Abstract
Purpose
Opioid-based intravenous patient-controlled analgesia (IV PCA) is popular method of postoperative pain control, but many patients suffer from IV PCA-related postoperative nausea and vomiting (PONV). In this retrospective observational study, we have determined independent predictors of IV PCA-related PONV and predictive values of the Apfel's simplified risk score in pursuance of identifying high-risk patients.
Materials and Methods
We analyzed 7000 patients who received IV PCA with background infusion after elective surgery. Patients who maintained IV PCA for a postoperative period of 48 hr (completion group, n=6128) were compared with those who have discontinued IV PCA within 48 hr of surgery due to intractable PONV (cessation group, n=872). Patients, anesthetics, and surgical factors known for predicting PONV were evaluated by logistic regression analysis to identify independent predictors of IV PCA related intractable PONV.
Results
In a stepwise multivariate analysis, weight, background infusion dose of fentanyl, addition of ketolorac to PCA, duration of anesthesia, general anesthesia, head and neck surgery, and Apfel's simplified risk score were revealed as independent risk factors for intractable PONV followed by the cessation of IV PCA. In addition, Apfel's simplified risk score, which demonstrated the highest odds ratio among the predictors, was strongly correlated with the cessation rate of IV PCA.
Conclusion
Multimodal prophylactic antiemetic strategies and dose reduction of opioids may be considered as strategies for the prevention of PONV with the use of IV PCA, especially in patients with high Apfel's simplified risk scores.
Keywords: Apfel's simplified risk score, patient-controlled analgesia, postoperative nausea and vomiting
INTRODUCTION
Intravenous patient-controlled analgesia (IV PCA) is a widely used postoperative analgesic strategy for its effectiveness and safety as acute postoperative pain relief.1-3 However, postoperative analgesia using opioids is associated with a high incidence of postoperative nausea and vomiting (PONV), despite multimodal preventive approaches.4-7 Clinically, opioid-based IV PCA is occasionally discontinued because of patients' complaints of various opioid-induced side effects, such as PONV, dizziness, urinary retention, and pruritis.1,8 In extreme cases, intractable PONV may even be perceived as a failure of IV PCA as a pain management technique. The cessation of IV PCA due to intractable PONV might also be an unreasonable clinical decision made by the surgical team because opioid-based IV PCA was thought to play a larger role in PONV than evidence suggested.9 The relationship between opioid usage and PONV is complex, and applying various adjuvants with opioid sparing effects to multimodal analgesic regimens does not necessarily lead to a reduced incidence of PONV.9,10 Numerous studies have investigated the risk factors for predicting the development of PONV, including those related to the patient, the anesthesia, and the surgery.11-19 However, no such comprehensive data exist regarding to the association of risk factors and intractable PONV with the postoperative use of IV PCA with background infusion, which has mainly been based on the site of operation and the age, weight, and gender of the patient.3 Moreover, possible risk factors for IV PCA-related PONV should be identified because the cessation of IV PCA may not only lead to discomfort, but also dissatisfy patients due to poor postoperative pain management and, as a result, increasing hospital costs.20,21
We hypothesized that multiple factors affect the cessation of IV PCA caused by intractable PONV. To test our hypothesis, we analyzed elective surgical patients who received fentanyl-based IV PCA for their postoperative pain control, using our large observational database. The aim of this study was to investigate perioperative predictive factors for intractable PONV accompanied by the cessation of IV PCA, as well as to evaluate the predictive value of the Apfel's simplified risk score for the cessation of IV PCA due to intractable PONV.
MATERIALS AND METHODS
The Institutional Review Board of Severance Hospital (ref. 4-2011-0475) approved this study and written informed consent was waived. Since 2009, the PCA service team has prospectively collected comprehensive clinical data for all postoperative PCA management for the purpose of clinical audit and outcome assessment (more than 20000 cases at present). The sample population in this study was defined as elective surgical patients who received fentanyl-based IV PCA for postoperative pain control between September 2010 and September 2011 at Severance Hospital. Patients were excluded for the following: age <18 years, age >80 years, American Society of Anesthesiologists physical status class ≥III, requirement of postoperative ventilator support or intensive care, and having received total intravenous anesthesia (TIVA). Patients who maintained IV PCA for a postoperative period of 48 hr (completion group) were compared with those who discontinued IV PCA within 48 hr after surgery due to intractable PONV (cessation group).
IV PCA and PONV management
General anesthesia was induced with propofol and opioids (remifentanil or fentanyl), and maintained with volatile anesthetics (isoflurane, sevoflurane, or desflurane) with/without a continuous infusion of remifentanil (0.05-0.2 µg/kg/min). All patients used the same model of disposable PCA pump (Accufuser plus® P2015M; Woo Young Medical, Chungbuk, Korea), which was programmed to deliver 2 mL/hr as a background infusion and 0.5 mL per demand, with a 15-min lockout during a 48-hr period. The PCA regimen typically consisted of fentanyl (concentration 10-15 µg/mL) plus normal saline (total volume of 100 mL). At the discretion of the attending anesthesiologists, 90-120 mg of ketorolac was added as an adjuvant to the PCA regimen according to age, preoperative renal function, or type of surgery. Additionally, a 5-hydroxytryptamine receptor 3 (5-HT3) antagonists (ondansetron 4-8 mg or ramosetron 0.3 mg) were either given at the end of surgery, or dexamethasone 4 mg was administered intravenously after induction of anesthesia. All IV PCA were started at a post-anesthesia care unit.
