Abstract
We have developed a murine model of the Hematopoietic Syndrome of the Acute Radiation Syndrome (H-ARS) for efficacy testing of medical countermeasures (MCM) against radiation according to the FDA Animal Rule. Ten to 12 week old male and female C57BL/6 mice were exposed to the LD50/30-LD70/30 dose of total body irradiation (TBI, 137Cs, 0.62-0.67 Gy min-1) in the morning hours when mice were determined to be most radiosensitive, and assessed for 30 day survival and mean survival time (MST). Antibiotics were delivered in the drinking water on days 4-30 post-TBI at a concentration based on the amount of water that lethally-irradiated mice were found to consume. The fluoroquinolones, ciprofloxacin and levofloxacin, and the tetracycline doxycycline and aminoglycoside neomycin, all significantly increased MST of decedent mice, while ciprofloxacin (p=0.061) and doxycycline + neomycin (p=0.005) showed at least some efficacy to increase 30 day survival. Blood sampling (30uL/mouse every 5th day) was found to negatively impact 30 day survival. Histopathology of tissues harvested from non-moribund mice showed expected effects of lethal irradiation, while moribund mice were largely septicemic with a preponderance of enteric organisms. Kinetics of loss and recovery of peripheral blood cells in untreated mice and those treated with two MCM, granulocyte-colony stimulating factor and Amifostine, further characterized and validated our model for use in screening studies and pivotal efficacy studies of candidate MCM for licensure to treat irradiated individuals suffering from H-ARS.
INTRODUCTION
The increasing presence worldwide of radioactive material for therapeutic, energy, or weapon applications underscores the need for medical preparedness for effective treatment in the event of accidental or intentional radiation exposure. However, there are currently no medical countermeasures (MCM) approved to treat severely irradiated personnel. Physicians would likely rely on medications used to treat chemo- and radiotherapy-induced myelosuppression, such as granulopoietic cytokines, in addition to supportive care.
Efficacy and safety studies for new MCM will require the use of relevant, applicable and practical animal models adhering to the Food and Drug Administration’s (FDA) Animal Rule (AR). The AR was drafted in 2002 to meet the regulatory need for guidance in conducting studies when “adequate and well-controlled clinical studies in humans cannot be ethically conducted and field efficacy studies are not feasible” (Crawford 2002). T he FDA has also published a guidance document in January 2009, relative to use of the AR, entitled; “Guidance for Industry. Animal models – Essential Elements to Address Efficacy Under the Animal Rule” (http://www.fda.gov/cder/guidance/index.htm). These documents offer regulatory guidance for licensure of MCM to treat lethally-irradiated personnel based on demonstrated efficacy in appropriate animal models.
Since publication of the AR in 2002 and the Guidance document in 2009, efforts in our and other laboratories have focused on development of appropriate animal models for the Hematopoietic (H) Syndrome of the Acute Radiation Syndrome (H-ARS). Although low dose exposure of 1-2 Gy total-body irradiation (TBI) or less in humans is generally non-life threatening, exposures in the range of 2-10Gy affect primarily the hematopoietic system, resulting in H-ARS. H-ARS is characterized by life-threatening neutropenia and thrombocytopenia, and possible death due to infection and/or bleeding. The lethal radiation dose for 50% of humans at 60 days post-exposure (LD50/60) is estimated to be 3.5-4.5Gy (Lushbaugh 1969, Vriesendorp and Van Bekkum 1984), but can be significantly increased to ~6-7Gy when antibiotics and fluids are provided as supportive care (Dainiak 2002, Anno et al. 2003).
Mice have been selected as the animal model system for H-ARS in the current study for several reasons: 1) existence of a relatively large database utilizing mice in radiation studies that spans decades, 2) favorable logistics for housing and handling, 3) relatively economical, 4) nominal ethical considerations, 5) critical use for performing efficient screening protocols for candidate MCM, 6) efficient use for assessing MCM route, dose and schedule of administration prior to assessing in large animal models, 7) ease of performance of pharmacokinetic and pharmacodynamic studies of applicable MCM, 8) existence of well defined congenic strains, cross-bred strains (i.e. Diversity Outbred), and strains specific for lung injury that can be used to assess MCM with varying mechanism(s) of action in different sequelae of ARS (H-ARS and GI-ARS) relative to radiation effects, and 9) ability to investigate age-related radiation effects and mitigation by MCM. For these reasons, mice are the basic model to investigate MCM efficacy and all key criteria of the FDA AR.
The strain C57BL/6 was chosen for these studies due to their moderate degree of radiosensitivity compared to other strains (Kohn and Kallman 1956) and because their well-defined hematopoietic system allows performance of sophisticated hematopoietic stem cell (HSC) assays for mechanistic studies (such HSC studies are the focus of an accompanying paper in this issue by the Orschell group (Plett reference)). Furthermore, C57Bl/6 mice are one of two strains of mice recommended for testing of radiomitigators and radioprotectants by the 2008 Centers for Medical Countermeasures against Radiation Animal Models Workshop (Williams et al. 2010). All studies are performed on mice of the same age to avoid age-related changes in radiosensitivity (Grahn and Hamilton 1957, Grahn 1958, Yuhas and Storer 1967, Casarett 1968). Our murine model of H-ARS in C57Bl/6 mice has been extensively defined in this publication in terms of the radiation dose-response relationship (DRR) with and without supportive care, complete blood count (CBC) parameters, mean survival time of decedents, efficacy of several antibiotics, hydration requirements post TBI, chronosensitivity, histology and microbiology of various tissues post-exposure, effects of known radioprotectants and radiomitigators, and variables affecting radiosensitivity. All studies can be conducted under Good Laboratory Practices (GLP) when needed, as required by the FDA’s AR for GLP-compliant survival studies.
MATERIALS AND METHODS
Mice
Specific pathogen free (SPF) C57BL/6 mice (50/50 male/female; Jackson Laboratory, Bar Harbor, Maine) were received at 10 weeks of age, an age analogous to a “young adult” human. C57BL/6 mice exhibit a moderate degree of radiosensitivity compared to other strains (Kohn and Kallman 1956). Weights ranged from 15.0-21.5gm (females) and 19.0 28.0gm (males). Mice were identified by tail tattoo, ear punch, or tail marks. All studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Husbandry
Up to 5 mice per cage were housed in microisolator cages on sterilized direct contact bedding (Alpha Dri) and provided certified commercial extruded lab rodent chow (Harlan 2018SXC) ad libitum in cage hoppers and acidified water (pH 2.0-3.0) in sipper tube bottles. Autoclaved acidified water was provided on days 4-30 post-TBI in sipper tubes and wet feed. Animal rooms on a 12-hour light/dark cycle were maintained at 21±3°C with 30-80% relative humidity and at least 10 air changes per hour of 100% conditioned fresh air.
Irradiation and dosimetry
In the morning of study Day 0, mice were placed in single chambers of a Plexiglas irradiation apparatus and exposed to a single uniform total body dose of gamma radiation from a 137Cs radiation source (GammaCell 40; Nordion International, Kanata, Ontario, Canada) at an exposure rate of 0.63-0.68 Gy/minute. Each group of mice irradiated together was roughly divided among all treatment groups to ensure that each group received the same irradiation exposure conditions. Each exposure was confirmed using Inlight Dot dosimeters (Landauer Inc.) placed inside of a parafilm mouse phantom and irradiated along with the mice. Dosimeters were read using a validated Landauer microStar reader calibrated with standard Dot dosimeters exposed with a NIST-traceable 137Cs source (Battelle Memorial Institute, WA). Reproducibility of individual dots was 3±1% with accuracy of 4±2%, well within the 10% industry standard for experimental radiation dosimetry.
Antibiotics
In some studies (as detailed in Results), antibiotics were provided on days 4-30 post-exposure in autoclaved acidified water ad libitum from sipper tubes and also as wetted feed in Petri dishes set on the cage bottom. Antibiotic support consisted of either: 1) neomycin-treated water (2mg/mL) in sipper tubes and wet feed plus 0.0625% doxycycline chow in cage hoppers, 2) ciprofloxacin-treated water (0.67mg/mL) in sipper tubes and wet feed, or 3) levofloxacin-treated water (0.67mg/mL) in sipper tubes and wet feed. Controls received no antibiotics but did receive autoclaved acidified water and wet feed on days 4-30 post-exposure.
