Abstract
Purpose
We investigated the effects of proteinuria and renal insufficiency on all-cause mortality in patients with colorectal cancer, with special emphasis on cancer staging and cancer-related deaths.
Materials and Methods
We retrospectively studied a cohort of patients with colorectal cancer. In protocol 1, patients were classified into four groups based on the operability of cancer and proteinuria: group 1, early-stage cancer patients (colorectal cancer stage ≤3) without proteinuria; group 2, early-stage cancer patients with proteinuria; group 3, advanced-stage cancer patients without proteinuria (colorectal cancer stage=4); and group 4, advanced-stage cancer patients with proteinuria. In protocol 2, patients were classified into four similar groups based on cancer staging and renal insufficiency (eGFR <60 mL/min/1.73 m2). Between January 1, 1998 and December 31, 2009, 3379 patients were enrolled in this cohort and followed until May 1, 2012 or until death.
Results
The number of patients with proteinuria was 495 (14.6%). The prevalence of proteinuria was higher in advanced-stage cancer (n=151, 22.3%) than in early-stage cancer patients (n=344, 12.7%). After adjusting for age, gender and other clinical variables, the proteinuric, early-stage cancer group was shown to be associated with an adjusted hazard ratio of 1.67 and a 95% confidence interval of 1.38-2.01, compared with non-proteinuric early-stage cancer patients. However, renal insufficiency was not associated with colorectal cancer mortality.
Conclusion
Proteinuria is an important risk factor for cancer mortality, especially in relatively early colorectal cancer.
Keywords: Cancer, death, proteinuria, GFR, stage
INTRODUCTION
In Korea, colorectal cancer is the fourth most common cancer, with an age-adjusted incidence of 56.7 and 29.5 per 100000 men and women, respectively.1 Colorectal cancer incidence has increased by 6.9% in men and 5.2% in women annually, between 1999 and 2008,1 despite developments in early diagnostic and treatment strategies, including improved chemotherapeutics and surgical techniques.2
Chronic kidney disease (CKD) has been shown to be associated with a high risk of both cardiovascular and non-cardiovascular mortality.3-5 Recently, many studies have suggested that CKD is associated with poor prognosis, and independently predicts high mortality rates in cancer patients.6,7 CKD also is highly prevalent (incidence: 13.8%) in Korea. By far, very little research has addressed the association between renal insufficiency and colorectal cancer mortality.
Albuminuria has been observed in patients with different types of cancer, such as lung, breast, renal cell, and colorectal cancers and non-Hodgkin lymphoma.8-19 Some studies have indicated that the extent of albuminuria reflects disease severity, showing high levels in patients with metastatic disease and large tumor burden. Therefore, albuminuria has been suggested as a nonspecific malignancy marker, reflecting microvascular responses and altered glomerular permeability in response to malignant cell products such as cytokines.8,20 However, studies assessing the relationship between proteinuria and all-cause mortality in patients with colorectal cancer are scarce. Such clinical studies would enable proper allocation of medical resources and help develop better prevention guidelines. In addition, while colorectal cancer staging is an important predictor of mortality, the effect of proteinuria or renal insufficiency at various cancer stages has not been fully examined.
This study aimed to investigate the impacts of proteinuria and renal insufficiency on colorectal cancer mortality, with special emphasis on cancer staging and cancer-related deaths.
MATERIALS AND METHODS
Study population
This study comprised a retrospective cohort of patients who had been admitted to Chonnam National University Hospital and diagnosed with colorectal cancer. Patients were classified according to the diagnostic C-code in the International Classification of Disease, Tenth Revision codes. In total, 3381 patients presenting with colorectal cancer were enrolled from January 1, 1998 to December 31, 2009. Among these, patients who were receiving long-term dialysis were excluded, and the remaining 3379 patients were finally enrolled. The study was approved by the institutional review board of Chonnam National University Hospital.
Protocol 1: retrospective analysis for the association of proteinuria and cancer specific mortality
Patients were divided into four groups based on the operability of cancer and proteinuria: group 1, patients without proteinuria and with operable stage (early stage) cancer (colorectal cancer stage ≤3); group 2, patients with proteinuria and early stage cancer; group 3, patients without proteinuria and with advanced stage cancer (colorectal cancer stage 4); and group 4, patients with proteinuria and advanced stage cancer.
