Abstract
Background:
Retinopathy of prematurity (ROP) is a serious complication of prematurity treatment and can lead to blindness unless recognized and treated early.
Objective:
To estimate the incidence of ROP in preterm infants in our NICU, to identify the risk factors which predispose to ROP, and to assess the outcome of these cases.
Materials and Methods:
ROP prospective screening survey was performed enrolling all prematures admitted to the NICU from January 2009 to December 2010, with a gestational age of 32 weeks or less at birth and a birth weight of 1500 g or less. Infants whom gestational age was >32 weeks or birth weight was >1500 g were included if they exposed to oxygen therapy for more than 7 days. Also infants who were born between 32 and 34 weeks gestational age were examined if they had a course of instability (like sepsis, asphyxia or ventilation). A total of 172 infants (88 females) had retinal evaluation by indirect ophthalmoscopy from the 4th postnatal week and followed up periodically. Perinatal risk factors for ROP were assessed using univariate and multivariate analysis. Infants who progressed to stage 3 ROP with plus disease were given laser therapy.
Results:
Out of the studied 172 infants, 33 infants (19.2%) developed ROP in one or both eyes; 18 (54.5%) cases stage 1, 9 (27.3%) cases stage 2 and 6 (18.2%) cases stage 3 with plus disease. None of the studied neonates presented ROP at stages 4 or 5. The six cases diagnosed as ROP stage 3 with plus disease underwent laser ablative therapy. Laser was effective in treatment and decreasing the progression of ROP. Univariate analysis showed that there was a significant relationship between the occurrence of ROP and gestational age (P=0.000), sepsis (P=0.004), oxygen therapy (P=0.018), and frequency of blood transfusions (P=0.030). However, nonsignificant relationship was found between the occurrence of ROP and sex, mode of delivery, birth weight, respiratory distress syndrome, patent ductus arteriosus, intraventricular hemorrhage, hypotension, phototherapy, duration of oxygen therapy, mechanical ventilation, and CPAP (all P>0.05). Gestational age, sepsis, oxygen therapy and frequency of blood transfusions remained significant variables after logistic regression analysis.
Conclusion:
The incidence of ROP in this study was 19.2%; low gestational age, sepsis, oxygen therapy and frequent blood transfusions were significant risk factors for ROP. Laser was effective in treatment and decreasing the progression of ROP. As this is a unit-based study, a comprehensive countrywide survey on ROP in Egypt is recommended to determine any regional differences in disease incidence.
Keywords: Oxygen therapy, prematurity, risk factors, retinopathy of prematurity
INTRODUCTION
Retinopathy of prematurity (ROP) is an important cause of preventable blindness in children.[1] In the Royal Blind School of Edinburgh, it accounts for up to 10% of childhood blindness,[2] and it is believed to account for 6-18% of childhood blindness in developed countries.[1] Recent advances in neonatal care in the last decade, have improved the survival rates for premature infants.[3] Consequently, the incidence of ROP has increased in parallel. ROP is under constant epidemiological study around the world.[4]
Early identification of retinal damage and the institution of appropriate treatment prevent blindness and offer child better overall development.[5]
ROP is characterized by abnormal neovascular development in the retina of premature infants. These abnormal blood vessels are fragile and can leak or bleed, scarring the retina and pulling it out of position. This causes a tractional retinal detachment, which is the main cause of visual impairment and blindness in ROP.[6]
Three factors have shown consistent and significant association with ROP: low gestational age, low birth weight and prolonged exposure to supplementary oxygen following delivery.[7]
Other putative risk factors include mechanical ventilation,[8] sepsis,[9] intraventricular hemorrhage,[7] surfactant therapy,[10] anemia,[11] frequent blood transfusions,[11] and apnea.[8] The precise roles of these factors individually in the progression of the disease have not yet been determined.[12]
The aim of this prospective study was to estimate the incidence of ROP in preterm infants at the Neonatal Intensive Care Unit (NICU) of Al-Minya University Hospital, to identify the risk factors which predispose to ROP, and to assess the outcome of these cases.
