Abstract
The anthrax toxins lethal toxin (LT) and edema toxin (ET), are essential virulence factors produced by B. anthracis. These toxins act during two distinct phases of anthrax infection. During the first, prodromal phase, which is often asymptomatic, anthrax toxins act on cells of the immune system to help the pathogen establish infection. Then, during the rapidly progressing (or fulminant) stage of the disease bacteria disseminate via a hematological route to various target tissues and organs, which are typically highly vascularized. As bacteria proliferate in the bloodstream LT and ET begin to accumulate rapidly reaching a critical threshold level that will cause death even when the bacterial proliferation is curtailed by antibiotics. During this final phase of infection the toxins cause an increase in vascular permeability and a decrease in function of target organs including the heart, spleen, kidney, adrenal gland, and brain. In this review, we examine the various biological effects of anthrax toxins, focusing on the fulminant stage of the disease and on mechanisms by which the two toxins may collaborate to cause cardiovascular collapse. We discuss normal mechanisms involved in maintaining vascular integrity and based on recent studies indicating that LT and ET cooperatively inhibit membrane trafficking to cell-cell junctions we explore several potential mechanisms by which the toxins may achieve their lethal effects. We also summarize the effects of other potential virulence factors secreted by B. anthracis and consider the role of toxic factors in the evolutionarily recent emergence of this devastating disease.
Keywords: Anthrax, B. anthracis, B. cereus, Lethal Factor (LF), Edema Factor (EF), Macrophage, Dendritic Cell, Myeloid cells, Neutrophil, Vascular endothelium, Mural cells, Cardiac, Exocyst, Rab11, Sec15, Cadherin, Notch, cAMP, MAPKK/MKK/MEK, PKA, EPAC
1. Historical Overview
Anthrax holds a unique place in the history of infectious disease. Named by Hippocrates for the black skin lesions it causes in its cutaneous form, anthrax (Greek: “coal”) was well known in antiquity (1190-1491 BC in Europe and perhaps as far back as 3000 BC in China) and is featured in two of the ten plagues of the Old Testament (5-pestilence and 6-boils). During the 1830s-1850s several observers, including Delafond and Davaine in France and Pollender in Germany, reported small rods in the blood of anthrax-infected animals. In 1863, Davaine suggested these were the cause of anthrax, following his reading of Pasteur's work on the role of microorganisms in fermentation and putrification. In 1876, Robert Koch applied for the first time his three postulates for an infectious agent to conclusively demonstrate that B. anthracis was indeed the cause of anthrax disease [1]. Soon thereafter, Louis Pasteur showed that a drop anthrax-infected blood serially passaged one hundred times in culture (thus diluting out any other suspected factors from the original blood sample), still retained full infectivity and caused the full range of symptoms typical of anthrax. Further classic studies of Pasteur and colleagues provided compelling evidence that buried cadavers of animals that succumbed to anthrax were a prominent source of new infections and that spores were transported to the surface in great measure by the action of earth worms, which he showed carried and released spores by defecation. These studies culminated in one of the great moments for experimental medical science in which Pasteur held a public demonstration in May, 1881 of an attenuated anthrax vaccine at a farm in Pouilly-le-Fort [2, 3] providing unequivocal evidence for the protective effects of his vaccination program against infection with a fully virulent form of B. anthracis. All inoculated sheep survived (except for one which he showed died of complications associated with pregnancy), while all unvaccinated control sheep died, the majority within one or two days of challenge with virulent B. anthracis. These initial experiments and parallel findings by Jean-Joseph Henri Toussiant and William Smith Greenfield (in England) were followed by large scale inoculation programs by Pasteur in France over the next two years and then by programs throughout Europe and America thereby ushering in the modern era of medical microbiology (reviewed in [4-6], see also a full and lucid recounting of Pasteur's anthrax experiments in [7]).
2. Identification and isolation of anthrax toxins
Pasteur was the first to show that filtered blood from animals infected with anthrax contained substances that could reproduce an effect associated with infection, namely agglutination of red cells in uninfected blood (Fig. 3A), although such filtrates were not sufficiently concentrated to cause significant local disturbances when injected into animals [8]. In the early through mid-1900s studies of Marmier, Bail, and Cromartie identified toxic factors produced by B. anthracis in culture [9] and in extracts of anthrax lesions [10] that could closely mimic the local effects of cutaneous infection with live B. anthracis [11]. Bail also found these extracts could immunize animals against infection by B. anthracis [12] and explicit recognition of protective antigen (PA) as a potent protective immunogen was made by Gladstone [13], thus revealing an important role of secreted toxic factors in the etiology of anthrax disease. Smith and Keppie then showed that guinea pigs infected with anthrax bacteria and treated with antibiotics would still die once they had passed a critical juncture, a conceptual advance indicating that secreted toxins were largely responsible for the systemic and lethal effects of anthrax infection. Extensive analysis by the Smith and Thorne/Strange groups led to the identification of three purified toxic factors corresponding to PA, lethal factor (LF), and edema factor (EF) (reviewed retrospectively by Smith in [14]). These studies provided the framework for anthrax toxemia by demonstrating that PA+LF (lethal toxin, LT) caused lethality when injected into animals, while subcutaneous administration of PA+EF (edema toxin, ET) caused edema. They also found that non-lethal doses of ET could nearly double the lethality caused by submaximal doses of LT [15] providing the first evidence for synergy between the two toxins.
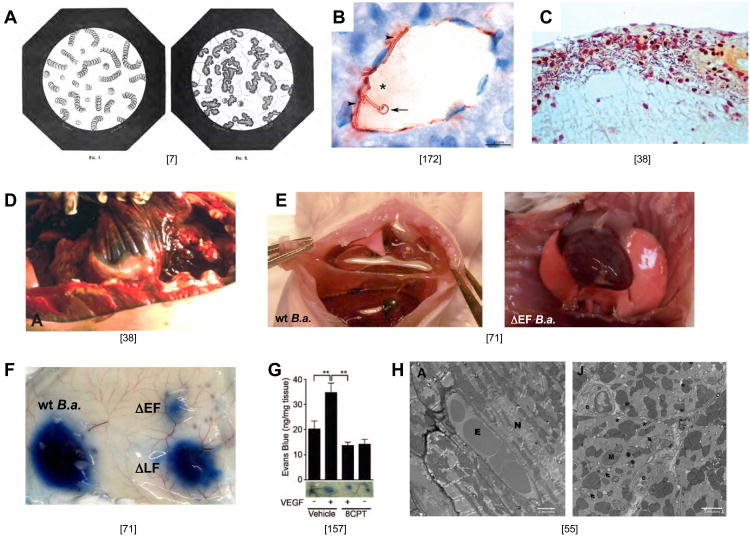
Figure 3. The fulminant phase of anthrax infection.
A) Left panel: drawing of a blood smear from an uninfected animal showing stacks of red cells (Fig. 1 from “Le charbon et la vaccination charbonneuse d'apres les traveau recents de M. Pasteur by C.H. Chamberland, 1883 [7]). Right panel (Fig. 2 from same source): Blood smear from an animal infected with B. anthracis showing aggregated masses of material and rod shaped bacteria. This phenotype could be reproduced by adding a drop of filtered cell-free liquid from blood of an anthrax infected animal to normal blood may be related to hemoconcentration or coagulopathies that have been reported in human cases of anthrax indicating the activity of a secreted substance. In Pasteur's own words: “Also, the filtered blood could be injected with impunity into the body (of a second animal) without producing anthrax or any local disturbance. However, this filtered anthrax blood, when placed in contact with fresh blood, rapidly generated agglutinated globules in as great or larger number than observed in (animals with) anthrax disease, possibly due to the presence of an enzyme formed by the bacteria.” The basis for this agglutination phenotype and whether ET or LT or neutral proteases such as InhA [184] contribute to it remains unknown. In his great summary of Pasteur's work, C.H. Chamberland also included a detailed description of the stages of anthrax disease provided by Delafond and Pasteur, which is remarkably similar to modern descriptions of the disease course in humans. These passages have been translated and condensed as follows: Delafond: “The earliest disease symptoms in a flock, which curiously would first appear in animals in their prime, were a more pronounced pink tinge of the nose and ear and small hemorrhages of blood vessels deep in the eye. During this phase lasting 2-4 days animals typically still ate and behaved normally. Blood drawn from the jugular vein as such symptoms appeared was black (a sign of anoxia), thick and agglutinated, and clotted more rapidly than normal blood (3-4 minutes versus 6-7 minutes). Next, signs of malaise were manifest: outstretched necks, dilated nostrils, difficulty breathing (dyspenea), bloody urine and moist sometimes bloody stool, and bloated stomachs after eating. The behavioral symptoms would generally subside temporarily during which time sheep would lick the rails of their enclosures or consume salt. After this short respite, disease symptoms would typically return, animals would stop eating, become lethargic, have rapid labored breath, look wild-eyed, discharge blood from their nostrils, fall to the ground, experience convulsions of all four members, and expel bloody urine and stool. Once such serious symptoms became evident, animals would expire within 5 minutes to three hours. Not all animals followed this progressive course, however. In some cases, animals that appeared to be in fine health with vigorous appetite would suddenly stop eating, lie or fall down, suffer convulsions, bleed profusely from the nose, and die in a period as brief as five minutes, seemingly by asphyxiation.” Chamberland: “In uninoculated animals similar overt signs of illness became manifest with death invariably following rapidly. In some cases, however, edema at the point of inoculation would resolve, body temperature would drop back to normal and the animals would make a full recovery, in which case the animals were almost always immune to reinfection, even with much larger doses of bacteria. The immunity of these surviving animals inspired Pasteur to conceive of vaccinating animals against anthrax with attenuated strains of bacteria.” As anthrax infected animals died, Pasteur and colleagues noted that “the cadavers had bloated abdomens, fluids leaked from all body orifices, and that they rapidly decomposed. Upon dissection of the cadavers they found lesions and hemorrhage in nearly all tissues and organs including the skin, subcutaneous tissues, lymphoid ganglia, intestinal mucosa, lungs, pancreas, kidneys, thymus, brain, choroid plexus, region surrounding the parotid and viscera cerebellum, had become swollen and gelatinous, having lost the normal morphological organization. Furthermore, all these organs while largely normal in gross structure were swollen by edema and their capillary vessels were filled with blood cells or distended with liquid. Blood leaked from larger vessels filling spaces under membranes surrounding organs such as the bronchia, intestinal mucosa, kidney, and bladder while in highly vascularized organs such as the spleen, kidney, lung, lymphoid ganglia, pancreas, thymus and choroid plexus, blood not only distended the organs engorging its vessels but escaped from the interior forming brown stains, bruises, effusion, and hemorrhaging rendering the organ a consistency of molasses that was easily torn and rent apart by pressure, oozing a dark thick blood.” B) Encapsulated B. anthracis adhere tightly to the wall of a blood vessel in the liver of an infected mouse (Fig. 3F from [172]). C) Invasion of B. anthracis into the subarachnoid space of meninges in an infected human brain (Fig. 14 from [38]). D) Gelatinous edema spreading from the mediastinum along the dorsal costal parietal pleura (Fig. 9A from [38]). E) Pleural effusion in lungs of CD-1 mice infected by IV infection with the Sterne strain of B. anthracis. Left panel WT B.a.; right panel, B.a mutant deleted for the gene encoding EF (Supp. Fig. 11C,D from [71]). F) Vascular effusion in response to subcutaneous infection with wild-type (WT) Sterne strain B. anthracis or mutants lacking the genes encoding LF (ΔLF) or EF (ΔEF) (Fig. 3p from [71]). G) Vascular effusion induced by subcutaneous injection of VEGF is reversed by activating the EPAC branch of the cAMP response revealing an in vivo barrier promoting role of cAMP. (Fig. 7D from [157]). H) Pathological changes in the hearts of LF treated mice. Left panel: EM section of heart from an untreated mouse with intact myocytes and endothelium (E = erythrocyte, N= myocyte nucleus). Right panel: Heart from an LT treated mouse (55 hours after treatment) exhibiting endothelial cell swelling, mitochondrial degeneration (arrows), and multiple swollen SR cisternae (*); M= mitochondria and e = endothelial cell. (Fig. 7A,J from [55]).
3. Basic Biochemistry and Structure of the Anthrax Toxins
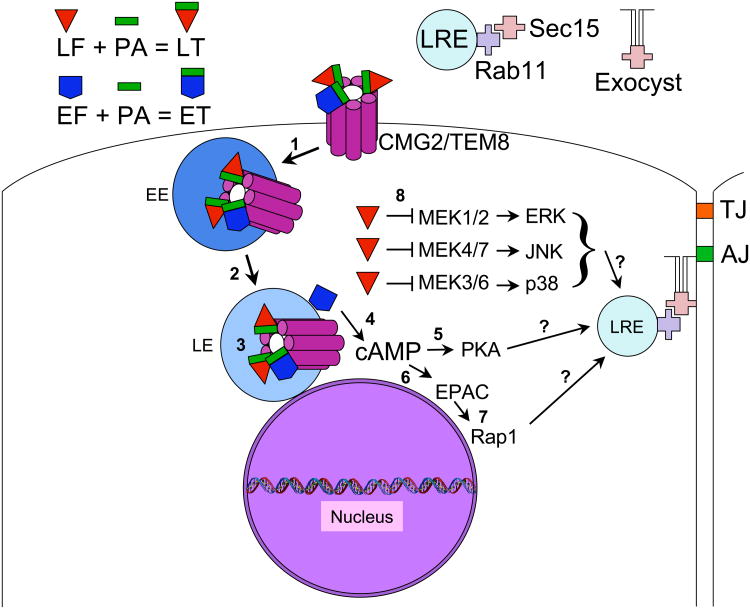
LT and ET are A/B type exotoxins comprised of separate polypeptides specialized for catalytic and toxic activities (A subunit = EF or LF) and for toxin delivery to cells (B subunit = PA) (Fig. 1). PA is synthesized as an 83 Kd precursor polypeptide that can bind two widely expressed cell surface receptors (TEM8 and CMG2). PA is cleaved by cell surface Furin type proteases to generate an active 63 Kd product, which then assembles into a heptameric ring and binds three subunits of EF and/or LF. The toxin complexes are endocytosed and trafficked to late endosomes where a change in pH triggers a conformational change creating a pore through which unfolded forms of EF and LF are translocated into the cytoplasm (reviewed in [16]). Biochemical analysis and in vivo imaging of toxin uptake into cells using GFP-tagged forms of LF and EF indicates that the two toxins travel together to the late endosomal compartment, whereupon LF is ejected into the cytoplasm, while EF remains associated with late endosomal membranes that surround the nucleus in a perinuclear necklace [17, 18].
Figure 1. Anthrax toxins: Entry into host cells and mechanism of action.
The enzymatic toxin moieties LF and EF associate with a proteolytically cleaved fragment of PA (PA 63Kd) and bind to host transmembrane anthrax receptors (CMG2 or TEM8) at the cell surface with a stoichiometry of 3 EF/PA or LF/PA per heptamer of receptor. Numbers in the diagram indicate the following: 1) Receptor-mediated endocytosis of the receptor toxin complex and trafficking into early endosomes (EE), 2) transport of the toxin-receptor complex into late endosomes, 3) a drop in pH in the late endosome leads to cytoplasmic translocation of the enzymatic portions of the toxins through the receptor pore (receptor toxin complexes actually reside in multivesicular bodies within the endosomal compartment which then fuse with endosomal membrane to liberate their contents into the cytoplasm), 4) following cytoplasmic translocation, the EF toxin remains associated with late endosomal membranes which are localized in a perinuclear pattern and via its highly active Calmodulin dependent adenylate cyclase activity generates a high local concentration of cAMP near the nucleus, 5) the catalytic subunit of protein kinase A (PKA) is one cAMP effector, which prior to activation resides in an inactive complex with a regulatory subunit (cAMP binds to the regulatory subunit releasing the active catalytic subunit that then phosphorylates downstream target proteins to alter their function), 6) a second cAMP effector is EPAC, a guanine nucleotide exchange factor (GEF) for the small RAS-related GTPase Rap1, 7) Rap1 once activated by EPAC binds to various effectors in the cell including RalA and proteins associated with the adherens junction (AJ) such as the Maguk protein MAG-1, the cAMP-independent Rap1 GEF Tiam, or CCM1 which when mutated in humans causes cerebral cavernous malformations (CCM) and forms a complex with other CCM proteins, 8) LF is a Zn++ metalloprotease that cleaves MEKs (also known as MAPKKs or MKKs) acting upstream of ERK mediated signaling (MEK1,2) and the JNK (MEK4,7) and p38 (MEK3,6) stress signaling pathways. Question marks indicate that links between the effectors of EF (PKA and EPAC) and LF (MEKs or possibly yet unknown effectors) and the exocyst remain to be determined. Abbreviations not defined above are: LRE = late recycling endosome and TJ = tight junction.
