Abstract
Aging and a sedentary lifestyle conspire to reduce bone quantity and quality, decrease muscle mass and strength, and undermine postural stability, culminating in an elevated risk of skeletal fracture. Concurrently, a marked reduction in the available bone-marrow-derived population of mesenchymal stem cells (MSCs) jeopardizes the regenerative potential that is critical to recovery from musculoskeletal injury and disease. A potential way to combat the deterioration involves harnessing the sensitivity of bone to mechanical signals, which is crucial in defining, maintaining and recovering bone mass. To effectively utilize mechanical signals in the clinic as a non-drug-based intervention for osteoporosis, it is essential to identify the components of the mechanical challenge that are critical to the anabolic process. Large, intense challenges to the skeleton are generally presumed to be the most osteogenic, but brief exposure to mechanical signals of high frequency and extremely low intensity, several orders of magnitude below those that arise during strenuous activity, have been shown to provide a significant anabolic stimulus to bone. Along with positively influencing osteoblast and osteocyte activity, these low-magnitude mechanical signals bias MSC differentiation towards osteoblastogenesis and away from adipogenesis. Mechanical targeting of the bone marrow stem-cell pool might, therefore, represent a novel, drug-free means of slowing the age-related decline of the musculoskeletal system.
Introduction
Pressure, gravity, waves, temperature and electric and magnetic fields have been omnipresent physical signals since the beginning of time. Unsurprisingly, the capacity of biologic systems to adapt to physical signals is a common attribute for essentially all forms of life,1–4 and the cellular machinery responsible for sensing and responding to mechanical signals might be even more evolved than those processes that are regulated by complex macromolecules. After all, single-celled life-forms from 3 billion years ago were constantly subjected to a range of physical challenges, and an organism's ability to persist, if not thrive, was largely based on its ability to accommodate, acclimate and adapt to changes in its physical environment.
Mammalian cells have conserved mechanosensory and mechanoresponse mechanisms, the aggregate of which provides an important regulatory component for the maintenance and repair of subcellular, cellular, tissue and organ systems throughout the organism's lifespan.5 The adaptive capacity of musculoskeletal tissues provides clear evidence of the phenotypic impact of mechanical loading, a regulatory process that is further emphasized by the consequences of removing physical signals, as reflected by the rapid onset of osteopenia and sarcopenia.
Functional loading of the skeleton is critical to achieving and maintaining adequate bone quantity and quality. Physiologic levels of loading promote coordinated, site-specific activation of osteoblast and osteoclast populations, and orchestrate focal modeling and remodeling of the bone tissue. This highly regulated, mechanically mediated, targeted bone turnover allows for adaptive changes and repair of damage in bone structure. Reductions in functional loading as a result of a lessactive lifestyle might manifest through metabolic (for example, obesity, diabetes), disease-induced (chronic bedrest), injury (paraplegia, cast immobilization), job-related (spaceflight) or natural (aging) means, culminating in a weakened bone structure that is more susceptible to frank failure. The goals of this Review are to consider the types of mechanical load that are necessary to achieve and maintain skeletal quantity and quality as demonstrated by both animal and human studies, and describe the cell types and processes that are involved in regulating this response.
Bone adaptation to exercise
It is well accepted that bone structure is compromised by disuse and enhanced by exercise, providing the ‘use it or lose it’ tenet of bone physiology referred to as Wolff 's Law.6 Retrospective studies illustrate the response of bone to physical extremes: astronauts enduring microgravity lose up to 2% of hip bone density each month,7 whereas professional tennis players possess up to 35% more bone in the dominant arm than the arm that tosses the ball into the air.8 Indeed, a range of site-specific benefits can be correlated to the special tasks of elite sportsmen and women trained over extended periods.9
Several prospectively designed trials indicate that new loading challenges can also induce focal accretions of bone mass. Intense exercise in young army recruits stimulated increases in bone mineral density (BMD),10 while a 10-month, high-impact strength-building regimen in children significantly increased femoral neck BMD.11 Despite the apparent anabolic nature of the mechanical signal, moderate exercise regimens generally result in only modest, if any, increases in bone mass; for example, a 1-year high-resistance strength-training study in young women significantly increased muscle strength but failed to influence bone mass.12 In parallel, it is also important to consider the role of a range of non mechanical issues, including genetic factors,13 sex,14 ethnicity,15 age16 and diet17 in pronouncing—or suppressing—the ability of mechanical signals to drive bone formation. Nevertheless, the inherent complexity of exercise-generated mechanical challenge to the skeleton indicates that some components of the load-bearing regimen might be more influential than others. Equally important, before mechanical signals evolve into clinically effective interventions for the treatment of bone disease or repair, we must identify the cells and the molecular mechanisms by which mechanically derived signals control bone formation and resorption.
In trying to understand the biological basis of Wolff 's Law, some success has been realized by characterizing the ability of the resident bone cell populations—osteoblasts, osteocytes and osteoclasts—to respond to physical signals. In addition, the mechanical sensitivity of the bone-marrow-derived stem-cell population and, in particular, mesenchymal stem cells (MsCs), has a marked influence on the bone, and fat, phenotype.18 Importantly, the mechanical influence on stem-cell activity is critical not only to tissue health, but to the regenerative capacity of organ systems. For example, if the mechanical signals that arise from weight bearing disappear during long-term spaceflight and result in osteoporosis for an astronaut, the consequences of microgravity might be compounded if an injury were to occur (for example, hip fracture from a fall), as the progenitor cell population critical to bone repair might have collapsed in the absence of mechanical signals.19 Therefore, interventions that are being designed to retain and/or reestablish a healthy, functional skeleton by targeting existing bone cells might also define the fate of the stem-cell niche, ensuring the capacity of these cells to proliferate and differentiate to higher-order connective tissues.
Identifying anabolic mechanical signals
Bone homeostasis
The skeleton carries out a diverse range of functional, developmental and metabolic requirements: it functions as a protective cage for internal organs and a safe niche for marrow, facilitates locomotion, and is the principal reservoir of minerals.20 The constant remodeling cycle of formation and resorption facilitates both the rapid repair of bone microdamage and the replacement of dead osteocytes, and orchestrates changes in mass and morphology to meet any changing demands of mechanical loads or metabolic need. In response to an increased mechanical demand (for example, during exercise), the balance in bone turnover favors anabolism (net formation) through osteoblast recruitment and activity, and enhances the strength of bone by adding matrix to resist new loading challenges. By contrast, decreased mechanical loading induces catabolism (resorption) by promoting osteoclastogenesis, while both bone formation and osteoblastogenesis are suppressed.