The PCA service team, which was comprised of anesthesiologists and PCA nurse specialists, monitored patients at 1-6, 6-24, and 24-48 hr intervals after surgery to inquire about the occurrence of adverse events, and the need for rescue analgesics and/or antiemetics. Fentanyl-based IV PCA-related adverse events included PONV, sedation, dizziness, headache, hypotension, and pruritis. In addition to these parameters, the incidence of PONV, severity of nausea, and pain intensity scores were recorded at the aforementioned time points. The intensity of nausea was graded on verbal rating scales using an 11-point scale (0=no nausea to 10=worst possible nausea). Pain intensity scores were measured on a visual analog scale (0 mm=no pain to 100 mm=worst pain imaginable). IV PCA related PONV management in a general ward was conducted in regular sequence by institutional guidelines. 1) Patients are given IV rescue antiemetic (ondansetron 4 mg) if moderate to severe nausea (verbal rating score ≥4) lasting ≥15 min and/or emetic symptoms (vomiting, retching) and/or upon the request of the patient; 2) If PONV is persistent 1 hr after rescue antiemetic treatment, IV PCA is temporarily stopped for approximately 2 hr to identify the cause of PONV; 3) If PONV is persistent or relieved PONV develop by resumed IV PCA, additional IV antiemetic (ondansetron 4 mg or ramosetron 0.3 mg) is given to patients; 4) The IV PCA was completely discontinued when intractable PONV persisted despite additional IV rescue antiemetic. Thus, IV PCA-related intractable PONV was defined as persistent nausea or emetic symptoms not relieved by appropriate antiemetic therapy when IV PCA was maintained. All prophylactic and rescue antiemetics that were administrated during the perioperative period were recorded.
The PCA service team kept tract of the reason for cessation, time of cessation, adverse side effects, and residual volume of IV PCA if it was discontinued within 48 hr of surgery. Additional patient data includes age, gender, height, weight, medical history, duration of anesthesia, type of anesthesia, type of surgery, smoking history, and previous history of motion sickness or PONV.
Statistical analysis
Continuous variables are shown as mean±standard deviation, and categorical variables are shown as number (percentage). The variables used for analysis included demographic data (age, gender, weight) and known risk factors associated with PONV (background infusion dose of fentanyl in PCA, addition of ketorolac in PCA, type of anesthesia, type of surgery, duration of anesthesia, diabetes mellitus, adjuvant chemotherapy, Apfel's simplified risk score). Patient and anesthetic characteristics were analyzed by independent t-test, χ2 test, or Fisher's exact test, where appropriate. Logistic regression was used to compute crude odds ratios (ORs) with 95% confidence intervals (CIs) for variables associated with the cessation of IV PCA due to IV PCA-related intractable PONV. Variables that had a p-value <0.05 were included in the multivariate logistic regression analysis estimating adjusted ORs with 95% CIs. The trend of the cessation rate of IV PCA according to Apfel's simplified risk score was analyzed using the Cochran-Armitage trend test. Additionally, a receiver operating characteristics (ROC) curve was drawn. The area under the ROC-curve (AUC) was used to estimate the discriminating power of the Apfel risk score for prediction of IV PCA related PONV and the cut-off point for a predictive risk of IV PCA related PONV was calculated. Pearson correlation analysis was used to test the associations between weight and background infusion dose of fentanyl in PCA. Statistical analysis was performed with SAS (version 9.2; SAS Institute, Inc., Cary, NC, USA). Values of p<0.05 were considered as statistically significant.
RESULTS
A total of 9221 patients were assessed for study eligibility, of which 7073 initially met the study criteria. IV PCA was ceased within 48 hr after surgery due to fentanyl-based IV PCA-related adverse events for 945 patients. After the exclusion of patients with other reasons for cessation of IV PCA, including headache and/or dizziness, hypotension, sedation, urinary retention and pruritis, 872 patients were included in the cessation group due to IV PCA-related intractable PONV. In the cessation group, IV PCA was ceased in 7%, 65%, and 28% of patients 1-6, 6-24, and 24-48 hr after surgery, respectively. The final study population included 7000 patients in this observational study (6128 patients in the completion group and 872 patients in the cessation group) (Fig. 1).
Fig. 1.
Study profile. TIVA, total intravenous anesthesia; PONV, postoperative nausea and vomiting; IV PCA, intravenous patient-controlled analgesia; ASA, American Society of Anesthesiologists.
