Abstract
Post-transplant lymphoproliferative diseases (PTLD) associated with Epstein-Barr virus (EBV) infection often develop after organ and haematopoietic stem-cell transplantation. These lymphoproliferative diseases are tumours that usually express all latent EBV viral proteins, and are therefore amenable to T-cell-based immune therapies, such as donor lymphocyte infusions and the adoptive transfer of EBV-specific cytotoxic T-lymphocytes (CTLs). In this Review, we describe current strategies of T-cell-based therapies to treat PTLD, and describe strategies that improve the feasibility of such treatment.
Introduction
Epstein-Barr virus (EBV)-associated lymphoproliferative disease occurring after haemopoietic stem-cell transplant or solid organ transplantation is a potentially life-threatening condition .1, 2 The ability of EBV to persist and become reactivated in B-cells is a unique characteristic of gamma herpesviruses and is fundamental to the pathogenesis of post-transplant lymphoproliferative diseases (PTLD), which occurs when the T-cell immune response to EBV is ablated or severely compromised by immunosupression.3 Despite the availability of therapies that target EBV-infected B cells, PTLD can occur rapidly and is potentially lethal.1. An attractive strategy for the treatment and prevention of this devastating complication is based on the use of ex vivo-derived EBV-specific cytotoxic T lymphocytes (EBV-CTL) to reconstitute the immune response to EBV1, 4 and several recent studies have confirmed the promise of this strategy.5–7 . The rationale for exploring T cell therapy for this disease is to restore the underlying immune defect which leads to the development of PTLD without causing bystander organ toxicity and/or further immune suppression. In this article we will discuss EBV biology and the pathogenesis of PTLD to emphasise the importance of the cell mediated immune response for the control of this disease. We will then discuss the advances in T cell based therapies for PTLD including the advantages and disadvantages of each approach. Finally we will highlight key strategies that are currently being used to broaden the application of this therapy beyond single centers.
Epstein-Barr virus
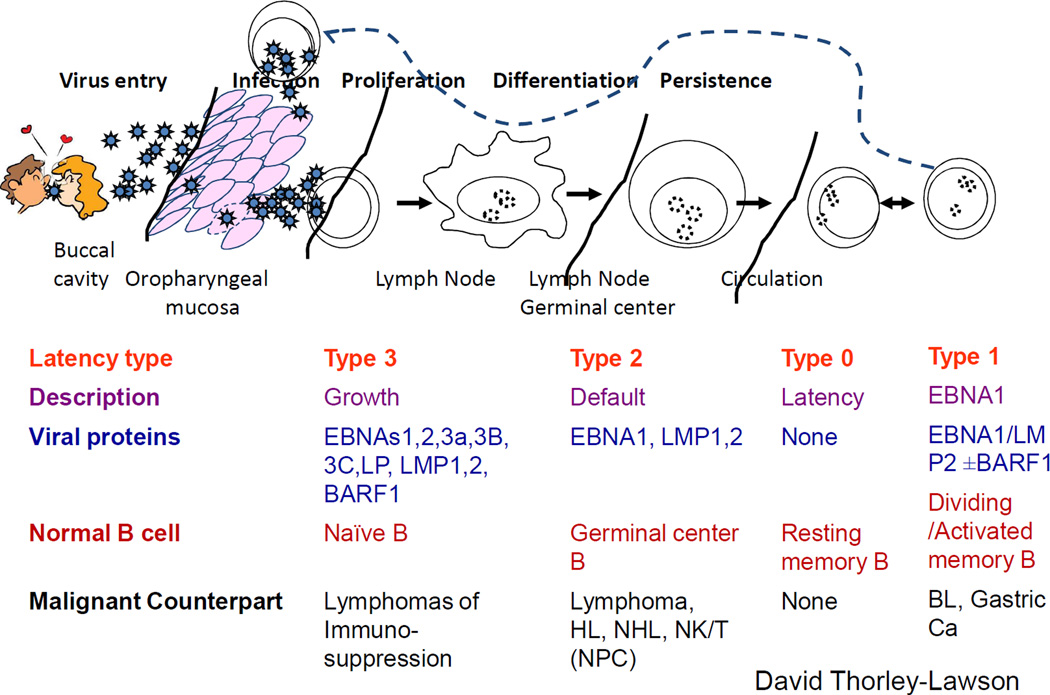
Epstein-Barr virus is a ubiquitous human herpesvirus that enters its human host via a mucosal route, infects and replicates in oropharyngeal epithelial cell and then infects B-lymphocytes in the oropharynx(Figure 1).8 Infected B-cells do not produce infectious virus, but instead express viral latency-associated proteins that induce B-cell proliferation and then drive the infected B-cell into ‘true latency’, in which no viral genes are expressed.9 These infected B-cells are phenotypically indistinguishable from normal memory B-cells and are ‘invisible’ to the immune response and, therefore, persist long-term. 8, 10 The virus life cycle is completed when infected B-cells circulate through the oropharynx where the virus reactivates from latency, and infectious virus is released into the saliva to infect new hosts.10 The transition from newly-infected B-cell to infected memory B-cell carrying virus in ‘true latency’ involves three stages of ‘transitional’ latency and each latency type is associated with specific B-cell malignancies(Box 1). 8, 10 Virus replication is important for virus transmission and to maintain the pool of infected memory B-cells.10 The role of the lytic cycle in established PTLD in minimal, and drugs such as acyclovir that inhibit viral replication have no impact on established tumours.11 However, viral replication and spread seem to be important for transmission of the virus from infected donor B-cells in an organ graft to recipient B-cells.12 This is evident in seronegative transplant recipients who become infected with EBV from seropositive donors. Recipients of intestinal grafts carrying a high B-cell load are at particularly high risk for PTLD, and this situation can be ameliorated by pre-emptive use of replication cycle inhibitors during the peritransplant period.12
Figure 1. Epstein-Barr virus life cycle, latency states and lymphoma.
The Epstein-Barr virus (EBV) life cycle involves at least five distinct stages and four are associated with disease. During primary infection, EBV infects naïve B-cells and expresses its entire latency gene complex, including 10 proteins and 2 small RNAs (type III latency). Type III latency drives B-cell transformation and proliferation, but because the cells are highly immunogenic, they are rapidly eliminated by EBV-specific T-cells. The virus survives in B-cells by downregulating its immunogenic proteins in two phases. Initially B-cells enter lymphoid follicles where they proliferate and express only three viral proteins (type III latency). Finally they exit the lymph node and downregulate viral proteins altogether (type 0 latency), and thus are invisible to the immune response. If circulating infected B-cells divide homeostatically they express a single viral protein (EBNA1, type I latency) that ensures that the virus genome divides with the cell genome. When infected B-cells circulate through the oropharynx they transfer the virus to epithelial cells, where it is replicated to infect new hosts by salivary transfer and where it infects new B cells to maintain the infected B-cell pool. With the exception of type I latency, each latency state is observed in specific types of lymphoma. Abbreviations: EBNA, Epstein-Barr nuclear antigen; EBV, Epstein-Barr virus
Box 1. Epstein-Barr virus latency states in post-transplant lymphoproliferative disease.
