Abstract
The human GT198 gene (gene symbol PSMC3IP) is located at chromosome 17q21, 470 kb proximal to BRCA1, a locus previously linked to breast and ovarian cancer predisposition. Its protein product (also known as TBPIP and Hop2) has been shown to regulate steroid hormone receptor–mediated gene activation and to stimulate homologous recombination in DNA repair. Here, we screened germline mutations in GT198 in familial and early-onset breast and ovarian cancer patients. We have identified 8 germline variants in a total of 212 index patients including reoccurring nonsense mutation c.310C>T (p.Q104X) and 5′ UTR mutation c.-37A>T, each found in 2 unrelated families. Most identified index patients from cancer families had early onsets with a median age of 35 years. c.310C>T was absent in a total of 564 control individuals analyzed. GT198 gene amplification with an imbalanced mutant copy gain was identified in the blood DNA of one of the patients carrying c.310C>T. When tested, this truncating mutation abolished DNA damage–induced Rad51 foci formation. In addition, we have identified 15 somatic mutations in 2 tumors from 1 patient carrying germline mutation c.-37A>T. The presence of a somatic mutation on the wild-type allele showed that GT198 was biallelically mutated in the tumor. The somatic mutations identified near a splicing junction site caused defective alternative splicing and truncated the open reading frame. Therefore, distinct mutations may cause a similar consequence by truncating the full-length protein and inducing a loss of the wild type. Our study provides the first evidence of the presence of inactivating mutations in GT198 in familial and early-onset breast and ovarian cancer patients. Mutations in GT198, a gene regulating DNA repair, potentially contribute to an increased risk in familial breast and ovarian cancers.
Keywords: GT198, mutation, gene amplification, breast and ovarian cancer
Introduction
Breast and ovarian cancer susceptibility genes identified to date account for less than half of the hereditary breast and ovarian cancers, leaving additional candidate genes to be identified.1,2 BRCA1 and BRCA2 have been shown to regulate homologous recombination and participate in DNA double-strand break repair.3,4 The finding of germline mutations in the BRCA2-binding protein gene PALB2,5,6 in RAD51C,7,8 and in RAD51D9 in familial breast and ovarian cancers has also supported a close link between breast and ovarian cancer genes and DNA recombination pathways. In addition, while early studies of familial breast and ovarian cancers showed a linkage of Mendelian hereditary predisposition to the chromosome 17q21 locus,10,11 BRCA1 mutations were detected only in a proportion of families that were linked to 17q21.12 It is therefore possible that an additional unidentified gene is located close to BRCA1. During refined locus mapping near BRCA1 at 17q21, GT198 (gene symbol PSMC3IP, encoding a protein known as GT198, TBPIP, or Hop2) was initially identified as a cDNA clone.13 The GT198 protein was later characterized as a nuclear receptor co-regulator that regulates estrogen, androgen, and progesterone receptor–mediated gene activation.14,15 GT198 has also been extensively shown to be involved in DNA recombination pathways16 and to stimulate Rad51-mediated DNA strand exchange.17-19 GT198 was reported to possess DNA recombinase activity, an activity present in Rad51 homologs.20 A germline deletion in GT198 was recently identified in a diseased family of ovarian dysgenesis, supporting its critical role in ovarian development.21
GT198 mutations in breast and ovarian cancers have not been previously reported. In this study, we screened germline mutations in GT198 in familial and early-onset breast and ovarian cancers and have identified 8 heterozygous germline variants in a total of 212 index patients. We subsequently focused our study on 2 mutations, c.310C>T and c.-37A>T; both occurred in 2 unrelated families. We have identified gene amplification in blood DNA with an imbalanced gain of mutant copies carrying c.310C>T. The truncated protein displayed dominant-negative activity, thereby suppressing the wild type. We have identified 15 somatic mutations in tumors from a patient carrying c.-37A>T. The somatic mutations induced defective alternative splicing, resulting in truncation of the open reading frame. This is the first report that describes inactivating mutations in GT198 in familial and early-onset breast and ovarian cancers.
Results
Germline mutations in GT198
To screen for germline mutations in GT198, we performed Sanger sequencing using blood-derived genomic DNA in 199 unrelated index patients from cancer families, including 179 breast cancer families and 20 ovarian cancer families, that had been tested negative for the BRCA1/BRCA2 mutation. In addition, 13 early-onset (≤35 years) breast cancer individuals, without a family history and negative for the BRCA1/BRCA2 mutation, were also included because early-onset breast cancer is potentially hereditary.
