed as ratio to average of signals in control mice fed a high-salt diet. In both high- and low-salt diet groups, db/db mice had increased phosphorylated NCC. (n=5). **p<0.01.
OSR1/SPAK Kinases Are Involved in the Mechanism of Increased NCC Phosphorylation in Hyperinsulinemic db/db Mice
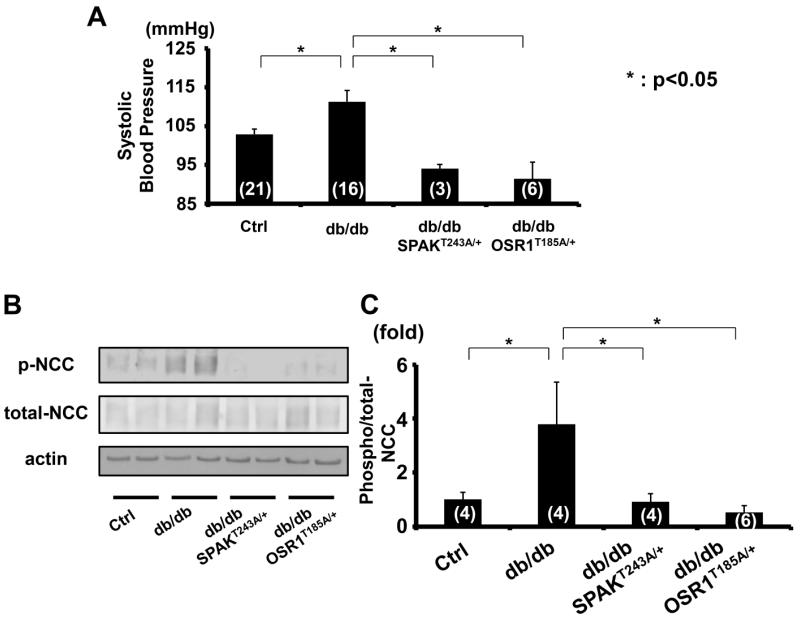
To confirm the contribution of WNK kinases and OSR1/SPAK to NCC phosphorylation in hyperinsulinemic db/db mice, they were mated with SpakT243A/+ and Osr1T185A/+ knock-in mice, which have reduced NCC phosphorylation and decreased blood pressure, as previously described.27 These SpakT243A/+ and Osr1T185A/+ knock-in mice in which the T-loop threonine residues in SPAK and OSR1 (243 and 185, respectively) were mutated to alanine to prevent activation by WNK kinases.27 Plasma insulin level, weight gain, blood glucose and lipid profile of these double knock-in db/db mice were not different from those of db/db mice, suggesting that SpakT243A/+ and Osr1T185A/+ knock-in did not affect metabolic characteristics of db/db mice (please see http://hyper.ahajournals.org Figures S3). However, as shown in Figures 6, increased blood pressure and NCC phosphorylation were completely corrected in these double knock-in mice, indicating that phosphorylation of SPAK and OSR1 by WNK kinases is required for generating these effects in db/db mice.
Figure 6. Phosphorylation of SPAK and OSR1 by WNK kinases is required for the high blood pressure and increased NCC phosphorylation of db/db mice.
A. Systolic blood pressure of SpakT243A/+ and Osr1T185A/+ knock-in db/db mice. Increased systolic blood pressure of db/db mice corrected in SPAKT243A/+ or OSR1T185A/+ knock-in mice (threonines in T-loop mutated to alanine to prevent activation by WNK kinases). Mean ± SEM. (n as indicated). *p<0.05.
B. Representative immunoblot of renal NCC phosphorylation in SPAKT243A/+ and OSR1T185A/+ knock-in db/db mice. Compared to db/db controls, NCC phosphorylation was decreased.
C. Densitometry analyses of renal NCC phosphorylation of NCC in SPAKT243A/+ and OSR1T185A/+ knock-in db/db mice. Values expressed as the ratio to the average of signals in vehicle group. Mean ± SEM. (n as indicated). *p<0.05.