LF is a Zn++ metalloprotease that cleaves and inactivates nearly all members of the MAPKK (or MKK/MEK) protein kinase family [19-21]. MEKs are linchpin upstream regulators of the ERK, JNK, and p38 signaling pathways involved in diverse cellular processes including growth, cell fate determination, apoptosis, and response to various forms of cellular stress (Fig. 1). LF is comprised of four partially related domains (reviewed in [22]): an N-terminal PA-binding domain (I) that is highly related to the PA-binding domain of EF (much of which derives from an ancestral domain IV that has lost key catalytic residues); a domain (II) involved in binding to residues in MEK substrates distant from the cleavage site (which folds in a pattern similar to the catalytic domain of VIP2, a C2 type of ADP-ribosylase from the sister species Bacillus cereus, that lacks the catalytic residues required for NAD binding) and contains an inserted helical domain (III) involved in binding to the cleavage site of MEK substrates; and a catalytic domain (IV) related to clostridial neurotoxins, which together with domain III binds to “D-motif” docking sequences at the N-terminus of MEK substrates. LF cleaves these N-terminal sequences from MEKs and inactivates them since the deleted D-motif domains are important for binding to and activating their MEK targets proteins [23].
EF is a highly active calmodulin (CaM)-dependent adenylate cyclase [24] (Fig. 1) sharing homology with adenylate cyclase toxins CyaA from Bordetella pertussis, and ExoY from Pseudomonas aeruginosa [25]. EF is comprised of three primary domains, an N-terminal PA-binding domain, which as mentioned above is highly similar to that of LF, a catalytic domain consisting of two subdomains that form the active site at their interface, and a C-terminal helical domain. In the absence of the host co-factor CaM, the helical domain associates with the catalytic domain and blocks its activity. CaM binds to the N-terminal portion of the helical domain of EF, causing a large conformational change that dislodges the helical domain from the catalytic domain. Intracellular Ca++ levels also regulate EF activity, but do so in a biphasic manner with low to moderate levels of [Ca++] activating EF via CaM, and high levels reducing EF activity by competition between Ca++ and Mg++ ion in the EF active site (reviewed in [25]). As a consequence of its perinuclear association with later endosomal membranes [17, 18], EF generates a gradient of cAMP emanating from the nucleus and diminishing toward the plasma membrane [26]. In contrast, endogenous host adenylate cyclases are localized to the plasma membrane and generate an oppositely oriented gradient of cAMP, which is highest at the cell surface [26, 27]. Further details regarding the structure and biochemistry of the anthrax toxins are provided in several excellent recent reviews [22, 25, 28-31].
4. Toxins play important roles during two distinct phases of anthrax infection
In phylogenetic terms, B. anthracis is a surprisingly recent pathogen, having diverged from the parent B. cereus lineage as little as 17,000-26,000 years ago. A particularly successful and globally dispersed clade of B. anthracis (A-clade) emerged contemporaneously with human domestication of herbivores. Herbivores are thought to be the primary natural host of this pathogen [32, 33], and anthrax ravaged livestock (sheep, cows, and horses) during the 18th and 19th centuries in Europe, killing up to 50% of animals in a herd during epidemics and 10-15% of animals during endemic periods [7] . Anthrax is still a major concern to livestock and wild herbivores in many areas of the world and can also infect other mammals including rodents, rabbits, herbivores, carnivores and primates (reviewed in [7, 34]), although the sensitivity to infection varies considerably among species [35].
Anthrax can infect hosts via cutaneous, intestinal, or pulmonary (inhalation) routes [34]. The cutaneous form of the disease, typically initiated by bacteria gaining access to wounded skin, causes initial swelling (edema) at the site of infection, which then progresses to the formation of large painless sores that form large black “coal” scars. Although cutaneous anthrax is usually limited to the dermis, like the other two forms, it can on occasion lead to systemic infection, in which case the disease is often fatal. Systemic anthrax infection itself involves two basic stages: a largely asymptomatic “prodromal” stage (generally 2-4 days, but sometimes considerably longer) in which phagocytic cells engulf and transport bacterial spores to lymph nodes (those nearest the port of entry), undergo apoptosis, and release spores that germinate to produce vegetative rod-like bacteria [36], followed by a rapidly progressing “fulminant” stage (often leading to death in 1-2 days) in which bacteria proliferate and are systemically disseminated to nearly all organs via the bloodstream. This sequence of events has been well documented in animals (e.g., see legend to Fig. 3A) and human patients [37, 38].
Human patients suffering from inhalation anthrax generally seek treatment during the onset of the fulminant stage, and commonly present with flu-like symptoms, chest pain, labored or irregular breathing, tachycardia, hypotension, headache or disorientation (a particularly bad prognostic indicator often associated with bacterial infiltration of the brain or meninges) ([37-42]). During this stage bacteria proliferate and disseminate to vascularized tissues throughout the body where they disrupt organ function, in part through secretion of LT and ET. Frequently affected organs include secondary lymph nodes, lung, spleen, kidney, liver (Fig. 3B), intestinal serosa, heart, meninges or the brain proper. Another common feature of late anthrax infection is compromised vascular integrity resulting in low pressure hemorrhage of the venous microvasculature (Fig. 3C) or high pressure hemorrhage of arterioles and small arteries often accompanied by fluid edema (Fig. 3D), which aids in the further dissemination of bacteria within the tissue, leading ultimately to death by asphyxiation, heart failure, or cerebral/meningeal hemorrhage.
Comparative studies of the effects of infection with B. anthracis to those of injection of anthrax toxins by intravenous, intraperatoneal, or subcutaneous routes various animals including guinea pigs [43, 44], rabbits [45], rodents [46-59], and primates [60-66] established that injection of anthrax toxins recapitulated many of the disease symptoms associated with the fulminant stage of systemic anthrax infection in those animals and paralleled those in infected humans [37-42]. In addition, these studies identified cases in which EF and LF acted synergistically to produce their effects [15, 49, 67-69]. Injection of toxins obviously can only approximate the normal production of toxin from an advancing wave of bacterial infection that progressively permeates the different organs and tissues of the host. Thus, effects of the toxins have also been assessed through genetic studies, in which mice are infected with wild-type versus mutant strains of B. anthracis, which lack either or both toxins in encapsulated strains (pXO2+) [69, 70] or in capsule-deficient mutants (Sterne, pXO2-) [67, 71]. In addition, mutant mice lacking the anthrax-receptor in specific cell types have been infected with Sterne or toxin deficient mutants to identify host cell types mediating different effects of the toxins [72, 73]. Cumulatively, these various in vivo studies of toxin function have identified two constellations of activity that parallel the two basic phases of systemic anthrax infection. First, during the prodromal establishment phase, LT and ET act on phagocytic and migratory cells of the myeloid lineage (macrophages, dendritic cells, and neutrophils) that engulf and transport bacterial spores from the lung to the lymph nodes, altering their migratory behavior, inducing their cell death, and dysregulating their production of immune cytokines. Then, during the fulminant stage, the toxins play a key role in causing toxic-shock like symptoms that culminate in death of the host. Here late stage antibiotic treatment is ineffective in humans, guinea pigs or other animal models. During this final disease phase, the cardiovasculature is thought to be the primary target of the toxins. We consider these two distinct roles of the toxins separately below. As current reviews have covered the role of the toxins in immune cells in considerable detail [74-77], we briefly summarize the key findings in this area and then focus primarily on the more recent analysis of toxin effects on the cardiovascular system.
5. Toxins are required during the prodromal phase of inhalation anthrax infection
The prodromal stage of inhalation anthrax infection can be subdivided into two phases during which the toxins are essential for silencing and altering immune cell functions wherein LT exerts the predominant effect and ET plays a contributing role, as observed in the original experiments of Pezard et al. [67] and more recently dissected by Liu et al. [72] (although a recent study using bioluminescent toxigenic encapsulated strains of B. anthracis points to a more substantial contribution of ET than previously thought, and an important role for this toxin in the dissemination of bacteria [70]). In the first phase, spores are transported from the lungs via lymphatic vessels to the mediastinal lymph nodes [36], which are located in the central chest between the lungs and drain lymph from the lungs. This generally accepted model is based on the pioneering studies of Ross in guinea pigs [36] (Fig. 2A), but may be an oversimplification as more recent studies with bioluminescent B. anthracis in mice [34, 70, 78] (Fig. 2B) indicate that spores may germinate earlier than previously appreciated and that there are alternative paths of primary dispersal of spores or vegetative bacteria to other lymph nodes. Thus, a component of spore transport to the mediastinal lymph nodes may occur secondarily from a splenic reservoir [70]. During the second establishment phase of infection, bacteria or germinating spores are transported to the lymph nodes where they lead to massive apoptotic cell death of immune cells [38] and hemorrhage of the lymph node [38, 42]. Macrophages and lymphocytes are among the few cell types that can be killed by exposure to LF, while EF is generally not cytotoxic to immune cells and seems to act primarily by altering their activation, migration, and/or production of cytokines (reviewed in [76, 77]). Several of these toxin activities are highlighted in the following sections and are treated more comprehensively in [74-77].
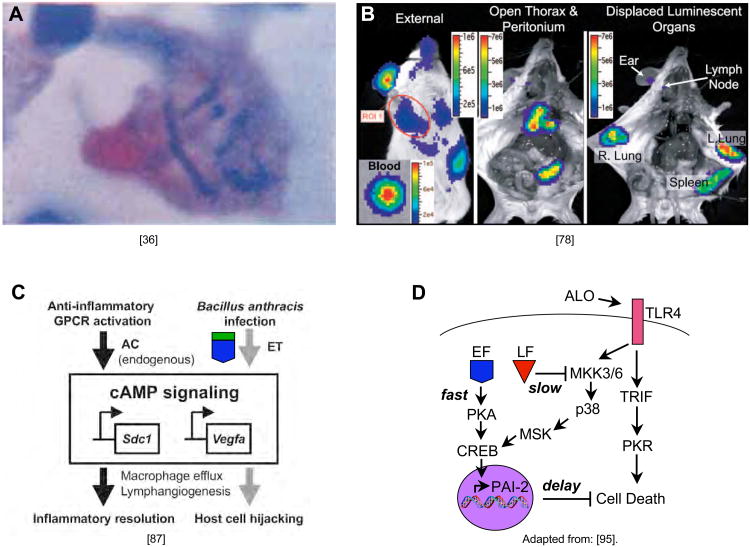
Figure 2. The prodromal phase of the disease: EF and LF help establish infection.
A) B. anthracis bacteria are transported from the lung to mediastinal lymph nodes in the guinea pig following infection by inhalation (Fig. 18 from [36]). B) Direct in vivo bioluminescence imaging of mice with a non-encapsulated strain of B. anthracis injected cutaneously into the ear reveals subsequent colonization of the lymph nodes, lung, and spleen (portion of Fig. 1C from [78]). C) EF mimics the anti-inflammatory activation of GPCRs in macrophages to induce migration of these cells, which in conjunction with the delay it creates in apoptosis (panel D), contributes to the “Trojan horse” delivery of spores and bacteria by macrophages to the lymph nodes (minor modification of Fig. 5 from [87]). D) A model for the coordinated effects of the pore forming toxins anthrolysin (ALO), LF and EF in inducing delayed apoptosis of infected macrophages (adapted and modified from Fig. 7 of [95]) in which ALO activates the TLR4 receptor which sends conflicting signals to induce (via PKR) and inhibit (via MEK3,6) apoptosis (by cleaving and inactivating MEKs, LF blocks the protective effect of MEK3,6 signaling shifting the balance of TLR4 signaling to cell death, but this effect is delayed by EF which acts via PKA/CREB to inhibit cell death, which presumably allows macrophages to migrate to lymph nodes where they then die and liberate their bacterial cargo).
5.1 Myeloid cells are a critical target of anthrax toxins
Recent experiments using conditional knockout mice lacking the ability to import toxins into cells of the myeloid lineage have cleanly delineated two distinct roles of anthrax toxins during infection [72]. In these elegant experiments, investigators from the Leppla group first showed that anthrax toxins gain access to myeloid cells (which include monocytes, macrophages and neutrophils) by binding to the CMG2 anthrax receptor and that the alternative receptor (TEM8) played little if any role in these cells. Mice with selective deletion of CMG2 function in myeloid cells were protected from doses of bacteria that were lethal to receptor-expressing control mice. Importantly, these mice were as susceptible as control mice to the individual and combined effects of LT and ET injection, indicating that the final lethal activities of the toxins were independent of their inhibition of immune cell function. This experiment (and others cited below) exclude the possibility that the fatal shock caused by the administration of toxins is merely a secondary consequence of a “cytokine storm” generated by infected monocytes/macrophages or released as a result of their lysis. However, the interaction of the two toxins to subvert the normal innate immune defense function of myeloid cells is central to the successful establishment of infection with B. anthracis.
5.2 Sensitivity of macrophages to LT killing
Perhaps one of the most complex and befuddling areas of anthrax research has been unraveling the mechanism and significance of macrophage sensitivity to killing by LT. This problem was first framed in rodents where it was observed that macrophages from certain inbred strains were highly sensitive to being killed by LT whereas those obtained from other strains were highly resistant. After a long pursuit of the genetic basis for this divergence, it was established that differing Nlrp1b (Nalp1b) alleles [79] were playing a central role. Nlrp1b is a component of the inflammasome pathway, which mediates cell death in LT sensitive macrophages through a rapid non-apoptotic mechanism known as pyroptosis. Cleavage of MEKs by LF still proceeds with equally efficiency in macrophages from both sensitive and insensitive mice [20, 80], provoking a slower classical apoptotic cell death pathway.
Another interesting finding is that different strains of mice exhibit varying sensitivities to systemic infection by B. anthracis, and surprisingly that this trait is generally inversely correlated to the in vitro susceptibility of their macrophages to killing by LT [35, 81, 82]. This observation has lead to the suggestion that premature killing of macrophages and associated inflammasome activation in Nlrp1b-sensitive strains may lead to a reduced dissemination of spores/bacteria due to killing of bacteria by neutrophils recruited in response to the IL1 release associated with inflammasome activation. Thus, macrophage sensitivity to toxin in mice is actually a host defense mechanism [83, 84]. Recently, the variable sensitivity of rats to LT, (sensitive strains can be killed in under 1 hour), was mapped to a Nlrp1b-homolog [85]. The linkage of rapid LT-mediated death to Nlrp1 in rats, which also controls rat macrophage sensitivity, may imply a direct relationship between macrophage sensitivity and animal death. Alternatively, rat Nlrp1 may control functions in cell types other than macrophages that are required for survival [85]. The relevance of Nlrp1 mediated killing of macrophages or other cells to anthrax disease in humans remains to be determined, however, since macrophages in monkeys [86] and all humans examined to date are insensitive to rapid LT-dependent killing, (i.e., this occurs only via the relatively slow apoptotic mechanisms). Thus, it will be interesting to examine whether other cell types relevant to anthrax pathogenesis in humans and animal models display Nlrp1b-dependent variations in sensitivity to LT killing.
