Abstract
Background
We developed and validated a heart failure (HF) risk score combining daily measurements of multiple device-derived parameters.
Methods
Heart failure patients from clinical studies with implantable devices were used to form two separate data sets. Daily HF scores were estimated by combining changes in intra-thoracic impedance, atrial fibrillation (AF) burden, rapid rate during AF, %CRT pacing, ventricular tachycardia, night heart rate, heart rate variability, and activity using a Bayesian model. Simulated monthly follow-ups consisted of looking back at the maximum daily HF risk score in the preceding 30 days, categorizing the evaluation as high, medium, or low risk, and evaluating the occurrence of HF hospitalizations in the next 30 days. We used an Anderson–Gill model to compare survival free from HF events in the next 30 days based on risk groups.
Results
The development data set consisted of 921 patients with 9790 patient-months of data and 91 months with HF hospitalizations. The validation data set consisted of 1310 patients with 10 655 patient-months of data and 163 months with HF hospitalizations. In the validation data set, 10% of monthly evaluations in 34% of the patients were in the high-risk group. Monthly diagnostic evaluations in the high-risk group were 10 times (adjusted HR: 10.0; 95% CI: 6.4–15.7, P < 0.001) more likely to have an HF hospitalization (event rate of 6.8%) in the next 30 days compared with monthly evaluations in the low-risk group (event rate of 0.6%).
Conclusion
An HF score based on implantable device diagnostics can identify increased risk for HF hospitalization in the next 30 days.
Keywords: Implantable device diagnostics, Heart failure, Ambulatory monitoring, Risk, Hospitalization
Background
Heart failure (HF) causes a significant economic burden, morbidity, and mortality.1 The primary cause of HF hospitalization (HFH) is volume overload which is treated using diuretic therapy.1 Further, ACE-inhibitors and β-blockers are known to reduce mortality in HF patients.1 Implantable medical devices, such as pacemakers, implantable cardioverter defibrillator (ICD), and cardiac resynchronization therapy defibrillator (CRT-D), can provide daily measurements of several ‘diagnostic’ parameters for possible evaluation of the HF status in patients. Earlier studies have shown that implantable device-measured ‘diagnostics’ such as intra-thoracic impedance (IMP),2 atrial fibrillation (AF) burden and rate control information,3 and night heart rate, heart rate variability, and patient activity4 can identify when patients are risk for HF events and could potentially be used unilaterally or in a combined fashion for informed patient management.5
In the past decade multiple studies have reported combining implantable device diagnostics to identify patients at risk of HF events and death.4–7 The objective of this study was to develop and validate a single-HF risk score derived by combining information from multiple device diagnostic parameters in a Bayesian Belief Network (BBN) framework to improve the ability to identify when patients are at risk for HFH. Multiple physiological processes interact in a complex manner during HF with a high degree of uncertainty in the severity of the manifestation of the disease that may or may not require hospitalization. The BBN approach8,9 allows for uncertain reasoning to estimate the probability of an HFH under a set of given diagnostic evidence. The BBN framework has been applied to other bio-medical applications.10
Methods
Data set and event definitions
The development set included data available from the OFISSER11 (n = 269), Italian ClinicalService Project12 (n = 174), and CONNECT13 (n = 478) studies. The validation set included data available from the PARTNERS-HF5 (n = 650), FAST14 (n = 134), PRECEDE-HF (n = 52), and SENSE-HF15 (n = 474) studies. Patient data were included in the data analysis cohorts if the patient had >90 days of device diagnostic data that includes intra-thoracic impedance monitoring. Details for each study and additional data inclusion criteria for this analysis are detailed in the Appendix. The studies were divided into development and validation data sets based on the chronological order in which data from the studies were made accessible for this investigation. The method for computing diagnostic information is the same in all the devices included for the data analysis. HFHs were used as the endpoint in the data analysis. Each cardiovascular hospitalization was carefully adjudicated for signs and symptoms of HF which included the administration of i.v. or oral diuretic during the hospitalization. Since a dynamic risk score for HFH was the focus of this study, death was not used as an endpoint in the data analysis.
