Abstract
A new library of small molecules with structural features resembling combretastatin analogs was synthesized and evaluated for anticancer activity against a panel of 60 human cancer cell lines. Three novel acrylonitrile analogs (5, 6 and 13) caused a significant reduction in cell growth in almost all the cell lines examined, with GI50 values generally in the range 10–100 nM. Based on the structural characteristics of similar drugs, we hypothesized that the cytotoxic activity was likely due to interaction with tubulin. Furthermore, these compounds appeared to overcome cell-associated P-glycoprotein (P-gp)-mediated resistance, since they were equipotent in inhibiting OVCAR8 and NCI/ADR-Res cell growth. Given that antitubulin drugs are among the most effective agents for the treatment of advanced prostate cancer we sought to validate the results from the 60 cell panel by studying the representative analog 6 utilizing prostate cancer cell lines, as well as exploring the molecular mechanism of the cytotoxic action of this analog.
Introduction
The microtubule system of eukaryotic cells is an important target for the development of anticancer agents. Chemicals which attack microtubules through tubulin interaction disrupt cellular microtubule structure and function, resulting in mitotic arrest.1 This action is accomplished by either preventing tubulin polymerization or preventing its depolymerisation.2–4 Examples of clinically used agents which prevent tubulin polymerization include vincristine, vinblastine, and combretastatin A4.5 Well-known microtubule stabilizing anticancer drugs include paclitaxel (Taxol), docetaxel (Taxotere), cabazitaxel (Jevtana), discodermolide, the epothilones, the eleutherobins, and laulimalide.6 These small molecules bind to different sites on tubulin, and thereby exert diverse effects on microtubule dynamics. However, despite their potent antitumor activities, all of these compounds have limitations resulting from high toxicity, poor oral bioavailability, difficulty of synthesis or isolation from natural sources, and are subject to multidrug resistance (MDR) cell mechanisms.7
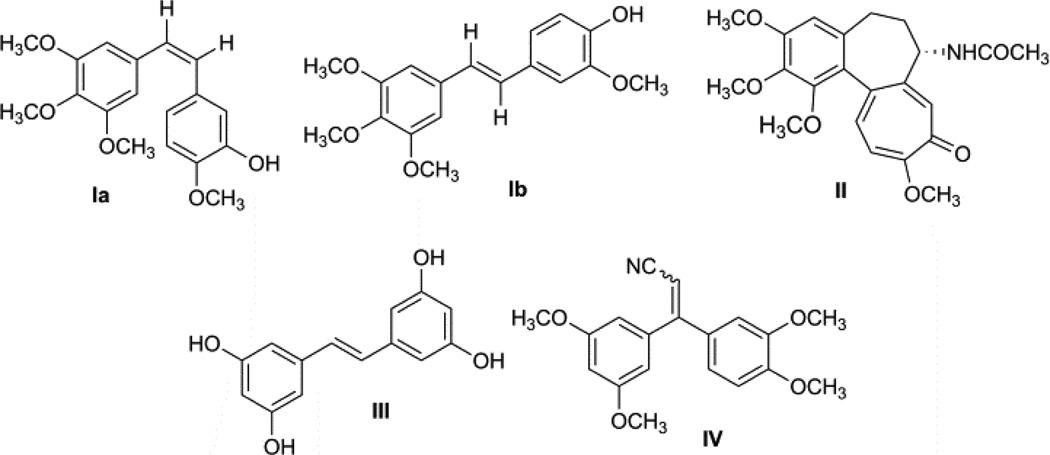
Thus, it is essential to develop new anticancer agents with fewer side effects, which are not substrates for MDR efflux proteins, and that exhibit cytotoxicity against cancers not effectively treated by existing anticancer drugs. The isolation of many stilbene derivatives, termed combretastatins, from the South African tree Combretum caffrum has been described.8 Many of these combretastatins (Fig. 1) were found to be cytotoxic, with combretastatin A-4 (Ia) being the most potent.9, 10 This compound was found to inhibit tubulin polymerization, and competitively inhibit the binding of radiolabeled colchicines (II) to tubulin. Investigation of combretastatins revealed that combrestastatin A-4 (Ia) was active against multidrug resistant (MDR) cancer cell lines.10 Combretastatin A-4 (Ia), as well as its trans-isomer Ib and a number of related substances have been found to cause mitotic arrest in cells in culture at cytotoxic concentrations. trans-Tetrahydroxystilbene (III) and a number of other structurally related substances were also reported to be cytotoxic agents.9 Recently, researchers at the Celgene Corporation reported a novel synthetic tubulin polymerization inhibitor, 3,3-diarylacrylonitrile analog (CC-5079, IV), for potential use in cancer chemotherapy.11 2,3-Diarylacrylonitriles are very important synthons that have been utilized in the synthesis of a wide spectrum of biologically active molecules.12 These compounds have been shown to possess spasmolytic, estrogenic, hypotensive, antioxidative, tuberculostatic, antitrichomonal, insecticidal and cytotoxic activities.13 Also, many natural products possessing a trimethoxyphenyl moiety, e.g., colchicines, and podophyllotoxin, are potent cytotoxic agents and exert their antitumor properties based on their antitubulin character. In view of the antitubulin activity of combretastatin A-4 (Ia), a large number of its derivatives have been synthesized and evaluated for antitubulin activity.14–17
Fig 1.
Chemical structures of naturally occurring antitubulin agents and their related analogs
In the current work, trimethoxyphenyl ring systems and stilbene structural fragments present in the combretastatin A-4 molecule have been connected to a benzothiophene heterocyclic ring system. The stilbene ring system has also been modified by introduction of an active acrylonitrile moiety, and a variety of different acrylonitrile analogs have been generated by incorporating monomethoxy, dimethoxy, or hydroxydimethoxy groups into the phenyl ring.