Data pertaining to patient characteristics, anesthesia, and surgery are described in Table 1. There were no significant differences between the completion group and the cessation group in age, presence of diabetes mellitus and neoadjuvant chemotherapy, concentration of fentanyl in PCA, and the number of incidence for intraoperative remifentanil infusion. All patients received prophylactic antiemetic therapy (5-HT3 antagonists) at the end of surgery. The percentage of patients receiving combination prophylactic antiemetic therapy (5-HT3 antagonist plus dexamethasone) was also similar between the two groups. There was a significant difference between the three volatile anesthetics used regarding to the overall cessation rate (9%, 15%, and 12% after isoflurane, desflurane, and sevoflurane, respectively; p<0.001), although the cessation rate during the initial 1-6-hr postoperative period did not differ among them (0.7%, 1.2%, and 1.1% after isoflurane, desflurane, and sevoflurane, respectively; p=0.669). In the Pearson correlation analysis, there was a statistically significant negative correlation between the background infusion dose of fentanyl in PCA and weight (r=-0.361, p<0.001).
Table 1.
Patient Characteristics, Anesthetic and Surgical Data
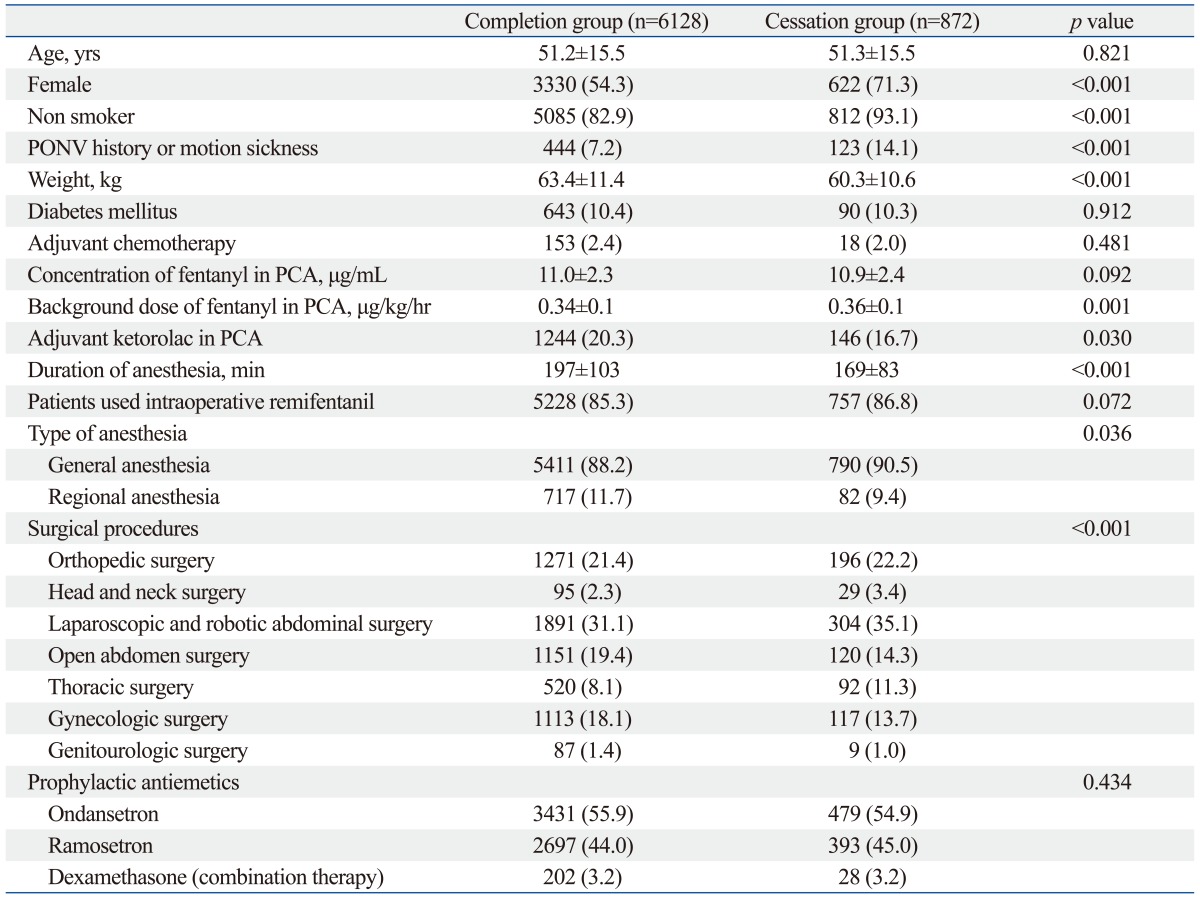
PCA, patient controlled analgesia; PONV, postoperative nausea and vomiting.
Values are number of patients (%) or means±standard deviation.