5.3 ET alters immune cell migration
Many cellular processes are altered by ET as a result of the greatly increased levels of intracellular cAMP. Relevant to spore transport during the prodromal phase of infection, ET can increase the overall motility of infected macrophages [87]. This effect is mediated at least in part at the transcriptional level by cAMP-dependent protein-kinase-A phosphorylation and activation of the transcription factor CREB. Key genes induced by ET included vascular endothelial growth factor (VEGF) and Syndecan-1, which are both also induced by host G-protein coupled receptor (GPCR)-dependent activation of cAMP synthesis by endogenous adenylate cyclases. ET therefore mimics normal chemotactic signaling in which GPCR activation by ligands such as prostaglandins or adenosine induces macrophages to migrate rapidly to lymph nodes near foci of infection. In this case, ET seems to have co-opted a host signaling pathway, facilitating transport of B. anthracis spores from portals of entry such as the lung to nearby lymph nodes during the early establishment phase of the disease [87] (Fig. 2C). Live imaging of bioluminescent toxigenic encapsulated strains of B. anthracis support a role of ET in increasing dissemination of bacteria since strains expressing only ET but not LT do not accumulate as they otherwise would in intermediate locations such as lymph nodes in transit to final target organs [70]. However, in other model systems ET has been found instead to inhibit directed chemotaxis of macrophages, neutrophils [88], and endothelial cells [89] toward defined attractants, or to act in concert with LT to perturb chemokine signal reception in T cells and macrophages [90]. Further research is required to clarify these contrasting results.
5.4 Combinatorial effects of LF and EF on immunity
Anthrax toxins also alter the response of immune cells to cytokines as well as their production of immune signals and bactericidal factors such as reactive oxygen species (ROS) and secreted phopholipase-A2 during bacterial infection (reviewed in [74-77]). Reported effects of the two toxins have varied on the cell type tested and on whether they are generated in vivo by B. anthracis bacteria or delivered as purified factors. In general, however, both toxins tend to suppress immune cell cytokine signaling and/or reduce expression of cell surface activation markers [52, 68, 69, 86, 91-94]. Typically the toxins act additively [52, 94], although there are instances in which they interact synergistically or in opposition. In this latter case, the opposing actions of LT and ET either cancel each other out, or the activity of one toxin dominates the other. One interesting example of such opposing toxin activities involves apoptosis of macrophages. As discussed above, all macrophages undergo a relatively slow apoptotic cell death in response to LT cleavage of MEKs, the delayed kinetics of which allows vegetative bacteria to be released following arrival at lymph nodes, from where they can ultimately disseminate. ET seems to play an important role in slowing the macrophage cell death by temporarily suppressing apoptosis [95] that would otherwise result from LT inactivating the MEK3,6/p38 pathway [20]. This mitigating effect of EF is mediated at least in part by CREB-dependent activation of the cell survival factor plasminogen activator 2 (PAI-2) [95] (Fig. 2D).
6. Anthrax toxins disrupt the cardiovascular system and endothelial barrier integrity
Death of the host during the fulminant stage of anthrax infection is often associated with toxic shock-like symptoms typified by severe respiratory dysfunction and hypotension followed by cardiac failure. These symptoms are similar in certain respects to those caused by cytokine storms such as those resulting from a runaway histamine response and anaphylactic shock. Thus, a prevalent proposal was that macrophages were a source of proinflammatory cytokines and that anthrax toxins induced expression of these factors to indirectly produce systemic shock.
A different view of late stage of anthrax pathogenesis has emerged over the past decade based on a variety of new evidence suggesting that anthrax toxins kill the host by direct actions on the cardiovascular system. Several excellent recent reviews summarize the evidence for this new view in detail [75, 76, 96-99] and highlight on the following observations: 1) LT and ET in general reduce the secretion of cytokines [52, 68, 92-94] and therefore oppose rather than induce a cytokine storm, 2) TNF-receptor, Caspase-1, and IL-1 receptor knock-out mice deficient in key inflammatory pathways are not resistant, but rather more sensitive, to anthrax infection than parental mouse strains [83, 100, 101], 3) agents used to treat inflammatory shock such as corticosteroids [102], vasopressive drugs [103], or volume replacement [40, 104] have proven ineffective in treating anthrax toxemia, 4) LT [51, 105, 106] and ET [56, 71]) directly decrease barrier integrity of vascular endothelial cells (VEC) in vivo and in cell culture (LT has also been reported to induce apoptosis in these cells [107, 108], although in vivo infection with B. anthracis or injection of LT into animals typically is not characterized by overt VEC death), 5) ET as well as LT cause severe cardiovascular dysfunction leading to death with ET primarily causing hypotension through increasing vascular permeability and inducing vasodilatation [48, 52, 56, 58, 59, 71, 76, 109-111] (Fig. 3 E,F) and LT acting directly on the heart to compromise cardiac structure [55] (Fig. 3H) and performance [50-55, 58, 59, 71, 76, 105, 106, 109-113] (there is an older alternative view, however, in which cardiovascular dysfunction is proposed to be a secondary consequence of a primary effect of anthrax toxins on the CNS in both rats and primates [114, 115]), and perhaps most conclusively, 6) selective elimination of toxin entry into myeloid cells, which prevents establishment of infection by B. anthracis (see above), does not protect against the lethal effects of LT and ET [72].
According to the new model, an important effect of LT and ET during the fulminant phase of infection is to breach the vascular barrier between the bloodstream and tissues, thereby leading to a variety of systemic effects that contribute to fatal outcomes including hypotension, tachycardia, hemoconcentration, coagulopathy, anoxia, cardiac failure, meningitis (or direct effects on CNS function). In the context of natural bacterial infection, LT and ET also contribute to the dissemination of bacteria into target organs (e.g., spleen, kidney, heart, adrenal gland, intestine, and brain) as well as penetrating highly vascularized membranes such as the pulmonary pleura and cerebral meninges. Proliferation of bacteria in the circulation and in tissues during this stage will also induce classical host sepsis pathophysiology that contributes to disease progression and adverse outcome [63]. Indeed in certain animal models, challenge with lethal toxin-deficient B. anthracis is sufficient to produce septicemic mortality [116]. This interplay between the effects of the toxins and the invading bacteria that produce them is highly coupled both spatially and temporally. Depending on the primary organ systems affected in specific individuals death can therefore result from vascular leakage induced respiratory shock, brain hemorrhage, or possibly disruption of autonomic CNS functions.
In this following section, we first summarize the basic cell biology underlying the formation of cell-cell junctions, then examine how LT and ET act in concert to breach these junctions, leading to increased vascular endothelial permeability in both cell culture and in vivo systems. Studies from various models including human cell culture, mice, zebrafish, and Drosophila have contributed to an emerging picture in which anthrax toxins disrupt intercellular junctions. Although the two toxins compromise intercellular junctions by several mechanisms, one point of convergence is inhibition of endocytic recycling, which normally plays a central role in targeting adhesion molecules and cell-cell signaling components to sites of cell-cell contact. We also address an intriguing unresolved paradox regarding the role of cAMP in promoting versus disrupting endothelial barrier function. Finally, we consider other vascular cell types and processes that may be involved in the barrier disrupting effects of anthrax toxins.
7. Cell-cell junctions
Polarized sheets of epithelial and endothelial cells are held together by an elaborate system of cell-cell junctions that form at discrete positions along the apical to basal axis of these cells. Cell polarity is initiated by the polarity proteins Par3 (Bazooka in Drosophila) and Par6 and atypical protein kinase C (aPKC), which were originally identified as being essential for establishing cell polarity in early C. elegans embryos (reviewed in [117]). These proteins interact with a complex of cytoplasmic scaffolding proteins including Pals, and Patj and the transmembrane protein Crumbs to define the most apical domain of the cell, and to exclude apical accumulation of proteins that act more basally such as Par1 [reviewed in [118, 119]]. In vertebrate cells, this most apical zone ultimately forms the zonula occludens or tight junction (TJ), which surrounds the cell as a continuous gasket limiting the exchange of small molecules between apical and basal domains of the cell (Fig. 4A). The key proteins of TJ regulating paracellular permeability (flow between cells) are transmembrane proteins in the Claudin family that form homodimeric and heteromeric complexes between adjacent cells and determine the size and charge of molecules that can cross the TJ. In addition, TJs include the transmembrane adhesive proteins Occludin and JAMs as well as intracellular scaffolding proteins such as ZO-1, that link membrane components of the TJ to the underlying actin cytoskeleton (Fig. 4B).
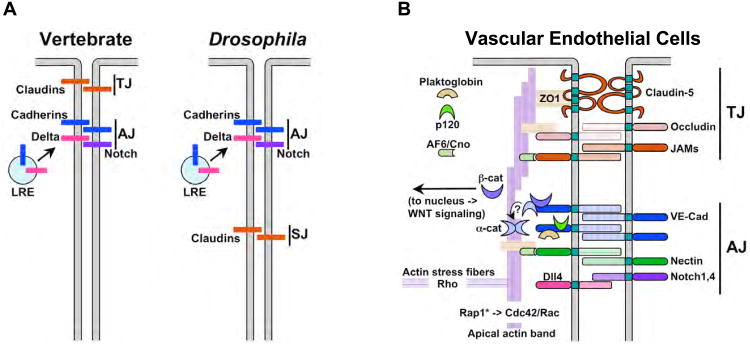
Figure 4. The endothelial barrier.
A) Diagram indicating the locations of the vertebrate tight junction (TJ) or its equivalent in Drosophila and other invertebrates (septate junction - SJ) relative the adherens junction (AJ). Key homophylic adhesion factors are also depicted (Claudins for the TJ and Cadherins for the AJ) as well as transport of cadherins and Notch ligands such as Delta to the AJ via the late recycling endosome (LRE). B) A more detailed diagram of the primary components of the AJ and TJ in VEC, which are not always restricted to distinct domains as in epithelial cells e.g., as depicted in panel A) but are intermingled in some VEC (reviewed in [124]). The TJ contains three adhesion molecules, Claudins (e.g., Claudin-5 in VEC), Occludin, and JAMs, which are linked via Claudins to the scaffolding protein ZO-1 and circumferential Actin cables. The AJ includes cadherins (e.g., the type-II VE-Cad in vascular endothelial cells) which bind linking proteins of the Armadillo family (β-catenin, Plakoglobin, p120) and α-catenin, as well as supportive adhesion factors (e.g., Nectin in vertebrate cells and Echinoid in Drosophila cells) and signaling components such as Delta-like4 (Dll4) and Notch1/Notch 4, the VEGF-Receptor2 (not shown in this figure, but see Fig. 5F). α-catenin (α-cat) can either bind to VE-CAD via an interaction with β-catenin (β-cat) or can form dimers that bind to and stabilize actin cables, but it cannot bind to both actin and cadherins at the same time. β-catenin that is not bound to cadherins can migrate to the nucleus where is acts as a transcriptional mediator of the WNT pathway. High levels of clustered cadherin expression at the AJ sequester nearly all available β-catenin, thus prevent it from participating in transcriptional regulation. Decreased barrier function, however, can result in a release of β-catenin that is then can translocate to the nucleus. Also shown is the adaptor protein Afadin 6 (Af6), known as Canoe (Cno) in Drosophila, which links Nectin or Echinoid molecules respectively to ZO1 and the actin cytoskeleton. In addition to circumferential actin cables that stabilize apical junctions, actin is also organized in perpendicular stress fibers (actin stress fibers) that may participate in membrane trafficking of cargo to the cell surface (see Fig. 5A).
Just basal to the TJ, or intermingled with it in VEC, is the adherens junction (AJ), which plays a central role in establishing and maintaining adhesion between cells [reviewed in [120]] (Fig. 4A,B). The structure and composition of AJs has been well conserved between vertebrates and invertebrates and depends primarily on the activity of cadherins, a highly conserved family of Ca++-dependent homophylic transmembrane adhesion proteins. Cadherins are localized to the AJ via interactions between their cytoplasmic tails and a complex of cytoplasmic scaffolding proteins including β-catenin, α-catenin, Plakoglobin, and p120. In addition, another cytoplasmic scaffolding protein Afadin links the AJ to the circumferential actin cytoskeleton via a second set of supporting homophylic molecules (e.g., Nectin in vertebrates and Echinoid in Drosophila). An apical circumferential band of actin helps stabilize both the TJ and AJ complexes, while actin/myosin based contraction of a perpendicularly arranged set of radial actin stress fibers, weakens cell junctions. The balance between these two actin superstructures is determined in part by the competing activities of the small GTPases Cdc42 and Rac1, which favor assembly of circumferential actin cables, versus RhoA, which promotes centripetal stress fiber formation. One mechanism dampening RhoA activity at the AJ is binding of the inactivating RhoGAP to p120 (which in turn is bound to the cytoplasmic tail of VE-Cad). RhoA is also involved, however, in targeting proteins to the AJ, so it's role in AJ assembly and maintenance is complex (reviewed in [121]). Finally, the basal region of the cell below the AJ is defined by the transmembrane proteins Neurexin IV and NRG, the scaffolding proteins Scribble, Dlg, Lgl, and Coracle, and the kinase Par1, which opposes recruitment of apical components such as Par3 to this zone [117]. Drosophila cells lack apically localized TJs and instead have basally located septate junctions that include Claudins and form a tight seal between apical and basal paracellular compartments [reviewed in [122]].
7.1 Establishment and maintenance of adherens junctions
Key AJ proteins such as cadherins are targeted to the appropriate location in the plasma membrane via vesicle trafficking and the stability of junctions is determined in part by the rate of cadherin endocytosis (reviewed in [120, 123, 124]). AJ-bound vesicles are comprised of late recycling endosomes fused to vesicles carrying primary Golgi cargo. The final step in this endocytic recycling process is mediated by a complex of eight proteins known as the exocyst, which was originally identified based on its requirement for secretion in yeast (reviewed in [125, 126]). Vesicle fusion with the plasma membrane is initiated by interaction between the small GTPase Rab11 (which is present on membranes from the late recycling endosome), and Sec15 from the exocyst complex (which connects via other components of the complex to the plasma membrane). These docked vesicles then fuse to the plasma membrane in a SNARE-dependent fashion delivering their cargo to the AJ. Delivery of cargo to the AJ may involve two distinct, albeit coupled, targeting mechanisms involving: 1) tethering of the exocyst components to interacting proteins at membrane delivery sites, and 2) actin mediated tracking of Rab11 tagged membrane vesicles to the membrane (which involves complexes with Af6/Cno at the AJ) [127] (Fig. 5E).
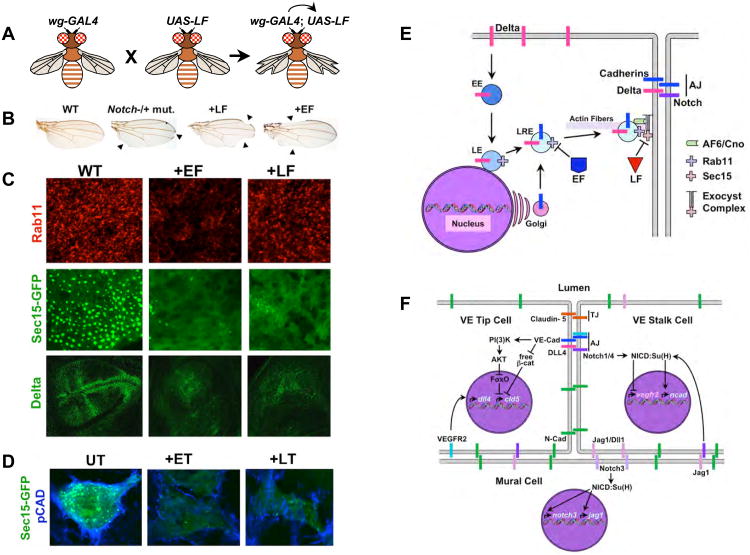
Figure 5. Anthrax toxins inhibit exocyst mediated trafficking to the AJ.