Chemistry
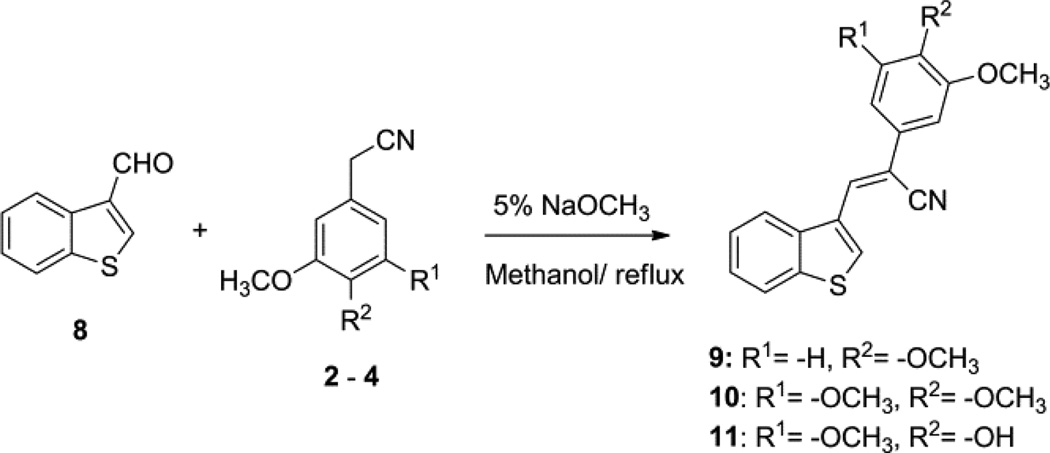
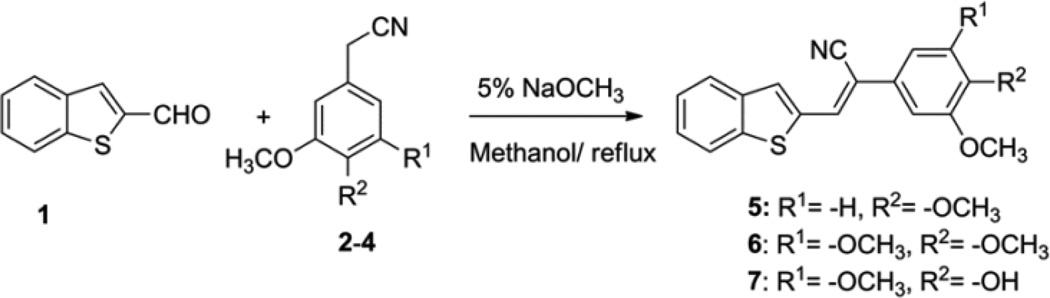
A series of diarylacrylonitrile analogs 5–7 and 9–11 were synthesized by refluxing either benzo[b]thiophene-2-carbaldehyde (1) or benzo[b]thiophene-3-carbaldehyde (8) with the following benzylnitriles: phenylacetonitrile, 3,4,5-trimethoxyphenylacetonitrile, 3,4-dimethoxyphenylacetonitrile, and 4-hydroxy-3,5-dimethoxyphenylacetonitrile in 5% sodium methoxide in methanol. Confirmation of the structure and purity of these analogs was obtained from 1H- & 13C-NMR and mass spectroscopic analysis. The geometry of the double bond (E or Z-isomers) in these molecules was established as the Z-configuration from single crystal X-ray crystallographic data.18 Compounds containing the acrylonitrile moiety that have Z-geometry can undergo conversion to the corresponding E isomer under certain conditions. This isomerization is dependent on the nature of the two groups linked across the double bond that carries the nitrile group, i.e. whether the groups are π-excessive (donating) or π-deficient (accepting). Normally, base-catalyzed condensation of aryl/hetero arylaldehydes with aryl/heteroaryl acetonitriles leads exclusively to the formation of the Z-isomer, however, Z to E isomerism can be achieved on exposure of the Z-isomer to UV light,19 and this can be readily monitored in solution by HPLC analysis.
Reaction of benzo[b]thiophene-3-carbaldehyde (8) with 3,4-dimethoxyphenylacetonitrile, 3,4,5-trimethoxyphenyl acetonitrile and 4-hydroxy, 3,5-dimethoxyphenylacetonitrile in 5% sodium methoxide in methanol afforded Z-3-(benzo[b]thiophen-3-yl)-2-(3,4-dimethoxyphenyl)acrylonitrile (9), (Z)-3-(benzo[b] thiophen-3-yl)-2-(3,4,5-trimethoxyphenyl)acrylonitrile (10) and Z-3-(benzo[b]thiophen-3-yl)-2-(4-hydroxy-3,5-dimethoxy phenyl) acrylonitrile (11) (Scheme 2).20
Scheme 2.
