Abstract
Purpose
The Mycobacterium tuberculosis complex comprises M. tuberculosis, M. bovis, M. bovis bacillus Calmette-Guérin (BCG) and M. africanum, and causes tuberculosis in humans and animals. Identification of Mycobacterium spp. and M. tuberculosis complex to the species level is important for practical use in microbiological laboratories, in addition to optimal treatment and public health.
Materials and Methods
A novel multiplex PCR assay targeting a conserved rpoB sequence in Mycobacteria spp., as well as regions of difference (RD) 1 and RD8, was developed and evaluated using 37 reference strains and 178 clinical isolates.
Results
All mycobacterial strains produced a 518-bp product (rpoB), while other bacteria produced no product. Virulent M. tuberculosis complex strains, M. tuberculosis, M. bovis and M. africanum, produced a 254-bp product (RD1), while M. bovis BCG, M. microti and nontuberculous mycobacteria produced no RD1 region product. Additionally, M. tuberculosis and M. africanum produced a 150-bp product (RD8), while M. bovis and M. bovis BCG produced a 360-bp product (deleted form of RD8). M. microti and nontuberculous mycobacteria produced no RD8 region product. This assay identified all Mycobacterium spp. and all M. tuberculosis complex strains to the species level.
Conclusion
The multiplex PCR assay of the present study could be implemented as a routine test in microbiology laboratories, and may contribute to more effective treatment and surveillance of tuberculosis stemming from the M. tuberculosis complex.
Keywords: M. tuberculosis complex, multiplex PCR, rpoB, RD1, RD8
INTRODUCTION
Tuberculosis is a global health problem, with approximately 8.8 million new cases and 1.4 million deaths reported in 2010.1 The disease is caused by members of the Mycobacterium tuberculosis (M. tuberculosis) complex, a group of closely related species and subspecies, which include M. tuberculosis, M. africanum, M. bovis, M. bovis bacillus Calmette-Guérin (BCG), M. microti, M. canettii, M. pinnipedii, and M. mungi.2 M. tuberculosis is the most common cause of tuberculosis in humans. M. bovis causes tuberculosis in humans and animals, and is responsible for 10 to 15% of new human tuberculosis cases in developing countries.3 M. bovis BCG, a live attenuated strain of M. bovis, is a widely used anti-tuberculosis vaccine, and is used for the treatment of bladder cancer.4 However, M. bovis BCG is known to lead to complications, such as osteomyelitis, abscesses, lymphadenitis, and dissemination of M. bovis BCG, most severely in human immunodeficiency virus-infected neonates and children. In M. bovis BCG-treated patients, complications such as pneumonitis, hepatitis and noncaseating granulomas have been reported.5
Identification of the M. tuberculosis complex to the species level is important for public health, epidemiology and treatment.6 In the laboratory, tuberculosis is diagnosed by acid-fast bacilli (Ziehl-Neelsen) staining and culturing of mycobacteria, followed by identification of the cultured mycobacteria. For more rapid identification, several polymerase chain reaction (PCR)-based methods have been developed, and PCR assay that targets M. tuberculosis-specific sequences, including IS6110 and the MPT64 gene, has been widely applied.7 However, while this assay can differentiate the M. tuberculosis complex from nontuberculous mycobacteria, it cannot identify the M. tuberculosis complex to the species level.7 For rapid and efficient identification of the M. tuberculosis complex, several PCR-based methods have been developed.8-10 However, these are rarely used as a routine test in diagnostic laboratories as they require two rounds of PCR reaction and electrophoresis to distinguish the M. tuberculosis complex from other mycobacteria and to identify M. tuberculosis complex to the species level.11,12 Moreover, identification of nontuberculous mycobacteria to the species level is of greater importance due to the increased incidence of infection with nontuberculous mycobacteria. Thus, more rapid and efficient methods of identification are needed.