There are four Epstein-Barr virus (EBV) latency states in B-cells3). Three of these are found in post-transplant lymphoproliferative disease (PTLDs) and each is associated with a different type of virus gene expression, different levels of immunogenicity, and different types of lymphoma11. In type III latency, the virus expresses all 10 of its latency-associated proteins that together induce B-cell transformation1 Tumours expressing type III latency are invariably of B-cell origin and develop early after transplant, during the period of most intense immune suppression. In recipients of solid organ transplants (SOT), B-cells expressing type III latency are frequently detected in lymph nodes and may be categorized as lymphoid hyperplasias that may never develop into full-blown lymphoma and that frequently respond to reduced immune suppression. However they may develop into diffuse large B cell lymphomas (DLBCLs) in both SOT and haematopoietic stem-cell transplant (HSCT) recipients.1 These lymphomas are highly immunogenic, generally susceptible to EBV-specific T-cell therapy and are never seen in immunocompetent individuals. Late after SOT, patients are susceptible to the less immunogenic type I and type II latency tumours that require additional oncogenic events to compensate for their reduced viral gene expression and can also arise in immunocompetent individuals.1 Burkitt’s lymphoma expresses type I latency viral genes—EB nuclear antigen 1(EBNA1) and latent membrane protein 2 (LMP2)—and is poorly susceptible to the immune response. Hodgkin lymphoma and non-Hodgkin lymphomas express type II latency (EBNA1, LMP1 and LMP2) and have intermediate immunogenicity. In the fourth type of latency, type 0 latency, no viral genes are expressed. Type 0 latency is not detected in tumours, as EBNA1 is required for the maintenance of the viral genome.
Pathobiology and presentation of PTLD
The normal balance between latently infected B-cells and the EBV-specific T-cells, both from either the donor or the recipient as a consequence of the immunocompromised state of patients after transplantation of solid organs or haematopoietic stem cells. This imbalance results in an increase of latently infected B-cells, which may develop into PTLD.13 This serious, and often life-threatening, disease is characterized by four major histological subtypes (early lesions, monomorphic, polymorphic, and classic Hodgkin lymphoma-type),14 which are all associated with EBV infection. Symptoms and signs of PTLD can include fever, lymphadenopathy, fulminant sepsis, and mass lesions in lymph nodes, spleen, or central nervous system.1 It can also present as a lymphomatous growth within the transplanted organ (for instance, small intestine, lung, liver or kidney).1
PTLD after HSCT
PTLD developing after haematopoietic stem-cell transplantation (HSCT) usually results from donor B-cells and appears within the first 6–12 months of the post-transplant period, when profound deficiencies of EBV-specific CTL are seen.15 However, in the setting of reduced-intensity conditioning regimens that include anti-thymocyte globulin (ATG) or alemtuzumab, some centres have described a high incidence of PTLD involving B-cells from recipient paediatric patients.16, 17 Risk factors for the development of PTLD include the use of a donor stem-cell product depleted of T-cells, a high degree of HLA-mismatch between donor and recipient, the degree and duration of immunosuppressive treatment after the transplant, and the use of ATG with reduced-intensity transplant conditioning.18 After HSCT, PTLD may present with a clinical picture similar to infectious mononucleosis with B symptoms (fevers, sweats, anorexia) and tonsilar enlargement and cervical lymphadenopathy. In highly immunosuppressed patients, the clinical presentation may also be more fulminant with diffuse multiorgan involvement and a clinical picture that is similar to sepsis or graft versus host disease (GVHD).
PTLD after solid organ transplant
Patients undergoing solid organ transplantation most commonly develop PTLD exhibiting type III latency in the first year, with the disease often arising from lymphoid cells of their own haematopoietic system (Box 1).19 The incidence can vary from 1% to 30% depending on the type of organ transplanted. The risk factors for this form of the disease include both the inability to develop an immune response against primary infection because of the EBV-seronegative status of the recipient prior to transplantation, and a higher dose of immunosuppressive treatment after receiving organs with more lymphoid tissue, such as small intestine or lung. Hence, children are particularly susceptible to PTLD, because they are more often EBV-seronegative at the time of transplantation.20
PTLD can be categorized into ‘early’ or ‘late’ lymphoma depending on the interval of its development after transplantation.21 This classification is closely linked to the intensity of immunosuppression occurring in the first period post-transplant. Early PTLD develops within the first year after transplantation and is invariably associated with EBV. By contrast, the so-called ‘late’ form, develops 2–5 years or more after transplantation and its pathogenesis is probably multi-factorial with a significant number of cases being EBV-negative.22 These late-onset lymphomas also have a much poorer prognosis than their earlier counterparts and require more-aggressive therapies. After transplantation of solid organs or haematopoietic stem cells, EBV viral load in peripheral blood should be monitored in patients at high risk of developing PTLD, according to published guidelines;23–25 if tracked over time, this sensitive—though non-specific—laboratory value can indicate likely viral reactivation.
Treatment of PTLD
Whether the intent is to prevent or to treat EBV-associated PTLD arising after solid organ and HSCT, the two approaches used are to remove the infected B-cells, and restore and expand an EBV-specific T-cell-mediated immune response. Several guidelines and algorithms for the sequential application of these strategies in patient with PTLD have been published.23, 26–29 In the setting of solid organ transplant, these guidelines suggest initial reduction of immune suppression if possible. If this manoeuver is unsuccessful, second-line therapies include rituximab—a chimeric murine/human monoclonal antibody against CD20, an antigen expressed in the surface of B-cells—either alone or in combination with chemotherapy such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) in patients with more extensive or rapidly progressive disease.23, 29 Toxicity associated with chemotherapy is a concern in patients with co-morbidities and for this reason less-intensive regimens are often used, particularly in children.20, 29
After HSCT, reduction in immune suppression rarely results in sufficient recovery of EBV-specific immunity to eradicate PTLD. Rituximab has proved effective both as pre-emptive therapy in patients with high EBV viral loads and as treatment for patients with established PTLD, producing response rates of 55–100% in both scenarios.30–32 Nevertheless, PTLD often recurs because cellular immunity against EBV has not been restored.33 Rituximab also increases the risk of infection in a population that is already immunosuppressed and can be ineffective as some tumours do not express CD20. Thus, strategies to restore immunity by infusing T-cells that target EBV antigens have been developed and are being used by several groups often after failure of immunotherapy reduction and rituximab. Because many PTLDs express type III latency, this setting serves as a good model for testing T-cell-based therapies designed to target EBV antigens that are presented on the cell surface by major histocompatibility molecules.