Synthesis of Z-benzo[b]thiophene-3-yl acrylonitriles
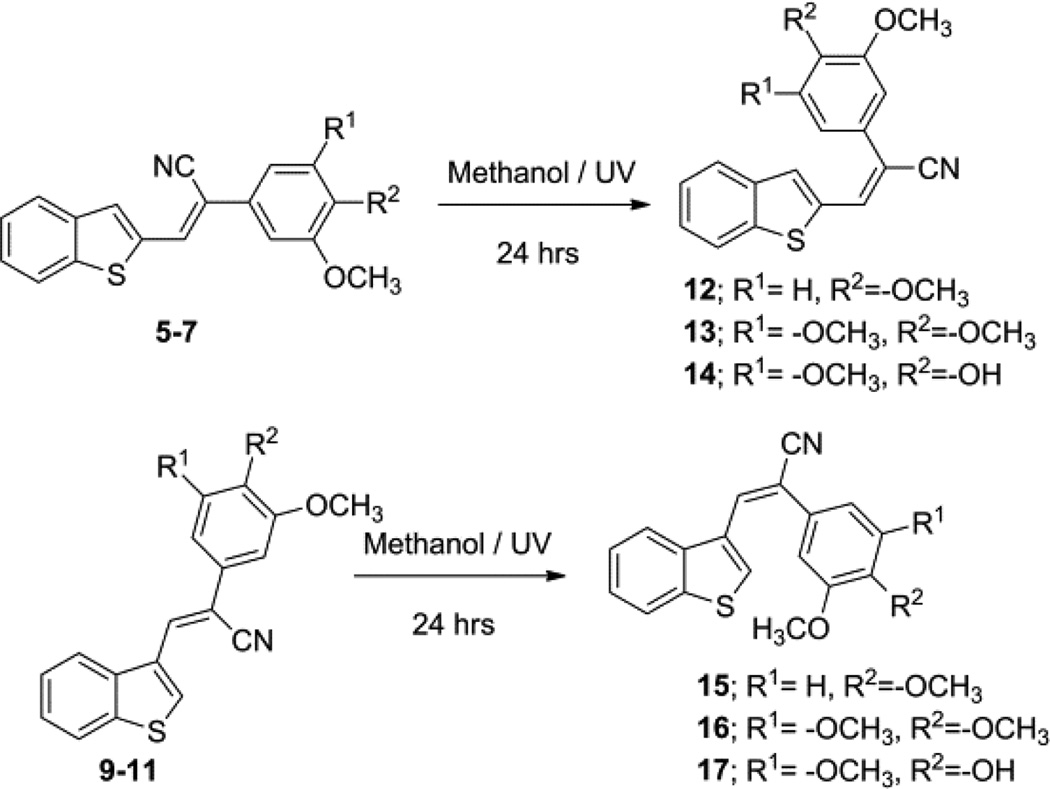
The E-isomers of compounds 5–7 and 9–11 were obtained (structures 12–14 and 15–17, respectively; Scheme 3) when each of these compounds was refluxed in methanol under ultraviolet (UV) light for 24 hrs.19
Scheme 3.
Synthesis of E-benzo[b]thiophene acrylonitrile analogs from their corresponding Z-isomers
Biological Evaluation
A. In vitro growth inhibition and cytotoxicity
Compounds 5–7, 9–11 and 12–17 were evaluated for their cytotoxic potency in a preliminary screen against a panel of 60 human cancer cell lines (NCI-60 panel). The compounds were considered to be active if they reduced the growth of any of the cancer cell lines to 60% or more in at least eight of the cell lines screened. From the preliminary screen, compounds 5, 6 and 13 were selected as leads for more comprehensive studies designed to determine GI50 and LC50 values, which represent the molar drug concentration required to cause 50% growth inhibition, and the concentration that kills 50% of the cells, respectively. These three analogs (5, 6 and 13) exhibited nanomolar level growth inhibition in subsequent five dose screening against all 60 human cancer cell lines in the panel. The growth inhibition results of these three potent compounds (5, 6 and 13) were presented in Table 1.
Table 1.
Antitumor activity (GI50/ nM)a data of the compounds selected for 5 dose studies for the NCI 60-cell lines screen
| Panel/cell line | 5 | 6 | 13 |
|---|---|---|---|
| GI50 (nM) |
GI50 (nM) |
GI50 (nM) |
|
| Leukemia | |||
| CCRF-CEM | 28.6 | 25.1 | <10.0 |
| HL-60(TB) | 22.2 | 24.4 | <10.0 |
| K-562 | 35.4 | 30.4 | <10.0 |
| MOLT-4 | 53.5 | 39.7 | <10.0 |
| RPMI-8226 | 29.8 | 28.6 | <10.0 |
| SR | 10.0 | 46.6 | <10.0 |
| Lung Cancer | |||
| A549/ATCC | 70.0 | 41.2 | <10.0 |
| EKVX | 90.8 | 52.1 | na |
| HOP-62 | 47.0 | 98.9 | <10.0 |
| HOP-92 | >100 | 17.9 | <10.0 |
| NCI-H226 | >100 | >100 | 17.9 |
| NCI-H23 | 57.9 | 52.3 | <10.0 |
| NCI-H322M | 31.5 | 51.7 | 18.3 |
| NCI-H460 | 35.4 | 35.9 | <10.0 |
| NCI-H522 | 10.0 | 29.7 | na |
| Colon Cancer | |||
| COLO 205 | 36.1 | 46.9 | <10.0 |
| HCC-2998 | 66.5 | 16.3 | 19.2 |
| HCT-116 | 41.1 | 49.4 | <10.0 |
| HCT-15 | 48.2 | 46.1 | <10.0 |
| HT29 | 31.8 | 31.9 | <10.0 |
| KM12 | 10.0 | 22.2 | <10.0 |
| SW-620 | 24.2 | 40.8 | <10.0 |
| CNS Cancer | |||
| SF-268 | 45.2 | 46.7 | <10.0 |
| SF-295 | 10.0 | 33.7 | na |
| SF-539 | 10.6 | 24.8 | <10.0 |
| SNB-19 | 44.4 | 44.0 | 39.1 |
| SNB-75 | 13.7 | 41.5 | <10.0 |
| U251 | 64.0 | 50.0 | <10.0 |
| Melanoma | |||
| LOX IMVI | 60.7 | 48.7 | <10.0 |
| MALME-3M | >100.0 | 23.3 | <10.0 |
| M14 | 61.1 | 37.9 | <10.0 |
| MDA-MB-435 | 10.0 | 23.5 | <10.0 |
| SK-MEL-2 | >100.0 | 41.6 | <10.0 |
| SK-MEL-28 | >100.0 | >100 | <10.0 |
| SK-MEL-5 | 21.0 | 34.4 | <10.0 |
| UACC-257 | >100.0 | NA | >100 |
| UACC-62 | 45.9 | NA | <10.0 |
| Ovarian Cancer | |||
| IGROV1 | 61.8 | NA | <10.0 |
| OVCAR-3 | 22.0 | 23.0 | <10.0 |
| OVCAR-4 | >100.0 | >100 | <10.0 |
| OVCAR-5 | 50.0 | 61.5 | <10.0 |
| OVCAR-8 | 25.1 | 23.5 | <10.0 |
| NCI/ADR-RES | 10.5 | 27.5 | <10.0 |
| SK-OV-3 | 63.4 | 47.2 | <10.0 |
| Renal Cancer | |||
| 786-0 | 33.7 | 12.2 | <10.0 |
| A498 | 26.6 | 28.8 | <10.0 |
| ACHN | 85.4 | 73.2 | <10.0 |
| CAKI-1 | 82.7 | 51.4 | 37.8 |
| RXF 393 | 90.9 | 24.9 | <10.0 |
| SN12C | 13.6 | 68.4 | <10.0 |
| TK-10 | 45.2 | 36.3 | >100 |
| UO-31 | 18.7 | 52.4 | <10.0 |
| Prostate Cancer | |||
| PC-3 | 29.7 | 45.0 | <10.0 |
| DU-145 | 36.6 | 21.1 | <10.0 |
| Breast Cancer | |||
| MCF7 | 47.8 | 40.2 | <10.0 |