A well-validated probe for the Mycobacterium genus targets the conserved sequence of the rpoB gene, which encodes the β subunit of RNA polymerase, and not only facilitates identification of all Mycobacterium spp., but also distinguishes Mycobacterium spp. at the species level by restriction enzyme analysis.13 In a comparative genomics study of M. tuberculosis H37Rv, 16 regions of the genome (regions of difference, RD) were shown to be deleted in M. bovis and M. bovis BCG.10 One of them, RD1, is present in virulent M. tuberculosis complex strains, including M. tuberculosis and M. bovis, but is absent in M. bovis BCG, allowing for M. tuberculosis and M. bovis to be differentiated from M. bovis BCG.11,12 Additionally, a novel RD8 is present in M. tuberculosis and M. africanum that is deleted in M. bovis and M. bovis BCG and absent in M. microti and nontuberculous mycobacteria.14
In this study, we developed a rapid and efficient multiplex PCR assay that enables not only the identification of the genus Mycobacterium using the rpoB sequence conserved in all mycobacteria, but also the identification of the M. tuberculosis complex to the species level, using RD1 and RD8 sequences. The assay was shown to be simple and efficient in identifying Mycobacterium genus and distinguishing the M. tuberculosis complex to the species level.
MATERIALS AND METHODS
Bacterial strains
The bacterial strains used in this study are listed in Table 1. Seventy-six clinical M. tuberculosis isolates from humans and 52 clinical M. bovis isolates from cattle were provided kindly from Yonsei University College of Medicine, Seoul, Korea. Twenty-seven clinical M. avium isolates and 23 clinical M. intracellulare isolates from humans were provided by the Korea Institute of Tuberculosis, Osong, Korea.
Table 1.
Bacterial Strains Used in This Study
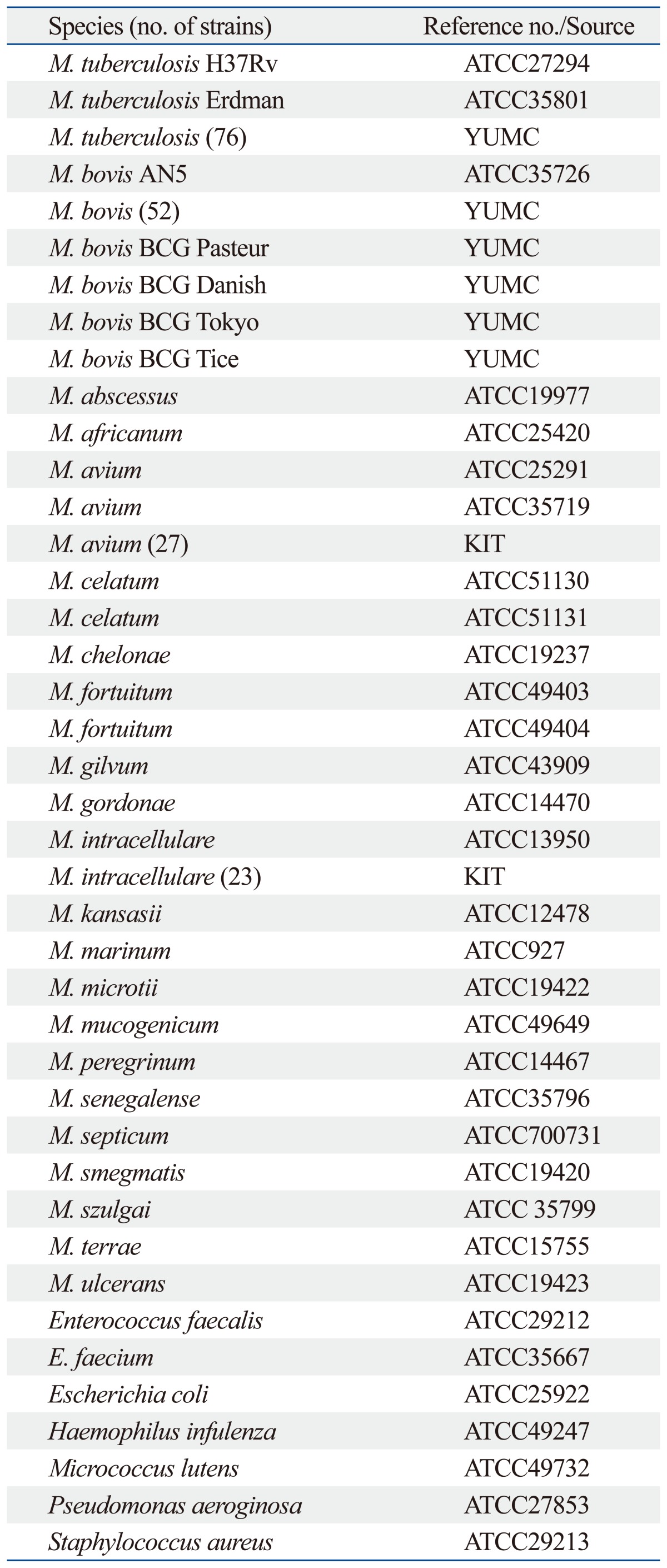
YUMC, Yonsei University College of Medicine, Seoul, Korea; KIT, Korea Institute of Tuberculosis, Osong, Korea; BCG, bacillus Calmette-Guérin.