Diagnostic parameters
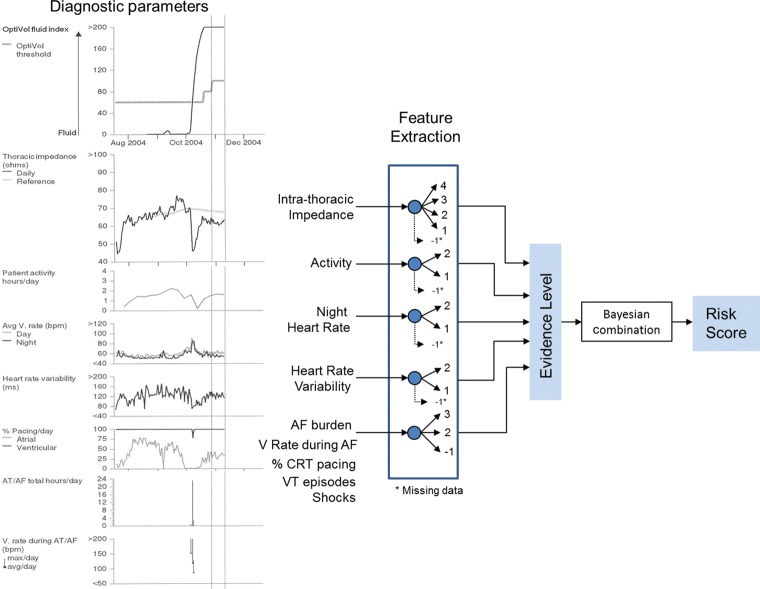
Implanted medical devices monitor several clinical diagnostic parameters that may include IMP, AF burden, ventricular rate during atrial fibrillation (VRAF), ventricular tachycardia (VT) episodes, patient activity (ACT), day and night heart rate (NHR), and heart rate variability (HRV) (Figure 1). These parameters are monitored continuously and the device stores sample data points for each parameter daily. IMP is a surrogate measure for blood volume or pulmonary capillary wedge pressure, with an increase in fluid volume leading to a reduction in IMP2. HRV is the standard deviation of 5 min median of atrial intervals during a 24 h period, with reducing HRV implying increases in sympathetic tone. NHR is the average heart rate between midnight and 4 am and is a measure for resting heart rate. ACT is the number of minutes in a 24 h period the patient is active and is a surrogate of functional capacity. AF burden is measured as total duration of fast atrial rate during a 24 h period, with atrio-ventricular conduction ratio ≥2:1. VRAF is the average ventricular rate during AF over a 24 h period. The device also records the % of CRT pacing delivered in a day, number of VT episodes and whether the patient received a defibrillation shock.
Figure 1.
The schematic for computation of the combined risk score using the different HF-related diagnostic variables in the Medtronic CRT-D system.
Combined diagnostics
Features were extracted from the diagnostics parameters to ascertain an evidence level for each diagnostic parameter on a daily basis (Appendix). A higher value of OptiVol fluid index implied a higher level of evidence for HF. Low or decreasing trend in ACT or HRV and high or increasing trend in NHR were considered as evidence for HF. If any two of the five arrhythmia/therapy related criteria were met it identified a higher evidence level for worsening HF. Absolute measurement thresholds used for the different diagnostic parameters were determined in earlier studies.3–5 The thresholds for the trend indexes which look for sustained increases or decreases in the measurements of NHR, ACT, and HRV were determined in the development set data.
A BBN framework8,9 was used to combine the evidence from each diagnostic parameter (Figure 1). On any day a certain set of diagnostic criteria is met which is categorized to different evidence levels as shown in Appendix. The evidence level for each diagnostic parameter is then used to generate the HF risk score for the day using a lookup table defined by the BBN model using data from the development set.
Statistical analysis
Monthly evaluations were simulated every 30 days, similar to the evaluation used in the PARTNERS-HF5 study, beginning on the 60th day from start of available diagnostic data. Each monthly evaluation included: (i) a retrospective look at maximum value of the diagnostic risk score in the last 30 days to ascertain the patient status into the diagnostic evaluation groups, and (ii) a prospective assessment for the first HFH in the next 30 days. A monthly evaluation was included only if there was >30 days of device data and clinical follow-up following the diagnostic evaluation, thus excluding deaths from the analysis. The risk score was categorized into three diagnostic evaluation groups: high, medium, and low. The first natural break after the top 10% of the risk score in the development set was chosen as the threshold for the high group. The rest of the risk scores were divided into two similar sized groups at a natural breakpoint with the HFH event rate <0.5% in the low group in the development set. The high and medium monthly diagnostic evaluation groups were compared with the low group for time to first HFH in the next 30 days using the Anderson–Gill model, an extension of the Cox proportional hazards model that accounts for multiple evaluations in patients. The model was adjusted for baseline variables (age, gender, NYHA, history of coronary artery disease, MI, AF, diabetes, and hypertension) and baseline medications (ACE-I/ARB, diuretics, β-blockers, and anti-arrhythmic drugs) in the validation data set.
A sensitivity and specificity analysis was performed for the combined diagnostic score using the same monthly evaluation scheme. Sensitivity (and specificity) is defined as the number of evaluations with score ≥ (or <) threshold and HFH (or no HFH) event in next 30 days divided by the total number of evaluations with HFH (without HFH) in next 30 days. The sensitivity and specificity computations are adjusted for multiple evaluations in patients using generalized estimating equation (GEE) with an exchangeable correlation structure. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
The development data set consisted of 921 patients with an average follow-up duration of 10.6 ± 5.8 months with 28 deaths and 68 patients (7.4%) with HFHs at a rate of 0.14 per patient year. A total of 9790 patient-months of data was analysed of which there were 91 months with HFHs providing an event rate of 0.9%. The validation data set consisted of 1310 patients with an average follow-up duration of 8.1 ± 5.0 months with 33 deaths and 110 patients (8.4%) with HFHs at a rate of 0.22 per patient year. A total of 10 655 patient-months of data was analysed of which there were 163 months with HFHs providing an event rate of 1.5%. The baseline characteristics of the patients in the study are shown in Table 1.
