Abstract
1-cys peroxiredoxin (1-cysPrx), a member of the peroxiredoxin superfamily, can protect cells against membrane oxidation through glutathione (GSH)-dependent reduction of phospholipid hydroperoxides to corresponding alcohols. However, purified native or recombinant enzyme in vitro generally lacks GSH peroxidase (GPx) activity because of oxidation of its single conserved cysteine. Reduction of the resultant oxidized cysteine is difficult because of its protected location within the homodimer formed by the oxidized protein monomers. Partial purification of 1-cysPrx from bovine lung revealed the presence of πGST in an active preparation, while purification to homogeneity yielded enzyme that inactivated with time. We show that heterodimerization of 1-cysPrx with GSH-saturated πGST results in glutathionylation of the oxidized cysteine in 1-cysPrx followed by subsequent spontaneous reduction of the mixed disulfide and restoration of enzymatic activity. Maximum activation of 1-cysPrx occurred with a 1:1 molar ratio of GSH-saturated πGST and a 2:1 molar ratio of GSH to 1-cysPrx. Liposome-mediated delivery of oxidized recombinant enzyme into NCI-H441 cells that lack 1-cysPrx but express πGST resulted in 1-cysPrx activation, whereas activation in MCF7 cells required co-delivery of πGST. Our data indicate a physiological mechanism for glutathionylation of the oxidized catalytic cysteine of 1-cysPrx by its heterodimerization with πGST followed by its GSH-mediated reduction and enzyme activation.
Peroxiredoxins are a superfamily of nonheme and nonselenium peroxidases that are widely distributed throughout all phyla (1-4). Of the six mammalian peroxiredoxins, five (Prx I-V) contain two conserved cysteines that participate in intramolecular disulfide/sulfhydryl redox cycling with thioredoxin resulting in reduction of H2O2 and organic hydroperoxides into corresponding alcohols (2). By contrast, 1-cys peroxiredoxin (1-cysPrx or Prx VI)† has a single conserved cysteine (5) and does not use thioredoxin as reductant (5, 6). This peroxiredoxin is expressed in all tissues but at particularly high levels in brain, eye, testes, and lung (2, 7, 8). Expression of 1-cysPrx protein or mRNA is decreased in a mouse that is susceptible to experimental atherosclerosis (9) and is elevated in brains of patients with Parkinsonian dementia (10), sporadic Creutzfeldt-Jacob disease (11), and Pick disease (12), in lungs from newborns (13), in malignant mesothelioma (14), in the healing edge of skin wounds (15), and in experimental cellular premature senescence (16). 1-cysPrx can reduce phospholipid and other hydroperoxides (6) and protects against cellular membrane damage (17, 18). This enzyme has phospholipase A2 activity (19), participates in the activation of neutrophil NADPH oxidase through its interaction with p67phox (20), and prevent methemoglobin formation in erythrocyte hemolysates (21).
1-cysPrx catalysis results in the peroxide-mediated oxidation of its Cys-47 to sulfenic acid as deduced from 1-cysPrx crystallization (22). Reduction of the sulfenic acid requires reaction with two thiols to form first a mixed disulfide and then a sulfhydryl (5). Cysteine-sulfenic acid is reactive and, thus, short lived (23). However, the Cys-47-sulfenic acid of 1-cysPrx is stable because of its inaccessibility within a globular protein; homodimer formation of oxidized monomers (22) further limits its accessibility. The two oxidized Cys-47 in the homodimer are separated by ≈32 Å, preventing their interaction (22). Native or recombinant purified 1-cysPrx lost activity after interaction with peroxides (5, 24, 25), presumably reflecting generation of the stable oxidized homodimer (22).
Prior studies have not definitely identified the physiological reductant for 1-cysPrx. Native protein isolated from the bovine eye (26) and rat olfactory mucosa (24) showed activity with glutathione (GSH). However, subsequent studies with native rat protein (25) or recombinant human or bovine protein (5, 27) found that GSH was ineffective. Previous studies from our laboratory also were inconsistent because recombinant human protein showed GSH-mediated activity (6), but our subsequent unpublished studies with recombinant human or rat protein could not reproduce these results. Thus, the role of GSH in the reaction mechanism remains equivocal.
The present communication describes a physiological mechanism for the glutathionylation of the oxidized cysteine in 1-cysPrx by its heterodimerization with GSH-loaded πGST. Glutathionylation, presumably through its perturbation of protein structure, facilitates access for GSH, resulting in spontaneous reduction of the mixed disulfide to the sulfhydryl and thereby activating the enzyme.
Materials and Methods
Reagents. G[35S]H was purchased from NEN. ProJect protein delivery kit and sulfo-SBED {sulfosuccinimidyl[2,6-(biotinamido)-2-(p-azidobenzamido)-hexanamido]ethyl-1,3-dithiopropionate}, a trifunctional crosslinker with cleavable biotin handle, were purchased from Pierce. PLPCOOH (1-palmitoyl-2-linolenoyl hydroperoxide-sn-glycero-3-phosphocholine) was prepared by peroxidation of 1-palmitoyl-2-linolenoyl-sn-glycero-3-phosphocholine (Sigma) with 15-lipoxygenase (Cayman Chemical, Ann Arbor, MI) according to a standard protocol (6). Bovine liver GST and molecular mass standards were purchased from Sigma. Recombinant πGST (P1-1) and a polyclonal antibody to this protein were obtained from Oxford Biomedical Research (Oxford, MI).
