Abstract
Fragile X syndrome (FXS) is the leading known inherited cause of intellectual disability and the most common known biological cause of autism. Approximately 25% to 50% of males with FXS meet full diagnostic criteria for autism. Despite the high comorbidity between FXS and autism and the ability to diagnose FXS prenatally or at birth, no studies have examined indicators of autism in infants with FXS. The current study focused on indices of visual attention, one of the earliest and most robust behavioral indicators of autism in idiopathic (non-FXS) autism. Analyses revealed lower HR variability, shallower HR decelerations, and prolonged look durations in 12-month old infants with FXS that were correlated with severity of autistic behavior but not mental age.
Keywords: fragile X, autism, early detection, heart rate, visual attention, high-risk infants
Introduction
In the field of idiopathic (non-FXS) autism, there has been a focus on earlier detection of autism in the first two years of life. These efforts are based on increasing prevalence rates (Kogan, Blumberg, Schieve, Boyle, Perrin, Ghandour, et al., 2009), evidence that developmental disruption can be detected before 3 years of age (Landa & Garrett-Mayer, 2006; Zwaigenbaum, Bryson, Rogers, Roberts, Brian, & Szatmari, 2005) and research on behavioral intervention and neuroplasticity suggesting that amelioration of the symptoms or even prevention of the disorder is plausible given detection and treatment of infants before the full syndrome is developed (Dawson, 2008; Landa, 2008; Vismara, Colombi, & Rogers, 2007). The identification of early markers of autism is crucial to promote early diagnosis and treatment for the child and family.
Autism is a behavioral diagnosis based on observed impairment in the development of selective social and communication skills and the presence of stereotyped and repetitive behavior and restricted interests. Despite evidence that early diagnosis leads to early intervention, which is shown to optimize outcomes (Bryson, Rogerts, & Fombonne, 2003; Rogers, 1998) there is often a significant time lapse between the onset of autism symptoms and the diagnosis of autism. Parental reports and retrospective videos suggest that autism symptoms are present in up to 50% of infants during the first year with 80% of parents reporting abnormalities by the time the child is 24 months of age (Dahlgren & Gillberg, 1989; DeGiacomo & Fombonne, 1998). However, children are initially evaluated at a mean of 4.0 years (Wiggins, Baio, & Rice, 2006) and the median age of autism diagnosis is 5.7 years (Shattuck, Durkin, Maenner, Newschaffer, Mandell, Wiggins, et al., 2006) Given the gap between onset of symptoms and diagnosis, recent efforts to systematically identify early indicators of autism in the first two years of life and develop screening and assessment tools targeted to detect autism earlier in life have accelerated, and diagnostic instruments that yield a reliable diagnosis of autism at 2 years of age are now available (Lord, Risi, Lambrecht, Coock, Leventhal, DiLavore, et al., 2000; Luyster, Gotham, Guthrie, Coffing, Petrak, Pierce, et al., 2009). Earlier diagnosis of autism can lead to earlier entry into intervention, onset of more targeted interventions, and inform family planning with regard to recurrence risk. Thus, early diagnosis and treatment of autism has the potential to affect the child and family in a dynamic and likely reciprocal manner.
Recent efforts have utilized prospective longitudinal designs primarily involving infants with an older sibling diagnosed with autism (hereafter referred to as “infant siblings”) given the high familial recurrence risk (Garon, Bryson, Zwaigenbaum, Smith, Brian, Roberts, et al., 2009; Rivto, Jorde, Mason-Brothers, Freeman, Pingree, Jones, et al., 1989). This work has shown that behavioral indicators of autism are detectable within the first year of life. One of the most consistent, robust and earliest emerging feature of autism identified in the first two years is impaired attention. Infants later diagnosed with autism have been shown to display increased gaze fixation to their mother's mouth relative to her eyes as young as 4 to 6 months of age (Merin, Young, Ozonoff, & Rogers, 2007), longer disengagement latencies at 10 months of age (Elsabbagh, Volein, Holmboe, Tucker, Csibra, Baron-Cohen, et al., 2009) and increased visual fixation at 12 months (Zwaigenbaum et al., 2005). Also, blunted developmental change in the disengagement of visual attention between 6 to 12 months has been shown to predict later autism diagnoses (Zwaigenbaum et al., 2005).
