Abstract
Hyperglycemia and insulin resistance are key players in the development of atherosclerosis and its complications. A large body of evidence suggest that metabolic abnormalities cause overproduction of reactive oxygen species (ROS). In turn, ROS, via endothelial dysfunction and inflammation, play a major role in precipitating diabetic vascular disease. A better understanding of ROS-generating pathways may provide the basis to develop novel therapeutic strategies against vascular complications in this setting. Part I of this review will focus on the most current advances in the pathophysiological mechanisms of vascular disease: (i) emerging role of endothelium in obesity-induced insulin resistance; (ii) hyperglycemia-dependent microRNAs deregulation and impairment of vascular repair capacities; (iii) alterations of coagulation, platelet reactivity, and microparticle release; (iv) epigenetic-driven transcription of ROS-generating and proinflammatory genes. Taken together these novel insights point to the development of mechanism-based therapeutic strategies as a promising option to prevent cardiovascular complications in diabetes.
Keywords: Diabetes, Vascular disease, Pathophysiology
Introduction
The number of people with diabetes mellitus is alarmingly increasing due to the growing prevalence of obesity, genetic susceptibility, urbanization, and ageing.1,2
Type 2 diabetes, the most common form of the disease, may remain undetected for many years and its diagnosis is often made incidentally through an abnormal blood or urine glucose test. Hence, physicians often face this disease at an advanced stage when vascular complications have already occurred in most of patients. Macrovascular complications are mainly represented by atherosclerotic disease and its sequelae. Diabetes-related microvascular disease such as retinopathy and nephropathy are major causes of blindness and renal insufficiency.1
Based on this scenario, a better understanding of the mechanisms underlying diabetic vascular disease is mandatory because it may provide novel approaches to prevent or delay the development of its complications. This review will focus on the most current advances in the pathophysiology of vascular disease (Part I) and will address clinical manifestations and management strategies of patients with diabetes (Part II).
Hyperglycemia, oxidative stress, and vascular disease
The alterations in vascular homeostasis due to endothelial and smooth muscle cell dysfunction are the main features of diabetic vasculopathy favouring a pro-inflammatory/thrombotic state which ultimately leads to atherothrombosis. Macro- and microvascular diabetic complications are mainly due to prolonged exposure to hyperglycemia clustering with other risk factors such as arterial hypertension, dyslipidemia as well as genetic susceptibility.3 Interestingly, nephropathy, retinopathy, and diabetic vascular disease are in line with the notion that endothelial, mesangial, and retinal cells are all equipped to handle high sugar levels when compared with other cell types.4 The detrimental effects of glucose already occur with glycemic levels below the threshold for the diagnosis of diabetes. This is explained by the concept of ‘glycemic continuum’ across the spectrum of prediabetes, diabetes, and cardiovascular risk.5–8 Early disglycemia caused by obesity-related insulin resistance or impaired insulin secretion is responsible for functional and structural alterations of the vessel wall culminating with diabetic vascular complications.
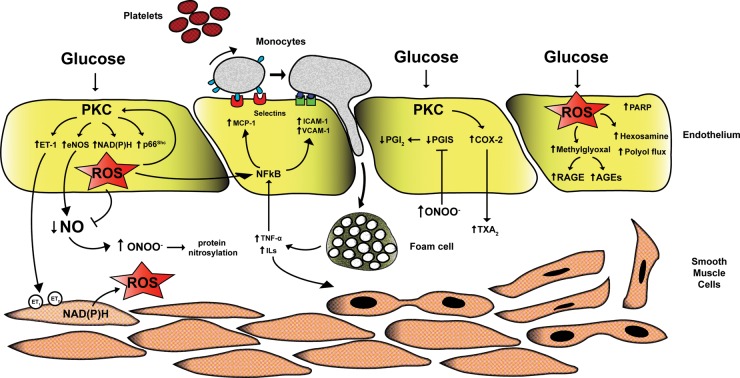
The initial trigger whereby high glucose concentrations alter vascular function is the imbalance between nitric oxide (NO) bioavailability and accumulation of reactive oxygen species (ROS), leading to endothelial dysfunction.9 Indeed, hyperglycemia-induced generation of superoxide anion (O2−) inactivates NO to form peroxynitrite (ONOO−), a powerful oxidant which easily penetrates across phospholipid membranes and induces substrate nitration.9 Protein nitrosylation blunts activity of antioxidant enzymes and endothelial NO synthase10 (eNOS, Figure 1). Importantly, reduced NO bioavailability is a strong predictor of cardiovascular outcomes.10,11
Figure 1.
Mechanisms of hyperglycemia-induced vascular damage. High intracellular glucose concentrations lead to PKC activation and subsequent ROS production by NADPH oxidase and p66Shc adaptor protein. Increased oxidative stress rapidly inactivates NO leading to formation of the pro-oxidant ONOO− responsible for protein nitrosylation. Reduced NO availability is also due to PKC-dependent eNOS deregulation. Indeed, PKC triggers enzyme up-regulation thus enhancing eNOS uncoupling and leading to a further accumulation of free radicals. On the other hand, hyperglycemia reduces eNOS activity blunting activatory phosphorylation at Ser1177. Together with the lack of NO, glucose-induced PKC activation causes increased synthesis of ET-1 favouring vasoconstriction and platelet aggregation. Accumulation of superoxide anion also triggers up-regulation of pro-inflammatory genes MCP-1, VCAM-1, and ICAM-1 via activation of NF-kB signalling. These events lead to monocyte adhesion, rolling, and diapedesis with formation of foam cells in the sub-endothelial layer. Foam cell-derived inflammatory cytockines maintain vascular inflammation as well as proliferation of smooth muscle cells, accelerating the atherosclerotic process. Endothelial dysfunction in diabetes also derives from increased synthesis of TXA2 via up-regulation of COX-2 and inactivation of PGIS by increased nitrosylation. Furthermore, ROS increase the synthesis of glucose metabolite methylglyoxal leading to activation of AGE/RAGE signalling and the pro-oxidant hexosamine and polyol pathway flux. PKC, protein kinase C; eNOS, endothelial nitric oxide synthase; ET1, endothelin 1; ROS, reactive oxygen species; NO, nitric oxide; MCP-1, monocyte chemoattractant protein-1; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intracellular cell adhesion molecule-1; AGE, advanced glycation end product.