MATERIALS AND METHODS
This prospective cohort study was conducted in NICU of Al-Minya University Hospital (a tertiary referral hospital) in cooperation between the Departments of Neonatology and Ophthalmology. Two-hundred and twenty-two preterm neonates were screened to be included in the study. All preterm infants admitted to the NICU from January 2009 to December 2010, with a gestational age of 32 weeks or less at birth and a birth weight of 1500 g or less. Infants whom gestational age was >32 weeks or birth weight was >1500 g were included if they were exposed to oxygen therapy for more than 7 days.[8] Also infants who were born between 32 and 34 weeks gestational age were examined if they had a course of instability (like sepsis, asphyxia or ventilation). Neonates who died before the first ophthalmological examination were excluded (n=24). Infants with congenital anomalies, chromosomal abnormalities, inborn errors of metabolism were excluded from the study (n=26). While 172 preterm neonates continued the study, all neonates included in this study were subjected to the following:
-
History: Perinatal history; presence of risk factors as prematurity, sepsis (offensive liquor, premature rupture of membrane >18 hours, maternal urinary tract infection, and intrapartum fever >38°C) and perinatal asphyxia.
Present history; includes the most common symptoms of respiratory distress requiring oxygen therapy, sepsis, phototherapy, congenital heart disease and blood transfusion
Clinical Examination: Weight, length, skull circumference, gestational age using new Ballard score, vital signs, primitive neonatal reflexes, neurological manifestations, respiratory manifestations and circulatory manifestations
Local Eye Examination: All infants were examined regularly by the same ophthalmologist at 1-2 weeks intervals from the 4th postnatal week onwards. The eyes were dilated with a combination of cyclopentolate 0.2% and phenylephrine 1% eye drops applied 1 hour before the examination.
Indirect ophthalmoscopy with a 28 dioptre lens was performed with speculum and scleral depression. Retinal examination by the ophthalmologist with retinal drawing and RetCam 2 fundus imaging was done when indicated.
ROP was classified by location on the retina (zone 1-3), and severity (stage 1-5), according to the International Committee for Classification of ROP.[13] All patients diagnosed with stage 3 ROP with plus disease were treated with laser photocoagulation. Treatment with laser was performed within 72 hours of detecting this finding after family consent. The ophthalmological examinations were initiated at the fourth week of life and were repeated weekly or biweekly, using the schedule for follow-up recommended by AAP, AAO and AAPO,[14] until full vascularization of the retina reached zone 3 (the most peripheral temporal retinal zone), or until full remission of ROP after treatment.
In this study, we examined a series of suspected pre- and postnatal risk factors for ROP to identify independent risk factors associated with the development of mild and severe forms of this disease in our NICU conditions. The prenatal variables were gestational age, birth weight, sex and mode of delivery. The after-birth variables were respiratory distress syndrome, oxygen therapy (through nasal catheter, mask, CPAP, or mechanical ventilation), phototherapy for jaundice, frequency of blood transfusions, sepsis (by clinical diagnosis, with either C-reactive protein greater than 6.0 mg/dl, or blood culture positive cases), hypotension (as identified by the standard mean for age and weight), intraventricular hemorrhage (as identified by cranial ultrasound) and patent ductus arteriosus (as identified by echocardiography).
Our study was carried out after approval by the ethical committee of the university. Informed consents were obtained from the parents of the subjects.
Statistical analysis
Data were analyzed by the Statistical Package for the Social Sciences (SPSS for windows, version 13.0). Descriptive statistics included the mean and standard deviation for numerical variables, and the percentage of different categories for categorical variables. The incidence rate of ROP was described in simple proportion. Group comparisons were done by the chi-squared (χ2) test or Fisher's exact test for categorical variables. A logistic regression model was performed and the adjusted OR (95% CI) was obtained for the risk factors which had been shown to be significant in the univariate analysis. A probability (P) of less than 0.05 was considered significant.