While no defining pathophysiological mechanisms causing autism have been unambiguously identified, genetic factors clearly are involved (Moldin, Rubenstein, & Hyman, 2006) and abnormal prenatal and postnatal brain development is highly associated. Preliminary evidence from MRI studies suggest that total brain volume is enlarged in children with autism at 2 to 4 year of age, and retrospective head circumference studies suggest that increased brain volume occurs post-natally as enlarged head circumference emerges between 6 and 12 months of age (Courchesne, Karns, Davis, Ziccardi, Carper, Tigue, et al., 2001; Hazlett, Poe, Gerig, Smith, Provenzale, Ross, et al., 2005). These data suggest that brain overgrowth may be associated with the emergence of autism features in the first year of life. Specifically, the anterior cingulate and areas of the prefrontal cortex are implied as part of the executive attentional network that shows rapid development during the first years of life (Rothbart, Posner, & Rosicky, 1994). In one of the few physiological studies with infant siblings, event-related-potentials indicated that 10-month-old infant siblings displayed a slower P400 response to photos of direct gaze but no difference to age matched controls in response to photos of averted gaze, and infant siblings showed a pattern of faster response to averted than direct gaze that was not evident in controls (Elsabbagh, Volein, Csibra, Holmboe, Garwood, Tucker, et al., 2009). These findings suggest that neural patterns reflecting detection of reference and modulation of attention as well as atypical neural connectivity may be potential biomarkers associated with high risk infants later diagnosed with autism.
Taken together, evidence suggests that attention deficits in infant siblings emerge during the first year of life and are associated with specific neural sources that may be predictive of later autism diagnosis. These findings suggest that impairments in attention reflect lower-order system function that may lead to secondary deficits or increased severity over time associated with altered neural system development consistent with experience-expectant stimulation. This theory supports that infants later diagnosed with autism possess a selective deficit in social attention that could then give rise to more severe or pervasive deficits observed later in development. However, caution is warranted given that a number of studies report early indicators of autism in high-risk groups without diagnostic categorization at later ages. Further there is preliminary evidence that autism associated features in high-risk infants may change over time and be differentially related to diagnostic outcomes of autism versus a communication or general developmental delay (Young, Merin, Rogers, & Ozonoff, 2009).
Fragile X syndrome (FXS) is the leading known heritable cause of intellectual disabilities affecting approximately 1 in 2,500 individuals (Hagerman, 2008). Fragile X syndrome is a single gene disorder associated with a CGG trinucleotide repeat expansion on the 5’ UTR of the fragile X mental retardation gene (FMR1). Alleles with the full mutation typically result in hypermethylation of the promoter region and silencing of the FMR1 gene resulting in reduced FMR1 protein (FMRP). The inheritance pattern is complex with successive generations at increased risk for expansion through maternal transmission. In normal individuals, there are between 5 and approximately 45 – 55 CGG repeats. Approximately 1:259 females and 1:813 males have the “premutation” of FMR1 which is characterized by approximately 55 to 200 repeats(Dombrowski, Levesque, Morel, Rouillard, Morgan, & Rousseau, 2002; Hagerman, Berry-Kravis, Kaufmann, Ono, Tartaglia, Lachiewicz, et al., 2009). The largest expansion of the CGG region, >200 repeats, is termed full mutation and, by definition, corresponds to FXS.
The most well documented phenotypic feature in FXS is the high association with autism in terms of increased core and associated features of autism as well as diagnostic categorization. A co-morbid diagnosis of autism in FXS is associated with debilitating effects across multiple domains. Up to 90% of males with the full mutation display one or more features of autism including social anxiety, abnormal communication, sensory processing deficits, social avoidance, anxiety, attention deficits, and gaze avoidance (Hagerman, 2006; Clifford, Dissanayake, Bui, Huggins, Taylor, & Loesch, 2007). Recent evidence indicates that 25% to 50% of males with FXS meet DSM-IV based criteria for autism, and 35% to 74% meet criteria for the autism spectrum (Hall, Lightbody, & Reiss, 2008; Harris, Hessl, Goodlin-Jones, Ferranti, Bacalman, Barbato, et al., 2008; Kaufmann, Cortell, Kau, Bukelis, Tierney, Gray, et al., 2004). Young children with FXS also exhibit elevated rates of autism with 25% to 27% of 1 to 8 year-olds meeting criteria for autism, and 18% to 33% of 1 to 8 year olds meeting criteria for pervasive developmental disorder using DSM IV based measures (Zingerevich, Greiss-Hess, Lemons-Chitwood, Harris, Hessl, Cook, et al., 2009). In a recent longitudinal study that included the largest sample of infants and toddlers with FXS to date (n=55, aged 8 to 48 months, 189 observations), 31% of the children scored in the mild to severe autism range of the Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Renner, 1988). Importantly, elevated autistic behavior has been shown to negatively affected the rate of development over time (Roberts, Mankowski, Sideris, Goldman, Hatton, Mirrett, et al., 2009). These studies unambiguously demonstrate that there is a high association with autism in young children with FXS, and evidence suggests that the profile of autism in FXS appears highly similar to idiopathic (non-FXS) autism (Bailey, Mesibov, et al., 1998; Baranek, Danko et al., 2005; Dissanayke et al., 2009; Rogers, Wehner et al., 2001).