T-cell therapy for PTLD
Understanding the latency states of EBV is important for the appropriate design of immunotherapies that target viral antigens for the eradication of tumour cells. EBV-transformed B-lymphoblastoid cell lines (LCLs) are ideal antigen-presenting cells (APCs) for the activation of T-cells used in immunotherapy for type III malignancies because they express the same 10 viral antigens. They also express high levels of class I/class II HLA and co-stimulatory molecules, are readily generated from healthy donors, and grow to large numbers3. EBV-specific CTLs are also easily activated from healthy EBV-seropositive HSCT donors using LCLs as APCs, and have been effective in recipients1. In solid organ recipients, in vivo T-cell expansion is compromised by the continued immunosuppression required by these patients. Nevertheless, EBV-specific T-cells can be expanded from patients ex vivo, and have been used with some success34–36. For patients with late PTLDs expressing type II latency, T-cells must be focused on the three viral proteins expressed—latent membrane proteins 1 and 2 (LMP1 and LMP2), and Epstein–Barr virus nuclear antigen (EBNA1)—so LCLs that express all 10 latency proteins are not ideal APCs. However, monocytes and LCLs expressing LMP1 and LMP2 from adenoviral vectors can reactivate LMP-specific T-cells that are effective for the treatment of type II latency tumors.37 Since EBNA1 is poorly immunogenic, there has so far been little clinical experience in targeting type I latency tumors (Burkitt’s lymphoma) with T-cells.38
Donor unmanipulated lymphocyte infusions
Because most HSCT donors are EBV seropositive, EBV-specific T-cell response can be increased in patients who develop PTLD in the allogeneic transplant setting by infusing unmanipulated donor lymphocytes.39, 40 This strategy has shown efficacy in more than 70% of patients with PTLD after HSCT.6 However, this strategy is associated with an increased incidence of severe or fatal GVHD, due to the presence of alloreactive T-cells in the infused cell product.39, 40 This problem could potentially be overcome by genetically modifying the T-cells with asuicide gene, such as the herpes simplex virus-derived thymidine kinase (TK) gene. If GVHD were to occur, T-cells transduced with this gene can then be killed by infusion of ganciclovir. This approach has shown efficacy in early phase studies and is currently being evaluated in a phase III licensing trial in Europe.41 Nonetheless, concerns with this approach include the development of an immune response against the xenogeneic thymidine kinase (TK) antigen and the removal of ganciclovir as an option for treating viral infections, such as cytomegalovirus after transplantation. These considerations have generated interest in using alternative suicide genes, such as inducible human caspase 9.42
Donor EBV-specific CTLs in HSCT recipients
An alternative approach to reducing alloreactivity of donor T-cells is to selectively expand T-cell lines directed against EBV antigens, using EBV-LCLs that, similar to PTLDs, express type III latency. Such T-cell lines usually contain both CD4+ and CD8+ T-cells that recognize multiple latent and early lytic cycle viral antigens and, when infused into patients, can reconstitute an immune response to EBV and eliminate PTLD without inducing GVHD. Our group recently completed studies in 114 patients who received EBV-specific CTLs to prevent or treat PTLD at three institutions (Table 1).5 No patients developed de novo GVHD after CTL infusion, and the most significant adverse effect was localized, but reversible, swelling at sites of disease during the therapeutic response in four patients with active disease.5,43 Among all patients, 101 received CTL infusions as prophylaxis resulting in up to a 4-log expansion of infused CTLs. None of these patients developed PTLD. Of the 13 patients who were treated for active disease, 11 patients achieved complete remissions that were sustained without recurrence. One patient died very early after treatment of progressive disease and a second patient did not respond because her tumour had a deletion of the two epitopes in the EBNA3B recognized by the infused line.44 The first 26 patients of the 114 patients received cells that had been genetically modified with a retroviral vector encoding the neomycin resistance gene as a marker, which allowed us to track the infused T-cells for up to 9 years.5 We concluded from this study that adoptively-transferred donor-derived EBV-specific CTLs is a safe and effective prophylactic approach or treatment method for PTLD, and that the manufacturing methodology is robust and reproducible, and can be used in other institutions.5
Table 1.
Clinical trials using EBV-CTLs for type III latency malignancies
| Study | n | Type of transplant |
GVHD | Other serious adverse events possibly attributable to CTLs |
Antiviral effects |
|---|---|---|---|---|---|
| St Jude, Baylor College of Medicine and Great Ormond St5, 75–77 | 113 | T-depleted HSCT | 8/51 with recurrence of previous acute GVHD post CTLs . 13/108 evaluable patients developed chronic GVHD (11 limited, 2 extensive) | Local inflammation during therapeutic response in 4 patients |
Prophylaxis: None of 101 developed PTLD Treatment: Induced CR in 11/13 patients |
| Memorial Sloan– Kettering 6, 69, 78 | 14 | HSCT | No patient in the EBV-CTL group developed de novo acute or chronic GVHD or a flare of pre-existing GVHD | None | CR in 10 patients, 4 with progressive disease |
| Gustafsson et al. (2000)79 | 6 | T-depleted HSCT or ATG/OKT3 conditioning | None reported | None | Decreased EBV DNA levels in 5 patients; 1 patient died of PTLD |
| Lucas et al. (1998)80 | 1 | T-depleted HSCT | 1 patient had limited reactivation of acute skin GVHD post CTLs | None | Patient attained CR |
| Imashsuki (1998)81 | 1 | Mismatched HSCT | None | None | Patient did not respond |
| Comoli et al. (2007)82 | 4 | Haploidentical | None | None | CR in 3 patients with recurrent PTLD post rituximab; decrease in EBV DNA in patient without overt PTLD |
| Moosmann et al. (2010)51 | 6 | HSCT (haploidentical in 5 patients) | None | None | CR in 3 patients; no response in 3 others |
Abbreviations: ATG, antithymocyte globulin; CR, complete remission; CTL, cytotoxic T-lymphocyte; EBV, Epstein-Barr virus; GVHD, graft versus host disease; HSCT, haematopoietic stem-cell transplantation; PTLD, post-transplant lymphoproliferative disease.
A second large study was recently reported by researchers based at the Memorial Sloan–Kettering Cancer Center and led by Richard J. O'Reilly. Their results in 47 patients with EBV-PTLD treated with donor lymphocyte infusions (DLI), donor derived EBV-CTLs or third party EBV-CTLs showed an overall response rate of 68% without evidence of de novo or recurrent GVHD.6 Analysis in vitro of the cases of patients who did not respond to CTL therapy showed three cases in which the CTL generated from the donor—by using donor LCLs transformed with EBV-producing marmoset B-cell line (B95-8)—did not lyse LCLs expanded from the patient’s blood or biopsied tumour. By contrast, CTL generated using LCL lines transformed with the patients own strain of EBV derived from the recipient could lyse LCLs from either the recipient or the donor. In another case, in which donor and recipient were a 7/10 match and the recipient had not responded to CTL therapy, the PTLD was proved to be of recipient origin, and the infused cell line was selectively restricted by HLA*A1101, present only in the donor. This patient subsequently received a partially HLA-matched third-party EBV-CTL line that had significant activity restricted by an HLA allele expressed by the patient’s tumour and achieved a complete response. Together, these cases, and those observed by our group,44 suggest that a lack of response to CTL therapies may reflect an infused line with a restricted specificity or antigenic differences between the EBV strain causing the PTLD and the B95-8 strain. Use of EBV-CTL lines from another donor should be considered in this situation. Several other smaller studies (Table 1) show response rates similar to those obtained by the Baylor and Sloan Kettering groups.