Overproduction of ROS by mitochondria is considered as a causal link between elevated glucose and the major biochemical pathways involved in the development of vascular complications of diabetes.12 Indeed, hyperglycemia-induced ROS production triggers several cellular mechanisms including polyol and hexosamine flux, advanced glycation end products (AGEs), protein kinase C (PKC) activation, and NF-kB-mediated vascular inflammation.12,13 One of the main sources of ROS in the setting of hyperglycemia is represented by PKC and its downstream targets. The hyperglycemic environment induces a chronic elevation of diacyglycerol levels in endothelial cells with subsequent membrane translocation of conventional (α, β1, β2) and non-conventional (δ) PKC isoforms. Once activated, PKC is responsible for different structural and functional changes in the vasculature including alterations in cellular permeability, inflammation, angiogenesis, cell growth, extracellular matrix expansion, and apoptosis.14 An important consequence of PKC activation is ROS generation. In vascular endothelial cells, hyperglycemia-induced activation of PKC increases superoxide production via NADPH oxidase15 (Figure 1). Indeed, treatment with a PKCβ inhibitor suppresses NADPH-dependent ROS generation.16
More recently, it has been reported that glucose-induced activation of PKC β2 isoform phosphorylates p66Shc at serine 36 leading to its translocation to the mitochondria, cytochrome c oxidation and accumulation of ROS into the organelle.17,18 The p66Shc adaptor protein functions as a redox enzyme implicated in mitochondrial ROS generation and translation of oxidative signals into apoptosis.17 Interestingly, diabetic p66Shc−/− mice are protected against hyperglycemia-induced endothelial dysfunction and oxidative stress.19 The relevance of p66Shc in the clinical setting of diabetes is supported by the notion that p66Shc gene expression is increased in peripheral blood mononuclear cells obtained from patients with type 2 diabetes and correlates with plasma 8-isoprostane levels, an in vivo marker of oxidative stress.20 Moreover, p66Shc protein has recently emerged as an upstream modulator of NADPH activation further strengthening its pivotal role in ROS generation.21,22
PKC affects NO availability not only via intracellular accumulation of ROS but also by decreasing eNOS activity.23–25 PKC also leads to increased production of endothelin-1 (ET-1) favouring vasoconstriction and platelet aggregation14 (Figure 1). The role of ET-1 in the pathophysiology of diabetic complications is confirmed by the observation that the activity of endogenous ET-1 on ET(A) receptors is enhanced in the resistance vessels of patients with diabetes.26
In the vessel wall, PKC-dependent ROS production also participates in the atherosclerotic process by triggering vascular inflammation.13,27 Indeed, ROS lead to up-regulation and nuclear translocation of NF-kB subunit p65 and, hence, transcription of pro-inflammatory genes encoding for monocyte chemoattractant protein-1 (MCP-1), selectins, vascular cell adhesion molecule-1 (VCAM-1), and intracellular cell adhesion molecule-1 (ICAM-1). This latter event facilitates adhesion of monocytes to the vascular endothelium, rolling, and diapedesis in the sub-endothelium with subsequent formation of foam cells (Figure 1). Secretion of IL-1 and TNF-α from active macrophages maintains up-regulation of adhesion molecules by enhancing NF-kB signalling in the endothelium and also promotes smooth muscle cells growth and proliferation10 (Figure 1). Consistently, inhibition of PKC β2 isoform blunts VCAM-1 up-regulation in human endothelial cells upon glucose exposure.27
Endothelial dysfunction in diabetes is not only the result of impaired NO availability but also of increased synthesis of vasocontrictors and prostanoids.10 PKC-mediated cyclooxygenase-2 (COX-2) up-regulation is associated with an increase of thromboxane A2 and a reduction of prostacyclin (PGI2) release28 (Figure 1). These findings suggest that PKC is the upstream signalling molecule affecting vascular homeostasis in the setting of hyperglycemia28 (Figure 1). Mitochondrial ROS also increase intracellular levels of the glucose metabolite methylglyoxal and AGEs synthesis.12,29,30 In experimental diabetes, methylglyoxal is a key player in the pathophysiology of diabetic complications through oxidative stress, AGEs accumulation, and endothelial dysfunction.29,31 Generation of AGEs leads to cellular dysfunction by eliciting activation of the AGEs receptor (RAGE).30,32 AGE-RAGE signalling in turn activates ROS-sensitive biochemical pathways such as the hexosamine flux.13 In the hyperglycemic environment, an increased flux of fructose-6-phosphate activates a cascade of events resulting in different glycosilation patterns which are responsible for deregulation of enzymes involved in vascular homeostasis. Specifically, O-GlcNAcylation at the Akt site of eNOS protein leads to reduced eNOS activity and endothelial dysfunction.13,33 Moreover, glycosylation of transcription factors causes up-regulation of inflammatory (TGFα, TGFβ1) and pro-thrombotic genes (plasminogen activator inhibitor-1).33,34 Glucose induced-ROS production also activates the polyol pathway flux involved in vascular redox stress.12,35 Accordingly, hyperactivation of this pathway has been associated with increased atherosclerotic lesions in diabetic mice.36
Insulin resistance and atherothrombosis
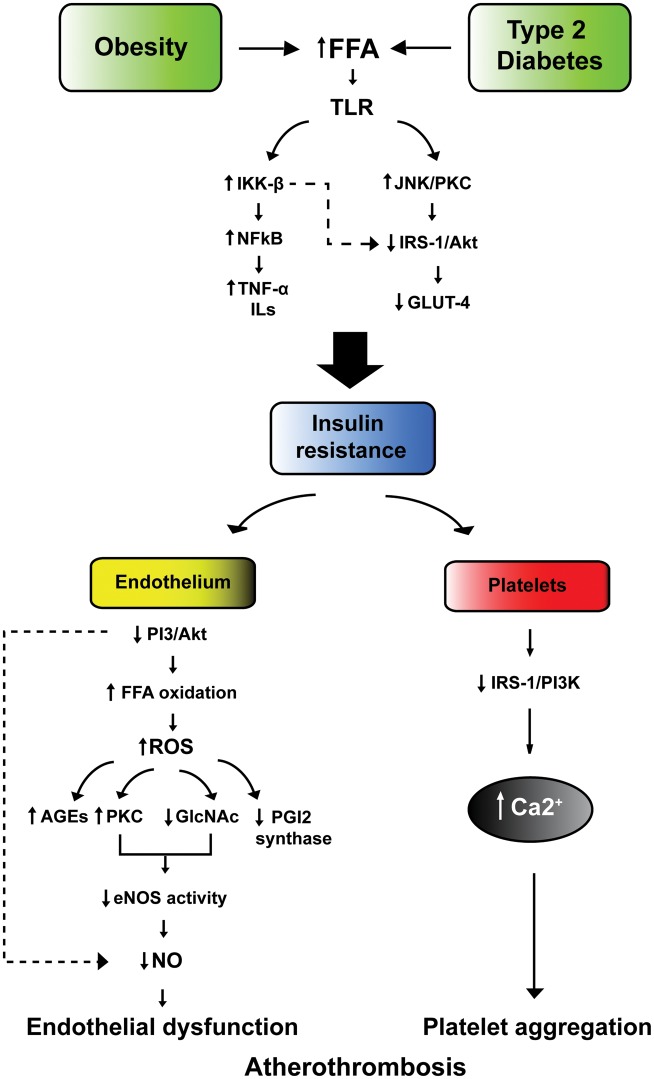
Insulin resistance is a major feature of type 2 diabetes and develops in multiple organs, including skeletal muscle, liver, adipose tissue, and heart.37 The onset of hyperglycemia and diabetes is often preceded by many years of insulin resistance. Obesity plays a pivotal role in this phenomenon providing an important link between type 2 diabetes and fat accumulation.38 Indeed, a substantial proportion of diabetic patients are obese.39 Obesity is a complex disorder leading to alterations in lipid metabolism, deregulation of hormonal axes, oxidative stress, systemic inflammation, and ectopic fat distribution. Adipose tissue is an active source of inflammatory mediators and free fatty acids (FFAs).40 Accordingly, obese patients with type 2 diabetes display increased plasma levels of inflammatory markers.41 Free fatty acids bind Toll-like receptor (TLR) activating NF-kB through degradation of the inhibitory complex IkBα by IKKβ-kinase.42 As a result, NF-kB triggers tissue inflammation due to up-regulation of inflammatory genes IL-6 and TNF-α.
