BACKGROUND
Hypoxic-ischemic injury remains an important cause of perinatally acquired brain injury in full-term infants. The best predictor of mortality and long-term outcome following perinatal injury is the presence of neonatal encephalopathy. If moderate encephalopathy is present, the risk of death is less than 10% and as many as one-third of the survivors have physical disabilities. With severe encephalopathy, mortality is higher (as much as 60%) and many, if not all, survivors are handicapped. The benefit of induced hypothermia in post-asphyxia encephalopathy has been proven in high-quality randomized controlled trials to be safe. In addition, it reduces the incidence of death and disability at 18-22 months of age. The current evidence does not support cooling of infants with mild hypoxic-ischemic encephalopathy (HIE) or those born before 35 weeks.
PURPOSE
To promote and facilitate standardization and consistency of practice, using the most updated evidence-based information
To provide direction to clinicians regarding therapeutic hypothermia for neonates of 36 weeks of gestation or greater with HIE.
STANDARD OF CARE
The aim is to cool infants with moderate or severe HIE within 6 h of birth to a body temperature between 33.5°C and 34.5°C and maintain this degree of cooling without interruption for 72 h.
This would be followed by slow re-warming over at least 4 h at a rate of 0.5°C per hour until their rectal temperature reaches the desired range (36.5-37°C).
EQUIPMENT / SUPPLIES
Cold packs or other alternative cooling devices
Rectal temperature probe (thermometer)
Temperature cable (appropriate for monitor)
Cerebral function monitor (CFM) if available
Cardio-respiratory monitor
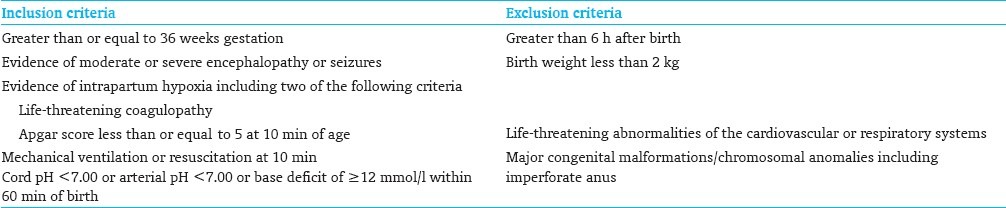
ELIGIBILITY CRITERIA FOR INFANT COOLING
Infants of gestational age greater than or equal to 36 weeks must meet both physiological and neurological criteria [Table 1].
Table 1.
Inclusion and exclusion criteria for therapeutic hypothermia
Physiological criteria
Evidence of intrapartum hypoxia, including at least two of the following:
Apgar score 5 or less at 10 min
Needing mechanical ventilation and/or ongoing resuscitation at 10 min
Metabolic or mixed acidosis defined as arterial cord gas, or any blood gas within the first hour of life showing pH of 7 or less, or base deficit of ≥12 mmol/l.
Other qualifying criteria
If no cord blood gas is available and the initial blood gas within 60 min of birth shows a postnatal pH of <7.10 with a base deficit of ≥16 mmol/l, plus an acute perinatal event (abruptio placenta, cord prolapse, or severe fetal heart rate (HR) abnormalities; variable or late decelerations) requires resuscitation, plus either (a) or (b)
-
a)
Apgar less than 5 at 10 min
-
b)
Continued need for ventilation initiated at birth and continued for at least 10 min
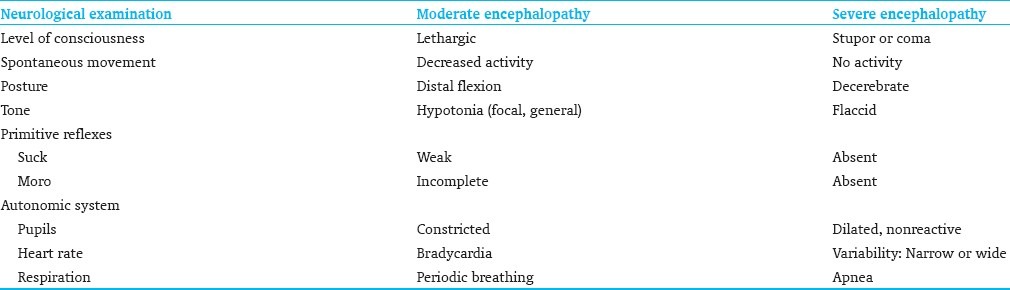
Neurological criteria
One of the following:
The presence of seizures is an automatic inclusion
Evidence of encephalopathy suggested by amplitude-integrated EEG (a-EEG)
Physical examination consistent with moderate to severe encephalopathy [Table 2]
Table 2.
