Abstract
The steroid hormone aldosterone maintains sodium homeostasis and is therefore important in control of blood volume and pressure. Angiotensin II (AngII) and elevated extracellular potassium concentrations ([K+]e), the prime physiologic regulators of aldosterone secretion from adrenal glomerulosa cells, activate phospholipase D (PLD) in these cells. The role of Ca2+ in the activation by these agents is unknown, although nitrendipine, a voltage-dependent Ca2+ channel antagonist, does not inhibit AngII-elicited PLD activation, despite the fact that this compound blocked elevated [K+]e-stimulated PLD activity. PLD activation triggered by AngII was also unaffected by the T-type calcium channel inhibitor nickel. Nevertheless, Ca2+ influx was required for AngII-induced PLD activation in both primary cultures of bovine adrenal glomerulosa cells and a glomerulosa cell model, the NCI H295R adrenocortical carcinoma cell line. The involvement of store-operated Ca2+ (SOC) influx and Ca2+ release-activated Ca2+ (CRAC) influx pathways in PLD activation was investigated using thapsigargin, an endoplasmic reticulum Ca2+ pump inhibitor that empties the store to induce SOC influx, and the SOC inhibitor YM-58483 (BTP2), as well as a CRAC inhibitor, tyrphostin A9. In bovine glomerulosa cells tyrphostin A9 inhibited AngII-induced PLD activation without affecting elevated [K+]e-stimulated enzyme activity. On the other hand, differences were observed between the bovine adrenal glomerulosa and H295R cells in the involvement of Ca2+ influx pathways in PLD activation, with the involvement of the SOC pathway suggested in the H295R cells. In summary, our results indicate that Ca2+ entry only through certain Ca2+ influx pathways is linked to PLD activation.
Keywords: NCI H295R cell line, protein kinase C, diacylglycerol, phosphatidic acid, thapsigargin, aldosterone, steroidogenesis
INTRODUCTION
The steroid hormone aldosterone controls sodium homeostasis and thus blood volume and pressure. Aldosterone is secreted from glomerulosa cells in the adrenal cortex in response to two major regulators, angiotensin II (AngII) and elevated extracellular potassium concentration ([K+]e). AngII induces aldosterone secretion by binding to the AngII Type 1 receptor, which is coupled to phosphatidylinositol 4,5-bisphosphate hydrolysis resulting in the production of inositol 1,4,5-trisphosphate and diacylglycerol (DAG) [reviewed in (Barrett et al., 1989; Foster, 2004; Rainey et al., In press; Spät et al., 2004)]. These second messengers, in turn, increase cytosolic calcium (Ca2+) levels (to activate Ca2+/calmodulin-dependent protein kinases) and stimulate protein kinase C (PKC), respectively [reviewed in (Rainey et al., In press)]. Elevated [K+]e, on the other hand, triggers glomerulosa cell depolarization, which opens voltage-dependent Ca2+ channels and also increases cytosolic Ca2+ levels [reviewed in (Rainey et al., In press)]. We previously showed that not only AngII but also elevated [K+]e activates phospholipase D (PLD) (Betancourt-Calle et al., 2001), the activity of which can produce lipid messengers including phosphatidic acid and DAG [reviewed in (Bollag et al., 2005)]. Furthermore, inhibition of PLD-mediated signal generation inhibits both AngII- and elevated [K+]e–elicited aldosterone secretion (Betancourt-Calle et al., 2001; Bollag et al., 2002; Zheng et al., 2003), suggesting the importance of this signaling enzyme to steroid hormone production. Therefore, it is important to understand the mechanisms that regulate PLD activity in response to AngII and elevated [K+]e.
The ability of both AngII and elevated [K+]e to activate PLD suggests a possible involvement of Ca2+ in the process, since an increase in the cytosolic Ca2+ concentration is common to these two agonists [reviewed in (Rainey et al., In press)]. However, the role of Ca2+ in PLD activation in other systems is unclear, and, in fact, Exton (Exton, 1999) indicates that “direct control of the enzyme by physiological changes in cytosolic Ca2+ seems unlikely.” Investigators have shown an ability of cytosolic Ca2+ concentration to modulate PLD activity, with chelation of intracellular Ca2+ inhibiting PLD activation in response to some agonists and Ca2+ ionophores increasing enzyme activity [reviewed in (Exton, 1999)]. The mechanism by which Ca2+ regulates PLD activity is not known but may involve calmodulin and/or the Ca2+-sensitive PKC isoenzymes [reviewed in (Exton, 1999)]. On the other hand, in primary bovine glomerulosa cells the inability of the Ca2+ ionophores ionomycin or A23187 to activate PLD (Bollag et al., 2002) suggests that changes in cytosolic Ca2+ levels alone are not sufficient to stimulate PLD activity. Furthermore, the fact that elevated [K+]e, which functions through voltage-dependent Ca2+ channels, activates PLD in bovine adrenal glomerulosa cells (Betancourt-Calle et al., 2001) suggests a possible involvement of Ca2+ influx in regulating PLD activity. However, the lack of inhibition of AngII-induced PLD activation by nitrendipine (Bollag et al., 2002), a voltage-dependent Ca2+ channel antagonist, at a dose that inhibits aldosterone secretion (Kojima et al., 1985b), supports the idea that only certain Ca2+ pathways may be important in stimulating PLD in response to particular agonists.
The objectives of this study were to determine the role of Ca2+ influx pathways in activating PLD in response to AngII as well as to elevated [K+]e in primary cultures of bovine adrenal glomerulosa cells. In addition, we sought to compare the effects of modulating Ca2+ influx on PLD activation and acute aldosterone secretion in this bovine system with the responses observed in the NCI H295R human adrenocortical carcinoma cell line.
MATERIALS AND METHODS
Materials
The following were obtained from Sigma (St. Louis, MO): PMA, AngII and 22(R)-hydroxycholesterol. UltroSer G was acquired from BioSepra (France) under a permit from the U.S. Department of Agriculture. [3H]Oleic acid was purchased from Dupont NEN (Boston, MA). Silica gel 60 thin-layer chromatography plates with concentrating zones were obtained from EM Science through VWR (West Chester, PA) and phosphatidylethanol and phosphatidic acid standards from Avanti Polar Lipids (Alabaster, AL). ITS+ premix (12.5 mg insulin, 12.5 mg transferrin, 12.5 μg selenous acid, 10.7 μg linoleic acid and 2.5 mg BSA) was purchased from BD Biosciences (San Jose, CA). Thapsigargin, YM-58483 (BTP2) and tyrphostin A9 were obtained from Calbiochem (San Diego, CA).
Primary Culture of Bovine Adrenal Glomerulosa Cells
Bovine adrenal glomerulosa cells were prepared and cultured as described in (Bollag et al., 2007). Briefly, the glomerulosa layer was dissected from adrenal glands of near-term fetal calves obtained from a local meat-packing plant. Glomerulosa cells were released from tissue slices by enzymatic and mechanical means and collected by centrifugation. Cells were plated in Primaria plates (BD Falcon, Franklin Lakes, NJ) overnight in a medium consisting of DMEM/Ham’s F12 (1:1 vol:vol) containing 10% horse serum, 2% fetal bovine serum, 100 μM ascorbate, 1.2 μM alpha-tocopherol, 0.05 μM Na2SeO3, 50 μM butylated hydroxyanisole, 5 μM metyrapone, 100 U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL fungizone. The cells were then incubated for 20-24 hours in serum-free DMEM/Ham’s F12 (containing 0.2% BSA, 100 μM ascorbate, 1.2 μM alphat-ocopherol, 0.05 μM Na2SeO3, 50 μM butylated hydroxyanisole, 5 μM metyrapone and antibiotics) prior to experimentation.