Given the strong association of FXS with autism, infants with FXS constitute a population at very high risk for autism. Despite their high risk for autism and the ability to diagnose FXS prenatally or at birth, no study has examined the emergence and stability of autism or autistic features in infants with FXS. Doing so would help illuminate important risk factors associated with a later diagnosis of autism to facilitate diagnosis and target treatment efforts.
Several studies have identified aberrant visual attention in infants with FXS supporting the need for further examination. In a study examining visual contrast detection in 27 infants with FXS (mean age 24 months), data suggest that infants with FXS display dorsal stream deficits reflected in higher detection thresholds for the second-order (contrast-defined) gratings (Farzin, Hagerman, & Rivera, 2008) which is consistent with reports with older aged children with FXS (Cornish, Munir, & Cross, 1999; Kogan, Bertone, Cornish, Boutet, Der Kaloustian, Andermann, et al., 2004; Kogan, Boutet, Cornish, Boutet, Der Kaloustian, Andermann, et al., 2004). Studies of attention in toddlers with FXS (aged 1 to 3 years) suggest poor oculomotor control (inhibition of saccades) and poor response inhibition (increased repetitive errors) compared to mental age typical controls (Scerif, Cornish, Wilding, Driver, & Karmiloff-Smith, 2004; Scerif, Karmiloff-Smith, Campos, Elsabbagh, Driver, & Cornish, 2005) which is consistent with inhibitory deficits observed in older children. While these studies of visual attention are helpful to identify the early emergence of aberrant visual attention in FXS, none of this work has examined the association of attention to autistic behavior or diagnoses. Considering the potential for implementation of universal newborn screening for FXS and recent advances in the detection of autism during the first two years of life, a study of the emergence and stability of autism in FXS is critical to characterize the infant phenotype of FXS and develop targeted interventions, including those shown to be effective in autism, to reduce the severity or prevent the occurrence of autism in children with FXS
The overall goal of this study was to examine behavioral and physiological indicators of visual attention over time in infants with FXS from both normative and individual differences perspectives. Our focus on visual attention in infants with FXS was based on several findings in the literature. First, aberrant attention is a hallmark feature of FXS across the lifespan with rather severe and deleterious effects typically associated including psychotopic medication use, extensive family and educational supports, and lowered quality of life (Bailey, Raspa, Holiday, Bishop, & Olmstead, 2009; Sullivan, Hatton, Hammer, Sideris, Hooper, Ornstein, et al., 2006). Second, evidence has emerged that atypical visual attention may be an early marker for autism in high-risk infant siblings as young as 4 to 6 months of age (Merin, Young, Ozonoff, & Rogers, 2007; Yirmiya, Gamliel, Pilowsky, Feldman, Baron-Cohen, & Sigman, 2006; Zwaigenbaum et al., 2005). Our specific hypotheses were as follows:
Twelve-month-old infants with FXS will display atypical behavioral and physiological responses to a visual attention task compared to normative trends and to an age and gender matched typically developing control group. Specifically, infants with FXS will display prolonged duration of attention coupled with decreased latency to disengage attention and elevated and less variable heart rate compared to the control group. However, heart rate is expected to reflect the expected developmental trends over time.
Severity of autistic behavior will be associated with visual attention and heart activity in infants with FXS over time. Increased severity of autistic behavior at 18 months of age will be associated with increased look duration and longer latency to disengage visual attention and higher and less variable heart activity over time.
Method
We utilized a mixed methods design that incorporated a prospective longitudinal design with assessments at 9, 12, and 18 months of age in infants with FXS and a cross-sectional component with typically-developing infants at 12 months. Data were not collected from the typically-developing comparison group across time due to limited resources, and the 12 month age was determined to be of primary importance given work showing that group differences can be detected across multiple markers at this age (Zwaigenbaum et al., 2005). We used a combined behavioral, both standardized and laboratory measures, and biomarker approach.
Participants
Infants with FXS
Thirteen boys with the full mutation of FXS participated in this study. While we had hoped to enroll all participants at 9 months of age, two boys were not referred to the study until they were 12 months of age. Therefore, 11 entered the study at 9 months of age, and 2 entered at 12 months of age. These participants were recruited through several sources including a national listserv for parents of children with FXS, a database of participants in our longitudinal studies, and web-based advertisements posted on FXS organizations. Families that participated in this study lived in 13 states across the continental United States, all children had a positive family history of FXS and had undergone genetic testing prior to study entry.
Typically developing infants
Ten 12- month-old typically developing (TD) children participated in this study. Typical development was defined as having a developmental quotient score within or above the average range and no documented or suspected disability. These children were recruited from local child care programs, and all lived in a southeastern community where the study took place.
