Abstract
We describe herein a case of life-threatening hypoglycemia due to spurious elevation of glucose concentration during the administration of ascorbic acid in a type 2 diabetic patient. A 31-year-old female was admitted for proliferative diabetic retinopathy treatment and prescribed high dose ascorbic acid. During hospitalization, she suddenly lost her consciousness and her glucose concentration was 291 mg/dL, measured using self-monitoring blood glucose (SMBG) device, while venous blood glucose concentration was 12 mg/dL. After intravenous injection of 50% glucose solution, the patient became alert. We reasoned that glucose measurement by SMBG device was interfered by ascorbic acid. Physicians should be aware of this interference; high dose ascorbic acid may cause spurious elevation of glucose concentration when measuring with SMBG devices.
Keywords: Self-monitoring of blood glucose, ascorbic acid, hypoglycemia
INTRODUCTION
Diabetic treatment with self-monitoring of blood glucose (SMBG) devices contributes to the improvement of glycemic control and reduction of diabetes-related morbidity.1,2 Although some studies reported that SMBG is not associated with glycemic control benefits,3 SMBG devices are still used for rapid detection of hypoglycemic events and the measurement of blood glucose concentration in hospital or at home. Therefore, the accuracy of SMBG devices is critical. However, analytical errors and safety concerns have been reported about SMBG devices.4,5 Several factors, such as hematocrit, temperature, humidity, and several drugs and substances, have been reported to affect the accuracy of SMBG device.6-8 Also, certain SMBG devices could be inaccurate when some agents, such as icodextrin and maltos, are used simultaneously.9,10 Here, we describe a case of life-threatening hypoglycemia due to spurious elevation of glucose concentration during the administration of high dose ascorbic acid in a patient with type 2 diabetes mellitus on hemodialysis.
CASE REPORT
A 31-year-old female was admitted to the department of Ophthalmology for proliferative diabetic retinopathy (PDR) treatment. She had suffered from type 2 diabetes for 12 years and had been on hemodialysis due to diabetic nephropathy. She had been treated with multiple-dose insulin injection therapy using a basal-bolus regimen. On the day after the admission, she was referred to the department of Endocrinology and Metabolism for the loss of consciousness and discrepancies between capillary and venous blood glucose levels. The physical examination showed blood pressure 140/90 mm Hg, heart rate 100/min, and body temperature 36.5℃. Brain computed tomography was normal. Her glucose level measured using Breeze 2 (Bayer HealthCare LLC, Elkhart, IN, USA) was 291 mg/dL.
However, the venous blood glucose concentration (Modular Analytics DP, Roche Diagnostics, Mannheim, Switzerland) of the blood sample collected simutaneously was 12 mg/dL. After an intravenous injection of 50 mL of 50% glucose solution, the patient became alert. At the time, she was on 10-day medication of high dose ascorbic acid (10 g per day). Because it has been known that glucose measurement reading in SMBG device could be interfered by ascorbic acid, the high dose ascorbic acid treatment was stopped immediately.
Table 1 shows the results of glucose concentration measured with Breeze 2, Accu-Chek Active (Roche Daignostics, GmbH, Mannheim, Germany) and corresponding venous blood glucose concentration in this patient. The glucose concentrations measured with Breeze 2 and Accu-Chek Active upon cessation of the ascorbic acid was also different from the venous glucose concentration. It may be due to the half-life or clearance of ascorbic acid in the body. The Breeze 2 and Accu-Check Acitve meet the international accuracy guideline for blood glucose monitoring systems.11
Table 1.
Comparison of Glucose Concentrations Reading of Breeze 2, Accu-Chek Active, and Venous Blood Glucose Concentration in the Patient after Discontinuation of Ascorbic Acid Administration
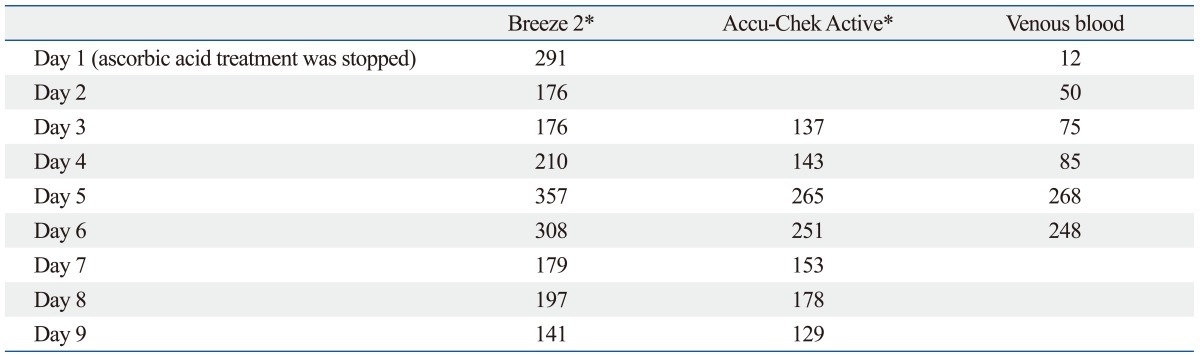
SMBG, self-monitoring of blood glucose.
