Abstract
Disseminated neonatal herpes simplex virus (HSV) infection is characterized by progressive multiple organ failure and high mortality rates up to 85% for untreated neonates. It can result from infection with either HSV-1 or HSV-2. We report the first known case of disseminated neonatal herpes associated with fulminant liver failure caused by HSV-2 who survived without liver transplant.
Keywords: Disseminated herpes simplex, liver failure, neonate
INTRODUCTION
Neonatal herpes simplex virus (HSV) infection, although relatively rare, has a high mortality and morbidity. It is estimated that 1 neonate in 3200 live births has HSV infection.[1] Most (85%) neonatal HSV infections are acquired during delivery, although in utero (5%) and postnatal (10%) infections do occur.[2] Neonatal herpes can be localized, skin, eyes, and mouth (SEM) infection, central nervous system (CNS) disease, or can cause disseminated infection involving multiple organs. Disseminated infection is the most severe form of neonatal herpes, with a mortality rate of 85% for untreated neonates.[3] Intravenous acyclovir given in a high dose and early in the course of the disease significantly improves prognosis.[4] Early diagnosis may be difficult as the characteristic vesicular rash is absent in up to 40% of the neonates who acquire the infection. Early symptoms are often nonspecific and the majority of their mothers lack a history of genital herpes infection.[5–7] Severe hepatitis may cause potentially fatal, acute liver failure in neonates with disseminated disease. Liver transplantation has been carried out successfully in a few reported neonates with fulminant hepatic failure associated with disseminated neonatal HSV disease.[8,9] Approximately, 80% of survivors of disseminated neonatal HSV disease may have normal neurologic development. [4,10,11] The risk of neurodevelopmental abnormalities is increased among infants with seizures at or before the initiation of antiviral therapy.[10] We report a case of disseminated HSV with fulminant liver failure who survived without liver transplantation and with a good neurodevelopmental outcome.
CASE REPORT
A 3-kg male was born at term to a healthy mother after a normal pregnancy. The mother did not have a history or signs of HSV infection. The infant was born after 17 h of ruptured membrane with an Apgar score of 9 and 9 at 1 and 5 min, respectively, and physical examination at birth was unremarkable.
On the fourth day of life, the infant developed fever, lethargy, and moderate respiratory distress. He was admitted to the pediatric intensive care unit, and sepsis workup was performed. He was treated with ampicillin and cefotaxime along with oxygen therapy through a nasal cannula. Initial laboratory investigations showed a normal leukocyte count (10200 cells/μL), a slightly elevated C- reactive protein level (18 mg/L), and elevated levels of liver enzymes (AST: 270 U/L, ALT: 161 U/L), and chest X-ray showed bilateral infiltrates. On the same day, a vesicular eruption in the left groin area with redness of the umbilical stump was noted.
On the fifth day of life, he was still febrile and the severity of the respiratory distress was increasing requiring a nasal CPAP; then subsequently, he was intubated and placed on conventional mechanical ventilation. However, due to severe desaturation and respiratory failure, he was shifted to high-frequency oscillatory ventilation (HFOV). Moreover, he developed hypotension for which dopamine and dobutamine infusions were started. Subsequent laboratory investigations showed thrombocytopenia (49,000 cells/μL), leukopenia (2000 cells/μL), elevated C-reactive protein level (33 mg/L), AST 6763 U/L, ALT: 1174, and marked coagulopathy.
The skin eruption, respiratory failure, fulminate hepatic failure, in combination with leukopenia, thrombocytopenia, and fever, raised the possibility of viral infection such as disseminated herpes simplex virus infection. Intravenous acyclovir treatment 20 mg/kg/dose every 8 h was commenced on day 2 of admission after obtaining the blood and CSF sample for the HSV PCR test. In addition to acyclovir, the infant was treated with the infusion of FFP, platelet, PRBC, clotting factors, GCSF, and IVIG. On day 6 of admission, the result of blood and CSF HSV PCR was positive for HSV-II virus. Retrospectively, both mother and father were tested for HSV and both came as positive for HSV-II antibody.
