Highlights
-
•
The epidemiological impact and cost-effectiveness of ‘MenB’ vaccination was assessed.
-
•
Routine infant vaccination could prevent 27% of cases over the lifetime of a cohort.
-
•
This policy could be cost-effective at £9 per vaccine dose.
-
•
Substantial disease reductions are predicted if the vaccine also prevents carriage.
-
•
In this case infant vaccination and catch-up could reduce disease by 71% in 10 years.
Abbreviations: MCC, meningococcal serogroup C conjugate; MenB, capsular group B meningococci; QALYs, quality adjusted life years
Keywords: Meningococcal, Vaccine, Model, Cost-effectiveness
Abstract
Background
Meningococcal disease remains an important cause of morbidity and mortality worldwide. The first broadly effective vaccine against group B disease (which causes considerable meningococcal disease in Europe, the Americas and Australasia) was licensed in the EU in January 2013; our objective was to estimate the potential impact of introducing such a vaccine in England.
Methods
We developed two models to estimate the impact of introducing a new ‘MenB’ vaccine. The cohort model assumes the vaccine protects against disease only; the transmission dynamic model also allows the vaccine to protect against carriage (accounting for herd effects). We used these, and economic models, to estimate the case reduction and cost-effectiveness of a number of different vaccine strategies.
Results
We estimate 27% of meningococcal disease cases could be prevented over the lifetime of an English birth cohort by vaccinating infants at 2,3,4 and 12 months of age with a vaccine that prevents disease only; this strategy could be cost-effective at £9 per vaccine dose. Substantial reductions in disease (71%) can be produced after 10 years by routinely vaccinating infants in combination with a large-scale catch-up campaign, using a vaccine which protects against carriage as well as disease; this could be cost-effective at £17 per vaccine dose.
Conclusions
New ‘MenB’ vaccines could substantially reduce disease in England and be cost-effective if competitively priced, particularly if the vaccines can prevent carriage as well as disease. These results are relevant to other countries, with a similar epidemiology to England, considering the introduction of a new ‘MenB’ vaccine.
1. Introduction
Meningococcal disease is a leading infectious cause of death in young children in the UK [1] and remains an important cause of morbidity and mortality worldwide, despite improvements in critical care and the availability of vaccines against some capsular groups. Globally five capsular groups cause most disease (A, B, C, W, Y though X is increasing) and B and C are dominant outside Africa and Asia [2]. The key to reducing incidence is prevention through vaccination, because early signs of the disease can be non-distinct, the infection can progress rapidly, and can be fatal in 5–10% of cases even if treatment is initiated early [3].
Effective vaccines are available against capsular groups A, C, W and Y. The meningococcal serogroup C conjugate (MCC) vaccine was first introduced in the UK in 1999 [4] and subsequently by several other European countries, Australia and Canada [2]. MCC vaccination achieved high uptake rates, and has led to a considerable reduction in group C disease [5] due both to high vaccine effectiveness and protection against carriage, interrupting transmission and generating herd immunity [6]. Until recently there was no broadly effective vaccine against capsular group B (MenB) the most common cause of meningococcal disease in the UK and Europe [7] (MenB disease accounted for 89% of cases in England and Wales in 2009/10 [8]). Progress towards a MenB vaccine has been hindered because the serogroup B capsule shares homologous structures with human neural tissue, resulting in the polysaccharide being poorly immunogenic in people and concerns about a MenB capsular-based vaccine inducing auto-immunity [9]. New vaccines with the capacity to protect against MenB, based on protein antigens, are in advanced stages of development [10,11] and one, Bexsero, was granted an EU license in January 2013. Policy makers are now faced with decisions about if, and how, to introduce the vaccine.
To help inform policy decisions we developed mathematical and economic models to predict the potential impact of introducing a new vaccine in England, with the capacity to protect against MenB disease (henceforth referred to as a ‘MenB’ vaccine).
2. Methods
2.1. Model structures
It is unknown whether the new meningococcal vaccines will reduce carriage. Consequently, we developed two models (using Berkley Madonna software [12]) to assess the potential impact of these vaccines: a cohort model that assumes the vaccine prevents disease only, and a transmission dynamic model that also allows the vaccine to prevent carriage [13,14].
2.1.1. Details common to both models
The model populations are stratified into 100 single year of age classes. Incidence rates include all capsular groups of meningococcal disease because the new vaccines are not group specific. Following disease, individuals may survive with or without sequelae, or die. Survivors with sequelae are assumed to have a reduced quality of life and fatal cases lose the average life expectancy for the age at which they die. Individuals may die due to causes other than meningococcal disease; published mortality rates were adjusted to remove deaths due to meningococcal disease as these are explicitly modelled. Vaccinated individuals have a reduced risk of disease. Immunity from vaccination wanes over time, and individuals then have the same risks of infection as unvaccinated individuals. For each vaccination scenario the model results were compared to the situation without vaccination. Models were run for 100 years (time horizon) to capture the full benefits of vaccination and effects of invasive disease over the lifetimes of individuals.
2.1.2. Cohort model specific details
The cohort model was constructed using a Markov model, with monthly time steps. Individuals are born into a susceptible non-vaccinated state (Fig. 1). Meningococcal disease cases arise by multiplying the age-specific probability of disease (in a given interval) by the population. We assumed individuals only have disease once and are removed from the susceptible pool (instances of repeat invasive disease are rare and are associated with individuals with immune deficiencies and anatomical defects [15]). Years of life are weighted by the age-specific quality of life. The cohort sizes were based upon population figures for 2008.
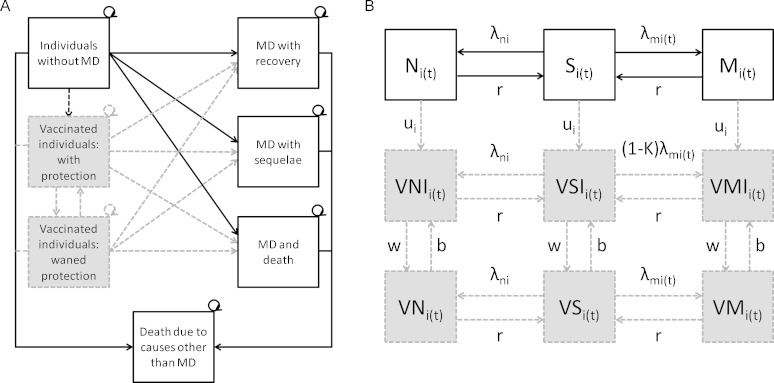
Fig. 1.