Load-driven adaptation of bone
What types of loading influence bone remodeling? First, the load must be dynamic (time varying) to initiate an anabolic response; large static loads are known to induce bone loss similar to that which occurs through disuse.21 Thus, astronauts should not expect Jupiter's gravitational pull (258% greater than that of the earth) to stimulate an anabolic response and reverse the osteoporosis caused by zero-gravity endured during prolonged spaceflight. Instead, to activate the mechanically driven adaptive response in bone, astronauts might have to struggle with exercise in this extreme gravitational pull even to maintain their bone mass. Conversely, even though the decreased gravitational pull of Mars (38% compared with that of earth) might be expected to accelerate bone loss, this environment might also allow exuberant and unconstrained dynamic activity, and thus promote bone formation.
Wherever astronauts land, they will have to be active to maintain their skeletal strength and, as illustrated, it cannot be presumed that it is simply the load magnitude that drives the adaptive response. naturally, any dynamic loading has magnitude and frequency components, all of which result in a range of strains (deformations normalized to geometry) and strain rates in the bone matrix.22 studies that have focused on the magnitude component of delivered strains show that these strains are efficient in inducing a tissue response directly, by matrix deformation, and indirectly, via the byproducts of strain, including fluid flow and streaming potentials.23,24
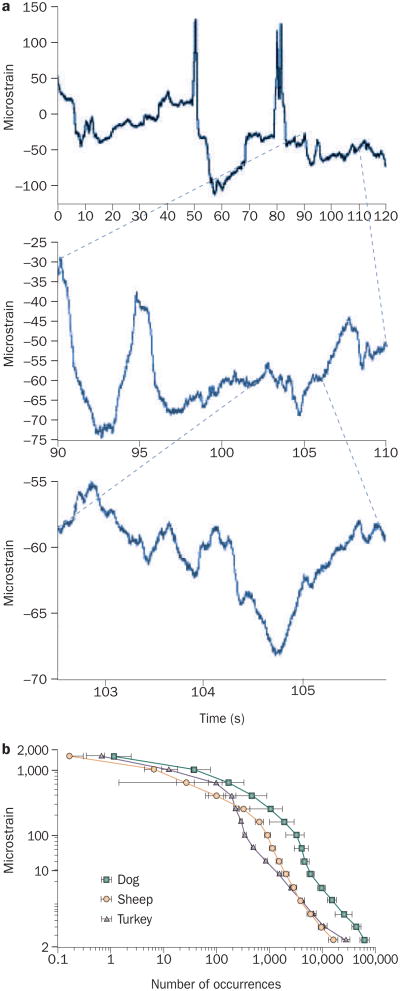
Over the daily course of functional challenges, bone will be subjected to exceptionally few high-strain (2,000–3,000 microstrain), low-frequency (1–3 Hz, or cycles per second) events, but to a persistent barrage of low-strain (<5 microstrain), high-frequency events (10–50 Hz), stemming from muscle contractions engaged to retain posture (Figure 1).25 The occurrence of omnipresent, high-frequency, low- magnitude mechanical events in the axial and appendicular skeleton decreases in parallel with the sarcopenia of aging or disuse,26 perhaps contributing to the etiology of the bone loss that correlates with the deterioration of muscle.
Figure 1.
Bone is subjected to a range of mechanical strains. a | A 2-minute recording from a strain gauge attached to a sheep tibia while the animal is standing (top panel) shows peak strains in the order of 200 microstrain. A 20-second section of that strain record (middle panel) shows peak strain events as large as 40 microstrain, occurring at a high frequency. Closer inspection of a 3-second period of the strain recording (bottom panel) illustrates events in the order of 5 microstrain occurring through the entire recording period. when the strain activity of a bone is recorded over a 12-hour period, it is clear that there are very few large strain events (>2,000 microstrain) and tens of thousands of small strain events (<10 microstrain). b | Strain recordings from the tibia of a diverse range of animals over a 12-hour period are remarkably similar. Reprinted from Journal of Biomechanics 33, Fritton, S. P., McLeod, K. J. and Rubin, C. T. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains, 317–325 © (2000), with permission from Elsevier.
‘Other than peak’ adaptive signals
At the level of the tissue, the potential of bone to adapt to changes in the mechanical loading environment is well documented. Animal models demonstrate that bone remodeling is sensitive to changes in strain magni-tude,27 the number of loading cycles,28 the distribution of loading,29 the rate of strain30 and the rate of fluid stress,31 and that the anabolic potential increases with the inclusion of rest periods between the mechanical events.32 Functional loading parameters that correlate with signal intensity imply that a ‘goal’ of mechanical adaptation is to minimize tissue strain for a given load, while simultaneously minimizing tissue mass. Alternatively, bone cells might actually be responding to ‘biologically relevant’ parameters of the functional milieu that are not necessarily linked to the magnitude of the signal.
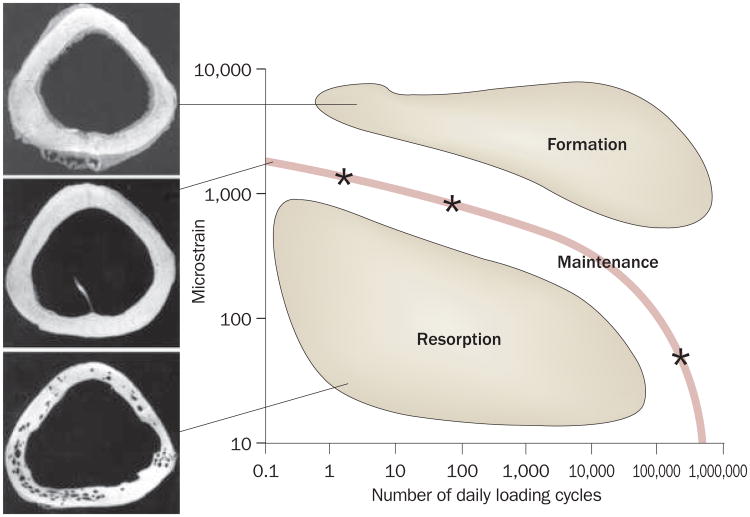
Several biologic systems are tuned to perceive and respond to ‘other than peak’ exogenous signals, such as vision, hearing and touch. It is well accepted that too much loading will damage the bone, leading to failure— just as too much light, noise or pressure will overwhelm sight, hearing and touch. And although the skeleton's primary responsibility is structural in nature, its overall responsibilities are broader than first presumed, and include, even, a critical role of the acoustic sensory organ in elephants.33 emphasizing this point, bone's adaptation to mechanical signals is nonlinear, such that it can be influenced by a very few high-magnitude strain events, or by many thousands of low-magnitude strain events (Figure 2).34
Figure 2.