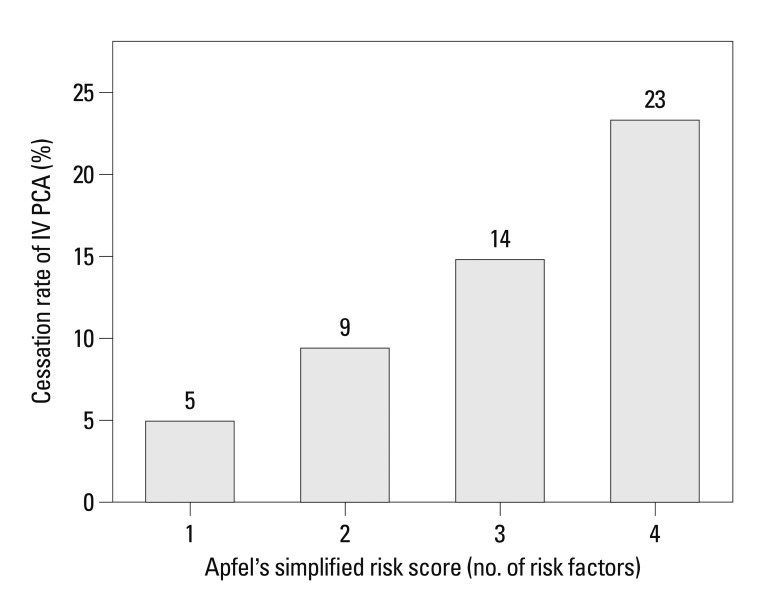
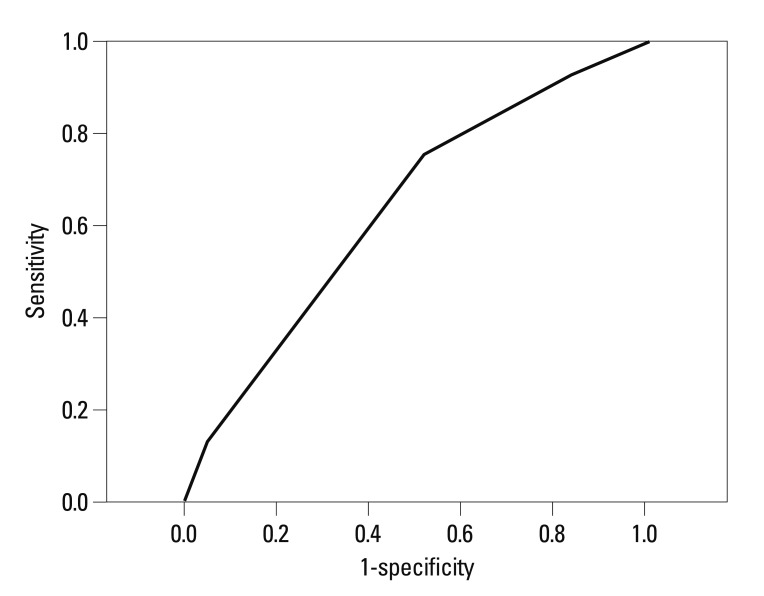
In the univariate analysis, we found that female gender, decreased weight, increased background infusion dose of fentanyl in PCA, non-addition of ketorolac in PCA, decreased duration of anesthesia, general anesthesia, head and neck surgery, and the risk factors of Apfel's simplified risk score were significantly associated with an increased incidence of IV PCA-related intractable PONV (Table 1). In the multivariate analysis of these variables, weight, background infusion dose of fentanyl, addition of ketorolac in PCA, duration of anesthesia, general anesthesia, head and neck surgery, and a high Apfel simplified risk score remained independent risk factors for IV PCA-related intractable PONV (Table 2). Additionally, the cessation rate of IV PCA due to intractable PONV was proportionally increased according to Apfel's simplified risk score with statistical significance based on the Cochran-Armitage trend test (p<0.001) (Fig. 2). The area under the ROC curve for the Apfel risk score was 0.671 (95% CI 0.661-0.686) and the optimal cut-off point was ≥3 risk factors (corresponding sensitivity=71%, specificity=58%) (Fig. 3).
Table 2.
Factors Associated with Cessation of IV PCA Due to PONV: Results of the Logistic Regression Analysis
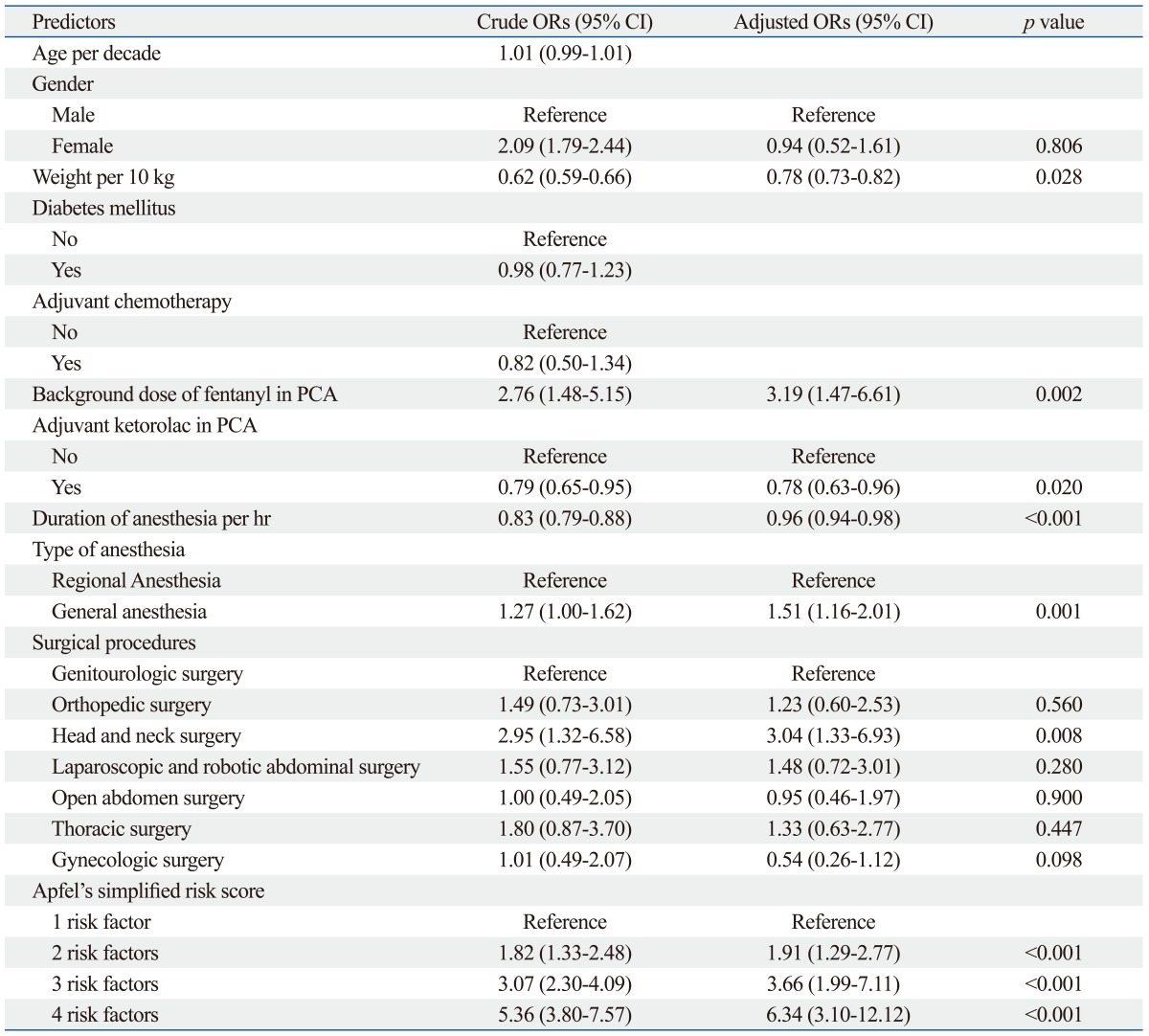
OR, odds ratio; CI, confidence interval; PCA, patient-controlled analgesia; PONV, postoperative nausea and vomiting.
Significant variables (p<0.05) in univariate analysis were selected for multivariate analysis.
Fig. 2.
The correlation between Apfel's simplified risk score and the cessation rate of IV PCA due to PONV. Data showed strong linear correlation between them based on Cochran-Armitage trend test (p<0.001). IV PCA, intravenous patient controlled analgesia; PONV, postoperative nausea and vomiting.
Fig. 3.