Cell Culture. NCI-H441 cells (American Type Culture Collection) and MCF7 cells (University of Pennsylvania Cell Center) were grown in standard MEM with 10% FBS plus 1% penicillin/streptomycin. Cells were grown to confluence in 60-mm Petri dishes in an incubator at 37°C with 5% CO2.
Isolation of 1-cysPrx from Bovine Lung. Bovine lung obtained from an abattoir was homogenized, and the supernatant was treated with saturated (NH4)2SO4. The pellet was redissolved and dialyzed overnight by using SnakeSkin (Pierce) dialysis tubing. The dialysate was loaded onto a DE-52 column, and the void volume was collected as described (28). This protein solution was frozen at -80°C in aliquots and used for further purification.
In Vitro Expression of Rat 1-cysPrx. The coding region of the rat 1-cysPrx cDNA (7) was amplified by PCR with Pfu DNA polymerase (Stratagene) in a GeneAmp PCR System 2400 (Perkin-Elmer). The forward primer was 5′-ATGCCGGAGGGCTGCTTCTC-3′ and the reverse primer was 5′-TTAAGGCTGGGGCGTATAACGGAGG-3′. The regions corresponding to the start codon (forward primer) and termination codon (reverse primer) are italicized. Amplification was performed for 30 cycles. In each cycle, denaturation was performed at 94°C for 1 min, annealing was at 55°C for 1 min, and elongation was at 72°C for 2 min. The amplified 675-bp fragment was inserted by blunt-end ligation into the EcoRV site of pETBlue-1 (Novagen). Ligated material was transformed into XL-10 Gold cells (Stratagene) and plasmid DNA was prepared by using a Qiagen kit (Qiagen, Valencia, CA). The DNA was transformed into Tuner (DE3) pLacI cells (Novagen) for protein expression. Cells were grown at 37°C in Terrific Broth (Sigma), induced at optical density of 0.8-1.0 with 0.5 mM IPTG and growth allowed to continue for 4 h. Bacteria were then centrifuged, and soluble proteins were extracted by using the BugBuster HT Protein Extraction Reagent (Novagen).
Purification of Bovine and Rat 1-cysPrx. The lysate of Escherichia coli 1-cysPrx-expressing cells or the partially purified material from bovine lung was centrifuged at 20,000 × g for 10 min. The supernatant was diluted 1:10 with elution buffer and was loaded onto a DEAE fast flow column (all columns from Amersham Biosciences). After elution with 50 mM Tris·HCl buffer [containing 1 mM EDTA and 5% glycerol (pH 5.0)], the target protein was found in the void volume. The pH of the eluate was adjusted to 8.0 and loaded onto a carboxymethyl Sepharose fast-flow column. The void volume containing the target protein was concentrated by using a Centricon (Millipore) YM-10 concentrator and stored at 4°C until use. A GSTrap fast-flow affinity column was used to separate bovine 1-cysPrx from πGST.
Size Exclusion Chromatography. Size exclusion chromatography was performed by using an HPLC Alliance 2695 separation unit, a PDA detector, and a YMC-Pack Diol column (500 × 8 mm ID, 5-μm particle and 120-Å pore sizes, Waters). The column has a linear calibration with a standard mixture of proteins (R2 = 0.99). The samples of homogeneous 1-cysPrx or πGST P-1 (7.7 and 8.3 μM, respectively) or their mixture were eluted isocratically (1.0 ml/min) with 0.2 M phosphate buffer (pH 7.0) containing 0.15 M NaCl. In some experiments, the proteins were incubated with 10× molar excess of GSH for 30 min at 4°C before injection.
Immunoblot Analysis. Protein concentration in samples was measured by using the Bradford assay (Bio-Rad). For analysis by SDS/PAGE (12% Tris-Glycine, 1 mm) with the Nu-PAGE system (Invitrogen), protein was solubilized with 4× sample buffer and boiled for 5 min before loading. After electrophoretic resolution, the proteins were electroblotted to Immobilon-P membranes (Millipore) and blocked for 1 h in Tris-buffered saline with 0.05% Tween 20 (TBST) containing 5% nonfat dry milk, and membranes were probed with either a monoclonal or a polyclonal antipeptide antibody against recombinant protein (7, 17).
Enzymatic Activity Assays. Peroxidase activity was measured by a GSH reductase/GSH/NADPH-coupled assay with PLPCOOH as the substrate by using spectrophotometric and fluorimetric detection (6). GST activity was measured spectrophotometrically (340 nm) with 1-chloro-2,4-dinitrobenzene as substrate with excess of GSH (6). Fluorescence and absorbance were detected with a PTI (Photon Technology International, Lawrenceville, NJ) spectrofluorometer equipped with a single-photon counting system for emission detection and absorbance detection channel with excitation and emission slits at 5 nm and the absorbance slit at 0.5 nm, respectively.