Culture of NCI H295R Human Adrenocortical Carcinoma Cells
NCI H295R cells were cultured as described in (Zheng et al., 2003). Briefly, cells were grown in DMEM/Ham’s F12 (1:1 vol:vol) containing 1% ITS+ (12.5 mg insulin, 12.5 mg transferrin, 12.5 μg selenous acid, 10.7 μg linoleic acid and 2.5 mg BSA from BD Biosciences, ), 2% UltroSer G, 100 U/mL penicillin,100 μg/mL streptomycin and 0.25 μg/mL fungizone, to approximately 70-75% confluence. The cells were then incubated for 20-24 hours in serum-free medium (consisting of DMEM/Ham’s F12 containing 0.01% BSA and antibiotics) prior to experimentation.
Measurement of Aldosterone Secretion
Cells were incubated for the indicated times with the appropriate agents in bicarbonate-buffered Kreb’s Ringer containing 2.5 mM sodium acetate (KRB+). Aldosterone release into the medium was then assayed using a radioimmunoassay kit from Diagnostic Products Corporation (Los Angeles, CA) as described in (Bollag et al., 2007). For stimulation with an elevated [K+]e, KCl was substituted isoosmotically for NaCl, as in (Betancourt-Calle et al., 2001).
PLD Activity Assay
Bovine glomerulosa or H295R cells were prelabeled with 2.5-5 μCi/mL [3H]oleate in the appropriate serum-free medium for 20-24 hours. The cells were then preincubated for 30 minutes in KRB+ prior to stimulation in the presence of 0.5% ethanol with and without AngII in KRB+ containing or lacking 1.2 mM CaCl2 or in the presence and absence of 2 μM thapsigargin or 10 μM tyrphostin A9 (or DMSO as the vehicle control) for 5 (H295R cells) or 30 minutes (bovine adrenal glomerulosa cells). Again, stimulation with elevated [K+]e was performed by isoosmotic substitution. Phospholipids were extracted with chloroform/methanol and separated by thin-layer chromatography, and radiolabeled phosphatidylethanol was quantified by liquid scintillation spectrometry as described in (Bollag, 1998).
Statistical Analysis
Experiments were performed a minimum of three times in duplicate or triplicate. Data were analyzed by analysis of variance with a Student-Newmann-Keuls post-hoc test using the computer program Instat (Graphpad, San Diego, CA), except where indicated in the figure legends. A p value less than or equal to 0.05 was considered significant.
RESULTS
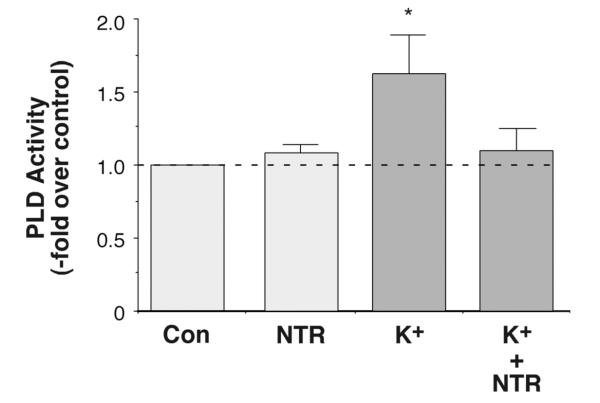
We have previously shown that elevated [K+]e stimulates PLD activity (Betancourt-Calle et al., 2001). Because of the importance of PLD to sustained aldosterone secretion (Bollag et al., 2002; Zheng et al., 2003), we wanted to characterize the mechanism by which elevated [K+]e and AngII activate PLD. We previously showed that the voltage-dependent Ca2+ channel antagonist, nitrendipine, does not alter AngII-induced PLD activation (Bollag et al., 2002), even at a concentration (1 μM) reported to inhibit both transient, or T-type, and long-lasting, or L-type, Ca2+ channels (Barrett et al., 1995). The role of voltage-dependent Ca2+ channels in elevated [K+]e–induced PLD activation was determined by monitoring the effect of nitrendipine on the elevated [K+]e–stimulated increase in the levels of [3H]phosphatidylethanol (PEt), a specific marker of PLD activity (Thompson et al., 1991), in [3H]oleate-prelabeled primary bovine adrenal glomerulosa cells (Bollag et al., 1990; Bollag et al., 1992). Nitrendipine (1 μM) completely abolished elevated [K+]e-induced changes in PLD activity (Figure 1), although it had no effect on either basal [Figure 1 and (Bollag et al., 2002)] or AngII-stimulated PLD activity (Bollag et al., 2002), indicating that Ca2+ influx through voltage-dependent Ca2+ channels is absolutely required for the effect of elevated [K+]e on PLD activity.
Figure 1.
Inhibition of Ca2+ Influx with Nitrendipine Completely Inhibited Elevated [K+]e-elicited PLD Activation. [3H]Oleate-prelabeled cells were incubated for 30 minutes with KRB+ containing 3.5 mM KCl (3.5 mM K+-KRB+, control), 1 μM nitrendipine (NTR) in 3.5 mM K+-KRB+, 15 mM K+-KRB+ or 1 μM NTR in 15 mM K+-KRB+ in the presence of 0.5% ethanol. [Note that NaCl in the medium was substituted for by KCl to maintain osmolarity in the 15 mM K+-KRB+ as in (Betancourt-Calle et al., 2001).] Reactions were terminated by the addition of 0.2% SDS containing 5 mM EDTA, and [3H]phosphatidylethanol was extracted, separated by thin-layer chromatography and quantified as in (Jung et al., 1998). Values are expressed as -fold over the control and represent the means (± SEM) of 5 separate experiments; *p<0.05 versus the control value.
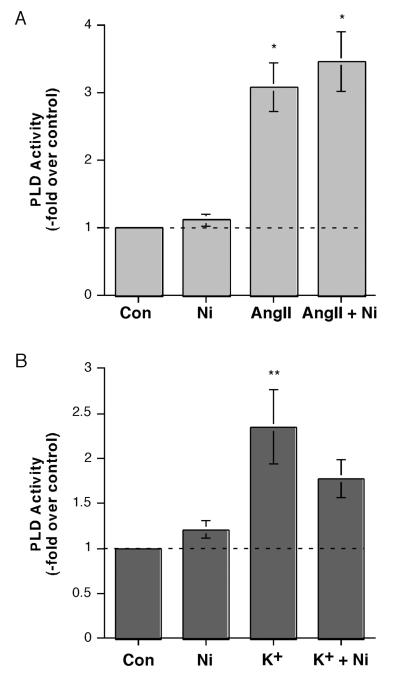
To further define the involvement of T-type versus L-type channels in the PLD response to AngII and elevated [K+]e, we used the T-type channel inhibitor, nickel, at a dose reported to inhibit T-type Ca2+ influx, specifically through alpha1H T-type Ca2+ channels (Schrier et al., 2001). We observed no effect of 50 μM nickel on PLD activity either under basal conditions or upon stimulation with AngII (Figure 2A). On the other hand, elevated [K+]e stimulated PLD activity, and nickel returned this increase to a level not significantly different from the control value, causing an approximate 43% inhibition (Figure 2B). Together these results are consistent with the idea that voltage-dependent Ca2+ channels are not involved in AngII-induced PLD activation but are critical for the activity stimulated by elevated [K+]e.