Culture media and DNA extraction
Bacterial culture and chromosomal DNA isolation were performed as previously described.13 Briefly, bacteria were cultured on Middlebrook 7H9 media (Difco, Detroit, MI, USA) supplemented with 10% Middlebrook OADC Enrichment media (BBL, Sparks, MD, USA) at 37℃ for 2 to 4 weeks. Bacterial cells were collected by centrifugation at 10000×g, resuspended in distilled water, and boiled for 10 min. Cell debris was sedimented by centrifugation for 2 min at 10000×g, and the resultant DNA concentration was measured using a spectrophotometer (260 nm, Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). DNA was stored at -20℃ prior to use.
PCR primer design
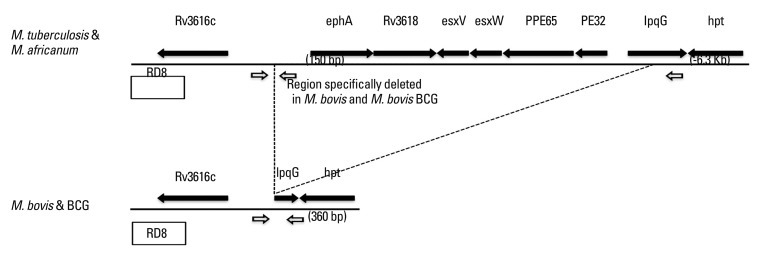
The primers used in this study are listed in Table 2. The genome sequence of M. tuberculosis H37Rv was imported into the Vector NTI® Software (Life technologies, Grand Island, NY, USA), and rpoB, RD1 and RD8 were created according to the genome address. Primers were selected by analysis of the appropriate genomic regions using Primer 3 software (http://frodo.wi.mit.edu/) (Fig. 1, Table 2).
Table 2.
Primers Used in This Study
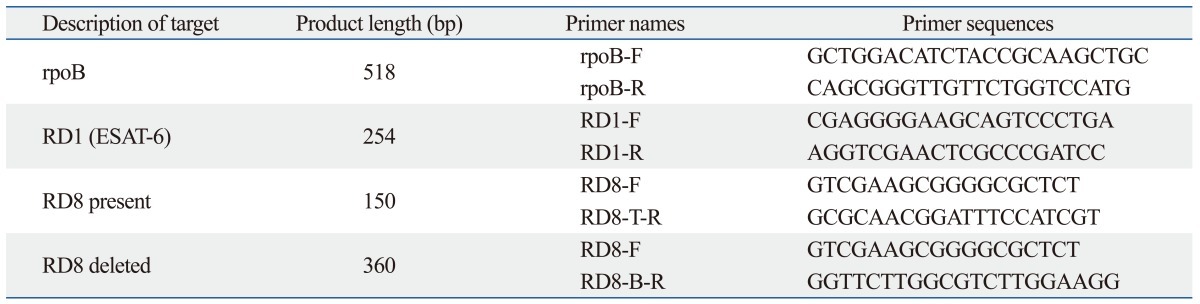
RD, regions of difference.
Fig. 1.