| MDA-MB-231/ATCC | 26.9 | 33.0 | <10.0 |
| HS 578T | 30.0 | 42.7 | <10.0 |
| BT-549 | 28.3 | 36.6 | <10.0 |
| T-47D | >100 | >100 | >100 |
| MDA-MB-468 | na | na | <10.0 |
na: Not analyzed;
GI50 50% Growth inhibition, concentration of drug resulting in a 50% reduction in net cell growth as compared to cell numbers on day 0.
Compound 5 [Z-3-(benzo[b]thiophen-2-yl)-2-(3,4-dimethoxyphenyl)acrylonitrile] exhibited GI50 values ranging from 10.0 nM to 90.9 nM in 85% of the cancer cell lines screened, and showed good growth inhibition properties in all six leukemia cell lines, all seven colon cancer cell lines, all six CNS cancer cell lines, and both prostate cancer cell lines, with GI50 values in the range 10–66.5 nM. Compound 5 also exhibited good growth inhibition against non-small cell lung cancer cell lines NCI-H522, NCI-H322M and NCI-H460 (GI50=10–35.4 nM); melanoma cancer cell lines MDA-MB-435, SK-MEL-5 and UACC-62 (GI50=10–45.9 nM); ovarian cancer lines NCI/ADR-RES, OVAR-3, and OVAR-8 (GI50= 10.5–25.1 nM); renal cancer cell lines 786-0, A498, TK-10 and UO-31 (GI50= 26.6–45.2 nM); and breast cancer cell lines MCF7, MDA-MB-231/ATCC, HS 578T, and BT-549 (GI50= 28.3–47.8 nM).
Analog 6 [Z-3-(benzo[b]thiophen-2-yl)-2-(3,4,5-trimethoxyphenyl)acrylonitrile] exhibited GI50 values ranging from 21.1 nM to 98.9 nM in 96% of the cancer cell lines screened and showed good growth inhibition properties in all six leukemia cell lines, all six CNS cancer cell lines, and both prostate cancer cell lines, with GI50 values in the range 21.2–50.0 nM. This compound also showed potent cytotoxicity against A549/ATCC, EKVX, HOP-92, NCI-H23, NCI-H322M, NCI-H460, and NCI-H522 lung cancer cell lines (GI50=17.9–52.3 nM); colon cancer cell lines COLO 205, HCT-116, HCT-15, HT29, KM12 and SW-620 (GI50 = 22.2–49.4 nM); melanoma cell lines LOX IMVI, MALME-3M, M14, and SK-MEL-5 (GI50=23.3–48.7 nM); ovarian cancer cell lines OVCAR-3, OVCAR5, OVCAR-8, NCI/ADR-RES, and SK-OV-3 (GI50=23.0–61.5 nM); renal cancer cell lines A498, CAKI-1, RXF 393, SN12C, TK-10, and UO-31 (GI50=24.9–68.4 nM); and breast cancer cell lines MCF7, MDA-MB-231/ATCC, HS 578T, and BT-549 (GI50=33.0–42.7 nM).
Importantly, the E-isomer, 13, [E-3-(benzo[b]thiophen-2-yl)-2-(3,4,5-trimethoxyphenyl)acrylonitrile] exhibited very potent growth inhibition (GI50= < 10.0 nM) in all but six of the human cancer lines examined, and of these six cell lines, five exhibited GI50 values ranging from 17.9–39.1 nM, while one cell line (breast cancer cell line T-47D) afforded a GI50 value of >100 nM.
Previous SAR studies of combretastatin analogs showed modification of the trimethoxybenzene with groups of higher lipophilicility could lead to decreased cytotoxicity and increased antitubulin activity.17 The structural similarity compounds with trimethoxybenzene moiety (6 and 13) and the compound with modification of the trimethoxybenzene group (5) showed relative potency in the NCI-60 cell line panel. We hypothesized that the cytotoxic activity of these novel Z-3-(benzo[b]thiophen-2-yl)-2-(phenyl)acrylonitrile analogs was likely due to their interaction with tubulin. Furthermore, these compounds appeared to overcome P-glycoprotein (P-gp)-mediated resistance, since they were equipotent in OVCAR8 and NCI/ADR-Res cells. Given that antitubulin drugs are among the most effective agents for the treatment of advanced prostate cancer we sought to validate the results from the NCI-60 panel of cancer cells using prostate cancer cell lines, and to explore the molecular mechanism of the cytotoxic action of the representative compound 6.