RESULTS
The study population included 172 neonates; all preterms with a gestational age of 32 weeks or less at birth and a birth weight of 1500 g or less and preterm infants whom gestational age was >32 weeks or birth weight was >1500 g with unstable condition, during the duration from January 2009 to December 2010.
Out of the 172 neonates; 88 (51.2%) were females. The mean gestational age was 33.02±1.72 weeks; 24 were ≤32 weeks and 148 were >32 weeks. The birth weight ranged from 940 to 2010 g with a mean of 1510±245 g. Seventy-two cases (41.9%) were delivered vaginally and 100 (58.1%) cases were delivered by Cesarean section [Table 1].
Table 1.
Demographic data of the studied cases

Out of the 172 neonates; 33 (19.2%) cases developed ROP in one or both eyes classified as: 18 (54.5%) cases stage 1, 9 (27.3%) cases stage 2, and 6 (18.2%) cases stage 3 with plus disease. None of the studied neonates presented ROP at stages 4 or 5.
[Table 2] shows the relationship between ROP and risk factors. There was a significant relationship between the occurrence of ROP and gestational age (P=0.000), sepsis (P=0.004), oxygen therapy (P=0.018), and frequency of blood transfusions (P=0.030). On the other hand, there was no significant relationship between the occurrence of ROP and sex, mode of delivery, birth weight, respiratory distress syndrome, patent ductus arteriosus, intraventricular hemorrhage, hypotension, phototherapy, duration of oxygen therapy, mechanical ventilation, and CPAP (all P>0.05).
Table 2.
Relationship between retinopathy of prematurity and risk factors
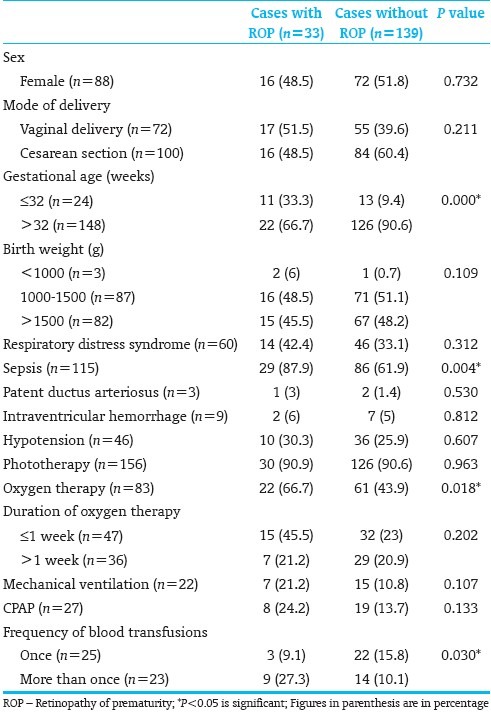
[Table 3] shows the relationship between gestational age and stages of ROP. There was no significant relationship between the gestational age and stages of ROP (P=0.325).
Table 3.
Relationship between gestational age and stages of ROP
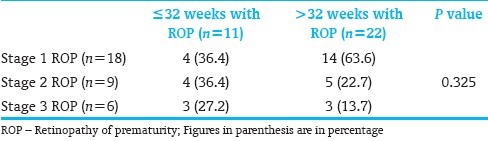
[Table 4] shows the relationship between oxygen therapy and stages of ROP. There was no significant relationship between oxygen therapy and stages of ROP (P=0.687).
Table 4.
Relationship between oxygen therapy and stages of ROP
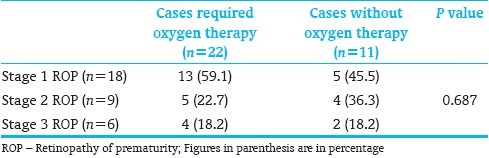
Those variables that were statistically significant after univariate analysis were analyzed using logistic regression analysis. Gestational age, sepsis, oxygen therapy, and frequency of blood transfusions remained significant variables [Table 5].