Autologous EBV-CTLs in solid organ recipients
Are the successes with EBV CTLs in HSCT recipients reproducible in recipients of solid organ transplant? In this setting, PTLD most commonly arises from recipient B-cells and the donor is often not accessible. Moreover, because the donor and recipient are rarely HLA-matched, donor-derived T-cells would need to have activity through shared antigens to be effective. Although patients usually remain on long-term immunosuppression, several groups have successfully generated recipient CTLs and tested them for activity in vivo (Table 2). Our group has administered autologous EBV-specific CTLs to 10 patients with persistently high or rising EBV DNA levels after solid organ transplant.36 Although the CTL infusions failed to decrease the viral load in a consistent manner, none of the patients developed PTLD. Treatment of two patients with active disease yielded a complete response and a partial response that persisted for up to 1 year.36 This experience is consistent with reports of other investigators who have used similar preparations to treat patients with elevated EBV viral load or active disease (Table 2).34, 35, 45 Overall, these studies have helped to allay concerns that autologous EBV-specific CTL might induce rejection of the transplanted solid organ.35, 36 This progress notwithstanding, the expansion and persistence of the CTLs were less than observed after HSCT, likely because of the need to continue immunosuppressive treatment for patients after solid organ transplant and because the lymphodepletion associated with HSCT is highly conducive to in vivo T-cell expansion.46 The type I and II latency malignancies that occur years after solid organ transplantation are less immunogenic and more immunosuppressive than classic PTLDs and may require T-cells that are genetically modified to resist immunosuppressive molecules47 and/or immunosuppressive drugs. Combination therapies with drugs that modify the tumor environment may also be beneficial but will likely be evaluated first outside of the transplant setting.
Table 2.
Clinical trials using autologous EBV CTLs in solid organ transplant
| Study | n | Prophylaxis or therapy |
Serious adverse events possibly attributable to CTLs |
Outcome |
|---|---|---|---|---|
| Comoli et al. (2002)35 | 7 | Prophylaxis | None | No PTLD |
| Haque et al. (1998)83 | 3 | Prophylaxis | None | No PTLD |
| Khanna et al. (1999)34 | 1 | Therapy | None | Significant regression |
| Sherrit et al. (2003)45 | 1 | Therapy | None | Complete remission |
| Comoli et al. (2005)84 | 5 | Therapy | None | Complete remission (used as adjuvant after chemotherapy and rituximab) |
| Savoldo et al. (2006)36 | 12 | Prophylaxis and Therapy | None | No PTLD;1 of 2 patients treated with PTLD attained complete remission and the other a partial remission |
Abbreviations: CTL, cytotoxic T-lymphocyte; EBV, Epstein-Barr virus; PTLD, post-transplant lymphoproliferative disease.
Simplifying the use of EBV-CTLS
In general, the manufacturing process of EBV CTL takes approximately 8–12 weeks, and half of this time is required for the generation of the antigen presenting cells (EBV–LCL). The other half of the time is required for the generation and expansion of the EBV-specific CTL to obtain the cell numbers which are required for clinical use as well as product testing.48. The widespread use of T-cells to prevent or treat EBV-PTLD is limited by this relatively long production time, as they cannot be generated in response to a patient developing PTLD. However, several groups have explored the development of more rapid approaches for the production of virus-specific T-cells.49–54 Three such methods have been used clinically;51, 55, 56 firstly, multimer selection, which selects T-cells directed against specific viral peptides in the context of a specific HLA class I molecule; secondly, IFN-γ capture, in which cells responding to stimulation by viral antigens are selected on the basis of their secretion of IFN-γ; and lastly, faster CTL culture methods that use peptides or plasmid-derived viral antigens expressed in dendritic cells to stimulate T-cells in the presence of cytokines. These strategies essentially enable the enrichment of virus-specific CTL derived from a small starting population of antigen specific T-cells. Despite the small numbers of T-cells infused using this approach, the highly specific T-cell populations have the potential to expand exponentially in vivo when they encounter antigen thereby providing potent antiviral activity.
Rapid ex vivo culture
To reduce the time to manufacture and develop a strategy that avoids the use of live viruses in APC, Gerdemann et al.,57 have developed an approach to generate virus-specific T-cells (rCTL). This method consists of a single stimulation of T-cells with dendritic cells previously transfected (using nucleofection) with DNA plasmids encoding immunogenic EBV (LMP2, EBNA1, BZLF1), as well as antigens from adenovirus and cytomegalovirus (CMV), and later expansion of the stimulated cells in the presence of interleukin 4 (IL-4) and IL-7 in a gas permeable culture device (G-Rex) which is used to promote gas exchange, improve the nutrient supply and prevent waste accumulation in the T cell cultures. In preliminary results ‘rapid-CTLs’ have been able to control infections including EBV-PTLD with no GVHD.58 Virus-derived peptides can also produce rapid T-cell expansion from peripheral blood mononuclear cells in the presence of cytokines and further reduce the culture time by eliminating the requirement for dendritic cells.59 However, this approach has yet to be tested clinically.
Tetramer selection
Cobbold and colleagues39 were the first to report the use of tetrameric HLA-peptide constructs (tetramers) to select a virus-specific T-cell population for clinical use.54 This group infused patients with CMV peptide-specific CD8+ T-cells isolated directly from the donor’s peripheral blood by incubating these cells with tetramers, followed by selection of T cells bound to the tetramers with magnetic beads.54 They were able to detect CMV specific T-cell function in the patient within 10 days of infusion despite the small number of virus-specific CD8+ cells infused. In a subsequent study, Schmitt and co-workers42 exploited a novel streptamer technology to select CMV-specific T-cells for transfer into patients with recurrent high CMV antigenemia after HSCT. This technique combines the teramer isolation approach with “Strep-tag/Strep-Tactin” technology, which results in a reversibility of the TCR-MHC interaction and enables more effective purification of antigen specific T-cells without affecting their functional state. They reported a dramatic expansion of CMV-specific CD8+ effector T-cells in association with clearance of CMV antigenemia.60 An additional case illustrates how this approach can be applied to EBV.56 Tetramers were used to select T-cells specific for two EBV antigens recognized in the context of HLA A2 from a haploidentical parent donor.60 A small dose of 104 CTLs/kg was infused into the recipient, who shared HLA A2 and had developed PTLD after a cord blood transplant. A dramatic expansion of T-cells coincided with a clinical response. A second infusion, administered in response to a probable early relapse (development of fever and recurrent detection of EBV in blood and tonsils) at 12 months post transplantation, also induced a response. Although these results are encouraging, the restricted specificity could result in tumour evasion, and such a strategy is limited to donors who express HLA alleles for which viral peptides are available, and for which sufficient circulating T-cells specific for the peptide can be isolated from the donor.