Models used to assess the impact of meningococcal vaccination in England. The ‘no vaccination’ model consists of white boxes and solid arrows; the ‘with vaccination’ model includes shaded boxes and dashed arrows in addition. (A) Cohort model structure: MD, meningococcal disease. (B) Dynamic model structure: Once individuals are carriers they have a chance of developing disease, with the same outcomes as shown in (A) S, susceptible non-vaccinated; M, infected carrier of a vaccine preventable meningococcal strain; N, infected carrier of a non-vaccine preventable meningococcal strain; VSI, susceptible vaccinated and immune; VMI, infected carrier of a vaccine preventable meningococcal strain, vaccinated and immune; VNI, infected carrier of a non-vaccine preventable meningococcal strain, vaccinated and immune; VS, susceptible vaccinated not immune; VM, infected carrier of a vaccine preventable meningococcal strain, vaccinated not immune; VN, infected carrier of a non-vaccine preventable meningococcal strain, vaccinated not immune; λm, force of infection for vaccine preventable meningococcal strains; λn, force of infection for non-vaccine preventable meningococcal strains; κ, vaccine efficacy against carriage; u, vaccine uptake; w, waning vaccine protection; b, vaccination booster; i, age; t, time.
2.1.3. Transmission dynamic model specific details
Individuals can have multiple episodes of asymptomatic carriage of meningococci in their lifetimes [16,17], therefore we used a Susceptible-Infected-Susceptible (SIS) model, with a daily time step, to represent the transmission dynamics of carriage in the population (Fig. 1). Individuals are born susceptible. They may then become carriers of a meningococcal strain (vaccine preventable or non-vaccine preventable), from which they recover and return to the susceptible state. We did not consider co-infection in the model because current evidence suggest carriage of multiple meningococcal strains is rare [18,19]. Cases of invasive disease are not explicitly included, but are generated from the number of new carriers arising over time (see Supplementary Material) using an age-specific case: carrier ratio. This ratio captures changes in disease risk given carriage acquisition across ages, which could be due to a number of factors including maturation of the immune system, physical changes in the pharynx, exposure to other pathogens and immunity following meningococcal carriage. Vaccinated individuals with vaccine induced immunity can have a reduced risk of becoming a carrier in addition to a reduced risk of disease.
2.2. Model parameters
Data sources used to estimate the parameters in the models, are summarised below and in Table 1 with further details provided in the Supplementary Material.
Table 1.
Base case parameters used in the models.
| Parameter | Base case | Distributiona | References |
|---|---|---|---|
| Epidemiological parameters | |||
| Carriage prevalence | Variable by age | NA | [20,21,55] |
| Disease incidence (per 100,000)b | 3.17 (variable by age) | Normal (variable by age) | [56–60]b |
| Case fatality rate (proportion) | 0.04 (variable by age) | Beta (variable by age) | b |
| Years of life lost | Variable by age and model year | Scenario variation | c |
| Natural mortality rates | Variable by age and model year | Scenario variation | [56–60]c |
| Population | Variable by age | NA | d |
| Acute treatment parameters | |||
| Proportion of patients requiring ambulance transfer to hospital | 0.48 | Beta (261.48; 279.52) | e |
| Hospitalisation rate (percentage) | 100.0 | Not varied | Assumed |
| Length of stay in hospital (days) | 9.7 (variable by age) | Normal (variable by age) | b |
| Proportion who require HDU | 0.10 (variable by age) | Beta (variable by age) | b |
| Proportion who require ITU | 0.14 (variable by age) | Beta (variable by age) | b |
| Length of stay in HDU (days) | 2.7 (variable by age) | Gamma (variable by age) | b |
| Length of stay in ITU (days) | 4.9 (variable by age) | Gamma (variable by age) | b |
| Long-term effects of meningococcal disease | |||
| Proportion of survivors with minor sequelae | 0.02 | Beta (1.94; 82.29) | [24] |
| Proportion of survivors with major sequelae | 0.07 (variable by age) | Beta (52.41; 665.54) | [24]f |
| QALY utilities | |||
| QALY utility for susceptibles and survivors of MD without sequelae | 0.86 (variable by age) | Scenario variation (variable by age) | [28] |
| QALY loss for survivors with sequelae | 0.20 | Beta (1.22; 4.89) | Assumed, based upon [25–27] |
| Vaccination parameters | |||
| Vaccination coverage – routine immunisation (%) | 91.00 | Scenario variation (range 85–91%) | [61] |
| Vaccination coverage – 1–17 years catch-up (%) | Variable by age | Scenario variation (variable by age) | [30] |
| Effective vaccine efficacy | 0.75 | Scenario variation (range 0–0.90) | Assumed |
| Rate of mild reactions (number of vaccine doses resulting in 1 reaction) | 1471.00 | Gamma (5.9; 249.5) | [62,63] |
| Rate of anaphylactoid reactions (number of vaccine doses resulting in 1 reaction) | 719,790.00 | Normal (719,790; 112,140) | [62,63] |
| Cost of treatment | |||
| Cost of ambulance transfer to hospital (£) | 169.23 | Gamma (53.66; 3.15) | [22]e |
| Cost per spell in hospital, (£) | 2715.93 | Gamma (8.03; 338.14) | [22] |
| Cost per HDU day, neonatal (≤28 days) (£) | 759.31 | Gamma (15.23; 49.84) | [22] |
| Cost per HDU day, paediatric (29 days to ≤18 years) (£) | 922.38 | Gamma (4.76; 193.82) | [22] |
| Cost per ITU day, neonatal (<29 days) (£) | 1081.31 | Gamma (13.74; 78.71) | [22] |
| Cost per ITU day, paediatric 29 days to ≤18 years (£) | 2056.20 | Gamma (26.31; 78.15) | [22] |
| Cost per critical care day, adult (19≥ years)(£) | 1149.11 | Gamma (12.95; 88.73) | [22] |
| Cost of follow-up appointment, paediatric (≤18 years)(£) | 221.82 | Gamma (2.50; 88.90) | [22] |
| Cost of follow-up appointment, adult (19≥ years)(£) | 292.91 | Gamma (7.36; 39.81) | [22] |
| Cost of hearing assessment | 57.56 | Gamma (6.63; 8.69) | [22] |
| Public health response | |||
| Cost of public health response to a case, excluding vaccine costs (£) | 68.00 | Gamma (27.5; 2.5) | [29]g |
| Long-term effects of meningococcal disease | |||
| Cost of support/care for those with mild sequelae (annual, £) | 500.00 | Gamma (6.25; 80.00) | Assumed |
| Cost of support/care for those with severe sequelae (annual, £) | 10,000.00 | Gamma (1.28; 7832.44) | Assumed |
| Vaccination | |||
| Cost per vaccine dose (£) | 40.00 | Scenario variation (range 5–40) | Assumed |
| Cost of administration – school (per dose, £) | 5.60 | Gamma (3.92; 1.43) | [31,32] |
| GP consultation cost (£) | 31.00 | Normal (31.00; 16.73) | [33,34] |
| Nurse consultation cost (£) | 6.51 | Gamma (4.98; 1.31) | [33,34]h |
| Adverse reaction (anaphylaxis), hospitalisation cost (£) | 421.07 | Gamma (5.15; 81.79) | [22] |
For the probabilistic cohort model, 1000 simple random samples from the distributions given were run through the model.