Detection of 1-cysPrx Glutathionylation with G[35S]H. To detect 1-cysPrx glutathionylation, native and denatured (6 M guanidinium HCl) protein samples were incubated with 3× molar excess of G[35S]H [10 μl, ≈6.67 μM, specific activity ≈10 nCi/μl (1 Ci = 37 GBq)] in PBS (pH 7.6) at 37°C for 1 h, washed, and concentrated by using fresh PBS and Centricon YM-10 concentrators. The concentrated protein solution (20 μl) was analyzed by SDS/PAGE that was developed with Simply Blue (Invitrogen). An identical gel run in parallel was imaged with the Personal FX phosphoimager (Bio-Rad).
Detection of 1-cysPrx-π GST Interaction with Sulfo-SBED. Rat 1-cysPrx was incubated with 10× sulfo-SBED reagent in the dark for 30 min under constant stirring. After removal of excess reagent by gel-filtration with BioSpin 6 (Bio-Rad), an aliquot of the preparation was mixed in equimolar amounts with intact 1-cysPrx or πGST (P1-1) and illuminated at 365 nm for 20 min. The resultant mixtures were analyzed by SDS/PAGE under reducing (2 mM DTT) and nonreducing conditions, followed by reaction with horseradish peroxidase-conjugated streptavidin.
1-cysPrx Delivery into Cells. Rat 1-cysPrx in PBS (pH 7.4) at 38.5 μM alone or with equimolar πGST was added to dry ProJect reagent and incubated for 5 min at 20°C, then added to serum free medium in Petri dishes with confluent NCI-H441 or MCF7 cells, incubated for 2 h at 37°C, and washed three times with PBS. Treated and control cells were scraped, lysed with water, subjected to three freeze/thaw cycles, and centrifuged at 10,000 × g for 10 min. The supernatant after concentration with a Centricon YM10 was centrifuged, and the pellet was analyzed by SDS/PAGE and Western blot.
Results
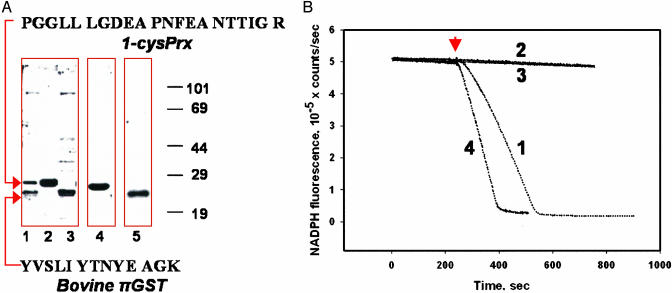
Use of the DE52 column for isolation of 1-cysPrx from bovine lung generated an enzymatically active semipure protein with two major bands on SDS/PAGE (Fig. 1 A, lane 1, and B, trace 1). Proteins from these bands were identified as 1-cysPrx and bovine πGST, respectively, after their digestion with trypsin and analysis of the peptides by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS (Fig. 1A). The presence of πGST was confirmed by Western blot (Fig. 1A, lanes 3 and 5). Further purification of 1-cysPrx by using a GST-trap column resulted in a homogeneous protein (Fig. 1A, lanes 2 and 4). This protein demonstrated GPx activity initially (not shown) which was lost during storage for 1 week at 4°C (Fig. 1B, trace 2). The semipure enzyme has been stored at 4°C for ≈1 year without significant loss of activity. Activity of the purified bovine lung 1-cysPrx was restored by addition of GSH-loaded πGST either as the concentrated retentate from the GST-trap column (not shown) or as recombinant human enzyme (P1-1) (Fig. 1B, trace 4). The πGST has no peroxidase activity with PLPCOOH (Fig. 1B, trace 3). A GST fraction from bovine liver which lacks the π- isoform had no effect on activity of purified bovine lung 1-cysPrx (not shown).
Fig. 1.
Activation of 1-cysPrx by incubation with πGST. (A) Lanes 1-3, SDS/PAGE under reducing conditions (2 mM DTT) and stained with Simply Blue. Lane 1, starting material for GST Trap column; lane 2, concentrated eluate from the column; lane 3, concentrated retentate after elution of the column with 10 mM GSH. Arrows indicate the bands that were analyzed by MS. The sequences of the analyzed peptides and their identification by blast analysis are shown. Western blot: lane 4, lane 2 probed with 1-cysPrx mAb; lane 5, lane 3 probed with πGST pAb. (B) Peroxidase activity by NADPH/GSH reductase/GSH-coupled assay with PLPCOOH (addition indicated by arrow) as substrate. Activities were 5.0 ± 0.4 μmol/min/mg of protein for partially purified bovine lung enzyme (trace 1), zero for purified 1-cysPrx after storage for 1 week at 4°C (trace 2) and for πGST alone (trace 3), and 4.5 ± 0.4 μmol/min/mg of protein (mean ± SE, n = 3) after GSH-saturated πGST addition to the purified inactive protein (1:1 molar ratio) (trace 4).