Table 5.
Logistic regression analysis
[Table 6] shows the outcome of ROP in studied cases. Intervention with laser was necessary for the 6 cases diagnosed as stage 3 with plus disease, and patients showed improvement on follow-up getting a complete remission. Laser photocoagulation was found to be very effective in regressing ROP. Three of the 6 cases with stage 3 with plus disease were ≤32 weeks with a birth weight <1500 g. The remaining three of the six cases with stage 3 with plus disease were >32 weeks with a birth weight >1500 g. All the six cases with stage 3 with plus disease suffered from sepsis, received oxygen therapy for >1week, and received blood transfusion frequently. The other 27 cases with ROP regressed spontaneously without intervention.
Table 6.
Outcome of ROP in studied cases

DISCUSSION
Retinopathy of prematurity is a disorder of retinal vascular development in preterm infants. It continues to be a significant complication in preterm neonates despite advances in neonatal care and remains a major cause of childhood blindness worldwide.[14]
Incidence
The incidence of ROP in this study was 19.2% which was lower than previously reported by other studies; 24% in India, [15] 29.2% in Singapore,[8] and 32.4% in Pakistan.[16] This can be explained by the fact that these studies involved only very low birth weight infants. However, it is higher than the study done in Beijing which involved infants with higher gestational age and birth weight (up to 2 kg and /or 34 weeks gestational age) and reported an incidence of 10.8%.[17]
Risk factors
ROP is a multifactorial disease. Low gestational age, low birth weight, sepsis, oxygen therapy, respiratory distress syndrome, and blood transfusion have been suspected to influence the incidence of ROP.[18] The most significant risk factors for development of ROP were low gestational age and low birth weight, as shown in many studies.[7,12,19] In our study, low gestational age, sepsis, oxygen therapy, and frequency of blood transfusions were found to be risk factors for development of ROP independently. Meanwhile, sex, mode of delivery, birth weight, respiratory distress syndrome, patent ductus arteriosus, intraventricular hemorrhage, hypotension, phototherapy, duration of oxygen therapy, mechanical ventilation, and CPAP were nonsignificant risk factors by using univariate analysis.
As regard the effect of low gestational age on occurrence of ROP, we found it the most important risk factor in ROP. This was in agreement with the results of studies done by Shah et al.,[8] Karna et al., [10] and Fortes et al.[20] This was explained by immaturity of vascularization that induces an increased susceptibility of the retina to oxidative damage and to a number of perinatal factors which include hyper- and hypoxia, blood transfusions, and sepsis. We found nonsignificant relationship between gestational age and the severity of ROP, but this was in disagreement with other studies, [8,18] showing that lower gestational age was significantly associated with severe ROP.
As regard the effect of birth weight on the occurrence of ROP, in agreement with Arroe and Peitersen,[21] we found that birth weight was nonsignificant factor for development of ROP. This was in disagreement with many studies,[8,20,22] which reported that lower birth weight was significantly associated with development of ROP, and explained that by more susceptibility for oxygen therapy, prolonged ventilation, sepsis, and blood transfusion in very low birth weight infants. In this work this may be related to the small number of patients (3 out of 172 cases) whose birth weight was less than 1000 g.
In this study, we found that sepsis was significantly associated with the development of ROP. This was in agreement with Shah et al.,[8] and Vinekar et al.,[23] which may be due to the effect of endotoxins on retinal blood vessels. On the other hand, this was in disagreement with the results of Chaudhari et al.,[24] and Smith.[25]
As regard oxygen therapy, in agreement with many studies,[8,15,26] it was an independent risk factor for development of ROP. We found significant relationship between the occurrence of ROP and use of oxygen therapy, but there was no significant relationship between oxygen therapy and stages of ROP. On the other hand, Palmer et al.,[27] reported that oxygen therapy was a nonsignificant factor for occurrence of ROP. They reported that ROP may develop in cases that did not receive oxygen therapy.