Figure 2.
Inhibition of alpha1H T-type Ca2+ Channels with Nickel Inhibited Elevated [K+]e-, but Not AngII-, elicited PLD Activation. (A) [3H]Oleate-prelabeled cells were incubated for 30 minutes with KRB+ containing 0.5% ethanol and with and without 10 nM AngII in the presence or absence of 50 μM nickel (Ni) for 30 minutes. Reactions were terminated by the addition of 0.2% SDS containing 5 mM EDTA, and [3H]phosphatidylethanol was extracted, separated by thin-layer chromatography and quantified as in (Jung et al., 1998). Values are expressed as -fold over the control and represent the means (± SEM) of 4 separate experiments; *p<0.05 versus the control value. (B) [3H]Oleate-prelabeled cells were incubated for 30 minutes with 3.5 mM K+-KRB+ or 15 mM K+-KRB+ containing 0.5% ethanol in the absence or presence of 50 μM nickel (Ni) for 30 minutes, and PLD activity monitored as above. Values are expressed as -fold over the control and represent the means (± S.E.M.) of 4 separate experiments; **p<0.01 versus the control value.
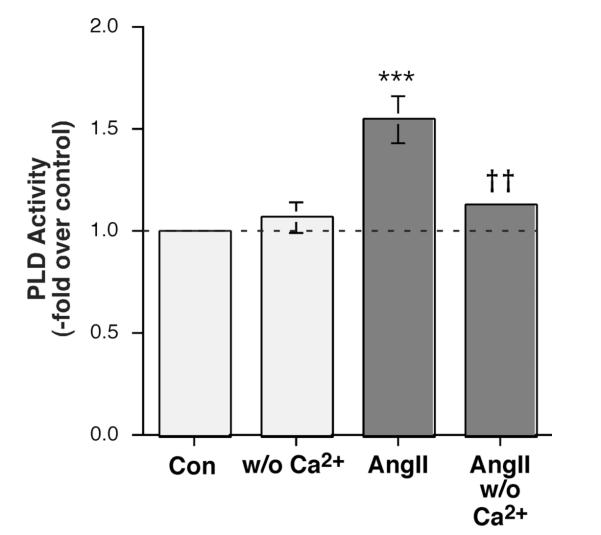
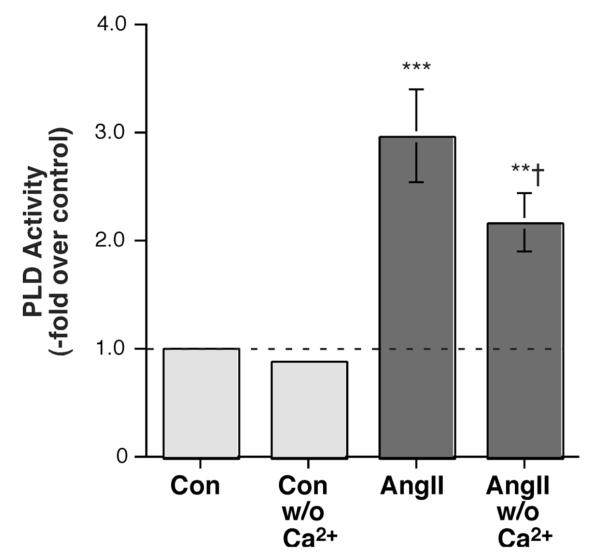
To determine whether Ca2+ influx was involved in AngII’s activation of PLD, AngII-stimulated PLD activity was then examined in the presence or absence of extracellular Ca2+. Interestingly, we observed a reduction in radiolabeled PEt production in the absence of Ca2+ in primary bovine adrenal glomerulosa cells (Figure 3). This result suggests that whereas elevated [K+]e requires Ca2+ influx through voltage-dependent Ca2+ channels, AngII-elicited PLD activation is independent of Ca2+ influx through voltage-dependent Ca2+ channels but nevertheless requires Ca2+ entry, presumably through store-operated or CRAC channels.
Figure 3.
AngII-induced PLD Activation was Modulated by Ca2+ in Primary Bovine Adrenal Glomerulosa Cells. Bovine adrenal glomerulosa cells were prelabeled for 20-24 hours with 2.5-5 μCi/mL [3H]oleate in serum-free medium. Cells were then stimulated with or without 10 nM AngII in the presence of 0.5% ethanol in medium containing 1.2 mM Ca2+ or no added Ca2+ for 30 minutes. Lipids were extracted and separated by thin-layer chromatography. PLD was measured as the radioactivity in phosphatidylethanol (PEt) relative to the control, and values are expressed as the means ± SEM of 4 separate experiments; ***p<0.001 versus the control value; ††p<0.01 versus the value in the presence of Ca2+.
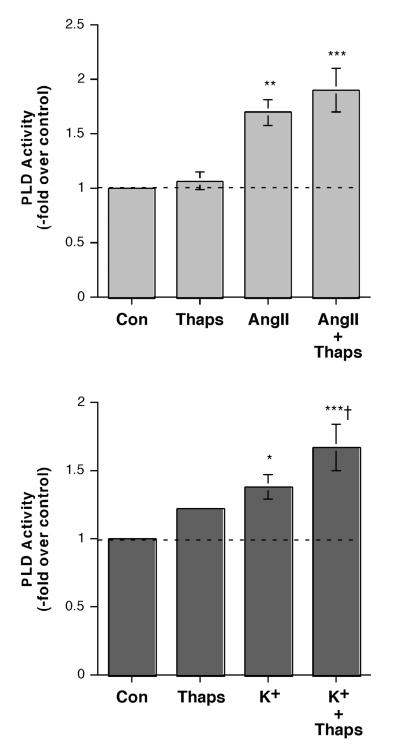
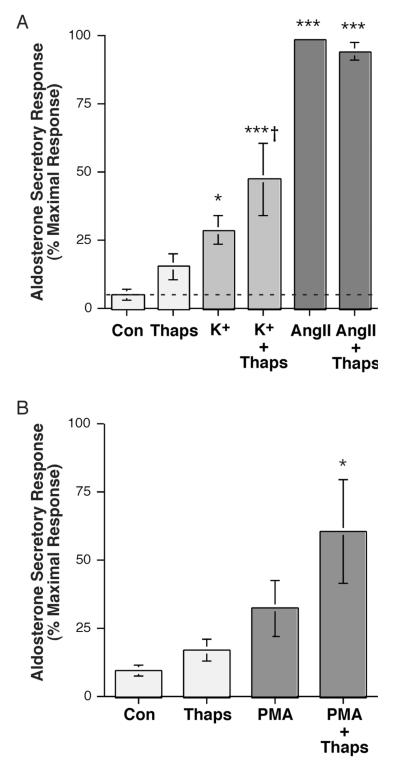
To test the role of store-operated Ca2+ influx in AngII- and elevated extracellular [K+]e–induced PLD activation, we determined the effects on PLD activity of thapsigargin, a pharmacologic agent that inhibits the sarcoplasmic/endoplasmic reticulum Ca2+ pump to release Ca2+ from these stores and activate store-operated Ca2+ influx [reviewed in (Spät et al., 2004)]. We selected a dose of thapsigargin (2 μM) that has previously been shown to increase SOC Ca2+ influx in primary cultures of bovine adrenal glomerulosa cells (Aptel et al., 1999; Burnay et al., 1998). We found that thapsigargin had little effect on AngII-induced PLD activation (Figure 4A) in primary bovine adrenal glomerulosa cells, whereas this compound stimulated elevated [K+]e–elicited PLD activity (Figure 4B). Interestingly, these results were mirrored by the effects of thapsigargin on aldosterone secretion in that the compound had little effect on AngII-induced steroidogenesis but augmented elevated [K+]e-elicited aldosterone secretion (Figure 5A), consistent with a previous report in bovine adrenal glomerulosa cells (Burnay et al., 1994). In addition, thapsigargin enhanced the aldosterone secretory response to the phorbol ester, phorbol 12-myristate 13-acetate (PMA), which mimics DAG action in primary bovine adrenal glomerulosa cells (Figure 5B). This latter result is consistent with the proposed requirement for two signals mediating sustained aldosterone secretion: a DAG second messenger and a Ca2+ influx signaling pathway [reviewed in (Rasmussen et al., 1995)].