Isolation of IFN-γ secreting T-cells
Selection of T-cells that secrete IFN-γ in response to antigen provides an alternative strategy for the immediate isolation of populations of virus-specific T-cells. Moosmann and colleagues51 used immunomagnetic separation to select cells that secreted IFN-γ in response to stimulation of donor mononuclear cells with 23 class I and II peptides derived from 11 EBV antigens. Six patients with PTLD—three of them with early stage disease—who received an average dose of 4 × 106 selected T-cells had complete responses, whereas three patients with more advanced disease did not respond to the treatment.51
This finding raises important issues. First, will CTLs that target selected EBV-peptides be as effective as CTLs activated using LCLs? Notably, CTLs activated with LCL antigens are physiologically processed, ensuring that the generation of CTL lines have high-avidity CTLs with broad reactivity against multiple CD4 and CD8 epitopes. However, and alternative approach is to use overlapping peptide pools from selected EBV antigens (e.g. EBNA1). Such a strategy has been recently shown to produce EBV specific T cells which have shown efficacy clinically.61 Second, which EBV antigens will induce protective T-cells? We have elected to use three EBV antigens: latent LMP2, which is subdominant, but recognized by a majority of donors and expressed in type II and type III latencies; EBNA1, which elicits CD4+ T-cells in a majority of donors and is expressed in all EBV-associated malignancies; and BZLF1, which is an immunodominant lytic cycle protein.62 This combination has proved effective in preliminary clinical studies58 Another concern regarding the attempts to broaden antigenic coverage is that it may increase competition for peptide binding to HLA, or generate EBV-specific T-cells with cross-reactivity against alloantigens.63, 64 However, de novo alloreactivity was not observed among 153 recipients who received CTLs generated using LCL as stimulator cells—including 73 patients for whom there was an HLA mismatch with the donor—and competition for HLA molecules did not reduce the repertoire of expanded T-cells in our preliminary studies.65 Strategies for direct selection of EBV-specific T-cells from peripheral blood require large blood volumes or apheresis products, which would be feasible only for related donors.56
Third party EBV-CTLs
EBV CTLs could be made rapidly available to a larger number of patients if there was a bank of characterized HLA-typed EBV-specific T-cell lines. This third-party approach was first tested in the clinic by Haque and colleagues,66 who manufactured a bank of polyclonal EBV CTL lines for the treatment of EBV-associated diseases in patients undergoing HSCT or solid organ transplantation. Eight patients with PTLD received closely HLA-matched EBV-specific CTL. The CTLlines were generated from unrelated third-party blood donors and selection of the CTL were based primarily on the best HLA match.67 Using this approach, 3 patients attained a complete remission without evidence of alloreactivity (Table 3). More recently, this group reported data from a larger phase II multicentre trial enrolling 33 patients with EBV positive PTLD from 19 transplant centres and who had not responded to conventional therapy and were given the best-HLA-matched ‘off-the-shelf’ product.7 Using this approach acheived overall response rates of 64% at 5 weeks, and 52% at 6 months. The best results wereobserved in patients who received donor T-cells which were best HLA matched with the recipient.7
Table 3.
Clinical trials using third party EBV CTLs
| Study | n | Type of transplant |
CTL line | Serious adverse events possibly attributable to CTLs |
Outcome |
|---|---|---|---|---|---|
| Haque et al. (2002)67 | 8 | SOT | Closely matched allogeneic EBV specific CTL | None reported | 3 attained CR; 2 did not respond, 3 did not complete treatment |
| Haque et al. (2007)7 | 33 | SOT and HSCT | Closely matched allogeneic EBV specific CTL | None reported | 14 attained CR, 3 had a PR and 16 had no response at 6 months |
| Gandhi et al. (2007)85 | 3 | SOT | Closely matched allogeneic EBV specific CTL | None reported | 2 attained CR |
| Sun et al. (2002)68 | 2 | SOT | Closely matched allogeneic EBV specific CTL | None reported | 2 attained CR (one also received radiotherapy) |
| Barker et al. (2010)69 Doubrovina et al.. (2012)6 | 5 | HSCT including cord | Closely matched allogeneic EBV specific CTL | None reported | 4 attained CR; 1 had progressive disease |
| Uhlin et al. (2010)56 | 1 | Cord | Haploidentical GLC-peptide separated CTLs | None reported | CR that recurred 9 months later and responded to 2nd infusion |
| Leen et al. (2010)71 | 5 | HSCT | Closely matched allogeneic trivirus specific CTL | 1 reactivation chronic GVHD | 3 attained CR or PR; 2 had no response |
Abbreviations: CR, complete remission; CTL, cytotoxic T-lymphocyte; EBV, Epstein-Barr virus; GVHD, graft versus host disease; HSCT, haematopoietic stem-cell transplantation; PR, partial remission; PTLD, post-transplant lymphoproliferative disease SOT, solid organ transplantation.
The above results are supported by reports from other centres. Sun et al.68 described two solid organ transplant recipients who responded to allogeneic CTLs with regressions of PTLD (Table 3). Complete responses were also achieved in four of five patients treated at the Memorial Sloan–Kettering Cancer Center who received third party EBV CTL lines.69, In the patient who did not respond, the infused line was selectively restricted by a class II HLA allele not shared with the transplant donor-derived PTLD, again illustrating the importance of matching activity in the infused line to antigens shared with tumour cells. We have also evaluated the use of third-party multivirus-specific CTLs to treat patients with refractory viral infections in a multicentre study that included five patients with EBV-associated PTLD. 6, 70,71 Although two of these five patients did not respond, the other three obtained a partial or complete response.
Interpretation of these results is not straightforward as many of the patients who responded also had their immunosuppressive treatments reduced and received other therapies.7, 71 Hence the encouraging benefits cannot be solely ascribed to the allogeneic CTLs. T-cell persistence was also reduced compared with donor-derived cells, and in some instances multiple infusions were required to control PTLD.6 Nevertheless, this approach has shown considerable promise, warranting further testing in definitive studies. If CTL banks are to succeed, it will be important to establish criteria that would provide broad coverage. There are potential advantages to using homozygous donors that facilitate characterization of antigen-specificity and HLA restriction. With respect to the use of third party cells, clinical protocols should allow for multiple infusions and for the substitution of donor lines if patients fail to respond.
We would recommend the use of directly selected T-cells for recipients of related donor HSCT, and the use of third party, banked CTLs for HSCT recipients with unrelated donors, and for solid organ recipients. This choice would provide time for the generation of ‘rapid CTLs’, from small amounts of donor cryopreserved blood at the time of transplant or from the solid organ recipients.