Schematic diagram showing regions flanking the direct repeat region in M. tuberculosis H37Rv, M. bovis and M. bovis bacillus Calmette-Guérin (BCG). This region is specifically deleted in M. bovis and M. bovis BCG, indicated by dotted lines. Primer positions are indicated by small arrows. RD, regions of difference.
PCR amplification
The amplification reaction mixture consisted of 10 mM Tri-HCl, 50 mM KCl, 1.5 mM MgCl2, 200 µM of each primer, 1.5 units of Taq DNA polymerase (Bioneer, Daejeon, Korea), and 20 ng of genomic DNA as the template. PCR amplification was performed in an automated thermal cycler (PTC-100 Programmable Thermal Controller, MJ Research, Inc., Waltham, MA, USA). The cycling parameters were 94℃ for 5 min, followed by 35 cycles of denaturation at 94℃ for 30 s, annealing at 68℃ for 30 s and a final extension at 72℃ for 7 min. Sterile distilled water was used as a negative control. Analysis of PCR products was performed by electrophoresis in ethidium bromide (0.5 µg/mL)-stained 1.7 % (w/v) agarose gels.
RESULTS
Validation of the PCR method
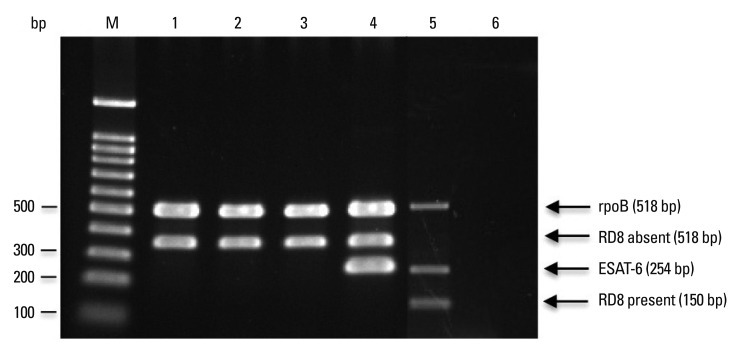
PCR conditions were optimized with four sets of primer pairs using M. tuberculosis H37Rv, M. bovis AN5 and M. bovis BCG Pasteur as representative strains (Figs. 1 and 2), and confirmed using all bacterial strains by gel electrophoresis. The PCR products for each target sequence from each set of primer pairs were also confirmed. All reference mycobacterial strains consistently yielded a single 518-bp product with primers rpoB-F and rpoB-R, but other bacterial strains yielded no products, indicating that all mycobacterial strains contained the specific rpoB gene (Table 3). M. tuberculosis H37Rv, M. africanum and M. bovis AN5 consistently yielded a single 254-bp product with RD1-F and RD1-R primers, but other bacterial strains, including all M. bovis BCG strains and M. microti, yielded no product with RD1-F and RD1-R primers, indicating the RD1 sequence is present in virulent M. tuberculosis complex strains. M. tuberculosis and M. africanum type strains consistently yielded a single 150-bp product with RD8-F and RD8-T-R primers, but yielded no product with RD8-F and RD8-T-R primers, indicating the presence of the RD8 sequence. Conversely, M. bovis and all M. bovis BCG strains consistently yielded a single 360-bp product with RD8-F and RD8-B-R primers, but yielded no product with RD8-F and RD8-T-R primers, indicating a deletion of the RD8 sequence in M. bovis, including M. bovis BCG (Fig. 1, Table 3). However, other bacteria, including nontuberculous mycobacteria and M. microti, yielded no product with either RD8-F and RD8-T-R or RD8-F and RD8-B-R primers.
Fig. 2.
Multiplex PCR for M. tuberculosis, M. bovis and M. bovis bacillus Calmette-Guérin (BCG) strains. PCR products were separated by electrophoresis and visualized. Lanes M: molecular size marker (100-bp ladder); 1: M. bovis BCG Pasteur; 2: M. bovis BCG Tice; 3: M. bovis BCG Tokyo; 4: M. bovis AN5; 5: M. tuberculosis H37Rv; 6: negative control. Expected sizes of PCR products are shown. RD, regions of difference; PCR, polymerase chain reaction; ESAT-6, early secreted antigenic target 6 kDa protein.