ROC-curve for the prediction of PONV for Apfel's simplified risk score (AUC 0.67, 95% CI 0.66-0.68). ROC-curve, receiver operating characteristic curve; PONV, postoperative nausea and vomiting; AUC, area under the ROC curve; CI, confidence interval.
During the entire PONV assessment period, the percentage of patients experiencing PONV and receiving rescue antiemetics was significantly higher in the cessation group than in the completion group (p<0.001), except for the percentage of patients receiving rescue antiemetics in the 24-48 hr postoperative period (p=0.377) (Fig. 4). The proportion of patients who experienced moderate-to-severe nausea (verbal rating score ≥4) was also significantly higher in the cessation group than in the completion group throughout the study period (1-6 hr: 5% vs. 38%; 6-24 hr: 4% vs. 34%; 24-48 hr: 1% vs. 19%; p<0.001). There were no differences among pain scores or the need for rescue analgesics between the two groups in each of the assessment intervals, except for the need for rescue analgesics in the 6-24 hr period, which was higher in the cessation group than the completion group (36% vs. 30%, p=0.008).
Fig. 4.
The percentages of patients experiencing PONV and receiving rescue antiemetics at 1-6, 6-24, and 24-48 hr after surgery. *p<0.001 versus completion group. PONV, postoperative nausea and vomiting.
DISCUSSION
This is the first study to analyze which patients, anesthetics, and surgical variables affect intractable PONV in the opioid-based IV PCA environment, resulting in the cessation of IV PCA. We identified the Apfel's simplified risk score, background infusion dose of fentanyl in PCA, addition of ketorolac in the PCA, type of surgery, anesthetic technique, and duration of anesthesia as significant predictors for the cessation of IV PCA caused by intractable PONV. In addition, the Apfel's simplified risk score, which demonstrated the highest OR among the predictors, was strongly correlated with the cessation rate of IV PCA.