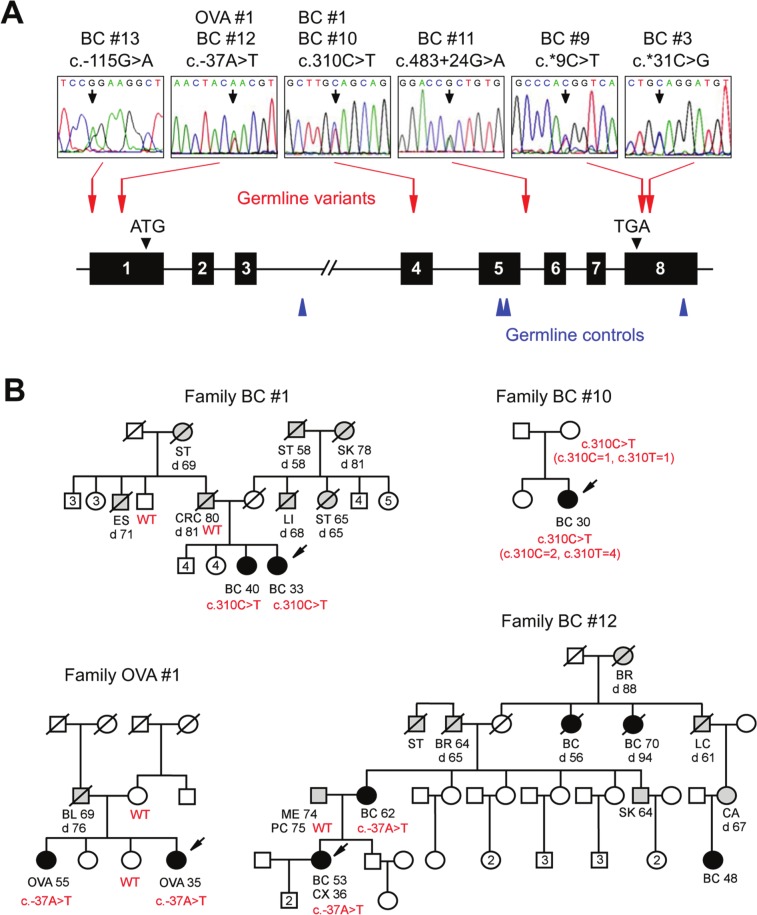
The human GT198 gene spans 5.5 kb and contains 8 exons (Fig. 1A). The entire GT198 gene was analyzed including exons, small introns, and splice junctions, except for the large intron 3. Eight index individuals were found to carry heterozygous variants in GT198. These included 6 breast cancer families (6/179), 1 early-onset breast cancer individual (1/13), and 1 ovarian cancer family (1/20) (Fig. 1B, Table 1, and Suppl. Fig. S1). c.310C>T (p.Q104X) and c.-37A>T were each identified in 2 unrelated index patients (Fig. 1B). Most identified familial patients carrying GT198 germline variants appeared to have early onsets with a median age of 35 years; however, the median age of onset was 46 years in the breast cancer family cohort and 47 years in the ovarian cancer family cohort. Several known single-nucleotide polymorphisms (SNPs) have also been detected in cancer families, and none of the identified variants were present in 94 ethnically matched control individuals (Suppl. Tables S1-3). The variant frequencies in controls were further evaluated using SNP databases including dbSNP, HapMap, Exome Variant Server, and USCS genome browser. We found that 3 germline variants, c.-115G>A, c.310C>T, and c.*31C>G, are completely absent in the population, and the other 3, c.-37A>T, c.483+24G>A, and c.*9C>T, are present at low frequencies in the Exome Variant Server database, which contains diseased but noncancerous individuals (Suppl. Table S3). Some rare variants may have pathogenic potential,22 which has been observed in cancer genes.23,24 To evaluate the functional impact, we focused our subsequent studies on 2 reoccurring mutations, c.310C>T and c.-37A>T. In summary, our data suggest that germline variants in GT198 are present at a low frequency in familial and early-onset breast and ovarian cancers.
Figure 1.
GT198 germline variants in familial and early-onset breast and ovarian cancers. (A) Schematic diagram of the human GT198 gene (not to scale). Introns are lines; 8 exons are numbered boxes. Translation start and stop codons are indicated by filled triangles. Colored arrows indicate the sites of germline mutations (red) and germline variations in controls (blue). Sequence traces of germline mutations are shown above. (B) Selected pedigrees with germline variants in GT198. Arrows indicate indexed patients. Genotypes are shown in red. Breast and ovarian cancer patients are filled, and other types of cancer are shaded. Numbers inside indicate additional healthy siblings. Slash symbols denote the deceased. Cancer types, age of diagnosis, and age of death (d) are shown when available. BC = breast cancer; OVA = ovarian cancer; CRC = colorectal cancer; ST = stomach cancer; SK = skin cancer; LI = liver cancer; ES = esophagus cancer; PC = prostate cancer; LC = lung cancer; BL = bladder cancer; ME = melanoma; BR = brain tumor; CX = cervical cancer; CA = cancer with unknown origin. Additional breast cancer pedigrees are shown in Supplementary Figure S1.
Table 1.
GT198 Germline Variants Identified in Breast and Ovarian Cancer Families and in Early-Onset Breast Cancer
| Family ID | Individual | Onset age, y | Nucleotide change | Protein change | Location | Frequency in control | Frequency in patient | Pathology diagnosis |
|---|---|---|---|---|---|---|---|---|
| BC #1 | BC33 | 33 | c.310C>T | Q104X | Exon 4 | 0/564 | 2/212 | BC ductal carcinoma (ER−PR−Her2−) |
| BC40 sister | 40 | c.310C>T | Q104X | Exon 4 | 0/564 | 2/212 | BC ductal carcinoma (ER−PR−Her2−) | |
| CRC80 father | 80 | WT | — | — | — | — | Colon cancer | |
| Uncle | — | WT | — | — | — | — | — | |
| BC #3 | BC35 | 35 | c.*31C>G | No | 3′ UTR | 0/94 | 1/212 | BC ductal carcinoma (ER−PR−Her2−) |
| BC #9 | BC35 | 35 | c.*9C>T | No | 3′ UTR | 0/94 | 1/212 | BC ductal carcinoma (ER+PR−Her2−) |
| BC #10 | BC30 | 30 | c.310C>T | Q104X | Exon 4 | 0/564 | 2/212 | BC ductal carcinoma (ER+PR+Her2−) |
| Mother | — | c.310C>T | Q104X | Exon 4 | 0/564 | 2/212 | — | |
| BC #11 | BC30 | 30 | c.483+ 24G>A | No | Intron 5 | 0/94 | 1/212 | BC ductal carcinoma (ER+PR−Her2−) |
| BC #12 | BC53 | 53 | c.-37A>T | No | 5′ UTR | 0/94 | 2/212 | BC lobular carcinoma (ER+PR+Her2−) |
| BC62 mother | 62 | c.-37A>T | No | 5′ UTR | 0/94 | 2/212 | BC ductal carcinoma | |
| PC75 father | 75 | WT | — | — | — | — | Prostate cancer, melanoma | |
| BC #13 | BC41 | 41 | c.-115G>A | No | 5′ UTR | 0/94 | 1/212 | BC ductal carcinoma (ER+PR+Her2−) |
| OVA #1 | OVA35 | 35 | c.-37A>T | No | 5′ UTR | 0/94 | 2/212 | OVA serous papillary carcinoma |
| OVA55 sister | 55 | c.-37A>T | No | 5′ UTR | 0/94 | 2/212 | OVA clear cell carcinoma, endometrial and peritoneal cancer, endometriosis | |
| Mother | — | WT | — | — | — | — | — | |
| Sister | — | WT | — | — | — | — | — |
Note: A total of 179 breast cancer (BC) families, 20 ovarian cancer (OVA) families, 13 early-onset breast cancers (onset age ≤35 years, without family history), and 94 controls were analyzed in the GT198 gene using blood genomic DNA. A total of 564 control individuals were analyzed at the nucleotide c.310C. Dashes = not applicable. BC #10 was identified from early-onset breast cancers, OVA #1 from ovarian cancer families, and the rest from breast cancer families.