Health status monitoring
Irradiated mice were observed for morbidity or mortality twice daily by trained laboratory personnel and scored a scale of zero to three for signs meeting the criteria for early euthanasia based on three parameters: the severity of hunched posture, squinted/closed eyes, and decreased activity. Mice with scores of eight or nine underwent euthanasia by CO2 inhalation followed by cervical dislocation.
Complete blood counts (CBC)
Mice were restrained and the tail anesthetized with ethylene chloride. Two to three mm of the end of the tail was snipped and 40uL of blood collected into EDTA-coated capillary tubes. Complete blood counts were performed using a validated HEMAVET® 950FS Hematology System (Drew Scientific) at least 10 mins after collection but within 24hrs.
Neupogen and Amifostine radiomitigators
Amifostine was administered subcutaneously (SC) 30 min prior to TBI at a single dose of approximately 400 mg/kg (8 mg/mouse) (Yuhas et al. 1977). Yuhas et al. (Yuhas et al. 1977) showed that this dose of Amifostine successfully radio-protected normal tissues in mice and did not cause toxicity. Granulocyte-colony stimulating factor (G-CSF, Filgrastim; Amgen, Inc, Thousand Oaks, CA) was administered SC at a dose of approximately 125 μg/kg (2.5 μg/mouse) once a day starting 24 ± 4 hours after TBI for 16 days.
Study Design, Sample Size, & Statistical analyses
Each cage was randomized to a radiation exposure dose and individual mice were randomized to treatment groups by a study statistician. Studies testing MCM as mitigators or protectants were powered to detect a 30% reduction in mortality (i.e. 70% to 40% and 90% to 60%) with 80% power using a two-tailed 5% significance level (n=20 mice per group). The primary outcome, 30-day survival, was examined using logistic regression, which also included sex, radiation dose and interactions of treatment with dose and sex to examine differential effects. Since the randomization was by mouse, “cage effects”, if present, did not bias the results but the model was adjusted using a Generalized Estimating Equations (GEE) method. Secondary analysis included time to death and was examined using a Cox proportional hazards regression model, which was analogous to the logistic regression and included treatment, sex and radiation dose.
CBC data were analyzed using a t-test to compare groups at each time point, and then examined to see if a transformation was necessary to satisfy the assumptions of the t-test before the analysis was implemented. In addition, data for multiple time points were examined together in a two way analysis of variance model with time and treatment as factors. If no treatment by time interaction exists, this analysis has more power than the t-tests. Five mice per group yielded 80% power to detect a 2 standard deviation difference using a two-tailed 5% significance level.
Chronoradiosensitivity study
Mortality was analyzed using a GEE methods with factors for study, gender, cage, and time of day. Interactions were also tested. Kaplan-Meier survival analysis was used to compare survival time between morning, midday, and afternoon irradiation groups.
Drinking studies
A longitudinal linear mixed models analysis was used to compare total volume (mL) of water consumed per mouse over time. A compound symmetry variance-covariance structure was assumed. The group by day interaction was not significant (p=0.569); group by study interaction was significant (p=0.045) and thus was kept in the final model.
RESULTS
Drinking studies
Since the first line treatment for irradiated, neutropenic humans includes antibiotics, efficacy of different antibiotics to increase 30 day survival in lethally irradiated mice was evaluated. To avoid the added stress of daily injections and handling of irradiated mice, antibiotics were provided ad libitum in the drinking water and feed. Therefore, as a first step in comparing the efficacy of different antibiotics, water consumption in mice exposed to lethal irradiation was evaluated to understand how to use drinking water as a vehicle for antibiotic delivery.
To this end, consumption of water from sipper tubes fashioned from graduated cylinders was followed after exposure of C57BL/6 mice to the LD50/30 dose of 137Cs (Fig. 1). Overall water consumption significantly decreased as early as one day after irradiation (from 3.9 ml/day to 2.3 ml/day, p<0.001, n=49), and continued to decrease with onset of radiation-induced morbidity to 2-3 weeks post-TBI (p<0.001, comparing d8 to d18, n=39), at which time consumption began to increase in surviving mice (p<0.002, comparing d19 and d29, n=34). Of interest, when wet feed was present in Petri dishes set on cage bottoms, mice consumed as much as 70% less water from sipper tubes (p<0.001, n=29-35).
Figure 1. Lethally-irradiated C57BL/6 mice provided with wet feed consumed less water from sipper tube bottles than mice provided only sipper tube bottles.
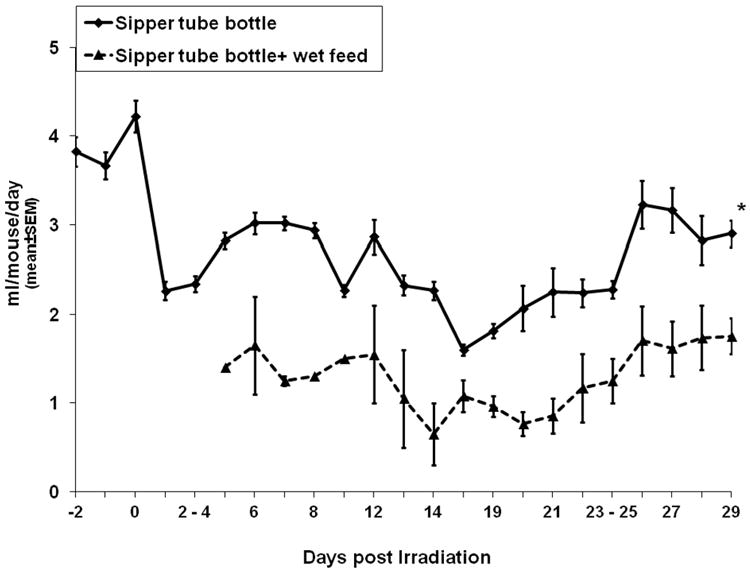
C57BL/6 mice were exposed to 7.96Gy and monitored for fluid consumption for 29 days. All mice were provided autoclaved acidified water for the duration of the study. Mice in the “sipper tube bottle + wet feed” group (broken line) were also provided wet feed set in Petri dishes on cage bottoms beginning on day 4 post-exposure. Data are from 1 to 2 separate experiments. *p<0.0001 comparing “Sipper tube bottle” to “Sipper tube bottle + wet feed”, n=29-35 mice/group. Data on days 2-4 and days 23-25 represent mean daily water intake over the weekend.
Water consumption in lethally-irradiated mice provided ciprofloxacin-containing water with or without added flavorings (grape, sweeteners) was found not to differ among the different treatment groups, so flavorings were deemed unnecessary to enhance antibiotic consumption (data not shown). In addition, there were no differences in water consumption between males and females (two-sided p-value = 0.244, data not shown).
Chronoradiosensitivity
C57BL/6 mice were exposed to the predicted LD50/30 dose of radiation at different times during the day and then analyzed for overall survival time for 30 days. Mice irradiated between 11am and 1pm (Fig. 2, Midday group) were found to be more radioresistant than mice irradiated in the morning or afternoon (p≤0.019). Lethality of mice irradiated in the morning was the closest to the predicted LD50/30 dose of radiation (54.4% lethality), therefore all mice in subsequent studies were irradiated in the morning to reduce chronoradiosensitivity effects between experiments.
Figure 2. Chronosensitivity of C57Bl/6 mice.
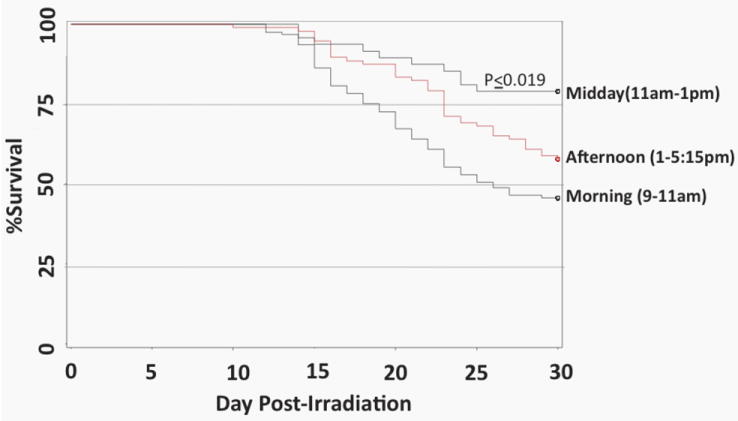
C57BL/6 mice were irradiated with the predicted LD50/30 at different times during the day and analyzed for survival time over 30 days. p≤0.019 comparing Midday group with either morning or afternoon. N=68 (morning group), n=10 (midday group), and n=41 (afternoon group).