PI3K and Akt Inhibitors Prevent NCC Phosphorylation by Acute Insulin Administration
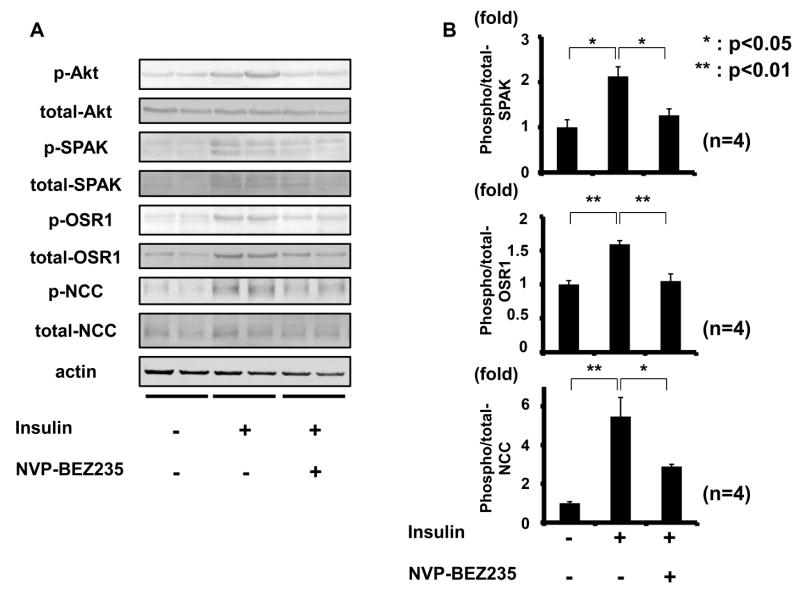
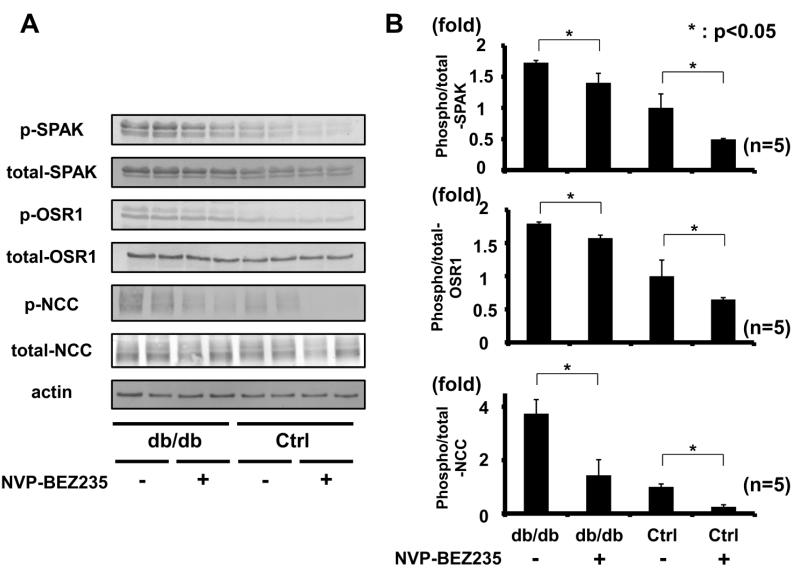
To investigate the mechanisms of action underlying increased NCC phosphorylation in db/db mice, attention was focused on PI3K and Akt, since renal phosphorylation of Akt at 473S, which accompanies with Akt activation,28,29 was clearly increased in these mice when fed both low- and high-salt diets (Figures 2 and 3), and because it is well known that Akt, a key downstream substrate of insulin signaling, is phosphorylated by insulin through PI3K.30 Moreover, Akt reportedly contributes to sodium reabsorption in the kidney,31 although the precise mechanisms involved have not been determined. In contrast, phosphorylation of SGK1 was not increased in db/db mice fed either low- or high-salt diets (please see http://hyper.ahajournals.org Figure S4), suggesting that SGK1 might not be involved in activating the WNK-OSR1/SPAK-NCC signaling cascade in this model. Therefore, the working hypothesis was that increased NCC phosphorylation in hyperinsulinemic db/db mice is regulated by the PI3K/Akt signaling cascade in the kidney. To verify this, the first investigation was into whether PI3K and Akt inhibitors could inhibit acute insulin-induced NCC phosphorylation by insulin injection. Since the classical PI3K inhibitors, LY294002 and wortmannin, were not tolerated for in vivo use due to their toxicities, NVP-BEZ235, a novel orally bioavailable imidazoquinoline derivative that inhibits PI3K activity by binding to the ATP binding cleft of these enzymes, was used.32-34 In addition, to increase specificity and reliability of inhibitor assays, GDC-0941 was used as a PI3K inhibitor, and MK-2206 as an Akt inhibitor. GDC-0941 is a selective oral inhibitor of class I PI3K with promising pharmaceutical properties, but without significant activity with regard to any other kinases.35,36 MK-2206 is an orally active and highly selective, allosteric, non-ATP competitive Akt inhibitor.37,38 Drugs were administered to mice by oral gavage, as previously reported.32,35 Since these PI3K and Akt inhibitors had not been tested in the kidney previously, renal phosphorylation of Akt was ascertained. Oral administration of these inhibitors was confirmed to suppress phosphorylation of Akt in mouse kidney (please see http://hyper.ahajournals.org Figure S5). Next, to see whether the acute insulin effect on NCC phosphorylation could be suppressed, insulin was injected to mice intraperitoneally with concurrent oral administration of the inhibitors. As shown in Figure 7, NVP-BEZ235 inhibited insulin-induced OSR1/SPAK and NCC phosphorylation in mouse kidney 30 min after insulin stimulation.
