Abstract
Purpose:
A hemodynamically significant Patent ductus arteriosus (HsPDA) in premature infants is known to be associated with significant morbidity. Recently brain natriuretic peptides and superior mesenteric artery (SMA)-resistive indices have been used to effectively diagnose HsPDA.
Objective:
To assess the sensitivity and specificity of N-terminal proBNP (NT-proBNP) in predicting an HsPDA diagnosed by clinical and echocardiographic criteria including pulsatility index (PI) of SMA.
Materials and Methods:
All preterm neonates <1500 g were evaluated with echocardiograms and NT-proBNP levels on the 3rd to 5th day of life and then every week until the echo showed either a closed PDA or non-HsPDA.
Results:
Sixty-nine babies with mean gestational age of 27 weeks were included in the study. NT-proBNP levels were significantly higher in the HsPDA group (n=22) with a mean±SEM of 24420±3190 compared to 3072±332 in the non-HsPDA group (n=47) (P<0.001). NT-pro BNP level of 5900 pg/ml had 96% sensitivity and 90% specificity of predicting HsPDA.
Conclusions:
With frequently changing hemodynamics in low-birth weight infants, including NT-proBNP and PI of SMA improve the ability of assessing the effects of a HsPDA and will help timing of intervention.
Keywords: Brain natriuretic peptide, patent ductus arteriosus, premature infants
INTRODUCTION
Patent ductus arteriosus (PDA) has been associated with significant morbidity in preterm infants, especially in very low-birth-weight infants (VLBW). In a hemodynamically significant PDA (HsPDA), there is significant shunting of blood from the systemic to the pulmonary circuit resulting in increased pulmonary blood flow with reduction in effective systemic blood flow. The diastolic runoff from the systemic circulation may lead to major complications like necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), and renal perfusion abnormalities.[1] The increased pulmonary blood flow contributes to the acute worsening of RDS and chronic lung disease later. Early treatment of an HsPDA has been shown to improve symptoms, the need for surgical ligation, duration of ventilator support and hospitalization in premature infants.[2,3]
Currently clinical, radiological and echocardiographic assessments used to diagnose a hemodynamically significant PDA have substantial limitations. Clinical findings like a continuous murmur and bounding pulses are not always present especially in VLBW infants on high ventilator support where the PDA could be ‘silent’. Although echocardiogram has been considered as the gold standard for the diagnosis,[4] it provides assessment at only one point of time and may not give a picture of the rapidly changing dynamic circulation especially in VLBW infants. There is also a lack of consensus in defining an HsPDA by echocardiographic criteria and size does not always correlate with the degree of shunting. Recently biomarkers like brain natriuretic peptides (BNP) have been used as indirect indicators of pulmonary blood flow and left ventricular volume overload.[5,6] The inactive fragment of BNP, NT-proBNP with longer half-life and no circadian variation may serve as a better marker than BNP for assessment of ventricular dysfunction and volume overload.[7] For assessing a reduction in the effective cardiac output, the resistive indices of the superior mesenteric artery (SMA) measured as Pulsatility Index (PI) have been used to indirectly assess the diastolic steal associated with an HsPDA.
Objective
This study was undertaken to assess the sensitivity and specificity of NT-proBNP in predicting an HsPDA diagnosed by clinical, traditional and functional echocardiographic criteria including a measure of the SMA blood flow PI in VLBW preterm infants.
MATERIALS AND METHODS
This was a prospective study conducted at the Brookdale University Hospital and Medical Center from January 2008 to September 2009. VLBW infants (less than 1500 g birth weight) were eligible for entry into the study. Infants with congenital heart defects, major congenital anomalies, confirmed sepsis, renal failure, persistent pulmonary hypertension and death within 3 days of admission to NICU were excluded. The study was approved by the institutional review board and consent was taken from parents before enrollment of the babies into the study.