A) A schematic summary of GAL4/UAS expression system [137]. Flies (left) carrying a transgenic construct (wg-GAL4) express the yeast GAL4 transactivator protein selectively in the wings. These flies have a normal phenotype, as do flies carrying a transgenic construct in which a cDNA encoding the enzymatic portion of LF has been placed under the control of the Up-Stream-Activating (UAS) sequence (middle). When these two strains of flies are crossed to each other, their progeny (right) inherit both the wg-GAL4 construct and UAS-LF construct to which GAL4 protein can bind and activate transcription of the UAS-LF transgene in the wing, resulting in a notched wing phenotype. B) Wings from adult fruit flies: Left panel, wild-type; second panel, a heterozygous Notch mutant with 50% of wild-type activity displaying a weak notching phenotype in which sections of the edge of the wing are missing (arrowheads); third panel, fly expressing LF in wing cells has gaps in the edge of the wing (arrowheads) similar to those observed in Notch mutants; right panel, fly expressing EF in wing cells also has gaps in the edge of the wing (arrowheads). C) Fields of developing cells in the Drosophila wing primordium from wild-type flies (WT, left panels), flies expressing EF in the wing (+EF, middle panels), and flies expressing LF in the wing (+LF, right panels). Cells were stained for expression of the endogenous Rab11 or Delta proteins or for expression of a GFP-Sec15 fusion protein associated with large secretory vesicles as indicated in the figure. EF reduces the levels and activity of Rab11 resulting in loss of cell surface Sec15-GFP expression, whereas LF does not affect Rab11, but does eliminate Sec15-GFP expression at the cell surface. Thus, EF acts by blocking Rab11 function (and indirectly Sec15-GFP expression), while LF blocks Sec15-GFP expression more directly. Inhibition of exocyst activity results in greatly reduced transport of the Notch ligand Delta and Drosophila E-cadherin (not shown here, but see [71]) to the cell surface. D) ET and LT inhibit exocyst mediated trafficking in human brain microvascular endothelial cells. Pan-cadherin (blue) and Sec15-GFP (green) staining are greatly reduced by treating cells with either ET or LT. Panels A-C are assembled from figures in [71]. E) Diagram summarizing the effects of EF and LF on the exocyst. Abbreviations are as in previous figures. F) Known roles of cadherins and Notch signaling in VEC that might be disrupted as a result of anthrax toxins inhibiting exocyst mediated trafficking of these proteins to the cell surface. The first Notch-dependent interaction involved vascular remodeling in which vein inducing signals such as VEGF acting via the VEGF-R2 RTK induce expression of the DLL4 ligand in microvascular endothelial cells leading these cells to initiate formation of a new vascular outgrowth. These DLL4 expressing cells become tip cells, disengage themselves transiently from their neighbors to organize vasculogenesis, and signal to their neighbors via Notch1/Notch4 to remain as existing stalk cells. One important element of Notch signaling in stalk cells is to repress expression of VEGF-R2, since signaling via this RTK activates DLL4 expression and induces the alternative tip cell fate. In addition, VEGF-R2 activity can be inhibited post-translationally in these cells by binding to VE-Cad at the AJ, which sequesters VEGF-R2 in an inactive form. Notch signaling is also important in a reciprocal form of signaling between vascular endothelial cells and mural cells (smooth muscle cells surrounding veins, venules, arteries and arterioles, or pericytes sealing junctions between microvascular cells in impermeant capillary beds such as those constituting the blood-brain-barrier). Adhesion between VEC and mural cells depends on the type-I N-cadherin, which is not clustered in at the AJ (in distinction to VE-Cad) but rather is distributed broadly over the cell surface. N-cadherin expression in VEC depends on Notch signaling (most likely mediated by the Jagged1 expressed in mural cells activating the Notch1/Notch4 receptors in VEC). Notch signaling is also required in mural cells to promote their differentiation and is mediated by the Jagged (Jag1) or Delta-like1 (Dll1) ligands expressed in VEC signaling via the Notch3 receptor in mural cells. One effect of this signaling is to maintain the Notch signaling network by activating expression of both the Notch3 and the jagged1 genes. There is also important cross-talk between the AJ and TJ in VEC. One well documented example of this type of junctional interaction is the VE-Cad-dependent activation of Claudin-5, an essential barrier component of the VEC TJ. Clustering of VE-Cad at the AJ, which provides a strong coordinated signal, results in activation of the PI(3)K/AKT phosphorylation cascade that terminates in inactivation of the transcriptional repressor FoxO. One target gene that is otherwise repressed by FoxO in VEC is the claudin-5 (cld5) gene. VE-Cad also relieves a second form of constitutive repression of cld5 expression mediated by β-catenin, which as indicated above is accomplished by VE-Cad sequestering this potential transcriptional co-repressor at the cell surface. Blocking both FoxO and β-catenin mediated repression of cld5 is then sufficient for activation of this gene in VEC.
In addition to mediating adhesive interactions between adjacent cells in epithelial or endothelial sheets, AJs provide an important site of cell-cell communication. For example, activity of the VEGF-2 receptor tyrosine kinase (VEGF-R2) is inhibited by association with VE-cadherin at the AJ, while signaling by VEGF-R2, mediated by Src phosphorylation of VE-cadherin, negatively regulates VE-cadherin mediated adhesion and leads to barrier disruption (reviewed in [123, 128]). Another signaling system for which both the ligand and receptor are localized to the AJ is the Notch signaling pathway (reviewed in [129]). Notch signaling is involved in many binary cell fate choices during development and plays a central role in vertebrate vascular development both during embryogenesis (reviewed in [130]) as well as in vascular remodeling in the adult (reviewed in [131]). In this latter capacity, Notch signaling favors maintenance of the tight patent vasculature over the formation of new microvascular growth induced by tumors via VEGF signaling (reviewed in [132]).
Although beyond the scope of the current review, there are important interactions between proteins defining the various junctional domains (as well as the reciprocal inhibitory interactions between apically versus basolaterally localized Par protein complexes mentioned above [117]). For example, in MDCK cells, AJ assembly precedes that of TJs and AJs are required to initiate formation, but not maintenance of TJs [133]. One mechanism underlying cross-talk between the AJ and TJ has recently been elucidated in VEC [134]. When these cells undergo VE-cadherin-dependent contact, they induce expression of Claudin-5, which plays an essential role in maintaining vascular integrity at the blood-brain barrier. This activation occurs at the level of transcription of the cld5 gene, and is mediated by regulating the PI(3)K/AKT/FoxO pathway and sequestering the cadherin scaffolding protein/nuclear transcriptional cofactor β-catenin to the plasma membrane (Fig. 5F).
8. Anthrax toxins are active in flies
As mentioned above, studies of confluent VEC in culture have revealed that both LT [105] and ET [71] can act directly on these cells to increase barrier permeability. Consistent with the important role that cadherins play in adhesion between VEC [124], studies with LT revealed a decrease in VE-cadherin levels at the cell surface following toxin treatment [105]. In the case of LT, known MEK targets may mediate some but not all effects of this toxin, since small molecule inhibitors blocking various MEKs only partially reproduced the effect of LF. In vivo studies on mice [51] and zebrafish [106] also found that LT can trigger vascular leakage, and LT-induced leakage in zebrafish could be largely rescued by stage specific expression of an activated form of MEK1 [112]. MEK-dependent signaling has also been shown to play an important role in neovascularization [135]. While these studies provided strong evidence for a direct role of anthrax toxins in compromising vascular integrity the mechanism(s) by which the toxins achieved this effect remained unresolved.
To address the cell biological mechanism by which anthrax toxins compromise barrier integrity, experiments were conducted using the genetic model system Drosophila melanogaster, which offers many advantages for studying host-pathogen interactions [136]. These studies in the fruit fly revealed that LF and EF act convergently to block a common step in protein trafficking to AJs. As technical background for these experiments, transgenes encoding the catalytic moieties LF and EF can be expressed directly in the cytoplasm of Drosophila cells using the conditional GAL4/UAS transactivation system [137] (Fig. 5A). In this method, cDNAs encoding the toxins are placed under the control of a cis-acting element from yeast called the upstream activator element (UAS), and transgenic flies are obtained carrying insertions of these UAS-toxin constructs into their genomes. A large variety of fly stocks exist that express the yeast transactivating transcription factor GAL4 in various cell-type specific patterns. One can then cross such a GAL4 “driver” stock with fly stocks carrying UAS-LF or UAS-EF constructs, and the progeny of such a cross will express the toxin transgene in the same cells as the GAL4 driver. For example, the wg-GAL4 driver expresses GAL4 selectively in cells of the developing wing. If one crosses wg-GAL4 flies to flies carrying a UAS-LF transgene, their progeny express LF specifically in the wing (Fig. 5A), causing nicks to form along the edges of adult wings (Fig. 5B, see below).
When LF and EF were expressed in flies, phenotypes were observed in various tissues and stages of development that were consistent with their known biochemical mechanisms of action [138]. Moreover, genetic epistasis experiments indicated that the toxins acted at the predicted step in the various pathways (e.g., LF acted at the level of MEKs in the JNK and ERK pathways and EF activated PKA signaling by relieving PKA-R inhibition [138]). These experiments validated the fly as a system for examining the effects of anthrax toxins and opened the way to exploring for new and/or cooperative effects of these toxins.
9. Anthrax toxins cooperatively inhibit endocytic recycling to cell-cell junctions
In the course of analyzing the effects of expressing LF and EF in flies, a novel unexpected phenotype was observed upon expression of either toxin, which closely resembled the effect of mutations compromising activity of the Notch signaling pathway (e.g., notches in the edge of the wing and thickened wing veins) [71] (Fig. 5B). Moreover, LF and EF acted in a highly synergistic fashion to elicit this phenotype suggesting that these two toxins disrupted a common process [71]. Further analysis of these toxin effects pointed to defects in endocytic trafficking of the Notch ligand Delta to AJs (Fig. 5C) [71], consistent with previous studies showing that Delta must undergo endocytic recycling to activate Notch signaling [139]. Consistent with their genetic synergy, LF and EF interfered at the same step in the recycling process wherein late endosomal vesicles interact with the exocyst complex to tether cargo laden vesicles to the plasma membrane in preparation for vesicle fusion. EF reduced the level and activity of Rab11 (associated with late recycling endosomes), while LF disrupted cell surface expression of its exocyst partner Sec15 [71] (Fig. 5C). Inhibition of the exocyst by EF or LF also reduced junctional expression of E-cadherin (which was more severe in EF than LF expressing flies), consistent the known role of the exocyst in cadherin trafficking to the AJ [140].
The trafficking effects of LT and ET were also examined in mammalian VEC and, as in flies, they inhibited the exocyst and cadherin transport to the AJ [71] (Fig. 5D). ET also increased the permeability of confluent human VEC monolayers measured by transepithelial resistance (TER) [141] or dye leakage in transwell assays [71], and infection of mice with B. anthracis Sterne caused toxin-dependent pleural effusions in the lung (Fig. 3E) as well as vascular dye leakage at the site of subcutaneous inoculation in the Miles assay (Fig. 3F) [71], with EF having the primary effect and LF providing a supporting role. These findings contrast with a study in which purified toxins were injected subcutaneously in mice [51] where it was found that LT, but not ET, caused leakage in the Miles assay. In another study, however, purified ET did cause leakage using the Miles assay in rabbits [56], although in these experiments leakage was assayed after a longer period of time following the injection of toxin than in the mouse experiments. Whether these various results reflect differences in delivery (bacterially produced versus purified toxin), species differences (mice versus rabbits), or time course of the experiments requires further analysis. Different effects of LT treatment on permeability of endothelial cells in culture have also been reported. Thus, in one study, LT treatment decreased TER and increased albumin flux across primary lung microvascular endothelial cell monolayers [105], whereas LT did not decrease TER [141] or increase transwell dye permeability in brain microvascular endothelial cells [71]. These divergent responses may reflect cell type differences as cadherin levels were only modestly decreased in HBMECs by LT, whereas primary dermal microvascular endothelial cells displayed more substantial decreases in cadherin levels upon LT treatment [71]. None-the-less, in aggregate, these experiments provide strong evidence for ET and LT compromising endothelial barrier integrity, albeit with the two toxins contributing to varying degrees depending on the experimental setting and/or cell type tested.
9.1 Potential molecular mechanisms of LF and EF actions on the exocyst
A key issue regarding the inhibition of exocyst-mediated endocytic recycling by LF and EF is the precise molecular mechanism by which these two toxins act. Based on their known biochemical activities, several plausible pathways could be involved in their interference with exocyst trafficking. In the case of LF, Warfel and colleagues showed that known MKK targets of LF could be involved since small molecule inhibitors of ERK and JNK signaling caused a decrease in TER in confluent VEC cultures, which although weaker than LT treatment, had similar time courses [105]. However, inhibition of the p38 pathway had an opposite effect of increasing TER and counteracted the effect of ERK and JNK inhibitors rendering a cocktail containing all three inhibitors virtually neutral with regard to altering TER. In addition, treatment with the single inhibitors of ERK and JNK differed from LT in that they did not lead to significant barrier permeabilization to larger molecules. The authors thus speculated that LF might be working in part via non-MKK targets to increase barrier permeability. A role of MEKs in promoting vascular integrity is supported by experiments in zebrafish embryos, in which conditional expression of an activated form of MEK1 rescued vascular barrier disruption in vivo caused by LT treatment [112]. There also are links between MEKs and the exocyst pertaining to its various roles in JNK-dependent cell migration [142], cell survival [143], and ERK-dependent epithelial permeability in MDCK cells [144]. In the latter instance, it was found that Sec10 overexpression strengthened the epithelial barrier and increased ERK activation, while inhibition of ERK increased the barrier disrupting effect of hydrogen peroxide. In addition, the MEK1 binding protein p18 anchors the MEK-ERK pathway to Rab11 positive late endosomes [145]. Whether the primary effect of LT in VEC is to disrupt ERK/JNK signaling is an important unresolved issue for future investigation. If novel LT targets are involved in this process, it will be interesting to know whether they may also participate in Nlrp1/inflammasome activation by LF where non-MKK targets may mediate rapid cell death in macrophages and possibly other cell types.
There are also several strong connections between ET induced cAMP production and the exocyst. With regard to the classic cAMP effector PKA, the Rab11 binding protein Rip11 binds to PKA (which can phosphorylate Rip11), as well as the motor proteins myosin V and kinesin II. Indeed motor proteins have been suggested to play a role in transporting exocyst components to cell junctions [127], consistent with Rip11 being required for protein trafficking to the cell surface [146]. In addition, the AKAP-anchoring protein MyRip acts as a perinuclear scaffold to interact with PKA and the exocyst components Sec6 and Sec8 [147]. The second cAMP effector EPAC (a cAMP-dependent Rap1 GEF) is concentrated in a perinuclear pattern, and this localization is increased by cAMP [148]. When cAMP binds to EPAC it activates its primary known effector Rap1, which plays an essential role in re-establishing cellular junctions following cell division [149]. Activation of Rap1 (Rap1*) can be detected in vivo using a FRET construct [150] revealing two prominent pools of Rap1* in VEC. One Rap1* pool, present in cells undergoing VE-cadherin mediated adhesion, is located at the AJ where Rap1 interacts with the RAL-GDS, which activates the exocyst associated small GTPase RalA (reviewed in [151, 152]), as well as the scaffolding proteins Cno/Afadin [153, 154], MAG-1 [150] or CCM1, which is activated by PDZ-GEF or Tiam, and recruits KRIT1 [155] to stabilize AJ-dependent barrier formation [150, 156] (reviewed in [124, 152]). The majority of Rap1*, however, is localized in a perinuclear pool [150], which coincides with the site of high levels cAMP production by EF [26] (recall that following its translocation into the cytoplasm EF remains associated with perinuclear late endosomal membranes [146], as is EPAC [148], the cAMP-dependent GEF for Rap1). It will be interesting to determine whether these two pools of Rap1* exert different influences on AJ integrity or exist in a balanced equilibrium that may be altered by localized perinuclear production of cAMP by EF. Examining these and other possible scenarios should provide fertile grounds for future studies on how EF and LF collaborate to inhibit endocytic recycling.
9.2 The cAMP paradox
As summarized above, abundant in vivo and cell culture evidence suggests that ET causes vascular leakage (e.g., Fig. 3E,F). There is an important paradox hidden in these results, however, which is that an extensive prior literature suggests that increased levels of cAMP in various epithelial and VEC has the opposite effect of increasing in barrier function (for example, Fig. 3G, [157, 158] and reviewed in [159]). This barrier protective effect of cAMP elevation, which has been suggested to be mediated at least in part by a direct interaction between the EPAC effector Rap1 [150, 155, 156], has also been shown to counteract the effect of inflammatory agents such as TNF-α that decrease barrier integrity [159]. Several non-exclusive explanations may reconcile this apparent paradox including: 1) the peak levels of cAMP generated by endogenous pathways versus EF, 2) kinetics and/or duration of cAMP production, 3) subcellular localization of cAMP synthesis, which may differentially activate the effector molecules PKA and EPAC anchored to specific subcellular compartments by scaffolding proteins such AKAPs, 4) interaction of toxin generated cAMP with cellular machinery regulating endogenous cAMP levels, and 5) different cell types responding differently to a given cAMP stimulus based on expression of different effectors. There are known examples where each of these parameters has been implicated in determining the response of a specific cell to a particular mode of cAMP production, which are briefly summarized below.