Protocol 2: retrospective analysis for the association of renal insufficiency and cancer specific mortality
To further investigate the association of renal insufficiency and all cause mortality, patients were classified into four similar groups, based on the operability of cancer and renal insufficiency [estimated glomerular filtration rate (eGFR) of <60 mL/min per 1.73 m2]: group 1, patients without renal insufficiency and with early cancer stage; group 2, patients with renal insufficiency and early cancer stage; group 3, patients without renal insufficiency and with advanced cancer stage; and group 4, patients with renal insufficiency and advanced cancer stage.
Definition of cancer stages
Colorectal cancer stage was based on the American Joint Committee on Cancer (AJCC) TNM classification system.21 We divided the patients into two groups according to cancer stages. Early stage was defined as non metastatic colorectal cancer (AJCC colorectal cancer stage ≤3) and advanced stage was defined as distant metastatic colorectal cancer (AJCC colorectal cancer stage ≥4).22
Assessment of kidney function
Laboratory data were collected on the date of admission. The renal function was accessed based on eGFR. The abbreviated Modification of Diet in Renal Disease formula was used to estimate GFR in milliliters per minute per 1.73 m2 from the serum creatinine level.23 Renal insufficiency was defined as an eGFR <60 mL/min/1.73 m2. The presence of proteinuria was defined as albumin 1+ or greater.
Measurement of covariates
Baseline variables included demographic features (age and sex), medical history (diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease), examination findings (body mass index), laboratory data (WBC count, hemoglobin, hematocrit, platelet count, BUN, creatinine), and treatment history (operation, chemotherapy and radiotherapy). The demographic features were the baseline records, and the medical history and treatment history were evaluated by review of the medical records.
Statistical assessment
Continuous variables are demonstrated as either means (±SD) or medians, and categorical variables as numbers and percentages. We compared baseline characteristics of the groups using analysis of variance for continuous variables and by the χ2 statistic for categorical variables. Using Kaplan Meier method, event-free survival was estimated, and curves were compared with log-rank test. Cox proportional hazards analyses were used to assess relationships among the four groups and cancer specific mortality after adjusting for variables. The time to event was defined as the time from the time of diagnosis until the end of follow-up (May 1, 2012) or date of death if earlier. p-values less than 0.05 were deemed significant. Statistical analysis was conducted with the Statistical Package for Social Sciences software (SPSS 18.0 for Windows, SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
The number of patients with proteinuria was 495 (14.6%). The prevalence of proteinuria was higher in advanced-stage cancer (n=151, 22.3%) than in early-stage cancer patients (n=344, 12.7%, p<0.001). Table 1 lists the baseline clinical characteristics of the patients, categorized according to cancer staging and proteinuria status.
Table 1.
Baseline Clinical Characteristics
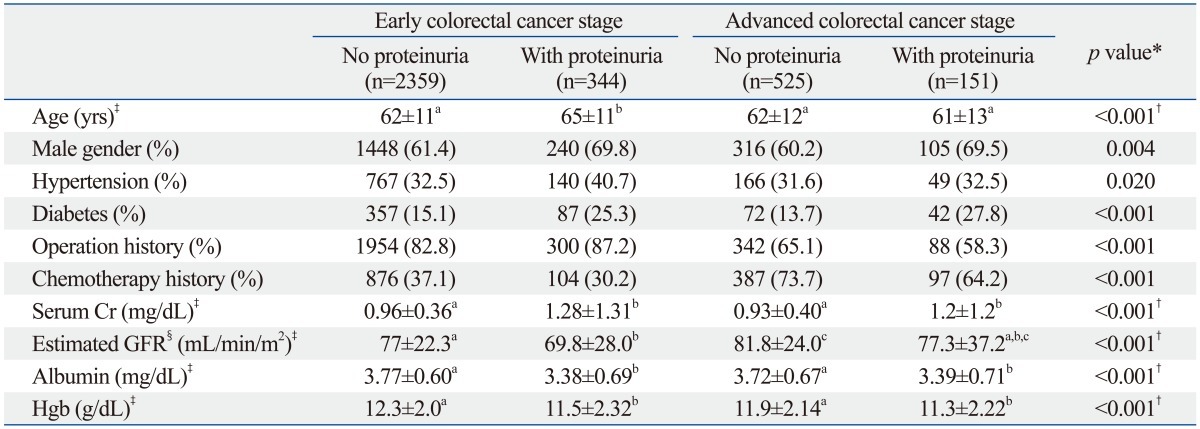
GFR, glomerular filtration rate; Cr, creatinine; BMI, body mass index.