Table 1.
Baseline demographics of patients in the development and validation sets
| Development set (n = 921) | Validation set (n = 1310) | |
|---|---|---|
| Mean age (SD) | 68 (11) | 67 (11) |
| Male gender (%) | 69 | 74 |
| NYHA (%) | ||
| I | 2 | 4 |
| II | 19 | 22 |
| III | 76 | 70 |
| IV | 3 | 4 |
| Ischaemic (%) | 63 | 61 |
| Myocardial infarction (%) | 43 | 48 |
| Hypertension (%) | 70 | 62 |
| Diabetes (%) | 37 | 38 |
| History of AF (%) | 21 | 32 |
| LVEF <35% (%) | 96 | 92 |
| Device type (%) | ||
| ICD | 0 | 4 |
| CRT-D | 100 | 96 |
| Baseline medications (%) | ||
| ACE/ARB | 70 | 84 |
| Beta-blockers | 87 | 88 |
| Diuretics | 77 | 87 |
| Digoxin | 29 | 33 |
| Aldosterone antagonist | 26 | 22 |
| AAD | 18 | 22 |
| Anti-platelet or anticoagulant | 86 | 61 |
| Warfarin | 33 | 25 |
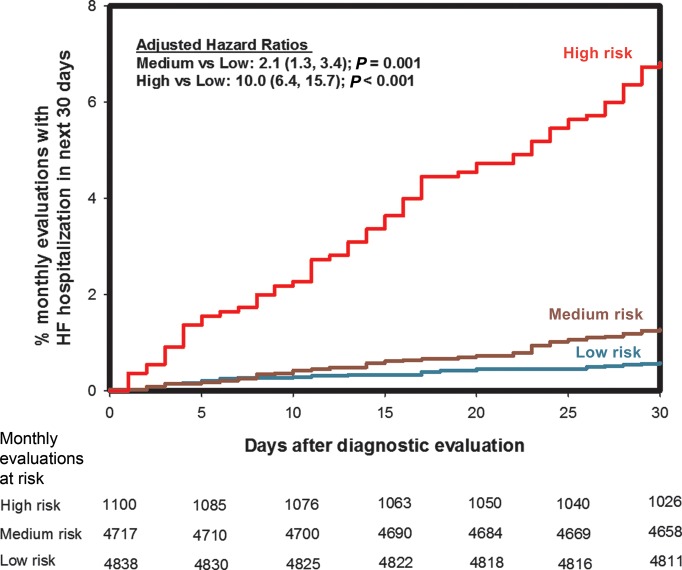
The event rates, expressed as a percentage of monthly evaluations that were followed by an HFH in the next 30 days, for the low, medium, and high evaluation groups in the development and the validation data sets are presented in Table 2. The hazard ratios for the comparison of the event rates in the medium and high groups with respect to the low group are also shown in Table 2. Figure 2 shows the Kaplan–Meier plot for time to first HFH in the 30 days following monthly diagnostic evaluation in the validation data set. In the validation data set, a total of 163 monthly evaluations (1.5%) were followed by an HFH in the next 30 days. Of the 1100 monthly evaluations when the risk score was in the high group, 75 (6.8%) were followed by an HFH in the next 30 days. The risk score was in the ‘high’ group in at least one monthly evaluation in 446 patients (34%). Monthly diagnostic evaluations with a risk score in the ‘high’ group were 10 times (HR: 10.0; 95% CI: 6.4–15.7, P < 0.001) more likely to have an HFH in the next 30 days compared with monthly evaluations with a risk score in the ‘low’ group. Results are similar if the model is adjusted for the presence of HFH in the last 30 days (HR: 8.2; 95% CI: 5.1–13.1, P < 0.001).
Table 2.
Comparison of event rates between different evaluation groups within the development and validation sets
| Data set | Evaluation groupsa | Evaluations (%) | Patients | HF hospitalizations (% of evaluations) | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Development (n = 921) | Low | 4525 (46) | 802 | 15 (0.3) | Reference | |
| Medium | 4018 (41) | 833 | 47 (1.2) | 3.7 (2.0, 6.7) | <0.001 | |
| High | 1247 (13) | 405 | 29 (2.3) | 6.2 (3.1, 12.3) | <0.001 | |
| Validation (n = 1310) | Low | 4838 (45) | 1085 | 28 (0.6) | Reference | |
| Medium | 4717 (44) | 1142 | 60 (1.3) | 2.1 (1.3, 3.4) | 0.001 | |
| High | 1100 (10) | 446 | 75 (6.8) | 10.0 (6.4, 15.7) | <0.001 |
aThe high group consisted of risk scores >20% and the low group consisted of risk scores ≤5%.
Figure 2.