With regard to levels, kinetics, and localized production of cAMP, comparative studies in which cells were treated with various cAMP elevating agents have reported marked differences in a variety of cellular responses including morphological transformation (e.g. cell rounding), transcriptional effects (e.g., CREB mediated gene expression), altered cell migration or chemotaxis, and induced ion fluxes (e.g., Cl-secretion mediated by the Cystic Fibrosis Transmembrane Receptor - CFTR - ion channel). These cAMP inducing agents include cAMP analogues such as 8Br-cAMP, agents inducing endogenous cAMP production (e.g., forskolin), or different cAMP producing toxins (reviewed in [160]) such as EF, CyaA toxin from Bordetella pertussis (also a CAM-dependent AC), pertussis toxin (an ADP-ribosylase that inactivates the Gαi, relieving inhibition of endogenous AC), and cholera toxin from Vibrio cholerae (an ADP-ribosylase that constitutively activates endogenous Gαs proteins, which in turn activates endogenous ACs).
One noteworthy study of T-cell activation suggested that cAMP levels were more critical than the subcellular or temporal patterns of cAMP production [161]. The authors found that low levels of the EF or CyaA toxins promoted differentiation of T-cells along the Th2 versus the Th1 lineage while high levels of either toxin blocked T-cell activation by antigen receptor ligation, which was dependent on a strong burst of endogenously produced cAMP. These investigators further proposed that high, but not low, levels of cAMP produced by the AC toxins swamped the incoming endogenous burst of receptor mediated cAMP production. Although EF and CyaA have different kinetics and subcellular distributions, these parameters did not have appreciable effects (EF is internalized slowly and remains associated with the late endosomes creating a gradient of cAMP that is highest in a perinuclear pattern [17, 18, 26], whereas CyaA is translocated directly across the plasma membrane where it rapidly begins producing cAMP near the cell surface [161, 162]). Another potential consideration regarding toxins generating high levels cAMP is that synthesis of this second messenger may deplete ATP pools and thus have indirect consequences on cell metabolism or signaling mediated by AMPK or other effectors.
Another study of T-cells treated with ET emphasized a kinetic contribution of delayed but maintained cAMP production and a potential contribution from the perinuclear source of cAMP [162]. The authors suggested that cAMP acts in a two-step process wherein a first phase of CREB activation is followed by a refractory period in which cells are unresponsive to inducing factors such as T-cell receptor cross-linking agents. Treatment of these same cells with CyaA, cholera toxin, or forskolin, all of which have rapid kinetics building quickly to peak cAMP levels (but decaying with differing kinetics), lead to a more transient activation of CREB phosphorylation and did not block activation in cells treated later with T-cell activators.
Studies performed with the cytoplasmically delivered ExoY toxin from Pseudomonas aeruginosa directly addressed the cAMP paradox [163]. In lung endothelial cells forskolin and ExoY lead to comparable high levels of cAMP production (≈ 800× normal) and both agents have similar rapidly peaking kinetics [162], yet only ExoY decreased barrier function. The most obvious difference between these two agents is the subcellular compartmentalization of cAMP production, which is cytoplasmic for ExoY and juxtamembranous for forskolin. Consistent with its effects on lung endothelial cells in culture, infection of mice with ExoY+ but not ExoY- strains of P. aeruginosa caused lung edema [163].
Cell type is also likely to play an important role in the response to cAMP. Thus, even within the relatively narrow category of VEC, which include macrovascular endothelial cells (e.g., human umbilical cord endothelial cells HUVECs) as well as venous or arterially derived microvascular endothelial cells, important differences have been reported depending on the precise derivation of the cell. For example, barrier function measured by TER is 10-fold greater for microvascular versus macrovascular endothelial cells in culture [164] and is also greater for lung arteriole versus venous microvascular endothelial cells in vivo [165], consistent with the higher density of tight junctions observed in these cells by EM [166]. Perhaps most importantly regarding the effect of EF, there are profound differences in both resting and induced cAMP levels in different VEC. Ca++-inhibited cAMP synthesis dominates in macrovascular cells whereas phosphodiesterase-dependent degradation of cAMP plays the key regulatory role in microvascular cells [167]. Variations in Ca++ sources and levels would also be expected to influence EF activity in various cells given that EF activity has a biphasic dependence on [Ca++] (see above). Indeed, there is evidence that the response to ET is cell type dependent. Thus, ET or CyaA treatment of macrovascular HUVECs leads to decreased transmembrane conductance [56], whereas ET treatment of human brain microvascular endothelial cells results in decreased TER [141], disruption of junctional ZO1 staining [141], increased dye permeability [71], and reduced cadherin levels [71], the latter also being observed in primary lung microvascular endothelial cells [71]. It is possible that the duration of toxin treatment also played a role in the latter experiments since decreases in cadherin expression were only observed after 48 hours of exposure to ET [71]. The opposing effects of EF versus the EPAC specific cAMP analog 8CPT in generating versus reversing vascular infusion, respectively (Fig. 3F,G), may also be related to timing since the former experiments [71] assayed leakage after six hours versus one hour for the latter [157].
Finally, ET may act by other mechanisms to increase ion flux across the vascular endothelium. In analogy to the action of cholera toxin in intestinal epithelial cells, the edema forming activity of ET may in part be mediated by the cAMP-dependent chloride secretion pathway. Although ET has been shown to act like Ctx to promote Cl- secretion in intestinal epithelial cells [168], surprisingly the edema-inducing activity of ET has not yet been scrutinized in VEC or pursued mechanistically.
Further studies are clearly needed to tease apart how various pathways such as exocyst mediated trafficking and Cl- secretion as well as cell type specific differences and temporal factors contribute to the effects of ET on permeability of the vascular endothelium. Such research will hopefully illuminate the different integrated facets of ET function and resolve the cAMP paradox.
10. Interactions between vascular endothelial and mural cells
When considering the actions of anthrax toxins on the vasculature, interactions between VEC and other cells including blood cells (e.g., platelets and neutrophils, which accumulate at points of barrier disruption) and mural cells (pericytes surrounding microvessels and smooth muscle cells surrounding larger vessels) also warrant attention (reviewed in [128]). For example, in capillary beds, where anthrax toxins are likely to exert their strongest effects on barrier integrity, there is an important interaction between endothelial cells and pericytes, which is particularly relevant in maintaining an intact blood-brain barrier. N-cadherin plays a key role in the adhesive interaction between cerebro-microvascular endothelial cells and pericytes and down-regulation of this adhesion molecule disrupts the functional interaction between these cell types leading to vascular leakage and hemorrhage. It has recently been shown that TGF-β and Notch signaling (mediated by Notch1 and Notch 4) coordinately activate N-cadherin expression in endothelial cells, and that blocking either pathway disrupts the interaction between endothelial cells and pericytes (reviewed in [130, 169]) (Fig. 5F). There is also a reciprocal interaction between these two cell types mediated by Notch that may be relevant wherein Notch3 signaling in mural cells is activated by the Dll4 ligand expressed in endothelial cells and leads to differentiation and maturation of mural cells as well as maintenance of Notch3 and Jagged1 expression (Fig. 5F). Given the ability of ET and LT to disrupt trafficking of Notch ligands (e.g., Dll4) and cadherins to the surface of microvascular endothelial cells, these toxins might be expected to interfere with reciprocal Notch signaling and adhesion between endothelial cells and pericytes, which could contribute importantly to vascular effusion and hemorrhage in vivo. VEGF produced at sites of vascular barrier breakdown (by platelets, neutrophils, or interstitial cells such as fibroblasts) may also contribute to disrupting interactions between pericytes and VEC since VEGF signaling negatively regulates PDGF activity, which is required for pericyte coverage of neovasculature [170]. As meningitis and cerebral hemorrhaging occurs in a large fraction of anthrax patients, and can be modeled in mice by infection with B. anthracis [57, 141], examining interactions between VEC, mural cells and other cell types during the fulminant stage of infection will be an interesting avenue of future investigation.
11. Interactions between paracellular and transcytosis permeability pathways
VEC permeability is determined by two seemingly distinct mechanisms -- namely regulation of transport of small molecules via the paracellular route as discussed above, and transport of bulk luminal contents and selected macromolecules such as proteins via vesicular transcytosis or the generation of transcellular channels or fenestrations of the vascular endothelium (reviewed in [121, 128]). Although detailed discussion of these various transcellular transport mechanisms is beyond the scope of the current review, we briefly summarize key features of transcytosis since this endocytic process might, in principle, be affected by anthrax toxins. Transcytosis is a caveoli-dependent form of endocytic transport of material from the apical luminal surface of VEC to the basal interstitial surface and is thought to contribute substantially to trafficking of large molecules across the endothelium since as much as 20% of the membrane in these cells is devoted to caveolar trafficking (reviewed in [121]). Caveoli carrying captured vascular fluid and/or ligands bound to receptors on lipid-raft microdomains pinch off from the luminal surface, move the short distance to the opposing basal surface, fuse to the plasma membrane via a SNAP/SNARE mechanism, and deliver their cargo to internal tissues by exocytosis of the caveolar contents. One highly relevant protein transported via the transcytosis pathway is albumin since the relative concentration of this multifunctional delivery protein is to determine the direction and extent of fluid flow into or out of the vasculature. Excessive albumin transport across VEC leads to fluid loss from the vasculature and hence to hypotension.
There is a strong coupling between the transcytotic and paracellular routes of transport as revealed in mouse cav-1 mutants lacking Caveolin-1, which is essential for transcytosis in VEC. In cav-1 mutants, paracellular permeability is greatly increased leading, paradoxically, to elevated overall transport of fluid and protein out of the vascular lumen into tissues. This compensatory coupling of the transcytotic and paracellular permeability pathways is mediated, at least in part, by eNOS that is sequestered by binding to Cav-1 in an inactive form and is released dynamically in response to caveolar trafficking. NO produced by the elevated levels free NOS present in Cav-1 mutant VEC covalently nitrosylates RhoGap bound to p120/VE-Cad at the AJ. This inhibition of RhoGap leads to increased RhoA activity, which in turn promotes stress fiber formation thereby weakening cellular junctions (reviewed in [121]). Since transcytosis may be inhibited by factors such as EF and LF that interfere with exocyst mediated trafficking (i.e., by blocking SNAP/SNARE-dependent, and possibly exocyst-dependent, docking of caveolar cargo to the basal cell surface) it will be interesting to examine whether such an effect could contribute to the increase in VEC paracellular permeability caused by these toxins. The increase in protein leakage from the vasculature resulting from inhibition of transcytosis, should also directly contribute to hypotension as indicated above. This loss of proteins such as albumin from the serum may be further aggravated by direct or indirect effects of LF on the liver that cause a decrease in albumin levels to a third of the normal levels [50]. Finally, it will be important to determine whether components of the transcytotic machinery contribute to the ability of B. anthracis to penetrate VEC barriers by crossing through the cells, a process that depends critically on production of LF [141]. Since components of the exocyst have been identified in genome-wide RNAi screens for host factors required for bacterial phagocytosis in Drosophila cells [171], it will also be valuable to examine the role of the exocyst in B. anthracis invasion of VEC.
In summary, several synergistic effects of EF and LF may contribute to the profound collaborative effect they have on increasing vascular permeability that result in fatal hypotension including: 1) inhibition of trafficking of cadherins to the AJ which directly weakens cell junctions, 2) inhibition of signaling mediated by Notch and other pathways (e.g., resulting in disruption of the interaction between VEC and mural cells), 3) potential disruption of transcytosis that could lead to compensatory increases in paracellular permeability and protein loss from the circulation, and 4) direct or indirect effects of toxins on liver cells leading to decreased production of serum proteins such as albumin).
12. Other candidate virulence factors secreted by B. anthracis
It is generally accepted that in addition to LT and ET, which are encoded on the same bacterial plasmid pXO1, the other primary virulence factor of B. anthracis is its poly-D-glutamate capsule, the synthesis and secretion of which is accomplished by proteins encoded by an operon carried on the pXO2 plasmid. Nonetheless, there are several other factors secreted by B. anthracis that may contribute to pathogenicity including an outer wall constituent of the bacterium (BslA), a pore forming toxin (anthrolysin O), and neutral proteases. We briefly summarize the known and proposed contributions of these additional candidate virulence factors.
12.1 Capsule
The Sterne strain of B. anthracis lacks the pXO2 plasmid and as a consequence has greatly attenuated virulence. Indeed this crippled strain has been used extensively for immunization of livestock worldwide and even for humans in Russia. The capsule is thought to act primarily by reducing phagocytosis of B. anthracis by monocytes thus allowing the bacteria to establish infection. Another recently proposed function of the capsule is to target the bacteria to the hepatic VEC wall [172] (Fig. 3B), where they presumably secrete EF, LF, and PA. Pretreatment of mice with isolated capsule material prior to infection with encapsulated (but not unencapsulated) B. anthracis bacteria significantly reduced the number of adherent bacteria in the liver [172]. Finally, the capsule has been found to form a complex with LT in the circulation of infected rabbits, guinea pigs and monkeys [173] and may modify the effect of LT or provide a protected reservoir of this toxin.
12.2 BslA
BslA, encoded on the pXO1 plasmid, is an S-layer surface protein that promotes B. anthracis adhesion to mammalian cells including HeLa [174] and brain microvascular endothelial cells. Infection of mice with BslA-deficient B. anthracis resulted in reduced mortality in guinea pigs [174] and decreased CNS infection in mice [141] compared to the parent strain. Analysis of the endothelial cell invasion phenotype showed the BslA adhesin promoted disruption of the tight junction protein ZO-1 [175].
12.3 Anthrolysin
Pore-forming toxins are produced by many bacterial pathogens and lead to severe cellular stress and activation of the JNK and p38 defense pathways [176]. B. anthracis produces one such pore forming toxin, anthrolysin O (ALO), which activates the TLR4 receptor in macrophages and induces apoptosis in conjunction with LF mediated inactivation of p38 [177]. Similarly, in one study, ALO activated ERK and p38 signaling in NMuMG epithelial cells, reducing junctional expression of cadherins [178]. In a second study, however, ALO altered the expression pattern of the TJ protein Occludin, but not E-cadherin in intestinal Caco-2 cells [179].
In addition to the known stress pathway responses, endocytic recycling has been shown to play an important role in clearing pore-forming toxins from the membrane [180]. Membrane recycling provides another potential point of convergence of ALO activity with EF and LF since clearance of pore-forming toxins depends critically on Rab11 as well as Rab5 activity [180]. An interesting prediction from these findings is that the effect of ALO should be highly dependent on EF and LF activity since these toxins would be expected to greatly retard elimination of the pore forming toxin from the plasma membrane by blocking Rab11/Sec15-dependent endocytic recycling.
12.4 Neutral proteases
Proteomic analysis of proteins secreted by B. anthracis have identified a variety of novel candidate virulence factors [181]. Two such proteins, which were purified from the culture supernatants of non-virulent pXO1-, pXO2- bacteria, are extracellular proteases [182]. Both of these proteases induce shedding of cell surface glycoproteins such as Syndecan, which often accompanies infection with a variety of bacterial pathogens. One of these proteases, InhA, disrupted barrier integrity of brain microvascular endothelial cells when they were infected with a strain of B. subtilis expressing InhA [183]. Associated with this reduction in barrier function, levels of the cytoplasmic TJ protein ZO-1 were significantly reduced by exposure to InhA. Furthermore, deletion of the inhA gene from an encapsulated (Ames) strain of B. anthracis resulted in a delay in the time to death of mice infected by the mutant versus wild-type strains, indicating that this factor contributes to pathogenesis in vivo [183].
Another potential role for neutral proteases such as InhA is to trigger the host coagulation cascade, which occurs in vivo surrounding clusters of B. anthracis bacteria [184]. InhA- mutant strains of B. anthracis lack the ability of the parental strain to induce clotting of human blood plasma [184], suggesting that neutral proteases may contribute to coagulopathy in anthrax patients, harkening back to original observations of Pasteur (Fig. 3A).