*Statistical significances for linear-by-linear association between categorical variables were tested using chi-square test for trend.
†Statistical significances were tested by analysis of variance among groups.
‡The same letters indicate non-significant difference between groups based on multiple comparison test.
§On the basis of abbreviated IDMS-MDRD (Modification of Diet in Renal Disease) study equation.
Hypertension was observed in 1122 patients (33.2%) and diabetes mellitus was observed in 558 patients (16.5%). In both early- and advanced-stage colorectal cancer groups, proteinuria was more prevalent among patients with hypertension or diabetes than among those without.
Colorectal cancer mortality is associated with proteinuria
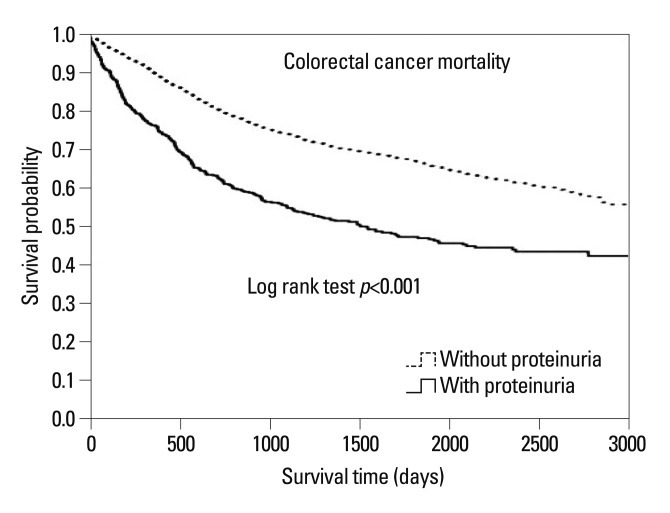
Between 1998 and 2012, over an average follow-up period of 3342 days, 1142 (37.7%) cancer patients died. We analyzed cancer-related mortality rates between the two groups (proteinuria versus non-proteinuria). Kaplan-Meier survival curves (Fig. 1) showed that subjects with proteinuria had a higher mortality risk than do those without proteinuria (log rank test p<0.001). Similarly, multivariate Cox proportional hazard regression analysis showed that subjects with proteinuria have a higher mortality risk due to colorectal cancer than do those without proteinuria. The hazard ratio (HR) of all-cause cancer deaths was higher in patients with proteinuria than in those without proteinuria [HR, 1.46; 95% confidence interval (CI), 1.26-1.69; p<0.001, data not shown].
Fig. 1.
Kaplan-Meier survival curves according to proteinuria. Patients with proteinuria showed increased mortality risk from colorectal cancer, as compared with those without proteinuria.
Assessment of patients with colorectal cancer based on cancer staging and proteinuria status
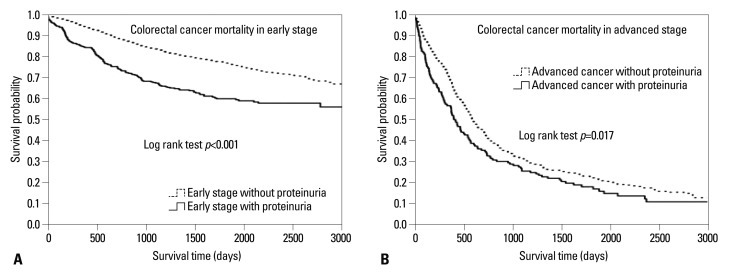
Kaplan-Meier survival curves of the four groups showed significant differences in mortality among the four groups (log-rank test, p<0.001). Proteinuric patients with advanced-stage cancer presented the lowest probability of event-free survival compared with all other groups. In addition, during the follow-up period, probability of death increased with advanced cancer staging and proteinuria.