Toll-like receptor activation by FFA leads to phosphorylation of insulin receptor substrate-1 (IRS-1) by c-Jun amino-terminal kinase (JNK) and PKC, thereby altering its ability to activate downstream targets PI3-kinase and Akt. These molecular events result in the down-regulation of the glucose transporter GLUT-4 and, hence, insulin resistance43 (Figure 2). Insulin resistance is critically involved in vascular dysfunction in subjects with type 2 diabetes.42 Indeed, down-regulation of PI3-kinase/Akt pathway leads to eNOS inhibition and decreased NO production.44 Together with reduced NO synthesis, intracellular oxidation of stored FFA generates ROS leading to vascular inflammation, AGEs synthesis, reduced PGI2 synthase activity, and PKC activation13,44 (Figure 2).
Figure 2.
Insulin resistance as trigger of atherothrombosis. In subjects with obesity or type 2 diabetes the increase in FFA activates TLR leading NF-kB nuclear translocation and subsequent up-regulation of inflammatory genes IL-6 and TNF-α. On the other hand, JNK and protein kinase C phosphorylate insulin receptor substrate-1 (IRS-1), thus blunting its downstream targets PI3-kinase and Akt. This results in down-regulation of glucose transporter GLUT-4 and, hence, insulin resistance. Impaired insulin sensitivity in the vascular endothelium leads to increased FFA oxidation, ROS formation, and subsequent activation of detrimental biochemical pathways such as AGE synthesis, PKC activation, protein glicosylation as well as down-regulation of PGI2. These events blunt eNOS activity thereby leading to endothelial dysfunction. Lack of insulin signalling in platelets impairs the IRS1/PI3K pathway resulting in Ca2+ accumulation and increased platelet aggregation. FFA, free fatty acids; TLR, toll-like receptor; JNK, c-Jun amino-terminal kinase; IRS-1, Insulin receptor substrate-1; NO, nitric oxide; eNOS, endothelial nitric oxide shyntase; IL-6, interleukin-6; TNF-α, tumor necrosis factor.
Increased ROS levels associated with insulin resistance scavenge NO production and produce peroxynitrite, with a further reduction of NO bioavailability. Reduced cellular levels of NO facilitate pro-inflammatory pathways triggered by increased cytokine production. Indeed, TNF-α and IL-1 increase NF-κB activity and expression of adhesion molecules. TNF-α also stimulates the expression of C-reactive protein which down-regulates eNOS and increases the production of adhesion molecules and endothelin-1.26,42 A recent study clearly demonstrated that loss of insulin signalling in the vascular endothelium leads to endothelial dysfunction, expression of adhesion molecules, and atherosclerotic lesions in mice.45
Although insulin resistance development has been attributed to adipocyte-derived inflammation, recent evidence is overturning the adipocentric paradigm.43 Indeed, inflammation and macrophage activation seem to primarily occur in non-adipose tissue in obesity.46,47 This concept is supported by the notion that suppression of inflammation in the vasculature prevents insulin resistance in other organs and prolongs lifespan.48 Consistently, transgenic mice with endothelium-specific overexpression of the inhibitory NF-kB subunit IkBα were protected from the development of insulin resistance. In these mice, obesity-induced macrophage infiltration of adipose tissue and plasma oxidative stress markers were reduced whereas blood flow, muscle mitochondrial content, and locomotor activity were increased, confirming the pivotal role of the transcription factor NFkB in oxidative stress, vascular dysfunction, and inflammation.48 Another study confirmed these findings, showing that genetic disruption of the insulin receptor substrate 2 (IRS-2) in endothelial cells reduces glucose uptake by skeletal muscle.49 These novel findings strengthen the central role of endothelium in obesity-induced insulin resistance, suggesting that blockade of vascular inflammation and oxidative stress may be a promising approach to prevent metabolic disorders. Notably, pharmacological improvement in insulin sensitivity in patients with type 2 diabetes and metabolic syndrome is associated with restoration of flow-mediated vasodilation.50–52
The atherogenic effects of insulin resistance are also due to changes in lipid profile such as high triglycerides, low HDL cholesterol, increased remnant lipoproteins, elevated apolipoprotein B (ApoB) as well as small and dense LDL.53 Once circulating FFA reach the liver, very low density lipoprotein (VLDL) are assembled and made soluble by increased synthesis of ApoB. VLDL are processed by cholesteryl ester transfer protein allowing transfer of triglycerides to LDL, which become small and dense and, hence, more atherogenic. Atherogenic dyslipidemia is a reliable predictor of cardiovascular risk and its pharmacological modulation reduces vascular events in subjects with type 2 diabetes and metabolic syndrome.54–56
Coronary events in patients with insulin resistance are triggered by virtue of a prothrombotic state. Under physiological conditions, insulin inhibits platelet aggregation and thrombosis via tissue factor (TF) inhibition and enhanced fibrinolytic action due to modulation of plasminogen activator inhibitor-1 (PAI-1) levels. Indeed, patients with acute myocardial infarction receiving fibrinolityic therapy plus 48 h insulin infusion displayed a marked decrease in PAI-1 levels.57 In contrast, insulin resistance facilitates atherothrombosis through increased cellular synthesis of PAI-1 and fibrinogen and reduced production of tissue plasminogen activator. In platelets, lack of insulin leads to a down-regulation of the IRS-1/Akt pathway resulting in calcium accumulation upon basal conditions58 (Figure 2). This latter mechanism may explain why platelets from diabetic patients show faster response and increased aggregation compared with those from healthy subjects.59 Moreover, platelet reactivity and excretion of tromboxane metabolites are increased in obese patients with insulin resistance and this phenomenon is reversed by weight loss or 3-week treatment with pioglitazone.60 Body weight as well as impaired insulin sensitivity may also account for the faster recovery of cyclooxygenase activity despite aspirin treatment.61 Indeed, higher body mass index was an independent predictor of inadequate suppression of tromboxane biosynthesis in non-diabetics subjects treated with aspirin.61 In this study, the increase of aspirin dosage was sufficient to warrant platelet inhibition. This clinical observation may explain the residual cardiovascular risk in obese patients treated with anti-platelet medications.