Radiation dose-response relationship (DRR) and LDXX/30
Fig. 3a shows the radiation dose relationship (DRR) using probit models of C57BL/6 mice exposed to various doses of radiation and provided ad libitum doxycycline & neomycin, ciprofloxacin, levofloxacin, or no antibiotic support from days 4-30 post-exposure. Mice receiving levofloxacin and the levofloxacin control group of “no antibiotic support” also received seven subcutaneous PBS injections every other day beginning on day 1 post-exposure. The purpose of the seven PBS injections during the study was to control for effects that the stress of handling (while administering medical countermeasures), or that the benefit of fluid replacement, may have on mortality of irradiated mice. Based on studies shown in Fig. 1 suggesting that irradiated sick mice drink at least 3mL of water per day, it is assumed that mice consumed at least 300mg/kg/day of neomycin, and 100mg/kg/day of ciprofloxacin or levofloxacin. Assuming that mice eat 4g/chow/day, they would have consumed 125mg/kg/day of doxycycline.
Figure 3. Radiation dose-response relationship (DRR) of C57Bl/6 mice.
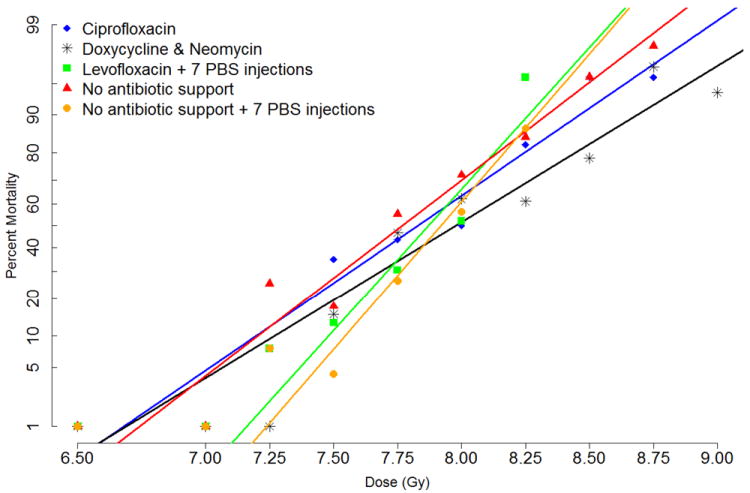
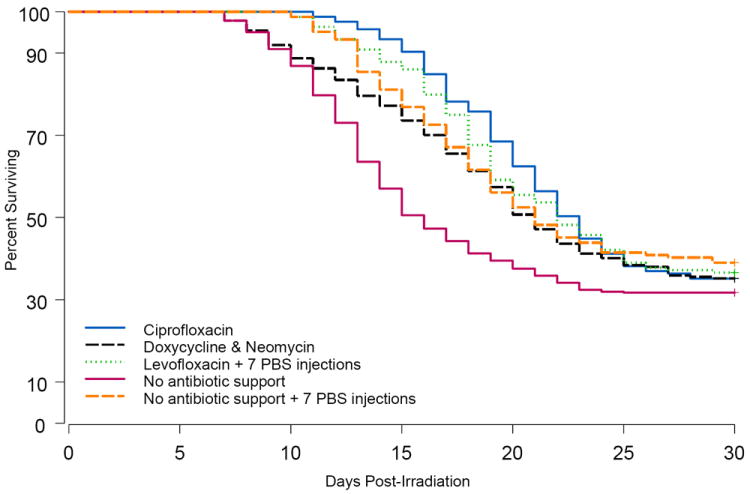
C57BL/6 mice were exposed to different doses of radiation and provided support on days 4 through 30 after irradiation as follows: one group received ciprofloxacin-treated water and chow wetted with ciprofloxacin-water (blue line). A second group received neomycin-treated water plus doxycycline chow in cage hoppers and also as wet feed (black line). A third group received levofloxacin-treated water and chow wetted with levofloxacin-water, plus seven subcutaneous injections of PBS every other day beginning on day one post-exposure (green line). Controls received no antibiotic support but did receive regular wet feed on days 4-30 either without (red line) or with the addition of seven PBS injections (orange line). Panel A shows the DRR using probit models. Survival at day 30 was analyzed at each dose of radiation and is shown as percent mortality on the Y-axis. Panel B are Kaplan-Meier survival curves showing the proportion of mice surviving at each time point for each treatment condition using all of the radiation doses combined.
P values for 30 day survival: p=0.005 comparing doxycycline and neomycin with No antibiotic support, p=0.061 comparing ciprofloxacin with No antibiotic support, p=0.461 comparing levofloxacin with No antibiotic support + 7 PBS injections.
P values for overall survival time: p<0.0001 comparing doxycycline and neomycin with No antibiotic support, p<0.0001 comparing ciprofloxacin with No antibiotic support, p=0.278 comparing levofloxacin with No antibiotic support + 7 PBS injections.
Thirty-day survival was calculated at each dose of radiation and is shown as percent mortality on the Y-axis. Doxycycline and neomycin significantly shifted the lethality curve to the right (p=0.005 compared with “No antibiotic support”), whereas ciprofloxacin was less effective at increasing 30 day survival (p=0.061 compared with “No antibiotic support”). Levofloxacin did not increase 30-day survival compared with control group “No antibiotic support + 7 PBS injections” (p=0.461). There were no statistical interactions between dose and treatment, gender and treatment, or dose and gender. Differences in the five DRR curves are illustrated by the LDXX/30 doses of radiation and 95% confidence intervals calculated from these data (Table 1).
Table 1.
LDXX/30 doses, 95% confidence intervals (C.I.), slopes of probit plots, and mean survival times (MST) from radiation dose response (RDD) experiments.
| aCipro | Doxy + Neo | No Support | Levo + 7 PBS | No Support + 7 PBS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% C.I. | 95% C.I. | 95% C.I. | 95% C.I. | 95% C.I. | |||||||||||
| LDXX/30 | Gy | Lo | Hi | Gy | Lo | Hi | Gy | Lo | Hi | Gy | Lo | Hi | Gy | Lo | Hi |
| LD10/30 | 7.19 | 6.94 | 7.44 | 7.25 | 7.02 | 7.48 | 7.22 | 7.08 | 7.35 | 7.50 | 7.35 | 7.65 | 7.57 | 7.43 | 7.71 |
| LD30/30 | 7.58 | 7.43 | 7.73 | 7.69 | 7.56 | 7.83 | 7.55 | 7.47 | 7.64 | 7.73 | 7.63 | 7.83 | 7.79 | 7.69 | 7.88 |
| LD50/30 | 7.82 | 7.70 | 7.94 | 7.96 | 7.86 | 8.07 | 7.76 | 7.69 | 7.83 | 7.88 | 7.79 | 7.96 | 7.92 | 7.84 | 8.01 |
| LD70/30 | 8.07 | 7.94 | 8.19 | 8.24 | 8.13 | 8.35 | 7.97 | 7.90 | 8.05 | 8.02 | 7.93 | 8.12 | 8.06 | 7.97 | 8.15 |
| LD90/30 | 8.47 | 8.26 | 8.69 | 8.70 | 8.51 | 8.90 | 8.32 | 8.21 | 8.44 | 8.26 | 8.12 | 8.41 | 8.29 | 8.15 | 8.42 |
| bMST (days) | 19.3 | 18.3 | 14.1 | 18.5 | 17.2 | ||||||||||
| Slope of Probit (±Standard Error) | 2.0259 (±0.2896) | 1.8071 (±0.2151) | 2.2669 (±0.1944) | 3.2765 (±0.4557) | 3.4185 (±0.4676) | ||||||||||
| Differential between LD90/30 and LD10/30 | 1.28 | 1.45 | 1.10 | 0.76 | 0.72 | ||||||||||
| LD90/30:LD10/30 ratio | 1.18 | 1.20 | 1.15 | 1.10 | 1.10 | ||||||||||
| Number of mice | 165 | 284 (pool of 3 experiments) | 463 (pool of 4 experiments) | 164 | 164 | ||||||||||
Cipro, Doxy+Neo, and No Support experiments were performed in 2006 and 2007. Levo and No Support + 7 PBS experiments were performed in 2011.
p values for MST: <0.0001 comparing ciprofloxacin with No support; p<0.0001 comparing doxycycline & neomycin with No antibiotic support; p=0.033 comparing Levofloxacin with No Support + 7 PBS; p=0.031 comparing ciprofloxacin with doxycycline & neomycin.