Some studies reported that a duration of oxygen therapy more than 7 days was a significant risk factor for development of ROP. [8,28] Meanwhile, in our study we found it nonsignificant which was in agreement with the results of Dutta et al.[29] We found that mechanical ventilation and CPAP were nonsignificant risk factors for ROP and this agreed with Murthy et al.[15] However, others observed that ventilatory support and CPAP were significantly associated with development of ROP.[8,24]
In our study, we found that the frequency of blood transfusions is an independent risk factor for development of ROP, and this agreed with Chawla et al.[30] This can be explained by the fact that, adult RBCs are rich in 2,3 DPG and adult hemoglobin which binds less firmly to oxygen, thus releasing excess oxygen to the retinal tissue. While Hirano et al.,[31] stated that it is controversial and iron overload rather than number of transfusions may contribute to the development of ROP.
Our study revealed nonsignificant relationship between sex and occurrence of ROP, in contrast to Darlow et al., [32] who found that male sex is a significant risk factor. In agreement with Seiberth and Lindarkomp,[33] we found nonsignificant relationship between the mode of delivery and occurrence of ROP. This was in disagreement with Shah et al.,[8] who found that Cesarean section delivery was significantly associated with occurrence of ROP.
Other risk factors including respiratory distress syndrome, patent ductus arteriosus, intraventricular hemorrhage, hypotension, and phototherapy showed nonsignificant relationship with the occurrence of ROP. Similarly Taqui et al.,[16] reported nonsignificant relation between ROP and patent ductus arteriosus and intraventricular hemorrhage, but observed a significant relation between respiratory distress syndrome and the development of ROP and related this to the fact that systemic hypoxia results in retinal hypoxia and more need for oxygen therapy. On the other hand, Shah et al.,[8] reported a significant relation between ROP development and patent ductus arteriosus, intraventricular hemorrhage and hypotension. Chaudhari et al.,[24] observed nonsignificant effect of phototherapy on ROP.
In multivariate analysis after logistic regression analysis, it was confirmed that low gestational age, sepsis, oxygen therapy and frequency of blood transfusions were significant risk factors for development of ROP.
Laser photocoagulation was found to be very effective in regressing ROP. In agreement with Coats et al.,[1] we found that the six cases that required laser intervention improved and ROP regressed with regular follow-up. Laser is now the preferred mode since the most severe forms of the disease are more easily treated with laser than with cryotherapy.
CONCLUSIONS
We are aware that a limitation of this study is the small number of patients. In conclusion, the incidence of ROP in this study was 19.2%, the data of this study suggest that low gestational age, sepsis, oxygen therapy and frequency of blood transfusions are independent risk factors in the development of ROP. Clinicians should be aware of the presence of the additional risk factors when monitoring preterm infants. The analysis of risk factors for ROP development will help to understand and predict it in preterm infants. The timely retinal screening of high-risk preterm infants is important to prevent the development of advanced ROP. Since ROP may produce serious sequelae up to complete blindness, all efforts must be made to prevent the development of advanced ROP through elimination of preterm births, changes in the neonatal care and improvement in detection of threatening ROP markers.