The results of Cox proportional hazards model adjusted for other clinical variables of mortality are shown in Table 2. After adjusting for other risk variables, including age, gender, hypoalbuminemia, anemia, history of hypertension and diabetes mellitus, and eGFR categories, early-stage cancer with proteinuria was shown to be associated with an adjusted hazard ratio of 1.67, compared with early-stage cancer group without proteinuria (Table 2, upper panel). Patients with advanced cancer stage and proteinuria had the highest rate of all-cause mortality compared with other groups (HR, 7.19; 95% CI, 5.91-8.75, p<0.001).
Table 2.
Unadjusted and Adjusted Cox Proportional Hazards Model for Mortality According to Cancer Stage and Proteinuria* and Stratified Analysis of Cox Proportional Hazards Model
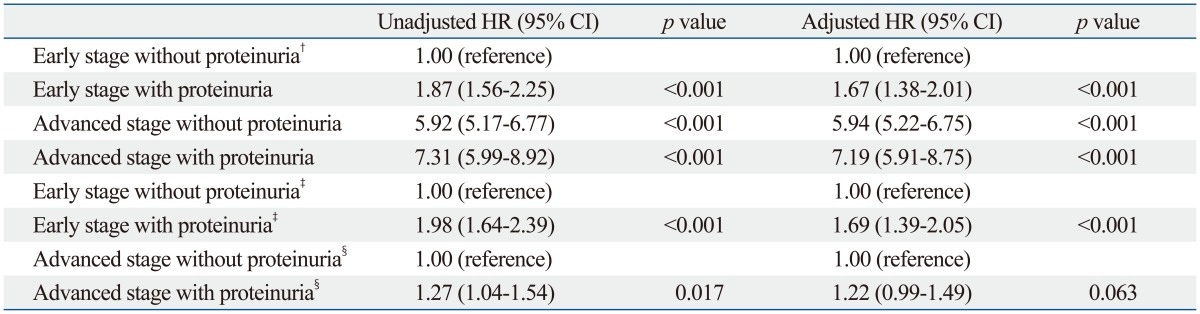
CI, confidence interval; HR, hazard ratio; eGFR, estimated glomerular filtration rate.
*The final model adjusted for age, gender, history of hypertension, diabetes, anemia, hypoalbuminemia, and eGFR categories.
†Proteinuria was defined by a reading of 1+ or more.
‡Stratified analysis of Unadjusted and adjusted Cox proportional hazards model for mortality in early cancer according to proteinuria.
§Stratified analysis of Unadjusted and adjusted Cox proportional hazards model for mortality in advanced cancer according to proteinuria.
Stratified analysis of all-cause mortality due to colorectal cancer associated with proteinuria
To exclude the influence of cancer staging, we compared the cancer mortality rates using a stratified analysis. We divided into patients of early stage and late stage and compared mortality in each stage separately (lower panel of Table 2). After adjusting covariates in the multivariate Cox's proportional hazards regression analysis, the HR of cancer deaths was higher in patients with proteinuria than in those without proteinuria in early-stage colorectal cancer (HR, 1.69; 95% CI, 1.39-2.05; p<0.001) (lower panel of Table 2). In contrast, in advanced-stage cancer, the mortality risk was the same between patients with and without proteinuria (lower panel of Table 2). Table 3 shows multivariate Cox's proportional hazard regression analysis of subdivided groups considering colorectal cancer staging. From stage 1 to 3, proteinuria was an independent risk factor in mortality. As shown in Kaplan-Meier survival curves, patients with proteinuria had a high mortality risk due to colorectal cancer than patients without proteinuria in both early and advanced cancer stage (Fig. 2).
Table 3.