Measures
Free play attention assessment
The Lab Based Assessment of Temperament (LabTAB; Goldsmith & Rothbart; 1996) is an observational measure of child temperament that involves the administration of standardized games for infants aged 6 months – 3 years. The toy play game involves unstructured toy play and was designed to measure attention in infants (Goldsmith & Rothbart, 1999). Prior to the presentation of the toy (e.g., set of toy keys), the infant is seated in a high chair or booster seat and placed in front of a table with no stimulus. The infant must be in a neutral state for at least 15 seconds (e.g., not crying, fussing, or yawning) prior to the toy presentation which is considered a baseline condition. Once the baseline is established, the infant is presented with a toy and allowed 3 minutes of unstructured toy play. The Lab-TAB is videorecorded to code behaviors offline based on stringent coding standards being met and maintained. The kappa for this study was .82. In this study, the toy play game was used to measure attention in infants. Two visual attention variables were coded from the session: the percent of time that the infants looked at the toy during the unstructured play time, and the latency for initial visual disengagement from the toy.
Heart activity
We collected heart activity as a biomarker of physiological activity for participants during completion of the visual attention experiment. The Mini-Logger 2000 (1994) is a telemetry system that uses a chest belt to transmit heart activity to a receiver that is placed within 3 feet of the child. This system has been shown to be tolerable to a wide age range of children with FXS and has shown adequate sensitivity and reliability across systems (Kaufman et al., 2004; Roberts, Boccia, Bailey, Hatton, & Skinner, 2001; Roberts, Boccia, Hatton, Skinner, & Sideris, 2006). Heart activity data were edited for artifacts then analyzed using the MxEdit program (MxEdit, 1989).
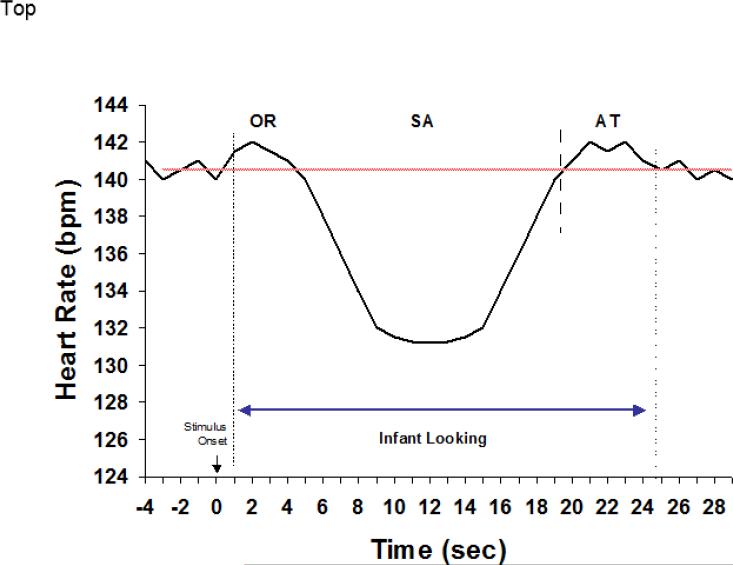
The HR data were analyzed in multiple ways by examining the mean and variability (standard deviation) during toy play then by adapting Richards’ framework to parse HR-defined phases of attention during infant looking. This approach has been used fruitfully in assessing at-risk groups (Colombo, Kannass, Shaddy, Kundurthi, Maikranz, Anderson, et al., 2004), longitudinal prediction (Colombo, Shaddy, Richman, Maikranz, & Blaga, 2004), and in various individual-difference analyses (Colombo, Richman, Shaddy, Greenhoot, & Maikranz, 2001). Figure 2 shows the general schematic for understanding the framework within the context of infant looking; based on the expectation that cognitive activity will be correlated with HR deceleration. Richards’ (1987) framework is generally used when HR measures are synchronized with behavioral indices of looking; HR data were not synchronized as such in this study so we analyzed HR in terms of a simpler scheme composed of decelerative and nondecelerative components. Decelerative phases were defined essentially as Richards’ (1987) sustained attention measure: a HR change below the median prestimulus period (here, we used the median baseline HR for this purpose) that lasts at least 5 consecutive beats before returning to the baseline median. Periods not meeting this criterion were classified as nondecelerative phases. We derived three variables based on the classification scheme of decelerative and nondecelerative phases: the proportion (percent) of time spent, the mean HR, and the standard deviation (variability) of HR during toy play. Means and standard deviations for these variables are shown in Table 3.
Figure 2.
Schematic representation of HR-defined phases of attention. Adapted from Richards and Casey (1990).