Figure 4.
Thapsigargin Had Little or No Effect on AngII-Induced PLD Activation, but Enhanced the Effect of Elevated [K+]e on PLD Activity in Bovine Adrenal Glomerulosa Cells. (A) Bovine adrenal glomerulosa cells were prelabeled for 20-24 hours with 2.5-5 μCi/mL [3H]oleate in serum-free medium. Cells were then stimulated with or without 10 nM AngII in the presence or absence of 2 μM thapsigargin (Thaps) or vehicle (0.1-0.2% DMSO) in the presence of 0.5% ethanol for 30 minutes. Lipids were extracted and separated by thin-layer chromatography. Values represent the radioactivity in phosphatidylethanol relative to the control and are expressed as the means ± S.E.M. of 9 separate experiments; *p<0.05 versus the control value. (B) [3H]Oleate-prelabeled bovine adrenal glomerulosa cells were incubated with 3.5 mM K+-KRB+ or 15 mM K+-KRB+ in the presence or absence of 2 μM thapsigargin (Thaps) or vehicle (0.1-0.2% DMSO) in the presence of 0.5% ethanol for 30 minutes. Lipids were extracted and separated by thin-layer chromatography. Values represent the radioactivity in phosphatidylethanol relative to the control and are expressed as the means ± S.E.M. of 5 separate experiments; **p<0.01 versus the control value; †p<0.05 versus K+ alone.
Figure 5.
Thapsigargin Had Little or No Effect Alone or on AngII-Stimulated Aldosterone Secretion but Enhanced Steroidogenesis in Response to Elevated [K+]e and PMA in Primary Cultures of Bovine Adrenal Glomerulosa Cells. (A) Bovine adrenal glomerulosa cells were incubated for 1 hour with a bicarbonate-buffered Kreb’s Ringer solution with 2.5 mM sodium acetate (KRB+) containing no additions (Con), 15 mM KCl (K+; iso-osmotic substitution with Na+) or 10 nM AngII in the presence or absence of 2 μM thapsigargin (Thaps) or vehicle (0.1-0.2% DMSO). Supernatants were collected and assayed for aldosterone secretion by radioimmunoassay. Values represent the means ± S.E.M. of 4-5 separate experiments; *p<0.05, **p<0.01 versus control by ANOVA and a Student-Newmann-Keuls post-hoc test. (B) Bovine adrenal glomerulosa cells were incubated for 1 hour with a bicarbonate-buffered Kreb’s Ringer solution with 2.5 mM sodium acetate (KRB+) containing no additions (Con) or 100 nM PMA in the presence or absence of 2 μM thapsigargin (Thaps) or vehicle (0.1-0.2% DMSO). Supernatants were collected and assayed for aldosterone secretion by radioimmunoassay. Values represent the means ± S.E.M. of 4-5 separate experiments, expressed as the percentage of the maximal aldosterone secretory response; *p<0.05, **p<0.01 versus control by ANOVA and a Student-Newmann-Keuls post-hoc test.
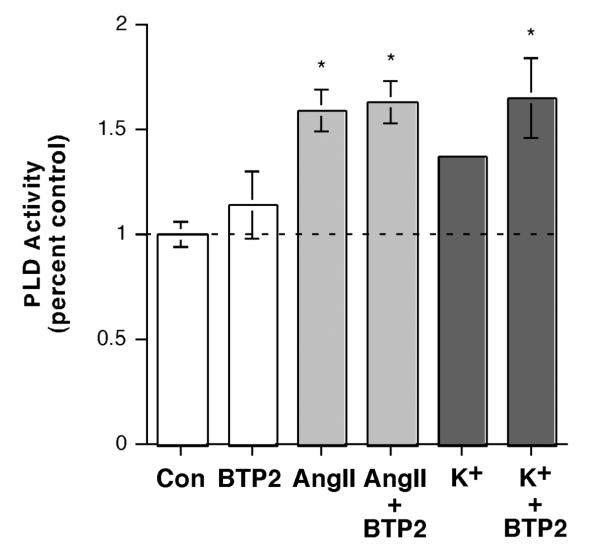
To further examine the role of SOC in AngII- and elevated [K+]e-induced PLD activation, we treated cells with these agonists in the presence and absence of the SOC inhibitor, YM-58483, also known as BTP2. We found that BTP2 had no effect on PLD activation elicited in response to AngII (Figure 6A). However, this agent actually seemed to increase elevated [K+]e-induced PLD activation, converting a non-significant response to elevated alone to a significant increase in the presence of BTP2 (Figure 6B). This result suggests that whereas SOC can enhance the activation of PLD, i.e. in conjunction with elevated [K+]e, it is not necessary for PLD activation in response to either agonist.
Figure 6.
Inhibition of Store-operated Ca2+ Influx with BTP2 (YM-58483) Had No Effect on AngII-induced, and Increased Elevated [K+]e-elicited PLD Activation. (A) [3H]Oleate-prelabeled cells were incubated for 30 minutes with KRB+ containing 0.5% ethanol and vehicle (0.1% DMSO) or 10 nM AngII in the presence or absence of 1 μM BTP2 for 30 minutes. Reactions were terminated by the addition of 0.2% SDS containing 5 mM EDTA, and [3H]phosphatidylethanol was extracted, separated by thin-layer chromatography and quantified as in (Jung et al., 1998). Values are expressed as -fold over the control and represent the means (± SEM) of 4 separate experiments; *p<0.05 versus the control value. (B) [3H]Oleate-prelabeled cells were incubated for 30 minutes with 3.5 mM K+-KRB+ or 15 mM K+-KRB+ containing 0.5% ethanol with vehicle (0.1% DMSO) or 1 μM BTP2 for 30 minutes, and PLD activity monitored as above. Values are expressed as -fold over the control and represent the means (± S.E.M.) of 4 separate experiments; *p<0.05 versus the control value.