Kaplan–Meier curves for time to first HF hospitalization after monthly diagnostic evaluation for the different risk score groups for the validation set.
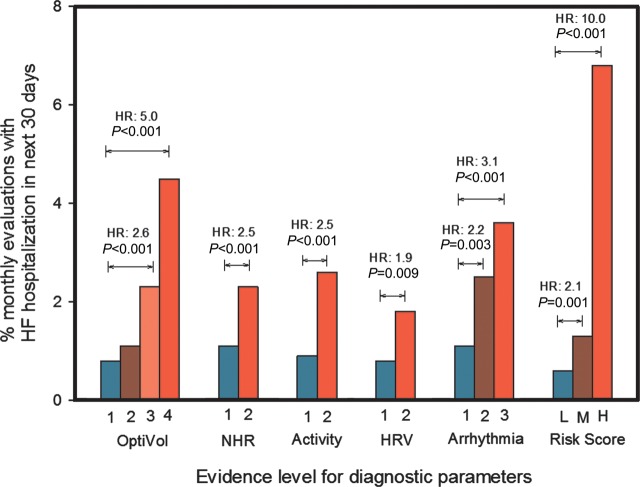
Figure 3 shows the event rates for individual diagnostic evidence levels described in Appendix and the combined risk score for the validation set. While each of the diagnostic element has the capability of stratifying patients at risk for HFHs, the combined risk score improves the ability to identify when patients are at a higher than normal risk and when patients are at lower than normal risk for HFHs.
Figure 3.
Event rates for different levels of evidence for each diagnostic parameter and the combined risk score.
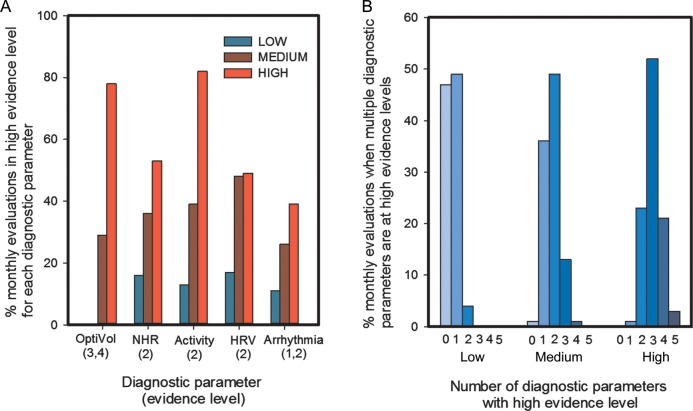
The number of times each of the individual diagnostic criteria was triggered as per cent of monthly evaluations with a risk score in the different risk score groups in the validation set is shown in Figure 4A. Each diagnostic parameter exceeded threshold significant proportion of times when the risk score is in the ‘high’ group with reduced patient activity (evidence level 2 in Appendix) and high OptiVol Fluid Index (evidence level 3 and 4 in Appendix) exceeding threshold most often. Figure 4B shows the number of diagnostic parameter that were triggered at the same time when the risk score was in the ‘low’, ‘medium’, and ‘high’ groups in the validation data set. The evidence level criteria for the trigger of the diagnostic parameters for Figure 4B were same as that used in Figure 4A. When the risk score was in the ‘low’ group, very often none of the diagnostic parameters triggered a high-evidence level, whereas three different diagnostic parameters triggered a high-evidence level most often when the risk score was in the ‘high’ group.
Figure 4.
(A) Per cent of monthly evaluations high-evidence level criteria was met for each diagnostic parameter in different risk score groups. (B) The distribution of number of diagnostic parameter that were triggered, based on evidence level criteria in (A), when the risk score was in the ‘low’, ‘medium’, and ‘high’ groups in the validation data set.
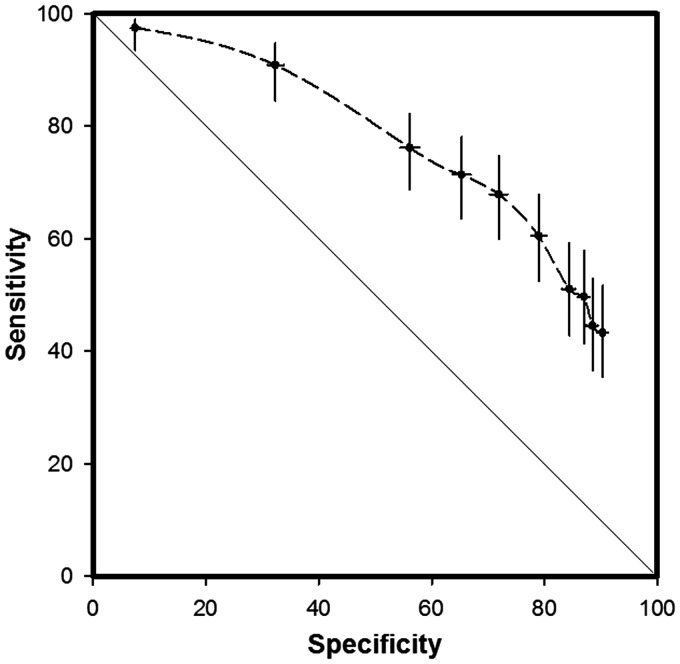
The 25-percentile, median, and 75-percentile of the risk score distribution in the validation set were 3.8, 5.5, and 11.5%, respectively. The receiver operating characteristic (ROC) curve, plotting the GEE estimates of sensitivity and specificity, for the validation set is shown in Figure 5. Evaluations done every 30 days in the monthly evaluation scheme contribute one data point for the sensitivity and specificity calculations. In the validation set, the threshold between the ‘low’ and ‘medium’ risk groups (score of 5%) had a sensitivity and specificity of 82.8 and 45.8%, respectively, and the threshold between the ‘medium-’ and ‘high’-risk groups (score of 20%) had a sensitivity of 46.0%, i.e. 46% of the months with HFHs were preceded by a ‘high’-risk score, and specificity of 90.2%, i.e. 10% of the months with no HFHs were also preceded by a ‘high’-risk score. At an HF risk score of 10% the sensitivity and specificity was 68.7 and 71.6%, respectively.