Hyperglycemia and insulin resistance alone may not explain the persistent cardiovascular risk burden associated with type 2 diabetes. Indeed, normalization of glycemia does not reduce macrovascular events suggesting that mediators of vascular risk other than glucose significantly participate to increase the residual cardiovascular risk in diabetic patients.62 In this regard, adipose tissue dysfunction, inflammation, and aberrant adipokine release may be particularly relevant.63 In patients with abdominal obesity, an increased lipid storage leads to hypoxia, chronic inflammation together with changes in the cellular components of adipose tissue, leading to an altered secretory profile. Adipokines linked to vascular disease are leptin, adipocyte fatty acid-binding protein, interleukins, and novel ones like lipocalin-2 and pigment epithelium-derived factor. These molecules may drive vascular dysfunction via increased proliferation/migration of smooth muscle cells, eNOS inhibition, and activation of NFkB signalling with subsequent expression of adhesion molecules and atherosclerosis.64 Future work will need to address the potential role of these molecules as biomarkers and/or drug targets.
MicroRNA and diabetic vascular disease
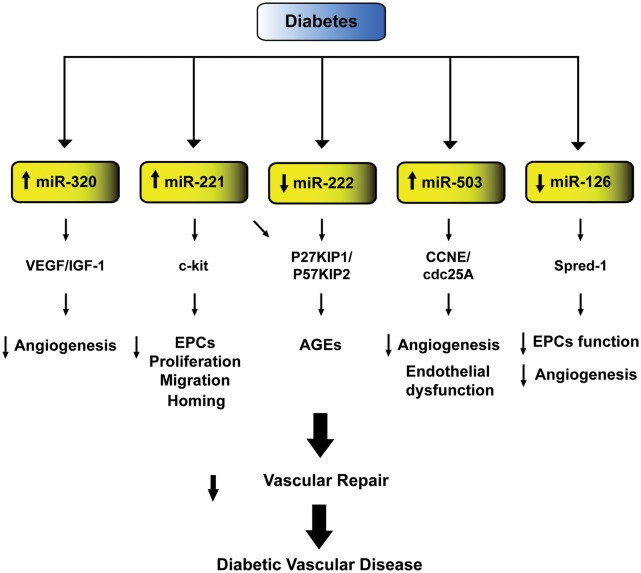
MicroRNAs (miRs) are a newly identified class of small non-coding RNAs emerging as key players in the pathogenesis of hyperglycemia-induced vascular damage.65,66 These small non-coding RNAs orchestrate different aspects of diabetic vascular disease by regulating gene expression at the post-transcriptional level. Microarray studies have shown an altered profile of miRs expression in subjects with type 2 diabetes.67–69 Indeed, diabetic patients display a significant deregulation of miRs involved in angiogenesis, vascular repair, and endothelial homeostasis.67 Over the last few years, different studies have explored the mechanisms whereby deregulation of miRs expression may contribute to vascular disease in subjects with diabetes. In endothelial cells exposed to high glucose miR-320 is highly expressed and targets several angiogenic factors and their receptors, including vascular endothelial growth factor and insulin-like growth factor-1 (IGF-1). Elevated levels of this miR are associated with decreased cell proliferation and migration, while its down-regulation restores these properties and increases IGF-1 expression, promoting angiogenesis and vascular repair70 (Figure 3).
Figure 3.
MicroRNAs involved in diabetic vascular disease. Schematic representation of microRNAs and their relative targets contributing to reduced vascular repair and, hence, diabetes-related vascular dysfunction. VEGF, vascular endothelial growth factor; IGF-1, insulin-like growth factor-1; ECs, endothelial cells; AGEs, advanced glycation end-products.
Hyperglycemia also increases the expression of miR-221, a regulator of angiogenesis targeting c-kit receptor which is responsible for migration and homing of endothelial progenitor cells (EPCs).71 miR-221 and 222 were also found to mediate AGE-induced vascular damage.72 Indeed, down-regulation of miR-222 both in human endothelial cells exposed to high glucose and in diabetic mice elicits AGE-related endothelial dysfunction via targeting, cyclin-dependent kinase proteins involved in cell cycle inhibition (P27KIP1 and P57KIP2).72 A recent study demonstrated that miR-503 is critically involved in hyperglycemia-induced endothelial dysfunction in diabetic mice and is up-regulated in ischaemic limb muscles of diabetic subjects.73 The detrimental effects of miR-503 in the setting of diabetes have been explained by its interaction with CCNE and cdc25A, critical regulators of cell cycle progression affecting endothelial cell migration and proliferation. Interestingly, miR-503 inhibition was able to normalize post-ischaemic neovascularization and blood flow recovery in diabetic mice. These findings provide the rationale to foresee a protective effect of the modulation of miR-503 expression against diabetic vascular complications.
Plasma miR profiling showed a profound down-regulation of miR-126 in a cohort of diabetic patients.67 Recent evidence suggest that reduced miR-126 expression levels are partially responsible for impaired vascular repair capacities in diabetes.74,75 miR-126 expression was reduced in EPCs isolated from diabetics and transfection with anti-miR-126 blunted EPCs proliferation and migration.74,75 In contrast, restored expression of this miR promoted EPCs-related repair capacities and inhibited apoptosis. miR-126 role in EPCs function is mediated by Spred-1, an inhibitor of Ras/ERK signalling pathway, a critical regulator of cell cycle.
Collectively, these studies support the notion that miRs drive complex signalling networks by targeting the expression of genes involved in cell differentiation, migration, and survival.