Interrelationship between loading cycles and bone adaptation. Using the turkey ulna model to determine the nonlinear interrelationship of cycle number and strain magnitude, bone mass can be maintained through a number of distinct strategies (line); bone is preserved with either four cycles per day of 2,000 microstrain, 100 cycles per day of 1,000 microstrain, or hundreds of thousands of cycles of signals of well below 10 microstrain (each represented as a star).34 These data indicate that falling below this ‘preferred strain history’ would stimulate bone loss, while exceeding this interrelationship would stimulate bone gain.
Even extremely-low-magnitude bone strains, three orders of magnitude below peak strains generated during strenuous activity, can be anabolic to bone when induced at high frequencies, in essence mimicking the spectral content of muscle contractibility.35 When considering the role of sarcopenia and diminished mechanical loading in the etiology of osteoporosis,36 the decay of muscle-based signal components would also suppress mechanically based regulatory signals and contribute as much to bone loss as a reduction in the sensitivity of bone tissue to mechanical signals.37,38 Thus, the potential to improve bone quality and quantity using specific components of the complex mechanical signal as a ‘surrogate’ for strenuous exercise and signals lost with aging seems feasible as a drug-free intervention against bone loss. Indeed, a mechanical strategy has unique advantages over pharmaceutical therapy, as mechanical signals are both self- targeted (maximum strain will occur in the weakest loci in the bone matrix) and self-optimizing (increased bone formation in the weak loci will reduce strain, and thus inherently reduce the signal).
Although loading increases bone density and structural morphology in a normal healthy skeleton, exercise might not be as efficacious in preventing disuse-induced osteopenia or reversing bone density following loss.39 Further, strenuous exercise, particularly in an already frail skeleton, might promote tissue microdamage or the fracture that the loading intervention was aimed to prevent.40 In that sense, an exercise-based strategy that incorporates high-magnitude strains to induce bone formation might be risky for elderly or disabled patients whose bones are already prone to failure, a risk compounded by the already compromised regenerative potential of the bone marrow.41 nevertheless, the identification of specific mechanical signals that can be used as ‘therapy’ could still harness the sensitivity of bone to mechanical signals, and enable the omission of damage-inducing components of the load.
Low-magnitude mechanical signals
Results from animal studies and preliminary clinical studies suggest the feasibility of replacing the regulatory mechanical signals that decay as a function of aging or disuse with exogenously delivered mechanical stimula-tion.42 The potential of using high-frequency, low-magnitude mechanical stimulation (LMMs) to improve the quantity and quality of skeletal tissue in animal and human studies is briefly summarized below; however, it is worth bearing in mind that these signals might also be anabolic to skeletal muscle,43 indicating that a ‘mechanical strategy’ might extend beyond bone to address frailty in the musculoskeletal system.
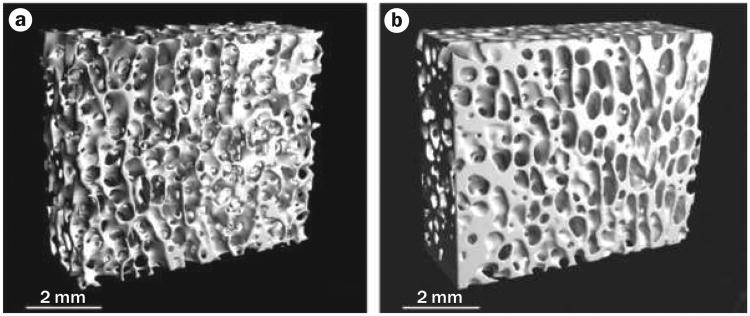
In a first ‘proof-of-principle’ assessment of LMMs, mature female sheep that were subjected to a 1-year treatment of brief (20 minutes per day), low-magnitude (0.3 g), high-frequency (30 Hz) mechanical signals attained 30% increases in the trabecular density and volume of the femur compared with controls,35 paralleled by an increase in bone stiffness and strength (Figure 3).44 These studies provided evidence that extremely small strains (<10 microstrain), far below those generated during strenuous activity,45 could readily serve as anabolic agents to bone.
Figure 3.
Low-magnitude mechanical signals are anabolic to bone. Microcomputed tomography of 1 cm cubes of trabecular bone from the distal femur of adult (8-year-old) sheep, comparing a | a control animal with b | an animal subjected to 20 minutes per day of 30 Hz (cycles per second) of a low-level (0.3 g) mechanical vibration for 1 year. The large increase in trabecular bone density results in enhanced bone strength, achieved via bone strain three orders of magnitude below those that cause tissue damage. These data suggest that mechanical signals need not be large to be anabolic to bone, and might represent a non-drug basis for the prevention or treatment of osteoporosis. Permission obtained from J. Bone Miner. Res. 17, 349–357 © (2002) American Society for Bone and Mineral Research.44
LMMs has also been shown to slow the bone loss caused by disuse in adult female rats.46 Following 1 month of disuse by hindlimb unloading, the proximal tibia showed bone formation had dropped to less than half that of age-matched controls. even ‘disuse’ animals allowed to bear weight on their hindlimbs for 10 minutes per day had a similar suppression of bone formation. By contrast, a daily 10-minute exposure to LMMs delivered by an oscillating platform (90 Hz, 0.25 g) restored bone formation to levels seen in age-matched, weight-bearing control animals.46
Is bone strain driving the response?