*All glucose concentration was measured with same SMBG devices.
Table 2 shows the glucose concentration measured with SMBG devices and venous glucose concentration in patients with type 2 diabetes mellitus on hemodialysis without ascorbic acid treatment. Overestimation in measurement using SMBG devices did not happen in these patients, suggesting that spurious elevation of glucose concentration in our patient was due to the administration of high dose ascorbic acid. Serum ascorbic acid concentration measured in the blood sample collected during the event in this patient was 1336.64 µg/mL (reference interval of our hospital: 1.90-15.00 µg/mL).
Table 2.
Result of Glucose Concentrations Reading of Breeze 2, Accu-Chek Active, and Venous Blood Glucose Concentration in the Patients with Type 2 Diabetes Mellitus on Hemodialysis without High Dose Ascorbic Acid Administration
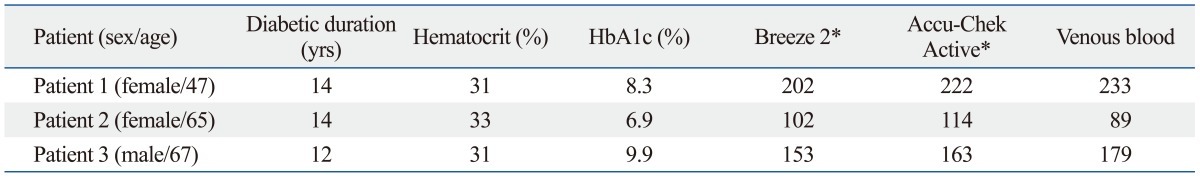
SMBG, self-monitoring of blood glucose.
*All glucose concentration was measured with same SMBG devices.
DISCUSSION
Our present case shows that high dose ascorbic acid treatment caused false-high glucose concentration reading in SMBG devices in the patient with diabetes.
Current diabetes care depends on measuring glucose concentration using SMBG devices and HbA1c to assess the quality of glycemic control and adjustment of its management. Therefore, the accuracy of SMBG devices is important for optimal glycemic control. Three enzyme systems are usually used to measure glucose: hexokinase, glucose oxidase (GOx) and glucose dehydrogenase (GDH).8 The hexokinase method is a reference method for glucose measurements in many clinical laboratories, because this method is highly specific for glucose.8 Our chemistry analyzer, Modular Analytics DP (Roche Diagnostics), is based on this method. The GOx and GDH methods are mostly adopted in SMBG devices. A variety of substance may interfere with the accuracy of SMBG devices.7 The suggested interference substances include acetaminophen, dopamine, ascorbic acid, and so on.7 There are few reports that icodextrin in peritoneal dialysis may interfere with glucose measurement of SMBG devices, especially when they are based on glucose dehydrogenase-pyrroloquinolinequinone (GDH-PQQ).12,13 Excessive ascorbic acid can also lead to false glucose reading in SMBG devices.7 Tang, et al.7 reported that these interferences occurred at both low glucose level (80-100 mg/dL) and at high glucose levels (200 mg/dL). The Food and Drug Administration suggested that the interference of ascorbic acid exists even at 3 mg/dL of ascorbic acid. The mechanism of ascorbic acid interference with SMBG devices based on electrochemical analysis, could be explained by the fact that ascorbic acid is oxidized at the electrode surface, resulting in the production of more electron and the generation of a greater current.14 Breeze 2 is based on GOx method, and Accu-Chek Active is based on GDH-PQQ method. Actually, most SMBG devices crossreact with ascorbic acid, and show higher or, rarely, lower glucose concentration than actual concentration.8 The low hematocrit (33%) or increased creatinine level of this patient may be associated with overestimation of glucose concentration.8 However, there was no interference in three of our patients with anemia and chronic kidney disease. We suggest that high ascorbic acid treatment is related with false elevation of glucose level.