Figure 7. PI3K inhibitor (NVP-BEZ235) supressed NCC phosphorylation by acute insulin administration.
A. Representative immunoblots of renal Akt, OSR1/SPAK and NCC phosphorylation in insulin-injected mice with or without administration of a PI3K inhibitor (NVP-BEZ235).
B. Densitometry analysis; NVP-BEZ235 inhibited acute insulin-induced phosphorylation of OSR1/SPAK and NCC. Mean ± SEM. (n=4). *p<0.05, **p<0.01.
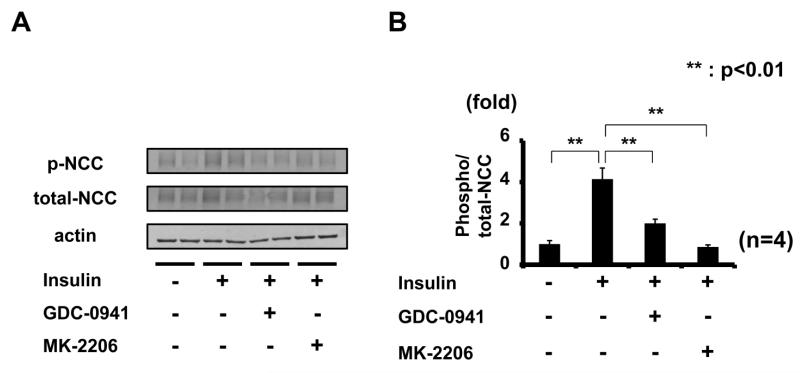
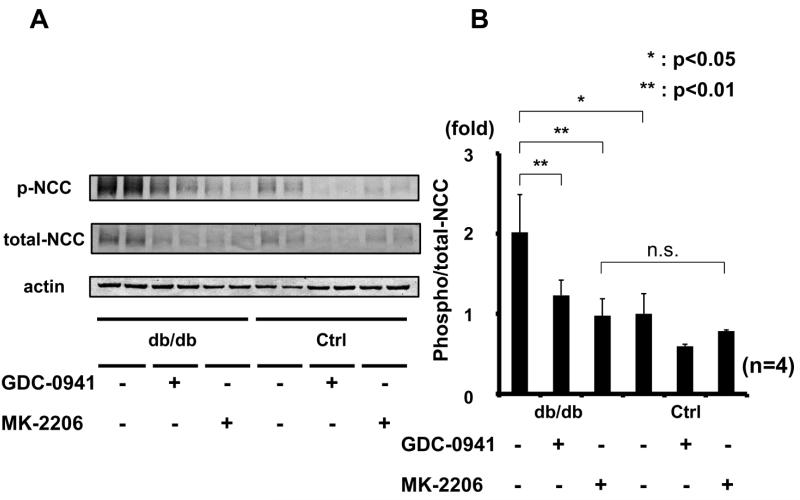
To confirm the involvement of PI3K/Akt in the mechanism of increased NCC phosphorylation by acute insulin stimulation, the effect of GDC-0941 and MK-2206 administration was determined. As shown in Figure 8, these inhibitors, like NVP-BEZ235, also inhibited insulin-induced NCC phosphorylation in mouse kidney. Considered together, these results indicate that PI3K and Akt are involved in insulin-induced NCC phosphorylation in vivo. In particular, the Akt inhibitor, MK-2206, suppressed NCC phosphorylation to the same level as that seen in controls, suggesting that insulin-induced NCC phosphorylation might be mainly activated by Akt.
Figure 8. Inhibition of insulin-induced NCC phosphorylation by PI3K inhibitor (GDC-0941) and Akt inhibitor (MK-2206).
A. Representative immunoblots of renal NCC phosphorylation in insulin-injected mice, with or without PI3K inhibitor (GDC-0941) and Akt inhibitor (MK-2206) administration.
B. Densitometry analysis; GDC-0941 and MK-2206 inhibited acute insulin-induced NCC phosphorylation. Mean ± SEM. (n=4). *p<0.05, **p<0.01.
PI3K and Akt Are Involved in Mechanisms Leading to Increased NCC Phosphorylation in db/db Mice
To investigate whether the PI3K/Akt signaling pathway regulates NCC phosphorylation in chronic hyperinsulinemic db/db mice, NVP-BEZ235, GDC-0941, or MK-2206 were administered. All these inhibitors decreased Akt phosphorylation in db/db mouse kidney, indicating that NVP-BEZ235 and GDC-0941 inhibited PI3K, and MK-2206 inhibited Akt in db/db mouse kidney, as expected (please see http://hyper.ahajournals.org Figure S6). Consistent with the previous reports,39 plasma insulin levels were increased in both control and db/db mice by these inhibitors due to feedback mechanisms, indicating that PI3K and Akt signaling pathway was properly blocked with these drugs (please see http://hyper.ahajournals.org Figure S7-8). As shown in Figure 9, NVP-BEZ235 suppressed NCC phosphorylation, suggesting that increased phosphorylation of NCC in db/db mice is regulated by PI3K. In addition, phosphorylation of OSR1/SPAK was suppressed by NVP-BEZ235 30 min after NVP-BEZ235 administration. As shown in Figure 10, the inhibitors GDC-0941 and MK-2206 also suppressed NCC phosphorylation in db/db and control mice as well as NVP-BEZ235, suggesting that regulation by the PI3K/Akt signaling cascade occurs. Similar to acute insulin administration, MK-2206 suppressed NCC phosphorylation in db/db mice to the same level as that seen in controls, suggesting that increased NCC phosphorylation in db/db mice is mainly activated by Akt.