Thrombosis and coagulation
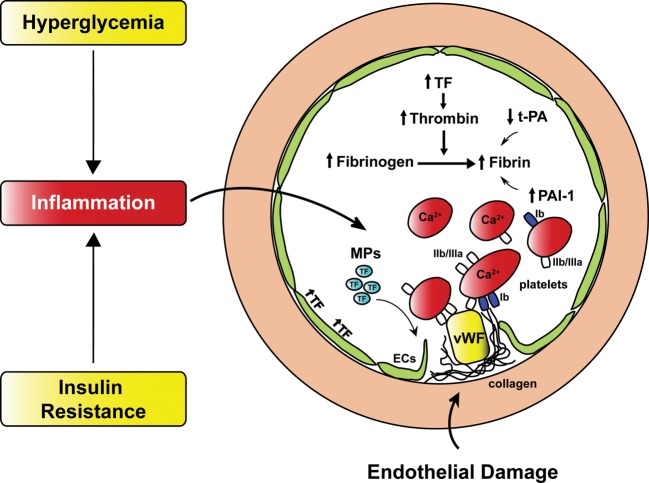
Individuals affected by diabetes display an increased risk of coronary events and cardiovascular mortality when compared with non-diabetic subjects.76–78 This phenomenon is largely explained by a deregulation of factors involved in coagulation and platelet activation.79,80 Both insulin resistance and hyperglycemia participate to the pathogenesis of this prothrombotic state.81 Insulin resistance increases PAI-1 and fibrinogen and reduces tissue plasminogen activator levels. The largest increase in PAI-1 has been reported in diabetic patients with poor glycemic control and treatment with glucose-lowering agents glipizide or metformin comparably decreased PAI-1.82 Hyperinsulinemia induces TF expression in monocytes of patients with type 2 diabetes leading to increased TF procoagulant activity and thrombin generation.83 These events are enhanced by hyperglycemia83,84 (Figure 4). Low-grade inflammation induces TF expression also in the vascular endothelium of diabetic subjects contributing to atherothrombosis.81,83
Figure 4.
Coagulation and platelet reactivity in diabetes. In patients with diabetes chronic hyperglycemia and insulin resistance determine a significant alteration in the coagulation factors as well as increased platelet aggregation, leading to a prothrombotic state. Diabetes-induced increase of TF levels activates thrombin converting fibrinogen into fibrin. Fibrin organization is further enhanced due to high PAI-1 and reduced t-PA levels. Increased Ca2+ content, thrombin stimulation as well as interaction with vWF via gpIIb/IIIa receptor lead to platelet shape change, granule release, and aggregation. Release of MPs from injured endothelium and circulating platelets contribute to accelerate thrombus development. Endothelial dysfunction precipitates rupture of the endothelial layer leading to exposure of collagen and vWF thereby activating platelets and favouring vascular thrombosis. TF, tissue factor; t-PA, tissue plasminogen activator; PAI-1, plasminogen activator inhibitor -1; MPs, microparticles; vWF, von Willebrand factor; ECs, endothelial cells.
Microparticles (MPs), vescicles released in the circulation from various cell types following activation or apoptosis, are increased in diabetic patients and predict cardiovascular outcome.85,86 Microparticles from patients with type 2 diabetes have shown to increase coagulation activity in endothelial cells85 (Figure 4). Moreover, MPs carrying TF promote thrombus formation at sites of injury representing a novel and additional mechanisms of coronary thrombosis in diabetes.85
Among the factors contributing to the diabetic prothrombotic state, platelet hyperreactivity is of major relevance.87 A number of mechanisms contribute to platelet dysfunction affecting adhesion, activation as well as aggregation phases of platelet-mediated thrombosis (Figure 4). Hyperglycemia alters platelet Ca2+ homeostasis leading to cytoskeleton abnormalities and increased secretion of proaggregant factors.58 Moreover, up-regulation of glycoproteins Ib and IIb/IIIa in diabetic patients triggers thrombus via interacting with Von Willebrand factor (vWF) and fibrin molecules (Figure 4).
Vascular hyperglycemic memory
Recent prospective clinical trials have shown that normalization of glycemia failed to reduce cardiovascular burden in the diabetic population.88–91 In these trials, intensive glucose-lowering therapy was started after a median duration of diabetes ranging from 8 to 11 years.88–91 In contrast, early treatment of hyperglycemia was shown to be beneficial.92,93 These findings support the concept that hyperglycemic environment may be remembered in the vasculature. Reactive oxygen species are probably involved in this phenomenon.94,95
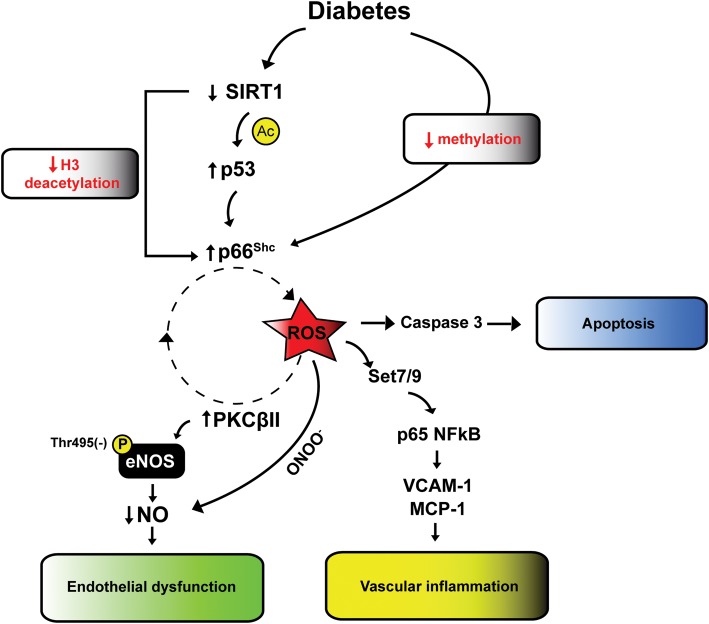
The persistence of hyperglycemic stress despite blood glucose normalization has recently been defined ‘hyperglycemic memory’. A substantial understanding of its mechanisms has been achieved only in recent years.96,97 It has been recently demonstrated that transient hyperglycemia activates NF-kB, and this effect persists despite subsequent normalization of glucose levels. This finding is explained by epigenetic changes occurring at the level of DNA and histone-binding promoter of pro-oxidant and pro-inflammatory genes. Specifically, methylation and acetylation are critical epigenetic mark modulated by the hyperglycemic environment. Methylation of p65/NFkB promoter by the ROS-dependent methyltransferase Set7/9 is indeed the mechanisms whereby vascular inflammation is not reverted by restoration of normoglycemia98 (Figure 5). We have recently identified the source of ROS perpetuating vascular dysfunction despite normoglycemia restoration.18
Figure 5.