Despite the early initiation of acyclovir and maximum medical support, he underwent complicated course of illness. He developed severe ARDS with bilateral pneumothorax with bilateral effusion necessitating HFOV and bilateral chest drains for almost 24 days. He had hypotension which needed inotropic support for 27 days. Moreover, he developed anasarca with abdominal ascites for which peritoneal drain was inserted. He had fulminated liver failure with cholestatic jaundice and hepatosplenomegaly. Abdominal ultrasound [Figure 1] showed derangement of the liver parenchymal architecture with fine nodular patterns best reflected on the liver surface with a hypertrophied left hepatic lobe; no intrahepatic duct dilatation was seen, the gallbladder was tortuous with sludge inside, the spleen was mildly enlarged, and there was an evidence of portal hypertension. He was treated with N-acetylcysteine, ursodiol, carnitine, cholestyramin with lactulose, and propranolol. His liver enzymes were trending down very slowly until almost normalized by the age of 5 months (AST: 56 U/L, ALT: 56, total bilirubin: 8 μmol/L). He was fed parentally for about 24 days, and enteral feed was introduced gradually till full feed through NGT. The patient was then shifted to oral feed as breast feeding. Repeated abdominal ultrasound at age of 5 months revealed a complete recovery of the hepatic hemodynamics and the nodularity of the liver surface and in the parenchyma was rudimentary with remaining prominence of the left hepatic lobe, complete recovery of the portal hypertension, and no gallbladder or bile duct pathology, and no sludge identified in the gallbladder.
Figure 1.
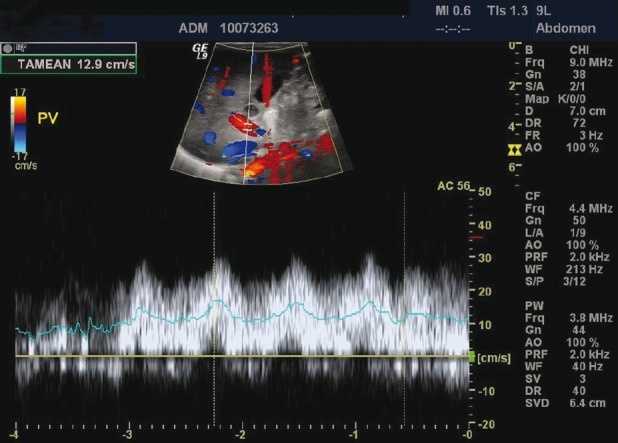
Liver ultrasound showed derangement of the liver parenchyma architecture with fine nodular patterns on the liver surface with a hypertrophied left hepatic lobe; no intra hepatic duct dilatation was seen
Fortunately, his renal function and urine output remained normal throughout the course of the illness apart from urinary tract infection with Klebsiella on day 39 of admission which was treated with meropenem and amikacin.
Neurologically, he did not develop any abnormal movement or seizure, and brain MRI was done and reported as normal along with fundoscopic examination of both eyes.
He completed almost a 4-week course of intravenous (IV) acyclovir and the last HSV PCR result from CSF and blood was negative. One week later, he started to have skin relapse in the form of skin eruption over the left groin, so IV acyclovir was restarted and HSV PCR from the skin lesion and blood was done, which came positive. He was continued on IV acyclovir for 2 weeks and was then shifted to oral acyclovir as suppressive treatment.
He was discharged at the age of 2 months on oral acyclovir and on full breast feeding. He was kept on oral acyclovir till the age of 6 months, but 3 days after stopping it, he had another skin relapse over his left foot, left arm, and over the pubic area, so oral acyclovir was started for 1 week and stopped. Today, he is off any treatment and with no more relapses documented and with normal neurodevelopment.
DISCUSSION
Disseminated HSV disease is the most lethal form of neonatal herpes infection. Mortality without treatment was 85%, and with treatment 57%.[11] All but few survivors have neurological impairment (abnormal neurologic status at 1 year was 92% in untreated patients and 86% in treated patients with disseminated disease). To the best of our knowledge, our patient is the first survived case of disseminated HSV with fulminant liver failure without liver transplant and with normal neurodevelopment at 1 year of age.