Figure 5.
Receiver operating characteristics curve plotting the sensitivity vs. specificity in a 30-day evaluation framework for the validation set.
When monthly evaluations were in ‘low’-risk group, <0.6% had HFHs in the next 30 days, i.e. a negative predictive value of 99.4% for the ‘low’-risk group. A high negative predictive value suggests that monthly evaluations with ‘low’ risk can be used to triage for patients who are at lower than average risk for HF requiring hospitalization. A very high-risk group, i.e. a group with high positive predictive value, can be formed for risk scores >40% that occurs in 2% of all monthly evaluations with 14.2% having HFHs in next 30 days. Similarly, another very high-risk group would be evaluations with a risk score in ‘high’ group for >14 of 30 days, which happens in 3% of the evaluations with 11.9% having HFHs in the next 30 days. The average number of days the risk was ‘high’ in the 30 days prior to months with HFH was 7 ± 11 days.
Discussion
The study presented the development and validation of a novel dynamic HF risk score derived from combining diagnostic parameters monitored in implantable devices. Patients who achieve a high-risk state on any day in the last 30 days are 10 times more likely to be hospitalized for HF in the next 30 days compared with patients who had a low risk on each of the last 30 days. Several HF risk scores have recently been developed and validated.16–19 Most of these risk scores identified a static risk at baseline or in an in-hospital setting, i.e. identify which patient is at risk for the development of HF or mortality. The dynamic HF risk score developed in this study, can identify when a high-risk patient is at higher risk of an HFH in an ambulatory setting, thus providing incremental information beyond what is provided by a static risk score. The dynamic HF risk score is time-varying and the same patient may be at high and low risk at different periods of time depending on the status of continuously monitored diagnostic parameters in the implanted device.
Management based on a dynamic risk score is similar in approach to recently reported intra-cardiac pressure20,21 or intra-thoracic impedance measurements.2,11,12,14 The key difference is incorporation of the multiple diagnostic parameters to form a combined diagnostic with the intention of improving the overall accuracy of the diagnostic. Long-term ambulatory monitoring using implantable devices featuring remote access and wireless alerting capabilities enables the dynamic assessment of the HF status, thus providing the opportunity to optimize treatment strategies for HF in a timely fashion. Like any diagnostic (weight, temperature, ECG, etc.), diagnostic information must be coupled with appropriate clinical actions in order to improve outcomes in HF patients.21 Whether therapeutic interventions based on the dynamic HF risk score is safe and effective in improving outcomes in HF patients need prospective evaluation. Several randomized controlled studies for management of HF patients based on diagnostic information have yielded inconsistent results.20–25
Each of the diagnostic parameters in implantable devices correspond to one or more of the basic HF assessment metrics such as fluid status, functional capacity, resting tachycardia, autonomic balance, arrhythmia, and non-adherence. Combining multiple parameters into a single-risk score makes it a simple to use triaging scheme indicating when a patient needs more attention in an ambulatory setting. A transition to high-risk state can proactively initiate collection of more clinical and symptomatic information over the telephone or in person to facilitate a diagnostic decision. Further, having information regarding which device parameters caused transition to high-risk state may lead to more clarity on treatment options. For example, a high-risk score caused by decrease in intra-thoracic impedance as well as new onset AF with poor rate control at the same time may indicate a very specific treatment plan: acute control of the fluid status, if necessitated by additional evidence, followed by improved chronic management of heart rate, once euvolaemia is achieved, to prevent future occurrences. A clinical action may not always need a medication adjustment; it can also be counselling for non-adherence or deciding to monitor the patient more often in the clinic.