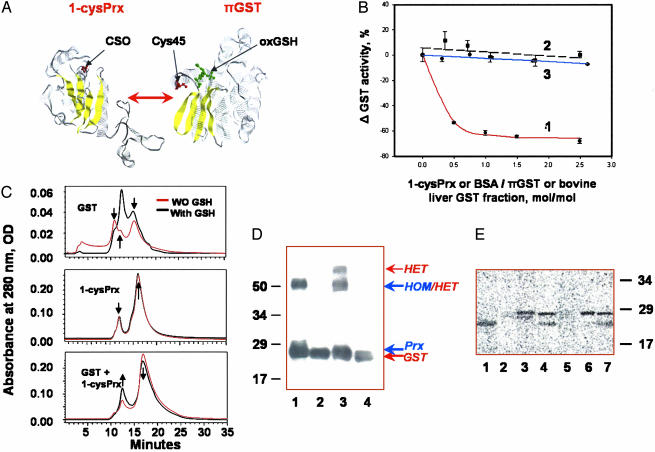
Purified recombinant protein was inactive but, after incubation with GSH-loaded πGST, its activity was similar to that of πGST-treated purified bovine lung enzyme (≈5.0 μmol/min/mg of protein). We propose that interaction between 1-cysPrx and πGST resulted in heterodimerization and glutathionylation of 1-cysPrx. The heterodimerization of proteins is illustrated in Fig. 2A, which shows the monomeric structures of 1-cysPrx (22) and πGST (29). This interaction was indicated by inhibition of πGST activity on addition of 1-cysPrx, whereas BSA (as a control) had no effect (Fig. 2B). 1-cysPrx had no effect on GST activity of the mixture from bovine liver that lacks the π-isoform (Fig. 2B). Interaction of 1-cysPrx with πGST was further evaluated by size exclusion HPLC with high-ionic-strength elution to enhance hydrophobic interactions (Fig. 2C). The elution profile of πGST (Fig. 2C Top) showed two peaks corresponding to the monomer (≈15.5 min, ≈23.5 ± 1.2 kDa) and multimer (11.0 min, ≈70 ± 3.4 kDa) with a shoulder corresponding to a homodimer (13.0 min, ≈49 ± 1.5 kDa). A molar excess of 10× GSH resulted in an increase in homodimer and a decrease in multimer content. The elution profile of 1-cysPrx (Fig. 2C Middle) showed two peaks corresponding to the monomer (≈15.3 min, ≈26 ± 1.4 kDa) and homodimer (12.5 min, ≈54 ± 1.6 kDa). Addition of GSH (10×) or equimolar amount of πGST (data not shown) had no effect on this elution profile. An addition of GSH-loaded πGST to 1-cysPrx resulted in increase of the dimer peak (12.8, min, ≈51 ± 3.0 kDa) and decrease of the monomer peak (15.3 min, ≈26 ± 1.4 kDa; Fig. 2C Bottom).
Fig. 2.
Heterodimerization and glutathionylation of 1-cysPrx. (A) Monomers of 1-cysPrx and πGST [with associated GSH sulfonate (oxGSH) in green] showing selected cysteine moieties in red (22, 27). Yellow indicates β-sheets. (B) GST enzymatic activity with 1-chloro-2,4-dinitrobenzene in the presence of increasing concentration of proteins: Trace 1, rat 1-cysPrx added to πGST; trace 2, BSA added to πGST; trace 3, rat 1-cysPrx added to liver GST that lacks the π isoform. (C) Size exclusion HPLC of a rat 1-cysPrx and πGST, and their equimolar mixture before (red trace) and after (black trace) preincubation with 10× excess of GSH. The arrows indicate characteristic peaks and their changes. The molecular mass standards used for calibration were chymotrypsinogen A (bovine pancreas) (25 kDa, RT = 15.5 min), chicken ovalbumin (44 kDa, RT = 13.25 min), BSA (67 kDa, RT = 11.25 min), and transferrin (81 kDa, RT = 10 min). (D) Detection of 1-cysPrx/πGST heterodimerization by crosslinking with sulfo-SBED. Proteins were separated by SDS/PAGE, transferred to Immobilon-P membrane, and detected with horseradish peroxidase-conjugated streptavidin. Lanes: 1, mixture of 1-cysPrx plus biotinylated 1-cysPrx; 2, same as lane 1 after reduction with DTT; 3, biotinylated 1-cysPrx plus πGST; 4, same as lane 3 after reduction with DTT. The molecular mass markers are shown on the left, and the presumed positions of the homo- (HOM) and heterodimer (HET) are shown on the right. (E) 35S autoradiogram of recombinant 1-cysPrx and πGST, and their combination after incubation with G[35S]H. Lanes: 1, authentic πGST; 2, oxidized rat 1-cysPrx (inactive); 3, oxidized and denatured rat 1-cysPrx; 4, rat 1-cysPrx plus πGST; 5, oxidized bovine (inactive) 1-cysPrx; 6, oxidized and denatured bovine 1-cysPrx; 7, bovine 1-cysPrx with GST. The molecular mass markers are indicated on the right.