Although ciprofloxacin and levofloxacin were unsuccessful to significantly increase 30day survival compared with their respective control groups, both antibiotic regimens did significantly increase overall survival time. Kaplan Meier survival curves (Fig. 3b) and Mean Survival Times (MST) of decedent mice (Table 1) show that the overall survival of mice treated with ciprofloxacin (MST=19.3 days) or levofloxacin (MST=18.5 days) was significantly greater than their respective controls (“No antibiotic support” MST=14.1 days; p<0.0001, and “No antibiotic support + 7 PBS” MST=17.2 days, p=0.033). MST of mice treated with doxycycline & neomycin (18.3 days) was significantly increased compared with “No antibiotic support” (p<0.0001). MST of ciprofloxacin mice was also significantly increased compared with MST of doxycycline & neomycin treated mice (p=0.031).
Of interest in these comparisons is that overall survival (Fig. 3b) and MST (Table 1) of “No antibiotic support + 7 PBS” mice appeared greater than “No antibiotic support” mice, suggesting that the fluid support provided via the seven injections may have enhanced survival. However, the steeper dose-response curve of the mice that received the seven PBS injections, illustrated by the increased slopes of the probit plots (Table 1), suggests that injections may have increased mortality of mice exposed to higher doses of radiation, possibly due to added stress of handling the sick mice.
Of particular note in these mouse studies is the overall steepness of all 5 curves, illustrated by the relatively small differential between the LD90/30 and LD10/30 doses of radiation (0.72 to 1.45 Gy, Table 1) compared to 2.45 Gy in the nonhuman primate (MacVittie, in press). The relatively low LD90/30:LD10/30 ratios [range 1.095-1.2 (Table 1) versus 1.39 in the nonhuman primate (MacVittie, in press)] also illustrate this point. Larger LD90/30:LD10/30 values, possibly approaching 2.00, would likely mimic the human population more than ratios generated from inbred mouse strains. The very steep curves mean that confidence intervals around the calculated LDXX/30 radiation doses span a larger fraction of the curve, thus pinpointing the LDXX/30 in subsequent experiments becomes difficult.
Moribundity and mortality of mice undergoing periodic blood sampling
Since pancytopenia is a major factor in radiation-induced morbidity and mortality, assessing recovery of hematopoietic cells after irradiation may be a useful secondary parameter to document mechanism of action of MCM. However, effects of longitudinal blood sampling on morbidity/mortality of lethally-irradiated mice is unknown. To investigate the effects of blood sampling on mortality, mice were exposed to the LD90/30 dose of ionizing radiation and were bled by tail snips once every 5 days (30uL per sample; a maximum of 6 samples/mouse over 30 days). Thirty day survival of mice undergoing periodic blood sampling was 3.2%, compared to 12.2% in non-bled mice (Fig. 4a, p=0.008). There was no difference in 30day survival between males and females (two-sided p=0.518). The MST of decedent mice bled every 5th day was significantly decreased by 1.6 days compared to non-bled mice (17.3 days versus 18.9 days, respectively; p=0.031). Cox Regression analysis of overall survival time of bled mice was significantly decreased compared to non-bled mice (p=0.005). Morbidity occurred earlier and was more prevalent in bled mice compared to non-bled (Fig. 4b). Taken together, these data suggest that either the small loss of blood or the stress of handling negatively impacted health and survival of lethally-irradiated mice.
Figure 4. Mortality and morbidity associated with periodic blood drawing.
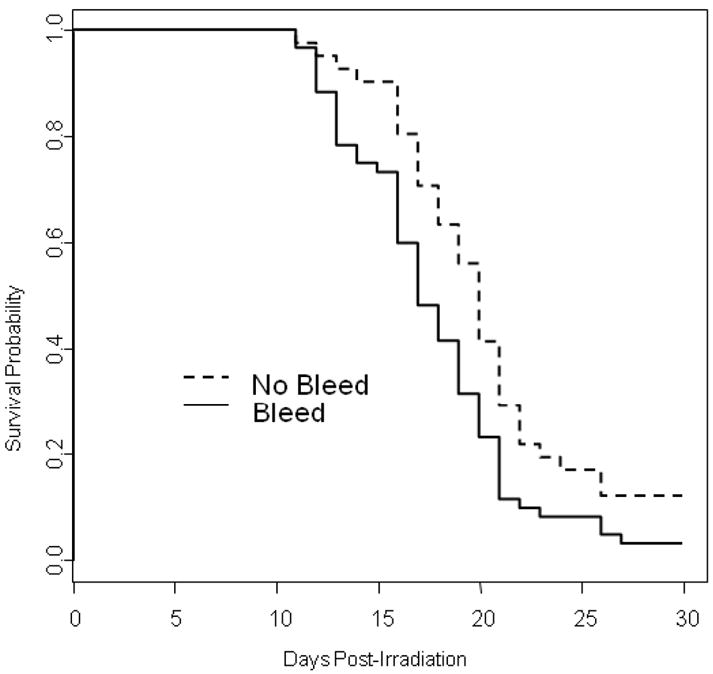
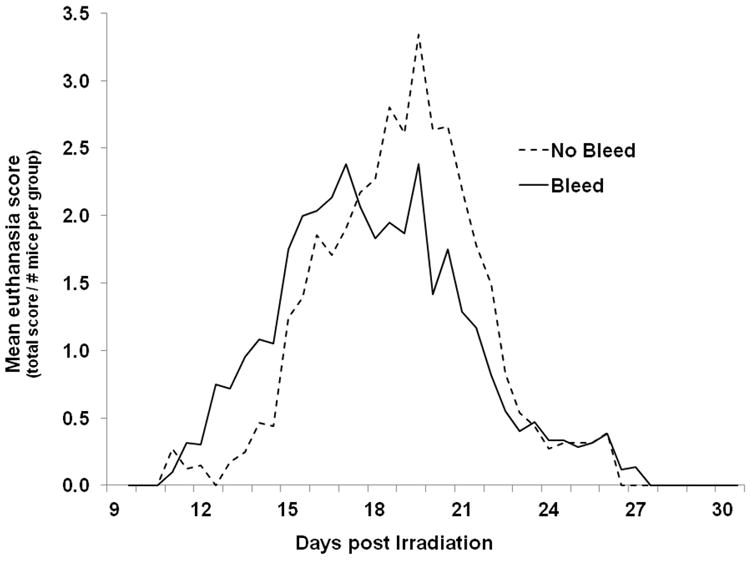
Mice were exposed to the LD90/30 dose of radiation and then bled by tail snips every 5 days (solid line), or not bled (dashed line). Mice were provided ciprofloxacin in their drinking water and wet feed ad libitum from day 4 through 30 post-exposure. Panel A shows Kaplan-Meier survival curves; panel B shows the mean euthanasia score per mouse in each group over time. n=60 mice in the bleed group and n=41 mice in the no bleed group. p=0.008 comparing 30 day survival of bled and not bled mice; p=0.031 comparing MST of bled and not bled mice, p=0.005 comparing overall survival time of bled and not bled mice.
Histology and microbiology of lethally-irradiated mice
To better understand mechanisms of radiation-induced death, moribund mice, and non-moribund mice culled on days 3, 8, and 17 post-8 Gy TBI, were humanely euthanized and underwent necropsy to assess microbial status and histopathology of various tissues (Table 2). Histology of the thymus and bone marrow (BM) on d3 (non-moribund mice) revealed classical effects of TBI: thymic atrophy, absence of cells in the cortex, and marked BM hypocellularity. Few myeloid cells were present in the BM, and sinusoids were dilated with red cells. At later time-points (d8 and 17), foci of myeloid cells appeared in the BM, sinusoidal congestion diminished, and adipocytes became numerous. Histology of spleens showed small germinal centers with indistinct mantle zones early after irradiation (d8), with discernible mantle zones and with foci of extramedullary hematopoiesis on later time-points (d17). Tissues from non-moribund mice terminated on day 31 were only mildly lymphopenic.
Table 2.