HES data from 1997/98 to 2005/06 were obtained from the University of Bristol (Davidson Ho, emailed personal communication, 18th March 2008); laboratory data for the same period from the Health Protection Agency (Mary Ramsay, emailed personal communication, 29th January 2008). In the sensitivity analysis the mean incidence from 1997/98–2005/06 was used. This was manipulated to account for the decline due to MCC vaccination by estimating the annual number of non-MenC cases and deaths (by applying serogroup proportions from laboratory confirmed cases to Hospital Episode Statistics admissions) and then adding on MenC cases seen in 2005/06 to each year to reflect current MenC levels.
National mortality rates and expectations of life by single year of age were obtained from the Office for National Statistics Centre for Demography (Nigel Henretty, emailed personal communication, 18th December 2009).
Population figures by single year of age were obtained from the Office for National Statistics Centre for Demography (Megan Elkin, emailed personal communication, 21st December 2009).
The proportion of patients with meningococcal disease requiring ambulance transfer to hospital was estimated from the 2003 Meningitis Research Foundation members survey (Laura Clark, emailed personal communication, 27th July 2011).
Unpublished data from [24] of the proportion of survivors with major sequelae by age from European studies (Andrew Clark, emailed personal communication, 18th June 2010).
The time spent on contact tracing and public health management following a case of meningococcal disease was based on the experience of an Academic Specialty Registrar in Public Health (Charlotte Chamberlain, emailed personal communication, 18th February 2010).
For vaccines given concomitantly in primary care, we assumed the appointment with the nurse would be extended by an average of 4 min. Where this was not the case (infant vaccination at 6 months of age, and 1–4 year and 16–17 year olds not in full time education as part of catch-up) we assumed the vaccines would be delivered by a nurse in a consultation lasting 9 min. These times were based upon the experience of vaccine research nurses (Dianne Web, emailed personal communication, 9th June 2010).
We used carriage prevalence estimates from a recent systematic review [20], with contact patterns estimated using a simple preferential mixing structure and recently published survey data on self-reported contacts [21]. Disease incidence naturally fluctuates over time; incidence peaked in the late 1990s and has declined since then. We therefore based disease incidence and case fatality upon hospital admissions from 2004/05–2005/06 to represent current low incidence. Data from 1997/98–2005/06 (adjusting for the decline in incidence due to MCC), which includes peak incidence years, were used to generate a ‘higher’ incidence comparator. We assumed all meningococcal disease cases were hospitalised and estimated those requiring augmented care from hospital admissions (1998/99–2005/06). We included published costs for time in hospital including augmented care [22], and all survivors of disease were assumed to have a hearing test and a follow-up review in line with recent NICE guidelines [23]. The proportion of survivors with minor and major sequelae following disease was estimated from a recent systematic review of sequelae following bacterial meningitis [24]. Those with sequelae were assumed to have a reduced quality of life (0.2 utility reduction [25–27]) compared to susceptible individuals, and survivors of disease without sequelae [28]. Long term costs of supporting those with mild and severe sequelae were estimated at £500 and £10,000 per year per individual respectively. For public health management we included costs of chemoprophylaxis (rifampicin for 3 adults and 2 children [29]) and staff time associated with contact tracing. Costs of outbreak control were not included.
Several vaccination strategies were considered (Table 2). Vaccination uptake for routine vaccination was assumed to equate to MCC in infants, and for catch-up cohorts, match the MCC catch-up programme [30]. Vaccine administration costs [31–34] were included separately from the cost of the vaccine itself, and were greater if given outside of current schedules. The full characteristics of the new meningococcal protein vaccines are not yet known; assumptions regarding vaccine effectiveness and duration of protection were based on data from trials, other meningococcal vaccines, such as the MCC or Outer Membrane Vesicle vaccines, and expert opinion. Data from trials of Bexsero have indicated, however, that the vaccine is immunogenic in infants [35], and adolescents [36], that responses are evident after two doses of the vaccine in infancy [37] and that it is possible to boost an individual's response [35]. Early genotypic estimates of strain coverage suggested 100% strain coverage was possible [38] however recent phenotypic approaches suggest strain coverage in England may be 73% (95% CI 57–87%), though these results are based on a method which may underestimate coverage [39]. In the base case model the vaccine was assumed to protect against all meningococcal strains. We included costs, but not quality of life losses, for adverse vaccine events. We assumed the vaccine cost £40 per vaccine dose in the base case, but varied this widely in the sensitivity analysis.
Table 2.
Vaccination strategies modelled.
| Routine strategy | [months protection] (Vaccine efficacy %)a | One-off catch-upb | [months protection] (Vaccine efficacy %)a | |
|---|---|---|---|---|
| A | Infant: 2,3,4 + 12 months of age | [18,36] (75) | ||
| B | Infant: 2,4,6 + 12 months of age | [24,48] (75) | ||
| C | Infant: 2,3,4 months of age | [18] (75) | ||
| D | Infant: 2,3,4 + 12 months of age | [18,36] (75) | 1–4 years: 0, 2, 6 | [60] (80) |
| E | Infant: 2,3,4 + 12 months of age | [18,36] (75) | 1–4 years: 0, 2, 6 | [60] (80) |
| 5–17 years: 0, 2 | [120] (80) | |||
| F | Adolescent: 0, 2, 6 schedule | [120] (80) | ||
| G | Adolescent: 0, 2, 6 schedule | [120] (80) | 13–17 years: 0, 2, 6 | [120] (80) |
Routine infant strategies (A–E) are presented according to the months of age the vaccines are received; adolescent strategies (F, G) are considered in the dynamic model only.