B. Mechanistic evaluation in prostate cancer cell lines
Biochemical assays to assess the interaction of 6 with tubulin were carried out using purified tubulin. Immunofluorescence staining for beta-tubulin was also evaluated in PC3 cells following drug treatment. Vinblastine and docetaxel were used to demonstrate tubulin destabilizing and stabilizing modes of action, respectively. Cytotoxicity assays (72 hour, GI50) were carried out in PC3 prostate cancer cells and in PC3-DR cells (docetaxel resistant PC3 cells). To assess the effect of P-gp, we used NCI/ADR-Res ovarian cancer cells and L-MDR1 porcine epithelial cells and their non-expressing parent lines. Assays were performed in 96-well plates using AlamarBlue cell viability dye. Western blot analyses were used to assess the effect of drug treatment on apoptotic pathway and on cell cycle proteins.
Results
Based on the similar in-vitro effectiveness against the OVCAR8 and NCI/ADR-RES cell lines (Table 1), we reasoned that these compounds are not P-gp substrates. PC3-DR cells were used as a docetaxel resistant clone of the prostate cancer cell line PC3. The NCI/ADR-RES cell line and OVCAR8 were also used to confirm the previous results (i.e., equal potency).
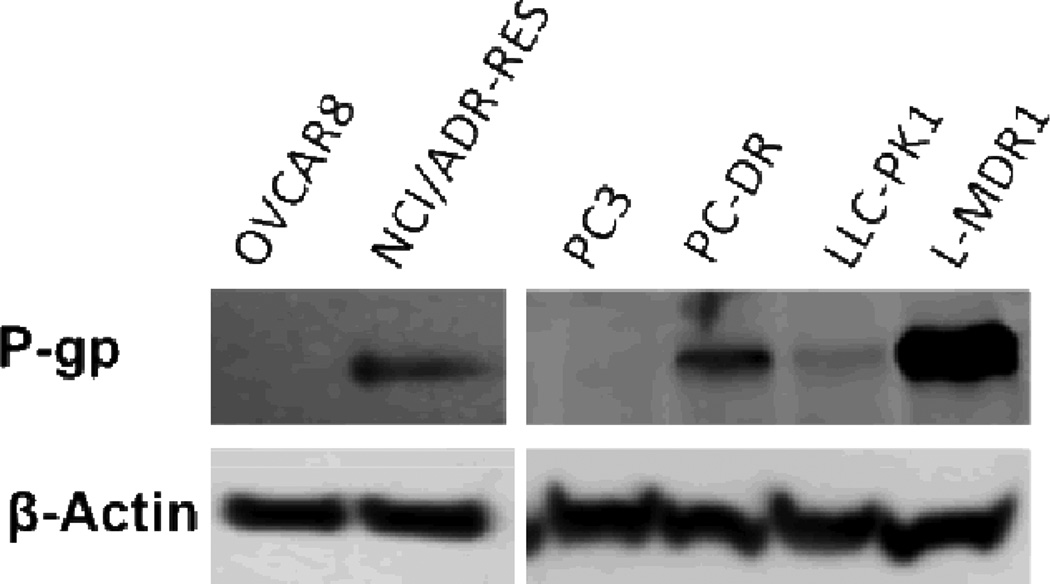
In addition, a porcine kidney cell line (LLC-PK1), which was engineered to express P-gp, was used as a positive control (L-MDR1). The expression of P-gp was verified in all cell lines by western blot (Fig. 2). Docetaxel and vinblastine were very potent cytotoxic agents in PC3, OVAR8 and LLC-PK1 cells. However, all cell lines expressing P-gp were resistant (22 to 627-fold) to docetaxel and vinblastine (Table 2). In contrast, the GI50 of compound 6 in all cell lines was in the low nanomolar range (3–16 nM) with no apparent resistance in P-gp expressing cells (Table 2).
Fig 2.
Western blot analysis for P-gp expression in cell lines used in the experiments described in Table 2. β-actin was used as a loading control.
Table 2.
Comparative GI50 (nM) values of compound 6, docetaxel and vinblastine in P-gp expressing cell lines
| Cell lines | 6 | Doc | Vin | |
|---|---|---|---|---|
| Prostate | PC3 | 8.6 | 2.5 | 5.2 |
| aPC3-DR | 7.6 | 54 (22) | n.a. | |
| Ovarian | OVCAR8 | 5.2 | 0.5 | 2.8 |
| NCI-ADR/Res | 3.2 | 196 (392) | 1402 (500) | |
| Kidney epithelium | LLC-PK1 | 9.7 | 0.17 | 1.5 |
| L-MDR1 | 11.7 (1.2) | 106.6 (627) | 86.8 (58) | |
na: Not analyzed,
PC3-DR: PC3 cells maintained in 3.6nM docetaxel. Numbers in parenthesis represent the relative resistance factor between P-gp expressing and non-expressing cells.
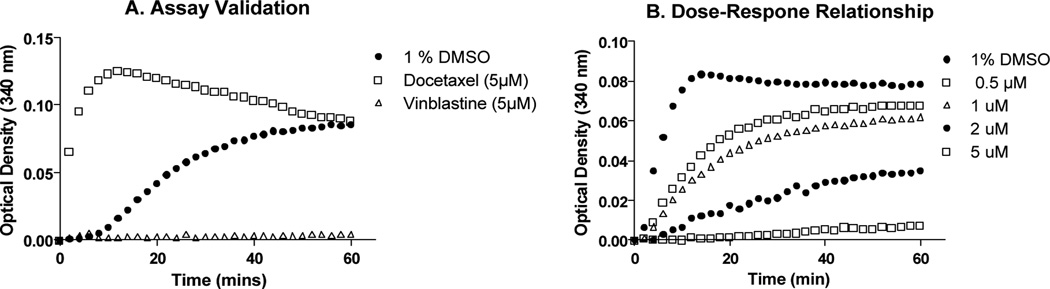
Compound 6 showed a concentration-dependent antitubulin interaction with a mode of action similar to vinblastine (Fig. 3). This suggests that these compounds act by interfering with the microtubule polymerization process.