Making CTLs resistant to immunosupression
In the setting of solid organ transplant, persistence of infused CTLs has been limited probably because these patients remain on long-term immune suppression. Similarly, CTLs would likely not persist after HSCT in patients on immune suppression due to GVHD. Hence, CTLs have been genetically modified to render them resistant to cyclosporine or tacrolimus or rapamycin in preclinical studies,72–74 although the efficiency of these strategies has not yet been evaluated in the clinic.
CONCLUSIONS
Cell-based immunotherapy with donor-derived lymphocytes or CTLs specific for EBV antigens has proved highly effective in treating PTLD in the HSCT setting. Current research is focused on broadening the application of this approach by optimizing manufacturing processes so that CTL products can be more rapidly available for infusion on a routine basis and to provide broad spectrum anti-viral coverage. Third-party CTL infusions targeting EBV lymphomas have also shown promise, and HLA-matching requirements and the factors associated with response are being defined to optimize this strategy further. Of note, all of these T-cell therapies, except DLI, are currently experimental and only available in the context of clinical trials although some approaches are being or will soon be tested in late phase or licensing studies after HSCT. Table 4 summarizes the T-cell approaches under evaluation, and lists the advantages and disadvantages of each of them. It will also be important eventually to undertake cost effectiveness analyses of different types of T-cell therapies compared with other options such as rituximab. Overall however, the implications of this work are that EBV-specific T cell therapy for PTLD can now move beyond a single center “boutique” approach and has the potential for mainstream use especially given the promise of the “off the shelf” third party CTL product. Future therapeutic strategies for PTLD may therefore now focus on combination immune based therapies with targeted T cell and antibody therapies. Hence, such an approach has the potential to minimize the use of untargeted cytotoxic chemotherapy for patients even with advanced stage disease thereby potentially not only preventing unacceptable toxicities but also an improved outcome for patients with EBV-associated PTLD.
Table 4.
Advantages and disadvantages of different T-cell therapies
| T-cell approach | Advantages | Disadvantages |
|---|---|---|
| DLI | Rapidly available from most HSCT donors Response rate of around 70% Can be given as standard of care |
Not usually an option after cord blood transplant Risk of GVHD |
| DLI with suicide gene | Safety switch if GVHD occurs | Requires gene transfer with integrating vector Only available on clinical trials |
| EBV CTLs (activated with LCLs) |
Response rates of 70–90% Can also be generated for some recipients of cord blood transplants |
Lengthy production (10–12 weeks) Only available on clinical trial |
| Rapid EBV CTLs | Available in 2–3 weeks Initial good response rate in a small number of patients |
Only available on clinical trials More data needed on response rate Not an option after cord transplant |
| Multimer selected EBV CTLs |
Available in 2–3 days Initial good response rate in a small number of patients |
Need for pheresis More data needed on response rate Risk of tumour evasion due to restricted specificity Not an option after cord transplant |
| Gamma capture-selected EBV CTLs |
Available in 5–7 days Initial good response rate in a small number of patients |
Need for pheresis More data needed on response rate Not an option after cord transplant |
| Third party CTLs | Available immediately Option for cord recipients Response rate 50–70% |
Response rate may be less than with donor-derived CTLs (more data needed) May be difficult to match some patients |
Abbreviations: CTL, cytotoxic T-lymphocyte; DLI, donor lymphocyte infusion; EBV, Epstein-Barr virus; GVHD, graft versus host disease; HSCT, haematopoietic stem-cell transplantation; LCL, lymphoblastoid cell lines; PTLD, post-transplant lymphoproliferative disease.
Review criteria
Information for this article was obtained by searching the PubMed, database as well as the clinicaltrials.gov site on 5 October 2011 (the approximate range when the articles discussed in this review were published is from 1990 to 2012).. The following MeSH terms “EBV”, “CTL”, “PTLD”, “lymphoma”, “T-cell”, and “immunotherapy”, were used in various combinations. The reference lists of retrieved articles as well as articles known to the authors were all reviewed. A final decision to include or exclude a given study was based on quality, relevance and clinical applicability of the article.
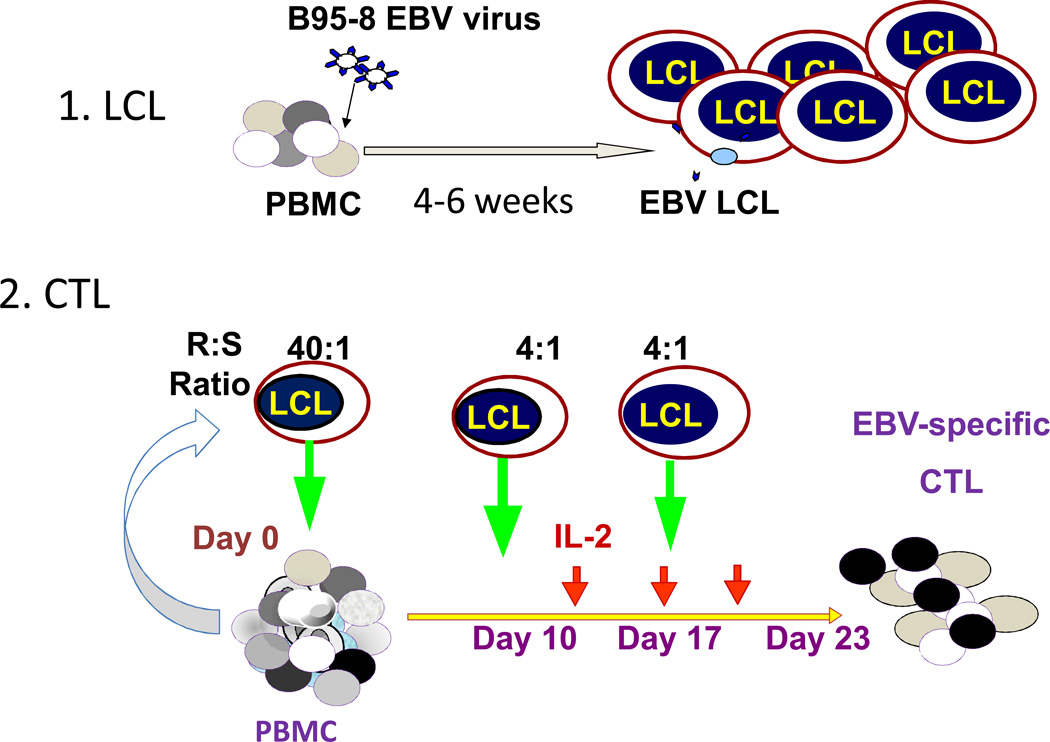
Figure 2. EBV-specific CTL production.