Table 3.
Signature Patterns of Positive and Negative Multiplex PCR Used to Discriminate Mycobacterial Strains
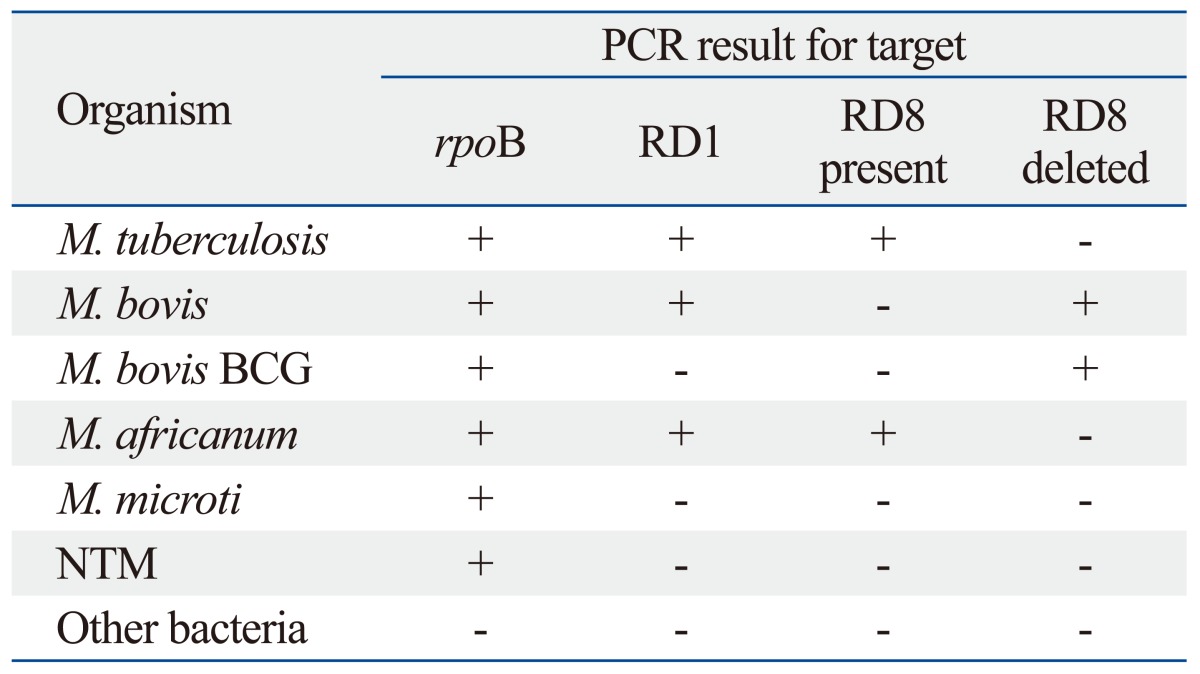
RD, regions of difference; BCG, bacillus Calmette-Guérin; PCR, polymerase chain reaction; NTM, non-tuberculous Mycobacteria.