The relevance of these and other possible contributing virulence factors in anthrax pathogenesis merits further investigation. In particular, it will be interesting to determine whether there are interactions between factors acting in similar capacities. For example, are there cooperative interactions between the capsule and BslA for bacterial targeting to endothelial membranes? Similarly, does inhibition of the exocyst by LF and EF enhance the effect of anthrolysin, and do proteases disrupting TJ stability such as InhA act in concert with EF and LF to promote vascular leakage?
13. Summary and perspectives
13.1 Two phases of anthrax toxin function
A variety of converging evidence points to two major biological effects of anthrax toxins as essential virulence factors of B. anthracis. The toxins first help establish infection by inhibiting the function of myeloid cells while at the same time hijacking these cells as vehicles for delivery of bacterial spores and germinating vegetative bacteria to lymph nodes. Accordingly, mice lacking monocyte/macrophage/neutrophil-specific expression of the anthrax toxin receptor CMG2 are protected from infection by B. anthracis. During this initial phase of the disease, LT playing a primary role acts via inhibition of MKK pathways to induce apoptosis and/or rapid cell death mediated by the Nlrp1/inflammasome pathway of macrophages (and perhaps other cells via Nlrp1), while EF acts to delay cell death and promote migration, thereby allowing macrophages to transport and deliver their bacterial cargo to distributing lymph nodes where the bacteria are then released following cell death of the host vehicles. This process is highly coordinated as revealed by the inverse relationship in mice between the sensitivity to Nlrp1b/inflammasome mediated cell death and systemic virulence. Throughout this early establishment phase of anthrax infection, the host typically displays few if any symptoms of disease.
The host first begins to experience symptoms at the onset of the second fulminant phase of anthrax infection, often involving the respiratory, cardiovascular, and gastrointestinal systems. Vegetative bacteria released from dying monocytes in lymph nodes begin to proliferate and then disseminated via a hematological route to various target tissues and organs, which are typically highly vascularized such as the lung, pulmonary parenchyma, cerebral meninges, spleen, liver, and intestine. The secreted LT and ET toxins begin to accumulate in the blood as bacteria proliferate and disseminate where they rapidly reach a critical threshold level that will cause death even when the bacterial proliferation is curtailed by antibiotics. During this final phase of infection the toxins cause an increase in vascular permeability and a decrease in function of target organs including the heart, spleen, kidney, adrenal gland, and brain. It has been proposed that ET plays a lead role with support from LT in inducing vascular leakage primarily by compromising the barrier function of VEC since these toxins have been shown to increase vascular permeability both in cell culture and in vivo. The primary late target of LT may be the heart itself and this effect, combined with compromised vascular integrity by ET and LT, may lead to the cataclysmic collapse of cardiovascular function leading to death. Barrier disruption in VEC is associated with a decrease in cadherin levels at the AJ and consequent reduction in expression of the TJ barrier protein Claudin-5. An important contributing role of other cells types, particularly vascular mural cells, has also been suggested in maintaining vascular integrity. Whether anthrax toxins disrupt critical interactions between the vascular endothelium and mural cells is likely to be a fruitful area for further investigation.
One way that anthrax toxins synergize to reduce barrier function of VEC is to inhibit endocytic membrane recycling mediated by the exocyst complex. An important challenge will be to delineate the precise mechanisms by which EF reduces the level and activity of Rab11 on late recycling endosomes and how LF blocks the function of Sec15, the exocyst component that binds Rab11. Another remaining question of fundamental importance is why cAMP-producing bacterial toxins such as EF result in increased vascular permeability while endogenous cAMP signaling has been tied to the opposite effect of barrier tightening. Issues to consider regarding this cAMP paradox include the subcellular distribution, magnitude, and kinetics of toxin induced cAMP synthesis as well as apparent cell-type specific responses that likely arise from differences in endogenous cAMP regulation (e.g., whether synthesis or degradation of host produced cAMP are limiting) and differences in the make up and organization of cell-type specific AJ and TJ associated factors.
A current challenge in the anthrax field is to understand more fully how LT, ET, and other potential secreted and membrane-associated virulence of B. anthracis contribute in vivo to the natural course of infection during the fulminant phase of the disease. A recurring theme at the organism level is that different cell types respond differently to the toxins and that such differences also vary based on the genotype(s) of the host. Thus, cells in the myeloid lineage (particularly macrophages) are most sensitive to the cytotoxic effects of LT, which is also species- and strain-dependent, and vascular endothelial barriers in general are much more prone to disruption than epithelial barriers (e.g., the intestine). Even among endothelial cells, there appear to be important differences in how they respond to anthrax toxin exposure. Presumably cell-type specific differences including the precise complement of synthetic and degradative enzymes involved in cAMP metabolism that are present, different combinations of scaffolding proteins (e.g., AKAPs or AJ associated proteins), varying combinations of receptors recruited to sites of cell-cell contact (e.g., Notch or RTKs), and differing neighboring cell types (e.g., mural cells interacting with VEC) contribute to different cellular responses to the toxins. An integrated understanding of bacterial dissemination and toxemia based on contributions from diverse cell specific characteristics and their responses to infection should help formulate a coherent integrated picture of disease pathogenesis.
13.2 The potential roles of anthrax toxins in the recent emergence of the B. anthracis lineage
Another fascinating question pertaining to anthrax toxins is how they may have contributed to the rapid evolution of B. anthracis from its closely allied ancestor, B. cereus [32]. Comparative evolutionary studies have raised several intriguing issues including the sequence of events leading to the assembly of the pXO1 and pXO2 plasmids and the key virulence factors they carry, and how these plasmids have apparently moved horizontally between B. anthracis, which is present only as spores outside of its hosts, and B. cereus, which can exist in a vegetative phase in the soil. Does plasmid transfer only occur during joint infection of a host with both bacterial species when vegetative forms of both bacteria can meet? Alternatively, are there other hosts in the soil such as the rhizosphere of grasses [185] (a primary food for herbivores) or other circumstances permitting at least brief vegetative contact of B. anthracis with B. cereus in the soil? These issues raise the related question of what are the key natural hosts of B. anthracis that have contributed to its evolution. There is strong evidence that herbivores, which are readily infected by B. anthracis, served as important primary hosts since co-evolution of B. anthracis with domesticated herbivores is strongly suggested by the coincidence of human dispersal and trade routes with fine structure geographical patterns of anthrax strain distributions [33, 186]. Such a link between humans and anthrax evolution is also consistent with the similar time scales for human domestication of sheep and cattle and the emergence of the particularly successful A-subtype of B. anthracis [186]. Given that migratory patterns of herbivores follow co-varying ecological trends such as the presence of grasslands, it is possible that soil organisms growing in the grass rhizosphere, such as protozoa that may feed on vegetative forms of the bacteria [185], could also have contributed evolution of B. anthracis pathogenesis strategies. Another interesting comparative evolution question is why the plcR master regulator of virulence factors in B. cereus has been mutated (in two separate events) in B. anthracis and in an anthracised strain of B. cereus that gained the pXO1 and pXO2 [32]. It has been suggested that conflicts may exist between the infectious programs of these two bacteria such as plcR mediated inhibition of pXO1-dependent sporulation [187] or plcR-dependent killing of macrophages [188], but further studies into this question are clearly warranted. Another intriguing discovery is that of Certhrax toxin in an isolate of B. cereus that caused fatal pneumonia in an otherwise healthy welder [189]. This strain of B. cereus also carries many genes from pXO1 encoding LF, EF and PA (but not pXO2) [190]. Certhrax toxin, a novel C2-toxin family member, consists of two domains related to the PA binding and ADP-ribosylation domains of LF, but lacking the metalloprotease domain of LF. In addition, the ADP-ribosylation domain of Certhrax is catalytically active unlike in LF in which critical residues have been mutated. The C2 A/B class ADP-ribosylases most closely related to Certhrax, such as iota and VIP2 toxin, ADP-ribosylate G-actin causing F-actin polymers to disassemble by mass action. It is also intriguing that the B-subunits of C2 toxins share sequence and structural similarity PA and, like PA, following proteolytic processing form heptameric prepores attached to host cell receptors.
Finally, a broader comparative question is whether there are common thematic grounds relating ET and LT as virulence factors in B. anthracis to cAMP producing toxins, MKK inhibiting factors, and additional barrier compromising factors (e.g., often inhibiting the activity of Rho family GTPases) in other pathogens such as V. cholerae (Ctx), B. pertussis (CyaA and Ptx), toxigenic E. coli (ExoY), and Y. pestis (secreted AC and YopJ)? Since these bacterial pathogens share a general strategy of colonizing and altering the barrier functions of various epithelia or endothelia (e.g., primary targeting of the vascular endothelium by B. anthracis, the airway epithelium by B. pertussis, and the secretory intestinal epithelium by V. cholerae) one may glean additional insights by comparing the specialized activities of the various toxins in the contexts of their primary cellular targets and final phenotypic effects (e.g. barrier permeabilization versus invasion). For example, why is profuse diarrhea resulting from stimulated fluid secretion from intestinal epithelial cells a primary characteristic of cholera, whereas only modest diarrhea accompanies anthrax infection in some patients, even though anthrax often results in massive submuccosal bacterial invasion of the intestine with associated edema? Reciprocally, despite the intestine being a highly vascularized tissue, why doesn't cholera toxin induce massive edema and vascular hemorrhage in the dense intestinal capillary beds underlying the intestinal epithelium?
Although great progress has been made in understanding anthrax pathogenesis since the pioneering studies of Delafond, Koch, and Pasteur, there are clearly many important questions remaining. Indeed the many fascinating facets of this disease and its relevance to broader questions of primary biological interest may have been sensed by the penetrating intuition of Pasteur, leading him to devote such a long period of his career to studying this pathogen. One might hope that these pioneers would have been gratified by current progress in the field. Hopefully, this new understanding will guide development of additional therapeutic strategies targeting specific cellular responses, which in principle should have fewer off-target effects. Pasteur's ultimate triumph would indeed be a translation of our current basic understanding of anthrax pathogenesis into an effective means of combating infection during the highly refractory and often fatal final phase of “le charbon”.
Acknowledgments
We thank Mahtab Moayeri, Jean-Nicolas Tournier, Elisabetta Dejana, Nina van Sorge, Theresa Koehler, Paul Keim, and members of Bier lab for helpful discussions and/or comments on the manuscript as well as the anonymous reviewers for thoughtful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koch R, editor. Beitrage zur Biologie der Pflanzen. Zweiter Band, Zweites Heft; Breslau: 1876. [Google Scholar]
- 2.Pasteur L, Chamberland C, Roux E. Compte-rendu sommaire des expériences faites à Pouilly-le-Fort, près Melun, sur la vaccination charbonneuse. Cr Acad Sci Paris. 1881;92:1393–1398. [Google Scholar]
- 3.Pasteur L, Chamberland C, Roux E. Summary report of the experiments conducted at Pouilly-le-Fort, near Melun, on anthrax vaccination. Yale J Biol Med. 2002;75:59–62. [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald JG. Louis Pasteur - his contribution to anthrax, vaccination and the evolution of a principle of active immunization. Cal State J Med. 1923;21:101–103. [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz M, Jekyll, Hyde a short history of anthrax. Mol Aspects Med. 2009;30:347–355. doi: 10.1016/j.mam.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Scorpio A, Blank TE, Day WA, Chabot DJ. Anthrax vaccines: Pasteur to the present. Cell Mol Life Sci. 2006;63:2237–2248. doi: 10.1007/s00018-006-6312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberland C, editor. Le charbon et la vaccination charbonneuse d'après les travaux récents de M Pasteur. Bernard Tignol; Paris: 1883. [Google Scholar]
- 8.Pasteur L, Joubert J. Charbon et septicémie. CR Acad Sci Paris. 1877;85:101–105. [Google Scholar]
- 9.Marmier L. Sur la toxine charbonneuse. Annales Inst Pasteur. 1895;9:533–574. [Google Scholar]
- 10.Bail O. Untersuchungen uber naturliche and kunstliche Milzbrandimmunitat. XI. Erster Bericht uber Milzbrandschutzimpfugen on Schafen. Centralbl f Bakteriol Abt I orig. 1904;37:270–280. [Google Scholar]
- 11.Cromartie WJ, Watson DW, et al. Studies on infection with Bacillus anthracis; II. The immunological and tissue damaging properties of extracts prepared from lesions of B. anthracis infections. J Infect Dis. 1947;80:14–27. doi: 10.1093/infdis/80.1.14. [DOI] [PubMed] [Google Scholar]
- 12.Bail O. Untersuchungen uber naturliche and kunstliche Milzbrandimmunitat. Zentrabl Bakt Parassiten Infektionskr I rig. 1904;36:262–272. [Google Scholar]
- 13.Gladstone GP. Immunity to anthrax: protective antigen present in cell-free culture filtrates. Br J Exp Pathol. 1946;27:394–418. [PMC free article] [PubMed] [Google Scholar]
- 14.Smith H. Discovery of the anthrax toxin: the beginning of studies of virulence determinants regulated in vivo. Int J Med Microbiol. 2002;291:411–417. doi: 10.1078/1438-4221-00147. [DOI] [PubMed] [Google Scholar]
- 15.Stanley JL, Smith H. Purification of factor I and recognition of a third factor of the anthrax toxin. J Gen Microbiol. 1961;26:49–63. doi: 10.1099/00221287-26-1-49. [DOI] [PubMed] [Google Scholar]
- 16.Abrami L, Reig N, van der Goot FG. Anthrax toxin: the long and winding road that leads to the kill. Trends Microbiol. 2005;13:72–78. doi: 10.1016/j.tim.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Guidi-Rontani C, Weber-Levy M, Mock M, Cabiaux V. Translocation of Bacillus anthracis lethal and oedema factors across endosome membranes. Cell Microbiol. 2000;2:259–264. doi: 10.1046/j.1462-5822.2000.00057.x. [DOI] [PubMed] [Google Scholar]
- 18.Zornetta I, Brandi L, Janowiak BJ, Dal Molin F, Tonello F, Collier RJ, Montecucco C. Imaging the cell entry of the anthrax oedema and lethal toxins with fluorescent protein chimeras. Cell Microbiol. 2010;12:1435–1445. doi: 10.1111/j.1462-5822.2010.01480.x. [DOI] [PubMed] [Google Scholar]