Developmental age
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) is a comprehensive standardized measure of development for children from birth to 68 months. It consists of five scales: visual reception, gross and fine motor, receptive and expressive language. T-scores, percentile ranks and age equivalents can be derived for each scale. The scales demonstrated satisfactory internal consistency (median reliability coefficients varying from .75 to .83 across the 5 scales). Test-retest reliabilities ranged from .76 to .96 (across scales and ages) and inter-rater reliabilities ranged from .91 to .96. In addition, the MSEL demonstrated strong concurrent reliability with other widely used developmental tests for the same age range. The Mullen was administered to the typically-developing infants and those with FXS at each assessment occasion. The Early Learning Composite was used to document typical development in the TD comparison group (M = 111, range 94 to 132). A mental age composite was derived and used as a continuous variable to examine the effect of mental age on visual attention in the FXS group. The mean mental age for the infants with FXS was 7.11 (SD 1.07) at 9 months, 9.33 (SD 1.07) at 12 months, and 12.54 (SD 1.88) at 18 months.
Autistic behavior
The CARS (Schopler, Reichler, & Renner, 1988) is a 15 item scale that provides a general rating of autistic behavior. The total score can be used as a continuum of autistic behavior or as a categorical indication of nonautistic (total score of 15-29.5), mildly or moderately autistic (total score of 30-36.5), and severely autistic (total score of 37 or higher). The CARS was only used with the infants with FXS at the 18 month assessment as it is not sufficiently sensitive to detect autistic behaviors at younger ages (Schopler, Reichler, & Renner, 1988). The total CARS score was used as a continuous variable in analyses.
Procedure
Once consent was obtained for study participation, the assessments were scheduled as close to the targeted age as possible. Two Ph.D. level investigators with over 5 years experience working with young children with FXS completed all the assessments with the infants with FXS, and one of these same investigators completed all the assessments with the typically-developing children with assistance from a trained research assistant. All assessments took place mid-morning to control for circadian effects on the HR data. The Mullen was completed as the first assessment activity as it allowed for informal and flexible interaction with the infant prior to the Lab-TAB. Per standardized guidelines, all children demonstrated interest in the toy, as reflected in physically reaching for the toy, before beginning the game. Heart activity data were continuously recorded while the participants completed the baseline and toy play visual attention procedures.
Results
Overview
The particular design of this study allowed for two specific types of comparisons. First, because all variables are available for both the FX and TD groups at the 12-month age point, it was possible to conduct group comparisons at this age. Second, since the FX groups were seen repeatedly at 9, 12, and 18 months, it was possible to conduct longitudinal analyses across these ages to determine age-related trends and the effect of chronological age, developmental age, and autistic behavior over time. As such, the results are organized in terms of these analyses. Within each of these divisions, we address analyses of both behavioral and physiological data. Due to the small sample size, nonparametric tests (Mann-Whitney U) were also conducted, but yielded the same results as the parametric analyses; thus, only the parametric tests are reported in the sections that follow.
Comparisons of FX and TD Groups at 12 Months
Visual attention
Table 1 presents the means, standard deviations and the results of simple t-tests for the proportion of time spent looking at the toy and the latency (in seconds) for the infant to initially disengage attention from the toy comparing infants’ performance at 12 months of age. Infants with FXS spent a significantly greater proportion of time looking at the toy than did their TD counterparts (73.9% versus 44.9% respectively). Latency to look away from the keys was, on average, nearly four times longer for infants with FXS than it was for TD infants. However, this mean difference was largely due to one FX outlier who had a latency of over 150 seconds. Removing this outlier reduced the mean difference to non-significance, although even after removal of the outlier infants with FXS still took twice as long to release looking from the keys (18.3 sec), relative to the TD infants (8.8 sec).
Table 1.
Group Differences at 12 months
| Variable | TD | FXS | t-testa | |||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | t | Sig | |
| Proportion in Gaze Stimulus | .45 | (.14) | .74 | (13.7) | −.32 | .005 |
| Latency to Gaze Away (s) | 8.8 | (6.2) | 32.2 | (17.0) | −1.39 | ns |
| Mean HR during keys | 133.9 | (12.8) | 136.8 | (6.5) | −0.63 | ns |
| SD of HR during keys | 9.13 | (3.52) | 6.27 | (1.324) | 2.49 | .024 |
Because of the small samples involved, nonparametric tests (Mann-Whitney U) were also conducted on these data. Because these tests yielded the same results as the parametric analyses, the parametric tests are reported here.