Fig 3.
In vitro tubulin polymerization assay. Purified tubulin was allowed to polymerize for 1 hour (A) in the presence of docetaxel and vinblastine or (B) in the presence of increasing concentrations of compound 6.
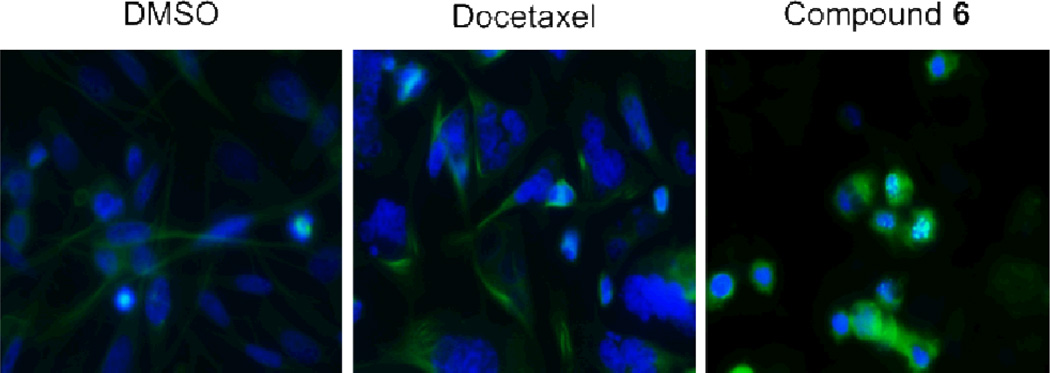
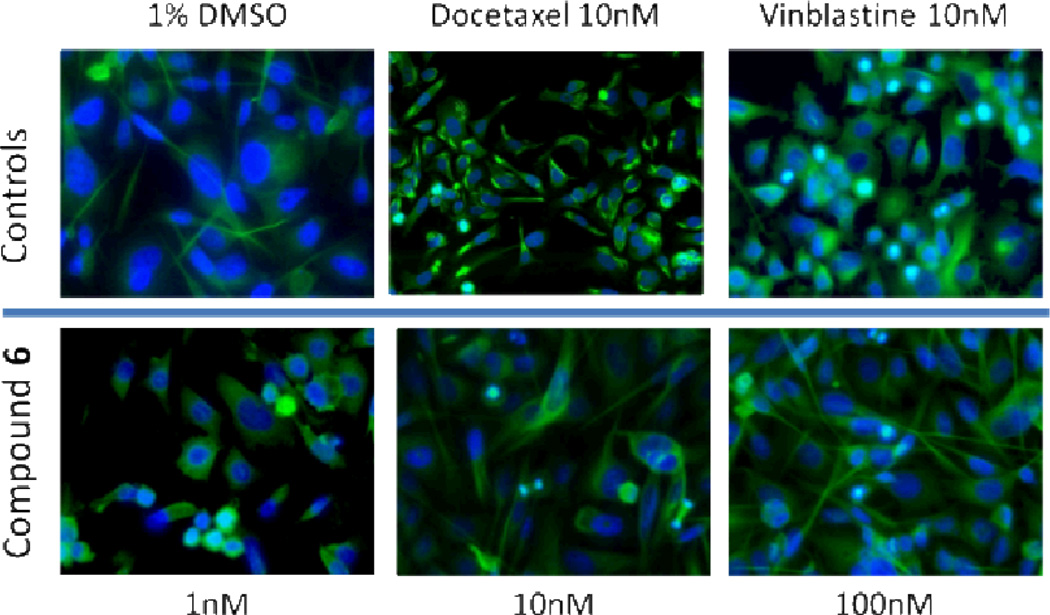
These results were corroborated by immunofluorescence staining for beta-tubulin in PC3 cells treated with increasing concentrations of compound 6 as well as with vinblastine and docetaxel (Fig. 4) for 24 hours. As expected, docetaxel stabilized tubulin and strong staining was observed in stabilized microtubules, whereas in the vinblastine and compound 6 treated cells the staining pattern was diffuse.
Fig 4.
Immunofluorescence staining for tubulin. Prostate cancer, PC3, cells were treated for 24 hours with 10nM docetaxel, 10 nM vinblastine, or increasing concentrations (1, 10, and 100 nM) of compound 6. Green staining denotes tubulin and blue staining (DAPI) denotes the cell nuclei.
Further investigation of the tubulin patterns in treated cells revealed that compound 6 treated cells underwent chromosome overduplication and multiple spindle-pole staining (Fig. 5).
Fig 5.
Tubulin immunofluorescence. Panel C demonstrates that compound 6 causes chromosome overduplication and formation of multiple spindle-poles. PC3 cells were treated with 100 nM docetaxel or 100 nM of compound 6 for 24 hours. Green staining denotes tubulin and blue staining (DAPI) denotes the cell nuclei.