Stratified Analysis of Unadjusted and Adjusted Cox Proportional Hazards Model for Mortality According to Cancer Stage with Proteinuria*
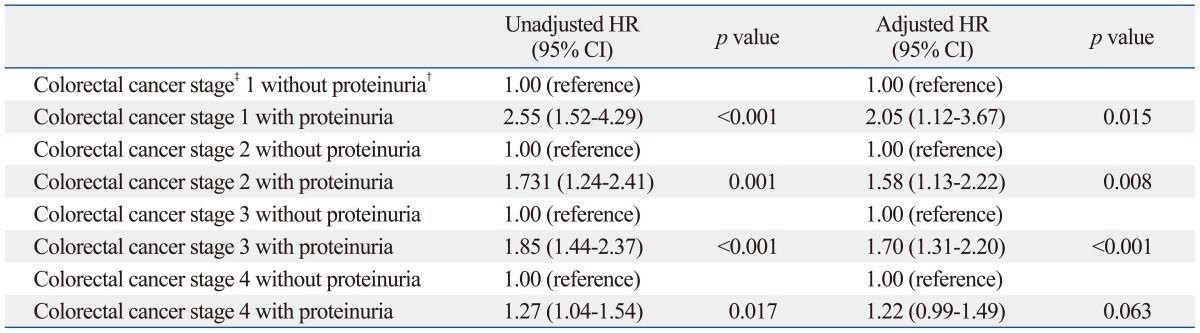
CI, confidence interval; HR, hazard ratio; AJCC, American Joint Committee on Cancer; eGFR, estimated glomerular filtration rate.
*The final model adjusted for age, gender, history of hypertension, diabetes, anemia, hypoalbuminemia, and eGFR categories.
†Proteinuria was defined by a reading of 1+ or more.
‡Colorectal cancer stage was based on TNM AJCC classification.
Fig. 2.
Kaplan-Meier survival curves according to cancer stage and proteinuria. Mortality from colorectal cancer, in both early (A) and advanced (B) cancer stages, increased incrementally with proteinuria.
Association of colorectal cancer mortality with renal insufficiency
To investigate the association between renal insufficiency and cancer-related mortality, patients were classified into 2 groups based on eGFR measurements. After adjusting covariates in the multivariate Cox's proportional hazards regression analysis, the mortality risk was not significantly different between patients with or without renal insufficiency (HR, 0.95; 95% CI, 0.84-1.10; p=0.449).
Assessing patients with colorectal cancer based on cancer staging and renal insufficiency
We divided the patients into 4 groups, based on their cancer staging and renal insufficiency, and compared the mortality among these groups. The results of Cox's proportional hazards model adjusted for other clinical variables of mortality are shown in Table 4. After adjusting for these other risk factors, there were no statistical differences between early-stage cancer groups with or without renal insufficiency (HR, 0.88; 95% CI, 0.75-1.04, p=0.143).
Table 4.
Unadjusted and Adjusted Cox Proportional Hazards Model for Mortality According to Cancer Stage and Renal Insufficiency* and Stratified Analysis of Cox Proportional Hazards Model
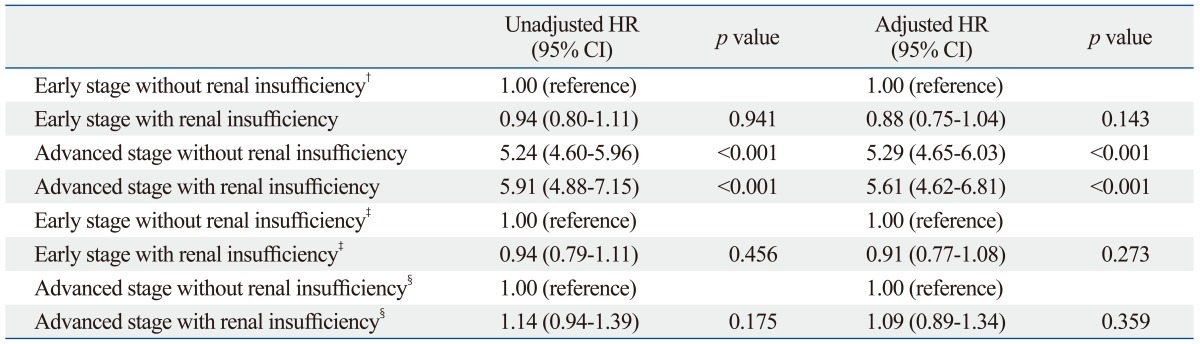
CI, confidence interval; HR, hazard ratio; eGFR, estimated glomerular filtration rate.