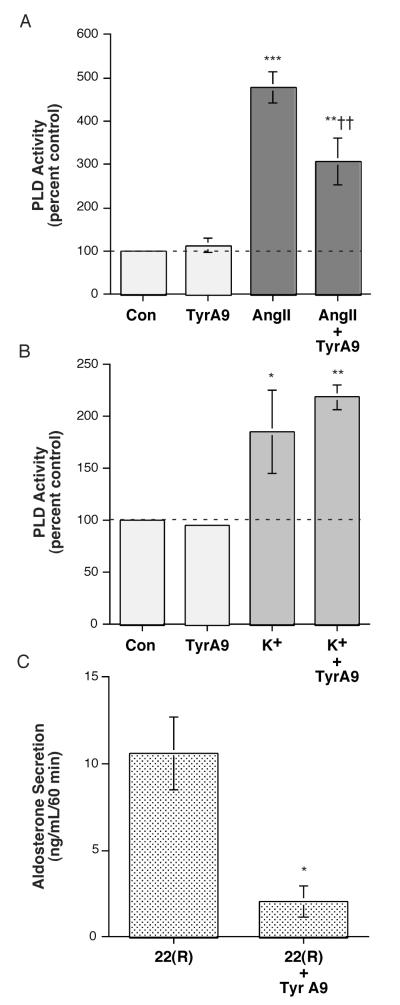
The archetypical SOC pathway is CRAC, mediated by Stim and Orai proteins [reviewed in (Potier et al., 2008)]. To examine the possible role of CRAC channels in AngII-induced PLD activation, we determined the effect of the CRAC channel inhibitor, tyrphostin A9 (Denys et al., 2004) on this signaling event. As shown in Figure 7A, we found that tyrphostin A9 inhibited AngII-elicited PLD activation in primary bovine adrenal glomerulosa cells, without affecting basal PLD activity. In contrast, tyrphostin A9 had no effect on elevated [K+]e-induced PLD activation in the primary glomerulosa cells (Figure 7B). In experiments to determine the ability of tyrphostin A9 to modulate the aldosterone secretory response, we found that tyrphostin A9 completely blocked AngII- and elevated [K+]e-induced aldosterone secretion (data not shown). However, we also observed that tyrphostin A9 significantly inhibited the ability of 22(R)-hydroxycholesterol to trigger steroidogenesis (Figure 7C). Because 22(R)-hydroxycholesterol can directly enter mitochondria to access the rate-limiting enzyme of aldosterone synthesis, thereby bypassing signaling mechanisms, inhibitory effects on secretion induced by this compound indicate that tyrphostin A9 either inhibits aldosterone biosynthetic enzymes or affects cell health. However, the fact that tyrphostin A9 did not alter basal or elevated [K+]e-elicited PLD activity indicates that the inhibitor is not simply cytotoxic and suggests instead that the compound inhibits an enzyme in the aldosterone synthetic pathway.
Figure 7.
Inhibition of Ca2+ Release-activated Ca2+ Influx with Tyrphostin A9 Inhibited AngII-, but not Elevated [K+]e-, elicited PLD Activation. (A) [3H]Oleate-prelabeled cells were incubated for 30 minutes with KRB+ containing 0.5% ethanol and vehicle (0.1% DMSO, control) or 10 nM AngII (+ 0.1% DMSO) in the presence or absence of 10 μM tyrphostin A9 (TA9) for 30 minutes. Reactions were terminated by the addition of 0.2% SDS containing 5 mM EDTA, and [ 3H]phosphatidylethanol was extracted, separated by thin-layer chromatography and quantified as in (Jung et al., 1998). Values are expressed as -fold over the control and represent the means (± SEM) of 5 separate experiments; **p<0.01, ***p<0.001 versus the control value, ††p<0.01 versus AngII alone. (B) [3H]Oleate-prelabeled cells were incubated for 30 minutes with 3.5 mM K+-KRB+ or 15 mM K+-KRB+ containing 0.5% ethanol and vehicle (0.1% DMSO, control) or 10 μM tyrphostin A9 (TA9) for 30 minutes, and PLD activity monitored as above. Values are expressed as -fold over the control and represent the means (± S.E.M.) of 4 separate experiments; *p<0.05, **p<0.01 versus the control value. (C) Bovine adrenal glomerulosa cells were incubated for 1 hour with KRB+ containing no additions (Con) or 10 μM 22(R)-hydroxycholesterol in the presence or absence of 10 μM tyrphostin A9 (TA9) or vehicle (0.1-0.2% DMSO). Supernatants were collected and assayed for aldosterone secretion by radioimmunoassay. Values represent the means ± S.E.M., expressed as the ng aldosterone/mL/60 minutes, of 3 separate experiments; *p<0.03 versus 22(R)-hydroxycholesterol alone, as determined using an unpaired Student’s t-test. Note that 22(R)-hydroxycholesterol induced a significant, approximately 11,000-fold increase over the control aldosterone secretory value.
We have previously shown that AngII activates PLD in H295R cells (Zheng et al., 2003), as in primary cultures of bovine adrenal glomerulosa cells (Bollag et al., 1990), and that this activity mediates, at least in part, the ability of AngII to induce steroidogenesis (Zheng et al., 2003), also as in the bovine cells (Bollag et al., 2002). However, in contrast to results in primary bovine adrenal glomerulosa cells, in which AngII-induced PLD activation is sustained (Jung et al., 1998), in H295R cells AngII elicits only transient PLD activation (Zheng et al., 2003). We wished to examine whether H295R cells exhibited other differences in the mechanism of PLD activation in response to aldosterone secretagogues. Initially, we examined the Ca2+ dependence of AngII-induced PLD activation in the H295R cells. Similarly to the primary bovine adrenal glomerulosa cells (Figure 2), a lack of extracellular Ca2+ also inhibited AngII-elicited PLD activation in H295R cells (Figure 6). Importantly, however, we were unable to detect PLD activation in response to elevated [K+]e in the H295R cells under a variety of conditions (data not shown), including at different time points and following pretreatment with aldosterone, reported to increase T-type channel expression (Lesouhaitier et al., 2001).
On the other hand and in contrast to the primary bovine adrenal glomerulosa cells (Figure 3), thapsigargin enhanced AngII-induced PLD activation in the H295R cells (Figure 7A). The effects on AngII-induced PLD activation in the H295R cells were not accompanied by comparable changes in aldosterone secretion, as thapsigargin had little or no effect on AngII-induced aldosterone production (Figure 7B), although this result was similar to that observed in the primary bovine adrenal glomerulosa cells (Figure 3). However, in contrast to the bovine cells, thapsigargin did not alter elevated [K+]e-elicited steroidogenesis in the H295R cells (Figure 7B). Taken together these results suggest that while similarities exist between the mechanisms of AngII-induced PLD activation in primary bovine adrenal glomerulosa cells versus the human adrenocortical carcinoma H295R cells, there are also distinct differences. However, which AngII-elicited PLD activation scenario is most similar to the situation occurring in vivo, i.e. in human patients, is at present unknown.