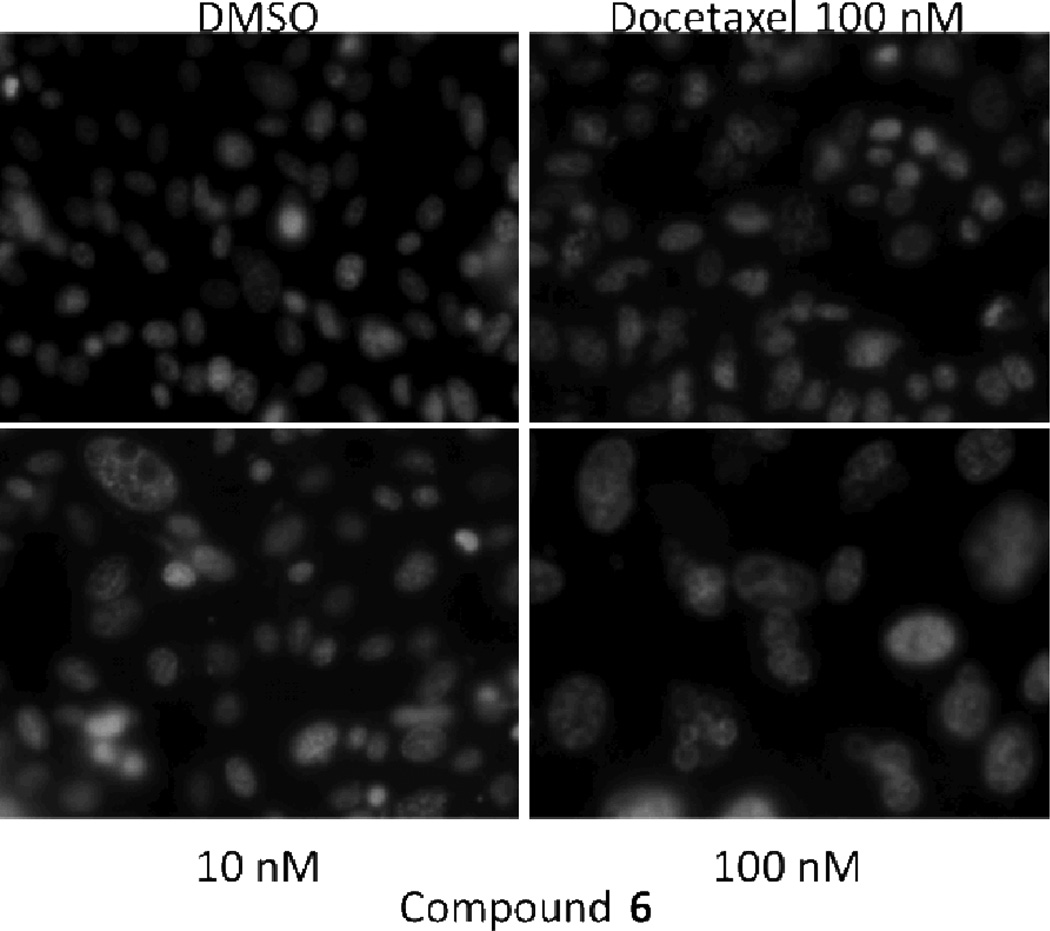
Upon longer treatment (72 hours) with compound 6, nuclear staining with Hoechst 33342 demonstrated the presence of enlarged nuclei/multinucleated cells, suggesting that mitotic catastrophe could be the primary mode of cell death (Fig. 6). This is consistent with the observation that exposure to compound 6 leads to centrosome overduplication and the presence of multiple spindle poles (Fig. 5).
Fig 6.
Nuclear staining of PC3 treated cells. PC3 prostate cancer cells were treated for 72 hours with docetaxel (100 nM) or compound 6 (10 and 100 nM). Nuclei were visualized with fluorescence imaging of cells treated with Hoechst 33342 nucleic acid stain.
Conclusion
Benzothiophene acrylonitrile analogs 5, 6 and 13 have potent anticancer properties that are likely mediated, at least in part, through interference with tubulin polymerization. Unlike taxanes and Vinca alkaloids, these compounds are not substrates for P-glycoprotein. Following treatment with compound 6, cells appear to be dying by an atypical apoptosis mode, which is consistent with mitotic catastrophe. This is significant, since many cancers have upregulated modes of apoptotic resistance that render them difficult to treat.
Supplementary Material
Scheme 1.
Synthesis of (Z)-benzo[b]thiophene-2-yl acrylonitriles
Acknowledgements
We are grateful to NCI/NIH (Grant Number CA 140409) and to the Arkansas Research Alliance (ARA) for financial support and to the NCI Developmental Therapeutic Program (DTP) for screening data.