Numbers in square brackets give the assumed average duration of protection, in months, following the priming and if applicable, booster doses. Numbers in rounded brackets give the assumed vaccine efficacy against disease.
Catch-up and adolescent strategies are presented according the spacing of vaccinations in months.
2.3. Scenario and sensitivity analysis
The cohort model was probabilistic, with distributions around the parameters reflecting uncertainty (Table 1). Where probabilistic analysis was not possible or appropriate (e.g. vaccine price will be fixed, but at a level currently unknown) we ran scenario analyses. Cost-effectiveness ratios from probabilistic results were calculated using the ratio of the means [40]. Cost-effectiveness acceptability curves were generated using a net benefit approach.
2.4. Costs and discounting
Three health outcomes were considered: cases averted; deaths averted; and quality adjusted life years (QALYs) gained. The cost-effectiveness (utility) analysis was undertaken from the perspective of the NHS and personal and social services according to NICE guidance [41]; the primary outcome was cost per QALY gained. Costs were measured in pounds sterling at 2008 prices, with previous years inflated to 2008 levels. All costs and benefits were assumed to occur at the start of the year with future costs and benefits discounted according to HM Treasury recommendations (discount rate of 3.5% for the first 30 years, 3.0% in years 31–75 and 2.5% in years 76–99) [42].
3. Results
3.1. Impact of vaccination assuming direct effects only
The model estimates 1799 cases of meningococcal disease (all capsular groups) could be expected in England over the lifetime of the 2008 birth cohort, resulting in 18,215 hospital bed days and 91 deaths. An estimated 484 (27%) cases (3756 bed days and 11 deaths) could be prevented by introducing routine early infant vaccination (strategy A Tables 2 and 3). Protection begins at 4 months, following two vaccine doses, and most cases are averted between the ages of one and two years (Supplementary Fig. 1). Due to waning vaccine immunity, averted cases decline rapidly after this.
Table 3.
Results of the cohort model of meningococcal disease and vaccination in England.
| Strategy | Undiscounted |
Discounted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Cases avoided | Deaths avoided | LYS | QALY gained | Cost without vaccination (£millions) | Cost with vaccination (£millions) | Cost per QALY gained (£) | ||
| A | 2,3,4 + 12 months | 1799 | 484 | 11 | 996 | 1600 | 102.9 | 176.5 | 162,800 |
| B | 2,4,6 + 12 months | 1799 | 496 | 11 | 1014 | 1633 | 102.9 | 177.9 | 164,100 |
| C | 2,3,4 months | 1799 | 339 | 8 | 696 | 1119 | 102.9 | 159.7 | 175,600 |
| D | Catch-up in 1–4 year olds | 5871 | 1261 | 28 | 2455 | 4075 | 323.6 | 614.3 | 238,500 |
| E | Catch-up in 1–17 year olds | 13,197 | 2615 | 74 | 5648 | 8836 | 690.8 | 1473.1 | 290,000 |
Strategies A–C are routine infant vaccination strategies without catch-up; strategies D and E are routine infant vaccination at 2,3,4 + 12 months in addition to the one-off catch-up presented (please see Table 2 for further details of the strategies modelled). Costs in GBP (£) assuming a vaccine cost per dose of £40 rounded to nearest £100.
Infants immunised later in childhood experience longer-lasting immunity, therefore, routine late infant vaccination could result in more averted cases despite protection starting later in life (strategy B Tables 2 and 3). Strategies including 12 month boosters result in more cases averted, compared to those without, because the duration of protection is assumed to be longer following the booster. Catch-up strategies result in further case reductions, but the effects are limited in this model assuming direct protection only.
Assuming the vaccine costs £40 per dose, early infant vaccination (strategy A) would cost £103·7 million for a single birth cohort and provide NHS savings of £30·1 million, resulting in a cost per QALY gained of £162,800. The cost per QALY of a late infant schedule (strategy B) remains high (£164,100), despite the greater number of cases avoided, due to the additional cost of calling children for vaccination at 6 months. The vaccine would need to cost around £9 per dose for these routine infant strategies to be considered cost-effective (<£30,000 per QALY). Catch-up campaigns are least cost-effective because these involve immunising a large number of people in ages where disease incidence is relatively low.
3.2. Impact of vaccination allowing for herd immunity
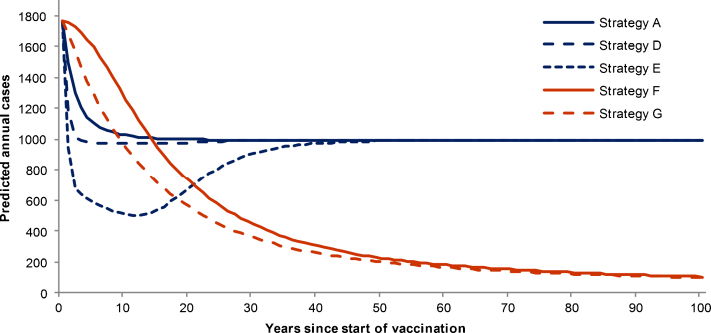
Routine early infant vaccination with a vaccine that offers 60% protection against carriage is estimated to prevent more cases than a strategy allowing for direct protection only (Fig. 2). However, a considerable disease burden remains because carriage is low in young children [20] thus the herd immunity effects generated are limited. Implementing a one-off large-scale catch-up could reduce the annual number of cases considerably. Ten years after the implementation of routine infant vaccination with 1–17 year old catch-up (strategy E), the annual number of cases is estimated to fall by 71%. Over time, however, cases increase as vaccine immunity wanes, and the catch-up cohorts age. Routinely vaccinating adolescents could result in a sustained reduction in cases in the long term, but is likely to be more effective in the short term in combination with catch-up.
Fig. 2.
Predicted meningococcal cases averted under different vaccination strategies (with herd immunity). Predicted all serogroup meningococcal cases over time under different vaccination strategies from the dynamic model. Please refere to Table 2 for further details of the vaccination strategies; all runs assume 60% vaccine efficacy against carriage.