That the deformation of the bone matrix generated in the tibia by LMMs was so small (<10 microstrain) yet these signals were both anabolic and anti-catabolic to bone suggested that tissue strain per se might not be the primary physical signal but might, instead, be masking the ‘real’ stimulus regulating bone cells. To examine whether bone strain was necessary to stimulate bone formation, adult mice were again hindlimb unloaded. While anesthetized and supine, the left hindlimbs of experimental mice were subjected to LMMs in the absence of weight-bearing by coupling the limb to an actuator that oscillated the limb back and forth at a high frequency (45 Hz). Trabecular bone in the proximal tibiae of these limbs showed significant increases in volume (17%) and stiffness (38%) compared with the contralateral control leg,47 indicating that bone was sensitive to mechanically derived signals other than deformation (strain) of the bone matrix. These data suggest that, in addition to mechanical information being transduced to the cell through distortion of the matrix,48 the cells in the bone can sense and respond to the acceleration and deceleration components of a motion in dependent of distortion of the surrounding tissue.49
Mechanical suppression of fat production
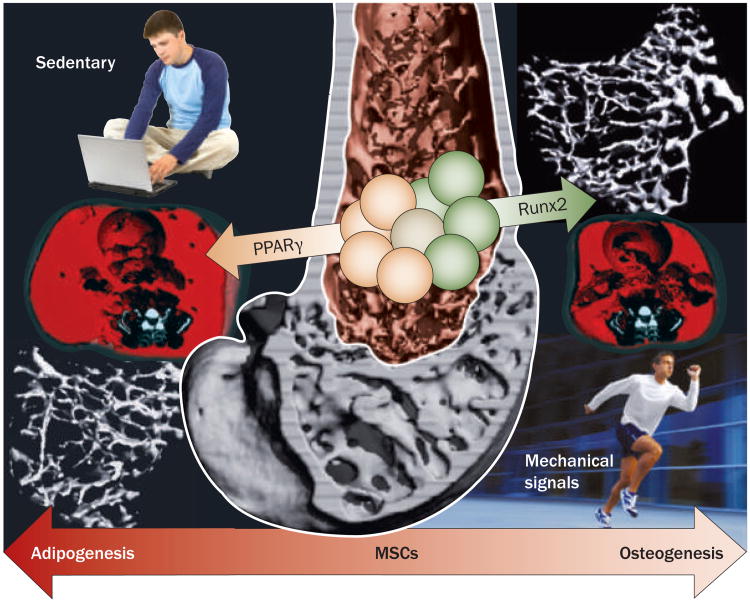
Considering the importance of exercise in stemming both osteoporosis and obesity, combined with the fact that MsCs are progenitors of both osteoblasts and adipocytes (fat cells), as well as the anabolic response of the skeletal system to LMMs, it was hypothesized that mechanical signals anabolic to bone would invariably cause a parallel decrease in fat production. seven-week-old C57BL/6J mice on a normal chow diet were randomized to undergo LMMs (90 Hz at 0.2 g for 15 minutes per day) or placebo treatment.50 At 15 weeks, with no differences in food consumption between groups, in vivo CT scans showed that the abdominal fat volume of mice subjected to LMMs was 27% lower than that of controls (P <0.01).51 Wet weights of visceral and subcutaneous fat deposits in LMMs mice were correspondingly lower. Furthermore, LMMs mice had a greater trabecular bone volume measured in the proximal tibia. Confirmed by fluorescent labeling and flow cytometry studies,50 these data indicated that mechanical signals influence not only the resident bone cell (osteoblast/osteocyte) population, but also their progenitors, biasing MsC differentiation towards bone (osteoblastogenesis) and away from fat (adipogenesis).
In a follow-on test of this hypothesis, mice fed a high-fat diet were subjected to LMMs or placebo treatment.52 suppression of adiposity by the mechanical signals was accompanied by a ‘mechanistic response’ at the molecular level showing that LMMs significantly influenced MsC commitment to either an osteogenic (Runx2, a transcription factor central to osteoblastogenesis) or adipogenic (peroxisome proliferator-activated receptor [PPAR]γ, a transcription factor central to adipogenesis) fate. Runx2 expression was greater and PPARγ expression was decreased in mice that underwent LMMs compared with controls (Figure 4). The PPARγ transcription factor, when absent or present as a single copy, facilitates osteogenesis at least partly through enhanced canonical Wnt signaling,53,54 a pathway critically important to MsC entry into the osteogenic lineage and expansion of the osteoprogenitor pool.55 notably, LMMs treatment also resulted in a 46% increase in the size of the MsC pool (P <0.05).52 These experiments, although not obviating a role for the osteoblast/osteocyte syncytium, provide evidence that bone marrow stem cells are capable of sensing exogenous mechanical signals and responding with an alteration in cell fate that ultimately influences both the bone and fat phenotype. Importantly, the inverse correlation of bone and fat phenotype has increasing support in the clinical literature.56 Although controversial, and despite the presumption that conditions such as obesity will inherently protect the skeleton owing to increased loading events, data in humans evaluating bone–fat interactions indicate that an ever-increasing adipose burden comes at the cost of bone structure and increased risk of fracture.57
Figure 4.
Mechanical loading influences MSC differentiation. The ability of mechanical signals to increase bone formation while inhibiting fat formation centers on the mechanical sensitivity of the common progenitor stem cell from which osteoblasts and adipocytes differentiate. Shown in reconstructed microcomputed images, low-magnitude mechanical signals bias the bone marrow stem-cell population towards osteoblastogenesis, resulting in greater bone density in the proximal tibia (upper right) and reduced visceral adiposity across the abdomen (red in the lower right image). These images are compared with those from placebo control animals who ate the same amount of food, yet over the same time period had significantly less trabecular bone (lower left) and more fat (upper left; reproduced at same scale). The phenotypic outcomes are mirrored by transcriptional changes, as reflected by increased Runx2 expression and reduced PPARγ expression in the marrow of the LMMS animals. Abbreviations: LMMS, low- magnitude mechanical signal; MSC, mesenchymal stem cell; PPARγ, peroxisome proliferator-activated receptor. Permission obtained from J. Bone Miner. Res.24, 50–61 © (2009) American Society for Bone and Mineral Research.52
Orchestrating the anabolic response
In order to facilitate bone adaptation and healing, cells involved in inflammation, repair and remodeling must function with a high degree of temporal and spatial orchestration. osteoblasts stem from MsCs to produce and mineralize matrix before final differentiation into the entombed osteocyte. simultaneously, unnecessary or damaged tissues are continuously perforated by resorbing osteoclasts, a large multinucleated cell type (>20 μm) that matures from the hematopoietic lineage, the activity of which is also susceptible to mechanical signals.58 This response is largely mediated through control of local expression of receptor activator of nuclear factor κB ligand and osteoprotegerin signaling by osteoblasts and stromal cells.59
Osteocytes are also ideally configured to perceive and orchestrate a modeling and/or remodeling response to mechanical signals. The fact that over 95% of the bone cells in the adult skeleton are osteocytes promotes consideration of their role in defining the mechanosensitivity of the skeleton.60 osteocytes maintain a dense network of connectivity with other osteocytes and bone-lining cells through cytoplasmic extensions that radiate outward from the central vascular canal within canaliculi. This canalicular matrix might allow chemical, electrical and stress-generated fluid communication through the dense bone matrix,61 and provide a means to ‘amplify’ even small signals by the very nature of its architecture.62
Burger and colleagues63 have shown that osteocytes are preferentially sensitive to shear strain over hydrostatic pressure, and that these mechanical signals can reduce the rate of osteocyte apoptosis, indicating the role of functional loading in the survival of these cells.64 However, it is also clear that too much strain will induce microdamage in the matrix and exacerbate the death of cells adjacent to the damaged matrix.65 This observation indicates the existence of a specific mechanical ‘window’ in which the strain signals would benefit the viability of the cell population, whereas too much, or too little, puts the cells in jeopardy.66