Histopathological analyses of tissues from non-moribund C57Bl/6 mice euthanized on various days post-lethal irradiation.
| Day post-TBI | Thymus | Bone Marrow | Spleen | Testis | Salivary gland | Small intestine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (#pos /total) | Description | % (#pos /total) | Description | % (#pos /total) | Description | % (#pos /total) | Description | % (#pos /total) | Description | % (#pos /total) | Description | |
| Day 3 | 100% a(5/5) 3/3 F 2/2 Mb | Marked atrophy; cortex is absent; small foci of lymphocytes scattered throughout the medulla; foci of lymphocytolysis | 100% (6/6) 3/3 F 3/3 M | Marked hypocellularity of myeloid tissues (all lineages involved); sinusoids dilated with red cells; small numbers of megakaryocytes & megakaryoblasts scattered throughout medullary cavity | N.D. | N.D. | N.D. | N.D. | ||||
| Day 8 | Not presentc | 100% (6/6) 3/3 F 3/3 M | Marked hypocellularity of myeloid tissues; moderate sinusoidal congestion; foci of undifferentiated (mononuclear) blasts repopulating marrow | 67% (4/6) 3/3 F 1/3 M | Few to multiple small germinal centers with thin or indistinct mantle zone | 100% (3/3) M | Focal seminiferous tubular degeneration | 100% (6/6) | Normal | 100% (6/6) | Normal | |
| 33% (2/6) 2/3 M | Marked hypocellularity of white pulp; germinal centers not readily identified | |||||||||||
| Day 17 | Not presentc | 57% (4/7) 1/3 F 3/4 M | Marked hypocellularity of myeloid tissues; abundant fat: one or more small foci of undifferentiated blast cells (mononuclear) repopulating marrow | 43% (3/7) 1/3 F 2/4 M | Few small germinal centers and thin mantle zones; focus of extra- medullary hematopoiesis | 100% (3/3) M | Moderate seminiferous tubular degeneration; reduced layers of tubular epithelia and vacuolation; reduced sperm in lumina | 14% (1/7) 0/3 F 1/4 M | Multilobular acinar cell degeneration of salivary gland | 100% (6/6) | Normal | |
| 29% (2/7) 1/3 F 1/4 M | Moderate hypocellularity of myeloid tissues; large area of undifferentiated blast cells (mononuclear and multinucleated) repopulating marrow | 57% (4/7) 2/3 F 2/4 M | Prominent germinal centers and thin mantle zones; multifocal extra- medullary hematopoiesis | 86% (6/7) 3/3 F 3/4 M | Normal | |||||||
| 14% (1/7) 1/3 F 0/4 M | Extensive repopulation of the marrow; all lineages involved | |||||||||||
indicates number of mice positive / total number analyzed. Number of females (F) and males (M) follow.
tissue from one male mouse could not be evaluated.
thymus could not be isolated from necropsied tissue presumably due to the severe atrophy.
Small intestines at all time-points evaluated showed no gross abnormalities, inflammation, ulceration, or bleeding, although villi blunting and modest crypt loss was evident (Table 2). Crypt regeneration was observed relative to non-irradiated controls. These findings are consistent with modest GI damage within the LD80/30 range of H-ARS.
A profound discoloration of the liver associated with cytoplasmic vacuolation (fatty change) was noted at early time points, but was absent in mice surviving to day 31. Radiation-mediated degeneration of seminiferous tubules was noted in testis, while salivary glands remained mostly normal, despite occasional acinar cell degeneration (Table 2).
Septicemia and bacteremia were more prevalent in moribund mice than in non-moribund mice at all time-points analyzed. At early time points (days 8-16), 88-100% of tissues from moribund mice were positive for microbes, whereas only 0-33% of tissues from non-moribund mice showed similar results (Table 3). By days 17-23, 25-75% of tissues from non-moribund mice were positive, compared to 83-100% of tissues of moribund mice. Similar numbers of bacterial strains were identified in moribund and non-moribund mice and ranged from 1-9/mouse with an average of 2.6±0.4 organisms/mouse (mean±SEM, n=23). Over 75% of the organisms found in harvested tissues were of enteric origin, with Klebsiella oxytoca and Enterococcus faecalis being the most prominent organisms isolated (Table 4).
Table 3.
Percentage of tissues from lethally-irradiated C57Bl/6 mice with positive microbiological cultures.
| Health Status of mice undergoing analysisa | Days post-irradiation | Tissue | ||||
|---|---|---|---|---|---|---|
| Blood | Heart | Kidney | Spleen | Liver | ||
| Non-moribund | 8 | 0% | 0% | 33% | 17% | 17% |
| 17 | 25% | 75% | 75% | 75% | 75% | |
| Moribund | b11-16 | 100% | 88% | 100% | 100% | 100% |
| 17-23 | 83% | 83% | 100% | 100% | 83% | |
n=4-8 mice at each time point.
data from moribund mice euthanized on days 11 through 16 were pooled, as were data from moribund mice euthanized on 17 through 23.
Table 4.
Incidence of organisms isolated from mouse blood and tissues.
| Organism | Gram stain | aIncidence |
|---|---|---|
| Klebsiella oxytoca | - | 48% |
| Enterococcus faecalis | + | 13% |
| Enterococcus species | + | 7% |
| Staphylococcus xylosus | + | 9% |
| Lactobacillus acidophilus | + | 4% |
| Coagulase Negative Staphylococcus | + | 6% |
| Staphylococcus epidermidis | + | 5% |
| Propionibacterium granulosum | + | 2% |
| Staphylococcus hominis | + | 1% |
| Un-identified G-ve rods | - | 5% |
| Un-identifed G+ve rods | + | 1% |
incidence represents the percentage of cultures that were positive for the given organism among all the tissues undergoing microbial analyses.
CBC
To provide evidence that the pathophysiological mechanism of toxicity of H-ARS is reproduced in the murine model, and to validate our mouse model of H-ARS with a known radiomitogator (Neupogen) and radioprotectant (Amifostine), the kinetics of hematopoietic recovery in lethally irradiated mice treated with either Neupogen or Amifostine was followed for 30 days post-exposure. Groups of 30 mice were exposed to the LD70/30 dose of radiation and treated with either Amifostine (400mg/kg) 30mins prior to exposure, Neupogen (125ug/kg) beginning 24hr post exposure and continuing daily to d16, or remained untreated. Amifostine and Neupogen significantly increased survival from 30% to 100% and 67%, respectively (Fig. 5a). The white blood cell (WBC) and absolute neutrophil count (ANC) hit their nadir around day 6-10 and did not begin to recover until after day 20 (Fig. 5b and 5c, respectively). The absolute lymphocyte count (ALC) reached nadir by day 2, and did not recover to levels seen in non-irradiated age-matched controls by day 30 (Fig. 5d). Erythrocytes and platelets were slower to reach their nadir (day 22 for RBC and day 17 for platelets), and both recovered to almost normal levels by day 30 (Fig. 5e and 5f). Apparent in all figures is the minimal hematopoietic suppression in mice treated with Amifostine, especially in RBC and platelet parameters, and recovery from the initial drop in WBC, ANC, and ALC. Mice treated with Neupogen were hematopoietically suppressed in all parameters for approximately 15 days, but recovered more quickly than untreated control mice. These data support and expand upon those previously reported (Patchen et al. 1992, Patchen and MacVittie 1994, Patchen 1995) by illustrating the temporal changes in hematopoietic parameters post-exposure and effects of a known radiomitigator (G-CSF) and known radioprotectant (Amifostine) to enhance the recovery blood cells and platelets.
Figure 5. Survival and CBC parameters in mice exposed to lethal radiation and treated with Amifostine or G-CSF.

Groups of 30 mice were exposed to the LD70/30 dose of radiation and treated with either Amifostine (400mg/kg, green lines) 30 minutes prior to exposure, Neupogen (125ug/kg, red lines) beginning 24 hr post exposure and continuing daily to day 16, or remained untreated (blue lines). Black lines are from non-irradiated age-matched controls. Mice were bled by tail snips every 5th day for CBC analyses using a validated HEMAVET® 950FS Hematology Systems. Panel A shows the Kaplan-Meier survival curves. Panels B through F show the white blood cell count (WBC), absolute Neutrophil count (ANC), absolute Lymphocyte count (ALC), red blood cell count (RBC) and platelet count (plt), respectively. Green asterisks indicate statistically significant difference comparing Amifostine to untreated control, p<0.0001; red asterisks indicate statistically significant difference comparing Neupogen group to untreated control, p<0.0367. n=30 mice/group.