Adolescent vaccination either with, or without, catch-up is the most favourable decision economically (though still above a £30,000 per QALY threshold at £39,000 and £40,200 respectively, Table 4). In the base case, this model suggests early routine vaccination (strategy A) could be cost-effective at a willingness to pay of £30,000 per QALY if the vaccine were to cost £15 per dose. Routine adolescent vaccination with catch-up (strategy G) could be cost-effective at £32 per dose.
Table 4.
Results of the dynamic model of meningococcal disease and vaccination in England.
| Discounted cost per QALY gained (£) |
|||||
|---|---|---|---|---|---|
| Discounting of costs | Base casea | 3.5% | 5.0% | 6.0% | |
| Discounting of benefits | 3.5% | 5.0% | 1.5% | ||
| A | 2,3,4 + 12 months | 96,000 | 116,200 | 158,000 | £27,900 |
| B | 2,4,6 + 12 months | 91,800 | 111,700 | 153,100 | £26,800 |
| D | Routine infant 2,3,4 + 12 months of age plus catch-up in 1–4 year olds (0,2,6 schedule) | 97,600 | 117,700 | 161,800 | £30,700 |
| E | Routine infant 2,3,4 + 12 months of age plus catch-up in 1–4 year olds (0,2,6 schedule) and 5–17 year olds (0,2 schedule) | 83,400 | 97,900 | 135,000 | £30,600 |
| F | Adolescent: 0, 2, 6 schedule | 40,200 | 54,000 | 84,500 | £12,600 |
| G | Routine adolescent (0,2,6 schedule) plus catch-up in 13–17 year olds (0,2,6 schedule) | 39,200 | 51,600 | 81,100 | £13,800 |
Results from the base case dynamic model showing the effect of varying the discount rates on the cost per Quality Adjusted Life Year; 60% vaccine effectiveness against carriage, 75% vaccine effectiveness against disease in infants, 80% vaccine effectiveness against disease in catch-up cohorts, £40 per vaccine dose, costs in GBP (£) rounded to nearest £100. Please see Table 2 for further details of the strategies modelled.
3.5% for the first 30 years, 3.0% for years 31–75, 2.5% for years 76 to 100.
3.3. Scenario and sensitivity analysis
Results were most sensitive to changes in disease incidence and case-fatality; with higher incidence and case-fatality, vaccination prevents more cases and deaths and is more economically favourable (Supplementary Table 2). In the probabilistic analysis of the cohort model, the results were sensitive to changes in the parameters with the greatest uncertainty, such as the annual cost of care for those with sequelae and the quality of life loss for those individuals (Supplementary Fig. 2).
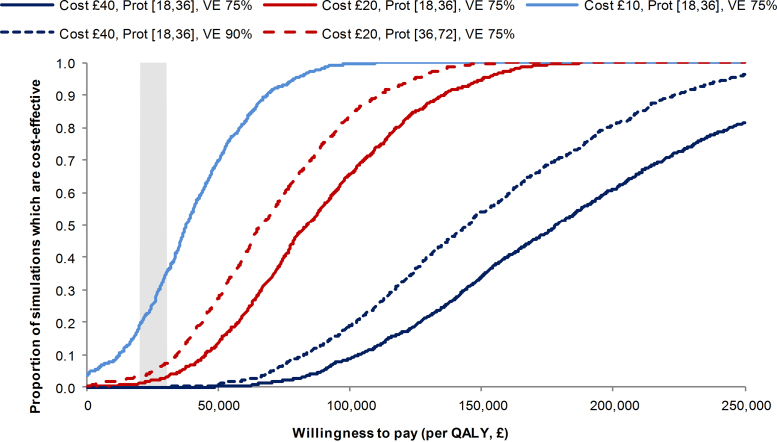
More cases and deaths are averted through vaccination when the carriage prevalence is lowered (Supplementary Fig. 3) and the discounted cost per QALY is reduced to £69,700 and £86,300 for routine infant vaccination with (strategy E) and without (strategy A) large-scale catch-up, respectively. The predicted annual cases were similar when using a simple preferential population mixing structure or one using self reported leisure contacts. However, cases increased more quickly once immunity from large-scale catch-up had waned, in the model using self reported contacts (Supplementary Fig. 3). Reducing the proportion of vaccine-preventable strains from 100% to 75% in the dynamic model resulted in a higher cost per QALY gained (£131,800) for routine infant vaccination (strategy A). Models were also sensitive to varying vaccine effectiveness, duration of protection and particularly vaccine cost (Fig. 3 and Supplementary Tables 2 and 3).
Fig. 3.
Cost-effectiveness acceptability curves for the cohort model in scenarios analyses. Curves show results under routine infant vaccination (strategy A) of the effect of varying cost (per dose) of the vaccine, vaccine duration of protection (given in months following the priming and booster doses) and vaccine efficacy (VE).
The discount rate has a large impact on the cost per QALY gained (Table 4). None of the strategies considered were cost-effective at a willingness to pay of £30,000 using Treasury recommended discount rates (at £40 per vaccine dose). Using differential discounting, with a lower discount rate for health benefits compared to costs (which allows for increasing value of health benefits), did result some in strategies appearing cost-effective (assuming a 60% vaccine efficacy against carriage).
4. Discussion
These are the first models to comprehensively assess the potential impact of introducing vaccines which have the capacity to protect against capsular group B meningococcal disease in England. Our results indicate that introducing a ‘MenB’ vaccine, which provides direct protection only, into the routine infant schedule, could prevent 27% of meningococcal cases per birth cohort, and that this could be cost-effective at £9 per vaccine dose. Substantial impact occurred if the vaccine disrupted carriage as well as preventing disease. In this scenario, the most efficient programme in the short term appears to be routine infant vaccination with catch-up, which after 10 years could reduce annual cases by 71% and be cost-effective at £17 per dose. Models were sensitive to assumptions around: disease incidence and case fatality; vaccine cost, duration of protection and efficacy; quality of life losses from the disease; and the cost of caring for those with sequelae.