Encephalopathy score and neurological examination
INFANTS NOT ELIGIBLE FOR COOLING
Birth weight less than 2000 g
Gestational age less than 36 weeks
Inability to initiate cooling by 6 h of age
Suspected coagulopathy
Life-threatening abnormalities of the cardiovascular or respiratory systems such as complex congenital heart disease and persistent pulmonary hypertension of the newborn (PPHN)
Major congenital malformations, imperforate anus, suspected neuromuscular disorders, or presence of known lethal chromosomal anomaly
Death appears inevitable
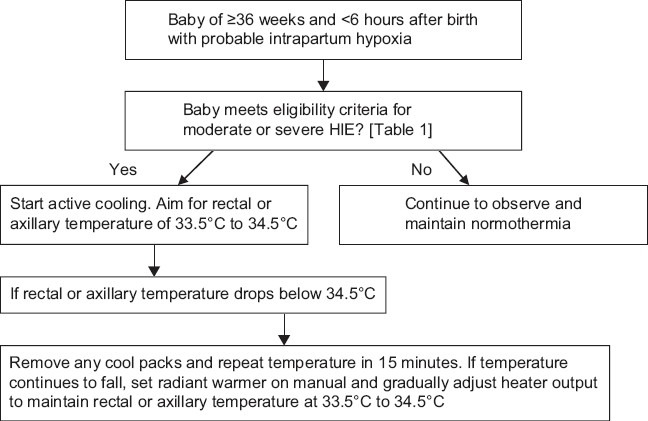
COOLING PROCEDURE
Within 6 h of birth, eligible infants will undergo whole body cooling therapy to achieve and maintain Core body temperature between 33.5°C and 34.5°C. (Core temperature is monitored continuously using esophageal or rectal temperature probe)[Figure 1].
Figure 1.
Flow chart for commencing therapeutic hypothermia
Where cooling is advised, start active cooling as below:
Protocol for active cooling
Nurse the infant naked on a radiant warmer with the warmer switched off. Do not nurse in an incubator
Use cold packs from the refrigerator (around 10°C). Never use frozen packs
Cold packs should be wrapped in cotton or equivalent [Table 3]. They should never be applied directly to the skin
The cold packs can be placed under the shoulders/upper back, under the head, and/or across the chest/body. Use of a fan to continue cooling may be considered [Table 4]
If continuous rectal temperature monitoring is used, insert rectal thermostat/probe into the anus at 5 cm and fix it to the thigh. It is very important that the probe is inserted to this depth to accurately measure the core temperature
Connect rectal probe to cable, temperature module, and monitor
Set temperature alarm limits at 33.5°C (low) and 34.5°C (high) during cooling
Record time of initiating active cooling and monitor rectal temperatures every 15 min
Although not ideal, if intermittent axillary temperature measurements are used, then ensure that observations are taken at least every 15 min
If rectal or axillary temperature drops below 34.5°C, remove all the cool packs and reassess temperature in 15 min. If the temperature continues to fall, set radiant warmer on manual and gradually adjust the heater output to maintain the temperature at 33.5-34.5°C
The aim is to achieve the target temperature range within 1 h, but more importantly, continue to manage airway, breathing, and circulation
If nursed using head box oxygen, do not humidify or warm the air/oxygen gas mixture
If ventilated, use normal humidifier settings
Advise/reassure parents about the baby's appearance and that he/she will feel cool to touch.
Table 3.
Temperature algorithm – Aim for 33 – 34.5°C
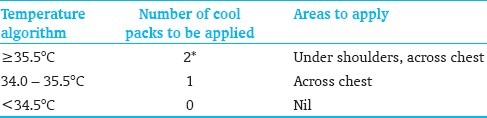
Table 4.
Cooling and rewarming procedures
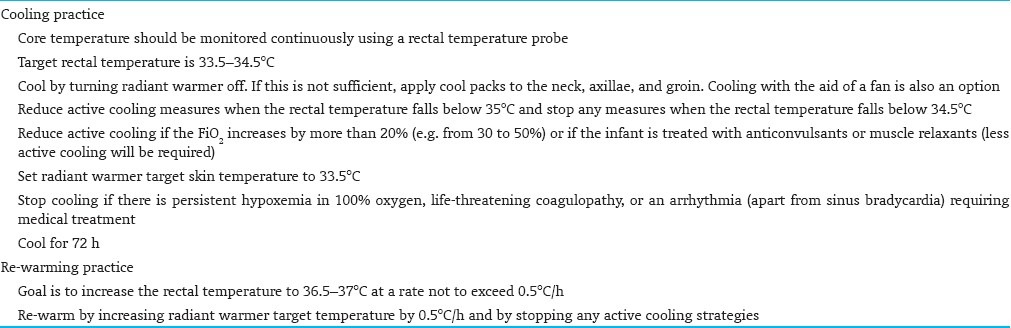
Reduce active cooling measures (cool packs) in case of the following [Table 4]:
The rectal temperature falls below 35°C
Significant FiO2 requirement, defined as: increment by more than 20% (e.g. from 30 to 50%) after the initiation of procedure. Other pathologies should be excluded first
The infant is treated with anticonvulsants or muscle relaxants
Stop active cooling measures when:
the rectal temperature falls below 34.5°C
the infant has persistent hypoxemia on 100% oxygen
there is life-threatening coagulopathy
there is an arrhythmia requiring medical treatment. Use re-warming procedure
Monitor temperature every 30 min after stopping active cooling until it reaches 35°C.