CBC were also performed on moribund mice (n=51) prior to euthanasia, and compared to CBC from irradiated non-moribund mice (n=242). Hematocrit and platelet counts of moribund mice were found to be only 20-24% of those of non-moribund mice (hematocrit = 5.6 ±1.60 versus 23.0 ±1.51, respectively, and platelets = 46 ±12.73 versus 215 ±25.99, respectively, mean±SEM). However, absolute neutrophil and lymphocyte counts were similar between moribund and non-moribund mice (ANC = 0.58 ±0.23 versus 0.51 ±0.10, respectively, and ALC = 1.1 ±0.50 versus 1.2 ±0.15, respectively, mean±SEM).
DISCUSSION
This paper describes the establishment of a mouse model that adheres to the FDA’s Animal Rule (AR) (Crawford 2002) and which can thus be used for efficacy testing and product licensure of MCM for the treatment of H-ARS. Following the AR, the FDA will rely on data from animal studies as evidence of MCM effectiveness when: 1) the animal model possesses a well understood pathophysiological mechanism of toxicity (of radiation) and its amelioration by the MCM, 2) the effect is demonstrated in more than one animal species and in a manner predictive of the human response (unless the effect can be demonstrated in a single animal species that represents a sufficiently well characterized animal model for the human response 3) the outcome of the animal study is clearly related to the desired benefit in human (i.e., reduced morbidity/mortality), and 4) data on pharmacokinetics and pharmacodynamics of the product in animals and humans allows selection of an effective dose in humans (Crawford 2002). In addition, the AR requires that efficacy studies for MCM undergoing final confirmation for FDA approval are to be done as GLP compliant pivotal trials using well-characterized animal models.
As a first step in developing our mouse model in accordance with the AR, the radiation dose-response relationship (RDD) for mortality from H-ARS was defined. Of the MCM currently available, antibiotic therapy is first line treatment for radiation exposure (Waselenko et al. 2004) and thus was included in studies establishing the RDD. Since the stress of injections would likely accelerate morbidity and mortality of lethally irradiated mice, antibiotics were provided ad libitum through the drinking water. It was thus important to first determine water consumption of irradiated mice, so that an appropriate amount of antibiotics could be added to water to achieve the desired dose through drinking. Our studies showed that irradiated mice drink approximately 3 mL of water per day, and less when wet feed is present. Although fluid consumption varied significantly after irradiation, we assumed that mice consumed at least the equivalent of 3mL of water per day from the sipper tube and from chow wetted with antibiotic water, and ate 4g of chow per day.
Three antibiotic regimens were examined in the current study: 1) the broad spectrum tetracycline antibiotic doxycycline administered with the aminoglycoside neomycin (the antibiotics routinely used at our institution for irradiated mice), 2) the broad spectrum fluoroquinolone ciprofloxacin, and 3) the fluoroquinolone levofloxacin, which possesses broader gram-positive coverage than ciprofloxacin. Based on our water consumption studies, it was estimated that irradiated mice consumed 300mg/kg/day of neomycin, 100mg/kg/day of ciprofloxacin, 100mg/kg/day of levofloxacin, and 125mg/kg/day of doxycycline (assuming that all mice in the same cage drank approximately the same amount of water). All these doses are well within the therapeutic range for irradiated mice. It should also be noted that females, which weighed approximately 23% less than males, drank approximately the same amount of water, so systemic levels of antibiotics may be greater in females than in males.
While all three antibiotic regimens significantly increased MST in our mouse model, only ciprofloxacin and doxycycline+neomycin significantly increased overall survival time as well. Furthermore, only doxycycline and neomycin significantly increased 30 day survival, while ciprofloxacin moderately increased 30 day survival (p=0.061). Doxycycline and neomycin were subsequently omitted from our investigations since neither is warranted for treatment of irradiated humans. The fluoroquinolones ciprofloxacin and levofloxacin are attractive for use in our model for the following reasons: both are absorbed systemically and would be useful for septicemia, both are warranted for treatment of humans after irradiation (D’Antonio et al. 1994), and both have efficacy in rodents (Brook et al. 1990, Brook and Elliott 1991, Brook and Ledney 1991, D’Antonio et al. 1994, Brook et al. 2005). In addition, ciprofloxacin is widely used in humans, does not affect glucose levels in mice observed with other fluoroquinolones (Hori et al. 2006), and would likely be the first line antibiotic used to treat humans in a radiation accident setting.
The broader gram positive coverage of levofloxacin may make it a more attractive antibiotic in our model than ciprofloxacin. Indeed, our data evaluating the microbiological profile of different tissues in lethally-irradiated mice (Table 4) revealed a large number of gram positive bacteria, suggesting that levofloxacin may be preferred over ciprofloxacin as a single antimicrobial agent. Since experiments evaluating ciprofloxacin and levofloxacin were performed 4-5 years apart, comparisons of data between the two should be viewed with caution. Ciprofloxacin was shown to significantly increase the MST of decedents by 5 days over controls, whereas levofloxacin increased MST by only 1 day. The increase in “alive days” effected by antibiotics allows an extension of the “window of opportunity” for MCM to show effectiveness for increased survival.
Evident through development of our animal model of H-ARS has been the variability in the predicted survival of irradiated mice from experiment to experiment. Despite controlling for such things as: room humidity, cage rack position, time of day of irradiation, time of year of the experiment, number of vehicle injections, age of animals, use of antibiotics versus no use, and type of antibiotic, it remains difficult to achieve the predicted LDXX/30. The observed variability is likely consequent to the steepness of the lethality curve, a characteristic of inbred strains such as C57Bl/6 mice. This steepness results in 95% confidence intervals (CI) that span a larger fraction of the dose response curve around the calculated LDXX/30 doses. Such broad, inclusive CI make pin pointing the LDXX/30 in subsequent experiments difficult. This observation has been previously acknowledged by Cerveny et al (Cerveny et al. 1989), where he notes: “the more inbred and homogenous the population, the steeper the slope of the lethality curve”. Others have made similar statements: “it seems possible to conclude that the doses giving between 90%-95% mortality in most animal experiments are about twice those giving 5%-10% mortality” (Baverstock and Ash 1983).
Examination of LD90/30:LD10/30 ratios in Table 1 reveals values of 1.10 to 1.20 for the inbred mouse strain C57Bl/6, whereas ratios for non-human primates are 1.39 (MacVittie, in press). Ratios approaching 2 are predicted for humans. A much smaller differential between the LD90/30 and LD10/30 in the C57Bl/6 DRR (0.72 to 1.45 Gy) also exists compared to that of nonhuman primates (2.45 Gy, MacVittie, in press). These differences illustrate the more shallow dose-response curves of outbred species, and exemplify the inherent problems associated with performing radiation lethality experiments using inbred strains. Whether outbred mice would allow generation of more stable DRR than inbred strains remains to be determined. Additionally, if “outbred” can be equated with variability (in the sense of genetic variability), then one could postulate that as laboratory systems become more standardized and streamlined, and experimental variables systematically reduced, that DRR may become even more steep.
Regardless, most radiation studies are done using inbred mouse strains (most commonly BALB/C, C3H/HeN, B6D2F1/J and C57Bl/6 (Williams et al. 2010)), which differ greatly in their radiosensitivities due to genetic variations. BALB/C, for example, are the most radiosensitive strain (LD50/30=6.4 (Kohn and Kallman 1956)) likely due to a double-strand DNA repair defect (Okayasu et al. 2000). C57BL/6 are the most radioresistant of the four strains listed above, but are moderate compared to a larger group of 18 strains (Kohn and Kallman 1956). Their moderate radiosensitivity, along with being one of the recommended strains for radiation studies by the 2008 Centers for Medical Countermeasures against Radiation Animal Models Workshop (Williams et al. 2010), as well as the availability of congenic strains that allow the performance of competitive transplantation studies for HSC function, make C57Bl/6 mice a particularly desirable strain for development of a model of H-ARS.
As one means of reducing variability in our model, we examined whether chronoradiosensitivity was affecting mortality of our irradiated mice. Chronoradiosensitivity of mice and rats has been recognized since the early 1960s (Pizzarello et al. 1963, Pizzarello et al. 1964, Nelson 1966, Hellwig and Rosenkranz 1968, Haus et al. 1974, Haus 2002), and has been suggested to be a useful guide to time delivery of radiotherapy in humans for malignancy. In rats and mice, mortality was shown to be higher when irradiation was delivered during the night time hours (Nelson 1966) and/or during the early light hours (Pizzarello et al. 1964, Hellwig and Rosenkranz 1968, Haus 2002). Our findings showed the highest mortality in the morning hours and are in agreement with these studies. It has been suggested that circadian rhythms controlling HSC cycling may be responsible for chronoradiosensitivity, similar to that shown for intestinal stem cells (Potten et al. 1977), but this has not been definitively shown. Circadian rhythms controlling HSC trafficking between BM and PB have, however, been recently documented (Méndez-Ferrer et al. 2008, Méndez-Ferrer et al. 2009).