ACNOWLEDGEMENT
We appreciate all members of Neonatology and Ophthalmology Departments of Al-Minya University Hospital, and also to the parents of the studied neonates for their patience and compliance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Coats DK, Miller AM, Hussein MA, McCreery KM, Holz E, Paysse EA. Involution of Retinopathy of Prematurity after Laser Treatment: Factors Associated with Development of Retinal Detachment. Am J Ophthalmol. 2005;140:214–22. doi: 10.1016/j.ajo.2004.12.106. [DOI] [PubMed] [Google Scholar]
- 2.Fleck BW, Dangata Y. Causes of visual handicap in the.Royal Blind School, Edinburgh, 1991-2. Br J Ophthalmol. 1994;78:421. doi: 10.1136/bjo.78.5.421-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominico R, Davis K, Davis O. Documenting the NICU design dilemma: Comparative patient progress in open-ward and single family room units. J Perinatol. 2011;31:281–8. doi: 10.1038/jp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tasman W. Retinopathy of prematurity: Do we still have a problem?: The Charles L. Schepens lecture. Arch Ophthalmol. 2011;129:1083–6. doi: 10.1001/archophthalmol.2011.192. [DOI] [PubMed] [Google Scholar]
- 5.Fanaroff AA, Martin RJ, editors. 7th ed. Louis: Mosby; 2002. Neonatal Perinatal Medicine; pp. 676–745. [Google Scholar]
- 6.Azad R, Chandra P. Retinopathy of prematurity. J Indian Med Assoc. 2005;103:370–2. [PubMed] [Google Scholar]
- 7.Kim TI, Sohn J, Pi SY, Yoon YH. Postnatal risk factors of retinopathy of prematurity. Paediatr Perinat Epidemiol. 2004;18:130–4. doi: 10.1111/j.1365-3016.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 8.Shah VA, Yeo CL, Ling YL, Ho LY. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore. Ann Acad Med Singapore. 2005;34:169–78. [PubMed] [Google Scholar]
- 9.Gupta VP, Dhaliwal U, Sharma R, Gupta P, Rohatgi J. Retinopathy of prematurity-risk factors. Indian J Pediatr. 2004;71:887–92. doi: 10.1007/BF02830827. [DOI] [PubMed] [Google Scholar]
- 10.Karna P, Muttineni J, Angell L, Karmaus W. Retinopathy of prematurity and risk factors: A prospective cohort study. BMC Pediatr. 2005;5:18. doi: 10.1186/1471-2431-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englert JA, Saunders RA, Purohit D, Hulsey TC, Ebeling M. The effect of anemia on retinopathy of prematurity in extremely low birth weight infants. J Perinatol. 2001;21:21–6. doi: 10.1038/sj.jp.7200511. [DOI] [PubMed] [Google Scholar]
- 12.Akkoyun I, Oto S, Yilmaz G, Gurakan B, Tarcan A, Anuk D, Akgun S, et al. Risk factors in the development of mild and severe retinopathy of prematurity. J AAPOS. 2006;10:449–53. doi: 10.1016/j.jaapos.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 13.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology and American Association for Pediatric Ophthalmology and Strabismus. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatr. 2006;117:572–6. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 15.Murthy KR, Nagendra, Babu K, Benakappa N, Niranjan, Murthy PR. Analysis of risk factors for the development of ROP in preterm infants at a tertiary referral hospital in South India. Acta Medica Litunica. 2006;13:147–51. [Google Scholar]
- 16.Taqui AM, Syed R, Chaudhry TA, Ahmad K, Salat MS. Retinopathy of Prematurity: Frequency and Risk Factors in a Tertiary Care Hospital in Karachi, Pakistan. J Pak Med Assoc. 2008;58:186–90. [PubMed] [Google Scholar]
- 17.Chen Y, Li XX, Yin H, Gilbert C, Liang JH, Jiang YR, et al. Risk factors for retinopathy of prematurity in six neonatal intensive care units in Beijing China. Br J Ophthalmol. 2008;92:326–30. doi: 10.1136/bjo.2007.131813. [DOI] [PubMed] [Google Scholar]