Resume active cooling when the temperature reaches 35°C and the patient is clinically stable.
For monitoring during cooling phases, [Table 5].
Table 5.
Monitoring during cooling and re-warming practices

What to expect during body cooling?
-
a)
Decreased HR
-
b)
Increased blood pressure (BP) initially due to increase in peripheral vasoconstriction
-
c)
Increase in urine output initially
-
d)
Decrease in magnesium, sodium, and potassium
-
e)
Labile glucose levels due to relative insulin resistance, decreased metabolic rate, and shivering
The total period of cooling will be 72 h. Upon completion of 72 h, infants will be gradually re-warmed over at least 4 h.
SPECIFIC SUPPORTIVE TREATMENT DURING HYPOTHERMIA
Respiratory support
Most infant will require assisted ventilation. Keep Oxygen and carbon dioxide within normal ranges Hypocarbia due to decrease Co2 producation induced by hypothermia could worsen the outcome (because of cerebral vasoconstriction) and should be avoided.
Although persistent pulmonary hypertension of the newborn (PPHN) was used as an exclusion criterion for all trials entry, pooled analysis from many trials including the 3 randomised controlled studies (CoolCap, National Institute of Child Health and Human Development (NICHD) and total body hypothermia for neonatal and encephalopathy (TOBY)) found no difference in the prevalence of PPHN between the Normothemic and hypothermic groups, in addition hypothermia would not worsen nor induce PPHN and therefore it should not be considered as contraindication for therapeutic hypothermia
Keep oxygen saturations between 92 and 98% as cooled infants are at risk of PPHN. Use humidified and heated gas as normal. More frequent suction with saline and regular re-positioning might be necessary as secretions tend to be more “sticky” when cold.
Positioning and skin care
Change the position every 6 h during care: flat- supine, right or left side to avoid pressure sores on cold edematous skin. Cyanosis of the hands and feet is common and usually transient.
Cardiovascular support
Hypothermia usually cause asymptomatic sinus bradycardia without cardiac dysfunction. At 33.5°C, the average HR is ~80-100 beats per minute (bpm). HR changes by 15 bpm per 1°C change in temperature.
Assess cardiac function clinically (BP, HR, and skin perfusion) and echocardiographically which also helpful to assess the degree of cardiac filling especially during inotropic or volume support. Hypothermic infants are at risk of hypovolumia as cold can induce water displacement into tissues. If inotropic support is required, the following regime is suggested:
Start with dopamine up to 10 μg/kg/min
If still hypotensive or ill perfuse, add dobutamine up to 10 μg/kg/min
If further support is required at this stage, start hydrocortisone or dexamethason
Fluid and electrolyte management
Start with 50-60 ml/kg/day and adjust according to the urine output and insensible water loss. Renal function is commonly impaired after Hypoxia ,therefore measurements of creatinine, electrolytes, daily weight and urine output will guide fluid management.insert urinary catheter to measure urine output more accurately hypothermic infants may need more volume due to redistribution of fluid into the tissues and increased diueresis induced by hypothermia.
Sodium and chloride
Check sodium and chloride levels which could fall duet o increased renal loss in hypothermia and replace deficits as required watch for hypernatremia during the re-warming phase.
Magnesium
Adult data suggest that higher levels (>1 mmol/l) of magnesium are of benefit for neuroprotection and to prevent excess shivering during cooling. Early neonatal data are encouraging but not conclusive. We recommend maintaining magnesium levels more than 0.8 mmol/l.
Calcium
In general hypothermia does not affect calcium levels.
Glucose
Measure blood glucose regularly and especially when fluid restricted.
Feeding
Due to an increased risk of necrotizing enterocolitis, feeds should be withholded and introduced cautiously at the rewarming phase and Total parenteral nutrition (TPN) should be commenced till full enteral intake is established.