Although bone matrix strains two orders of magnitude below those generated by strenous activity are anabolic to the tissue,67 the means by which such low-magnitude mechanical strains cause bone formation is still not clear. The multitude of functionally induced forces generated in the bone marrow cavity where the MsC population resides includes strain, pressure, fluid flow, electric potentials and acceleration.68 The number of studies examining the therapeutic potential of MsCs has rapidly increased,69 but only in the past 5 years has the idea that these cells are mechanically active emerged.70 Indeed, in addition to the biochemical factors that might be capable of promoting stem-cell viability, mechanical signals are recognized to have key roles in defining the differentiation pathway and proliferative status of the stem-cell niche.71
Departing from a matrix-deformation-dependent pathway of mechanotransduction, the sensitivity of the adaptive system of bone to the frequency of the signal points towards a more fundamental pathway by which mechanical signals might be recognized by cells.72 The physical acceleration/deceleration of a cell during loading could represent a more generic signal that can transmit physical challenges by altering the relationship between the cytoskeleton, membrane, matrix and organ-elles.73 As such, MsCs, as well as other cells throughout an organism, might be able to respond to low-magnitude mechanical signals in the absence of matrix deformation through induced motion of intracellular content.48
Transducing mechanical signals
Identifying mechanoreceptors
Even the most strenuous activity will generate peaks of only 0.3% strain (3,000 microstrain) in loaded bones. Perfectly coupled to the matrix, a 10-μm-long cell subjected to such a strain would cause deformations in the order of Angstroms, necessitating an exquisitely sensitive system to sense these subtle physical challenges. Alternatively, the mechanoreceptor machinery senses by-products of load, such as pressure or fluid shear on the apical membrane. Although there are examples in sensory organs of channels that are regulated by movement of mechanosensory bristles, or by tension waves,74,75 a unified model of proximal events inducing intracellular signal transduction in nonsensory tissues such as bone does not yet exist.
Ion channels
Ion channel activity in osteoblasts stimulated by stretch and/or strain of the membrane or by parathyroid hormone has been associated with elevated bone cell activity76,77 Patch-clamp techniques have demonstrated the presence of at least three classes of mechanosensitive ion channels.78 In limb bone cultures, gadolinium chloride, which blocks some stretch and/or shear-sensitivecation channels, blocked load-related increases in the release of prostacyclin and nitric oxide.79
Integrins
Membrane deformation and shear across the membrane,80 as well as pressure transients, are transmitted to the cytoskeleton and ultimately to the cell-adhesion proteins that anchor the cell in place.81 Both membrane- spanning integrins, which couple the cell to the extracellular matrix, and a large number of adhesion-associated linker proteins, are potential molecular mechano-transducers. The architecture of the cyto skeleton, with its micro filamentous and microtubular networks that link adhesion receptors to the cell nucleus, might also be important in perceiving small deformations and directly transducing them to the nucleus.4
Lipid rafts
Cells possess a complex organizational structure that supports compartmentalization of signals within an equally complex plasma membrane that contains several phases of liquid-ordered and liquid-disordered lipid.82 The organized lipid rafts might sense mechanical signals. In endothelial cells, shear stress causes signaling molecules to translocate to caveolar lipid rafts and, if caveolae are disassembled, both proximal and downstream mechanical signals, including the mitogen-activated protein kinase pathway, are abrogated.83
Wnt-β-catenin signaling
Mechanical loading, whether through tissue or substrate deformation or through fluid flow shear stress, is known to affect osteoblast and osteocyte function.84 Although many of the responses of osteocytes are similar to those of their osteoblast progenitors, they are not entirely the same. An excellent example is the expression of the osteocyte-specific protein sclerostin, which binds to LDL-receptor-related protein 5/6 and antagonizes canonical Wnt signaling.85 Mechanical loading seems to reduce sclerostin levels in bone, suggesting that mechanical regulation of bone mass must induce osteocytes to secrete less of this inhibitory protein.86 In vitro experimentation with osteocyte-like cells also shows that pulsating fluid flow increases the expression of proteins that function in the canonical Wnt pathways.87 As such, osteocytes have an important role in regulating bone mass and morphometry, and must not be overlooked.
As mentioned earlier, physical signals can promote MsC differentiation towards an osteoblastic phenotype through canonical Wnt–β-catenin signaling. not surprisingly, then, activation and nuclear trans location of β-catenin occurs in MsCs within minutes of the mechanical stimulus.88 β-catenin activation requires that the strains somehow suppress the activity of glycogen synthase kinase-3β, allowing for an increase in the available β-catenin pool, thereby resulting in increased osteoblast differentiation and a greater commitment to the musculoskeletal system.89
The critical role of β-catenin in early selection of the osteoprogenitor lineage and the ability of mechanical signals to activate cellular β-catenin beg the question of whether mechanical signals might influence osteoblast differentiation at very early stages. Primary marrow stem cells subjected to daily bouts of mechanical strain express bone lineage markers at double the rate of those devoid of deformation.90 simultaneously influencing adipo-genesis, although a progressive decrease in both active and total β-catenin accompanies adipogenic differentiation of MsCs, mechanical signals serve to stem such decreases.91 Mechanical stimuli, via their effects on critical intra cellular pathways, have been shown to control differentiation of MsCs, resulting in profound effects on musculoskeletal and fat morphology.50,52,71,89
Clinical use of mechanical signals
Having outlined results indicating that bone is highly sensitive to mechanical signals, including low-magnitude mechanical signals, it is ultimately important to determine whether these signals can be used in the clinic to prevent or reduce the progression of osteoporosis. The costs of osteoporosis alone, a disease afflicting 50% of postmenopausal women, increasing the risk of bone fracture, are projected to exceed US$250 billion within the next 50 years.92
The notion that mechanical signals in general, and LMMs in particular, could serve as an anabolic agent in the clinic, and thus help to prevent osteopenia, was first tested in 64 postmenopausal women in a randomized, double-blind, placebo-controlled pilot study.93 Thirty-two women underwent mechanical loading for two 10-minute periods per day, through floor-mounted devices that produced a 0.2 g mechanical stimulus at 30 Hz; the control group (n = 32) received inactive placebo devices. evaluating those in the highest quartile of compliance (86% compliant), the placebo group lost 2.13% in femoral neck BMD over the year, whereas the active cohort was associated with a gain of 0.04%, reflecting a 2.17% relative benefit of treatment (P = 0.06). In this high-compliance quartile, the spine of lighter women (<65 kg) exhibited a relative benefit from LMMs of 3.35% greater BMD (P = 0.009) over the year; whereas the group that was above average in compliance realized a 2.73% benefit (P = 0.02).