The Apfel's simplified risk score for predicting PONV consists of four predictors: female gender, nonsmoking, history of PONV or motion sickness, and the use of postoperative opioids. When none, one, two, three, or four of these risk factors were present, it was found that the incidences of PONV were 10%, 21%, 39%, 61%, and 79%, respectively.18 Furthermore, the Apfel's simplified risk score can predict the risk of PONV with more favorable discrimination and calibration properties than other PONV scoring systems.14,17,22 In our multivariate analysis, the Apfel's simplified risk score was the strongest predictive factor for IV PCA-related intractable PONV among the numerous patients, anesthetics and surgical parameters that we assessed. In addition, the Apfel's simplified risk score was proportionally correlated with an increase in the cessation rate of IV PCA due to PONV. The value of AUC for prediction of PONV using the Apfel's simplified risk score (AUC=0.67) was similar to that of multi-center studies conducted in non-IV PCA environments (AUC=0.63 to 0.72).15,17 All of the patients in the present study had more than one risk factor of the Apfel's score (use of postoperative opioid) and received a prophylactic antiemetic therapy. These reasons may affect the PONV predictive power of the Apfel's score in our study. Female gender is the strongest single predictor for PONV among four predictors in Apfel's simplified risk score.15 We thus included female gender in the multivariate analysis as a separate variable despite the possibility of interactions in variables of the Apfel's model. Female gender was a significant independent predictor of IV PCA related intractable PONV in the univariate analysis, although this factor did not remain significant in the multivariate analysis. This result was consistent with a previous study, which found that the Apfel's simplified risk score model with four predictors was superior to a single predictor model with female gender alone for estimating individual risk for PONV.15
Next in importance to the Apfel's simplified risk score for predicting IV PCA-related intractable PONV was the infusion dose of fentanyl. Our results were consistent with previous studies that demonstrated a strong dose-response relationship between postoperative opioid use and PONV.23,24 In our study, ketorolac, nonsteroidal anti-inflammatory drug, were combined with fentanyl as an adjuvant therapy in the IV PCA for approximately 20% of the analyzed patients. Previously, the addition of ketorolac to an opioid-based IV PCA has been reported to reduce postoperative opioid requirements and opioid related side effects.10 We similarly found that the addition of ketorolac was associated with a reduced dose of fentanyl, which led to a decrease in the cessation rate of IV PCA (11% vs. 13%, p=0.014) compared with the use of fentanyl alone. In our study, the IV PCA device was programmed to deliver a continuous background infusion, which may increase the total amount of opioid delivered and the incidence of side effects.25 Although the consumption of fentanyl could not be assessed in each of the time intervals because of the simplicity of our balloon type PCA device, postoperative pain scores were similar between the two groups during the 48-hrpostoperative period. However, the need for rescue analgesics in the 6-24 hr period was higher in the cessation group than the completion group because the cessation of IV PCA might require additional pain treatment, especially in the acute postoperative pain phase.
In general, the IV PCA opioid use has primarily been based on gender, age, body weight, cancer, and surgical site.3 Body weight was found to be a significant predictor for IV PCA demand, although body weight and BMI appeared to have little influence on the incidence of PONV.3,26 By logistic regression analysis, low body weight were significant factors for predicting the cessation of IV PCA due to intractable PONV in our study. This result may be explained by the close correlation between weight and the background infusion dose of fentanyl in the PCA despite a similar concentration of fentanyl between the two groups. In a previous study, the effect of weight on IV PCA morphine requirements increased gradually from 10th percentile to 80th percentile of weight but decreased at the 90th percentile.3 Likewise, a careful determination of fentanyl dosage in the IV PCA should be considered especially for patients with low weight. On the other hand, age was not a significant predictor for the cessation of IV PCA due to intractable PONV in our study. Although the incidence of PONV is the highest in young female adults, the known effects of age are not strong enough for its incorporation in the risk model for PONV.12,18,22
The effect of the type of surgery on the incidence of PONV has been debated in the literature. Some studies have suggested that the type of surgery was associated with a high incidence of PONV, whereas others have suggested that differences in the incidence of PONV were mainly due to patient- or anesthesia-related factors.27 In our study, we categorized the type of surgery as anatomical groups of procedures to exclude the bias of narrow classifications such as gender, anesthetic technique, or particular surgical procedure.28 We found that patients who underwent head and neck surgery were the highest risk for the cessation of IV PCA in a fentanyl-based IV PCA environment. In our categorization, head and neck surgery included extensive procedures, such as facial reconstruction surgery with wired jaw and radical neck dissection, which required a long duration of balanced anesthesia. In addition, head and neck surgery incorporated multiple factors related to the pathogenesis of PONV, such as activation of target receptors in the chemoreceptor trigger zone and vestibular system, which might also explain our results.29
The duration of anesthesia is also a possible risk factor for PONV,11,27 although this association is not yet well established.18,22 Interestingly, our analysis showed that a prolonged duration of anesthesia was associated with a reduction in the frequency of IV PCA-related intractable PONV and the decrease in the cessation rate of IV PCA due to PONV. Duration of anesthesia can be affected by various factors, such as type of surgery and anesthesia technique. The duration of anesthesia still remained an independent predictor for IV PCA related PONV in multivariate analysis with adjustment of these factors. This result might be explained by the characteristics of our study population distribution. In our study, patients with high Apfel's scores underwent a shorter duration of anesthesia (1 risk factor: 218±98 min, 2 risk factors: 215±103 min, 3 risk factors: 178±98 min, 4 risk factors: 174±90 min, respectively; p<0.001). Also, most of our study population is limited to patients with ≥90 min prolonged duration of anesthesia (86% of all patients), and there was no difference in the cessation rate of IV PCA due to PONV between patients with <90 min and ≥90 min duration of anesthesia (13.6% vs. 12.3%, p=0.213), which should be considered for the interpretation of the data.