Sedation
For non-ventilated babies who appear distressed should receive sedation as there is experimental evidence that stress could negates the neuroprotective effects of hypothermia.
Start treatment using a low dose of morphine and watch for oversedation due to possible accumulation, especially if phenobarbitone has been given.
For ventilated babies, the following should be followed: Give a loading dose of morphine. then start an infusion at a rate of 10-20 μg/kg/h. Consider early weaning after 12 h. At 48 h, discontinuation of morphine should be considered to reduce the risk of accumulation and toxicity. Morphine should be made up in 10% dextrose to avoid hypoglycemia.
Coagulation
In general threpuatic hypothermia (33.5°C - 34.5°C.) cause mild derangement of blood viscosity and coagulation (~30% prolongation of the international normalized ratio INR) which require no treatment if bleeding is suspected however a rapid treatment with fresh frozen plasma should be ordered while waiting for clotting results.
Drugs
Any drug metabolized by the liver has prolonged metabolism in hypothermia, especially phenobarbitone (half-life is 300 h vs. 100 h in non-cooled babies). Avoid continuous infusions of paralytic agents or anticonvulsants where possible, using boluses instead.
RE-WARMING PROCEDURE
After completion of 72 h of total body cooling, the goal is to increase the rectal temperature to 36.5-37°C at a rate not to exceed 0.5°C per hour. Re-warm by stopping any active cooling strategies and increasing the incubator temperature by 1°C per hour or increasing the radiant heat source temperature setting by 0.5°C per hour [Table 4].
The final temperature goal is 36.5°C and should take about 7 h to achieve.
Monitoring during re-warming phases [Table 5]
Record rectal temperatures every 30 min until temperature goal is achieved
Record HR, respiratory rate (RR), oxygen saturation, and BP (every 15 min for the first 2 h, then hourly until re-warmed)
Document vital signs every 3 h once the temperature goal is achieved and continue rectal temperature monitoring for another 24 h
-
Obtain the following laboratory results once the temperature goal is achieved:
-
a)Glucose
-
b)Coagulation profile (INR, PTT, fibrinogen)
-
c)Complete blood counts (CBC)
-
d)Arterial blood gas (ABG )with lactate
-
a)
What to expect during re-warming?
-
a)
Increase in HR
-
b)
Decrease in BP due to decrease in peripheral vascular resistance
-
c)
Decrease in urine output
-
d)
Electrolyte imbalance, as renal clearance rates change
FOR FURTHER READING
Perlman JM, Davis P, Wyllie J, Kattwinkel J. Therapeutic hypothermia following intrapartum hypoxia-ischemia. An advisory statement from the Neonatal Task Force of the International Liaison Committee on Resuscitation. Resuscitation 2010;81:1459-61.
Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349-58.
Anderson M, Longhofer TA, Phillips WM, Ray DE. Passive cooling to initiate hypothermia for transported encephalopathic newborns. J Perinatol 2007; 27:592-3.
Rutherford M, Azzopardi D, Whitelaw A, Cowan F, Renowden S,Edwards AD, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics 2005;116:1001-6.
Shankaran S, Laptook AR, Ehrencranz RA, Tyson JE, McDonald SA,Donovan EF, et al. National Institute of Child Health and Development Neonatal Research Network . Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. New Engl J Med 2005;353:1574-84.
Zanelli S, Naylor M, Dobbins N, Quigg M, Goodkin HP, Matsumoto JA, et al. Implementation of a ′Hypothermia for HIE′ program: 2-year experience in a single NICU. J Perinatol 2008;28:171-5.
Fairchild K, Sokora D, Scott J, Zanelli S. Therapeutic hypothermia on neonatal transport: 4-year experience in a single NICU. J Perinatol 2008;30:324-9.
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomized trial. Lancet 2005;365:663-70.
Evans N. Royal Prince Albert Newborn Care Guidelines. Moderate systemic hypothermia for neonatal hypoxic ischaemic encephalopathy (HIE). Sydney, Australia: New South Wales Government, 2007. Available from: http://www.sswahs.nsw.gov.au/rpa/neonatal/html/docs/hypothermia.pdf [Last accessed on 2011 Mar 14].
Whole Body Cooling. McMaster University Hospital Neonatal Guidelines. Hamilton, Ontario, Canada: McMaster University Hospital 2007.
Jackson A. Greater Glasgow and Clyde Neonatal Guidelines. Therapeutic hypothermia for hypoxic-ischaemic encephalopathy - cooling centre guidelines. Scotland: NHS Greater Glasgow and Clyde, 2010. Available from: http://www.clinicalguidelines.scot.nhs.uk/PD%20guidelines/GGC_Cooling_Neonates.pdf
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