GT198 gene amplification with an imbalanced mutant copy gain
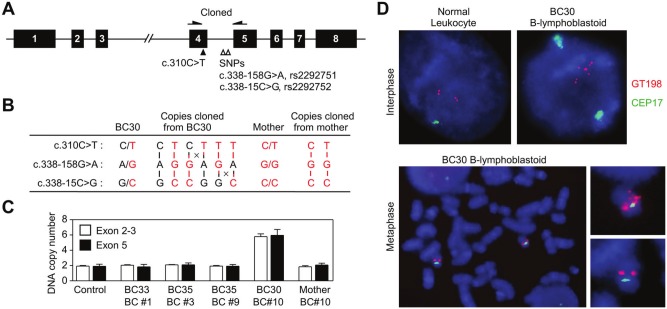
A heterozygous nonsense mutation c.310C>T (p.Q104X) in exon 4 was found in 2 unrelated breast cancer patients (Fig. 1B). The mutation was absent in a total of 564 ethnically matched control individuals analyzed at the location of c.310C, suggesting its potential association with breast cancer risk. The 2 sisters in family BC #1 were affected at 33 and 40 years of age. Their father and uncle are wild type, suggesting that their mother is an unaffected carrier. Patient BC30 with breast cancer diagnosed at age 30 years in family BC #10 was selected on the basis of early onset and carried the same c.310C>T mutation. Her mother is also an unaffected carrier. There are 2 linked common SNPs in intron 4 (c.378-158G>A, rs2292751 and c.338-15C>G, rs2292752), located in close vicinity to c.310C>T in exon 4 (Fig. 2A). To examine the genotype difference of the mutant alleles from the 2 families, we took advantage of these SNPs by cloning PCR fragments amplified from blood genomic DNA into plasmid vectors, followed by sequencing of individually cloned alleles. The results suggested that the mutant alleles from both families carried the same SNP genotypes G (rs2292751) and C (rs2292752). Thus, whether the 2 families acquired the mutation independently or had the same ancestry is currently undetermined. Unexpectedly, while patient BC30 from family BC #10 has SNP genotypes A/G and G/C, a total of 6 different copies of the gene were cloned from her blood DNA sample, including 2 wild-type and 4 mutant copies distinguished by a combination of SNPs (Fig. 2B). The haplotype details are not yet determined. However, gene duplication until amplification should have occurred with DNA recombination between c.310C>T and 2 SNPs and also between each SNP, resulting in unusual SNP genotypes G and G or A and C (Fig. 2B). The unusual SNP genotypes were absent in all other individuals analyzed. The copy number gain was confirmed by quantitative real-time PCR analysis using blood DNA samples, showing that daughter BC30 but not the mother has 6 copies of the GT198 gene (Fig. 2C). We further performed fluorescent in situ hybridization (FISH) analysis using a GT198 bacterial artificial chromosome (BAC) probe on immortalized B-lymphoblastoid cells from patient BC30. Extra signals on interphase chromosomes representing additional copies were identified when compared to normal leukocytes (Fig. 2D). These extra copies are distant from the original ones, indicating gene translocation to distant locations. On metaphase chromosomes, FISH results showed extra signals on one copy of chromosome 17 but not the other (Fig. 2D), indicating gene amplification within 1 chromosome 17. Together with cloning data, it appears that gene amplification in conjunction with DNA recombination resulted in an imbalanced gain of mutant copies, which were translocated outside the original gene context. In conclusion, we identified a mutant copy gain of the GT198 gene in germline DNA of a breast cancer patient.
Figure 2.
GT198 gene amplification with an imbalanced mutant copy gain. (A) Diagram illustrates the GT198 gene with a cloned region harboring c.310C>T and 2 common SNPs. (B) Two wild-type c.310C and 4 mutant c.310T copies were identified by cloning blood DNA from index patient BC30 of family BC #10. Genotypes in maternal copies are shown in red, and genotypes in paternal copies are shown in black. The predicted locations of DNA recombination are denoted by crosses. (C) Quantitative real-time PCR analysis of copy number changes using blood DNA in both the 5′ (exons 2 and 3) and 3′ (exon 5) ends of the GT198 gene. Normal human genomic DNA is used as a control. (D) FISH analysis of B-lymphoblastoid cells of index patient BC30 of family BC #10. Chromosome 17 centromere probe CEP17 is in green, GT198 BAC probe RP11-400F19 in red, and DAPI in blue. Normal human leukocyte is a control, showing 2 sets of doublet signals on sister chromatids in the interphase nucleus. Extra signals distant to 2 doublets are present in B-lymphoblastoid cells of patient BC30. In metaphase chromosomes, extra signals are present on a single copy of chromosome 17 with enlarged images shown at the right.