Figure 9. PI3K inhibitor (NVP-BEZ235) suppressed increased NCC phosphorylation in db/db mouse kidney.
A. Representative immunoblots of phosphorylation of Akt, OSR1/SPAK and NCC from db/db and control mouse kidney, with or without administration of a PI3K inhibitor (NVP-BEZ235).
B. Densitometry analysis; NVP-BEZ235 suppressed increased OSR1/SPAK and NCC phosphorylation in db/db mouse kidney. Mean ± SEM. (n=4). *p<0.05, **p<0.01.
Figure 10. Inhibition of increased NCC phosphorylation in db/db mice by PI3K inhibitor (GDC-0941) and Akt inhibitor (MK-2206).
A. Representative immunoblots of phosphorylation of NCC in db/db mice kidney, with or without administration of PI3K inhibitor (GDC-0941) and Akt inhibitor (MK-2206).
B. Densitometry analysis; GDC-0941 and MK-2206 inhibited increased phosphorylation of NCC in db/db mouse kidney. Mean ± SEM. (n=4). *p<0.05, **p<0.01.
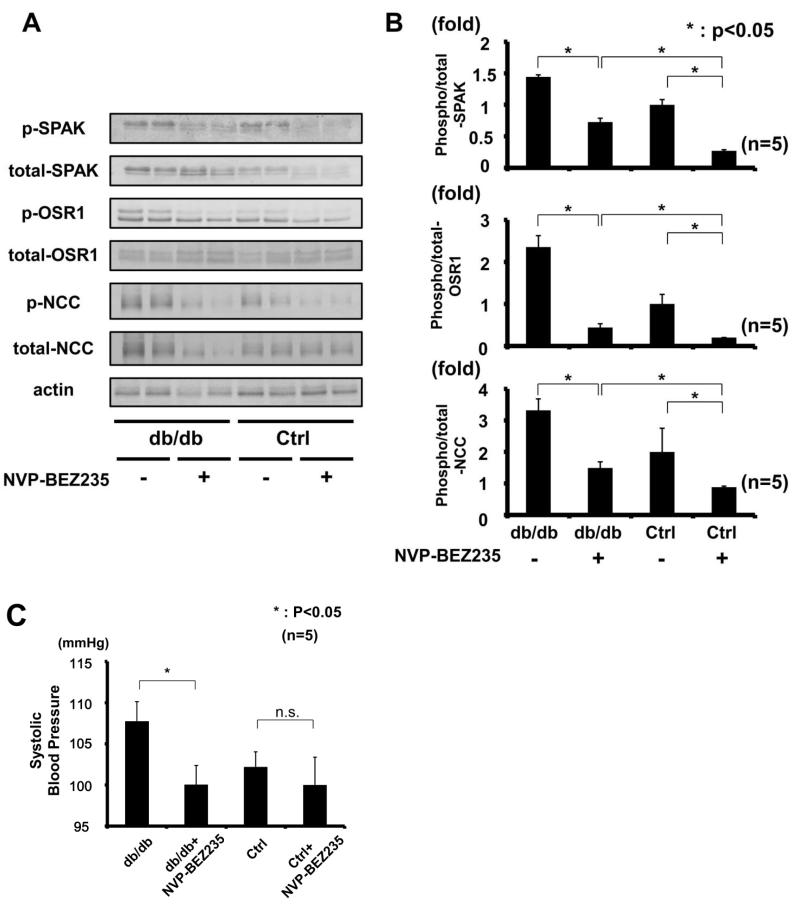
To see chronic effect of PI3K inhibitor on WNK-OSR1/SPAK-NCC phosphorylation cascade in db/db mice, we also performed chronic administration of NVP-BEZ235 to mice. As expected, chronic treatment with NVP-BEZ235 decreased phosphorylation of OSR1/SPAK and NCC in db/db mice kidney (Figure 11 A-B). In addition, chronic treatment with NVP-BEZ235 decreased blood pressure only in db/db mice, but not in control mice (Figure 11C), indicating that PI3K signaling cascade significantly contributed to the mechanisms of hypertension in db/db mice.
Figure 11. Chronic treatment of PI3K inhibitor (NVP-BEZ235) suppressed increased NCC phosphorylation and blood pressure in db/db mice.