EBV-transformed B-cell LCLs are prepared from the CTL donor by infection of PBMCs with a clinical grade laboratory strain of EBV (B95-8) in the presence of cyclosporin A. Once the LCL is established (about 6 weeks), it is irradiated and used to stimulate PBMCs from the same donor and a 40:1 ratio of PBMC to LCL. From 9 to 12 days later and weekly thereafter, the T-cells are re-stimulated with the LCL at a 4:1 ratio. IL-2 is added 3 days after the second stimulation and twice weekly thereafter. The CTLs should kill autologous LCLs but not autologous PHA blasts. Their specificity is donor dependent and they may have specificity for any of the 10 latency associated antigens and or for early lytic cycle proteins that are express by a small fraction of the LCLs, which are grown in acyclovir to prevent the production of infectious virus by blocking the viral thymidine kinase. Abbreviations: CTL, cytotoxic T-lymphocyte; EBNA, Epstein-Barr nuclear antigen; EBV, Epstein-Barr virus; IL-2, Interleukin-2; LCL, lymphoblastoid cell lines; PHA, phytohaemagglutinin, PBMCs, peripheral blood mononuclear cell.
KEY POINTS.
Uncontrolled growth of EBV-infected B-cells in patients after haemopoietic or solid organ transplants due to immunosuppression or depletion of virus-specific T-cells can result in development of high-grade EBV lymphomas
Transplantation of EBV-specific cytotoxic T-lymphocytes (CTLs) derived from the donor has effectively prevented EBV-associated post-transplant lymphoproliferative disease (PTLD), inducing complete responses in over 70% of patients with this complication
Treatment of using closely matched EBV-CTLs from third-party donors can lead to responses in over 50% of such cases
Failure to respond to CTLs occurs when T-cells of restricted specificity are infused, recipients lack HLA antigens through which EBV activity in the line is restricted or tumours express variants of EBV antigens used to stimulate CTLs
Novel methods for the rapid production of EBV-specific T-cells and increased commercial interest should make EBV-specific T-cells more readily available to transplant recipients in the future
Acknowledgements
This work was supported by grants P01CA094237, P50CA126752 and U54HL08100 from the NIH and a SCOR award from the Leukemia and Lymphoma Society
Footnotes
Author contributions
All authors contributed to researching and writing the article and editing the final manuscript.
Competing interests statement
The authors declare no competing interests.
Reference List
- 1.Gottschalk S, Rooney CM, Heslop HE. Post-Transplant Lymphoproliferative Disorders. Annu. Rev. Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JI. Epstein-Barr virus infection. N. Engl. J. Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 3.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 4.Khanna R, Moss D, Gandhi M. Technology insight: Applications of emerging immunotherapeutic strategies for Epstein-Barr virus-associated malignancies. Nat. Clin Pract. Oncol. 2005;2:138–149. doi: 10.1038/ncponc0107. [DOI] [PubMed] [Google Scholar]
- 5.Heslop HE, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doubrovina E, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque T, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 8.Long HM, Taylor GS, Rickinson AB. Immune defence against EBV and EBV-associated disease. Curr. Opin. Immunol. 2011;23:258–264. doi: 10.1016/j.coi.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA. Epstein-barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med. 1999;190:567–576. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorley-Lawson DA, Duca KA, Shapiro M. Epstein-Barr virus: a paradigm for persistent infection - for real and in virtual reality. Trends Immunol. 2008;29:195–201. doi: 10.1016/j.it.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Elmagd KM, et al. Lymphoproliferative disorders and de novo malignancies in intestinal and multivisceral recipients: improved outcomes with new outlooks. Transplantation. 2009;88:926–934. doi: 10.1097/TP.0b013e3181b7509c. [DOI] [PubMed] [Google Scholar]
- 13.Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA. Epstein-barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J. Exp. Med. 1999;190:567–576. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swerdlow SH, Webber SA, Chadburn A, Ferry J. Post Transplant Lymphoproliferative Disorders. International Agency for Research on Cancer; 2008. [Google Scholar]
- 15.Meij P, et al. Impaired recovery of Epstein-Barr virus (EBV)-specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood. 2003;101:4290–4297. doi: 10.1182/blood-2002-10-3001. [DOI] [PubMed] [Google Scholar]
- 16.Brunstein CG, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen J, et al. Increased incidence of EBV-related disease following paediatric stem cell transplantation with reduced-intensity conditioning. Br J Haematol. 2005;129:229–239. doi: 10.1111/j.1365-2141.2005.05439.x. [DOI] [PubMed] [Google Scholar]
- 18.Landgren O, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swinnen LJ, et al. Prospective study of sequential reduction in immunosuppression, interferon alpha-2B, and chemotherapy for posttransplantation lymphoproliferative disorder. Transplantation. 2008;86:215–222. doi: 10.1097/TP.0b013e3181761659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross TG, Savoldo B, Punnett A. Posttransplant lymphoproliferative diseases. Pediatr. Clin North Am. 2010;57:481–503. doi: 10.1016/j.pcl.2010.01.011. table. [DOI] [PubMed] [Google Scholar]
- 21.Dotti G, et al. Epstein-Barr virus-negative lymphoproliferate disorders in long-term survivors after heart, kidney, and liver transplant [see comments] Transplantation. 2000;69:827–833. doi: 10.1097/00007890-200003150-00027. [DOI] [PubMed] [Google Scholar]
- 22.Dotti G, et al. Lymphomas occurring late after solid-organ transplantation: influence of treatment on the clinical outcome. Transplantation. 2002;74:1095–1102. doi: 10.1097/00007890-200210270-00007. [DOI] [PubMed] [Google Scholar]
- 23.Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009;114:4002–4008. doi: 10.1182/blood-2009-07-143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker A, et al. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients - BCSH and BTS Guidelines. Br. J Haematol. 2010;149:675–692. doi: 10.1111/j.1365-2141.2010.08161.x. [DOI] [PubMed] [Google Scholar]
- 25.Gulley ML, Tang W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin Microbiol. Rev. 2010;23:350–366. doi: 10.1128/CMR.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker A, et al. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients - BCSH and BTS Guidelines. Br. J Haematol. 2010;149:693–705. doi: 10.1111/j.1365-2141.2010.08160.x. [DOI] [PubMed] [Google Scholar]
- 27.Styczynski J, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43:757–770. doi: 10.1038/bmt.2008.386. [DOI] [PubMed] [Google Scholar]
- 28.Tomblyn M, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol. Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zelenetz AD, et al. NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin's lymphomas. J. Natl. Compr. Canc. Netw. 2010;8:288–334. doi: 10.6004/jnccn.2010.0021. [DOI] [PubMed] [Google Scholar]
- 30.Kuehnle I, et al. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood. 2000;95:1502–1505. [PubMed] [Google Scholar]
- 31.van Esser JW, et al. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood. 2002;99:4364–4369. doi: 10.1182/blood.v99.12.4364. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter PA, et al. A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graft-versus-host disease. Blood. 2002;99:2712–2719. doi: 10.1182/blood.v99.8.2712. [DOI] [PubMed] [Google Scholar]
- 33.Savoldo B, et al. Cellular immunity to Epstein-Barr virus in liver transplant recipients treated with rituximab for post-transplant lymphoproliferative disease. Am. J Transplant. 2005;5:566–572. doi: 10.1111/j.1600-6143.2004.00693.x. [DOI] [PubMed] [Google Scholar]
- 34.Khanna R, et al. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci U S A. 1999;96:10391–10396. doi: 10.1073/pnas.96.18.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comoli P, et al. Infusion of autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for prevention of EBV-related lymphoproliferative disorder in solid organ transplant recipients with evidence of active virus replication. Blood. 2002;99:2592–2598. doi: 10.1182/blood.v99.7.2592. [DOI] [PubMed] [Google Scholar]