GT198 truncation abolishes Rad51-mediated DNA repair
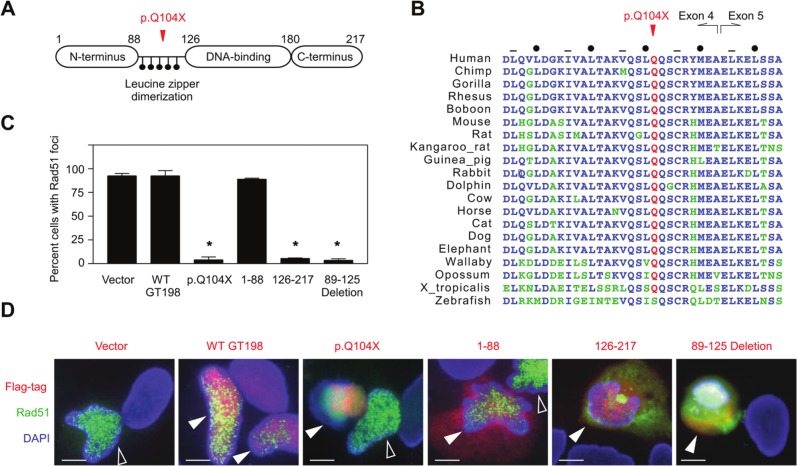
To understand why mutant copy gain would affect GT198 function, we analyzed GT198 protein activity induced by the c.310C>T (p.Q104X) truncation. GT198 encodes a small protein with 217 amino acids (Fig. 3A).14,19 The DNA binding domain binds both single- and double-stranded DNA and is essential for DNA repair activities.17,19 The leucine zipper domain is required for GT198 dimerization, protein-protein interactions, and transcriptional activity.14 The truncation at p.Q104X is within the leucine zipper encoded by exons 4 and 5. Protein sequence alignment among 20 vertebrate species showed highly conserved heptad leucine and hydrophobic residues (Fig. 3B), suggesting a conserved function of the leucine zipper. Truncation by p.Q104X within this domain is predicted to produce protein fragments unable to dimerize. We tested p.Q104X using a Rad51-mediated foci formation assay for DNA repair activity in which GFP-tagged Rad51 can form nuclear foci upon γ-irradiation during the DNA repair process. Foci formation serves as a readout to test proteins that influence Rad51-mediated DNA repair. Wild-type GT198, mutant p.Q104X encoded by full-length cDNA carrying c.310C>T, and GT198 protein fragments were co-transfected together with GFP-Rad51. Upon γ-irradiation, co-transfected cells were counted for the presence of foci, and representative images were examined. Compared to the vector control, wild-type GT198 did not disrupt Rad51 foci, while the p.Q104X mutant completely abolished Rad51 foci formation, causing diffused Rad51 expression (Fig. 3C and 3D). Abnormal Rad51 expression can also be seen in the cytoplasm in p.Q104X-transfected cells. The DNA-binding C-terminal fragment and the leucine zipper deletion, but not the N-terminus, abolished foci formation similar to the p.Q104X mutant (Fig. 3D). Phosphorylated histone H2AX (γH2AX) also forms foci during the repair of DNA damage. Diffuse staining rather than focal γH2AX signals was observed in the p.Q104X-transfected nucleus (Suppl. Fig. S2). These data suggest that the DNA binding domain, in the absence of dimerization, possibly competes with wild-type GT198, causing a potential dominant-negative effect. p.Q104X is capable of expressing a C-terminal protein fragment due to an internal ATG start codon located at amino acid 126 in exon 5. Indeed, the expression of a C-terminal Flag-tagged protein by p.Q104X can be detected by fluorescent staining (Fig. 3D). Nonetheless, the p.Q104X truncation did not merely result in a functional loss but exerted a strong inhibitory effect in DNA repair. These results provide a plausible explanation for the observed mutant copy gain. While a wild-type allele is present, a mutant allele could moderately inhibit the wild type in an unaffected mother. In a daughter, gene amplification with 4 mutant copies would lead to stronger inhibition of the wild-type protein and potentially increase the risk of cancer.
Figure 3.
Truncating mutation c.310C>T (p.Q104X) in GT198 impaired Rad51-mediated foci formation during DNA repair. (A) Schematic diagram of GT198 protein domain structures. Numbers indicate amino acids. Leucine residues in the leucine zipper domain are depicted as black dots. (B) Protein sequence alignment of 20 GT198 vertebrate homologs in the leucine zipper domain. Amino acid residues identical to humans are shown in blue, nonidentical in green, and p.Q104 in red. The leucine zipper domain is encoded by exons 4 and 5 as indicated. Dots and hyphens denote conserved heptad leucine and hydrophobic residues, respectively.14 (C, D) HeLa cells were co-transfected with GFP-Rad51 and Flag-tagged GT198 plasmids encoding wild-type GT198, the p.Q104X mutant, the N-terminus (1-88), the DNA-binding C-terminus (126-217), and the leucine zipper deletion (89-125 deletion) as indicated. Cells were γ-irradiated before foci-positive nuclei were counted in >200 cells for double-transfected cells (green foci in red nuclei), except in the empty vector control (green only). Nuclei were counterstained with DAPI. Data in the graph are shown as the mean ± SEM of triplicate experiments (n = 3). *P < 0.05 by the Student t test. In p.Q104X, the foci were disrupted in the co-transfected nucleus but not in the adjacent nucleus with Rad51 only. Open arrows indicate GFP-Rad51–transfected nuclei only. Filled arrows indicate double-transfected nuclei.