A. Representative immunoblots of phosphorylation of OSR1/SPAK and NCC from db/db and control mouse kidney, with or without administration of a PI3K inhibitor (NVP-BEZ235).
B. Densitometry analysis; NVP-BEZ235 suppressed increased OSR1/SPAK and NCC phosphorylation in db/db mouse kidney. Mean ± SEM. (n=4). *p<0.05, **p<0.01.
C. Systolic blood pressure is decreased only in db/db mice with NVP-BEZ235, compared to vehicle treated db/db mice. Mean ± SEM. (n as indicated). *p<0.05.
Discussion
The metabolic syndrome is related to an increased risk for cardiovascular disease, chronic kidney disease, and mortality.40 Furthermore, salt-sensitivity is associated with an increased risk of cardiovascular disease and premature death.41,42 Therefore, the mechanisms underlying the salt sensitivity seen in hyperinsulinemic patients are potentially important therapeutic targets. It has been reported that hyperinsulinemia enhances salt sensitivity, which in turn leads to salt-sensitive hypertension.12,17,42 However, to the best of our knowledge, the molecular mechanisms involved have not been well established. The results of this study demonstrate that phosphorylation of OSR1/SPAK and NCC is increased in a mouse model of the metabolic syndrome. Moreover, use of kinase-dead SpakT243A/+ and Osr1T185A/+ knock-in db/db mice clarified that phosphorylation of OSR1 and SPAK by WNK kinase(s) is required to effect increased NCC phosphorylation. The current results clearly indicate that the WNK-OSR1/SPAK-NCC phosphorylation cascade plays an important role in development of salt-sensitive hypertension in hyperinsulinemic conditions.
Although it is widely accepted that metabolic syndrome pathophysiology involves insulin resistance, renal insulin resistance does not develop in the same manner as in glucogenic tissues like muscle and adipose tissue.43-45 Consistent with a previous report,43 the current study demonstrated that phosphorylated Akt is increased in kidneys from db/db mice, indicating that, although their activities were decreased in muscle and adipose tissue,46-48 PI3K and Akt were activated in the kidney, compared to control mice. It was shown that PI3K and Akt inhibitors suppressed increased phosphorylation of NCC in db/db mice. Therefore, as previously suggested,12,13,49 direct insulin stimulation appears to activate PI3K/Akt signaling and cause inappropriate sodium reabsorption and salt-sensitive hypertension through activation of the WNK-OSR1/SPAK-NCC signaling cascade.
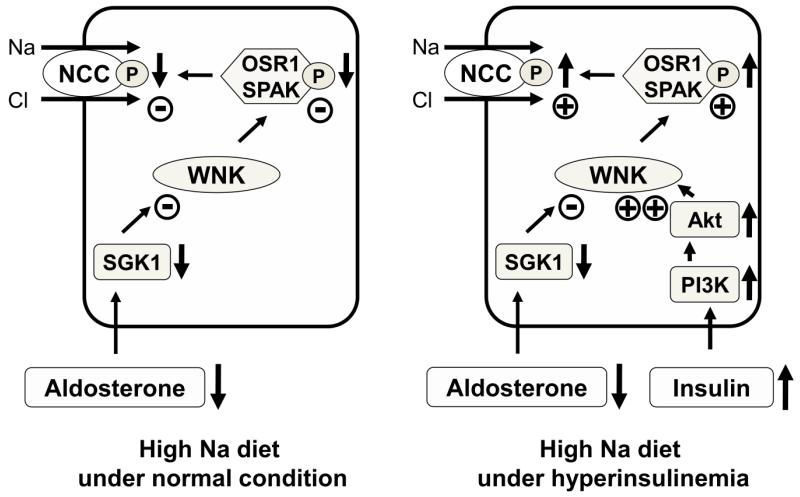
As shown in Figure 5, db/db mice fed a high-salt diet had increased phosphorylated NCC equal to the level seen in control mice fed a low-salt diet. Considering that phosphorylated NCC in mice fed a low-salt diet is highly increased by the physiological response to salt depletion, this increased level of renal phosphorylated NCC could explain the salt-sensitivity occurring in the hyperinsulinemic state. Moreover, these data indicate that the insulin effect on NCC phosphorylation is independent of aldosterone. In normal mice, as previously reported,6 a high-salt diet results in down-regulation of the WNK-OSR1/SPAK-NCC phosphorylation cascade through a low plasma aldosterone level. Also previously reported is the fact that constitutive activation of this cascade even with a high-salt diet—that is, lack of down-regulation—causes hypertension in PHAII.5 Because the insulin signal appears to be independent of aldosterone, hyperinsulinemia could bring about a situation similar to PHAII via the PI3K/Akt signaling pathway even during a high-salt diet. Hyperinsulinemia prevents proper down-regulation of NCC phosphorylation by high-salt intake and thus causes salt-sensitive hypertension (Figure 12).