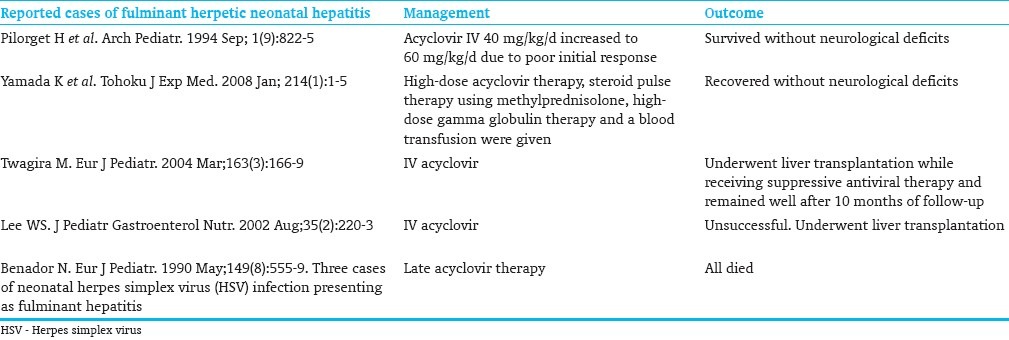
HSV infection should be considered in the differential diagnosis of acutely unwell neonate. Effective antiviral therapy exists but is often not started early in the clinical course. Moreover, the indications for the initiation of empiric acyclovir have not been standardized yet. Early and prompt administration of acyclovir improves survival and outcome.[4,12,13] In our case, prompt diagnosis was difficult initially because of the early appearance of nonspecific symptoms and signs, but due to clinical deterioration and multiple organ failure in a very short period, in addition to skin lesions, acyclovir was started early at the course of illness. There are some case reports of successful treatment of fulminant neonatal hepatitis caused by disseminated HSV [Table 1].
Table 1.
Cases of fulminant hepatitis caused by disseminated HSV
Disseminated and CNS disease should be treated for a minimum of 21 days, because the persistence of HSV DNA in the cerebrospinal fluid (CSF) is associated with poor outcome;[14] repeat lumbar puncture to obtain CSF HSV DNA PCR near the end of therapy is recommended to make sure that HSV DNA PCR is negative and that CSF parameters have returned to normal.[10,15–19]
Even after successful parenteral treatment, the recurrence of HSV can occur and may be a lifelong problem for the patient and family. Fortunately, the recurrence of CNS disease is rare. However, recurrent vesicles at sites in the skin, eyes, and mouth are common and occur in 60-80% of neonates, with 1-12 episodes in the first year of life. Therefore, some experts recommend long-term suppressive therapy with oral acyclovir to reduce skin or eye recurrences during infancy.[15] The recurrence of CNS disease, as well as emergence of acyclovir-resistant HSV mutants, has occurred in neonates who were receiving oral acyclovir suppression.[20,21] Although our patient received IV acyclovir for 4 weeks, and CSF and the blood samples were negative for the HSV PCR test, he developed skin relapse, for which he needed a prophylaxis course of oral acyclovir till the age of 6 months, but even then he continued to have skin relapses after 6 months of age till 1 year of age.
The effectiveness of long-term suppression with oral acyclovir to reduce the risk of CNS recurrence after neonatal HSV disease is unknown, and CNS recurrence in neonates receiving long-term oral suppression has been documented.[20] Oral acyclovir suppression therapy is associated with dose-dependent reversible neutropenia in one-half to two-thirds of infants, and an emergence of HSV mutants that are acyclovir resistant has been documented.[12,15,20,21]
Severe hepatitis, caused by either HSV strains, may cause potentially fatal, acute liver failure in neonates with disseminated disease. Liver transplantation has been carried out successfully in a few reported neonates with fulminant hepatic failure associated with disseminated neonatal HSV disease.[8,9,22] Our patient developed fulminant liver failure and he was a candidate for liver transplant, but with full medical support his liver failure was almost completely resolved, and he is the first known case to the best of our knowledge who survived disseminated HSV with fulminant liver failure without liver transplant.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203–9. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 2.Fidler KJ, Pierce CM, Cubitt WD, Novelli V, Peters MJ. Could neonatal disseminated herpes simplex virus infections be treated earlier? J Infect. 2004;49:141–6. doi: 10.1016/j.jinf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Kimberlin D. Herpes simplex virus, meningitis and encephalitis in neonates. Herpes. 2004;11:65A–76A. [PubMed] [Google Scholar]