All babies enrolled in the study were evaluated with echocardiograms on the 3rd – 5th day of life and then every week until the echo showed either a closed PDA or non-HsPDA. A Phillips 5500 ultrasonography machine (Phillips Medical Systems, NA, Bothell, WA) incorporating 2-dimensional, color flow, pulsed and continuous wave Doppler was used. If a PDA was diagnosed the point of maximal constriction of the color flow jet was measured in millimeters by frame by frame analysis to assess ductal size. The left atrium and aortic root dimensions were measured in the parasternal short axis view at the level of the aortic valve and the LA: AO (left atrial: aortic) ratio was calculated. In addition left ventricular dimensions in systole and diastole, tricuspid regurgitant jet gradients to assess systolic pulmonary artery pressures and systolic function by % shortening fraction (SF) were measured. A sagittal subcostal view was used to image the superior mesenteric artery (SMA). The PI of the SMA calculated as peak systolic velocity - end diastolic velocity / time averaged mean velocity (VTI) was measured as an index of change in the local vascular resistance and reduction in effective cardiac output. All measures were performed based on the American Society of Echocardiography guidelines and an average of two readings were taken and reviewed by a single cardiologist blinded to the clinical status or laboratory data of the patient.
HsPDA was defined as PDA size of >1 mm with at least two additional features of PDA (continuous murmur, pulse pressure >25 mmHg, worsening respiratory status, LA/AO ratio >1.4, PI of SMA >6, base excess >-5) .[8,9] Infants with HsPDA were treated with indomethacin, ibuprofen or surgery depending on the clinical status of the infant and discretion of the neonatologist.
Blood samples (0.5 ml) for plasma NT-proBNP were collected by arterial or venous catheter aspiration or by venous blood sampling along with other routine blood sampling to avoid extra needle sticks and excessive blood sampling. It was collected on the same day that the echocardiogram was done and analyzed immediately. Plasma NT-proBNP was measured with VITROS NT-proBNP reagent pack using Intellicheck technology (Ortho Clinical Diagnostics, a Johnson and Johnson Company).
Data including body weight changes, fluid intake and output, respiratory and cardiovascular status were collected every week and data on the length of stay, prevalence of NEC, bronchopulmonary dysplasia (BPD as defined by need for supplemental oxygenation at 36 weeks' postmenstrual age), IVH, and mortality were collected from the charts and neonatal database.
Statistical analysis
Analysis was performed using SPSS, version 15 (SPSS Inc, Chicago, IL). Demographics and clinical characteristics were compared between HsPDA and non-HsPDA groups using Fishers exact and Chi-square tests. Mean and standard deviation were calculated for all continuous variables. T-test and Mann-Whitney U test were used to compare continuous variables. Pearson′s correlation coefficient was used to test for correlations between plasma NT-proBNP levels and other continuous clinical and echocardiographic variables. The receiver operator characteristic (ROC) curve was created to select the best cut-off point of NT-proBNP for detection of HsPDA. P values less than 0.05 were considered statistically significant.
RESULTS
Sixty-nine babies with mean gestational age (GA) of 27±2.6 weeks were included in the study for whom a total of 121 echocardiographic studies were done. The HsPDA group had 22 patients while the non-HsPDA group had 47 patients. The HsPDA group had a significantly lower gestational age and birth weight compared to the non-HsPDA group [Table 1]. The HsPDA group had significantly higher NT-proBNP levels [Figure 1], lower base excess, wider pulse pressure and lower mean and diastolic blood pressures. The fluids given each day were higher for HsPDA but not significantly different.
Table 1.