- 19.Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K, Paull KD, Vande Woude GF. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 20.Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- 21.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 22.Tonello F, Montecucco C. The anthrax lethal factor and its MAPK kinase-specific metalloprotease activity. Mol Aspects Med. 2009;30:431–438. doi: 10.1016/j.mam.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Bardwell AJ, Frankson E, Bardwell L. Selectivity of docking sites in MAPK kinases. J Biol Chem. 2009;284:13165–13173. doi: 10.1074/jbc.M900080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang WJ, Guo Q. The adenylyl cyclase activity of anthrax edema factor. Mol Aspects Med. 2009;30:423–430. doi: 10.1016/j.mam.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dal Molin F, Tonello F, Ladant D, Zornetta I, Zamparo I, Di Benedetto G, Zaccolo M, Montecucco C. Cell entry and cAMP imaging of anthrax edema toxin. EMBO J. 2006;25:5405–5413. doi: 10.1038/sj.emboj.7601408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dal Molin F, Zornetta I, Puhar A, Tonello F, Zaccolo M, Montecucco C. cAMP imaging of cells treated with pertussis toxin, cholera toxin, and anthrax edema toxin. Biochem Biophys Res Commun. 2008;376:429–433. doi: 10.1016/j.bbrc.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Turk BE. Manipulation of host signalling pathways by anthrax toxins. Biochem J. 2007;402:405–417. doi: 10.1042/BJ20061891. [DOI] [PubMed] [Google Scholar]
- 29.Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Schubert RL, Bugge TH, Leppla SH. Anthrax toxin: structures, functions and tumour targeting. Expert Opin Biol Ther. 2003;3:843–853. doi: 10.1517/14712598.3.5.843. [DOI] [PubMed] [Google Scholar]
- 31.Mourez M. Anthrax toxins. Rev Physiol Biochem Pharmacol. 2004;152:135–164. doi: 10.1007/s10254-004-0028-2. [DOI] [PubMed] [Google Scholar]
- 32.Kolsto AB, Tourasse NJ, Okstad OA. What sets Bacillus anthracis apart from other Bacillus species? Annu Rev Microbiol. 2009;63:451–476. doi: 10.1146/annurev.micro.091208.073255. [DOI] [PubMed] [Google Scholar]
- 33.Van Ert MN, Easterday WR, Huynh LY, Okinaka RT, Hugh-Jones ME, Ravel J, Zanecki SR, Pearson T, Simonson TS, U'Ren JM, Kachur SM, Leadem-Dougherty RR, Rhoton SD, Zinser G, Farlow J, Coker PR, Smith KL, Wang B, Kenefic LJ, Fraser-Liggett CM, Wagner DM, Keim P. Global genetic population structure of Bacillus anthracis. PLoS One. 2007;2:e461. doi: 10.1371/journal.pone.0000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goossens PL. Animal models of human anthrax: the quest for the Holy Grail. Mol Aspects Med. 2009;30:467–480. doi: 10.1016/j.mam.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Lincoln RE, Walker JS, Klein F, Rosenwald AJ, Jones WI., Jr Value of field data for extrapolation in anthrax. Fed Proc. 1967;26:1558–1562. [PubMed] [Google Scholar]
- 36.Ross JM. The pathogenesis of anthrax following the administration of spores by the respiratory route. J Pathol Bacteriol. 1957;73:485–494. [Google Scholar]
- 37.Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci U S A. 1993;90:2291–2294. doi: 10.1073/pnas.90.6.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grinberg LM, Abramova FA, Yampolskaya OV, Walker DH, Smith JH. Quantitative pathology of inhalational anthrax I: quantitative microscopic findings. Mod Pathol. 2001;14:482–495. doi: 10.1038/modpathol.3880337. [DOI] [PubMed] [Google Scholar]
- 39.Albrink WS, Brooks SM, Biron RE, Kopel M. Human inhalation anthrax. A report of three fatal cases. Am J Pathol. 1960;36:457–471. [PMC free article] [PubMed] [Google Scholar]
- 40.Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med. 2006;144:270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 41.Holty JE, Kim RY, Bravata DM. Anthrax: a systematic review of atypical presentations. Ann Emerg Med. 2006;48:200–211. doi: 10.1016/j.annemergmed.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 42.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross JM. On the histopathology of experimental anthrax in the guinea-pig. Br J Exp Pathol. 1955;36:336–339. [PMC free article] [PubMed] [Google Scholar]
- 44.Smith H, Keppie J. Observations on experimental anthrax; demonstration of a specific lethal factor produced in vivo by Bacillus anthracis. Nature. 1954;173:869–870. doi: 10.1038/173869a0. [DOI] [PubMed] [Google Scholar]
- 45.Zaucha GM, Pitt LM, Estep J, Ivins BE, Friedlander AM. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch Pathol Lab Med. 1998;122:982–992. [PubMed] [Google Scholar]
- 46.Beall FA, Dalldorf FG. The pathogenesis of the lethal effect of anthrax toxin in the rat. J Infect Dis. 1966;116:377–389. doi: 10.1093/infdis/116.3.377. [DOI] [PubMed] [Google Scholar]
- 47.Eckert NJ, Bonventre PF. In vivo effects of Bacillus anthracis culture filtrates. J Infect Dis. 1963;112:226–232. [Google Scholar]
- 48.Firoved AM, Miller GF, Moayeri M, Kakkar R, Shen Y, Wiggins JF, McNally EM, Tang WJ, Leppla SH. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol. 2005;167:1309–1320. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Firoved AM, Moayeri M, Wiggins JF, Shen Y, Tang WJ, Leppla SH. Anthrax edema toxin sensitizes DBA/2J mice to lethal toxin. Infect Immun. 2007;75:2120–2125. doi: 10.1128/IAI.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gozes Y, Moayeri M, Wiggins JF, Leppla SH. Anthrax lethal toxin induces ketotifen-sensitive intradermal vascular leakage in certain inbred mice. Infect Immun. 2006;74:1266–1272. doi: 10.1128/IAI.74.2.1266-1272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui X, Li Y, Li X, Laird MW, Subramanian M, Moayeri M, Leppla SH, Fitz Y, Su J, Sherer K, Eichacker PQ. Bacillus anthracis edema and lethal toxin have different hemodynamic effects but function together to worsen shock and outcome in a rat model. J Infect Dis. 2007;195:572–580. doi: 10.1086/510856. [DOI] [PubMed] [Google Scholar]
- 53.Cui X, Moayeri M, Li Y, Li X, Haley M, Fitz Y, Correa-Araujo R, Banks SM, Leppla SH, Eichacker PQ. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R699–709. doi: 10.1152/ajpregu.00593.2003. [DOI] [PubMed] [Google Scholar]
- 54.Culley NC, Pinson DM, Chakrabarty A, Mayo MS, Levine SM. Pathophysiological manifestations in mice exposed to anthrax lethal toxin. Infect Immun. 2005;73:7006–7010. doi: 10.1128/IAI.73.10.7006-7010.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moayeri M, Crown D, Dorward DW, Gardner D, Ward JM, Li Y, Cui X, Eichacker P, Leppla SH. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS) PLoS Pathog. 2009;5:e1000456. doi: 10.1371/journal.ppat.1000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tessier J, Green C, Padgett D, Zhao W, Schwartz L, Hughes M, Hewlett E. Contributions of histamine, prostanoids, and neurokinins to edema elicited by edema toxin from Bacillus anthracis. Infect Immun. 2007;75:1895–1903. doi: 10.1128/IAI.01632-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Sorge NM, Ebrahimi CM, McGillivray SM, Quach D, Sabet M, Guiney DG, Doran KS. Anthrax toxins inhibit neutrophil signaling pathways in brain endothelium and contribute to the pathogenesis of meningitis. PLoS One. 2008;3:e2964. doi: 10.1371/journal.pone.0002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson LE, Kuo SR, Katki K, Dang T, Park SK, Dostal DE, Tang WJ, Leppla SH, Frankel AE. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS One. 2007;2:e466. doi: 10.1371/journal.pone.0000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watson LE, Mock J, Lal H, Lu G, Bourdeau RW, Tang WJ, Leppla SH, Dostal DE, Frankel AE. Lethal and edema toxins of anthrax induce distinct hemodynamic dysfunction. Front Biosci. 2007;12:4670–4675. doi: 10.2741/2416. [DOI] [PubMed] [Google Scholar]
- 60.Fritz DL, Jaax NK, Lawrence WB, Davis KJ, Pitt ML, Ezzell JW, Friedlander AM. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab Invest. 1995;73:691–702. [PubMed] [Google Scholar]
- 61.Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J Hyg (Lond) 1956;54:28–36. doi: 10.1017/s0022172400044272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klein F, Lincoln RE, Dobbs JP, Mahlandt BG, Remmele NS, Walker JS. Neurological and physiological responses of the primate to anthrax infection. J Infect Dis. 1968;118:97–103. doi: 10.1093/infdis/118.1.97. [DOI] [PubMed] [Google Scholar]
- 63.Stearns-Kurosawa DJ, Lupu F, Taylor FB, Jr, Kinasewitz G, Kurosawa S. Sepsis and pathophysiology of anthrax in a nonhuman primate model. Am J Pathol. 2006;169:433–444. doi: 10.2353/ajpath.2006.051330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vasconcelos D, Barnewall R, Babin M, Hunt R, Estep J, Nielsen C, Carnes R, Carney J. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2003;83:1201–1209. doi: 10.1097/01.lab.0000080599.43791.01. [DOI] [PubMed] [Google Scholar]
- 65.Albrink WS, Goodlow RJ. Experimental inhalation anthrax in the chimpanzee. Am J Pathol. 1959;35:1055–1065. [PMC free article] [PubMed] [Google Scholar]
- 66.Klein F, Hodges DR, Mahlandt BG, Jones WI, Haines BW, Lincoln RE. Anthrax toxin: causative agent in the death of rhesus monkeys. Science. 1962;138:1331–1333. doi: 10.1126/science.138.3547.1331. [DOI] [PubMed] [Google Scholar]
- 67.Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect Immun. 1991;59:3472–3477. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paccani SR, Tonello F, Ghittoni R, Natale M, Muraro L, D'Elios MM, Tang WJ, Montecucco C, Baldari CT. Anthrax toxins suppress T lymphocyte activation by disrupting antigen receptor signaling. J Exp Med. 2005;201:325–331. doi: 10.1084/jem.20041557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drysdale M, Olson G, Koehler TM, Lipscomb MF, Lyons CR. Murine innate immune response to virulent toxigenic and nontoxigenic Bacillus anthracis strains. Infect Immun. 2007;75:1757–1764. doi: 10.1128/IAI.01712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dumetz F, Jouvion G, Khun H, Glomski IJ, Corre JP, Rougeaux C, Tang WJ, Mock M, Huerre M, Goossens PL. Noninvasive imaging technologies reveal edema toxin as a key virulence factor in anthrax. Am J Pathol. 2011;178:2523–2535. doi: 10.1016/j.ajpath.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guichard A, McGillivray SM, Cruz-Moreno B, van Sorge NM, Nizet V, Bier E. Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst. Nature. 2010;467:854–858. doi: 10.1038/nature09446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu S, Miller-Randolph S, Crown D, Moayeri M, Sastalla I, Okugawa S, Leppla SH. Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe. 2010;8:455–462. doi: 10.1016/j.chom.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu S, Crown D, Miller-Randolph S, Moayeri M, Wang H, Hu H, Morley T, Leppla SH. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci U S A. 2009;106:12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Banks DJ, Ward SC, Bradley KA. New insights into the functions of anthrax toxin. Expert Rev Mol Med. 2006;8:1–18. doi: 10.1017/S1462399406010714. [DOI] [PubMed] [Google Scholar]
- 75.Frankel AE, Kuo SR, Dostal D, Watson L, Duesbery NS, Cheng CP, Cheng HJ, Leppla SH. Pathophysiology of anthrax. Front Biosci. 2009;14:4516–4524. doi: 10.2741/3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med. 2009;30:439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tournier JN, Rossi Paccani S, Quesnel-Hellmann A, Baldari CT. Anthrax toxins: a weapon to systematically dismantle the host immune defenses. Mol Aspects Med. 2009;30:456–466. doi: 10.1016/j.mam.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Glomski IJ, Corre JP, Mock M, Goossens PL. Noncapsulated toxinogenic Bacillus anthracis presents a specific growth and dissemination pattern in naive and protective antigen-immune mice. Infect Immun. 2007;75:4754–4761. doi: 10.1128/IAI.00575-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 80.Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- 81.Klein F, Haines BW, Mahlandt BG, Dearmon IA, Jr, Lincoln RE. Dual nature of resistance mechanisms as revealed by studies of anthrax septicemia. J Bacteriol. 1963;85:1032–1038. doi: 10.1128/jb.85.5.1032-1038.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Welkos SL, Keener TJ, Gibbs PH. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect Immun. 1986;51:795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moayeri M, Crown D, Newman ZL, Okugawa S, Eckhaus M, Cataisson C, Liu S, Sastalla I, Leppla SH. Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 2010;6:e1001222. doi: 10.1371/journal.ppat.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, LeVine SM, Bradley KA. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Newman ZL, Printz MP, Liu S, Crown D, Breen L, Miller-Randolph S, Flodman P, Leppla SH, Moayeri M. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 2010;6:e1000906. doi: 10.1371/journal.ppat.1000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ribot WJ, Panchal RG, Brittingham KC, Ruthel G, Kenny TA, Lane D, Curry B, Hoover TA, Friedlander AM, Bavari S. Anthrax lethal toxin impairs innate immune functions of alveolar macrophages and facilitates Bacillus anthracis survival. Infect Immun. 2006;74:5029–5034. doi: 10.1128/IAI.00275-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim C, Wilcox-Adelman S, Sano Y, Tang WJ, Collier RJ, Park JM. Antiinflammatory cAMP signaling and cell migration genes co-opted by the anthrax bacillus. Proc Natl Acad Sci U S A. 2008;105:6150–6155. doi: 10.1073/pnas.0800105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szarowicz SE, During RL, Li W, Quinn CP, Tang WJ, Southwick FS. Bacillus anthracis edema toxin impairs neutrophil actin-based motility. Infect Immun. 2009;77:2455–2464. doi: 10.1128/IAI.00839-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong J, Doebele RC, Lingen MW, Quilliam LA, Tang WJ, Rosner MR. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J Biol Chem. 2007;282:19781–19787. doi: 10.1074/jbc.M700128200. [DOI] [PubMed] [Google Scholar]
- 90.Paccani SR, Tonello F, Patrussi L, Capitani N, Simonato M, Montecucco C, Baldari CT. Anthrax toxins inhibit immune cell chemotaxis by perturbing chemokine receptor signalling. Cell Microbiol. 2007;9:924–929. doi: 10.1111/j.1462-5822.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 91.Agrawal A, Lingappa J, Leppla SH, Agrawal S, Jabbar A, Quinn C, Pulendran B. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature. 2003;424:329–334. doi: 10.1038/nature01794. [DOI] [PubMed] [Google Scholar]
- 92.Chou PJ, Newton CA, Perkins I, Friedman H, Klein TW. Suppression of dendritic cell activation by anthrax lethal toxin and edema toxin depends on multiple factors including cell source, stimulus used, and function tested. DNA Cell Biol. 2008;27:637–648. doi: 10.1089/dna.2008.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Comer JE, Chopra AK, Peterson JW, Konig R. Direct inhibition of T-lymphocyte activation by anthrax toxins in vivo. Infect Immun. 2005;73:8275–8281. doi: 10.1128/IAI.73.12.8275-8281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tournier JN, Quesnel-Hellmann A, Mathieu J, Montecucco C, Tang WJ, Mock M, Vidal DR, Goossens PL. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J Immunol. 2005;174:4934–4941. doi: 10.4049/jimmunol.174.8.4934. [DOI] [PubMed] [Google Scholar]
- 95.Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 96.Goldman DL, Casadevall A. Anthrax-associated shock. Front Biosci. 2008;13:4009–4014. doi: 10.2741/2988. [DOI] [PubMed] [Google Scholar]
- 97.Golden HB, Watson LE, Lal H, Verma SK, Foster DM, Kuo SR, Sharma A, Frankel A, Dostal DE. Anthrax toxin: pathologic effects on the cardiovascular system. Front Biosci. 2009;14:2335–2357. doi: 10.2741/3382. [DOI] [PubMed] [Google Scholar]
- 98.Sherer K, Li Y, Cui X, Eichacker PQ. Lethal and edema toxins in the pathogenesis of Bacillus anthracis septic shock: implications for therapy. Am J Respir Crit Care Med. 2007;175:211–221. doi: 10.1164/rccm.200608-1239CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y, Sherer K, Cui X, Eichacker PQ. New insights into the pathogenesis and treatment of anthrax toxin-induced shock. Expert Opin Biol Ther. 2007;7:843–854. doi: 10.1517/14712598.7.6.843. [DOI] [PubMed] [Google Scholar]
- 100.Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011;35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalns J, Scruggs J, Millenbaugh N, Vivekananda J, Shealy D, Eggers J, Kiel J. TNF receptor 1, IL-1 receptor, and iNOS genetic knockout mice are not protected from anthrax infection. Biochem Biophys Res Commun. 2002;292:41–44. doi: 10.1006/bbrc.2002.6626. [DOI] [PubMed] [Google Scholar]
- 102.Moayeri M, Webster JI, Wiggins JF, Leppla SH, Sternberg EM. Endocrine perturbation increases susceptibility of mice to anthrax lethal toxin. Infect Immun. 2005;73:4238–4244. doi: 10.1128/IAI.73.7.4238-4244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mina B, Dym JP, Kuepper F, Tso R, Arrastia C, Kaplounova I, Faraj H, Kwapniewski A, Krol CM, Grosser M, Glick J, Fochios S, Remolina A, Vasovic L, Moses J, Robin T, DeVita M, Tapper ML. Fatal inhalational anthrax with unknown source of exposure in a 61-year-old woman in New York City. JAMA. 2002;287:858–862. doi: 10.1001/jama.287.7.858. [DOI] [PubMed] [Google Scholar]