In a parallel study involving children with disabling conditions such as cerebral palsy (4–19 years, 10 children per group), it was hypothesized that LMMs (5 days per week, 10 minutes per day, 90 Hz, 0.3 g) would be able to substitute for the reduced functional loading in this cohort, and restore bone quantity in the weight-bearing skeleton.94 After 6 months, the placebo group lost 11.9% of volumetric trabecular BMD in the proximal tibia, whereas the treatment group gained 6.3%, representing a 17.7% ‘benefit’ of treatment to BMD (P = 0.003).
In a third clinical trial, 48 young (16–21 years) women whose BMD was in the lowest quartile of that age group and who had already sustained a fracture were randomized to receive LMMs or serve as controls.95 Following a 1-year trial and using an intention-to-treat analysis, increases in cancellous and cortical bone were 2.0% (P = 0.06) and 2.3% (P = 0.04) greater, respectively, in the experimental group (n = 24) compared with controls (n = 24). Interestingly, the cross-sectional area of paraspinous musculature was 4.9% greater in the experimental group versus controls (P <0.01). Gains in both muscle and bone strongly correlated to a threshold in compliance, where the benefit of the intervention was realized once subjects used the device for at least 2 minutes per day, suggesting a biologic ‘trigger’ was activated, rather than a reparative response initiated by the need to repair damage. In this per-protocol analysis, women who used the device for at least 2 minutes per day (n = 18) showed a 2.9% increase in the cortical bone of the femur, 3.9% increase in the spine and 7.2% increase in the paraspinous musculature, compared with controls and poor compliers (n = 30; P <0.01 for all comparisons). Importantly, the morphologic responses measured in the clinic in both bone and muscle indicate the potential of mechanical signals in general to influence risk factors for osteoporosis beyond just bone mass.
Of course, LMMs is not the only means of mechanically challenging the skeleton. Although high-magnitude (>10 g) whole body vibration has been used extensively in gyms and sports medicine clinics to build muscle mass,96 these extreme signals, meant to mimic strenuous activity and work through tetanus of the musculature, have also been shown to slow bone loss that follows the menopause and osteopenia that accompanies bed rest.97,98 As with any other intervention, physical or chemical, extreme caution must be employed when using mechanical signals, to ensure that the benefits outweigh the possible consequences, particularly given the extensive research indicating that even brief exposure to high-magnitude vibration can promote circulatory disorders, low back pain, cartilage destruction, bone matrix failure, and even percussive injuries to the brain.99
Conclusions
The critical role of mechanical signals in the achievement and maintenance of bone quantity and quality is clear. What is surprising, perhaps, is that these signals need neither be large nor endured over long periods of time to have a significant benefit on skeletal health. not only are these mechanical factors essential for preserving an effective structure in the intact skeleton, but they also have the potential for accelerating bone repair following injury, such as in fracture healing or osseointegration.100,101
In contrast to systemic pharmaceutical interventions, the advantages of a mechanically delivered strategy are manifold: mechanical signals are native to bone tissue, safe at low intensities,102 incorporate all aspects of the remodeling cycle, and will ultimately induce production of lamellar bone.103 As mechanical signals influence tissues beyond the skeleton, including musculature, they might provide a more ‘systems level’ intervention for osteoporosis, fall risk, and the age-related decline of the musculoskeletal system. The widespread use of mechanical stimuli in the treatment of skeletal disorders will not be fully possible, however, until we achieve a better understanding of the physical and biologic mechanisms by which exquisitely small signals can markedly influence musculoskeletal physiology and phenotype.58
Mechanical signals persist as a normal outcome of loading. These mechanical signals are present in the cranial, axial and appendicular skeleton,104 and persist essentially at all times, including during passive actions such as standing and speaking.25 Indeed, the sarcopenia that parallels the aging process and, more specifically, the attenuation of the 20–50 Hz spectral content of muscle contraction, suggests that the absence of these signals could indicate the absence of a key regulatory stimulus to the bone tissue. Although osteopenia, to a certain extent, might arise through an age or disease-related diminished response of bone cells to mechanical stimuli, there might also be a decline in key regulatory signals caused by muscle wasting. Further complicating matters, deterioration of the regenerative pool of MsCs that occurs with aging and/or disuse will jeopardize the ability of the skeleton to protect itself. Ultimately, just as life began bathed in physical signals that helped define organismal function and morphology, perhaps physical signals can be used to mitigate the complications endured through life.
Key points.
Mechanical signals are anabolic to bone while their removal is permissive to osteoporosis
Mechanical signals need not be large to stimulate bone formation
Clinical studies suggest that low-magnitude mechanical signals can increase bone mineral density
Differentiation of mesenchymal stem cells towards osteoblastogenesis simultaneously suppresses adipogenesis
Mechanical signals can stem osteoporosis and augment and/or accelerate the healing of bone
Review criteria.
A PubMed search was carried out using the following words alone or in combination: “bone”, “bone marrow cells”, “mechanotransduction”, “mechanical loading”, “disuse”, “aging”, “tissue regeneration”, “mesenchymal stem cells”, “obesity”, “adiposity” and “treatment”. Articles published in English from January 1980 to June 2009 were used for the Review. Additional descriptive review articles were included, when appropriate, to provide scientific and/or clinical background.
Acknowledgments
This work was supported by National institutes of Health Grant AR 43498.
Footnotes
Competing interests: C. T. Rubin declares an association with the following company: Marodyne Medical. See the article online for full details of the relationship. The other authors declare no competing interests.
Contributor Information
Engin Ozcivici, Department of Biomedical Engineering, Stony Brook University, Stony Brook, New York, NY 11794-2580, USA.
Yen Kim Luu, Department of Biomedical Engineering, Stony Brook University, Stony Brook, New York, NY 11794-2580, USA.
Ben Adler, Department of Biomedical Engineering, Stony Brook University, Stony Brook, New York, NY 11794-2580, USA.
Yi-Xian Qin, Department of Biomedical Engineering, Stony Brook University, Stony Brook, New York, NY 11794-2580, USA.
Janet Rubin, Department of Medicine, University of North Carolina, Chapel Hill, NC 27599, USA.
Stefan Judex, Department of Biomedical Engineering, Stony Brook University, Stony Brook, New York, NY 11794-2580, USA.