Figure 12. Proposed NCC-mediated mechanism of increased salt sensitivity in hyperinsulinemic conditions.
In mice fed a high-salt diet, the WNK-OSR1/SPAK-NCC phosphorylation cascade is down-regulated through a low plasma aldosterone level (left panel). However, this newly-described insulin signaling pathway, which is independent of aldosterone, increases phosphorylation of Akt through PI3K and activates the WNK-OSR1/SPAK-NCC phosphorylation cascade. As a result, phosphorylation of NCC in hyperinsulinemic mice fed a high-salt diet is not down-regulated effectively (right panel).
The detailed mechanisms underlying WNK activation in db/db mice remain to be determined. Considering that PI3K/Akt inhibitors suppressed increased phosphorylation of NCC in the kidney, it is clear that the PI3K/Akt signaling pathway plays a key role. One possible mechanism is that Akt activated by insulin/PI3K signaling modifies the function of WNK4, since WNK4 has several Akt phosphorylation motifs, and phosphorylation at one of these sites has been reported to alter WNK4 kinase activity.50,51 It is also possible that WNK1 might be involved in the mechanism of increased NCC phosphorylation in db/db mice, since Thr-60 of WNK1 is phosphorylated by insulin and Akt.52 Activation of WNK1 could result in OSR1, SPAK and NCC phosphorylation by insulin as well.
Finally, we briefly mention the limitation of our study. We only used db/db mice in this study as a model animal showing hyperinsulinemia. Since the phenotypes of db/db mice essentially come from the defect of leptin signaling,21 it is possible that activation of the WNK-OSR1/SPAK-NCC cascade in the kidney might be caused not only by hyperinsulinemia but also by inactivation of leptin signaling. Therefore, analyses on other model animals showing hyperinsulinemia may be necessary. Very recently, activation of NCC in Zucker obese rats was reported,53 although the detailed mechanism in kidney was not investigated in vivo. We also have to be cautious about the interpretation of data from conventional knockout and knock-in mice since significant compensatory mechanisms might occur in those mice. In this respect, our data obtained from OSR1 and SPAK knock-in mice should be confirmed by other genetically engineered mice such as conditional knockout mice of OSR1 and SPAK.
Perspectives
In summary, it was determined that increased NCC phosphorylation in hyperinsulinemic db/db mice is regulated by the PI3K/Akt signaling pathway, indicating that the PI3K-Akt-WNK-OSR1/SPAK-NCC signaling cascade plays an essential physiological role in this phenomenon. Since the same mechanism has been proven to be the cause of human hypertension, this cascade may be one factor operating in the development of salt-sensitive hypertension in human hyperinsulinemic conditions. Future investigations are warranted to further elucidate details of the underlying mechanisms involved.
Supplementary Material
Novelty and Significance.
1. What Is New?
It was determined that increased NCC phosphorylation in db/db mice is regulated by the PI3K/Akt signaling pathway. Moreover, the PI3K-Akt-WNK-OSR1/SPAK-NCC signaling cascade plays an essential physiological role in salt-sensitive hypertension under hypersinsulinemic conditions.
2. What Is Relevant?
It has been reported that the metabolic syndrome enhances salt-sensitivity, leading to salt-sensitive hypertension. The metabolic syndrome causes hyperinsulinemia as a result of insulin resistance, and hyperinsulinemia causes an aberrant increase in sodium reabsorption by the kidney. We discovered that hyperinsulinemia in db/db mice can activate NCC through the PI3K-Akt-WNK-SPAK/OSR1-NCC signaling cascade. Since the same mechanism has been proven to be the cause of human hypertension (PHAII), this cascade may be one factor operating in the development of salt-sensitive hypertension in human hyperinsulinemic conditions.
3. Summary
In this paper, we report the PI3K/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. This mechanism may play a role in the pathogenesis of salt-sensitive hypertension in human hyperinsulinemic conditions such as the metabolic syndrome.
Acknowledgements
We thank C. Iijima and C. Iinuma for help in the experiments and T. Suganami and Y. Ogawa for helpful discussion.
Sources of Funding
This study was supported in part by Grants-in-Aid for Scientific Reaserch on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grants-in-Aid for Scientific Reaserch on Creative Scientific Reaserch from the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science, Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Salt Science Research Foundation (#1228), Takeda Science Foundation, Kanae Foundation for the Promotion of Medical Science, and The Nakajima Foundation.