The PARTNERS-HF5 study used a heuristic method for combining multiple diagnostic variables. The chosen diagnostic criteria were optimized for simplicity of a quick visual review of each diagnostic variable. The BBN approach also combines multiple diagnostic parameters, but it does so in a more rigorous decision-making framework that mimics clinical decision-making with elements of uncertain reasoning, causal relationships, and differential reasoning in the same framework. The BBN approach generates a single-risk score that can be used for initial triage without having to go through each diagnostic variable, thus improving the simplicity. The risk score is a continuous number allowing for the choice of flexible thresholds for obtaining optimal performance. Further, feature set definition for individual diagnostic variables can be improved without compromising the simplicity, e.g. relative change in activity, NHR, and HRV can be incorporated in addition to absolute thresholds. Finally, more diagnostic variables with orthogonal information, e.g. biomarkers, intra-cardiac pressures, can be easily added into the framework without needing data from all the variables in the same study to create a revised framework.
The absolute risk of an HFH in a 30-day period following a monthly evaluation with a high-risk state was only 6.8%. Thus, a high-risk state should not be used unilaterally to make treatment decisions as it may lead to over-reaction as it happened in the DOT-HF study.25 The absolute risk is low primarily because of the low rate of HFHs in this ambulatory monthly evaluation framework (overall event rate of 1.5%), the denominator being all monthly evaluations in all patients. When the event rate is higher, for example, in evaluating readmissions for HF,26 the absolute risk is also higher. The absolute risk for events with milder symptoms of HF not requiring hospitalization will be higher. The HF risk score categorizes the baseline risk of 1.5% into three groups, one with a higher risk (6.8%) and one with a lower risk (0.6%), with the middle group being similar to the overall risk of 1.5%. Although the absolute risk is low, the relative risk between the high- and low-risk groups is high. Thus, the risk score can be used as a tool to triage patients who may need more attention (e.g. more frequent follow-up). Randomized control studies of intensive follow-up-based HF management have yielded varying results;23,24 however, a risk score-based follow-up may improve the efficiency of disease management programmes by spending more resources on patients in a high-risk state and fewer resources on patients in a low-risk state.
Limitations
The retrospective analysis was done by pooling data from multiple studies in order to increase the sample size for the development and validation sets. With the exception of the FAST study, most of the data included in the study was from within the first year of the device life, thus the results may not reflect the performance of the risk score during the later years of the device life. Serial assessment of clinical diagnostic data related to HF, such as weight, blood-pressure, and BNP, was not performed in a consistent manner in all the studies. Thus, comparison of the dynamic risk score to previously described clinical risk scores could not be performed, and the adjustment for other clinical variables in the statistical analysis was limited to baseline history collected in the studies. The incremental value of an HF risk score over clinical diagnostic measurements cannot be established in this data set. It is hypothesized that the dynamic assessment of an HF risk score provides an ambulatory triage mechanism to indicate when to gather additional clinical information to evaluate the patient status in a timely manner to improve the efficacy of disease management programmes.
Conclusions
We developed and validated a method for combining multiple device-derived diagnostic parameters into a single-dynamic HF risk score which may be evaluated in an ambulatory setting to triage patients at a higher risk for HF events in the next 30 days. Future studies are needed to prospectively evaluate whether timely clinical actions initiated by the stratification of HF patients using the HF risk score on a regular basis can reduce HFHs.
Funding
M.C. salary is supported by the National Institute for Health Research Biomedical Research Unit at the Royal Brompton Hospital, London. Funding to pay the Open Access publication charges for this article was provided by Medtronic Inc.
Conflict of interest: Dr Cowie: research grants from Medtronic, and honoraria from Medtronic, St Jude Medical and Boston Scientific. Dr Sarkar and Ms Koehler are employees of Medtronic. Dr Whellan: research grants from Medtronic and consultant for Medtronic. Dr Crossley: consultant for Medtronic, Boston Scientific, and Cardiac Control systems; lecturing income from Medtronic, Boston Scientific and Sanofi; research support from Medtronic, Boston Scientific and St Jude Medical. Dr Tang: Consultant for Medtronic, received honoraria from Medtronic. Dr Abraham: consulting fees from Medtronic, St Jude Medical, Biotronik, CardioMEMS, and Novartis. Dr Sharma: employee of Medtronic. Dr Santini: consultant for Medtronic. V.S., S.S., and J.K. have paid from Medtronic, Inc., for stock/stock options. W.H.W.T.: consultant for St Jude Medical and employed for Clevland Clinic. W.H.W.T.: grants from National Institutes of Health and Abbott Laboratories. J.K.: patents (planned, pending, or issued) from Medtronic. G.C.: granted from Medtronic and received payment for lectures including service on speakers bureaus from Medtronic. M.C.: consultant for Medtronic and received payments for lectures including service on speakers bureaus from Medtronic.
Acknowledgements
The authors would like to thank all investigators in the studies included in this data analysis. The authors would also like to thank Douglas Hettrick and Eduardo Warman for their intellectual contributions in the development of the risk score.
Appendix
.