Intraoperative opioids and volatile anesthetics have also been suggested as possible risk factors for PONV,22 but these factors mainly affect PONV in the early postoperative period, including the time in the post-anesthesia care unit.16,30,31 In our study, the cessation rate during the initial 1-6-hr postoperative period represented a relatively small portion of the study population, and this did not differ among the three volatile anesthetics. Thus, we did not include intraoperative opioids or the type of volatile anesthetics in our logistic regression analysis. However, general volatile or balanced anesthesia was associated with a higher incidence of PONV than was regional anesthesia in our study, which is consistent with previous studies.13,32 Thus, reducing baseline risk factors associated with anesthesia technique should be kept in mind for prevention of IV PCA related PONV.
We acknowledge several limitations of this study. Being observational in nature, this study also used the real-world clinical practice model in which attending physicians from various surgical departments decided on rescue antiemetics, as well as the use and the cessation of IV PCA on the general ward, according to institutional guidelines. Therefore, the decision to administer antiemetics or to cease IV PCA could have been influenced by the attending physician's judgment and preconceptions concerning IV PCA-related PONV. Furthermore, combination antiemetic prophylaxis was conducted in only 6% of all patients. Finally, we could not collect data on patients who underwent TIVA, which was conducted mainly by neurosurgery in our hospital. Hence, we were unable to study an entire surgical population, including patients uninfluenced by combination antiemetic prophylaxis or who underwent TIVA. Therefore, further studies will be needed to take these limitations into consideration.
In conclusion, our results suggest that anesthesiologists can predict an increased risk of IV PCA-related intractable PONV in patients with high Apfel's scores and those undergoing head and neck surgery. Thus, patients with these strong predictors might need careful attention in the selection of intraoperative anesthetic technique, dose of fentanyl used in IV PCA, and prophylactic antiemetic strategies. These results may offer practical information for the prevention of IV PCA-related PONV and the improvement of the quality of pain control.
ACKNOWLEDGEMENTS
We wish to thank PCA nurse practitioner Won-Hee Baek and Sun-Young Oh for their expert assistance in collecting data. In addition, statistical help from Associate Professor Jieun Kim and Research Assistant Min-Woong Kang at the Biostatistics Collaboration Unit of Yonsei University College of Medicine was greatly appreciated.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36–46. doi: 10.1093/bja/87.1.36. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne JC, Carr DB, Chalmers TC, Dear KB, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: meta-analyses of initial randomized control trials. J Clin Anesth. 1993;5:182–193. doi: 10.1016/0952-8180(93)90013-5. [DOI] [PubMed] [Google Scholar]
- 3.Yen CR, Tsou MY, Mandell MS, Chan CT, Chan KH, Chen TH, et al. An analysis of patient variables that influence intravenous patient-controlled analgesic use of morphine with quantile regression. Anesthesiology. 2010;112:688–695. doi: 10.1097/ALN.0b013e3181cbd1f3. [DOI] [PubMed] [Google Scholar]
- 4.Song JW, Park EY, Lee JG, Park YS, Kang BC, Shim YH. The effect of combining dexamethasone with ondansetron for nausea and vomiting associated with fentanyl-based intravenous patient-controlled analgesia. Anaesthesia. 2011;66:263–267. doi: 10.1111/j.1365-2044.2011.06648.x. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa Y, Imashuku Y, Kitagawa H, Kawamoto S, Yuasa M, Nosaka S. [Evaluation of the side effects of intravenous patient controlled analgesia after spine surgery] Masui. 2011;60:920–923. [PubMed] [Google Scholar]
- 6.Choi DK, Chin JH, Lee EH, Lim OB, Chung CH, Ro YJ, et al. Prophylactic control of post-operative nausea and vomiting using ondansetron and ramosetron after cardiac surgery. Acta Anaesthesiol Scand. 2010;54:962–969. doi: 10.1111/j.1399-6576.2010.02275.x. [DOI] [PubMed] [Google Scholar]
- 7.Tramèr MR, Walder B. Efficacy and adverse effects of prophylactic antiemetics during patient-controlled analgesia therapy: a quantitative systematic review. Anesth Analg. 1999;88:1354–1361. doi: 10.1097/00000539-199906000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell LG, Kaufmann SC, Bitzer S, Jackson EV, Jr, McGready J, Kost-Byerly S, et al. The effects of a small-dose naloxone infusion on opioid-induced side effects and analgesia in children and adolescents treated with intravenous patient-controlled analgesia: a double-blind, prospective, randomized, controlled study. Anesth Analg. 2005;100:953–958. doi: 10.1213/01.ANE.0000148618.17736.3C. [DOI] [PubMed] [Google Scholar]