Footnotes
Author Disclosures
Hidenori Nishida: No disclosures
Eisei Sohara: No disclosures
Naohiro Nomura: No disclosures
Motoko Chiga: No disclosures
Dario R Alessi: No disclosures
Tatemitsu Rai: No disclosures
Sei Sasaki: No disclosures
Shinichi Uchida: No disclosures
References
- 1.Achard JM, Disse-Nicodeme S, Fiquet-Kempf B, Jeunemaitre X. Phenotypic and genetic heterogeneity of familial hyperkalaemic hypertension (Gordon syndrome) Clin Exp Pharmacol Physiol. 2001;28:1048–1052. doi: 10.1046/j.1440-1681.2001.03575.x. [DOI] [PubMed] [Google Scholar]
- 2.Gordon RD. Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension. 1986;8:93–102. doi: 10.1161/01.hyp.8.2.93. [DOI] [PubMed] [Google Scholar]
- 3.Schambelan M, Sebastian A, Rector FC. Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): role of increased renal chloride reabsorption. Kidney Int. 1981;19:716–727. doi: 10.1038/ki.1981.72. [DOI] [PubMed] [Google Scholar]
- 4.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 5.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int. 2008;74:1403–1409. doi: 10.1038/ki.2008.451. [DOI] [PubMed] [Google Scholar]
- 7.Talati G, Ohta A, Rai T, Sohara E, Naito S, Vandewalle A, Sasaki S, Uchida S. Effect of angiotensin II on the WNK-OSR1/SPAK-NCC phosphorylation cascade in cultured mpkDCT cells and in vivo mouse kidney. Biochem Biophys Res Commun. 2010;393:844–848. doi: 10.1016/j.bbrc.2010.02.096. [DOI] [PubMed] [Google Scholar]
- 8.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl(−) cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 10.Naito S, Ohta A, Sohara E, Ohta E, Rai T, Sasaki S, Uchida S. Regulation of WNK1 kinase by extracellular potassium. Clin Exp Nephrol. 2011;15:195–202. doi: 10.1007/s10157-010-0378-9. [DOI] [PubMed] [Google Scholar]
- 11.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl-cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Lu F, Hu D, Rice T, Kelly TN, Hamm LL, Whelton PK, He J. GenSalt Collaborative Research Group. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet. 2009;373:829–835. doi: 10.1016/S0140-6736(09)60144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galletti F, Strazzullo P, Ferrara I, Annuzzi G, Rivellese AA, Gatto S, Mancini M. NaCl sensitivity of essential hypertensive patients is related to insulin resistance. J Hypertens. 1997;15:1485–1491. doi: 10.1097/00004872-199715120-00017. [DOI] [PubMed] [Google Scholar]
- 15.Shimamoto K, Hirata A, Fukuoka M, Higashiura K, Miyazaki Y, Shiiki M, Masuda A, Nakagawa M, Iimura O. Insulin sensitivity and the effects of insulin on renal sodium handling and pressor systems in essential hypertensive patients. Hypertension. 1994;23:I29–33. doi: 10.1161/01.hyp.23.1_suppl.i29. [DOI] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Goldberg M, Agus ZS. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976;58:83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari S, Nordquist L, Halagappa VK, Ecelbarger CA. Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol. 2007;293:F178–185. doi: 10.1152/ajprenal.00447.2006. [DOI] [PubMed] [Google Scholar]
- 19.Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol. 2006;290:F1055–1064. doi: 10.1152/ajprenal.00108.2005. [DOI] [PubMed] [Google Scholar]
- 20.Sohara E, Rai T, Yang SS, Ohta A, Naito S, Chiga M, Nomura N, Lin SH, Vandewalle A, Ohta E, Sasaki S, Uchida S. Acute insulin stimulation induces phosphorylation of the Na-Cl cotransporter in cultured distal mpkDCT cells and mouse kidney. PLoS One. 2011;6:e24277. doi: 10.1371/journal.pone.0024277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 22.Shibuya K, Kanasaki K, Isono M, Omata M, Sugimoto T, Araki S, Isshiki K, Kashiwagi A, Haneda M, Koya D. N-acetyl-seryl-aspartyl-lysyl-proline prevents renal insufficiency and mesangial matrix expansion in diabetic db/db mice. Diabetes. 2005;54:838–845. doi: 10.2337/diabetes.54.3.838. [DOI] [PubMed] [Google Scholar]
- 23.Chin M, Isono M, Isshiki K, Araki S, Sugimoto T, Guo B, Sato H, Haneda M, Kashiwagi A, Koya D. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am J Pathol. 2005;166:1629–1636. doi: 10.1016/s0002-9440(10)62473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun. 2009;380:44–49. doi: 10.1016/j.bbrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Rutkai I, Feher A, Erdei N, Henrion D, Papp Z, Edes I, Koller A, Kaley G, Bagi Z. Activation of prostaglandin E2 EP1 receptor increases arteriolar tone and blood pressure in mice with type 2 diabetes. Cardiovasc Res. 2009;83:148–154. doi: 10.1093/cvr/cvp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O’Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med. 2010;2:63–75. doi: 10.1002/emmm.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 29.Alessi DR, Downes CP. The role of PI 3-kinase in insulin action. Biochim Biophys Acta. 1998;1436:151–164. doi: 10.1016/s0005-2760(98)00133-7. [DOI] [PubMed] [Google Scholar]