- 4.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Corey L, Gruber WC The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Safety and efficacy of high dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001;108:230–8. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- 5.Ashley RL, Wald A. Genital herpes: Review of the epidemic and potential use of type-specific serology. Clin Microbiol Rev. 1999;12:1–8. doi: 10.1128/cmr.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337:509–15. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 7.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Frenkel LM, Gruber WC. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108:223–9. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- 8.Verma A, Dhawan A, Zuckerman M, Hadzic N, Baker AJ, Mieli-Vergani G. Neonatal herpes simplex virus infection presenting as acute liver failure: Prevalent role of herpes simplex virus type I. J Pediatr Gastroenterol Nutr. 2006;42:282–6. doi: 10.1097/01.mpg.0000214156.58659.4c. [DOI] [PubMed] [Google Scholar]
- 9.Egawa H, Inomata Y, Nakayama S, Matsui A, Yamabe H, Uemoto S, et al. Fulminant hepatic failure secondary to herpes simplex virus infection in a neonate: A case report of successful treatment with liver transplantation and perioperative acyclovir. Liver Transpl Surg. 1998;4:513. doi: 10.1002/lt.500040601. [DOI] [PubMed] [Google Scholar]
- 10.Kimberlin DW. Herpes simplex virus infections of the newborn. Semin Perinatol. 2007;31:19–25. doi: 10.1053/j.semperi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Whitley RJ, Nahmias AJ, Soong SJ, Galasso GG, Fleming CL, Alford CA. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics. 1980;66:495. [PubMed] [Google Scholar]
- 12.Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1. doi: 10.1128/CMR.17.1.1-13.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CA, Walker KS, Badawi N. Antiviral agents for treatment of herpes simplex virus infection in neonates. Cochrane Database Syst Rev. 2009:CD004206. doi: 10.1002/14651858.CD004206.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mejías A, Bustos R, Ardura MI, Ramírez C, Sánchez PJ. Persistence of herpes simplex virus DNA in cerebrospinal fluid of neonates with herpes simplex virus encephalitis. J Perinatol. 2009;29:290. doi: 10.1038/jp.2008.235. [DOI] [PubMed] [Google Scholar]
- 15.Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1. doi: 10.1128/CMR.17.1.1-13.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickering LK, editor. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village:IL: American Academy of Pediatrics; 2009. American Academy of Pediatrics. Herpes simplex; p. 363. [Google Scholar]
- 17.Kimura H, Futamura M, Kito H, Ando T, Goto M, Kuzushima K, et al. Detection of viral DNA in neonatal herpes simplex virus infections: Frequent and prolonged presence in serum and cerebrospinal fluid. J Infect Dis. 1991;164:289–93. doi: 10.1093/infdis/164.2.289. [DOI] [PubMed] [Google Scholar]
- 18.Troendle-Atkins J, Demmler GJ, Buffone GJ. Rapid diagnosis of herpes simplex virus encephalitis by using the polymerase chain reaction. J Pediatr. 1993;123:376–80. doi: 10.1016/s0022-3476(05)81735-4. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez PJ, Demmler-Harrison GJ. Viral infections of the fetus and neonate. In: Feigin RD, Cherry JD, Demmler-Harrison GJ, Kaplan SL, editors. Textbook of Pediatric Infectious Diseases. 6th ed. Philadelphia: Saunders; 2009. p. 895. [Google Scholar]
- 20.Fonseca-Aten M, Messina AF, Jafri HS, Sánchez , PJ Herpes simplex virus encephalitis during suppressive therapy with acyclovir in a premature infant. Pediatrics. 2005;115:804–9. doi: 10.1542/peds.2004-0777. [DOI] [PubMed] [Google Scholar]
- 21.Kimberlin D, Powell D, Gruber W, Diaz P, Arvin A, Kumar M, et al. Administration of oral acyclovir suppressive therapy after neonatal herpes simplex virus disease limited to the skin, eyes and mouth: results of a phase I/II trial. Pediatr Infect Dis J. 1996;15:247–54. doi: 10.1097/00006454-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Twagira M, Hadzic N, Smith M, Ramaswamy M, Verma A, Dhawan A, et al. Disseminated neonatal herpes simplex virus (HSV) type 2 infection diagnosed by HSV DNA detection in blood and successfully managed by liver transplantation. Eur J Pediatr. 2004;163:166–9. doi: 10.1007/s00431-003-1383-8. [DOI] [PubMed] [Google Scholar]