Demographic and clinical parameters
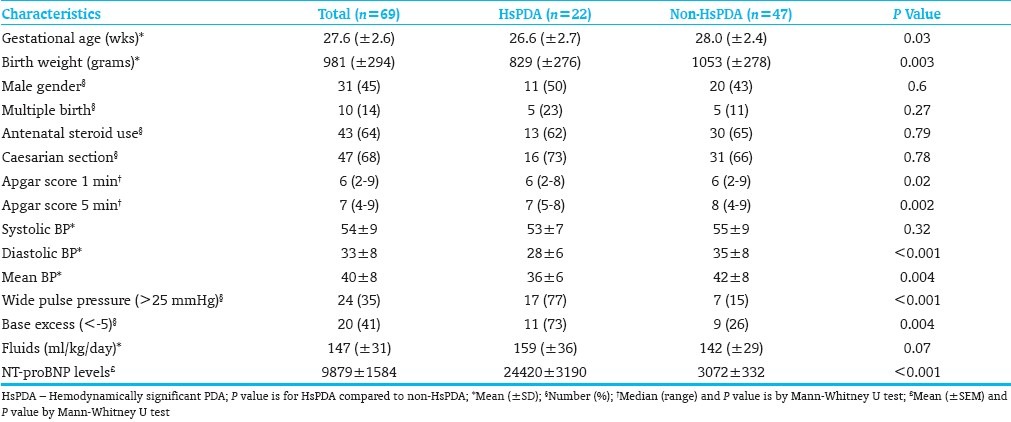
Figure 1.
Weekly NT-proBNP levels
Echo parameters
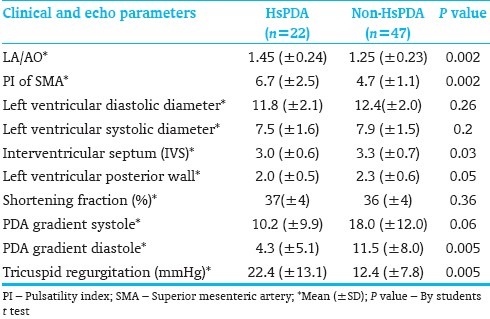
The HsPDA group had significantly higher estimated pulmonary artery pressures measured by the TR jet gradient and lower PDA systolic and diastolic pressure gradients indicating higher pulmonary artery pressures [Table 2].
Table 2.
Echocardiogram parameters
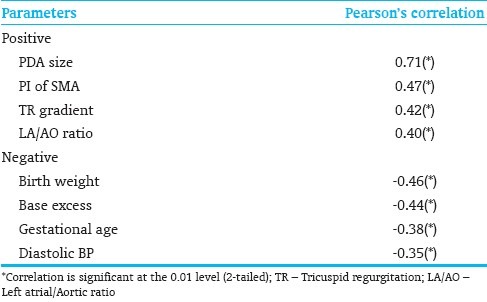
There were significant positive correlations of NT-proBNP with echo parameters of PDA size, LA/AO ratio, the resistive index of SMA blood flow and higher estimated PA pressures. Significant negative correlations were found with low birth weight, gestational age, diastolic blood pressure and base excess [Table 3].
Table 3.
Correlations with NT-proBNP
Clinical outcomes
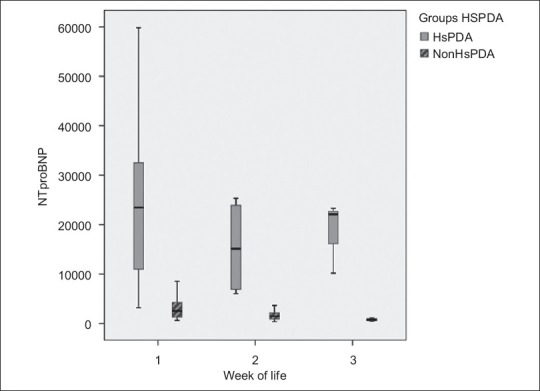
In the HsPDA group, 45% of the infants received intervention; 6 received ibuprofen, one received indomethacin and 3 underwent surgical ligation. In the HsPDA group, 6 infants (27%) died and the remaining 16 infants (73%) had PDA closure at a mean age of 2.7 weeks. In the non-HsPDA group, 13 infants (28%) had PDA of whom 2 infants (3%) died and the remaining had PDA closure at mean age of 2.1 weeks. The mean age of PDA closure was not significantly different between both groups. The HsPDA group had a higher, but not statistically significant incidence of IVH (27% in HsPDA group vs. 15% in the non-HsPDA group, P=0.2), chronic lung disease (36% in HsPDA group vs. 17% in the non-HsPDA group, P=0.1), and a significant increase in mortality within 2 weeks (27% in HsPDA group vs. 4% in the non-HsPDA group, P=0.01). The NT-proBNP levels continued to decline over time in both groups although the mean values remained higher at all times in the HsPDA group [Figure 1]. Using the receiver operating characteristic curve (ROC), NT-proBNP levels were a good predictor of an HsPDA with the area under the curve of 0.981 (P<0.001). Using a cut off of 5900 pg/ml was associated with a 96% sensitivity and 90% specificity of accurately diagnosing an HsPDA. On multivariate analysis, NT-proBNP levels and weight were significant independent predictors of HsPDA.