- 104.Sherer K, Li Y, Cui X, Li X, Subramanian M, Laird MW, Moayeri M, Leppla SH, Fitz Y, Su J, Eichacker PQ. Fluid support worsens outcome and negates the benefit of protective antigen-directed monoclonal antibody in a lethal toxin-infused rat Bacillus anthracis shock model. Crit Care Med. 2007;35:1560–1567. doi: 10.1097/01.CCM.0000266535.95770.A2. [DOI] [PubMed] [Google Scholar]
- 105.Warfel JM, Steele AD, D'Agnillo F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am J Pathol. 2005;166:1871–1881. doi: 10.1016/S0002-9440(10)62496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bolcome RE, 3rd, Sullivan SE, Zeller R, Barker AP, Collier RJ, Chan J. Anthrax lethal toxin induces cell death-independent permeability in zebrafish vasculature. Proc Natl Acad Sci U S A. 2008;105:2439–2444. doi: 10.1073/pnas.0712195105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kirby JE. Anthrax lethal toxin induces human endothelial cell apoptosis. Infect Immun. 2004;72:430–439. doi: 10.1128/IAI.72.1.430-439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paddle BM, Wong VK, Muller BD. The cytotoxic effect of anthrax lethal toxin on human lung cells in vitro and the protective action of bovine antibodies to PA and LF. J Appl Toxicol. 2006;26:162–168. doi: 10.1002/jat.1119. [DOI] [PubMed] [Google Scholar]
- 109.Hicks CW, Li Y, Okugawa S, Solomon SB, Moayeri M, Leppla SH, Mohanty A, Subramanian GM, Mignone TS, Fitz Y, Cui X, Eichacker PQ. Anthrax edema toxin has cAMP-mediated stimulatory effects and high-dose lethal toxin has depressant effects in an isolated perfused rat heart model. Am J Physiol Heart Circ Physiol. 2011;300:H1108–1118. doi: 10.1152/ajpheart.01128.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuo SR, Willingham MC, Bour SH, Andreas EA, Park SK, Jackson C, Duesbery NS, Leppla SH, Tang WJ, Frankel AE. Anthrax toxin-induced shock in rats is associated with pulmonary edema and hemorrhage. Microb Pathog. 2008;44:467–472. doi: 10.1016/j.micpath.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 111.Sweeney DA, Cui X, Solomon SB, Vitberg DA, Migone TS, Scher D, Danner RL, Natanson C, Subramanian GM, Eichacker PQ. Anthrax lethal and edema toxins produce different patterns of cardiovascular and renal dysfunction and synergistically decrease survival in canines. J Infect Dis. 2010;202:1885–1896. doi: 10.1086/657408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bolcome RE, 3rd, Chan J. Constitutive MEK1 activation rescues anthrax lethal toxin-induced vascular effects in vivo. Infect Immun. 2010;78:5043–5053. doi: 10.1128/IAI.00604-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kandadi MR, Hua Y, Ma H, Li Q, Kuo SR, Frankel AE, Ren J. Anthrax lethal toxin suppresses murine cardiomyocyte contractile function and intracellular Ca2+ handling via a NADPH oxidase-dependent mechanism. PLoS One. 2010;5:e13335. doi: 10.1371/journal.pone.0013335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vick JA, Lincoln RE, Klein F, Mahlandt BG, Walker JS, Fish DC. Neurological and physiological responses of the primate to anthrax toxin. J Infect Dis. 1968;118:85–96. doi: 10.1093/infdis/118.1.85. [DOI] [PubMed] [Google Scholar]
- 115.Fish DC, Klein F, Lincoln RE, Walker JS, Dobbs JP. Pathophysiological changes in the rat associated with anthrax toxin. J Infect Dis. 1968;118:114–124. doi: 10.1093/infdis/118.1.114. [DOI] [PubMed] [Google Scholar]
- 116.Levy H, Weiss S, Altboum Z, Schlomovitz J, Rothschild N, Blachinsky E, Kobiler D. Lethal factor is not required for Bacillus anthracis virulence in guinea pigs and rabbits. Microb Pathog. 2011 doi: 10.1016/j.micpath.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 117.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 119.Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- 120.Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778:562–571. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 122.Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- 123.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 124.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 125.He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 127.Bendezu FO, Martin SG. Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol Biol Cell. 2010;22:44–53. doi: 10.1091/mbc.E10-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 129.Bigas A, Robert-Moreno A, Espinosa L. The Notch pathway in the developing hematopoietic system. Int J Dev Biol. 2010;54:1175–1188. doi: 10.1387/ijdb.093049ab. [DOI] [PubMed] [Google Scholar]
- 130.Kume T. Novel insights into the differential functions of Notch ligands in vascular formation. J Angiogenes Res. 2009;1:8. doi: 10.1186/2040-2384-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 132.Dufraine J, Funahashi Y, Kitajewski J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene. 2008;27:5132–5137. doi: 10.1038/onc.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 135.Depeille PE, Ding Y, Bromberg-White JL, Duesbery NS. MKK signaling and vascularization. Oncogene. 2007;26:1290–1296. doi: 10.1038/sj.onc.1210198. [DOI] [PubMed] [Google Scholar]
- 136.Bier E, Guichard A. Deconstructing host-pathogen interactions in Drosophila. Dis Model Mech. 2011 doi: 10.1242/dmm.000406. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 138.Guichard A, Park JM, Cruz-Moreno B, Karin M, Bier E. Anthrax Lethal Factor and Edema Factor act on conserved targets in Drosophila. Proc Natl Acad Sci U S A. 2006;103:3244–3249. doi: 10.1073/pnas.0510748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 140.Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell. 2005;9:365–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 141.Ebrahimi CM, Sheen TR, Renken CW, Gottlieb RA, Doran KS. Contribution of lethal toxin and edema toxin to the pathogenesis of anthrax meningitis. Infect Immun. 2011;79:2510–2518. doi: 10.1128/IAI.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rosse C, Formstecher E, Boeckeler K, Zhao Y, Kremerskothen J, White MD, Camonis JH, Parker PJ. An aPKC-exocyst complex controls paxillin phosphorylation and migration through localised JNK1 activation. PLoS Biol. 2009;7:e1000235. doi: 10.1371/journal.pbio.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Balakireva M, Rosse C, Langevin J, Chien YC, Gho M, Gonzy-Treboul G, Voegeling-Lemaire S, Aresta S, Lepesant JA, Bellaiche Y, White M, Camonis J. The Ral/exocyst effector complex counters c-Jun N-terminal kinase-dependent apoptosis in Drosophila melanogaster. Mol Cell Biol. 2006;26:8953–8963. doi: 10.1128/MCB.00506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park KM, Fogelgren B, Zuo X, Kim J, Chung DC, Lipschutz JH. Exocyst Sec10 protects epithelial barrier integrity and enhances recovery following oxidative stress, by activation of the MAPK pathway. Am J Physiol Renal Physiol. 2010;298:F818–826. doi: 10.1152/ajprenal.00596.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nada S, Hondo A, Kasai A, Koike M, Saito K, Uchiyama Y, Okada M. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J. 2009;28:477–489. doi: 10.1038/emboj.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Horgan CP, McCaffrey MW. The dynamic Rab11-FIPs. Biochem Soc Trans. 2009;37:1032–1036. doi: 10.1042/BST0371032. [DOI] [PubMed] [Google Scholar]
- 147.Goehring AS, Pedroja BS, Hinke SA, Langeberg LK, Scott JD. MyRIP anchors protein kinase A to the exocyst complex. J Biol Chem. 2007;282:33155–33167. doi: 10.1074/jbc.M705167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qiao J, Mei FC, Popov VL, Vergara LA, Cheng X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem. 2002;277:26581–26586. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- 149.Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295:1285–1288. doi: 10.1126/science.1067549. [DOI] [PubMed] [Google Scholar]
- 150.Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, Nakaoka Y, Mochizuki N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–976. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boettner B, Van Aelst L. Control of cell adhesion dynamics by Rap1 signaling. Curr Opin Cell Biol. 2009;21:684–693. doi: 10.1016/j.ceb.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frische EW, Zwartkruis FJ. Rap1, a mercenary among the Ras-like GTPases. Dev Biol. 2010;340:1–9. doi: 10.1016/j.ydbio.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 153.Boettner B, Harjes P, Ishimaru S, Heke M, Fan HQ, Qin Y, Van Aelst L, Gaul U. The AF-6 homolog canoe acts as a Rap1 effector during dorsal closure of the Drosophila embryo. Genetics. 2003;165:159–169. doi: 10.1093/genetics/165.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–24103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- 155.Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol. 2008;294:H1188–1196. doi: 10.1152/ajpheart.00937.2007. [DOI] [PubMed] [Google Scholar]
- 157.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 159.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 160.Ahuja N, Kumar P, Bhatnagar R. The adenylate cyclase toxins. Crit Rev Microbiol. 2004;30:187–196. doi: 10.1080/10408410490468795. [DOI] [PubMed] [Google Scholar]
- 161.Paccani SR, Benagiano M, Capitani N, Zornetta I, Ladant D, Montecucco C, D'Elios MM, Baldari CT. The adenylate cyclase toxins of Bacillus anthracis and Bordetella pertussis promote Th2 cell development by shaping T cell antigen receptor signaling. PLoS Pathog. 2009;5:e1000325. doi: 10.1371/journal.ppat.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Puhar A, Dal Molin F, Horvath S, Ladant D, Montecucco C. Anthrax edema toxin modulates PKA- and CREB-dependent signaling in two phases. PLoS One. 2008;3:e3564. doi: 10.1371/journal.pone.0003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res. 2004;95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- 164.Blum MS, Toninelli E, Anderson JM, Balda MS, Zhou J, O'Donnell L, Pardi R, Bender JR. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am J Physiol. 1997;273:H286–294. doi: 10.1152/ajpheart.1997.273.1.H286. [DOI] [PubMed] [Google Scholar]
- 165.Qiao RL, Bhattacharya J. Segmental barrier properties of the pulmonary microvascular bed. J Appl Physiol. 1991;71:2152–2159. doi: 10.1152/jappl.1991.71.6.2152. [DOI] [PubMed] [Google Scholar]
- 166.Schneeberger EE. Segmental differentiation of endothelial intercellular junctions in intra-acinar arteries and veins of the rat lung. Circ Res. 1981;49:1102–1111. doi: 10.1161/01.res.49.5.1102. [DOI] [PubMed] [Google Scholar]
- 167.Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol. 1999;277:L119–126. doi: 10.1152/ajplung.1999.277.1.L119. [DOI] [PubMed] [Google Scholar]
- 168.Beauregard KE, Wimer-Mackin S, Collier RJ, Lencer WI. Anthrax toxin entry into polarized epithelial cells. Infect Immun. 1999;67:3026–3030. doi: 10.1128/iai.67.6.3026-3030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Winkler EA, Bell RD, Zlokovic BV. Lack of Smad or Notch leads to a fatal game of brain pericyte hopscotch. Dev Cell. 2011;20:279–280. doi: 10.1016/j.devcel.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 170.Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cherry S. Genomic RNAi screening in Drosophila S2 cells: what have we learned about host-pathogen interactions? Curr Opin Microbiol. 2008;11:262–270. doi: 10.1016/j.mib.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Piris-Gimenez A, Corre JP, Jouvion G, Candela T, Khun H, Goossens PL. Encapsulated Bacillus anthracis interacts closely with liver endothelium. J Infect Dis. 2009;200:1381–1389. doi: 10.1086/644506. [DOI] [PubMed] [Google Scholar]
- 173.Ezzell JW, Abshire TG, Panchal R, Chabot D, Bavari S, Leffel EK, Purcell B, Friedlander AM, Ribot WJ. Association of Bacillus anthracis capsule with lethal toxin during experimental infection. Infect Immun. 2009;77:749–755. doi: 10.1128/IAI.00764-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kern J, Schneewind O. BslA, the S-layer adhesin of B. anthracis, is a virulence factor for anthrax pathogenesis. Mol Microbiol. 2010;75:324–332. doi: 10.1111/j.1365-2958.2009.06958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ebrahimi CM, Kern JW, Sheen TR, Ebrahimi-Fardooee MA, van Sorge NM, Schneewind O, Doran KS. Penetration of the blood-brain barrier by Bacillus anthracis requires the pXO1-encoded BslA protein. J Bacteriol. 2009;191:7165–7173. doi: 10.1128/JB.00903-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, Aroian RV. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc Natl Acad Sci U S A. 2004;101:10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Park JM, Ng VH, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200:1647–1655. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Popova TG, Millis B, Bradburne C, Nazarenko S, Bailey C, Chandhoke V, Popov SG. Acceleration of epithelial cell syndecan-1 shedding by anthrax hemolytic virulence factors. BMC Microbiol. 2006;6:8. doi: 10.1186/1471-2180-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bishop BL, Lodolce JP, Kolodziej LE, Boone DL, Tang WJ. The role of anthrolysin O in gut epithelial barrier disruption during Bacillus anthracis infection. Biochem Biophys Res Commun. 2010;394:254–259. doi: 10.1016/j.bbrc.2010.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Los FC, Kao CY, Smitham J, McDonald KL, Ha C, Peixoto CA, Aroian RV. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe. 2011;9:147–157. doi: 10.1016/j.chom.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chitlaru T, Shafferman A. Proteomic studies of Bacillus anthracis. Future Microbiol. 2009;4:983–998. doi: 10.2217/fmb.09.73. [DOI] [PubMed] [Google Scholar]
- 182.Chung MC, Popova TG, Millis BA, Mukherjee DV, Zhou W, Liotta LA, Petricoin EF, Chandhoke V, Bailey C, Popov SG. Secreted neutral metalloproteases of Bacillus anthracis as candidate pathogenic factors. J Biol Chem. 2006;281:31408–31418. doi: 10.1074/jbc.M605526200. [DOI] [PubMed] [Google Scholar]
- 183.Mukherjee DV, Tonry JH, Kim KS, Ramarao N, Popova TG, Bailey C, Popov S, Chung MC. Bacillus anthracis protease InhA increases blood-brain barrier permeability and contributes to cerebral hemorrhages. PLoS One. 2011;6:e17921. doi: 10.1371/journal.pone.0017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kastrup CJ, Boedicker JQ, Pomerantsev AP, Moayeri M, Bian Y, Pompano RR, Kline TR, Sylvestre P, Shen F, Leppla SH, Tang WJ, Ismagilov RF. Spatial localization of bacteria controls coagulation of human blood by ‘quorum acting’. Nat Chem Biol. 2008;4:742–750. doi: 10.1038/nchembio.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Saile E, Koehler TM. Bacillus anthracis multiplication, persistence, and genetic exchange in the rhizosphere of grass plants. Appl Environ Microbiol. 2006;72:3168–3174. doi: 10.1128/AEM.72.5.3168-3174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kenefic LJ, Pearson T, Okinaka RT, Schupp JM, Wagner DM, Hoffmaster AR, Trim CB, Chung WK, Beaudry JA, Jiang L, Gajer P, Foster JT, Mead JI, Ravel J, Keim P. Pre-Columbian origins for North American anthrax. PLoS One. 2009;4:e4813. doi: 10.1371/journal.pone.0004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mignot T, Mock M, Robichon D, Landier A, Lereclus D, Fouet A. The incompatibility between the PlcR- and AtxA-controlled regulons may have selected a nonsense mutation in Bacillus anthracis. Mol Microbiol. 2001;42:1189–1198. doi: 10.1046/j.1365-2958.2001.02692.x. [DOI] [PubMed] [Google Scholar]
- 188.Sastalla I, Maltese LM, Pomerantseva OM, Pomerantsev AP, Keane-Myers A, Leppla SH. Activation of the latent PlcR regulon in Bacillus anthracis. Microbiology. 2010;156:2982–2993. doi: 10.1099/mic.0.041418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fieldhouse RJ, Turgeon Z, White D, Merrill AR. Cholera- and anthrax-like toxins are among several new ADP-ribosyltransferases. PLoS Comput Biol. 2010;6:e1001029. doi: 10.1371/journal.pcbi.1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hoffmaster AR, Hill KK, Gee JE, Marston CK, De BK, Popovic T, Sue D, Wilkins PP, Avashia SB, Drumgoole R, Helma CH, Ticknor LO, Okinaka RT, Jackson PJ. Characterization of Bacillus cereus isolates associated with fatal pneumonias: strains are closely related to Bacillus anthracis and harbor B. anthracis virulence genes. J Clin Microbiol. 2006;44:3352–3360. doi: 10.1128/JCM.00561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]