Because of the similarity in the molecular mass of the 1-cysPrx (25 kDa) and πGST (23 kDa) monomers, the above analysis could reflect either hetero- or homodimerization. To discriminate between these possibilities, 1-cysPrx surface amines were covalently labeled with sulfo-SBED crosslinker containing biotin and a photoactive azide group. Photoactivation of labeled protein during incubation with unlabeled 1-cysPrx or πGST resulted in biotinylation of the subsequently generated homo- and heterodimers. SDS/PAGE and Western blot (with horseradish peroxidase-conjugated streptavidin) showed labeling compatible with the presence of both the 1-cysPrx homodimer and the 1-cysPrx:πGST heterodimer (Fig. 2D, lanes 1 and 3). After reduction of the crosslinker disulfide, the transfer of biotin, derived from the sulfo-SBED reagent to the 1-cysPrx or πGST monomers, confirms homo- or heterodimerization (Fig. 2D, lanes 2 and 4).
35S-labeled GSH (G35SH) was used to detect πGST-mediated glutathionylation of rat and bovine 1-cysPrx (Fig. 2E). Incubation with G35SH resulted in 35S incorporation into πGST (Fig. 2E, lane 1) but not into 1-cysPrx (Fig. 2E, lanes 2 and 5). On the other hand, glutathionylation was observed after denaturation of 1-cysPrx (Fig. 2E, lanes 3 and 6). This confirms inaccessibility of oxidized cysteine in naturally folded protein. Both native bovine and recombinant rat 1-cysPrx when incubated with πGST in the presence of G35SH showed 35S incorporation (Fig. 2E, lanes 4 and 7). As a control, G35SH incorporation was not detected with recombinant native or denatured 1-cysPrx after mutation of Cys-47 to Ser (C47S) (not shown). Thus, πGST mediates glutathionylation of 1-cysPrx, indicating that heterodimerization overcomes the natural accessibility barrier.
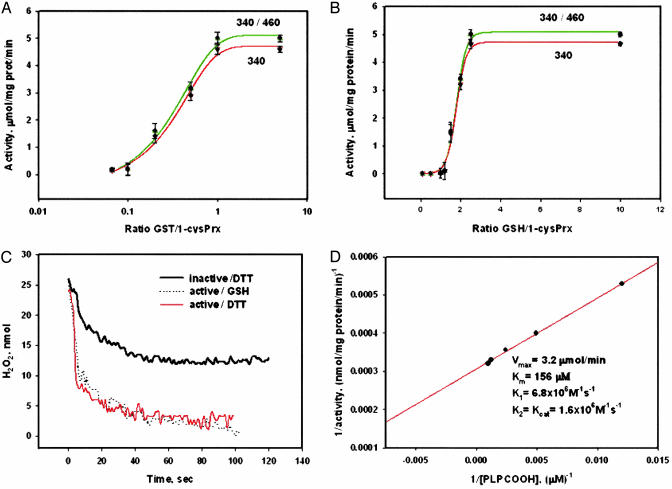
The effect of the 1-cysPrx-πGST interaction on GPx activity was studied by varying the ratio of the two proteins. Activity showed saturation (≈5 μmol/min/mg of protein) at a ≈1:1 ratio (Fig. 3A) in agreement with a heterodimer-mediated mechanism, as suggested by the data in Fig. 2B. Activity measured by either absorbance or fluorescence detection of NADPH oxidation was similar (Fig. 3A). To study the effect of the GSH/πGST ratio on 1-cysPrx activity (at a 1-cysPrx/πGST = 1:1), excess GSH and πGST were removed by passing the reaction mixture through BioSpin 6 (Bio-Rad) size exclusion and GST-trap affinity columns (Fig. 3B). Saturation of 1-cysPrx activity occurred at an ≈2:1 molar ratio of GSH/1-cysPrx, supporting the proposed mechanism of 1-cysPrx activation, which requires two GSH molecules per molecule of enzyme. The sharp increase of 1-cysPrx activity in the range of GSH/1-cysPrx between 1.0 and 2.5 corresponds with a first-order reaction. These data indicates that reduction of glutathionylated 1-cysPrx is a rate-limiting step of the proposed πGST-mediated mechanism of 1-cysPrx activation.
Fig. 3.
Biochemical characterization of 1-cysPrx activation. (A) enzymatic activity of recombinant 1-cysPrx as a function of GST/1-cysPrx as detected by NADPH fluorescence (340/460, green) or absorbance (340, red) with PLPCOOH as substrate (mean ± SE, n = 3). (B) Same as A but varying GSH/1-cysPrx with GST/1-cysPrx = 1. (C) Enzymatic activity of 1-cysPrx with H2O2 substrate detected by real-time absorbance (340 nm). Activity of 1-cysPrx in the presence of 88 μM DTT (black trace), and GST-activated enzyme with either 100 μM GSH (dot trace) or with 88 μM DTT (red trace). (D) Double reciprocal plot and kinetic constants of GST-activated 1-cysPrx with PLPCOOH as substrate.