- 9.Woodhouse A, Mather LE. Nausea and vomiting in the postoperative patient-controlled analgesia environment. Anaesthesia. 1997;52:770–775. doi: 10.1111/j.1365-2044.1997.144-az0148.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim SY, Kim EM, Nam KH, Chang DJ, Nam SH, Kim KJ. Postoperative intravenous patient-controlled analgesia in thyroid surgery: comparison of fentanyl and ondansetron regimens with and without the nonsteriodal anti-inflammatory drug ketorolac. Thyroid. 2008;18:1285–1290. doi: 10.1089/thy.2008.0007. [DOI] [PubMed] [Google Scholar]
- 11.Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology. 1999;91:109–118. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52:443–449. doi: 10.1111/j.1365-2044.1997.117-az0113.x. [DOI] [PubMed] [Google Scholar]
- 13.Stadler M, Bardiau F, Seidel L, Albert A, Boogaerts JG. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology. 2003;98:46–52. doi: 10.1097/00000542-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Pierre S, Benais H, Pouymayou J. Apfel's simplified score may favourably predict the risk of postoperative nausea and vomiting. Can J Anaesth. 2002;49:237–242. doi: 10.1007/BF03020521. [DOI] [PubMed] [Google Scholar]
- 15.Apfel CC, Kranke P, Greim CA, Roewer N. What can be expected from risk scores for predicting postoperative nausea and vomiting? Br J Anaesth. 2001;86:822–827. doi: 10.1093/bja/86.6.822. [DOI] [PubMed] [Google Scholar]
- 16.Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002;88:659–668. doi: 10.1093/bja/88.5.659. [DOI] [PubMed] [Google Scholar]
- 17.Apfel CC, Kranke P, Eberhart LH, Roos A, Roewer N. Comparison of predictive models for postoperative nausea and vomiting. Br J Anaesth. 2002;88:234–240. doi: 10.1093/bja/88.2.234. [DOI] [PubMed] [Google Scholar]
- 18.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Wallenborn J, Gelbrich G, Bulst D, Behrends K, Wallenborn H, Rohrbach A, et al. Prevention of postoperative nausea and vomiting by metoclopramide combined with dexamethasone: randomised double blind multicentre trial. BMJ. 2006;333:324. doi: 10.1136/bmj.38903.419549.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen PP, Chui PT, Ma M, Gin T. A prospective survey of patients after cessation of patient-controlled analgesia. Anesth Analg. 2001;92:224–227. doi: 10.1097/00000539-200101000-00043. [DOI] [PubMed] [Google Scholar]
- 21.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–658. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884–1898. doi: 10.1213/01.ANE.0000219597.16143.4D. [DOI] [PubMed] [Google Scholar]
- 23.Roberts GW, Bekker TB, Carlsen HH, Moffatt CH, Slattery PJ, McClure AF. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg. 2005;101:1343–1348. doi: 10.1213/01.ANE.0000180204.64588.EC. [DOI] [PubMed] [Google Scholar]
- 24.Buvanendran A, Kroin JS, Tuman KJ, Lubenow TR, Elmofty D, Moric M, et al. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. JAMA. 2003;290:2411–2418. doi: 10.1001/jama.290.18.2411. [DOI] [PubMed] [Google Scholar]
- 25.Doyle E, Robinson D, Morton NS. Comparison of patient-controlled analgesia with and without a background infusion after lower abdominal surgery in children. Br J Anaesth. 1993;71:670–673. doi: 10.1093/bja/71.5.670. [DOI] [PubMed] [Google Scholar]
- 26.Kranke P, Apfel CC, Papenfuss T, Rauch S, Löbmann U, Rübsam B, et al. An increased body mass index is no risk factor for postoperative nausea and vomiting. A systematic review and results of original data. Acta Anaesthesiol Scand. 2001;45:160–166. [PubMed] [Google Scholar]
- 27.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–2451. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz JR, Kee SS, Frenzel JC, Ensor JE, Selvan M, Riedel BJ, et al. The effect of an anatomically classified procedure on antiemetic administration in the postanesthesia care unit. Anesth Analg. 2010;110:403–409. doi: 10.1213/ane.0b013e3181a9d076. [DOI] [PubMed] [Google Scholar]
- 29.Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–243. doi: 10.2165/00003495-200059020-00005. [DOI] [PubMed] [Google Scholar]
- 30.Junger A, Hartmann B, Benson M, Schindler E, Dietrich G, Jost A, et al. The use of an anesthesia information management system for prediction of antiemetic rescue treatment at the postanesthesia care unit. Anesth Analg. 2001;92:1203–1209. doi: 10.1097/00000539-200105000-00023. [DOI] [PubMed] [Google Scholar]
- 31.Wallenborn J, Rudolph C, Gelbrich G, Goerlich TM, Helm J, Olthoff D. The impact of isoflurane, desflurane, or sevoflurane on the frequency and severity of postoperative nausea and vomiting after lumbar disc surgery. J Clin Anesth. 2007;19:180–185. doi: 10.1016/j.jclinane.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Borgeat A, Ekatodramis G, Schenker CA. Postoperative nausea and vomiting in regional anesthesia: a review. Anesthesiology. 2003;98:530–547. doi: 10.1097/00000542-200302000-00036. [DOI] [PubMed] [Google Scholar]