- 30.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 31.Zdychová J, Komers R. Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications. Physiol Res. 2005;54:1–16. doi: 10.33549/physiolres.930582. [DOI] [PubMed] [Google Scholar]
- 32.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, Garcia-Echeverria C. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 33.Cho DC, Cohen MB, Panka DJ, Collins M, Ghebremichael M, Atkins MB, Signoretti S, Mier JW. The efficacy of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235 compared with rapamycin in renal cell carcinoma. Clin Cancer Res. 2010;16:3628–3638. doi: 10.1158/1078-0432.CCR-09-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, Lifshits E, Chen Z, Maria SM, Garcia-Echeverria C, Wong KK, Engelman JA. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A. 2009;106:19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Martinez JM, Wullschleger S, Preston G, Guichard S, Fleming S, Alessi DR, Duce SL. Effect of PI3K- and mTOR-specific inhibitors on spontaneous B-cell follicular lymphomas in PTEN/LKB1-deficient mice. Br J Cancer. 2011;104:1116–1125. doi: 10.1038/bjc.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, Folkes A, Gowan S, Ds Haven Brandon A, Di Stefano F, Hayes A, Henley AT, Lensun L, Pergl-Wilson G, Robson A, Saghir N, Zhyvoloup A, McDonald E, Sheldrake P, Shuttleworth S, Valenti M, Wan NC, Clarke PA, Workman P. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther. 2009;8:1725–1738. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng J, Dai B, Fang B, Bekele BN, Bornmann WG, Sun D, Peng Z, Herbst RS, Papadimitrakopoulou V, Minna JD, Peyton M, Roth JA. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PLoS One. 2010;5:e14124. doi: 10.1371/journal.pone.0014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 39.Smith GC, Ong WK, Rewcastle GW, Kendall JD, Han W, Shepherd PR. Effects of acutely inhibiting PI3K isoforms and mTOR on regulation of glucose metabolism in vivo. Biochem J. 2012;442:161–169. doi: 10.1042/BJ20111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 41.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 42.Kuroda S, Uzu T, Fujii T, Nishimura M, Nakamura S, Inenaga T, Kimura G. Role of insulin resistance in the genesis of sodium sensitivity in essential hypertension. J Hum Hypertens. 1999;13:257–262. doi: 10.1038/sj.jhh.1000800. [DOI] [PubMed] [Google Scholar]
- 43.Feliers D, Duraisamy S, Faulkner JL, Duch J, Lee AV, Abboud HE, Choudhury GG, Kasinath BS. Activation of renal signaling pathways in db/db mice with type 2 diabetes. Kidney Int. 2001;60:495–504. doi: 10.1046/j.1523-1755.2001.060002495.x. [DOI] [PubMed] [Google Scholar]
- 44.Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int. 2003;64:2163–2171. doi: 10.1046/j.1523-1755.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 45.Sechi LA, Griffin CA, Giacchetti G, Zingaro L, Catena C, Bartoli E, Schambelan M. Abnormalities of insulin receptors in spontaneously hypertensive rats. Hypertension. 1996;27:955–961. doi: 10.1161/01.hyp.27.4.955. [DOI] [PubMed] [Google Scholar]
- 46.Shao J, Yamashita H, Qiao L, Friedman JE. Decreased Akt kinase activity and insulin resistance in C57BL/KsJ-Leprdb/db mice. J Endocrinol. 2000;167:107–115. doi: 10.1677/joe.0.1670107. [DOI] [PubMed] [Google Scholar]
- 47.Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, Maag D, Kim S, Huang AS, Dailey MJ, Saleh M, Snowman AM, Moran TH, Mezey E, Snyder SH. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121:4477–4490. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schnyder B, Pittet M, Durand J, Schnyder-Candrian S. Rapid effects of glucose on the insulin signaling of endothelial NO generation and epithelial Na transport. Am J Physiol Endocrinol Metab. 2002;282:E87–94. doi: 10.1152/ajpendo.00050.2001. [DOI] [PubMed] [Google Scholar]
- 50.Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest. 2009;119:2601–2612. doi: 10.1172/JCI38323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci U S A. 2007;104:4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitari AC, Deak M, Collins BJ, Morrice N, Prescott AR, Phelan A, Humphreys S, Alessi DR. WNK1, the kinase mutated in an inherited high-blood-pressure syndrome, is a novel PKB (protein kinase B)/Akt substrate. Biochem J. 2004;378:257–268. doi: 10.1042/BJ20031692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komers R, Rogers S, Oyama TT, Xu B, Yang CL, McCormick J, Ellison DH. [Accessed July 20, 2012];Enhanced phosphorylation of Na-Cl cotransporter in experimental metabolic syndrome - role of insulin. Clin Sci. 2012 May 31; doi: 10.1042/CS20120003. DOI: 10.1042/CS20120003 http://www.clinsci.org/cs/imps/abs/CS20120003.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.