DISCUSSION
We have previously shown that PLD-mediated signal generation is required for maximal AngII-induced secretion in both primary cultures of bovine adrenal glomerulosa cells and the NCI H295R human adrenocortical carcinoma cell line (Bollag et al., 2002; Zheng et al., 2003) [as well as elevated [K+]e-induced steroidogenesis in bovine cells (Betancourt-Calle et al., 2001)]. Because of the importance of PLD signal generation to sustained aldosterone secretion, we sought to determine the mechanism by which AngII and elevated [K+]e modulate PLD’s activity. Our results indicate that Ca2+ influx through voltage-dependent Ca2+ channels is absolutely required for elevated [K+]e-induced PLD activation. This finding is not particularly surprising considering that elevated [K+]e is thought to exert its effects on adrenal glomerulosa cells by depolarizing the cells, thereby opening voltage-dependent calcium channels [reviewed in (Rainey et al., In press)]. Perhaps a bit more unexpected is the observation that AngII-induced PLD activity also requires Ca2+ influx, although this Ca2+ influx is not mediated by voltage-dependent Ca2+ channels, as neither nitrendipine nor nickel has an inhibitory effect on AngII-induced PLD activation (Bollag et al., 2002). However, removal of extracellular Ca2+ inhibits AngII-elicited PLD activation in both primary bovine adrenal glomerulosa cells (Figure 3) and H295R cells (Figure 7), suggesting that Ca2+ influx plays some role in PLD activation. Nevertheless, the importance of Ca2+ in regulating PLD activity was somewhat unanticipated since we previously found no effect of Ca2+ ionophores on PLD activity in primary glomerulosa cells nor inhibition of AngII-induced enzyme activation by a voltage-dependent Ca2+ channel antagonist (Bollag et al., 2002). On the other hand, we have previously shown that in both cell models, PKC appears to mediate, at least in part, PLD activation in response to AngII (Bollag et al., 2002; Zheng et al., 2003), as has been reported for other cell systems [reviewed in (Exton, 1998; Frohman et al., 1996)]. Thus, exposure to PMA is sufficient to induce PLD activation in both bovine and H295R cells (Bollag et al., 2002; Zheng et al., 2003), and PKC inhibitors reduce AngII-stimulated PLD activity (Zheng et al., 2003). Thus, the requirement for Ca2+ influx may be related to activation of PKC, presumably a classical, Ca2+-dependent PKC isoenzyme, as suggested by the results of Kojima et al. (Kojima et al., 1994), who demonstrated that Ca2+ can, indeed, regulate PKC activity.
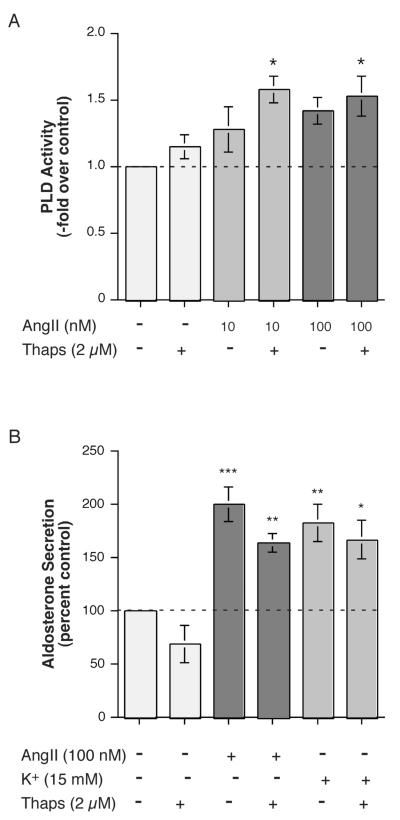
Although voltage-dependent Ca2+ channels do not appear to be involved in AngII-stimulated PLD activation (Bollag et al., 2002), AngII also increases Ca2+ influx through other pathways, such as SOC and CRAC [reviewed in (Spät et al., 2004)]. Stimulation of SOC by thapsigargin has no effect alone or together with AngII on PLD activation in primary bovine adrenal glomerulosa cells (Figure 3). We also observed no effect of thapsigargin on AngII-elicited aldosterone secretion, in contrast to a previous report in rat adrenal glomerulosa cells (Hajnoczky et al., 1991). The reason for this disparity is unclear but may be related to the species and/or the use of submaximal concentrations of AngII and thapsigargin in the rat cells (Hajnoczky et al., 1991). Nevertheless, the 2 μM thapsigargin dose used herein was sufficient to enhance the elevated [K+]e-stimulated PLD activity and aldosterone secretory rate (Figure 4), indicating its efficacy in promoting emptying of the intracellular Ca2+ store, as has been previously demonstrated in these same cells (Aptel et al., 1999; Burnay et al., 1998). On the other hand, however, thapsigargin was able to enhance AngII’s activation of PLD in H295R cells (Figure 7). It is not clear why there is the disparity between the two cell types, although we have previously reported that AngII induces a transient PLD activation in H295R cells (Zheng et al., 2003) but a sustained activity in bovine adrenal glomerulosa cells (Jung et al., 1998). Similarly, Bird et al. (Bird et al., 1993) have shown that the production of inositol bis- and trisphosphate in response to AngII in the H295R cells is also transient, whereas in bovine adrenal glomerulosa cells AngII elicits a sustained phosphoinositide hydrolysis response (Kojima et al., 1984). These data suggest that phosphoinositide-specific phospholipase C is only transiently activated in H295R cells by AngII, such that over time the endoplasmic reticulum Ca2+ pool might be expected to refill upon extinction of the transient signal, thereby terminating SOC. Thus, thapsigargin, by prolonging SOC influx could enhance PLD activation (Figure 8A) in response to AngII in the H295R cells, without affecting these parameters in primary bovine adrenal glomerulosa cells (Figures 4 and 5), which exhibit sustained AngII-elicited phospholipase C activation and therefore maintained (i.e., already maximal) SOC. However, despite its enhancement of AngII-induced PLD activation in H295R cells, thapsigargin did not alter AngII-(or elevated [K+]e-) stimulated aldosterone secretion in these cells (Figure 8B). Furthermore, the SOC inhibitor BTP2 (YM-5848) had no effect on AngII-induced PLD activation and appeared to increase that elicited by elevated [K+]e (Figure 6), suggesting that Ca2+ influx mediated by SOC is not necessary for PLD activation in response to either secretagogue.
Figure 8.
AngII-induced PLD Activation was Modulated by Ca2+ in NCI H295R Adrenocortical Carcinoma Cells. H295R cells were prelabeled for 20-24 hours with 2.5-5 μCi/mL [3H]oleate in serum-free medium. Cells were then stimulated with or without 100 nM AngII in KRB+ containing 1.2 mM Ca2+ or no added Ca2+ in the presence of 0.5% ethanol for 5 minutes. Lipids were extracted and separated by thin-layer chromatography. Values represent the radioactivity in phosphatidylethanol (PEt) relative to the control and are expressed as the means ± S.E.M. of 4 separate experiments; **p<0.01, ***p<0.01 versus the control value, †p<0.05 versus the value in the presence of Ca2+.
SOC Ca2+ influx occurs through a pathway exhibiting heterogenous characteristics, with BTP2 reported to inhibit transient receptor potential (TRP) channels, with a half-maximal inhibitory concentration of 0.1-0.3 μM (He et al., 2005). On the other hand, CRAC represents an archetypical SOC [reviewed in (Potier et al., 2008)]. Based on our results, CRAC may be a key AngII-induced Ca2+ influx pathway involved in regulating PLD activation in primary bovine adrenal glomerulosa cells. Thus, a CRAC inhibitor, tyrphostin A9 (Denys et al., 2004), inhibited AngII-stimulated PLD activity but had no effect on the basal or elevated [K+]e-induced value (Figure 7A and B). Tyrphostin A9 has also been reported to inhibit platelet-derived growth factor receptor tyrosine kinase activity (Marhaba et al., 1996); thus, the possibility remains that the effects of this compound on AngII-induced PLD activation may be related to inhibition of tyrosine kinase. However, in H295R cells we were previously unable to detect an effect of the general tyrosine kinase inhibitor, genistein (or the Janus kinase inhibitor AG490 or Src kinase inhibitor PP2), on AngII-stimulated PLD activity (Zheng et al., 2003), suggesting the likelihood that tyrphostin A9’s inhibition of CRAC mediates the inhibition of PLD activation in response to AngII. Unfortunately, it was not possible to determine if CRAC was important in AngII-elicited steroidogenesis. Although tyrphostin A9 completely inhibited AngII-induced aldosterone secretion, as well as that elicited in response to elevated [K+]e (data not shown), it also inhibited secretion in response to 22(R)-hydroxycholesterol (Figure 7C), indicating either effects on cell health or on one or more aldosterone biosynthetic enzymes. However, the inability of tyrphostin A9 to alter basal or elevated [K+]e-stimulated PLD activity (Figure 6) suggests that cytotoxicity was not responsible for the inhibition. An ability of inhibitors to affect aldosterone biosynthetic enzymes is not without precedent, as Rainey and colleagues have demonstrated that genistein inhibits 3β-hydroxysteroid dehydrogenase (Sirianni et al., 2001).