Intracellular signalling of vascular hyperglycemic memory. Hyperglycemia causes a deregulation of SIRT1 resulting in increased acetylation of histone 3-binding p66Shc promoter. Together with these changes, hypomethylation of p66Shc promoter leads to persistent overexpression of the adaptor protein despite glucose normalization. SIRT1 down-regulation also causes increased p53 activation further promoting p66Shc gene transcription. Overexpression of p66Shc causes mitochondrial ROS accumulation leading to vascular apoptosis, vascular inflammation (Set7/9-dependent methylation of p65 promoter and expression of inflammatory genes) and endothelial dysfunction via a detrimental vicious cycle involving ROS, PKCβ2 and eNOS inhibiting phosphorylation at Thr-495. H3, histone 3; ROS, reactive oxygen species; PKCβII, protein kinase C βII; NO, nitric oxide; MCP-1, monocyte chemoattractant protein 1; VCAM-1, vascular cell adhesion molecule 1.
In diabetic mice and human endothelial cells, glucose normalization did not revert up-regulation of p66Shc protein, a mitochondrial adaptor critically involved in ROS generation.18 Persistent p66Shc expression is driven by epigenetic changes as reduced promoter methylation and acetylation of histone 3 (Figure 5). Moreover, p66Shc-dependent ROS generation maintains up-regulation of PKCβII and inhibits eNOS activity, thus feeding a detrimental vicious cycle despite restoration of normoglycemia18 (Figure 5). Persistent oxidative stress is also responsible for sustained vascular apoptosis via caspase 3 activation. Gene silencing of p66Shc blunted persistent endothelial dysfunction and oxidative stress in the vasculature of diabetic mice, suggesting that this protein drives hyperglycemic memory18 (Figure 5). In addition, other studies have shown that both mammalian deacetylase SIRT-1 and tumour suppressor p53 have a strong memory effect despite glucose normalization.99,100
Interestingly enough, these findings are in line with the notion that both SIRT-1 and p53 control p66Shc transcription.101,102 Indeed, reduced SIRT-1 activity in diabetes favours acetylation of histone 3-binding p66Shc promoter. Moreover, increased p53 activity maintains p66Shc memory effect (Figure 5).101,102 All together, these pathways might be involved in self-perpetuating vascular damage of patients with diabetes despite optimal glycemic control.
Future perspectives
Oxidative stress plays a major role in the development of micro- and macrovascular complications. Accumulation of free radicals in the vasculature of diabetic patients is responsible for the activation of detrimental biochemical pathways, miRs deregulation, release of MPs, and epigenetic changes contributing to vascular inflammation and ROS generation. Since cardiovascular risk burden is not eradicated by intensive glycemic control associated with optimal multifactorial treatment, mechanism-based therapeutic strategies are in highly demand.88–91 Specifically, inhibition of key enzymes involved in hyperglycemia-induced vascular damage or activation of pathways improving insulin sensitivity may represent promising approaches.
Modulation of specific miRs might contribute to improve EPC-driven vascular repair. Moreover, the progressive identification of a complex scenario driven by epigenetic changes that modulate transcription of ROS-generating and pro-inflammatory genes may represent an attractive opportunity to dampen oxidative stress, vascular inflammation, and hence to prevent cardiovascular complications in patients with diabetes.
Funding
This work was supported by grants from the Swiss Heart Foundation, Fondazione Roma, Italy (to F.C.).
Conflict of interest: F.P is the recipient of a fellowship from the Italian Society of Hypertension.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 4.Naudi A, Jove M, Ayala V, Cassanye A, Serrano J, Gonzalo H, Boada J, Prat J, Portero-Otin M, Pamplona R. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res. 2012;2012:696215. doi: 10.1155/2012/696215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998;21:1167–1172. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 6.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 7.Rutter MK, Nesto RW. Blood pressure, lipids and glucose in type 2 diabetes: How low should we go? Re-discovering personalized care. Eur Heart J. 2011;32:2247–2255. doi: 10.1093/eurheartj/ehr154. [DOI] [PubMed] [Google Scholar]
- 8.Bartnik M, Cosentino F. Dysglycaemia, cardiovascular outcome and treatment. Is the jury still out? Eur Heart J. 2009;30:1301–1304. doi: 10.1093/eurheartj/ehp168. [DOI] [PubMed] [Google Scholar]
- 9.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 10.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 11.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 13.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 16.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase c and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 17.Cosentino F, Francia P, Camici GG, Pelicci PG, Luscher TF, Volpe M. Final common molecular pathways of aging and cardiovascular disease: role of the p66shc protein. Arterioscler Thromb Vasc Biol. 2008;28:622–628. doi: 10.1161/ATVBAHA.107.156059. [DOI] [PubMed] [Google Scholar]
- 18.Paneni F, Mocharla P, Akhmedov A, Costantino S, Osto E, Volpe M, Luscher TF, Cosentino F. Gene silencing of the mitochondrial adaptor p66shc suppresses vascular hyperglycemic memory in diabetes. Circ Res. 2012;111:278–89. doi: 10.1161/CIRCRESAHA.112.266593. [DOI] [PubMed] [Google Scholar]
- 19.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Luscher TF, Cosentino F. Genetic deletion of p66(shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci USA. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagnin E, Fadini G, de Toni R, Tiengo A, Calo L, Avogaro A. Diabetes induces p66shc gene expression in human peripheral blood mononuclear cells: relationship to oxidative stress. J Clin Endocrinol Metab. 2005;90:1130–1136. doi: 10.1210/jc.2004-1283. [DOI] [PubMed] [Google Scholar]
- 21.Tomilov AA, Bicocca V, Schoenfeld RA, Giorgio M, Migliaccio E, Ramsey JJ, Hagopian K, Pelicci PG, Cortopassi GA. Decreased superoxide production in macrophages of long-lived p66shc knock-out mice. J Biol Chem. 2010;285:1153–1165. doi: 10.1074/jbc.M109.017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Cosentino F, Camici GG, Akhmedov A, Vanhoutte PM, Tanner FC, Luscher TF. Oxidized low-density lipoprotein activates p66shc via lectin-like oxidized low-density lipoprotein receptor-1, protein kinase c-beta, and c-jun n-terminal kinase kinase in human endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:2090–2097. doi: 10.1161/ATVBAHA.111.229260. [DOI] [PubMed] [Google Scholar]