Handling of lethally-irradiated, sick mice, such as that necessary to administer MCM, can accelerate morbidity and mortality. Conversely, the “fluid replacement” gained through administration of MCM has been shown to enhance survival in the gastrointestinal subsyndrome of ARS (Booth, in press). However, this is less definitive in the H-ARS (Moccia et al. 2010). Since the possibility exists that both these variables can affect the DRR, we aimed to generate a radiation dose-response curve that controlled for both the stress of handling the mice and the possible benefit of fluid replacement that mice would receive during testing of injectable MCM. We chose to give seven subcutaneous injections every other day post-exposure since most MCM are given in 2-16 injections post-TBI. When the radiation dose response curve of the injected mice was compared with that of the non-injected mice, the curve of injected mice was found to be steeper. These data suggest that the stress of handling the mice during injections may have caused increased mortality of mice exposed to higher doses of radiation, while administration of fluids may have provided a survival benefit to mice exposed to lower doses of radiation. It should be noted that LD90/30:LD10/30 ratios of experiments where mice were injected appear less than those of experiments using non-injected mice.
Since neutropenia and thrombocytopenia, and subsequent infection and hemorrhage, are accepted as the cause of death in H-ARS, monitoring the recovery of blood parameters post-exposure may provide a desirable secondary parameter to assess efficacy and mechanism of action of putative MCM. In our study, we found that longitudinal blood sampling exhibited negative effects on morbidity and mortality of lethally-irradiated mice. Overall 30d survival and moribundity of mice undergoing periodic blood sampling were significantly less than non-bled mice. These data suggest that either the small amount of blood (30uL every 5th day) taken from the lethally irradiated mice, or the stress of handling during the tail snip/blood sampling, may have had negative impacts on their recovery. These data will guide development of FDA pivotal protocols assessing MCM in small animal models of H-ARS, and may necessitate performance of separate studies to assess secondary parameters such as kinetics of blood cell recovery.
The granulopoietic hematopoietic growth factors (HGF) G-CSF, GM-CSF, and peg G-CSF remain as the standard of treatment for drug- and radiation-induced neutropenia for their demonstrated efficacy to increase granulocyte regeneration in animals and humans (Zsebo et al. 1986, Shimamura et al. 1987, Morstyn et al. 1988, Sheridan et al. 1989, MacVittie et al. 1990, Patchen et al. 1992, Patchen 1995, Farese et al. 1996, Welte et al. 1996, Molineux et al. 1999, Bishop et al. 2000, Lord et al. 2001, Farese et al. 2012). Tanikawa et al. (Tanikawa et al. 1990) and MacVittie et al. (MacVittie et al. 2005) have demonstrated significantly increased survival in mice and canines, respectively, when G-CSF was administered after lethal irradiation. Both G-CSF and GM-CSF have been used in radiation accident victims and appear to have shortened the time to neutrophil recovery (Dainiak et al. 2003). Amifostine is a known radioprotectant and has also proven efficacy to increase 30 day survival in lethally irradiated mice (Patchen et al. 1990, Patchen et al. 1992). Both G-CSF and Amifostine significantly increased 30 day survival and various PB hematopoietic cell parameters in our model, thereby validating our murine model of H-ARS with known MCM.
CONCLUSIONS
This report describes our efforts to develop an animal model of H-ARS in C57Bl/6 mice according to the guidelines set forth by the FDA Animal Rule (Crawford 2002) and the FDA Guidance document (http://www.fda.gov/cder/guidance/index.htm). We have sufficiently characterized this model with respect to fluid consumption of sick mice post-exposure to estimate antibiotic intake, chronoradiosensitivity to radiation response, effects of different classes of antibiotics on the DRR, effects of longitudinal blood sampling on morbidity and mortality, histopathology and microbiology of moribund mice, and final validation of our model with known MCM that significantly increased survival and recovery of blood cells. This well-characterized murine model is suitable for screening studies of candidate MCM and final pivotal efficacy studies performed under GLP for licensure of MCM to treat lethally-irradiated personnel exposed to radiation doses resulting in H-ARS.
Acknowledgments
Funding:
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases (Contract numbers HHSN266200500043C and HHSN272201000046C) and from the National Heart, Lung, and Blood Institute (Award Number R01HL075660 to CMO), National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
No financial conflict of interest was declared by any of the authors.
LITERATURE
- Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships for acute ionizing-radiation lethality. Health Phys. 2003;84:565–75. doi: 10.1097/00004032-200305000-00001. [DOI] [PubMed] [Google Scholar]
- Baverstock K, Ash P. A review of radiation accidents involving whole body exposure and the relevance to the LD50/60for man. Br J Radiol. 1983;56:837. doi: 10.1259/0007-1285-56-671-837. [DOI] [PubMed] [Google Scholar]
- Bishop MR, Tarantolo SR, Geller RB, Lynch JC, Bierman PJ, Pavletic ZS, Vose JM, Kruse S, Dix SP, Morris ME, Armitage JO, Kessinger A. A randomized, double-blind trial of filgrastim (granulocyte colony-stimulating factor) versus placebo following allogeneic blood stem cell transplantation. Blood. 2000;96:80–5. [PubMed] [Google Scholar]
- Brook I, Elliott TB. Quinolone therapy in the prevention of mortality after irradiation. Radiat Res. 1991;128:100–3. [PubMed] [Google Scholar]
- Brook I, Elliott TB, Ledney GD. Quinolone therapy of Klebsiella pneumoniae sepsis following irradiation: comparison of pefloxacin, ciprofloxacin, and ofloxacin. Radiat Res. 1990;122:215–7. [PubMed] [Google Scholar]
- Brook I, Germana A, Giraldo DE, Camp-Hyde TD, Bolduc DL, Foriska MA, Elliott TB, Thakar JH, Shoemaker MO, Jackson WE, Ledney GD. Clindamycin and quinolone therapy for Bacillus anthracis Sterne infection in 60Co-gamma-photon-irradiated and sham-irradiated mice. J Antimicrob Chemother. 2005;56:1074–80. doi: 10.1093/jac/dki367. [DOI] [PubMed] [Google Scholar]
- Brook I, Ledney GD. Ofloxacin and penicillin G combination therapy in prevention of bacterial translocation and animal mortality after irradiation. 1991;35:1685–1687. doi: 10.1128/aac.35.8.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarett A. Radiation biology. Englewood, New Jersey: Prentice-Hall Inc; 1968. [Google Scholar]
- Cerveny T, MacVittie T, Young R. Acute Radiation Syndrome in Humans. Vol. 2. Falls Church, VA: Walker RI, TTM Publisher; 1989. pp. 17–36. [Google Scholar]
- Crawford L. New drug and biological drug products; Evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. 21 CFR parts 314 and 601, FDA, HHS; ACTION: Final Rule. 2002:37988–37998. [PubMed] [Google Scholar]
- D’Antonio D, Piccolomini R, Iacone A, Fioritoni G, Parruti G, Betti S, Quaglietta AM, Accorsi P, Dell’Isola M, Favalli C. Comparison of ciprofloxacin, ofloxacin and pefloxacin for the prevention of the bacterial infection in neutropenic patients with haematological malignancies. J Antimicrob Chemother. 1994;33:837–44. doi: 10.1093/jac/33.4.837. [DOI] [PubMed] [Google Scholar]
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology. 2003:473–96. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- Farese A, Cohen M, Stead R, Jackson W, III, MacVittie T. FPeg-filgrastim, administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose, radiation-induced meylosuppression in rhesus macaques. Radiat Res. 2012 doi: 10.1667/RR2900.1. In Press. [DOI] [PubMed] [Google Scholar]