Somatic splicing mutations in GT198 truncate the open reading frame
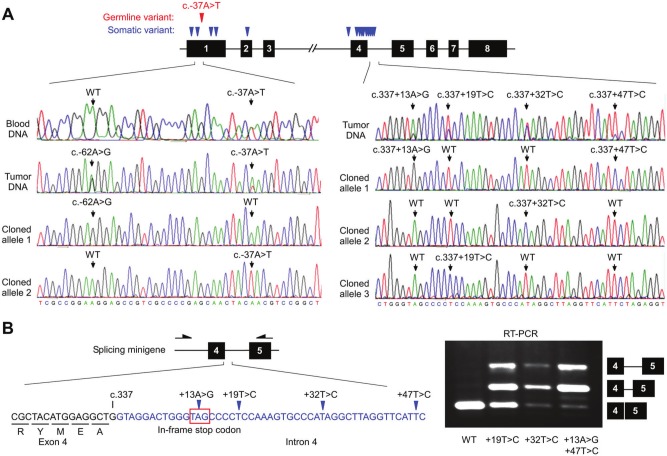
The germline variant c.-37A>T in the 5′ untranslated region (UTR) also occurred in 2 families, BC #12 and OVA #1 (Fig. 1B). c.-37A>T appeared to segregate with breast or ovarian cancer in both families (Fig. 1B). For the remaining 4 breast cancer families with other rare variants, segregation analysis was not attempted due to a lack of samples from family members. However, those index patients have very early onsets (Suppl. Fig. S1). Both patients OVA35 and OVA55 from family OVA #1 underwent 2 surgeries of hysterectomy and/or salpingo-oophorectomy (Suppl. Fig. S3). When verifying the germline mutation in tumors, we unexpectedly found a total of 15 somatic GT198 mutations in patient OVA55 in her 2 tumor samples that were available, including an endometrial tumor and peritoneal tumor in the omentum (Fig. 4, Suppl. Fig. S4, and Suppl. Table S4). These somatic mutations were absent from her blood DNA. The sequencing results were repeated and verified when tested together with a negative control tumor from her sister, OVA35, who carried only a germline variant c.-37A>T. Several somatic mutations are located in the 5′ UTR near or within the predicted transcription factor binding sites (Suppl. Fig. S4A). One of them, c.-62A>G, is present on the wild-type allele (Fig. 4A), showing the presence of biallelic GT198 changes in the tumor. In addition, more than 2 distinct mutant alleles were identified in 1 sample, suggesting that multiple clonal somatic changes in GT198 may be present in the tumor (Fig. 4A). Near the splice junction site of exon 4, there are 4 densely located mutations in intron 4 (Fig. 4B). The entire intron 4 encodes 12 in-frame stop codons. We speculated that defective splicing, resulting in intron 4 inclusion, would therefore cause functional loss by truncating wild-type GT198. To test this idea, we constructed splicing minigenes carrying mutated genomic sequences, encompassing exon 4 to exon 8. RT-PCR analysis of vector-expressed transcripts indeed detected defective splicing products, with partial intron 4 retention in mutants but not in the wild type (Fig. 4B). Noticeably, double mutations yielded relatively more incompletely spliced transcripts than single mutations. The data indicate that the somatic mutations in intron 4 are able to truncate the wild type. These genetic damages are likely the consequence of functional selection since they truncate the full-length transcript at the leucine zipper domain similar to the c.310C>T mutation. Our data may provide an explanation for the requirement of multiple, rather than single, genetic changes in tumors since a stronger splicing defect can be induced when multiple changes are present near the splice junction.
Figure 4.
Somatic GT198 mutations truncate the open reading frame through defective intron splicing. (A) The diagram at the top denotes somatic GT198 mutations in blue and germline mutations in red. Sequence traces of the 5′ UTR from blood and tumor DNA are shown for comparison at the left. A somatic mutation, c.-62A>G, is on one allele and germline c.-37A>T on the other, showing the presence of biallelic changes. Densely located intron 4 mutations in cloned alleles are shown at the right. A wild-type allele was also identified but is not shown. Multiple mutant alleles suggest the presence of clonal somatic changes. (B) Intron 4 contains a total of 12 in-frame stop codons, and 1 TAG is shown near the splice junction in intron 4, containing 4 somatic mutations. Exon 4 is shown in black and intron 4 in blue. The 3 cloned mutant sequences were transfected in HeLa cells using the wild type as a control. RT-PCR was carried out utilizing a vector-specific primer to detect minigene transcripts, which were analyzed by sequencing to confirm the presence of partial retentions of intron 4 in mutants.
Discussion
In this study, we have shown that GT198 germline and somatic mutations are present in familial and early-onset breast and ovarian cancer cases. Our data collectively suggest that the tested inactivating mutations truncate the wild-type protein and induce functional loss. It has been proposed that variants in DNA repair genes with low frequencies, such as in ATM, BRIP1, PALB2, and CHEK2, may have a higher risk than estimated in breast cancer patients with a strong family history and with early onsets.25 GT198 germline mutations appear to have a low frequency but are associated with patients with early onsets. Of 7 index patients with a family history that we identified, 5 have onset ages of ≤35 years (Table 1). This phenomenon implies that GT198 potentially belongs to the same group as one of the DNA repair genes. We did not perform statistical analysis for case-control comparison due to the relatively small number of samples analyzed and also due to the low mutation frequencies detected. However, analysis of existing SNP databases, which contain a large number of controls, has confirmed that identified GT198 germline variants are either completely absent from the databases or have rather low frequencies (Suppl. Table S3). Because the risk level can be mutation specific, the impact of rare mutations on cancer should be evaluated by functional studies, which may provide stronger evidence than statistical analysis alone. A better estimate of the overall risk of the GT198 gene in familial breast and ovarian cancers may require a much larger scale genetic study in the future.
Gene amplification has been proposed to be initiated through a breakage-fusion-bridge cycle26 in which one daughter cell loses a copy of a gene while the other daughter cell gains an extra copy during mitosis. Continued cell cycles with asymmetrical distributions of multiplied genes to daughter cells lead to gene amplification in the surviving cell populations that have gained growth advantage. Gene amplification will subsequently promote DNA rearrangement.27 The organization of resulting amplified segments can be highly complex including the presence of truncation, inversion, and fusion of genes.27,28 Our data suggest that GT198 gene amplification, DNA recombination between 2 homologous chromosomes (paternal allele carries maternal SNPs and vice versa), and DNA rearrangement to a distant location are all present in peripheral blood DNA in index patient BC30 of family BC #10 (Fig. 2). We show (Peng et al., in this issue) that wild-type GT198 is upregulated during early stages of stem cell differentiation. Therefore, a cell carrying a mutant GT198 copy may have reduced the differentiation capacity, thereby gaining growth advantage. Potentially, initial duplication of the mutant gene is through the breakage-fusion-bridge cycle. The subsequent DNA recombination between duplicated mutant genes relies on fragile, amplified DNA segments. Even though an extra wild-type copy was also acquired, we speculate that the activity would not be compensated due to the presence of 4 mutant copies (Fig. 2), which were selected by growth advantage. It is currently unclear whether extensive DNA recombination is due to defective GT198, an altered DNA repair gene itself. The boundaries of the multiplied GT198 gene have not been determined, but neighboring genes could be affected due to the rearrangement of amplified DNA segments.