- 23.Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997;96:25–28. doi: 10.1161/01.cir.96.1.25. [DOI] [PubMed] [Google Scholar]
- 24.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 25.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation. 2002;106:1783–1787. doi: 10.1161/01.cir.0000032260.01569.64. [DOI] [PubMed] [Google Scholar]
- 27.Kouroedov A, Eto M, Joch H, Volpe M, Luscher TF, Cosentino F. Selective inhibition of protein kinase cbeta2 prevents acute effects of high glucose on vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 2004;110:91–96. doi: 10.1161/01.CIR.0000133384.38551.A8. [DOI] [PubMed] [Google Scholar]
- 28.Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Luscher TF. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 29.Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, Stehouwer CD, De Mey JG, Schalkwijk CG. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010;53:989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan SF, Ramasamy R, Schmidt AM. The rage axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sena CM, Matafome P, Crisostomo J, Rodrigues L, Fernandes R, Pereira P, Seica RM. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol Res. 2012;65:497–506. doi: 10.1016/j.phrs.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding rage, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 33.Fulop N, Marchase RB, Chatham JC. Role of protein o-linked n-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006;290:E1–E8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 36.Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190–1200. doi: 10.1093/eurheartj/ehr453. [DOI] [PubMed] [Google Scholar]
- 39.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 40.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavelti-Weder C, Babians-Brunner A, Keller C, Stahel MA, Kurz-Levin M, Zayed H, Solinger AM, Mandrup-Poulsen T, Dinarello CA, Donath MY. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35:1654–1662. doi: 10.2337/dc11-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 43.Kim JK. Endothelial nuclear factor kappab in obesity and aging: is endothelial nuclear factor kappaB a master regulator of inflammation and insulin resistance? Circulation. 2012;125:1081–1083. doi: 10.1161/CIRCULATIONAHA.111.090134. [DOI] [PubMed] [Google Scholar]
- 44.Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOs activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein e null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 47.Gray S, Kim JK. New insights into insulin resistance in the diabetic heart. Trends Endocrinol Metab. 2011;22:394–403. doi: 10.1016/j.tem.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, Uno K, Gao J, Kaneko K, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Blockade of the nuclear factor-kappab pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012;125:1122–1133. doi: 10.1161/CIRCULATIONAHA.111.054346. [DOI] [PubMed] [Google Scholar]
- 49.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Vitale C, Mercuro G, Cornoldi A, Fini M, Volterrani M, Rosano GM. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med. 2005;258:250–256. doi: 10.1111/j.1365-2796.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 51.Naka KK, Papathanassiou K, Bechlioulis A, Pappas K, Kazakos N, Kanioglou C, Papafaklis MI, Kostoula A, Vezyraki P, Makriyiannis D, Tsatsoulis A, Michalis LK. Rosiglitazone improves endothelial function in patients with type 2 diabetes treated with insulin. Diab Vasc Dis Res. 2011;8:195–201. doi: 10.1177/1479164111408628. [DOI] [PubMed] [Google Scholar]
- 52.Wang TD, Chen WJ, Cheng WC, Lin JW, Chen MF, Lee YT. Relation of improvement in endothelium-dependent flow-mediated vasodilation after rosiglitazone to changes in asymmetric dimethylarginine, endothelin-1, and C-reactive protein in nondiabetic patients with the metabolic syndrome. Am J Cardiol. 2006;98:1057–1062. doi: 10.1016/j.amjcard.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Dellsperger KC, Zhang C. The link between metabolic abnormalities and endothelial dysfunction in type 2 diabetes: an update. Basic Res Cardiol. 2012;107:237. doi: 10.1007/s00395-011-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee M, Saver JL, Towfighi A, Chow J, Ovbiagele B. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: a meta-analysis. Atherosclerosis. 2011;217:492–498. doi: 10.1016/j.atherosclerosis.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 55.Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN, Kadowaki T, Lablanche JM, Marx N, Plutzky J, Reiner Z, Rosenson RS, Staels B, Stock JK, Sy R, Wanner C, Zambon A, Zimmet P. The residual risk reduction initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102:1K–34K. doi: 10.1016/S0002-9149(08)01833-X. [DOI] [PubMed] [Google Scholar]
- 56.Arca M, Montali A, Valiante S, Campagna F, Pigna G, Paoletti V, Antonini R, Barilla F, Tanzilli G, Vestri A, Gaudio C. Usefulness of atherogenic dyslipidemia for predicting cardiovascular risk in patients with angiographically defined coronary artery disease. Am J Cardiol. 2007;100:1511–1516. doi: 10.1016/j.amjcard.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 57.Chaudhuri A, Janicke D, Wilson MF, Tripathy D, Garg R, Bandyopadhyay A, Calieri J, Hoffmeyer D, Syed T, Ghanim H, Aljada A, Dandona P. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109:849–854. doi: 10.1161/01.CIR.0000116762.77804.FC. [DOI] [PubMed] [Google Scholar]
- 58.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24:1476–1485. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira IA, Mocking AI, Feijge MA, Gorter G, van Haeften TW, Heemskerk JW, Akkerman JW. Platelet inhibition by insulin is absent in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2006;26:417–422. doi: 10.1161/01.ATV.0000199519.37089.a0. [DOI] [PubMed] [Google Scholar]
- 60.Basili S, Pacini G, Guagnano MT, Manigrasso MR, Santilli F, Pettinella C, Ciabattoni G, Patrono C, Davi G. Insulin resistance as a determinant of platelet activation in obese women. J Am Coll Cardiol. 2006;48:2531–2538. doi: 10.1016/j.jacc.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 61.Rocca B, Santilli F, Pitocco D, Mucci L, Petrucci G, Vitacolonna E, Lattanzio S, Mattoscio D, Zaccardi F, Liani R, Vazzana N, Del Ponte A, Ferrante E, Martini F, Cardillo C, Morosetti R, Mirabella M, Ghirlanda G, Davi G, Patrono C. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost. 2012;10:1220–1230. doi: 10.1111/j.1538-7836.2012.04723.x. [DOI] [PubMed] [Google Scholar]
- 62.Sattar N, Wannamethee SG, Forouhi NG. Novel biochemical risk factors for type 2 diabetes: pathogenic insights or prediction possibilities? Diabetologia. 2008;51:926–940. doi: 10.1007/s00125-008-0954-7. [DOI] [PubMed] [Google Scholar]