Vaccination with MCC has shown that the impact of herd immunity effects can be extremely important when predicting the likely impact and cost-effectiveness of vaccination [43]. We therefore used two types of model, including a transmission dynamic model, in order to appropriately capture potential herd immunity effects [13]. Unlike previous meningococcal models, our carriage estimates were drawn from a recent systematic review and meta-analysis [20] and we assessed the impact of assuming different mixing patterns in the population using simple preferential mixing and mixing based on self-reported contacts [21]. Our transmission models differ to others previously used to model meningococcal disease [43–45] as they are not capsular group specific. This is not required as the vaccines in development, unlike their predecessors, do not target the capsular group. We chose not to explicitly include N. lactamica as the role of this commensal in affording protection remains unclear, and if N. lactamica remains at the current level, any protection is implicitly included through the use of a case: carrier ratio. That said, our results are consistent with previous models in that a vaccine able to disrupt carriage can have substantial added benefits in terms of disease prevention.
There are limited data available to inform some parameters, in particular relating to the nature of the new vaccines and the proportion, type and costs associated with sequelae following meningococcal disease. These results are nevertheless important because decisions regarding vaccination are starting to be considered and the scenarios presented cover the most likely features of the vaccine. We used hospital admission data from two time periods to calculate disease incidence. The ability of hospital data to accurately capture the ‘true’ number of cases in unknown, but other sources such as laboratory reports are likely to be less complete, and we feel the true incidence probably lies within the range explored. Assumptions regarding the proportion of survivors with sequelae come from a recent systematic review of meningococcal meningitis only [24], so we may be underestimating the proportion of sequelae following meningococcal disease. Sequelae costs were based on dichotomising individuals into those with mild and severe sequelae, when in fact there is a considerable range in severity. Previous models have faced the same paucity of data [43,46] and we therefore chose to investigate parameter uncertainty in the cohort model through the use of probabilistic analysis, with particularly wide distributions for sequelae parameters. We did not consider possible replacement effects or adverse effects due to the loss of natural boosting through reduced carriage in the dynamic model (of meningococci or other nasopharyngeal flora) due to the paucity of data. The dynamic results here, therefore, may be optimistic. There was concern in the UK about the possibility of serogroup replacement following MCC introduction, though there is no evidence to suggest that this has occurred [47]. However, serotype replacement has tempered the impact of the pneumococcal conjugate vaccine (introduced in the UK in 2006) [48]. The results from our models, therefore, should be taken as an indication to the relative merits of different strategies, particularly as the models have a long time horizon over which many factors can change, including strain distributions, disease levels and population structure.
In the UK, the Joint Committee on Vaccination and Immunisation can only recommend the introduction of a vaccine if it is deemed to be cost-effective [49]. Our models indicate that a ‘MenB’ vaccine could substantially reduce disease in England, and be cost-effective if competitively priced. The results are of relevance, to other countries with similar epidemiology considering the introduction of one of the new meningococcal vaccines, though reapplication of our models with country specific data may be desirable in some circumstances (for example, if there are considerable inter-country differences in vaccine implementation costs).
Our model results were sensitive to assumptions around the profile of the vaccine, disease incidence and case fatality and sequelae, including quality of life losses and costs of care. Further data on whether the new vaccines disrupt carriage should be forthcoming, and better information on the duration of protection will also emerge from the results of ongoing clinical trials [50,51], which will help to reduce some of the uncertainty in our models. Efforts are also being made to improve the information surrounding the impact of the disease in terms of QALY losses and care for those requiring sequelae [52–54].
5. Conclusion
Using cohort and dynamic models we have shown that a ‘MenB’ vaccine has the capacity to significantly reduce meningococcal disease in England and that vaccine programmes could be cost-effective if the vaccine is competitively priced.
Acknowledgements
We would like to thank the following individuals for providing information and assistance: Mary Ramsay, Ray Borrow and Dianne Webb, Health Protection Agency; Andrew Clark, London School of Hygiene and Tropical Medicine; Davidson Ho, Roy Maxwell, Laura Clark and Charlotte Chamberlain, University of Bristol; and Nigel Henretty and Megan Elkin, Office for National Statistics. The HES data were made available by the NHS Health and Social Care Information Centre. Funding: This work was supported by the National Institute for Health Research [RDA/03/07/014 and PDF-2012-05-245 to HC, PDA/02/06/088 to CT]. This work is produced by the authors under the terms of these research training fellowships issued by the NIHR. The views expressed in this publication are those of the authors and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflicts of interest: All authors: No reported conflicts.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2013.03.034.
Contributor Information
Hannah Christensen, Email: hannah.christensen@bristol.ac.uk.
Matthew Hickman, Email: matthew.hickman@bristol.ac.uk.
W. John Edmunds, Email: John.Edmunds@lshtm.ac.uk.
Caroline L. Trotter, Email: caroline.trotter@bristol.ac.uk, clt56@cam.ac.uk.
Appendix A. Supplementary data
Following are the Supplementary data to this article:
References
- 1.Mortality statistics: Deaths registered in 2010 (Series DR) Table 5: Office for National Statistics, Available from http://www.ons.gov.uk/ons/rel/vsob1/mortality-statistics--deaths-registered-in-england-and-wales--series-dr-/2010/dr-tables-2010.xls; 2011 [accessed 30.05.12].
- 2.Harrison L.H., Trotter C.L., Ramsay M.E. Global epidemiology of meningococcal disease. Vaccine. 2009;27:B51–B63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 3.Leake J.A.D., Perkins B.A. Meningococcal disease: challenges in prevention and management. Infect Med. 2000;17:364. [Google Scholar]
- 4.PL CPHO (1999)1: Introduction of immunisation against Group C Meningococcal infections: Department of Health, Available from http://www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/Professionalletters/Chiefpharmaceuticalofficerletters/DH_4066113; 1999 [accessed 30.05.12].
- 5.Campbell H., Andrews N., Borrow R. Updated postlicensure surveillance of the Meningococcal C Conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17:840–847. doi: 10.1128/CVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trotter C.L., Maiden M.C. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines. 2009;8:851–861. doi: 10.1586/erv.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Invasive Neisseria meningitidis in Europe. London: EU-IBIS Network Health Protection Agency, Available from http://www.hpa-bioinformatics.org.uk/euibis/documents/2006_meningo.pdf; 2011 [accessed 30.05.11].
- 8.Meningococcal Reference Unit isolates of Neisseria meningitidis: England and Wales, by serogroup & epidemiological year, 1998/99-2009/10: Health Protection Agency, Available from http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1234859711901; 2011 [accessed 12.11.12].