*The final model adjusted for age, gender, history of hypertension, diabetes, anemia, hypoalbuminemia, and eGFR categories.
†Renal insufficiency was defined as an eGFR <60 mL/min/1.73 m2.
‡Stratified analysis of Unadjusted and adjusted Cox proportional hazards model for mortality in early cancer according to renal insufficiency.
§Stratified analysis of Unadjusted and adjusted Cox proportional hazards model for mortality in advanced cancer according to renal insufficiency.
Stratified analysis of mortality associated with renal insufficiency in colorectal cancer
The lower panel of Table 4 shows the results of the Cox's proportional hazards model adjusted for other clinical risks of mortality due to renal insufficiency using a stratified analysis. After adjusting for other risk factors, there were no significant differences between early-stage and advanced-stage cancer. According to Kaplan-Meier survival curves, there was no association between renal insufficiency and mortality in patients with colorectal cancer (early-stage with renal insufficiency: log-rank test, p=0.455; advanced-stage with renal insufficiency: log-rank test, p=0.174, data not shown).
DISCUSSION
Our study demonstrated that proteinuria is a strong, independent prognostic factor for mortality in patients with colorectal cancer. Especially in the early-stage cancer patients, colorectal cancer mortality increased incrementally with comorbid proteinuria.
Microalbuminuria was originally thought to be an early sign of nephropathy in patients with diabetes; however, numerous other studies have found that microalbuminuria also predicts all-cause mortality in individuals not having diabetes.24 In this study, the prevalence of proteinuria was 14.6% in patients with colorectal cancer, and a higher prevalence was found in advanced-stage cancer rather than early-stage cancer (22.3% vs. 12.7%, p<0.001). In addition, all-cause mortality was higher in the proteinuria group than in the non-proteinuria group. These findings concur with those of other studies,25 and suggest proteinuria as a marker of generalized vascular dysfunction in malignancy besides glomerular dysfunction.26
A previous study by Lin, et al.25 demonstrated that albuminuria is associated with an increased risk of mortality in cancers of different origins, including lung and prostate cancers in men. Although cancer staging is an important risk factor for cancer mortality, Lin, et al. did not consider cancer staging in their study. In this context, we took into account cancer staging as a mortality risk factor while classifying cancer patients into four groups according to early or advanced cancer staging with or without proteinuria. Advanced-stage cancer patients showed higher mortality and higher HR than did early-stage cancer patients (advanced-stage cancer and proteinuria group: HR, 7.19; 95% CI, 5.91-8.75; p<0.001) (Table 2). To further analyze the effect of proteinuria on cancer mortality, we conducted a stratified analysis considering proteinuria in the early or advanced cancer stage. Presence of proteinuria was associated with increased cancer mortality in early-stage colorectal cancer, but not in advanced-stage cancer. These results suggest that proteinuria increases the risk of cancer-related deaths, especially in early-stage cancer, but not in advanced cancer. Therefore, cancer staging is concluded to be a powerful risk factor for colorectal cancer mortality.
Weng, et al.6 reported that deaths due to hepatic, renal, and urinary tract cancers increase incrementally with the severity of renal impairment. We adjusted for cancer staging in the multivariable analysis, and demonstrated no significant association between renal insufficiency (eGFR <60 mL/min/m2) and colorectal cancer mortality. The reason for discrepancies between previous studies and ours may be due to differences in study cases (e.g., combined different cancer types, including hepatic, renal, and urinary tract cancers versus single-entity colorectal cancer in this study). In addition, Weng, et al. did not include cancer staging as a risk variable while analyzing varied and multiple cancers. Cancer staging is a strong prognostic factor for cancer-specific mortality and should be included as a risk variable. These findings combined with our data suggest that renal insufficiency itself is not an independent risk factor for cancer mortality in colorectal cancer.