As expected, in primary bovine adrenal glomerulosa cells elevated [K+]e-induced PLD activation was dependent on voltage-dependent Ca2+ channels (Figure 1). Interestingly, SOC also appeared to stimulate elevated [K+]e-elicited PLD activation and aldosterone secretion in bovine adrenal glomerulosa cells, as shown by the ability of thapsigargin to stimulate these processes (Figures 4 and 5). There is some controversy as to whether elevated [K+]e triggers phosphoinositide hydrolysis (Betancourt-Calle et al., 2001; Ganguly et al., 1990; Hajnoczky et al., 1992; Hunyady et al., 1982; Kojima et al., 1985a; Underwood et al., 1988). Our data, however, would suggest that if it does so, elevated [K+]e is not particularly effective at inducing SOC, since thapsigargin can augment the effects of this ion on aldosterone secretion in primary bovine adrenal glomerulosa (Figure 5). On the other hand, we were unable to detect elevated [K+]e-induced PLD activation in H295R cells, under a variety of conditions (data not shown), suggesting differences in signal transduction, particularly with regards to the Ca2+ signal, in the bovine adrenal glomerulosa cells versus the H295R cells.
Together, our results suggest that: (1) PLD activation in response to elevated [K+]e, but not AngII, is dependent on Ca2+ influx via voltage-dependent Ca2+ channels but (2) AngII-induced PLD activation nevertheless requires Ca2+, likely entering the cell through Ca2+ release-activated Ca2+ (CRAC) channels. Furthermore, our results highlight the importance of the specific Ca2+ influx pathway in coupling of the aldosterone secretagogues, AngII and elevated [K+]e to PLD activation in adrenal glomerulosa cells.
Figure 9.
Thapsigargin Enhanced the Effect of AngII on PLD Activation Cells but Had No Effect on AngII- (or Elevated [K+]e-) Stimulated Aldosterone Secretion in H295R. (A) H295R cells were pre-labeled for 20-24 hours with 2.5-5 μCi/mL [3H]oleate in serum-free medium. Cells were then stimulated with or without 10 or 100 nM AngII in the presence or absence of 2 μM thapsigargin (Thaps) or vehicle (0.2% DMSO) in the presence of 0.5% ethanol. Lipids were extracted and separated by thin-layer chromatography. Values represent the radioactivity in phosphatidylethanol relative to the control and are expressed as the means ± S.E.M. of 3 separate experiments; *p<0.05 versus the control value. (B) H295R cells were incubated for 5 hours with medium containing no additions (Con), 15 mM KCl (K+) or 100 nM AngII in the presence or absence of 2 μM thapsigargin (Thaps) or vehicle (0.1-0.2% DMSO) as indicated. Supernatants were collected and assayed for aldosterone secretion by radioimmunoassay. Values represent the means ± S.E.M. of 4-5 separate experiments; *p<0.05, **p<0.01, ***p<0.001 versus the control value.
Acknowledgments
FUNDING This work was supported by a National Institutes of Health Award #HL70046 and American Heart Association Grant-in-Aid Award #0350166N.
Abbreviations used
- AngII
angiotensin II
- DAG
diacylglycerol
- CRAC
Ca2+ release-activated Ca2+ influx
- [K+]e
extracellular potassium concentration
- KRB+
bicarbonate-buffered Kreb’s Ringer solution containing 2.5 mM sodium acetate
- PEt
phosphatidylethanol
- PKC
protein kinase C
- PLD
phospholipase D
- PMA
phorbol 12-myristate 13-acetate
- SEM
standard error of the mean
- SOC
store-operated Ca2+ influx
- tyrphostin A9
[3,5-bis(1,1-Dimethylethyl)-4-hydroxyphenyl]methylene]propanedinitrile
- YM-58483 (BTP2)
4-methyl-4′-[3.5-bis(trifluoromethyl)-1H-pyrazol-1-yl]-1,2,3-thiadoazole-5-carboxanilide
Footnotes
DECLARATION OF INTEREST The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Aptel HB, Burnay MM, Rossier MF, Capponi AM. The role of tyrosine kinases in capacitative calcium influx-mediated aldosterone production in bovine adrenal zona glomerulosa cells. J Endocrinol. 1999;163(1):131–138. doi: 10.1677/joe.0.1630131. [DOI] [PubMed] [Google Scholar]
- Barrett PQ, Bollag WB, Isales CM, McCarthy RT, Rasmussen H. Role of calcium in angiotensin II-mediated aldosterone secretion. Endocr Rev. 1989;10(4):1–22. doi: 10.1210/edrv-10-4-496. [DOI] [PubMed] [Google Scholar]
- Barrett PQ, Ertel EA, Smith MM, Nee JJ, Cohen CJ. Voltage-gated calcium currents have two opposing effects on the secretion of aldosterone. Am. J. Physiol. 1995;268:C985–C992. doi: 10.1152/ajpcell.1995.268.4.C985. [DOI] [PubMed] [Google Scholar]
- Betancourt-Calle S, Jung EM, White S, Calle RA, Rasmussen H, Bollag WB. Elevated K + induces MARCKS phosphorylation and phospholipase D activation in glomerulosa cells. Mol. Cell. Endocrinol. 2001;184:65–76. doi: 10.1016/s0303-7207(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE. Human NCI-H295 adrenocortical carcinoma cells: A model for angiotensin-II-responsive aldosterone secretion. Endocrinology. 1993;133:1555–1561. doi: 10.1210/endo.133.4.8404594. [DOI] [PubMed] [Google Scholar]
- Bollag WB. Measurement of phospholipase D activity. Meth. Mol. Biol. 1998;105:151–160. doi: 10.1385/0-89603-491-7:151. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Barrett PQ, Isales CM, Liscovitch M, Rasmussen H. A potential role for phospholipase-D in the angiotensin-II-induced stimulation of aldosterone secretion from bovine adrenal glomerulosa cells. Endocrinology. 1990;127(3):1436–1443. doi: 10.1210/endo-127-3-1436. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Barrett PQ, Isales CM, Liscovitch M, Rasmussen H. Signal transduction mechanisms involved in carbachol-induced aldosterone secretion from bovine adrenal glomerulosa cells. Mol Cell Endocrinol. 1992;86(1-2):93–101. doi: 10.1016/0303-7207(92)90179-a. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Jung EM, Calle RA. Mechanism of angiotensin II-induced phospholipase D activation in adrenal glomerulosa cells. Mol. Cell. Endocrinol. 2002;192:7–16. doi: 10.1016/s0303-7207(02)00134-x. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Kent P, White S, Malinova M, Isales CM, Calle RA. Characterization and phospholipase D mediation of the angiotensin II priming response in adrenal glomerulosa cells. Endocrinology. 2007;148(2):585–593. doi: 10.1210/en.2006-0898. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Zheng X, editors. Trends in Protein Research. NovaScience Publishers; Hauppauge, New York: 2005. The role of phospholipase D in keratinocyte biology. [Google Scholar]