There were no misleading events of glucose concentration in this patient before the present episode. We suspected that her glucose concentration was too high to cause the develop hypoglycemia before the present event, and she did not take high dose of ascorbic acid. Her HbA1c level before admission was 7.8%. Steroid treatment for her PDR after admission elevated her glucose levels. With increasing insulin dosage, her glucose control state improved. On several days later, SMBG devices showed falsely elevated glucose concentration despite low glucose concentration, and an erroneous injection of increased insulin dosage led to her hypoglycemic events. Her HbA1c and fructosamine levels after the hypoglycemic episode were 6.8% (reference range 4.2-5.9%) and 69 µmol/L (reference range 0-285 µmol/L), respectively.
There has been a concern about the possibility that some medications or substances may interfere with the measurement of glucose concentration in a particular SMBG devices, and these interferences may pass unrecognized. Lyon, et al.15 reported that ascorbic acid produced statistically significant analytical errors with SMBG devices. These interferences may mask true hypoglycemia or hyperglycemia. Especially, false hyperglycemia can lead to life-threatening events due to increased insulin dosage based on the false reading. However, there are only a few reports about analytical errors with SMBG device for patient with ascorbic acid use. We confirmed in our case, that such interference can exist in clinical setting and cause life-threatening hypoglycemia. Therefore, physicians should recheck the glucose level with another testing system or in the central laboratories when SMBG device results are inconsistent with clinical judgment that points to suspected hypoglycemia.
ACKNOWLEDGEMENTS
This study was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education Science and Technology (NRF-2012-0470).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Martin S, Schneider B, Heinemann L, Lodwig V, Kurth HJ, Kolb H, et al. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49:271–278. doi: 10.1007/s00125-005-0083-5. [DOI] [PubMed] [Google Scholar]
- 2.Welschen LM, Bloemendal E, Nijpels G, Dekker JM, Heine RJ, Stalman WA, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care. 2005;28:1510–1517. doi: 10.2337/diacare.28.6.1510. [DOI] [PubMed] [Google Scholar]
- 3.Davis WA, Bruce DG, Davis TM. Is self-monitoring of blood glucose appropriate for all type 2 diabetic patients? The Fremantle Diabetes Study. Diabetes Care. 2006;29:1764–1770. doi: 10.2337/dc06-0268. [DOI] [PubMed] [Google Scholar]
- 4.Melker RJ. Test strips for blood glucose monitors are not always accurate. Diabetes Care. 2003;26:3190. doi: 10.2337/diacare.26.11.3190. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3:903–913. doi: 10.1177/193229680900300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabadjief D, Nichols JH. Assessing glucose meter accuracy. Curr Med Res Opin. 2006;22:2167–2174. doi: 10.1185/030079906X148274. [DOI] [PubMed] [Google Scholar]
- 7.Tang Z, Du X, Louie RF, Kost GJ. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol. 2000;113:75–86. doi: 10.1309/QAW1-X5XW-BVRQ-5LKQ. [DOI] [PubMed] [Google Scholar]
- 8.Heller A, Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem Rev. 2008;108:2482–2505. doi: 10.1021/cr068069y. [DOI] [PubMed] [Google Scholar]
- 9.Frias JP, Lim CG, Ellison JM, Montandon CM. Review of adverse events associated with false glucose readings measured by GDH-PQQ-based glucose test strips in the presence of interfering sugars. Diabetes Care. 2010;33:728–729. doi: 10.2337/dc09-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melamud B, Shilo S, Munter G. Life-threatening hypoglycemia due to false measurement of glucose in a peritoneal dialysis patient. Isr Med Assoc J. 2010;12:125–126. [PubMed] [Google Scholar]
- 11.International Organization for Standardization. In vitro diagnostic test systems-Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. Geneva, Switserland: International Organization for Standardiation; 2003. [Google Scholar]
- 12.Riley SG, Chess J, Donovan KL, Williams JD. Spurious hyperglycaemia and icodextrin in peritoneal dialysis fluid. BMJ. 2003;327:608–609. doi: 10.1136/bmj.327.7415.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disse E, Thivolet C. Hypoglycemic coma in a diabetic patient on peritoneal dialysis due to interference of icodextrin metabolites with capillary blood glucose measurements. Diabetes Care. 2004;27:2279. doi: 10.2337/diacare.27.9.2279. [DOI] [PubMed] [Google Scholar]
- 14.Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors (Basel) 2010;10:4558–4576. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyon ME, DuBois JA, Fick GH, Lyon AW. Estimates of total analytical error in consumer and hospital glucose meters contributed by hematocrit, maltose, and ascorbate. J Diabetes Sci Technol. 2010;4:1479–1494. doi: 10.1177/193229681000400624. [DOI] [PMC free article] [PubMed] [Google Scholar]