Clinton T. Rubin, Department of Biomedical Engineering, Stony Brook University, Stony Brook, New York, NY 11794-2580, USA
References
- 1.Kruse K, Julicher F. Oscillations in cell biology. Curr Opin Cell Biol. 2005;17:20–26. doi: 10.1016/j.ceb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Zhou XL, et al. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc Natl Acad Sci USA. 2003;100:7105–7110. doi: 10.1073/pnas.1230540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neel PL, Harris RW. Motion-induced inhibition of elongation and induction of dormancy in liquidambar Science. 1971;173:58–59. doi: 10.1126/science.173.3991.58. [DOI] [PubMed] [Google Scholar]
- 4.Ingber DE. Mechanical control of tissue growth: function follows form. Proc Natl Acad Sci USA. 2005;102:11571–11572. doi: 10.1073/pnas.0505939102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 7.Lang T, et al. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 8.Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59:204–208. [PubMed] [Google Scholar]
- 9.Heinonen A, et al. Bone mineral density in female athletes representing sports with different loading characteristics of the skeleton. Bone. 1995;17:197–203. doi: 10.1016/8756-3282(95)00151-3. [DOI] [PubMed] [Google Scholar]
- 10.Leichter I, et al. Gain in mass density of bone following strenuous physical activity. J Orthop Res. 1989;7:86–90. doi: 10.1002/jor.1100070112. [DOI] [PubMed] [Google Scholar]
- 11.McKay HA, et al. “Bounce at the Bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br J Sports Med. 2005;39:521–526. doi: 10.1136/bjsm.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinonen A, Sievanen H, Kannus P, Oja P, Vuori I. Effects of unilateral strength training and detraining on bone mineral mass and estimated mechanical characteristics of the upper limb bones in young women. J Bone Miner Res. 1996;11:490–501. doi: 10.1002/jbmr.5650110410. [DOI] [PubMed] [Google Scholar]
- 13.Judex S, Garman R, Squire M, Donahue LR, Rubin C. Genetically based influences on the site-specific regulation of trabecular and cortical bone morphology. J Bone Miner Res. 2004;19:600–606. doi: 10.1359/JBMR.040101. [DOI] [PubMed] [Google Scholar]
- 14.Peacock M, et al. Sex-specific and non-sex-specific quantitative trait loci contribute to normal variation in bone mineral density in men. J Clin Endocrinol Metab. 2005;90:3060–3066. doi: 10.1210/jc.2004-2143. [DOI] [PubMed] [Google Scholar]
- 15.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Differences in osteocyte and lacunar density between Black and white American women. Bone. 2006;38:130–135. doi: 10.1016/j.bone.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Heaney RP, et al. Peak bone mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 17.Weaver CM. The role of nutrition on optimizing peak bone mass. Asia Pac J Clin Nutr. 2008;17(Suppl. 1):135–137. [PubMed] [Google Scholar]
- 18.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 19.Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–2432. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- 20.Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 22.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Knothe Tate ML, Knothe U. An ex vivo model to study transport processes and fluid flow in loaded bone. J Biomech. 2000;33:247–254. doi: 10.1016/s0021-9290(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 24.Pollack SR, Salzstein R, Pienkowski D. The electric double layer in bone and its influence on stress-generated potentials. Calcif Tissue Int. 1984;36(Suppl. 1):S77–S81. doi: 10.1007/BF02406138. [DOI] [PubMed] [Google Scholar]
- 25.Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self–similarity of low-magnitude strains. J Biomech. 2000;33:317–325. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 26.Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci. 1999;54:B352–B357. doi: 10.1093/gerona/54.8.b352. [DOI] [PubMed] [Google Scholar]
- 27.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 28.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66:397–402. [PubMed] [Google Scholar]
- 29.Lanyon LE, Goodship AE, Pye CJ, MacFie JH. Mechanically adaptive bone remodelling. J Biomech. 1982;15:141–154. doi: 10.1016/0021-9290(82)90246-9. [DOI] [PubMed] [Google Scholar]
- 30.O'Connor JA, Lanyon LE, MacFie H. The influence of strain rate on adaptive bone remodelling. J Biomech. 1982;15:767–781. doi: 10.1016/0021-9290(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 31.Bacabac RG, et al. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun. 2004;315:823–829. doi: 10.1016/j.bbrc.2004.01.138. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17:1613–1620. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connell-Rodwell CE. Keeping an “ear” to the ground: seismic communication in elephants. Physiology (Bethesda) 2007;22:287–294. doi: 10.1152/physiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 34.Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998;16:482–489. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- 35.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism: Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(Suppl. 5):S990–S991. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 37.Lee WS, Cheung WH, Qin L, Tang N, Leung KS. Age-associated decrease of type iiA/B human skeletal muscle fibers. Clin Orthop Relat Res. 2006;450:231–237. doi: 10.1097/01.blo.0000218757.97063.21. [DOI] [PubMed] [Google Scholar]
- 38.Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int. 1992;50:306–313. doi: 10.1007/BF00301627. [DOI] [PubMed] [Google Scholar]
- 39.Vico L, et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–1611. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 40.Burr DB, et al. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 41.Augat P, Simon U, Liedert A, Claes L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int. 2005;16(Suppl. 2):S36–S43. doi: 10.1007/s00198-004-1728-9. [DOI] [PubMed] [Google Scholar]
- 42.Rubin C, et al. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28:2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 43.Xie L, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol. 2008;104:1056–1062. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]
- 44.Rubin C, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 45.Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol. 1984;107:321–327. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- 46.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low- magnitude mechanical stimuli. FASEB J. 2001;15:2225–2229. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 47.Garman R, Rubin C, Judex S. Small oscillatory accelerations, independent of matrix deformations, increase osteoblast activity and enhance bone morphology. PLoS ONE. 2007;2:e653. doi: 10.1371/journal.pone.0000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bacabac RG, et al. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J. 2006;20:858–864. doi: 10.1096/fj.05-4966.com. [DOI] [PubMed] [Google Scholar]