In a subgroup analysis of 12 patients who had NT-proBNP measured both with a HsPDA and later a non-HsPDA, the mean±SEM levels were significantly higher for HsPDA compared to non-HsPDA (24322±3857 pg/ml vs. 3381±578 pg/ml, respectively, P<0.001 by paired t-test).
DISCUSSION
This study supports the previously shown increased morbidity and mortality in VLBW premature infants with HsPDA.[2,3] Our HsPDA group showed a significantly higher prevalence of chronic lung disease, and early mortality compared to those without an HsPDA and similar findings have been shown by other investigators.[10,11] In spite of the high prevalence of a PDA in nearly 60-70% of VLBW infants and its associated morbidity there are no universally accepted methods to diagnose an HsPDA and no accepted indications for intervention or the timing of intervention.[12,13] One of the major reasons for this is the dynamic nature of the ductal tissue and its varied sensitivity to a number of vasodilators and vasoconstrictors in the preterm infant. Factors that alter the pulmonary and systemic vascular resistance will also influence ductal shunting irrespective of ductal size. Clinical signs such as a heart murmur, hyperdynamic precordium, bounding pulses and metabolic acidosis are not accurate predictors of a significant ductus in the first few days of life.[14,15] Even traditional echocardiographic parameters like the size of the ductus, left atrial dimensions, left atrial to aortic root ratio (LA:AO) are not specific for determining an HsPDA and may take a few days before these features are evident by echo.
To assess LV volume overload related to a PDA shunt we used NT-proBNP, a brain natriuretic peptide, a biomarker used in previous studies as an indicator of left ventricular volume overload.[5,6] BNP is mainly synthesized by the ventricles of the heart in response to volume and pressure loading and leads to diuresis, vasodilatation and inhibition of renin/aldosterone production. It is synthesized as a pro-hormone pro-BNP which is cleaved into the biologically active BNP (half-life of 20 min) and the inactive fragment NT-proBNP (half-life of 90 min).[7] NT-proBNP has no circadian variation and remains stable in blood sample. Therefore, it can be kept for later analysis and may serve as a better marker than BNP for assessment of ventricular dysfunction and volume overload. Previous studies in healthy full-term infants have shown that the plasma BNP level rapidly rises after birth, reaching its peak at day 2-3 of life suggesting a physiological role for BNP in the transitional neonatal circulation.[16,17] Recent studies have also shown high plasma BNP levels to correlate with degree of ductal shunting.[18,19] A decline of BNP level was also noted after PDA was closed by indomethacin. In this study we used serial NT-proBNP levels to assess correlation with echo and clinical features of a PDA over time. The first measurement was done on day 3-5 when the expected early adjustments in the premature transitional circulation would be over and the effect of the left to right PDA shunt more evident on the left ventricle volume status. Our data confirms the previous findings that NT-proBNP levels are significantly higher in patients with a HsPDA compared to the non-HsPDA group and also showed a significant drop in levels when the duct was closed or no longer hemodynamically significant. Previous investigators have shown similar trends in BNP levels with age and after use of indomethacin.[18,19] Patients identified with HsPDA were noted by echocardiogram to have significantly lower mean septal and posterior wall thickness although there was no difference in their mean LV diastolic and systolic dimensions. This implies that patients with an HsPDA may have an altered ventricular remodeling response to the volume overload. Previous studies have shown abnormal diastolic function in premature infants with a large PDA.[20]