- Burnay MM, Python CP, Vallotton MB, Capponi AM, Rossier MF. Role of the capacitative calcium influx in the activation of steroidogenesis by angiotensin-II in adrenal glomerulosa cells. Endocrinology. 1994;135:751–758. doi: 10.1210/endo.135.2.8033823. [DOI] [PubMed] [Google Scholar]
- Burnay MM, Vallotton MB, Capponi AM, Rossier MF. Angiotensin II potentiates adrenocorticotropic hormone-induced cAMP formation in bovine adrenal glomerulosa cells through a capacitative calcium influx. Biochem. J. 1998;330:21–27. doi: 10.1042/bj3300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys A, Aires V, Hichami A, Khan NA. Thapsigargin-stimulated MAP kinase phosphorylation via CRAC channels and PLD activation: inhibitory action of docosahexaenoic acid. FEBS Lett. 2004;564(1-2):177–182. doi: 10.1016/S0014-5793(04)00361-8. [DOI] [PubMed] [Google Scholar]
- Exton JH. Phospholipase D. Biochim Biophys Acta. 1998;1436:105–115. doi: 10.1016/s0005-2760(98)00124-6. [DOI] [PubMed] [Google Scholar]
- Exton JH. Regulation of phospholipase D. Biochim. Biophys. Acta. 1999;1439:121–133. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- Foster RH. Reciprocal influences between the signalling pathways regulating proliferation and steroidogenesis in adrenal glomerulosa cells. J Mol Endocrinol. 2004;32(3):893–902. doi: 10.1677/jme.0.0320893. [DOI] [PubMed] [Google Scholar]
- Frohman MA, Morris AJ. Phospholipid signalling: Rho is only ARF the story. Curr. Biol. 1996;6:945–947. doi: 10.1016/s0960-9822(02)00634-6. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Chiou S, Davis JS. Intracellular mediators of potassium-induced aldosterone secretion. Life Sciences. 1990;46:173–180. doi: 10.1016/0024-3205(90)90102-w. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Varnai P, Buday L, Farago A, Spat A. The role of protein kinase-C in control of aldosterone production by rat adrenal glomerulosa cells: activation of protein kinase-C by stimulation with potassium. Endocrinology. 1992;130(4):2230–2236. doi: 10.1210/endo.130.4.1547736. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Varnai P, Hollo Z, Christensen SB, Balla T, Enyedi P, Spat A. Thapsigargin-induced increase in cytoplasmic Ca2+ concentration and aldosterone production in rat adrenal glomerulosa cells: interaction with potassium and angiotensin-II. Endocrinology. 1991;128:2639–2644. doi: 10.1210/endo-128-5-2639. [DOI] [PubMed] [Google Scholar]
- He LP, Hewavitharana T, Soboloff J, Spassova MA, Gill DL. A functional link between store-operated and TRPC channels revealed by the 3,4-bis(trifluoromethyl)pyrazole derivative, BTP2. J. Biol. Chem. 2005;280:10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Balla T, Nagy K, Spät A. Control of phosphatidylinositol turnover in adrenal glomerulosa cells. Biochim. Biophys. Acta. 1982;713:352–357. doi: 10.1016/0005-2760(82)90253-3. [DOI] [PubMed] [Google Scholar]
- Jung EM, Betancourt-Calle S, Mann-Blakeney R, Foushee T, Isales CM, Bollag WB. Sustained phospholipase D activation in response to angiotensin II but not carbachol in bovine adrenal glomerulosa cells. Biochem. J. 1998;330:445–451. doi: 10.1042/bj3300445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I, Kawamura N, Shibata H. Rate of calcium entry determines the rapid changes in protein kinase C activity in angiotensin II-stimulated adrenal glomerulosa cells. Biochem. J. 1994;297:523–528. doi: 10.1042/bj2970523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I, Kojima K, Kreutter D, Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984;259(23):14448–14457. [PubMed] [Google Scholar]
- Kojima I, Kojima K, Rasmussen H. Intracellular calcium and adenosine 3′,5′-cyclic monophosphate as mediators of potassium-induced aldosterone secretion. Biochem. J. 1985a;228:69–76. doi: 10.1042/bj2280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I, Kojima K, Rasmussen H. Role of calcium fluxes in the sustained phase of angiotensin II-mediated aldosterone secretion from adrenal glomerulosa cells. J Biol Chem. 1985b;260(16):9177–9184. [PubMed] [Google Scholar]
- Lesouhaitier O, Chiappe A, Rossier MF. Aldosterone increase T-type calcium currents in human adrenocarcinoma (H295R) cells by inducing channel expression. Endocrinology. 2001;142:4320–4330. doi: 10.1210/endo.142.10.8435. [DOI] [PubMed] [Google Scholar]
- Marhaba R, Mary F, Pelassy C, Stanescu AT, Aussel C, Breittmayer JP. Tyrphostin A9 inhibits calcium release-dependent phosphorylations and calcium entry via calcium release-activated channel in Jurkat T cells. J. Immunol. 1996;157:1468–1473. [PubMed] [Google Scholar]
- Potier M, Trebak M. New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflugers Arch. 2008;457:405–415. doi: 10.1007/s00424-008-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE, Bollag WB, Isales CM. Aldosterone regulation. In: Singh AJ, Williams GH, editors. Textbook of Nephro-Endocrinology. Elsevier, Inc.; Atlanta, Ga: 2008. [Google Scholar]
- Rasmussen H, Isales CM, Calle R, Throckmorton D, Anderson M, Gasalla-Herraiz J, McCarthy R. Diacylglycerol production, Ca2+ influx, and protein kinase C activation in sustained cellular responses. Endocr. Rev. 1995;16:649–681. doi: 10.1210/edrv-16-5-649. [DOI] [PubMed] [Google Scholar]
- Schrier AD, Wang H, Talley EM, Perez-Reyes E, Barrett PQ. alpha1H T-type Ca2+ channel is the predominant subtype expressed in bovine and rat zona glomerulosa. Am. J. Physiol. Cell Physiol. 2001;280:C265–C272. doi: 10.1152/ajpcell.2001.280.2.C265. [DOI] [PubMed] [Google Scholar]
- Sirianni R, Sirianni R, Carr BR, Pezzi V, Rainey WE. A role for src tyrosine kinase in regulating adrenal aldosterone secretion. J. Mol. Endocrinol. 2001;26:207–215. doi: 10.1677/jme.0.0260207. [DOI] [PubMed] [Google Scholar]
- Spät A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol. Rev. 2004;84:489–539. doi: 10.1152/physrev.00030.2003. [DOI] [PubMed] [Google Scholar]
- Thompson NT, Bonser RW, Garland LG. Receptor-coupled phospholipase D and its inhibition. Trends Pharmacol. Sci. 1991;12:404–408. doi: 10.1016/0165-6147(91)90617-2. [DOI] [PubMed] [Google Scholar]
- Underwood RH, Greeley R, Glennon ET, Menachery AI, Braley LM, Williams GH. Mass determination of polyphosphoinositides and inositol triphosphate in rat adrenal glomerulosa cells with a microspectrophotometric method. Endocrinology. 1988;123:211–219. doi: 10.1210/endo-123-1-211. [DOI] [PubMed] [Google Scholar]
- Zheng X, Bollag WB. AngII induces transient phospholipase D activity in the H295R glomerulosa cell model. Mol. Cell. Endocrinol. 2003;206:113–122. doi: 10.1016/s0303-7207(03)00211-9. [DOI] [PubMed] [Google Scholar]