- 9.Finne J., Leinonen M., Makela P.H. Antigenic similarities between brain components and bacteria causing meningitis: implications for vaccine development and pathogenesis. Lancet. 1983;322:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher L.D., Bernfield L., Barniak V. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72:2088–2100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliani M.M., du-Bobie J., Comanducci M. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macey RI, Oster G. Berkeley Madonna. 8.3.14 ed, Available from http://www.berkeleymadonna.com/index.html; 2006.
- 13.Edmunds W.J., Medley G.F., Nokes D.J. Evaluating the cost-effectiveness of vaccination programmes: a dynamic perspective. Stat Med. 1999;18:3263–3282. doi: 10.1002/(sici)1097-0258(19991215)18:23<3263::aid-sim315>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Dynamic transmission modeling: A report of the ISPOR-SMDM modeling good research practices task force-5 (draft), Available from http://www.ispor.org/workpaper/modeling_methods/dynamic-transmission-modeling.asp; [accessed 12.11.12]. [DOI] [PMC free article] [PubMed]
- 15.Ginsberg L. Difficult and recurrent meningitis. J Neurol Neurosurg Psychiatry. 2004;75:i16–i21. doi: 10.1136/jnnp.2003.034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altmann G., Egoz N., Bogokovsky B. Observations on asymptomatic infections with Neisseria meningitidis. Am J Epidemiol. 1973;98:446–452. doi: 10.1093/oxfordjournals.aje.a121574. [DOI] [PubMed] [Google Scholar]
- 17.Ala’Aldeen D.A.A., Neal K.R., Ait-Tahar K. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J Clin Microbiol. 2000;38:2311–2316. doi: 10.1128/jcm.38.6.2311-2316.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen J., Berthelsen L., Jensen B.B. Dynamics of the meningococcal carrier state and characteristics of the carrier strains: a longitudinal study within three cohorts of military recruits. Epidemiol Infect. 1998;121:85–94. doi: 10.1017/s0950268898008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craven D.E., Frasch C.E., Mocca L.F. Rapid serogroup identification of Neisseria meningitidis by using antiserum agar: prevalence of serotypes in a disease-free military population. J Clin Microbiol. 1979;10:302–307. doi: 10.1128/jcm.10.3.302-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen H., May M., Bowen L. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–861. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 21.Mossong J., Hens N., Jit M. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Department of Health. National Schedule of Reference Costs 2008-09 for NHS Trusts and PCTs combined, Available from http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591; 2010 [accessed 20.05.11].
- 23.National Institute for Health and Clinical Excellence. CG102 Bacterial meningitis and meningococcal septicaemia: quick reference guide (amended), Available from http://guidance.nice.org.uk/CG102/QuickRefGuide/pdf/English; 2010 [accessed 20.05.11].
- 24.Edmond K., Clark A., Korczak V.S. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317–328. doi: 10.1016/S1473-3099(10)70048-7. [DOI] [PubMed] [Google Scholar]
- 25.Ruedin H.J., Ess S., Zimmermann H.P. Invasive meningococcal and pneumococcal disease in Switzerland: cost-utility analysis of different vaccine strategies. Vaccine. 2003;21:4145–4152. doi: 10.1016/s0264-410x(03)00562-0. [DOI] [PubMed] [Google Scholar]
- 26.Erickson L., De Wals P. Complications and sequelae of meningococcal disease in Quebec, Canada, 1990–1994. Clin Infect Dis. 1998;26:1159–1164. doi: 10.1086/520303. [DOI] [PubMed] [Google Scholar]
- 27.Buysse C.M., Raat H., Hazelzet J.A. Long-term health status in childhood survivors of meningococcal septic shock. Arch Pediatr Adolesc Med. 2008;162:1036–1041. doi: 10.1001/archpedi.162.11.1036. [DOI] [PubMed] [Google Scholar]
- 28.Kind P, Hardman G, Macran S. UK population norms for EQ-5D. Discussion paper 172, Available from http://www.york.ac.uk/inst/che/pdf/DP172.pdf; 1999 [accessed 15.05.08].
- 29.British National Formulary [Homepage on the Internet], Available from http://www.bnf.org/bnf/; [accessed 22.01.13].
- 30.Trotter C.L., Ramsay M.E., Kaczmarski E.B. Meningococcal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Commun Dis Public Health. 2002;5:220–225. [PubMed] [Google Scholar]
- 31.Wallace L.A., Young D., Brown A. Costs of running a universal adolescent hepatitis B vaccination programme. Vaccine. 2005;23:5624–5631. doi: 10.1016/j.vaccine.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 32.Trotter C.L., Edmunds W.J. Modelling cost effectiveness of meningococcal serogroup C conjugate vaccination campaign in England and Wales. Br Med J. 2002;324:809. doi: 10.1136/bmj.324.7341.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtis L. Unit Costs of Health and Social Care 2009, Available from http://www.pssru.ac.uk/pdf/uc/uc2009/uc2009.pdf; 2009 [accessed 20.05.11].
- 34.Hollinghurst S., Horrocks S., Anderson E. Comparing the cost of nurse practitioners and GPs in primary care: modelling economic data from randomised trials. Br J Gen Pract. 2006;56:530–535. [PMC free article] [PubMed] [Google Scholar]
- 35.Vesikari T., Esposito S., Prymula R. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381:825–835. doi: 10.1016/S0140-6736(12)61961-8. [DOI] [PubMed] [Google Scholar]
- 36.Santolaya M.E., O’Ryan M.L., Valenzuela M.T. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet. 2012;379:617–624. doi: 10.1016/S0140-6736(11)61713-3. [DOI] [PubMed] [Google Scholar]
- 37.Snape M., Dawson T., Oster P. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J. 2010;29:e71–e79. doi: 10.1097/INF.0b013e3181f59f6d. [DOI] [PubMed] [Google Scholar]
- 38.Lucidarme J., Comanducci M., Findlow J. Characterization of fHbp, nhba (gna2132), nadA, porA, and sequence type in Group B meningococcal case isolates collected in England and Wales during January 2008 and potential coverage of an investigational Group B meningococcal vaccine. Clin Vaccine Immunol. 2010;17:919–929. doi: 10.1128/CVI.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogel U., Taha M.-K., Vazquez J.A. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013 doi: 10.1016/S1473-3099(13)70006-9. [Epub ahead of print], http://www.sciencedirect.com/science/article/pii/S1473309913700069#. [DOI] [PubMed] [Google Scholar]
- 40.Stinnett A.A., Paltiel A.D. Estimating CE Ratios under second-order uncertainty. Med Decis Making. 1997;17:483–489. doi: 10.1177/0272989X9701700414. [DOI] [PubMed] [Google Scholar]
- 41.Guide to the methods of technology appraisal, Available from http://www.nice.org.uk/aboutnice/howwework/devnicetech/technologyappraisalprocessguides/guidetothemethodsoftechnologyappraisal.jsp; 2008 [accessed 23.06.08].