Gastrointestinal blood flow is affected in the early stages of an HsPDA secondary to ductal steal and redistribution of blood to vital organs. The superior mesenteric artery (SMA) supplies most of the intestines and changes in blood flow in this vessel reflect changes in bowel perfusion. There are several studies which have shown decreased intestinal blood flow and changes in resistive indices of the SMA in patients with a symptomatic PDA.[2,21] In this study, we used the SMA PI of greater than 6 to characterize an HsPDA based on our preliminary data correlating the currently accepted echo and clinical parameters with values of the PI giving a sensitivity and specificity of >80% in predicting an HsPDA. Including this as a feature for diagnosing an HsPDA added a measure of the effective systemic output and ductal steal in addition to the other echocardiographic and clinical parameters.
The limitations of this study were a small sample size and no standard protocol for management. Thus the natural history of an HsPDA could not be clearly defined. Also there are a few confounding factors that can influence the validity of NT-proBNP levels such as increased pulmonary vascular resistance, concomitant renal impairment, hydration status and sepsis.
The strengths of the study were that this was a prospective study performed on all premature infants less than 1500 grams in a single medical center, at preset time intervals thus reducing selection bias and one of the only studies to our knowledge that has used a measure of systemic blood flow and pulmonary blood flow to gauge an HsPDA.
Until further randomized control trials are done in VLBW infants to assess the effect of medical treatment and/ or surgical ligation vs. conservative management on short-term and long-term morbidity and mortality, frequent clinical assessments, directed functional echocardiography utilizing measures to assess both pulmonary and effective systemic blood flow and biomarkers like BNP or NT-proBNP together may help direct timing and treatment strategies for those patients with an HsPDA.
Use of biomarkers could also help determine the relative contribution of filling pressures to worsening respiratory status associated with intermittent reactive pulmonary hypertension in severe RDS. This marker may help direct management towards improving ventricular function and reducing the volume overload with the judicious use of inotropes, fluid restriction, diuretics and interventions to close the PDA.
CONCLUSIONS
The PDA and premature transitional circulation is dynamic and can lead to rapid and frequent changes in hemodynamic status. Serial NT-proBNP levels can be used as a simple screening test which is cost effective, easily available and can supplement the information obtained from functional echocardiography to aid in the diagnosis and management of an HsPDA.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125:1020–30. doi: 10.1542/peds.2009-3506. [DOI] [PubMed] [Google Scholar]
- 2.Fowlie PW, Davis PG. Prophylactic indomethacin for preterm infants: A systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2003;88:F464–6. doi: 10.1136/fn.88.6.F464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–72. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 4.Evans N. Diagnosis of patent ductus arteriosus in the preterm newborn. Arch Dis Child. 1993;68:58–61. doi: 10.1136/adc.68.1_spec_no.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pesonen E. Role of natriuretic hormones in the diagnosis of patent ductus arteriosus in newborn infants. Acta Paediatr. 2001;90:363–5. [PubMed] [Google Scholar]
- 6.Holmstrom H, Omland T. Natriuretic peptides as markers of patent ductus arteriosus in preterm infants. Clin Sci (Lond) 2002;103:79–80. doi: 10.1042/cs1030079. [DOI] [PubMed] [Google Scholar]