To further evaluate activation of recombinant 1-cysPrx, we compared GSH- and DTT-mediated reduction of H2O2 (Fig. 3C). πGST-activated 1-cysPrx had similar GSH- and DTT-mediated activities, which were about four times higher than previously reported DTT-mediated activity of recombinant enzyme (5). Kinetic constants for πGST-activated recombinant 1-cysPrx with PLPCOOH (Fig. 3D) are similar to our published results (6).
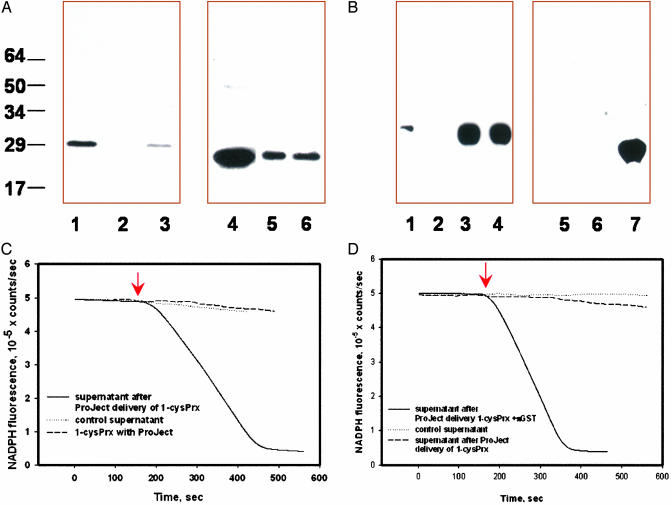
To evaluate 1-cysPrx activation under physiological conditions, we used the liposome-based ProJect system to deliver recombinant 1-cysPrx into H441 and MCF7 cells as confirmed by immunoblot analysis (Fig. 4 A and B). H441 cells express πGST but do not express 1-cysPrx as shown by Western blot (Fig. 4A, lanes 2 and 5) and by lack of GPx activity with PLPCOOH (Fig. 4C). Inactive recombinant enzyme was activated after delivery into H441 cells, as shown by assay with the cellular supernatant (Fig. 4 A and C). Delivery of recombinant 1-cysPrx into MCF7 cells that lack both endogenous 1-cysPrx and πGST (Fig. 4B, lanes 2 and 5) had no effect on activity of the supernatant. However, co-delivery of both 1-cysPrx and πGST (1:1 mol/mol) resulted in peroxidase activity (Figs. 4 B, lanes 4 and 7, and D).
Fig. 4.
Intracellular activation of 1-cysPrx. (A) ProJect-mediated delivery of rat recombinant 1-cysPrx into H441 cells as detected with mAb against 1-cysPrx (lanes 1-3) or after reprobing with pAb against πGST (lanes 4-6). Lanes: 1, 1-cysPrx standard; 2 and 5, supernatant of control H441 cells; 3 and 6, concentrated supernatant of H441 cells incubated with the ProJect/1-cysPrx complex; 4, πGST (P1-1). (B) ProJect-mediated delivery of rat 1-cysPrx or as a mixture with πGST (1:1, mol/mol) into MCF7 cells as detected with mAb against 1-cysPrx (lanes 1-4) or pAb against πGST (lanes 5-7). Lanes: 1, 1-cysPrx standard; 2 and 5, supernatant from control MCF7 cells; 3, 4, 6, and 7, supernatant of MCF7 cells incubated with ProJect/enzyme(s) complex. Lanes 3 and 6 are 1-cysPrx alone, and lanes 4 and 7 are 1-cysPrx and πGST. (C) 1-cysPrx activity (PLPCOOH substrate) in H441 cells. Rat 1-cysPrx mixed with ProJect as well as the supernatant of control H441 cells shows no activity. Supernatant of H441cells treated with ProJect/1-cysPrx had GPx activity of ≈3.4 ± 0.5 μmol/min/mg of protein (mean ± SE, n = 3). (D) 1-cysPrx activity in MCF7 cells. Supernatant of MCF7 cells before and after ProJect/1-cysPrx treatment shows no activity. Supernatant of MCF7 cells treated with Project/(1-cysPrx-πGST) has GPx activity ≈4.1 ± 0.5 μmol/mg of protein/min.
Discussion
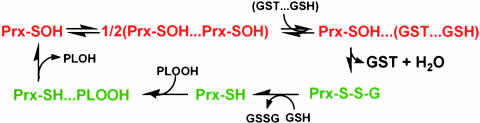
In this study, we have demonstrated (i) the presence of πGST in a semipure preparation of active bovine lung 1-cysPrx and loss of activity after further purification of the protein; (ii) that loss of the peroxidase activity of the enzyme is associated with oxidation of the catalytic cysteine; (iii) that incubation of inactive enzyme with GSH-loaded πGST results in heterodimer formation, glutathionylation of 1-cysPrx, and enzyme reactivation; and (iv) that delivery of exogenous oxidized 1-cysPrx into cells that normally lack 1-cysPrx but express πGST results in 1-cys-Prx activity. The delivery of 1-cysPrx into MCF7 cells, which do not express πGST (30), did not result in activity, whereas co-delivery of 1-cysPrx and πGST into these cells resulted in GPx activity. From these data, we propose that physiological activation of 1-cysPrx requires its heterodimerization with πGST, followed by glutathionylation of its oxidized Cys-47; subsequent dissociation of the heterodimer yields glutathionylated protein, which after spontaneous reduction by GSH becomes catalytically active.‡ This scheme (Fig. 5) is compatible with previous reports that multimer formation is essential for activation of bacterial alkyl hydroperoxide reductase (AhpC), another member of the peroxiredoxin family (31, 32). It is well known that GSH has an important role in the reduction of protein sulfenic acids; our model for reduction of 1-cysPrx proposes that πGST acts as a GSH carrier and does not alter the accepted pattern of electron flow.