Details of studies included in data analysis
| Development data set | ||||
| Study | OFISSER | Italian ClinicalService | CONNECT | |
| Design | Observational | Observational | Randomized | |
| Centres | Multiple, USA | Multiple, Italy | Multiple, USA | |
| Main inclusion | CRT-D device for 6 months | CRT-D device | CRT or ICD device | |
| Main exclusion | None | None | Permanent AF chronic warfarin Life expectancy <15 months |
|
| Inclusion for analysis | First 269 patients enrolled in study | OptiVol alerts turned OFF | CRT-D device Control arm |
|
| Access to data | Yes | Yes | Yes | |
| Audible or remote care alerts | No | No | No | |
| Validation data set | ||||
| Study | PARTNERS-HF | FAST | PRECEDE-HF | SENSE-HF |
| Design | Observational | Observational | Randomized | Observational |
| Centres | Multiple, USA | Multiple, USA | Multiple, USA | Multiple, Europe, Asia |
| Main inclusion | CRT-D device | CRT-D device or ICD device with EF <35% and NYHA class III or IV |
CRT-D or ICD device with HF event in last 12 months | CRT-D or ICD device with HF event in last 12 months |
| Main exclusion | Permanent AF CAI Heart transplant Renal disease |
Heart transplant Severe chronic obstructive pulmonary disease PAH Life expectancy <6 months |
Heart transplant CAI or MI Renal insufficiency |
Heart transplant Severe chronic obstructive pulmonary disease PAH Renal insufficiency |
| Inclusion for analysis | OptiVol diagnostics | None | Control arm | First phase data (first 6 months) |
| Access to data | Yes | No | No | No |
| Audible or remote care alerts | No | No | No | No |
PAH, pulmonary arterial hypertension; CAI, coronary artery intervention; MI, myocardial infarction.
.
Criteria for individual diagnostic parameters used to categorize the parameters into different evidence levels
| Diagnostic parameter | Diagnostic criteria | Evidence level |
| OptiVol | Fluid index ≥100 | 4 |
| 60≤ Fluid index <100 | 3 | |
| 30≤ Fluid index <60 | 2 | |
| 0≤ Fluid index <30 | 1 | |
| Data not available | −1 | |
| Night heart rate (NHR) | AvgNHR ≥85 b.p.m. OR AvgNHR ≤55 b.p.m. | 2 |
| NHRtrendIndex*≥NHR trend threshold | 2 | |
| If condition for evidence level 2 not met | 1 | |
| Data not available | −1 | |
| Patient activity (ACT) | AvgACT ≤60 min | 2 |
| ACTtrendIndex*≥ACT trend threshold | 2 | |
| If condition for evidence level 2 not met | 1 | |
| Data not available | −1 | |
| Heart rate variability (HRV) | AvgHRV ≤60 ms | 2 |
| HRV trend index*≥HRV trend threshold | 2 | |
| If condition for evidence level 2 not met | 1 | |
| Data not available | −1 | |
| Arrhythmia/pacing combination | VTepisodes ≥5 OR Shock = ‘True’ OR AF burden ≥1 h/day OR MeanVRAF ≥90 b.p.m. AND AF ≥6 h/day OR %Ventricular pacing ≤90% AND CRT device |
1 |
| Two or more of the above 5 arrhythmia conditions met | 2 | |
| No condition met OR data not available | −1 |
*Trend index is computed as a running cumulative sum of the difference between short- and long-term averages over a 14-day period. Trend Index is compared with thresholds which are related to the value of the long-term average.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. doi:10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 2.Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–848. doi: 10.1161/CIRCULATIONAHA.104.492207. doi:10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar S, Koehler J, Crossley GH, Tang WHW, Abraham WT, Whellan DJ. Burden of atrial fibrillation and poor rate control detected by continuous monitoring via implanted devices identifies when a patient is at risk for heart failure hospitalization. Am Heart J. 2012;164:616–624. doi: 10.1016/j.ahj.2012.06.020. doi:10.1016/j.ahj.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Adamson PB, Smith AL, Abraham WT, Kleckner KJ, Stadler RW, Shih A, Rhodes MM. InSync III Model 8042 and Attain OTW Lead Model 4193 Clinical Trial Investigators. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389–2394. doi: 10.1161/01.CIR.0000139841.42454.78. doi:10.1161/01.CIR.0000139841.42454.78. [DOI] [PubMed] [Google Scholar]
- 5.Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB, Pavri BB, O'Connor CM. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol. 2010;55:1803–1810. doi: 10.1016/j.jacc.2009.11.089. doi:10.1016/j.jacc.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 6.Page E, Cazeau S, Ritter P, Galley D, Casset C. Physiological approach to monitor patients in congestive heart failure: application of a new implantable device-based system to monitor daily life activity and ventilation. Europace. 2007;9:687–693. doi: 10.1093/europace/eum066. doi:10.1093/europace/eum066. [DOI] [PubMed] [Google Scholar]
- 7.Singh JP, Rosenthal LS, Hranitzky PM, Berg KC, Mullin CM, Thackeray L, Kaplan A. Device diagnostics and long-term clinical outcome in patients receiving cardiac resynchronization therapy. Europace. 2009;11:1647–1653. doi: 10.1093/europace/eup250. doi:10.1093/europace/eup250. [DOI] [PubMed] [Google Scholar]