- 7.Farombi-Oghuvbu I, Matthews T, Mayne PD, Guerin H, Corcoran JD. N-terminal pro-B-type natriuretic peptide: A measure of significant patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2008;93:F257–60. doi: 10.1136/adc.2007.120691. [DOI] [PubMed] [Google Scholar]
- 8.Nuntnarumit P, Khositseth A, Thanomsingh P. N-terminal probrain natriuretic peptide and patent ductus arteriosus in preterm infants. J Perinatol. 2009;29:137–42. doi: 10.1038/jp.2008.185. [DOI] [PubMed] [Google Scholar]
- 9.Chiruvolu A, Punjwani P, Ramaciotti C. Clinical and echocardiographic diagnosis of patent ductus arteriosus in premature neonates. Early Hum Dev. 2009;85:147–9. doi: 10.1016/j.earlhumdev.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Noori S, McCoy M, Friedlich P, Bright B, Gottipati V, Seri I, et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123:e138–44. doi: 10.1542/peds.2008-2418. [DOI] [PubMed] [Google Scholar]
- 11.Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O'Shea TM. Risk factors for chronic lung disease in the surfactant era: A North Carolina population-based study of very low birth weight infants.North Carolina Neonatologists Association. Pediatrics. 1999;104:1345–50. doi: 10.1542/peds.104.6.1345. [DOI] [PubMed] [Google Scholar]
- 12.Sehgal A, McNamara PJ. Does echocardiography facilitate determination of hemodynamic significance attributable to the ductus arteriosus? Eur J Pediatr. 2009;168:907–14. doi: 10.1007/s00431-009-0983-3. [DOI] [PubMed] [Google Scholar]
- 13.Evans N. Current controversies in the diagnosis and treatment of patent ductus arteriosus in preterm infants. Adv Neonatal Care. 2003;3:168–77. doi: 10.1016/s1536-0903(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 14.Davis P, Turner-Gomes S, Cunningham K, Way C, Roberts R, Schmidt B. Precision and accuracy of clinical and radiological signs in premature infants at risk of patent ductus arteriosus. Arch Pediatr Adolesc Med. 1995;149:1136–41. doi: 10.1001/archpedi.1995.02170230090013. [DOI] [PubMed] [Google Scholar]
- 15.Urquhart DS, Nicholl RM. How good is clinical examination at detecting a significant patent ductus arteriosus in the preterm neonate? Arch Dis Child. 2003;88:85–6. doi: 10.1136/adc.88.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshibayashi M, Kamiya T, Saito Y, Nakao K, Nishioka K, Temma S, et al. Plasma brain natriuretic peptide concentrations in healthy children from birth to adolescence: Marked and rapid increase after birth. Eur J Endocrinol. 1995;133:207–9. doi: 10.1530/eje.0.1330207. [DOI] [PubMed] [Google Scholar]
- 17.Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–8. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi BM, Lee KH, Eun BL, Yoo KH, Hong YS, Son CS, et al. Utility of rapid B-type natriuretic peptide assay for diagnosis of symptomatic patent ductus arteriosus in preterm infants. Pediatrics. 2005;115:e255–61. doi: 10.1542/peds.2004-1837. [DOI] [PubMed] [Google Scholar]
- 19.Attridge JT, Kaufman DA, Lim DS. B-type natriuretic peptide concentrations to guide treatment of patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2009;94:F178–82. doi: 10.1136/adc.2008.147587. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz L, Stiller B, Koch H, Koehne P, Lange P. Diastolic left ventricular function in preterm infants with a patent ductus arteriosus: A serial Doppler echocardiography study. Early Hum Dev. 2004;76:91–100. doi: 10.1016/j.earlhumdev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Freeman-Ladd M, Cohen JB, Carver JD, Huhta JC. The hemodynamic effects of neonatal patent ductus arteriosus shunting on superior mesenteric artery blood flow. J Perinatol. 2005;25:459–62. doi: 10.1038/sj.jp.7211294. [DOI] [PubMed] [Google Scholar]