Fig. 5.
Proposed scheme for activation of 1-cysPrx by interaction with πGST. Heterodimer formation of πGST with the oxidized enzyme leads to Cys-47 glutathionylation, followed by spontaneous reduction of the enzyme with GSH.
GSTs (EC 2.5.1.18) are ubiquitous enzymes that play a key role in cellular detoxification by conjugating toxicants to GSH and may serve as intracellular stoichiometric binding proteins for diverse lipophilic ligands. GSTs have been grouped into five isoenzymes. Four of them (alpha, pi, mu, and theta) comprise dimeric cytosolic enzymes. πGST binds GSH with low affinity (Ka ∼ 100 μM) and catalyzes the nucleophilic attack of the SH group of GSH on an electrophilic center of a lipophilic substrate. Although πGST is known to catalyze the conjugation of GSH to electrophilic substrates, its role in glutathionylation of oxidized protein cysteines represents a previously uncharacterized function. Importantly, naturally nucleophilic sulfhydryls become electrophiles because of their oxidation to sulfenic or sulfinic acid, thus satisfying substrate specificity for GST-mediated catalysis. In our experiments, the effect of 1-cysPrx on enzymatic activity of πGST was maximal at ≈1:1 molar ratio, indicating that glutathionylation of 1-cysPrx is collisional. The participation of πGST in activation of 1-cysPrx does suggest synergy of these two proteins in protection against cellular oxidative stress.
Acknowledgments
We thank Dr. D. Speicher for MS analysis, Dr. U. J. Zimmerman for assistance with protein isolation, Dr. I. Kotelnikova, Ms. L. Lu, and Mr. J. Haig for technical assistance, and Ms. J. Rossi for typing the manuscript. This work was supported by National Heart, Lung, and Blood Institute Grants HL 65543 and HL 19737.
This work was presented in part at Experimental Biology 2003, April 11-15, 2003, San Diego, CA [Manevich, Y., Feinstein, S. I. & Fisher, A. B. (2003) FASEB J. 17, A156 (abstr.)].
Abbreviations: 1-cysPrx, 1-cys peroxiredoxin; GSH, glutathione; PLPCOOH, 1-palmitoyl-2-linolenoyl hydroperoxide-sn-glycero-3-phosphocholine; sulfo-SBED, sulfosuccinimidyl[2,6-(biotinamido)-2-(p-azidobenzamido)-hexanamido]ethyl-1,3-dithiopropionate.
Footnotes
This protein also has been called antioxidant protein 2 (AOP2), nonselenium GSH peroxidase (NSPGPx), acidic Ca2+-independent phospholipase A2 (aiPLA2), and p67phox-binding protein. The corresponding cDNA has been called ORF6.
It is not clear whether the active form of 1-cysPrx is the monomer or the homodimer.
References
- 1.Hofmann, B., Hecht, H. J. & Flohé, L. (2002) Biol. Chem. 383, 347-364. [DOI] [PubMed] [Google Scholar]
- 2.Rhee, S. G., Kang, S. W., Chang, T. S., Jeong, W. & Kim, K. (2001) IUBMB Life 52, 35-41. [DOI] [PubMed] [Google Scholar]
- 3.Wood, Z. A., Schroder, E., Robin Harris, J. & Poole, L. B. (2003) Trends Biochem. Sci. 28, 32-40. [DOI] [PubMed] [Google Scholar]
- 4.Jacquot, J. P., Gelhaye, E., Rouhier, N., Corbier, C., Didierjean, C. & Aubry, A. (2002) Biochem. Pharmacol. 64, 1065-1069. [DOI] [PubMed] [Google Scholar]
- 5.Kang, S. W., Baines, I. C. & Rhee, S. G. (1998) J. Biol. Chem. 273, 6303-6311. [DOI] [PubMed] [Google Scholar]
- 6.Fisher, A. B., Dodia, C., Manevich, Y., Chen, W. & Feinstein, S. I. (1999) J. Biol. Chem. 274, 21326-21334. [DOI] [PubMed] [Google Scholar]
- 7.Kim, T. S., Dodia, C., Chen, X., Hennigan, B. B., Jain, M., Feinstein, S. I. & Fisher, A. B. (1998) Am. J. Physiol. 274, L750-L761. [DOI] [PubMed] [Google Scholar]
- 8.Singh, A. K. & Shichi, H. (1998) J. Biol. Chem. 273, 26171-26178. [DOI] [PubMed] [Google Scholar]