- 49.Ozcivici E, Garman R, Judex S. High-frequency oscillatory motions enhance the simulated mechanical properties of non-weight bearing trabecular bone. J Biomech. 2007;40:3404–3411. doi: 10.1016/j.jbiomech.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Rubin CT, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luu YK, et al. In vivo quantification of subcutaneous and visceral adiposity by micro-computed tomography in a small animal model. Med Eng Phys. 2009;31:34–41. doi: 10.1016/j.medengphy.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luu YK, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akune T, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol. 2006;26:5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnan V, Bryant HU, MacDougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 57.Taes YE, et al. Fat mass is negatively associated with cortical bone size in young healthy male siblings. J Clin Endocrinol Metab. 2009;94:2325–2331. doi: 10.1210/jc.2008-2501. [DOI] [PubMed] [Google Scholar]
- 58.Rubin J, Murphy T, Nanes MS, Fan X. Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. Am J Physiol Cell Physiol. 2000;278:C1126–C1132. doi: 10.1152/ajpcell.2000.278.6.C1126. [DOI] [PubMed] [Google Scholar]
- 59.Kim CH, et al. Trabecular bone response to mechanical and parathyroid hormone stimulation: the role of mechanical microenvironment. J Bone Miner Res. 2003;18:2116–2125. doi: 10.1359/jbmr.2003.18.12.2116. [DOI] [PubMed] [Google Scholar]
- 60.Cowin SC, Weinbaum S. Strain amplification in the bone mechanosensory system. Am J Med Sci. 1998;316:184–188. doi: 10.1097/00000441-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Fritton SP, Cowin SC, Weinbaum S. Fluid pressure relaxation depends upon osteonal microstructure: modeling an oscillatory bending experiment. J Biomech. 1999;32:663–672. doi: 10.1016/s0021-9290(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 62.Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci USA. 2004;101:16689–16694. doi: 10.1073/pnas.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burger EH, Klein-Nulend J, Veldhuijzen JP. Modulation of osteogenesis in fetal bone rudiments by mechanical stress in vitro. J Biomech. 1991;24(Suppl. 1):101–109. doi: 10.1016/0021-9290(91)90381-v. [DOI] [PubMed] [Google Scholar]
- 64.Noble BS, et al. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284:C934–C943. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 65.Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15:60–67. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 66.Frost HM. Perspectives: bone's mechanical usage windows. Bone Miner. 1992;19:257–271. doi: 10.1016/0169-6009(92)90875-e. [DOI] [PubMed] [Google Scholar]
- 67.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Qin YX, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003;36:1427–1437. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 69.Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 70.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 71.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garman R, Gaudette G, Donahue LR, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25:732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 74.Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci STKE. 2004;2004:re4. doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- 75.Morris CE. Mechanosensitive ion channels. J Membr Biol. 1990;113:93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- 76.Duncan RL, Hruska KA, Misler S. Parathyroid hormone activation of stretch- activated cation channels in osteosarcoma cells (UMR-106.01) FEBS Lett. 1992;307:219–223. doi: 10.1016/0014-5793(92)80771-8. [DOI] [PubMed] [Google Scholar]
- 77.Ferrier J, Ward A, Kanehisa J, Heersche JN. Electrophysiological responses of osteoclasts to hormones. J Cell Physiol. 1986;128:23–26. doi: 10.1002/jcp.1041280105. [DOI] [PubMed] [Google Scholar]
- 78.Davidson RM, Tatakis DW, Auerbach AL. Multiple forms of mechanosensitive ion channels in osteoblast-like cells. Pflugers Arch. 1990;416:646–651. doi: 10.1007/BF00370609. [DOI] [PubMed] [Google Scholar]
- 79.Rawlinson SC, Pitsillides AA, Lanyon LE. involvement of different ion channels in osteoblasts' and osteocytes' early responses to mechanical strain. Bone. 1996;19:609–614. doi: 10.1016/s8756-3282(96)00260-8. [DOI] [PubMed] [Google Scholar]
- 80.McGarry JG, Klein-Nulend J, Prendergast PJ. The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun. 2005;330:341–348. doi: 10.1016/j.bbrc.2005.02.175. [DOI] [PubMed] [Google Scholar]
- 81.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 82.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 83.Rizzo V, Sung A, Oh P, Schnitzer JE. Rapid mechanotransduction in situ at the luminal cell surface of vascular endothelium and its caveolae. J Biol Chem. 1998;273:26323–26329. doi: 10.1074/jbc.273.41.26323. [DOI] [PubMed] [Google Scholar]
- 84.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 86.Robling AG, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 87.Santos A, Bakker AD, Zandieh-Doulabi B, Semeins CM, Klein-Nulend J. Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. J Orthop Res. 2009;27:1280–1287. doi: 10.1002/jor.20888. [DOI] [PubMed] [Google Scholar]
- 88.Armstrong VJ. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007;282:20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 89.Case N, et al. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.David V, et al. Mechanical loading down regulates PPAR gamma in bone marrow stromal cells and favours osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 91.Sen B, et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carmona R. Bone Health and Osteoporosis: A Report of the Surgeon General. US. Dept of Health and Human Services, Public Health Service; Oct 10, 2004. pp. 1–404. [Google Scholar]
- 93.Rubin C, et al. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 94.Ward K, et al. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 95.Gilsanz V, et al. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 96.Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31:3–7. doi: 10.1097/00003677-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 97.Verschueren SM, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19:352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 98.Armbrecht G, et al. Resistive vibration exercise attenuates bone and muscle atrophy in 56 days of bed rest: biochemical markers of bone metabolism. Osteoporos Int. doi: 10.1007/s00198-009-0985-z. [DOI] [PubMed] [Google Scholar]
- 99.Kiiski J, Heinonen A, Jarvinen TL, Kannus P, Sievanen H. Transmission of vertical whole body vibration to the human body. J Bone Miner Res. 2008;23:1318–1325. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 100.Goodship AE, Lawes TJ, Rubin CT. Low-magnitude high-frequency mechanical signals accelerate and augment endochondral bone repair: preliminary evidence of efficacy. J Orthop Res. 2009;27:922–930. doi: 10.1002/jor.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rubin CT, McLeod KJ. Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin Orthop. 1994;298:165–174. [PubMed] [Google Scholar]
- 102.Carter DR, Caler WE, Spengler DM, Frankel VH. Fatigue behavior of adult cortical bone: the influence of mean strain and strain range. Acta Orthop Scand. 1981;52:481–490. doi: 10.3109/17453678108992136. [DOI] [PubMed] [Google Scholar]
- 103.Rubin CT, Gross TS, McLeod KJ, Bain SD. Morphologic stages in lamellar bone formation stimulated by a potent mechanical stimulus. J Bone Miner Res. 1995;10:488–495. doi: 10.1002/jbmr.5650100321. [DOI] [PubMed] [Google Scholar]
- 104.Rubin CT, Lanyon LE. Limb mechanics as a function of speed and gait: a study of functional strains in the radius and tibia of horse and dog. J Exp Biol. 1982;101:187–211. doi: 10.1242/jeb.101.1.187. [DOI] [PubMed] [Google Scholar]