GT198 is located at the chromosome 17q21 locus 470 kb proximal to BRCA1 and 15 kb distal to HSD17B1, a well-studied linkage marker that encodes 17β-hydroxysteroid dehydrogenase. Previously, it was speculated that additional candidate genes may exist at the 17q21 locus near BRCA1.29 Our finding is consistent with early studies and further reinforces the link between this locus and breast and ovarian cancer predisposition. It is important to note that the chromosome 17q21 locus has frequent genomic rearrangements in breast cancer, and BRCA1 germline rearrangements are significantly present.30 GT198 contains 7 Alu or L2 repeats in its large intron 3 and additional 2 Alu repeats upstream of its promoter, a relatively high density of Alu repeats in view of its small gene size of 5.5 kb. GT198 could be prone to rearrangements. Furthermore, GT198 is 2.9 Mb distal to the HER2/neu gene at 17q12, which is often amplified in breast cancer. An additional 2 breast and ovarian cancer susceptibility genes, RAD51C at 17q25 and RAD51D at 17q11,7,9 are also located at the long arm of chromosome 17. The potential interrelationships among these genes in an aberrant chromosome 17, particularly in tumors, remain to be elucidated. Further studies on large-scale genomic rearrangements involving GT198 may provide a better understanding of breast and ovarian cancers.
The finding of somatic mutations was initially unexpected. Thus, identified somatic mutations were extensively validated through the following steps: First, the somatic mutations in patient OVA55 were absent in blood DNA from the same individual. Second, these mutations were absent in the SNP databases (Suppl. Table S3). Third, the somatic mutations were absent in the tumor from her sister OVA35, who carried only the germline mutation c.-37A>T. Finally, allelic mutations can be confirmed by cloning. In addition, identified somatic mutations are not randomly distributed but are more clustered in the 5′ UTR and in the exon 4–intron 4 junction. The clustering phenomenon implies a consequence of functional selection for these somatic mutations. Supporting our finding, 7 verified somatic mutations in GT198 are present in the COSMIC database (Suppl. Table S5). Several of them in exons 2 and 4 are in close vicinity to the mutations that we identified. Two exon 4 mutations are identified also in endometrial cancers (Suppl. Table S5). The presence of multiple somatic mutations in GT198 has also been investigated (Peng et al., in this issue), suggesting that alternative splicing plays a predominant role in regulating the GT198 gene activity, which is consistent with the current study on somatic mutations (Fig. 4).
Four of 5 identified nontruncating GT198 germline variants are located in the 5′ UTR and 3′ UTR (Fig. 1A). In the 5′ UTR, identified variants are located within or near predicted transcription factor binding sites and thus may interfere with the regulation of expression (Suppl. Fig. S4A). In the 3′ UTR, a predicted 22-nucleotide mature microRNA binding site (miR-301a) is located 11 base pairs downstream of c.*31C>G and 33 base pairs downstream of c.*9C>T. The 5′ seeding sequence of mature miR-301a contains 8 nucleotides with a perfect match to the GT198 3′ UTR. It has been suggested that unstable RNA structures are needed in the vicinity of microRNA binding sites31; we speculate that the 2 variants adjacent to the binding site may modify the RNA structure and alter microRNA regulation. In principle, carriers with GT198 germline mutations must survive through embryonic stages when GT198 is expressed.14 Consequently, germline mutations may not be as deleterious as somatic mutations. This explains the existence of germline variants with a mild functional effect. Our study is also in concordance with the “common disease–rare variant” hypothesis,32 which suggests that rare mutations in multiple genes in a pathway underlie the susceptibility to a common disease. BRCA1 regulates DNA recombinase, and GT198 possesses DNA recombinase activity itself.20 Consistently, germline mutations in recombinase genes RAD51C and RAD51D were found at low frequency in familial breast and ovarian cancers.8,9 Germline mutations in GT198 are also observed at low frequency in patients with early onsets. Multiple DNA repair genes can act in concert and together contribute to breast and ovarian cancer predisposition.33 Future studies on GT198 may provide additional insights into this important question.
Materials and Methods
Germline mutation analysis
Genomic DNA isolated from peripheral white blood cells was obtained from 179 familial breast cancer families, 13 early-onset breast cancer individuals, and 94 control individuals with a Dutch ethnic background. Samples were provided by the VU University Medical Center (Department of Clinical Genetics, Amsterdam, the Netherlands). The selection criteria for breast cancer families include breast cancer patients with 1 or more first- or second-degree relative(s) affected with breast cancer. Some families contain ovarian cancer cases in addition to breast cancer. Early-onset breast cancer patients were selected for an onset age of ≤35 years. All control individuals analyzed in this study were anonymized, nonaffected first-degree relatives of patients with a genetically confirmed diagnosis of a hereditary disease unrelated to cancer. Both breast cancer cases and controls had been selected based on Dutch family names, thereby ensuring that a large proportion of the cancer cases were from the same ethnic background as controls. The nonsense mutation c.310C>T was further screened in a total of 564 Dutch control individuals. The peripheral blood genomic DNA samples from 20 ovarian cancer families with a mixed ethnic background were provided by the Gilda Radner Familial Ovarian Cancer Registry (Roswell Park Cancer Institute, Buffalo, NY). The 20 index patients are affected by ovarian cancer. Among the 20 families, 19 families have 2 to 5 cases of ovarian cancer in blood relatives. One family has 1 ovarian cancer, 1 breast cancer, and 2 prostate cancer cases. Some families contain breast cancer cases in addition to ovarian cancer. BRCA1 and BRCA2 have been previously analyzed by Sanger sequencing of all exons and intron/exon boundaries and with MLPA performed for BRCA1 to obtain a BRCA1 and BRCA2 mutation-negative cohort. Germline mutations in GT198 were screened by Sanger sequencing of the entire gene (c.-131G to c.*316C) including its exons, small introns, and splicing junctions, except for the large intron 3 (c.225+160A to c.226-96G). The analyzed GT198 sequence regions were verified by BLAST and BLAT searches as unique sequences in the human genome. The hg19 coordinates of identified variants, SNPs, and sequencing primers (Suppl. Table S6) are shown in the supplementary material.