- 9.Phelan, S. A., Beier, D. R., Higgins, D. C. & Paigen, B. (2002) Mamm. Genome 13, 548-553. [DOI] [PubMed] [Google Scholar]
- 10.Power, J. H., Shannon, J. M., Blumbergs, P. C. & Gai, W. P. (2002) Am. J. Pathol. 161, 885-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krapfenbauer, K., Yoo, B. C., Fountoulakis, M., Mitrova, E. & Lubec, G. (2002) Electrophoresis 23, 2541-2547. [DOI] [PubMed] [Google Scholar]
- 12.Krapfenbauer, K., Engidawork, E., Cairns, N., Fountoulakis, M. & Lubec, G. (2003) Brain Res. 967, 152-160. [DOI] [PubMed] [Google Scholar]
- 13.Kim, H.-S., Pak, J.-H., Gonzales, L. W., Feinstein, S. I. & Fisher, A. B. (2002) Am. J. Respir. Cell Mol. Biol. 27, 227-233. [DOI] [PubMed] [Google Scholar]
- 14.Kinnula, V. L., Lehtonen, S., Sormunen, R., Kaarteenaho-Wiik, R., Kang, S. W., Rhee, S. G. & Soini, Y. (2002) J. Pathol. 196, 316-323. [DOI] [PubMed] [Google Scholar]
- 15.Munz, B., Frank, S., Hubner, G., Olsen, E. & Werner, S. (1997) Biochem. J. 326, 579-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dierick, J. F., Kalume, D. E., Wenders, F., Salmon, M., Dieu, M., Raes, M., Roepstorff, P. & Toussaint, O. (2002) FEBS Lett. 531, 499-504. [DOI] [PubMed] [Google Scholar]
- 17.Manevich, Y., Sweitzer, T., Pak, J. H., Feinstein, S. I., Muzykantov, V. & Fisher, A. B. (2002) Proc. Natl. Acad. Sci. USA 99, 11599-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pak, J. H., Manevich, Y., Kim, H. S., Feinstein, S. I. & Fisher, A. B. (2002) J. Biol. Chem. 277, 49927-49934. [DOI] [PubMed] [Google Scholar]
- 19.Chen, J.-W., Dodia, C., Feinstein, S. I., Jain, M. K. & Fisher, A. B. (2000) J. Biol. Chem. 276, 28421-28427. [DOI] [PubMed] [Google Scholar]
- 20.Leavey, P. J., Gonzalez-Aller, C., Thurman, G., Kleinberg, M., Rinckel, L., Ambruso, D. W., Freeman, S., Kuypers, F. A. & Ambruso, D. R. (2002) J. Biol. Chem. 277, 45181-45187. [DOI] [PubMed] [Google Scholar]
- 21.Stuhlmeier, K. M., Kao, J. J., Wallbrandt, P., Lindberg, M., Hammarstrom, B., Broell, H. & Paigen, B. (2003) Eur. J. Biochem. 270, 334-341. [DOI] [PubMed] [Google Scholar]
- 22.Choi, H. J., Kang, S. W., Yang, C. H., Rhee, S. G. & Ryu, S. E. (1998) Nat. Struct. Biol. 5, 400-406 [DOI] [PubMed] [Google Scholar]
- 23.Giles, G. I. & Jacob, C. (2002) Biol. Chem. 383, 375-388. [DOI] [PubMed] [Google Scholar]
- 24.Peshenko, I. V., Novoselov, V. I., Evdokimov, V. A., Nikolaev, Y. V., Kamzalov, S. S., Shuvaeva, T. M., Lipkin, V. M. & Fesenko, E. E. (1998) Free Radical Biol. Med. 25, 654-659. [DOI] [PubMed] [Google Scholar]
- 25.Peshenko, I. V. & Shichi, H. (2001) Free Radical Biol. Med. 31, 292-303. [DOI] [PubMed] [Google Scholar]
- 26.Shichi, H. & Demar, J. C. (1990) Exp. Eye Res. 50, 513-520. [DOI] [PubMed] [Google Scholar]
- 27.Peshenko, I. V., Singh, A. K. & Shichi, H. (2001) J. Ocul. Pharmacol. Ther. 17, 93-99. [DOI] [PubMed] [Google Scholar]
- 28.Akiba, S., Dodia, C., Chen, X. & Fisher, A. B. (1998) Comp. Biochem. Physiol. 120, 393-404. [DOI] [PubMed] [Google Scholar]
- 29.Reinemer, P., Dirr, H. W., Ladenstein, R., Schaffer, J., Gallay, O. & Huber, R. (1991) EMBO J. 10, 1997-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moscow, J. A., Townsend, A. J. & Cowan, K. H. (1989) Mol. Pharmocol. 36, 22-28. [PubMed] [Google Scholar]
- 31.Reynolds, C. M., Meyer, J. & Poole, L. B. (2002) Biochemistry 41, 1990-2001. [DOI] [PubMed] [Google Scholar]
- 32.Bryk, R., Lima, C. D., Erdjument-Bromage, H., Tempst, P. & Nathan, C. (2002) Science 295, 1073-1077. [DOI] [PubMed] [Google Scholar]