Multiplex PCR on reference strains and clinical isolates
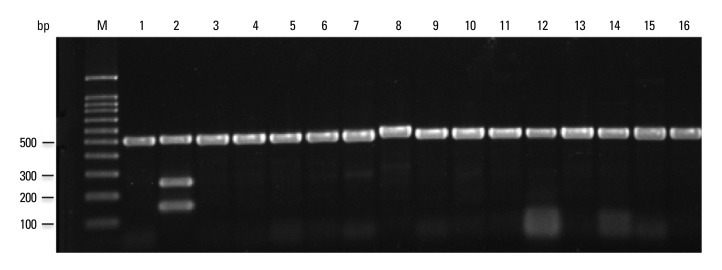
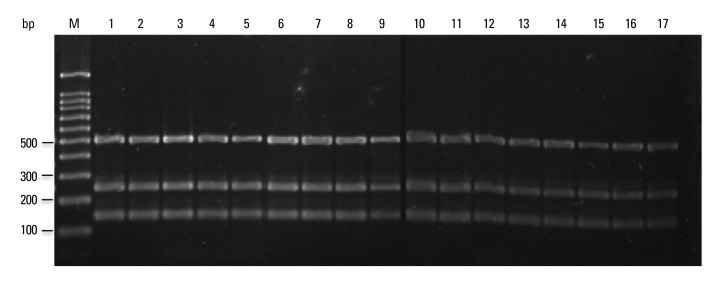
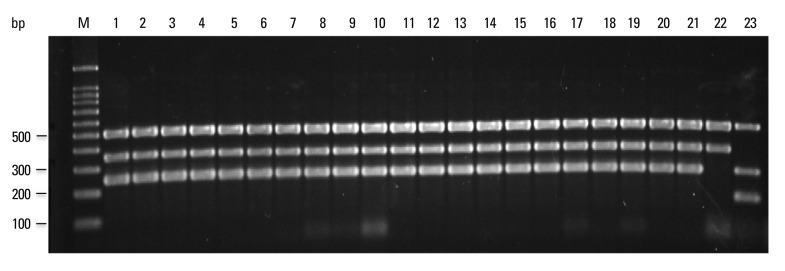
PCR products using the four sets of primer pairs were performed on 30 reference strains and 178 clinical isolates (Figs. 3, 4 and 5). Multiplex PCR of M. tuberculosis H37Rv and M. tuberculosis Erdman yielded three 518-, 254- and 150-bp products, indicating the presence of rpoB, RD1 and RD8, respectively (Fig. 4). Multiplex PCR of 76 clinical M. tuberculosis isolates provided results identical to the M. tuberculosis reference strain (Fig. 4). Multiplex PCR of M. africanum, a member of the M. tuberculosis complex, yielded results identical to M. tuberculosis (Fig. 3). However, multiplex PCR of M. microti yielded only a 518-bp product, similar to nontuberculous mycobacteria.
Fig. 3.
Multiplex PCR for the Mycobacterium species. PCR products were separated by electrophoresis and visualized. Lanes M: molecular size marker (100-bp ladder); 1: M. avium; 2: M. africanum; 3: M. abscesses; 4: M. celatum; 5: M. chelonae; 6: M. fortuitum; 7: M. gordonae; 8: M. intracellulare; 9: M. kansasii; 10: M. marinum; 11: M. microtii; 12: M. phlei; 13: M. septicum; 14: M. szulgai; 15: M. terrae; 16: M. ulcerans; PCR, polymerase chain reaction.
Fig. 4.
Multiplex PCR for clinical M. tuberculosis isolates. PCR products were separated by electrophoresis and visualized. Lanes M: molecular size marker (100-bp ladder); 1-15: M. tuberculosis isolates; 16: M. tuberculosis H37Rv; 17: M. tuberculosis Erdman; PCR, polymerase chain reaction.
Fig. 5.
Multiplex PCR for clinical M. bovis isolates. PCR products were separated by electrophoresis and visualized. Lanes M: molecular size marker (100-bp ladder); 1-20: M. bovis isolates; 21: M. bovis AN5; 22: M. bovis BCG Pasteur; 23: M. tuberculosis H37Rv. BCG, bacillus Calmette-Guérin; PCR, polymerase chain reaction.
Multiplex PCR on M. bovis AN5 and 52 clinical M. bovis isolates yielded 518-, 360- and 254-bp products, indicating the presence of rpoB and RD1, as well as the deletion of RD8 (Fig. 5). All four M. bovis BCG strains yielded 518- and 360-bp products, indicating the presence of rpoB, the absence of RD1 and the deletion of RD8. Multiplex PCR of 23 nontuberculous mycobacteria reference strains and 50 clinical nontuberculous mycobacteria isolates yielded only a 518-bp product, indicating the absence of RD1 and RD8. Multiplex PCR of other non-mycobacterial bacteria yielded no positive results. The data were consistently reproducible, and complete concordance between independent researchers was confirmed.