- Farese AM, Hunt P, Grab LB, MacVittie TJ. Combined administration of recombinant human megakaryocyte growth and development factor and granulocyte colony-stimulating factor enhances multilineage hematopoietic reconstitution in nonhuman primates after radiation-induced marrow aplasia. J Clin Invest. 1996;97:2145–51. doi: 10.1172/JCI118652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D. Acute radiation response of mice from a cross between radiosensitive and radioresistant strains. Genetics. 1958;43:835–843. doi: 10.1093/genetics/43.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D, Hamilton K. Genetic variation in the acute lethal response of four inbred mouse strains to whole body x-irradiation. Genetics. 1957;42:189–198. doi: 10.1093/genetics/42.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus E. Chronobiology of the mammalian response to ionizing radiation. Potential applications in oncology. Chronobiol Int. 2002;19:77–100. doi: 10.1081/cbi-120002592. [DOI] [PubMed] [Google Scholar]
- Haus E, Halberg F, Loken MK, Kim YS. Circadian rhythmometry of mammalian radiosensitivity. New York: Academic Press; 1974. [Google Scholar]
- Hellwig G, Rosenkranz J. Uber Circadiane Schwankungen der Strahlensensibilität von Ratten. Strahlentherapie. 1968:220–222. [PubMed] [Google Scholar]
- Hori S, Kizu J, Kawamura M. Effect of fluoroquinolones on plasma glucose levels in fasted and glucose-loaded mice. Journal of Infection and Chemotherapy. 2006;12:109–111. doi: 10.1007/s10156-006-0429-z. [DOI] [PubMed] [Google Scholar]
- Kohn H, Kallman K. The influence of strain on acute x-ray lethality in the mouse. I LD50 and death rate studies. Radiation Res. 1956;5:309–317. [PubMed] [Google Scholar]
- Lord BI, Woolford LB, Molineux G. Kinetics of neutrophil production in normal and neutropenic animals during the response to filgrastim (r-metHu G-CSF) or filgrastim SD/01 (PEG-r-metHu G-CSF) Clinical Cancer Research. 2001;7:2085–90. [PubMed] [Google Scholar]
- Lushbaugh C. Reflections on some recent progress in human radiobiology. Advances in Radiation Biology. 1969;277 [Google Scholar]
- MacVittie TJ, Farese AM, Jackson W., 3rd Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 2005;89:546–55. doi: 10.1097/01.hp.0000173143.69659.5b. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Monroy RL, Patchen ML, Souza LM. Therapeutic use of recombinant human G-CSF (rhG-CSF) in a canine model of sublethal and lethal whole-body irradiation. International Journal of Radiation Biology. 1990;57:723–36. doi: 10.1080/09553009014550891. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Chow A, Merad M, Frenette P. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16:235–42. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Lucas D, Battista M, Frenette P. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Moccia KD, Olsen CH, Mitchell JM, Landauer MR. Evaluation of Hydration and Nutritional Gels as Supportive Care after Total-Body Irradiation in Mice (Mus musculus) Journal of the American Association for Laboratory Animal Science. 2010;49:323–328. [PMC free article] [PubMed] [Google Scholar]
- Molineux G, Kinstler O, Briddell B, Hartley C, McElroy P, Kerzic P, Sutherland W, Stoney G, Kern B, Fletcher FA, Cohen A, Korach E, Ulich T, McNiece I, Lockbaum P, Miller-Messana MA, Gardner S, Hunt T, Schwab G. A new form of Filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Experimental Hematology. 1999;27:1724–34. doi: 10.1016/s0301-472x(99)00112-5. [DOI] [PubMed] [Google Scholar]
- Morstyn G, Campbell L, Souza LM, Alton NK, Keech J, Green M, Sheridan W, Metcalf D, Fox R. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988;1:667–72. doi: 10.1016/s0140-6736(88)91475-4. [DOI] [PubMed] [Google Scholar]
- Nelson RF. Variation in radiosensitivity of mice with time of day. Acta Radiol Ther Phys Biol. 1966;4:91–6. doi: 10.3109/02841866609133136. [DOI] [PubMed] [Google Scholar]
- Okayasu R, Suetomi K, Yu Y, Silver A, Bedford JS, Cox R, Ullrich RL. A Deficiency in DNA Repair and DNA-PKcs Expression in the Radiosensitive BALB/c Mouse. Cancer Research. 2000;60:4342–4345. [PubMed] [Google Scholar]
- Patchen ML. Amifostine plus granulocyte colony-stimulating factor therapy enhances recovery from supralethal radiation exposures: preclinical experience in animals models. European Journal of Cancer. 1995;31A(Suppl 1) doi: 10.1016/0959-8049(95)00147-b. [DOI] [PubMed] [Google Scholar]
- Patchen ML, MacVittie TJ. Granulocyte colony-stimulating factor and amifostine (Ethyol) synergize to enhance hemopoietic reconstitution and increase survival in irradiated animals. Seminars in Oncology. 1994;21:26–32. [PubMed] [Google Scholar]
- Patchen ML, MacVittie TJ, Souza LM. Postirradiation treatment with granulocyte colony-stimulating factor and preirradiation WR-2721 administration synergize to enhance hemopoietic reconstitution and increase survival. International Journal of Radiation Oncology, Biology, Physics. 1992;22:773–9. doi: 10.1016/0360-3016(92)90522-j. [DOI] [PubMed] [Google Scholar]
- Patchen ML, MacVittie TJ, Weiss JF. Combined modality radioprotection: the use of glucan and selenium with WR-2721. International Journal of Radiation Oncology, Biology, Physics. 1990;18:1069–75. doi: 10.1016/0360-3016(90)90442-m. [DOI] [PubMed] [Google Scholar]
- Pizzarello DJ, Isaak D, Chua KE, Rhyne AL. Circadian Rhythmicity in the Sensitivity of Two Strains of Mice to Whole-Body Radiation. Science. 1964;145:286–91. doi: 10.1126/science.145.3629.286. [DOI] [PubMed] [Google Scholar]
- Pizzarello DJ, Witcofski RL, Lyons EA. Variations in survival time after whole-body radiation at two times of day. Science. 1963;139:349. doi: 10.1126/science.139.3552.349. [DOI] [PubMed] [Google Scholar]
- Potten C, Al-Barwari S, Hume W, Searle J. Circadian rhythms of presumptive stem cells in three different epithelia of the mouse. Cell Tissue Kinet. 1977;10:557–568. doi: 10.1111/j.1365-2184.1977.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Sheridan WP, Morstyn G, Wolf M, Dodds A, Lusk J, Maher D, Layton JE, Green MD, Souza L, Fox RM. Granulocyte colony-stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet. 1989;2:891–5. doi: 10.1016/s0140-6736(89)91552-3. [DOI] [PubMed] [Google Scholar]
- Shimamura M, Kobayashi Y, Yuo A, Urabe A, Okabe T, Komatsu Y, Itoh S, Takaku F. Effect of human recombinant granulocyte colony-stimulating factor on hematopoietic injury in mice induced by 5-fluorouracil. Blood. 1987;69:353–5. [PubMed] [Google Scholar]
- Tanikawa S, Nose M, Aoki Y, Tsuneoka K, Shikita M, Nara N. Effects of recombinant human granulocyte colony-stimulating factor on the hematologic recovery and survival of irradiated mice. Blood. 1990;76:445–9. [PubMed] [Google Scholar]
- Vriesendorp H, Van Bekkum D. Susceptibility to total-body irradiation. Response of Different Species to Total Body Irradiaton. 1984 [Google Scholar]
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, Lowry P, Armitage JO, Dainiak N. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–51. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996;88:1907–29. [PubMed] [Google Scholar]
- Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, MacVittie TJ, Mason KA, Medhora MM, Moulder JE, Okunieff P, Otterson MF, Robbins ME, Smathers JB, McBride WH. Animal Models for Medical Countermeasures to Radiation Exposure. Radiation Research. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas JM, Storer JB. The effect of age on two modes of radiation death and on hematopoietic cell survival in the mouse. Radiat Res. 1967;32:596–605. [PubMed] [Google Scholar]
- Yuhas JM, Yurconic M, Morton MK, West G, Peterson DF. Combined Use of Radioprotective and Radiosensitizing Drugs in Experimental Radiotherapy. Radiation Research. 1977;70:433–443. [PubMed] [Google Scholar]
- Zsebo KM, Cohen AM, Murdock DC, Boone TC, Inoue H, Chazin VR, Hines D, Souza LM. Recombinant human granulocyte colony stimulating factor: molecular and biological characterization. Immunobiology. 1986;172:175–84. doi: 10.1016/S0171-2985(86)80097-3. [DOI] [PubMed] [Google Scholar]