Heart rate
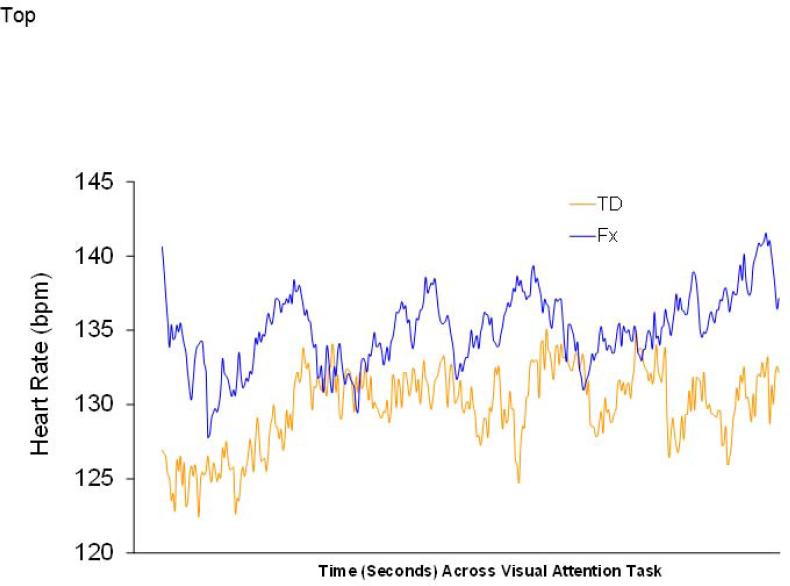
The HR data were analyzed in two steps. First, we analyzed the mean and standard deviation of HR during toy play. Next, we analyzed the amount of time, mean HR during, and standard deviation of HR during the decelerative and nondecelerative HR categories during toy play. Figure 1 shows the overall beat-by-beat pattern of responses for TD infants and infants with FXS at 12 months across the baseline and toy play epochs. The mean and standard deviation for infants’ heart rate during toy play was analyzed as a function of group membership. Means, standard deviations, and t-test results for the two groups are presented in Table 1 and graphically displayed in Figure 1. During the toy play epoch, t-tests indicated significant differences (p = .02) on HR variability, with FX infants having lower HR variability than TD infants. There were no differences on mean HR during toy play. When the mean and standard deviation during the toy play epoch were entered together in a multivariate analysis, the effect of Group did attain statistical significance (multivariate F(2, 16) = 7.89, p = .004).
Figure 1.
Heart rate patterns during baseline and keys task for TD and FX infants at 12 months.
In the second step, we analyzed HR by decelerative versus nondecelerative categories. Results indicated no group differences in the proportion of time during toy play spent in decelerative and nondecelerative phases. However, the analysis for mean HR yielded a significant main effect for Phase (multivariate F(1, 15) = 132.8, p = .000), which was qualified by a significant Group × Phase interaction (multivariate F(1, 15) = 5.89, p = .028). As expected, both groups showed decelerations as a function of the two Phases involved; this produced the main effect described above. However, the interaction was produced by TD infants having lower HRs during the decelerative phase (i.e., deeper decelerations) than the infants with FXS; HRs during the nondecelerative phases were not appreciably different. Analyses of the standard deviation of HR during decelerative and nondecelerative phases yielded a significant main effect for Group (multivariate F(1, 14) = 5.53, p = .034) but no significant interactions.
It is worth noting that the main effect for Group on variability of HR was not attributable simply to the lowered HRs observed for the TD infants during the decelerative phase and described in the previous paragraph. If we repeat the analysis of HR variability described above with the mean HR during the decelerative phase covaried, the Group effect remained statistically reliable (multivariate F(1,13) = 22.2, p = .05). Thus, the Group effect on SD is independent of the difference in mean HR between the two groups in the decelerative phase. Finally, as expected from the t-tests earlier, infants with FXS had lower variability than TD infants. The lower variability in FX infants, however, was not specific to either the decelerative or nondecelerative phase, as the Group × Phase interaction did not attain statistical significance (multivariate F(1, 14) = 1.86, ns).
Developmental Trends for FX Infants from 9 to 18 Months
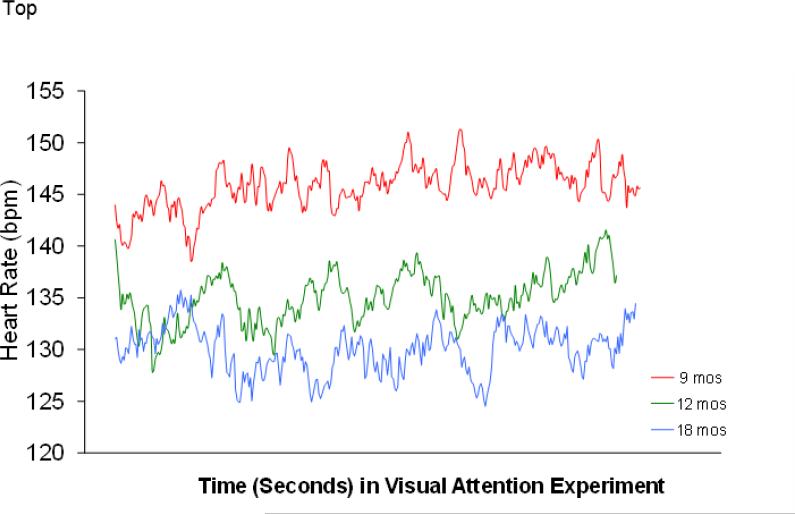
Mixed-model (HLM) analyses were conducted on the available longitudinal data for FX infants from 9 to 18 months of age. All of the behavioral and physiological variables described above were subjected to these analyses, but statistically significant change was noted only for mean HR during the toy play epoch (p = .024). Table 2 shows descriptive data over time, and figure 3 shows a beat-by-beat graph of infants with FXS during object attention as a function of age; the age effects were clear in that older infants had lower mean HRs (and thus, fewer beats within the same constrained time period). However, significant age effects were absent for the HR variability, duration of visual attention, and latency to disengage visual attention (all p's > .05). Table 3 displays means and standard deviations of these variables across time. For the infants with FXS, there were no significant changes across the 9, 12, and 18 month age points. While we do not have a TD control group at 18 months, It is interesting to note that differences between FXS and TD infants observed at 12 months showed no evidence of disappearing at 18 months; infants with FXS at that age showed longer visual attention to the toy than the typically developing infants at 12 months (68% versus 45%) and longer latency to disengage attention (19 seconds versus 9 seconds).