- 36.Savoldo B, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs) Blood. 2006;108:2942–2949. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bollard CM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorley-Lawson DA, Allday MJ. The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat. Rev. Microbiol. 2008;6:913–924. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- 39.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N. Engl. J. Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 40.O'Reilly RJ, et al. Biology and adoptive cell therapy of Epstein-Barr virus-associated lymphoproliferative disorders in recipients of marrow allografts. Immunol. Rev. 1997;157:195–216. doi: 10.1111/j.1600-065x.1997.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 41.Ciceri F, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 42.Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heslop HE, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottschalk S, et al. An Epstein-Barr virus deletion mutant that causes fatal lymphoproliferative disease unresponsive to virus-specific T cell therapy. Blood. 2001;97:835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 45.Sherritt MA, et al. Reconstitution of the latent T-lymphocyte response to Epstein-Barr virus is coincident with long-term recovery from posttransplant lymphoma after adoptive immunotherapy. Transplantation. 2003;75:1556–1560. doi: 10.1097/01.TP.0000058745.02123.6F. [DOI] [PubMed] [Google Scholar]
- 46.Peggs KS, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 47.Bollard CM, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 48.Smith CA, et al. Production of genetically modified EBV-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease. J Hematother. 1995;4:73–79. doi: 10.1089/scd.1.1995.4.73. [DOI] [PubMed] [Google Scholar]
- 49.Peggs KS, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin. Infect. Dis. 2011;52:49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- 50.Feuchtinger T, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 51.Moosmann A, et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010;115:2960–2970. doi: 10.1182/blood-2009-08-236356. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt A, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51:591–599. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 53.Neudorfer J, et al. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol. Methods. 2007;320:119–131. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Cobbold M, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp. Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerdemann U, et al. Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol. Ther. 2009;17:1616–1625. doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uhlin M, et al. A novel haplo-identical adoptive CTL therapy as a treatment for EBV-associated lymphoma after stem cell transplantation. Cancer Immunol. Immunother. 2010;59:473–477. doi: 10.1007/s00262-009-0789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerdemann U, et al. Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol. Ther. 2009;17:1616–1625. doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerdemann U, et al. Adoptive Transfer of Rapidly-Generated Multivirus-Specific T Cells to Treat Adenovirus, EBV and CMV Infections of Hematopoietic Stem Cell Transplant Recipients. Biol Blood Marrow Transplant. 2012;18(2):S219. Ref Type: Abstract. [Google Scholar]
- 59.Gerdemann U, et al. Rapidly-generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Molecular Therapy. 2012 doi: 10.1038/mt.2012.130. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitt A, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51:591–599. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 61.Tcheva V, et al. Adoptive transfer of EBNA1-specific T cells as a treatment of Epstein-Barr-Virus reactivation and lymphoproliferative disorders following allogeneic stem cell transplantation. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.39.8495. Ref Type: In Press. [DOI] [PubMed] [Google Scholar]
- 62.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 63.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179:1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amir AL, et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 65.Melenhorst JJ, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010;116:4700–4702. doi: 10.1182/blood-2010-06-289991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haque T, et al. Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells. Transplantation. 2001;72:1399–1402. doi: 10.1097/00007890-200110270-00012. [DOI] [PubMed] [Google Scholar]
- 67.Haque T, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360:436–442. doi: 10.1016/S0140-6736(02)09672-1. [DOI] [PubMed] [Google Scholar]
- 68.Sun Q, Burton R, Reddy V, Lucas KG. Safety of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for patients with refractory EBV-related lymphoma. Br J Haematol. 2002;118:799–808. doi: 10.1046/j.1365-2141.2002.03683.x. [DOI] [PubMed] [Google Scholar]
- 69.Barker JN, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116:5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leen AM, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nature Medicine. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 71.Leen AM, et al. Most Closely HLA-Matched Allogeneic Virus Specific Cytotoxic T-Lymphocytes (CTL) to Treat Persistent Reactivation or Infection with Adenovirus, CMV and EBV After Hemopoietic Stem Cell Transplantation (HSCT) Blood. 2010;829(Suppl 1) Ref Type: Abstract. [Google Scholar]
- 72.Brewin J, et al. Generation of EBV-specific cytotoxic T cells that are resistant to calcineurin inhibitors for the treatment of posttransplantation lymphoproliferative disease. Blood. 2009;114:4792–4803. doi: 10.1182/blood-2009-07-228387. [DOI] [PubMed] [Google Scholar]
- 73.De Angelis B, et al. Generation of Epstein-Barr virus-specific cytotoxic T lymphocytes resistant to the immunosuppressive drug tacrolimus (FK506) Blood. 2009;114:4784–4791. doi: 10.1182/blood-2009-07-230482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huye LE, et al. Combining mTor inhibitors with rapamycin-resistant T cells: a two-pronged approach to tumor elimination. Mol. Ther. 2011;19:2239–2248. doi: 10.1038/mt.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rooney CM, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 76.Heslop HE, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Medicine. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 77.Rooney CM, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 78.Papadopoulos EB, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N. Engl. J. Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 79.Gustafsson A, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95:807–814. [PubMed] [Google Scholar]
- 80.Lucas KG, et al. Semiquantitative Epstein-Barr virus (EBV) polymerase chain reaction for the determination of patients at risk for EBV-induced lymphoproliferative disease after stem cell transplantation. Blood. 1998;91:3654–3661. [PubMed] [Google Scholar]
- 81.Imashuku S, et al. Unsuccessful CTL transfusion in a case of post-BMT Epstein-Barr virus-associated lymphoproliferative disorder (EBV-LPD) Bone Marrow Transplant. 1998;20:337–340. doi: 10.1038/sj.bmt.1700883. [DOI] [PubMed] [Google Scholar]
- 82.Comoli P, et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am. J Transplant. 2007;7:1648–1655. doi: 10.1111/j.1600-6143.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- 83.Haque T, et al. Reconstitution of EBV-specific T cell immunity in solid organ transplant recipients. J Immunol. 1998;160:6204–6209. [PubMed] [Google Scholar]
- 84.Comoli P, et al. Treatment of EBV-Related Post-Renal Transplant Lymphoproliferative Disease with a Tailored Regimen Including EBV-Specific T Cells. Am. J. Transplant. 2005;5:1415–1422. doi: 10.1111/j.1600-6143.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 85.Gandhi MK, et al. Immunity, homing and efficacy of allogeneic adoptive immunotherapy for posttransplant lymphoproliferative disorders. Am. J Transplant. 2007;7:1293–1299. doi: 10.1111/j.1600-6143.2007.01796.x. [DOI] [PubMed] [Google Scholar]