Notes and references
- 1.Paull KD, Lin CM, Malspeis L, Hamel E. Cancer Res. 1992;52:3892–3900. [PubMed] [Google Scholar]
- 2.Schiff PB, Horwitz SB. Proc Natl Acad Sci., USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan A, Hadfield JA, Lawrence NJ, McGown AT. Med. Res. Rev. 1998;18:259–296. doi: 10.1002/(sici)1098-1128(199807)18:4<259::aid-med3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Jang YC, Chi YC, Ching CK, Li-Tzong C, Yung-Shung W, Yueh-Hsiung K. Mol. Pharmacol. 2004;65:77–84. [Google Scholar]
- 5.Ohsumi K, Nakagawa R, Fukuda Y, Hatanaka T, Morinaga Y, Nihei Y, Ohishi K, Suga Y, Akiyama Y, Tsuji T. J.Med.Chem. 1998;41:3022–3032. doi: 10.1021/jm980101w. [DOI] [PubMed] [Google Scholar]
- 6.Kavallaris M, Verills NM, Hill BT. Drug Resist. Updates. 2001;4:392–401. doi: 10.1054/drup.2002.0230. [DOI] [PubMed] [Google Scholar]
- 7.Wood KW, Cornwell WD, Jackson JR. Curr. Opin. Pharmacol. 2001;1:370–377. doi: 10.1016/s1471-4892(01)00064-9. [DOI] [PubMed] [Google Scholar]
- 8.Pettit GR, Rhodes MR, Herald DL, Chaplin DJ, L. Stratford MR, Hamel E, Pettit RK, Chapuis JC, Oliva D. Anti-Cancer Drug Des. 1998;13:981–993. [PubMed] [Google Scholar]
- 9.Lin CM, Singh SB, Chu PS, Dempcy RO, Schmidt JM, Pettit GR, Hamel E. Mol. Pharmacol. 1988;34:200–208. [PubMed] [Google Scholar]
- 10.Cushman M, Nagarathnam D, Gopal D, Chakraborti AK, Lin CM, Hamel E. J.Med.Chem. 1991;34:2579–2588. doi: 10.1021/jm00112a036. [DOI] [PubMed] [Google Scholar]
- 11.Zhang LH, Wu L, Raymon HK, Chen RS, Corral L, Shirley MA, Narla RK, Gamez J, Muller GW, Stirling DI, Bartlett JB, Schafer PH, Payvandi F. Cancer Res. 2006;66:951–959. doi: 10.1158/0008-5472.CAN-05-2083. [DOI] [PubMed] [Google Scholar]
- 12.Loupy A, Pellet M, Petit A, Vo-Thanh G. Org. Biomol. Chem. 2005;3:1534–1540. doi: 10.1039/b418156e. [DOI] [PubMed] [Google Scholar]
- 13.Saczewski F, Reszka P, Gdaniec M, Grunert RJ, Bednarski PJ. J.Med.Chem. 2004;47:3438–3449. doi: 10.1021/jm0311036. [DOI] [PubMed] [Google Scholar]
- 14.Rasolofonjatovo E, Provot O, Hamze A, Rodrigo J, Bignon J, Bakala JW, Desravines D, Dubois J, Brion JD, Alami M. Euro. J. Med. Chem. 2012;52:22–32. doi: 10.1016/j.ejmech.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Lu X, Chen K, Yan R, Li QS, Zhu HL. Bioorg Med Chem. 2012;20:903–909. doi: 10.1016/j.bmc.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 16.Gaukroger K, Hadfield K, Lawrence JA, Nolan NJ, McGown ATS. Org. Biomol. Chem. 2003;1:3033. doi: 10.1039/b306878a. [DOI] [PubMed] [Google Scholar]
- 17.Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA. J.Med.Chem. 2006;49:3033–3037. doi: 10.1021/jm0512903. [DOI] [PubMed] [Google Scholar]
- 18.Sonar VN, Parkin S, Crooks PA. Acta Cryst. 2007;C63:o743–o745. doi: 10.1107/S0108270107054959. [DOI] [PubMed] [Google Scholar]
- 19.Pettit GR, Anderson CR, Herald DL, Jung MK, Lee DJ, Hamel E, Pettit RK. Journal of Medicinal Chemistry. 2003;46:525–531. doi: 10.1021/jm020204l. [DOI] [PubMed] [Google Scholar]
- 20.General synthetic procedure: Synthesis of Z-benzo[b]-thiophene acrylonitriles(5–7 and 9–11): A mixture of benzo[b]thiophene-3-carbaldehyde 8 (1 mole) and the appropriate substituted phenylacetonitrile reactant (1.1 mole equivalent) in 5% sodium methoxide was heated under reflux for 4 to 6 hrs. The resulting solution was then cooled to room temperature and poured into ice-cold water to afford a crude yellow solid. The solid was filtered off, washed with water, and finally washed with cold methanol. The obtained crude solid was recrystallized from ethyl acetate to afford the desired condensation product as a pure crystalline solid. Synthesis of E-benzo[b]thiophene acrylonitriles (12–17): Each of the above Z-isomer 5–7 and 9–11 was dissolved in methanol under reflux conditions and stirred for 24 hrs under UV light (254 nm). The yellow colored solution gradually changed to colorless solution. After the reaction was complete, the reaction mixture was cooled to room temperature, and allowed to crystallize from methanol to afford the corresponding E-benzo[b]thiophenephenyl) acrylonitrile as white crystalline product. Analytical data. Compound 5: 1H NMR (DMSO-d6): 3.81 (s, 3H), 3.87 (s, 3H), 7.07–7.09 (d, J=8.4 HZ, 1H), 7.26–7.29 (dd, J=8Hz, 1H), 7.36 (s, 1H), 7.44–7.47 (m, 2H), 7.95–7.99 (m, 2H), 8.06–8.08 (d, J=6.8Hz, 1H), 8.32 (s, 1H). 13C NMR (DMSO-d6): 56.13, 56.23, 108.80, 109.35, 112.29, 118.27, 119.66, 123.15, 123.20, 125.12, 126.19, 131.30, 134.29, 138.14, 138.67, 140.58, 149.64, 150.51. Compound 6: 1H NMR (DMSOd6): 3.72 (s, 3H), 3.88 (s, 6H), 7.04 (s, 2H), 7.43–7.52 (m, 2H), 7.99 (dd, 1H), 8.03 (s, 1H), 8.09 (dd, 1H), 8.40 (s, 1H); 13C NMR (DMSO-d6): 56.12, 60.18, 103.25, 108.75, 117.66, 122.67, 124.69, 125.18, 126.70, 128.67, 131.48, 135.52, 137.30, 138.01, 138.42, 140.17, 153.11. Compound 13: 1H NMR (DMSO-d6): δ 3.76 (s, 3H), 3.78 (s, 6H), 6.81 (s, 2H), 7.36–7.37 (t, 2H), 7.85–7.89 (m, 3H), 8.05 (s, 1H) ppm; 13C NMR (DMSO-d6): δ 56.45, 61.32, 106.53, 112.34, 120.10, 122.42, 124.65, 125.13, 126.85, 127.01, 131.71, 136.93, 137.94, 138.10, 139.64, 141.94, 154.15 ppm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.