- 63.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;302:H2148–H2165. doi: 10.1152/ajpheart.00907.2011. [DOI] [PubMed] [Google Scholar]
- 64.Li ZY, Wang P, Miao CY. Adipokines in inflammation, insulin resistance and cardiovascular disease. Clin Exp Pharmacol Physiol. 2011;38:888–896. doi: 10.1111/j.1440-1681.2011.05602.x. [DOI] [PubMed] [Google Scholar]
- 65.Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res. 2012;93:583–593. doi: 10.1093/cvr/cvr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. Circ Res. 2012;110:508–522. doi: 10.1161/CIRCRESAHA.111.247445. [DOI] [PubMed] [Google Scholar]
- 67.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microrna profiling reveals loss of endothelial mir-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 68.Karolina DS, Armugam A, Tavintharan S, Wong MT, Lim SC, Sum CF, Jeyaseelan K. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS One. 2011;6:e22839. doi: 10.1371/journal.pone.0022839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dehwah MA, Xu A, Huang Q. MicroRNAs and type 2 diabetes/obesity. J Genet Genomics. 2012;39:11–18. doi: 10.1016/j.jgg.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2009;36:181–188. doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Song YH, Li F, Yang T, Lu YW, Geng YJ. MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochem Biophys Res Commun. 2009;381:81–83. doi: 10.1016/j.bbrc.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Togliatto G, Trombetta A, Dentelli P, Rosso A, Brizzi MF. Mir221/mir222-driven post-transcriptional regulation of p27kip1 and p57kip2 is crucial for high-glucose- and age-mediated vascular cell damage. Diabetologia. 2011;54:1930–1940. doi: 10.1007/s00125-011-2125-5. [DOI] [PubMed] [Google Scholar]
- 73.Caporali A, Meloni M, Vollenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH, Agami R, le Sage C, Condorelli G, Madeddu P, Martelli F, Emanueli C. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 74.Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Downregulation of microrna-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene SPRED-1. J Mol Cell Cardiol. 2012;53:64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Wang DE. MicroRNA regulation and its biological significance in personalized medicine and aging. Curr Genomics. 2009;10:143. doi: 10.2174/138920209788185216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 77.Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J. 2011;32:2748–2757. doi: 10.1093/eurheartj/ehr305. [DOI] [PubMed] [Google Scholar]
- 78.Radke PW, Schunkert H. Diabetics with acute coronary syndrome: advances, challenges, and uncertainties. Eur Heart J. 2010;31:2971–2973. doi: 10.1093/eurheartj/ehq347. [DOI] [PubMed] [Google Scholar]
- 79.Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007;262:157–172. doi: 10.1111/j.1365-2796.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 80.Vazzana N, Ranalli P, Cuccurullo C, Davi G. Diabetes mellitus and thrombosis. Thromb Res. 2012;129:371–377. doi: 10.1016/j.thromres.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 81.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 82.Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JB. Hyperglycemia: a prothrombotic factor? J Thromb Haemost. 2010;8:1663–1669. doi: 10.1111/j.1538-7836.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- 83.Boden G, Rao AK. Effects of hyperglycemia and hyperinsulinemia on the tissue factor pathway of blood coagulation. Curr Diab Rep. 2007;7:223–227. doi: 10.1007/s11892-007-0035-1. [DOI] [PubMed] [Google Scholar]
- 84.Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes. 2006;55:202–208. [PubMed] [Google Scholar]
- 85.Tsimerman G, Roguin A, Bachar A, Melamed E, Brenner B, Aharon A. Involvement of microparticles in diabetic vascular complications. Thromb Haemost. 2011;106:310–321. doi: 10.1160/TH10-11-0712. [DOI] [PubMed] [Google Scholar]
- 86.Sinning JM, Losch J, Walenta K, Bohm M, Nickenig G, Werner N. Circulating cd31+/annexin v+ microparticles correlate with cardiovascular outcomes. Eur Heart J. 2011;32:2034–2041. doi: 10.1093/eurheartj/ehq478. [DOI] [PubMed] [Google Scholar]
- 87.Linden MD, Tran H, Woods R, Tonkin A. High platelet reactivity and antiplatelet therapy resistance. Semin Thromb Hemost. 2012;38:200–212. doi: 10.1055/s-0032-1301417. [DOI] [PubMed] [Google Scholar]
- 88.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 90.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 92.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 93.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 94.Ihnat MA, Thorpe JE, Kamat CD, Szabo C, Green DE, Warnke LA, Lacza Z, Cselenyak A, Ross K, Shakir S, Piconi L, Kaltreider RC, Ceriello A. Reactive oxygen species mediate a cellular ‘memory’ of high glucose stress signalling. Diabetologia. 2007;50:1523–1531. doi: 10.1007/s00125-007-0684-2. [DOI] [PubMed] [Google Scholar]
- 95.Ceriello A, Esposito K, Ihnat M, Zhang J, Giugliano D. Simultaneous control of hyperglycemia and oxidative stress normalizes enhanced thrombin generation in type 1 diabetes. J Thromb Haemost. 2009;7:1228–1230. doi: 10.1111/j.1538-7836.2009.03445.x. [DOI] [PubMed] [Google Scholar]
- 96.Ceriello A. Hypothesis: The ‘metabolic memory’, the new challenge of diabetes. Diabetes Res Clin Pract. 2009;86(Suppl 1):S2–S6. doi: 10.1016/S0168-8227(09)70002-6. [DOI] [PubMed] [Google Scholar]
- 97.Keating ST, El-Osta A. Chromatin modifications associated with diabetes. J Cardiovasc Transl Res. 2012;5:399–412. doi: 10.1007/s12265-012-9380-9. [DOI] [PubMed] [Google Scholar]
- 98.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng Z, Chen H, Li J, Li T, Zheng B, Zheng Y, Jin H, He Y, Gu Q, Xu X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the lkb1/ampk/ros pathway and therapeutic effects of metformin. Diabetes. 2012;61:217–228. doi: 10.2337/db11-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schisano B, Tripathi G, McGee K, McTernan PG, Ceriello A. Glucose oscillations, more than constant high glucose, induce p53 activation and a metabolic memory in human endothelial cells. Diabetologia. 2011;54:1219–1226. doi: 10.1007/s00125-011-2049-0. [DOI] [PubMed] [Google Scholar]
- 101.Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, Liu JJ, Lu YB, Zhang ZQ, Yang RF, Zhang R, Cai H, Liu DP, Liang CC. Repression of p66shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109:639–648. doi: 10.1161/CIRCRESAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 102.Kim CS, Jung SB, Naqvi A, Hoffman TA, DeRicco J, Yamamori T, Cole MP, Jeon BH, Irani K. P53 impairs endothelium-dependent vasomotor function through transcriptional upregulation of p66shc. Circ Res. 2008;103:1441–1450. doi: 10.1161/CIRCRESAHA.108.181644. [DOI] [PubMed] [Google Scholar]