DISCUSSION
We developed a simple and effective multiplex PCR assay that enabled the differentiation of Mycobacteria spp. from other bacteria and identified the M. tuberculosis complex to the species level. Our analysis using reference strains and clinical isolates suggested that the multiplex PCR assay is specific for M. tuberculosis, M. bovis and M. bovis BCG, as well as nontuberculosis mycobacteria. The conserved rpoB sequence of the mycobacteria was used to differentiate the genus Mycobacterium from other bacteria, resulting in data specific for all Mycobacteria spp. tested.13 Moreover, the fragment length polymorphism of the 518-bp PCR products targeting rpoB were effective for species identification (data not shown). This sequence offers advantages over other target sequences, including IS6110 and 16S RNA. IS6110 can be used for the detection of M. tuberculosis complex, but cannot differentiate members of the M. tuberculosis complex.7 16S RNA has been used for the identification of Mycobacteria spp., but is unable to provide identification of M. tuberculosis complex to the species level, due to their high similarity.15 The present assay was based on the detection of RD1 and RD8 in order to distinguish members of the M. tuberculosis complex. Using this assay, RD1 was detected in all M. tuberculosis complex strains, including M. tuberculosis, M. bovis and M. africanum, but not M. bovis BCG or M. microti, in accordance with previous reports.11,12 RD8 was detected in M. tuberculosis and M. africanum, but a deleted form of RD8 was detected in M. bovis and M. bovis BCG. Interestingly, this sequence was absent in M. microti even though M. microti belongs to the M. tuberculosis complex. This result was in agreement with previous reports that demonstrated the presence of RD8 in M. tuberculosis, but not M. microti.16 In our study, the M. africanum reference strain (ATCC 25420) contained RD8; however, investigation using other M. africanum strains would be necessary since Vasconcellos, et al.17 reported RD8 to be present in the West African-1 lineage, but absent from the West African-2 lineage. Our assay using RD8 possessed advantages over other RDs, including RD4 and RD9, since RD8 can distinguish mycobacteria to three groups, M. tuberculosis and M. africanum, M. bovis and M. bovis BCG, and M. microti and nontuberculous mycobacteria, by determining the presence of RD8, the deletion of RD8 or the absence of RD8, respectively. Conversely, RD4 and RD9 can distinguish between M. tuberculosis and M. bovis, including M. bovis BCG, but not between M. africanum and M. microtii.11 Multiplex PCR assays that target RD8 combined with RD1 effectively distinguish the M. tuberculosis complex to the species level.
Several methods of identifying the M. tuberculosis complex currently exist, including high-performance liquid chromatography,18 restriction fragment length polymorphism,7,8 and spoligotyping.19 However, these assays are generally not used routinely in clinical microbiology laboratories as they are time consuming and costly. Recently, multiplex PCR assays have been proposed as a diagnostic method due to a single reaction and multiple targets.7,11,12 The present assay is the first multiplex PCR assay shown to identify the Mycobacterium genus and distinguish the M. tuberculosis complex to the species level by targeting the rpoB sequence, as well as RD1 and RD8 sequences, respectively.