- 8.Pearl J. Probabilistic Reasoning in Intelligent Systems. San Mateo, CA: Morgan Kaufman; 1988. [Google Scholar]
- 9.Jensen FV, Nielsen TD. Bayesian Networks and Decision Graphs. 2nd ed. New York: Springer; 2007. [Google Scholar]
- 10.Lucas PJ, van der Gaag LC, Abu-Hanna A. Bayesian Networks in biomedicine and health-care. Artif Intell Med. 2004;30:201–214. doi: 10.1016/j.artmed.2003.11.001. doi:10.1016/j.artmed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Small RS, Wickemeyer W, Germany R, Hoppe B, Andrulli J, Brady PA, Labeau M, Koehler J, Sarkar S, Hettrick DA, Tang WH. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: clinical utility of implanted device monitoring without a patient alert. J Card Fail. 2009;15:475–481. doi: 10.1016/j.cardfail.2009.01.012. doi:10.1016/j.cardfail.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Perego GB, Landolina M, Vergara G, Lunati M, Zanotto G, Pappone A, Lonardi G, Speca G, Iacopino S, Varbaro A, Sarkar S, Hettrick DA, Denaro A. Implantable CRT device diagnostics identify patients with increased risk for heart failure hospitalization. J Interv Card Electrophysiol. 2008;23:235–242. doi: 10.1007/s10840-008-9303-5. doi:10.1007/s10840-008-9303-5. [DOI] [PubMed] [Google Scholar]
- 13.Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–1189. doi: 10.1016/j.jacc.2010.12.012. doi:10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Abraham WT, Compton S, Haas G, Foreman B, Canby RC, Fishel R, McRae S, Toledo GB, Sarkar S, Hettrick DA. Superior performance of intrathoracic impedance-derived fluid index versus daily weight monitoring in heart failure patients: results of the Fluid Accumulation Status Trial (FAST) congest. Heart Fail. 2011;17:51–55. doi: 10.1111/j.1751-7133.2011.00220.x. [DOI] [PubMed] [Google Scholar]
- 15.Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, Cowie MR. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J. 2011;32:2266–2273. doi: 10.1093/eurheartj/ehr050. doi:10.1093/eurheartj/ehr050. [DOI] [PubMed] [Google Scholar]
- 16.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. doi:10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. doi:10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 18.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The seattle heart failure model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. doi:10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 19.Peterson PN, Rumsfeld JS, Liang L, Albert NM, Hernandez AF, Peterson ED, Fonarow GC, Masoudi FA. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3:25–32. doi: 10.1161/CIRCOUTCOMES.109.854877. doi:10.1161/CIRCOUTCOMES.109.854877. [DOI] [PubMed] [Google Scholar]
- 20.Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM, Jr, Magalski A, Zile MR, Smith AL, Smart FW, O'Shaughnessy MA, Jessup ML, Sparks B, Naftel DL, Stevenson LW. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073–1079. doi: 10.1016/j.jacc.2007.10.061. doi:10.1016/j.jacc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. doi:10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 22.Kimmelstiel C, Levine D, Perry K, Patel AR, Sadaniantz A, Gorham N, Cunnie M, Duggan L, Cotter L, Shea-Albright P, Poppas A, LaBresh K, Forman D, Brill D, Rand W, Gregory D, Udelson JE, Lorell B, Konstam V, Furlong K, Konstam MA. Randomized, controlled evaluation of short- and long-term benefits of heart failure disease management within a diverse provider network: the SPAN-CHF trial. Circulation. 2004;110:1450–1455. doi: 10.1161/01.CIR.0000141562.22216.00. doi:10.1161/01.CIR.0000141562.22216.00. [DOI] [PubMed] [Google Scholar]
- 23.Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45:1654–1664. doi: 10.1016/j.jacc.2005.01.050. doi:10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Jaarsma T, van der Wal MH, Lesman-Leegte I, Luttik ML, Hogenhuis J, Veeger NJ, Sanderman R, Hoes AW, van Gilst WH, Lok DJ, Dunselman PH, Tijssen JG, Hillege HL, van Veldhuisen DJ. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Arch Intern Med. 2008;168:316–324. doi: 10.1001/archinternmed.2007.83. doi:10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 25.van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Aguilera RM, Lunati M, Yu CM, Gerritse B, Borggrefe M. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–1726. doi: 10.1161/CIRCULATIONAHA.111.043042. doi:10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 26.Whellan DJ, Sarkar S, Koehler J, Small RS, Boyle A, Warman EN, Abraham WT. Development of a method to risk stratify patients with heart failure for 30-day readmission using implantable device diagnostics. Am J Cardiol. 2013;111:79–84. doi: 10.1016/j.amjcard.2012.08.050. doi:10.1016/j.amjcard.2012.08.050. [DOI] [PubMed] [Google Scholar]