Table 2.
Behavioral and physiological changes over time in the infants with FXS
| FXS (n=11) | FXS (n=13) | FXS (n=13) | |
|---|---|---|---|
| Variable | 9 months | 12 months | 18 months |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Proportion in Gaze Stimulus | .65 (.28) | .73 (.21) | .68 (.19) |
| Latency to Gaze Away | 21.24 (19.96) | 32.52 (43.92) | 19.06 (21.65) |
| Mean HR during keys | 146.26 (13.36) | 136.77 (6.53) | 130.1 (11.15) |
| SD of HR during keys | 6.56 (1.03) | 6.27 (1.32) | 6.74 (1.46) |
Figure 3.
Heart rate patterns during baseline and keys task for FX infants from 9 to 18 months.
Relationships of Autistic Behavior and Developmental Age to Attention and HR in FXS
The relationship of autistic behavior and developmental age to attention and HR in FXS was examined through a series of Pearson correlations.
Developmental age
Pearson correlations indicated that developmental age, as measured by the mean mental age from the Mullen, was not associated with any of the visual attention or HR variables across all 3 assessment occasions (9, 12, and 18 months).
Autistic behavior
Autistic behavior was measured using the CARS total score at the 18-month assessment. Results indicated that increased time spent looking at the toy was moderately, related to elevated CARS scores at 12 months of age (r = .49, p = .09) and at 18 months of age (r = .55, p = .08) as reflected in the scatterplot of Figure 4. Also, increased latency to disengage attention between the 9 and 12 month assessment (change score) was moderately related to elevated CARS scores (r = .46, p = .09). However, latency to disengage looking at the toy, mean HR during the keys, and variability (standard deviation) at 9, 12, and 18 months were not related to the 18-month CARS scores.
Figure 4.
Scatterplot of CARS and proportion of time looking at object for infants with FXS at 18 months of age.
Discussion
This is the first prospective, longitudinal study of visual attention and its association with autistic behavior in infants with FXS at 9, 12 and 18 months of age. We used multiple methods including standardized and experimental measures of behavior along with physiological measures to increase our understanding of the developmental trajectory, predictors and profile of visual attention in infants FXS. Increased understanding of visual attention in FXS very early in development has the potential to facilitate early identification and identify potential causal developmental pathways to inform treatment efforts. Based on existing work documenting the high comorbidity of autism with FXS and similar clinical profiles between children with idiopathic (non-FXS) autism compared to those with autism associated with FXS, we hypothesized that infants with FXS would display atypical behavioral and physiological indices of visual attention as has been reported in infants at high risk for autism by virtue of having an older sibling diagnosed with autism. Given that up to 50% of males with FXS meet diagnostic criteria for autism and up to 74% meet diagnostic criteria for autism spectrum disorder using DSM IV criteria (Hernandez, Feinberg, Vaurio, Passanate, Thompson, & Kaufmann, 2009; Zingerevich et al., 2009), identifying early signs of autism in FXS is critical to facilitate early identification of both autism and FXS in this population.
Consistent with our hypothesis, we found that infants with FXS displayed atypical visual attention across multiple measures over time. In comparison to an age and gender matched TD control group, 12-month-old infants with FXS displayed significantly longer look durations and twice as long to shift attention, albeit not a significant difference, during an experimental procedure. These findings of atypical attention were supported by the longitudinal analysis of the infants with FXS showing some atypical trends. Rather than displaying significant changes in attention that were expected based on normative data (Bornstein, 1998; Courage, Reynolds, & Richards, 2006; Zwaigenbaum et al., 2005), infants with FXS displayed an overall flat trajectory from the first to the second year.