- 18.Fortes Filho JB, Barros CK, Lermann VL, Eckert GU, Costa MC, Procianoy RS. Prevention of blindness due to retinopathy of prematurity at Hospital de Clinicas de Porto Alegre, Brazil: Incidence, risk factors, laser treatment and outcomes from 2002 to 2006. Acta Medica Lituanica. 2006;13:130–6. [Google Scholar]
- 19.Dammann O, Brinkhaus MJ, Bartels DB, Dördelmann M, Dressler F, Kerk J, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: A multi-hit hypothesis. Early Hum Dev. 2009;85:325–9. doi: 10.1016/j.earlhumdev.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Fortes Filho JB, Eckert GU, Procianoy L, Barros CK, Procianoy RS. Incidence and risk factors for retinopathy of prematurity in very low and in extremely low birth weight infants in a unit-based approach in southern Brazil. Eye (Lond) 2009;23:25–30. doi: 10.1038/sj.eye.6702924. [DOI] [PubMed] [Google Scholar]
- 21.Arroe M, Peitersen B. Retinopathy of Prematurity: Review of a seven year period in a Danish neonatal intensive care unit. Acta Pediatr. 1994;83:501–5. doi: 10.1111/j.1651-2227.1994.tb13067.x. [DOI] [PubMed] [Google Scholar]
- 22.Flores-Santos R, Hernández-Cabrera MA, Henández-Herrera RJ, Sepúlveda-Cañamar F. Screening for retinopathy of prematurity: Results of a 7-year study of underweight newborns. Arch Med Res. 2007;38:440–3. doi: 10.1016/j.arcmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Vinekar A, Dogra MR, Sangtam T, Narang A, Gupta A. Retinopathy of prematurity in Asian Indian babies weighting greater than 1250 gram at birth; ten years data from tertiary care center in a developing country. Indian J Ophthalmol. 2007;55:331–6. doi: 10.4103/0301-4738.33817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhari S, Patwardhan V, Vaidya U, Kadam S, Kamat A. Retinopathy of Prematurity in a Tertiary Care Center, Incidence, Risk Factors and Outcomes. Indian Pediatr. 2009;46:219–24. [PubMed] [Google Scholar]
- 25.Smith LE. Pathogenesis of retinopathy of prematurity. Acta Paediatr Suppl. 2002;437:26–8. doi: 10.1111/j.1651-2227.2002.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 26.Weinberger B, Laskin DL, Heck DE, Laskin JD. Oxygen toxicity in premature infants. Toxicol Appl Pharmacol. 2002;181:60–7. doi: 10.1006/taap.2002.9387. [DOI] [PubMed] [Google Scholar]
- 27.Palmer EA, Hardy RJ, Dobson V, Phelps DL, Quinn GE, Summers CG, et al. Cryotherapy for retinopathy of prematurity cooperative group. Outcomes following threshold retinopathy of prematurity. Final results from the multicenter trial of Cryotherapy for Retinopathy of Prematurity. Arch Ophthalmol. 2005;123:311–18. doi: 10.1001/archopht.123.3.311. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda H, Kuriyama S. Risk factors for retinopathy of prematurity requiring photocoagulation. Jpn J Ophthalmol. 2004;48:68–71. doi: 10.1007/s10384-003-0015-1. [DOI] [PubMed] [Google Scholar]
- 29.Dutta S, Narang S, Narang A, Dogra M, Gupta A. Risk factors of threshold retinopathy of prematurity. Indian Pediatr. 2004;41:665–71. [PubMed] [Google Scholar]
- 30.Chawla D, Agarwal R, Deorari A, Paul VK, Chandra P, Azad RV. Retinopathy of Prematurity. Indian J Pediatr. 2012;79:501–9. doi: 10.1007/s12098-010-0279-7. [DOI] [PubMed] [Google Scholar]
- 31.Hirano K, Morinobu T, Kim H, Hiroi M, Ban R, Ogawa S, et al. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;84:188–93. doi: 10.1136/fn.84.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darlow BA, Hutchinson JL, Henderson-Smart DJ, Donoghue DA, Simpson JM, Evans NJ. Australian and New Zealand Neonatal Network.Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005;115:990–6. doi: 10.1542/peds.2004-1309. [DOI] [PubMed] [Google Scholar]
- 33.Seiberth V, Lindarkomp O. Risk factors in retinopathy of prematurity: A multivariate statistical analysis. Ophthalmologica. 2000;214:131–5. doi: 10.1159/000027482. [DOI] [PubMed] [Google Scholar]