FISH analysis
FISH analysis on index patient BC30 of family BC #10 was carried out using an Epstein-Barr virus–immortalized B-lymphoblastoid cell line. Peripheral blood leukocyte from a normal donor was used as a control. Cultured cells were incubated with 0.1 µg/mL colcemid for 30 minutes before harvest. A BAC clone (RP11-400F19, 170 kb) (Children’s Hospital Oakland Research Institute, Oakland, CA) containing the GT198 gene, confirmed by PCR, was labeled with SpectrumRed dUTP (Nick translation kit, Vysis Inc., Des Plains, IL, USA) according to the manufacturer’s protocol to produce the FISH probe. SpectrumGreen dUTP–labeled chromosome 17 centromeric α satellite probe CEP17 (Vysis) was applied simultaneously for dual-color visualization. Cells were hybridized using Vysis reagents and counterstained with DAPI II before visualization with fluorescence microscopy.
Cloning and real-time PCR analyses
Genomic DNA from the blood of index patient BC30 and her mother from family BC #10 was PCR amplified. The PCR products containing c.310C>T and 2 SNPs (c.338-158G>A, rs2292751 and c.338-15C>G, rs2292752) were cloned into a pGEM-T Easy TA cloning vector (Promega, Fitchburg, WI). Multiple isolated clones were sequenced individually. Real-time PCR quantification of the GT198 copy number in blood genomic DNA was performed using SYBR Green dye in duplicate reactions (Mx3000P, Stratagene, La Jolla, CA). A standard curve for each primer pair was generated using serial, diluted normal genomic DNA (Clontech, Mountain View, CA). An unrelated thyroid hormone receptor α gene was used as an internal control for concentration normalization. In a 25-µL reaction, samples were denatured at 94°C for 5 minutes and amplified for 45 cycles (94°C for 45 seconds, 55°C for 45 seconds, 72°C for 1 minute) with data collected at each cycle. DNA concentration was calculated using standard curves.
Rad51 foci formation and immunofluorescence
HeLa cells were co-transfected with GFP-Rad51 (green) and Flag-tagged GT198 plasmids (red) encoding wild-type GT198, the p.Q104X mutant, the N-terminus (1-88), the DNA-binding C-terminus (126-217), and the leucine zipper deletion (89-125). GFP-Rad51 plasmid contains full-length Rad51 in the pEGFP-C3 vector (Clontech). Expression plasmid carrying c.310C>T cDNA was C-terminal Flag-tagged. Cells were γ-irradiated at 8 Gy and recovered for 4 hours before methanol-fixed for immunofluorescence. Immunofluorescence staining was carried out using mouse anti-Flag antibody (1:1,000) (Sigma, St. Louis, MO). Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) was applied at a dilution of 1:200. Sections were counterstained with DAPI. The foci-positive nuclei were counted in double-transfected cells (green foci in red nuclei), except in the empty vector control (green only). Data in Figure 3C are shown as the mean ± SEM of triplicate experiments (n = 3). Over 200 cells were counted in each experiment, and *P < 0.05 was obtained by the Student t test. The representative fluorescent images of Rad51 foci formation were taken containing nontransfected (blue), single (green), and double (red and green)–transfected cells.
Analysis of splicing mutations.
Three GT198 mutant sequences with alternative splicing capacity were constructed in a pcDNA3 vector using sequences containing the 3′ half of the GT198 gene (c.226-120T in intron 3 to c.664G in exon 8). The minigenes carried either single mutation c.337+19T>C, c.337+32T>C, or double mutations c.337+13A>G and c.337+47T>C. Wild-type minigene was used as a control. RT-PCR analysis in HeLa cells utilized a forward primer in the vector and a reverse primer in exon 5 to detect the minigene transcripts but not the endogenous transcripts. The vector-expressed splicing products were gel-purified and analyzed by sequencing to confirm the presence of aberrant splicing with partial retentions of intron 4.
Somatic mutation analysis
GT198 somatic mutations were initially identified during verification of germline mutations in ovarian cancer family OVA #1 using available formalin-fixed and paraffin-embedded tumor tissues. The sections were deparaffinized in xylene and 100% ethanol, and genomic DNA was isolated by the DNeasy Tissue Kit (Qiagen, Hilden, Germany). Allelic germline and somatic mutations were examined by cloning amplified PCR products into the pGEM-T Easy TA cloning vector (Promega) and by sequencing each clone individually.
Acknowledgments
The authors acknowledge the Gilda Radner Familial Ovarian Cancer Registry at Roswell Park Cancer Institute. They thank Drs. John K. Cowell, Hao Zhang, Patricia V. Schoenlein, Hernan Flores-Rozas, Shuang Huang, Dongjing Fu, and Dorothy Tuan for their help.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Lan Ko, the corresponding author, is the inventor of patent applications concerning potential diagnosis and drug development using the GT198 gene. No financial agreement or licensing has been involved.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article. This work is supported in part by the Georgia Cancer Coalition Distinguished Cancer Scholar Award (to L.K.), by CCA/V-ICI Amsterdam (to J.L.B.), and by the National Institutes of Health (CA062130 and CA132640 to N.F.M.).
Supplementary material for this article is available on the Genes & Cancer website at http://ganc.sagepub.com/supplemental.