- 42.Treasury H.M. The Stationery Office; London: 2003. The green book. Appraisal and evaluation in central government. [Google Scholar]
- 43.Trotter C.L., Edmunds W.J. Reassessing the cost-effectiveness of meningococcal serogroup C conjugate (MCC) vaccines using a transmission dynamic model. Med Decis Making. 2006;26:38–47. doi: 10.1177/0272989X05284109. [DOI] [PubMed] [Google Scholar]
- 44.Coen P.G., Cartwright K., Stuart J. Mathematical modelling of infection and disease due to Neisseria meningitidis and Neisseria lactamica. Int J Epidemiol. 2000;29:180–188. doi: 10.1093/ije/29.1.180. [DOI] [PubMed] [Google Scholar]
- 45.Guzzetta G., Manfredi P., Gasparini R. On the relationship between meningococcal transmission dynamics and disease: remarks on humoral immunity. Vaccine. 2009;27:3429–3434. doi: 10.1016/j.vaccine.2009.01.092. [DOI] [PubMed] [Google Scholar]
- 46.Ortega-Sanchez I.R., Meltzer M.I., Shepard C. Economics of an adolescent meningococcal conjugate vaccination catch-up campaign in the United States. Clin Infect Dis. 2008;46:1–13. doi: 10.1086/524041. [DOI] [PubMed] [Google Scholar]
- 47.Trotter C.L., Andrews N.J., Kaczmarski E.B. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–367. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 48.Current Epidemiology of Invasive Pneumococcal Disease (IPD): Health Protection Agency, Available from http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/EpidemiologicalDataPneumococcal/CurrentEpidemiologyPneumococcal/; 2010 [accessed 30.05.11].
- 49.Joint Committee on Vaccination and Immunisation. Minutes of the meeting held on 18 February 2009: Joint Committee on Vaccination and Immunisation, Available from http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_097411.pdf; 2009 [accessed 30.05.11].
- 50.Novartis Vaccines. A Phase 3 observer blind randomized, multi-center, controlled study to evaluate the effect of Novartis vaccine's Meningococcal B Recombinant and MenACWY conjugate vaccines on pharyngeal carriage of N. meningitidis in young adults. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US), Available from http://www.clinicaltrials.gov/ct2/show/NCT01214850; NLM Identifier: NCT01214850 [accessed 07.03.13].
- 51.Novartis Vaccines. Persistence of antibody levels and response to fifth or third Meningococcal B Recombinant Vaccine in 4-year old healthy children who previously participated in study V72P12E1. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from http://www.clinicaltrials.gov/ct2/show/NCT01717638; NLM Identifier: NCT01717638 [accessed 07.03.13].
- 52.Viner R.M., Latham S., Hudson L. Outcomes of meningococcal serogroup B disease: findings from a nationally representative case-control study. Arch Dis Child. 2010;95:A5. [Google Scholar]
- 53.Wright C, Wordsworth R, Glennie L. Counting the cost: a severe case of bacterial menigitis, Available from http://www.meningitis.org/assets/x/53379; 2011 [accessed 24.03.12].
- 54.Wright C, Wordsworth R, Glennie L. Counting the cost: a severe case of meningococcal septicaemia. Available from http://www.meningitis.org/assets/x/53382; 2011 [accessed 24.03.12].
- 55.Wallinga J., Teunis P., Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epidemiol. 2006;164:936–944. doi: 10.1093/aje/kwj317. [DOI] [PubMed] [Google Scholar]
- 56.Table 2.1 Deaths: underlying cause, sex and age-group, 2004: Chapter I Certain infectious and parasitic diseases. Mortality Statistics. Cause [document available on the Internet], Available from www.statistics.gov.uk/downloads/theme_health/Dh2_31/DH2No31.pdf; 2005 [accessed 15.01.09].
- 57.Table 2.1 Deaths: underlying cause, sex and age-group, 2005: Chapter I Certain infectious and parasitic diseases. Mortality Statistics. Cause [document available on the Internet], Available from www.statistics.gov.uk/downloads/theme_health/Dh2_32/DH2_No32_2005.pdf; 2006 [accessed 15.01.09].
- 58.Table 5.1 Deaths: underlying cause, sex and age-group, 2006: Chapter I Certain infectious and parasitic diseases. Mortality statistics. Deaths registered in 2006 [document on the Internet], Available from www.statistics.gov.uk/downloads/theme_health/DR-2006/DR_06Mort_Stats.pdf; 2008 [accessed 15.01.09].
- 59.Table 5.1 Deaths: underlying cause, sex and age-group, 2007: Chapter I Certain infectious and parasitic diseases. Mortality statistics. Deaths registered in 2007 [document on the Internet], Available from www.statistics.gov.uk/downloads/theme_health/DR2007/DR_07_2007.pdf; 2008 [accessed 15.01.09].
- 60.Table 5.1 Deaths: underlying cause, sex and age-group, 2008: Chapter I Certain infectious and parasitic diseases. Mortality statistics. Deaths registered in 2008 [document on the Internet], Available from http://www.statistics.gov.uk/downloads/theme_health/DR2008/DR_08.pdf; 2009 [accessed 21.07.10].
- 61.The Information Centre for Health and Social Care. NHS Immunisation Statistics England 2008-09, Available from http://www.ic.nhs.uk/statistics-and-data-collections/health-and-lifestyles/immunisation/nhs-immunisation-statistics-england-2008-09; 2009 [accessed 20.05.11].
- 62.Safety of meningococcal C conjugate vaccines. Current Problems in Pharmacovigilance. 2000; 26:14
- 63.McNicholas A., Galloway Y., Stehr-Green P. Post-marketing safety monitoring of a new Group B Meningococcal vaccine in New Zealand, 2004-2006. Hum Vaccin. 2007;3:196–204. doi: 10.4161/hv.3.5.4458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.