This assay can be used as a routine diagnostic test in microbiology laboratories and may contribute to determination of an optimal treatment regimen, improve the surveillance of tuberculous disease due to members of the M. tuberculosis complex, and aid in the identification of the M. tuberculosis complex and nontuberculous mycobacteria.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A010024, HYL) and by the Yonsei University Research Fund of 2000 (in part).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Raviglione M, Marais B, Floyd K, Lönnroth K, Getahun H, Migliori GB, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet. 2012;379:1902–1913. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- 2.Ernst JD, Trevejo-Nuñez G, Banaiee N. Genomics and the evolution, pathogenesis, and diagnosis of tuberculosis. J Clin Invest. 2007;117:1738–1745. doi: 10.1172/JCI31810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Rua-Domenech R. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb) 2006;86:77–109. doi: 10.1016/j.tube.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Lamm DL, Stogdill VD, Stogdill BJ, Crispen RG. Complications of bacillus Calmette-Guerin immunotherapy in 1,278 patients with bladder cancer. J Urol. 1986;135:272–274. doi: 10.1016/s0022-5347(17)45606-0. [DOI] [PubMed] [Google Scholar]
- 5.Kamphuis JT, Buiting AG, Miseré JF, van Berge Henegouwen DP, van Soolingen D, Rensma PL. BCG immunotherapy: be cautious of granulomas. Disseminated BCG infection and mycotic aneurysm as late complications of intravesical BCG instillations. Neth J Med. 2001;58:71–75. doi: 10.1016/s0300-2977(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis. 1995;76(Suppl 1):1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 7.Sankar S, Ramamurthy M, Nandagopal B, Sridharan G. An appraisal of PCR-based technology in the detection of Mycobacterium tuberculosis. Mol Diagn Ther. 2011;15:1–11. doi: 10.1007/BF03257188. [DOI] [PubMed] [Google Scholar]
- 8.Frothingham R. Differentiation of strains in Mycobacterium tuberculosis complex by DNA sequence polymorphisms, including rapid identification of M. bovis BCG. J Clin Microbiol. 1995;33:840–844. doi: 10.1128/jcm.33.4.840-844.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjeldsen MK, Bek D, Rasmussen EM, Priemé A, Thomsen VØ. Line probe assay for differentiation within Mycobacterium tuberculosis complex. Evaluation on clinical specimens and isolates including Mycobacterium pinnipedii. Scand J Infect Dis. 2009;41:635–641. doi: 10.1080/00365540903127425. [DOI] [PubMed] [Google Scholar]
- 10.Parsons LM, Brosch R, Cole ST, Somoskövi A, Loder A, Bretzel G, et al. Rapid and simple approach for identification of Mycobacterium tuberculosis complex isolates by PCR-based genomic deletion analysis. J Clin Microbiol. 2002;40:2339–2345. doi: 10.1128/JCM.40.7.2339-2345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halse TA, Escuyer VE, Musser KA. Evaluation of a single-tube multiplex real-time PCR for differentiation of members of the Mycobacterium tuberculosis complex in clinical specimens. J Clin Microbiol. 2011;49:2562–2567. doi: 10.1128/JCM.00467-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinsky BA, Banaei N. Multiplex real-time PCR assay for rapid identification of Mycobacterium tuberculosis complex members to the species level. J Clin Microbiol. 2008;46:2241–2246. doi: 10.1128/JCM.00347-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol. 2000;38:2966–2971. doi: 10.1128/jcm.38.8.2966-2971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon SV, Eiglmeier K, Garnier T, Brosch R, Parkhill J, Barrell B, et al. Genomics of Mycobacterium bovis. Tuberculosis (Edinb) 2001;81:157–163. doi: 10.1054/tube.2000.0269. [DOI] [PubMed] [Google Scholar]
- 15.Dobner P, Feldmann K, Rifai M, Löscher T, Rinder H. Rapid identification of mycobacterial species by PCR amplification of hypervariable 16S rRNA gene promoter region. J Clin Microbiol. 1996;34:866–869. doi: 10.1128/jcm.34.4.866-869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frota CC, Hunt DM, Buxton RS, Rickman L, Hinds J, Kremer K, et al. Genome structure in the vole bacillus, Mycobacterium microti, a member of the Mycobacterium tuberculosis complex with a low virulence for humans. Microbiology. 2004;150(Pt 5):1519–1527. doi: 10.1099/mic.0.26660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasconcellos SE, Huard RC, Niemann S, Kremer K, Santos AR, Suffys PN, et al. Distinct genotypic profiles of the two major clades of Mycobacterium africanum. BMC Infect Dis. 2010;10:80. doi: 10.1186/1471-2334-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler WR, Jost KC, Jr, Kilburn JO. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991;29:2468–2472. doi: 10.1128/jcm.29.11.2468-2472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streicher EM, Victor TC, van der Spuy G, Sola C, Rastogi N, van Helden PD, et al. Spoligotype signatures in the Mycobacterium tuberculosis complex. J Clin Microbiol. 2007;45:237–240. doi: 10.1128/JCM.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]