References
- 1. Rahman N, Seal S, Thompson D, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007;39:165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nathanson KL, Wooster R, Weber BL. Breast cancer genetics: what we know and what we need. Nat Med. 2001;7:552-6 [DOI] [PubMed] [Google Scholar]
- 3. Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25:5864-74 [DOI] [PubMed] [Google Scholar]
- 4. Welcsh PL, Owens KN, King MC. Insights into the functions of BRCA1 and BRCA2. Trends Genet. 2000;16:69-74 [DOI] [PubMed] [Google Scholar]
- 5. Erkko H, Xia B, Nikkila J, et al. A recurrent mutation in PALB2 in Finnish cancer families. Nature. 2007;446:316-9 [DOI] [PubMed] [Google Scholar]
- 6. Xia B, Sheng Q, Nakanishi K, et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719-29 [DOI] [PubMed] [Google Scholar]
- 7. Akbari MR, Tonin P, Foulkes WD, et al. RAD51C germline mutations in breast and ovarian cancer patients. Breast Cancer Res. 2010;12: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meindl A, Hellebrand H, Wiek C, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42:410-4 [DOI] [PubMed] [Google Scholar]
- 9. Loveday C, Turnbull C, Ramsay E, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43:879-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall JM, Lee MK, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250:1684-9 [DOI] [PubMed] [Google Scholar]
- 11. Narod SA, Feunteun J, Lynch HT, et al. Familial breast-ovarian cancer locus on chromosome 17q12-q23. Lancet. 1991;338:82-3 [DOI] [PubMed] [Google Scholar]
- 12. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rommens JM, Durocher F, McArthur J, et al. Generation of a transcription map at the HSD17B locus centromeric to BRCA1 at 17q21. Genomics. 1995;28:530-42 [DOI] [PubMed] [Google Scholar]
- 14. Ko L, Cardona GR, Henrion-Caude A, Chin WW. Identification and characterization of a tissue-specific coactivator, GT198, that interacts with the DNA-binding domains of nuclear receptors. Mol Cell Biol. 2002;22:357-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satoh T, Ishizuka T, Tomaru T, et al. Tat-binding protein-1 (TBP-1), an ATPase of 19S regulatory particles of the 26S proteasome, enhances androgen receptor function in cooperation with TBP-1-interacting protein/Hop2. Endocrinology. 2009;150:3283-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petukhova GV, Romanienko PJ, Camerini-Otero RD. The Hop2 protein has a direct role in promoting interhomolog interactions during mouse meiosis. Dev Cell. 2003;5:927-36 [DOI] [PubMed] [Google Scholar]
- 17. Enomoto R, Kinebuchi T, Sato M, et al. Stimulation of DNA strand exchange by the human TBPIP/Hop2-Mnd1 complex. J Biol Chem. 2006;281:5575-81 [DOI] [PubMed] [Google Scholar]
- 18. Pezza RJ, Voloshin ON, Vanevski F, Camerini-Otero RD. Hop2/Mnd1 acts on two critical steps in Dmc1-promoted homologous pairing. Genes Dev. 2007;21:1758-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Enomoto R, Kinebuchi T, Sato M, et al. Positive role of the mammalian TBPIP/HOP2 protein in DMC1-mediated homologous pairing. J Biol Chem. 2004;279:35263-72 [DOI] [PubMed] [Google Scholar]
- 20. Petukhova GV, Pezza RJ, Vanevski F, et al. The Hop2 and Mnd1 proteins act in concert with Rad51 and Dmc1 in meiotic recombination. Nat Struct Mol Biol. 2005;12:449-53 [DOI] [PubMed] [Google Scholar]
- 21. Zangen D, Kaufman Y, Zeligson S, et al. XX ovarian dysgenesis is caused by a PSMC3IP/HOP2 mutation that abolishes coactivation of estrogen-driven transcription. Am J Hum Genet. 2011;89:572-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorlov IP, Gorlova OY, Frazier ML, Spitz MR, Amos CI. Evolutionary evidence of the effect of rare variants on disease etiology. Clin Genet. 2011;79:199-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frank B, Hemminki K, Wirtenberger M, et al. The rare ERBB2 variant Ile654Val is associated with an increased familial breast cancer risk. Carcinogenesis. 2005;26:643-7 [DOI] [PubMed] [Google Scholar]
- 24. Gudmundsson J, Sulem P, Gudbjartsson DF, et al. A study based on whole-genome sequencing yields a rare variant at 8q24 associated with prostate cancer. Nat Genet. 2012;44:1326-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Byrnes GB, Southey MC, Hopper JL. Are the so-called low penetrance breast cancer genes, ATM, BRIP1, PALB2 and CHEK2, high risk for women with strong family histories? Breast Cancer Res. 2008;10: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coquelle A, Pipiras E, Toledo F, Buttin G, Debatisse M. Expression of fragile sites triggers intrachromosomal mammalian gene amplification and sets boundaries to early amplicons. Cell. 1997;89:215-25 [DOI] [PubMed] [Google Scholar]
- 27. Coquelle A, Rozier L, Dutrillaux B, Debatisse M. Induction of multiple double-strand breaks within an hsr by meganucleaseI-SceI expression or fragile site activation leads to formation of double minutes and other chromosomal rearrangements. Oncogene. 2002; 21:7671-9 [DOI] [PubMed] [Google Scholar]
- 28. Storlazzi CT, Lonoce A, Guastadisegni MC, et al. Gene amplification as double minutes or homogeneously staining regions in solid tumors: origin and structure. Genome Res. 2010;20:1198-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vogelstein B, Kinzler KW. Has the breast cancer gene been found? Cell. 1994;79:1-3 [DOI] [PubMed] [Google Scholar]
- 30. Mazoyer S. Genomic rearrangements in the BRCA1 and BRCA2 genes. Hum Mutat. 2005;25:415-22 [DOI] [PubMed] [Google Scholar]
- 31. Bentwich I. Prediction and validation of microRNAs and their targets. FEBS Lett. 2005;579:5904-10 [DOI] [PubMed] [Google Scholar]
- 32. Schork NJ, Murray SS, Frazer KA, Topol EJ. Common vs. rare allele hypotheses for complex diseases. Curr Opin Genet Dev. 2009;19:212-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turnbull C, Rahman N. Genetic predisposition to breast cancer: past, present, and future. Annu Rev Genomics Hum Genet. 2008;9: 321-45 [DOI] [PubMed] [Google Scholar]