Proteinuria is a strong marker of increased cardiovascular mortality as well as CKD progression.27 Previous research has shown that immunological changes are common in the kidneys of patients who have died of malignancy.28-30 Although the underlying mechanisms are unknown, albuminuria has been regarded as a paraneoplastic phenomenon that is an outcome of inflammatory processes. Increased glomerular permeability and, thus, albuminuria can be due to the presence of neoplastic cells, which secrete elevated cytokine levels. We found that the higher death rate in early-stage cancer patients may be associated with proteinuria. Proteinuria was associated with an increase in overall cancer patient mortality rates, independent of other known risk factors (HR, 1.79; 95% CI, 1.476-2.171; p<0.001). This observation suggests that assessing, monitoring and managing proteinuria in cancer patients are crucial because of its potential effect on survival, especially in early-stage colorectal cancer.
Even though these factors may explain the relationship between proteinuria and cancer prognosis, few studies have assessed the incidence and clinical effects of proteinuria in patients with colorectal cancer.25 Therefore, understanding the effect of proteinuria on cancer mortality due to colorectal cancer will assist with the development of guidelines and follow-up care protocols for patients with advanced colorectal cancer. Furthermore, even with a relatively normal renal function, proteinuria can present at the early stages of renal impairment or GFR loss. We concluded that proteinuria is an important prognostic marker of mortality in patients with colorectal cancer.
Our study has several limitations. We used only a dipstick test to measure proteinuria. Twenty-four hour urine collections should be used to measure its constituents and albumin-to-creatinine ratios to quantify proteinuria better, confirm its prevalence,31 verify the prognostic importance and determine its incidence.
In conclusion, the present study showed that proteinuria is an important risk factor for cancer specific mortality, especially in early stage colorectal cancer. In colorectal cancer patients, early diagnosis and appropriate management of proteinuria may improve anticancer treatment and outcomes.
ACKNOWLEDGEMENTS
This research was supported by a grant from the Ministry of Science & Technology (MoST)/Korea Science & Engineering Foundation (KOSEF) (2010-0021808) and by the National Research Foundation of Korea (NRF) grant (MRC for Gene Regulation, 2011-0030132) funded by the Korea government (MSIP).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012;44:25–31. doi: 10.4143/crt.2012.44.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 3.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 4.Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM South Tees Diabetes Mortality Study. Cause-specific mortality in a population with diabetes: South Tees Diabetes Mortality Study. Diabetes Care. 2002;25:43–48. doi: 10.2337/diacare.25.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol. 2003;157:1092–1100. doi: 10.1093/aje/kwg100. [DOI] [PubMed] [Google Scholar]
- 6.Weng PH, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol. 2011;6:1121–1128. doi: 10.2215/CJN.09011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Na SY, Sung JY, Chang JH, Kim S, Lee HH, Park YH, et al. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol. 2011;33:121–130. doi: 10.1159/000323740. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen LM, Milman N. Microalbuminuria in patients with lung cancer. Eur J Cancer. 1998;34:76–80. doi: 10.1016/s0959-8049(97)10003-x. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen LM, Johnsen HE. Microalbuminuria is associated with impaired glomerular permselectivity in lymphoma patients. Scand J Clin Lab Invest. 2005;65:477–484. doi: 10.1080/00365510510025827. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen LM, Sørensen PG. Mediators of inflammation correlate with microalbuminuria in patients with non-Hodgkin's lymphoma. Br J Haematol. 2003;121:275–279. doi: 10.1046/j.1365-2141.2003.04285.x. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen LM, Milman N. Prevalence and prognostic significance of proteinuria in patients with lung cancer. Acta Oncol. 1996;35:691–695. doi: 10.3109/02841869609084000. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen LM, Sørensen PG. Increased urinary albumin excretion rate in breast cancer patients. Acta Oncol. 2000;39:145–149. doi: 10.1080/028418600430699. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen LM, Sørensen PG. Clinical significance of urinary albumin excretion in patients with non-Hodgkin's lymphoma. Br J Haematol. 1999;107:889–891. doi: 10.1046/j.1365-2141.1999.01772.x. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen LM, Sørensen PG. Urinary albumin excretion is a predictor of response to treatment and disease progression in low-grade non-Hodgkin's lymphoma. Leuk Lymphoma. 2004;45:547–551. doi: 10.1080/10428190310001593049. [DOI] [PubMed] [Google Scholar]
- 15.Sawyer N, Wadsworth J, Wijnen M, Gabriel R. Prevalence, concentration, and prognostic importance of proteinuria in patients with malignancies. Br Med J (Clin Res Ed) 1988;296:1295–1298. doi: 10.1136/bmj.296.6632.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roumen RM, Wijnen MH. Proteinuria: a frequent paraneoplastic phenomenon in colorectal cancer? Eur J Cancer. 1998;34:206–207. doi: 10.1016/s0959-8049(97)00346-8. [DOI] [PubMed] [Google Scholar]
- 17.Puolijoki H, Mustonen J, Pettersson E, Pasternack A, Lahdensuo A. Proteinuria and haematuria are frequently present in patients with lung cancer. Nephrol Dial Transplant. 1989;4:947–950. doi: 10.1093/ndt/4.11.947. [DOI] [PubMed] [Google Scholar]
- 18.Georgiannos SN, Weston PM, Goode AW. Correlation between albuminuria and positively charged amino acids in gastrointestinal cancer. Int Surg. 1995;80:49–52. [PubMed] [Google Scholar]
- 19.Vaglio A, Buzio L, Cravedi P, Pavone L, Garini G, Buzio C. Prognostic significance of albuminuria in patients with renal cell cancer. J Urol. 2003;170(4 Pt 1):1135–1137. doi: 10.1097/01.ju.0000085984.90991.9a. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen LM, Terslev L, SŁrensen PG, Stokholm KH. Urinary albumin excretion and transcapillary escape rate of albumin in malignancies. Med Oncol. 2000;17:117–122. doi: 10.1007/BF02796206. [DOI] [PubMed] [Google Scholar]
- 21.Greene FL. Current TNM staging of colorectal cancer. Lancet Oncol. 2007;8:572–573. doi: 10.1016/S1470-2045(07)70185-7. [DOI] [PubMed] [Google Scholar]
- 22.Engstrom PF, Arnoletti JP, Benson AB, 3rd, Chen YJ, Choti MA, Cooper HS, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw. 2009;7:778–831. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Karalliedde J, Viberti G. Microalbuminuria and cardiovascular risk. Am J Hypertens. 2004;17:986–993. doi: 10.1016/j.amjhyper.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Lin YS, Chiu FC, Lin JW, Hwang JJ, Caffrey JL. Association of albuminuria and cancer mortality. Cancer Epidemiol Biomarkers Prev. 2010;19:2950–2957. doi: 10.1158/1055-9965.EPI-10-0617. [DOI] [PubMed] [Google Scholar]
- 26.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 27.Gorriz JL, Martinez-Castelao A. Proteinuria: detection and role in native renal disease progression. Transplant Rev (Orlando) 2012;26:3–13. doi: 10.1016/j.trre.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland JC, Markham RV, Jr, Mardiney MR., Jr Subclinical immune complexes in the glomeruli of kidneys postmortem. Am J Med. 1974;57:536–541. doi: 10.1016/0002-9343(74)90003-5. [DOI] [PubMed] [Google Scholar]
- 29.Pascal RR, Iannaccone PM, Rollwagen FM, Harding TA, Bennett SJ. Electron microscopy and immunofluorescence of glomerular immune complex deposits in cancer patients. Cancer Res. 1976;36:43–47. [PubMed] [Google Scholar]
- 30.Beaufils H, Jouanneau C, Chomette G. Kidney and cancer: results of immunofluorescence microscopy. Nephron. 1985;40:303–308. doi: 10.1159/000183483. [DOI] [PubMed] [Google Scholar]
- 31.Wu MT, Lam KK, Lee WC, Hsu KT, Wu CH, Cheng BC, et al. Albuminuria, proteinuria, and urinary albumin to protein ratio in chronic kidney disease. J Clin Lab Anal. 2012;26:82–92. doi: 10.1002/jcla.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]