In addition to somewhat atypical attention using behavioral measures, we found atypical psychophysiological functioning that was associated with autistic behavior. Infants with FXS displayed elevated mean HR and less variability (standard deviation) in comparison with typically developing infants. This is consistent with reported patterns of systemic hyperarousal in FXS (Hall, Lightbody, & Reiss, 2008; Roberts, Boccia, Bailey, Hatton, & Skinner, 2001). In addition to general mean HR and variability, we were interested in patterns of heart activity reflecting decelerative phases during which active cognitive processing is believed to occur (Colombo, Kannass, et al., 2004; Richards, 1987). Our data suggest that infants with FXS do not differ in the amount of time spent in decelerative heart rate phases; however, their mean heart rate during the decelerative phases is higher likely reflecting the elevated systemic arousal reported above. Infants with FXS also exhibit lower variability during the decelerative phases even when mean heart rate is controlled for. Taken together, these results suggests that infants with FXS may have lowered capacity to modulate arousal than typically developing controls which has been reported in studies with older children (Roberts, Boccia, Bailey, Hatton, & Skinner, 2001) and adults with FXS (Rivera et al., 2002; Roberts, Boccia, Hatton, Skinner, & Sideris, 2006). Ours is the first, however, to report this pattern in infants with FXS.
Indeed, recent evidence has documented an association between reduced variability and elevated sympathetic and reduced vagal tone to autism in individuals with FXS 5 to 20 years of age (Hall, Lightbody, & Reiss, 2008) which confirmed earlier preliminary evidence by our group (Roberts, Boccia, Bailey, Hatton, & Skinner, 2001). In the current study, we report that elevated autistic behavior as measured by the CARS was associated with lower HR during the visual attention experiment at 9 months of age but not at the 12 and 18 month assessment and not with HR variability at any age. Collectively, our findings suggest that physiological hyperarousal is an endogenous characteristic of persons with FXS likely the result of abnormal gene function and reduced FMRP. However, the developmental pathway regarding the relationship between physiological hyperarousal and aberrant behavior is unclear particularly in the first years of life.
Our results suggest a number of parallels to findings of high risk infant siblings later diagnosed with autism. First, our finding that infants with FXS displayed longer look durations and extended latency to disengage visual attention is consistent with results of high risk infant siblings who show longer latencies to disengage visual attention (Bryson, Czapinski, Landry, McConnell, Rombough, & Wainwright, 2004; Bryson, Rogers, & Fombonne, 2003; Zwaigenbaum et al., 2005) and increased object fixation (Zwaigenbaum et al., 2005). Also, we report a relationship of increased severity of autistic behavior on the CARS to increased look duration in the infants with FXS at 12 and 18 months of age providing further evidence of the parallel behavioral patterns of aberrant visual attention between infants with FXS and infants later diagnosed with autism. Second, we report a slight increase in latency to disengage attention between 9 and 12 months of age which was moderately correlated with elevated severity of autistic behavior in the infants with FXS. Zwaigenbaum and colleagues (2005) report a similar finding with longer latencies to disengage visual attention between 6 and 12 months of age predictive of autism spectrum diagnoses.
In conclusion, our study of visual attention in infants with FXS has identified a pattern of potential neurodevelopmental abnormalities detectable in the first year of life. These include prolonged visual attention to objects, longer latencies to disengage attention, less variability of HR, and tempered HR deceleration, compared to TD infants. Remarkably, the behavioral markers of visual attention in infants with FXS in the present study are highly consistent with findings reported with high risk infant siblings later diagnosed with autism. The consistency of visual attention abnormalities between infants with FXS and high risk infant siblings coupled with correlations of the severity of autistic behavior to specific behaviors and biomarkers in the infants with FXS suggest a common profile among these two etiologically distinct groups. However, given the preliminary nature of this study, these patterns and profiles need to be examined in larger samples with more precise diagnostic measures.
This work has a number of important clinical and research implications. Of primary clinical importance is the need for early and differential diagnosis in FXS to initiate treatment and provide family support. According to Bailey and colleagues (2009) a common pathway to diagnosis in FXS in the absence of a positive family history starts with concern about the child's developmental skills (X of 12 months), proceeds with diagnosis of a developmental delay (X of 20 months of age), leads to initiation of early intervention (X of 22 months of age), and ends with a diagnosis of FXS (X of 36 months). While initiation of early intervention services is of primary concern to the affected child with regard to optimizing outcomes, the diagnosis of FXS has a number of unique family ramifications including risk of recurrence with 64% of uninformed families have a second child with FXS (Bailey, Raspa, Holiday, Bishop, & Olmstead, 2009).
Clearly, early identification has unique importance in FXS; however, there are a number of barriers to facilitating identification at earlier ages including findings that developmental delays may be subtle or absent in the first year with only 50% of 9 and 12 month olds with FXS are projected to meet criteria for early intervention (Roberts et al., 2009). Thus, identification of FXS within the first year is likely to continue to be challenging if reliant on traditional developmental markers such as motor or language milestones. Given recent reports of an increased prevalence of autism to 1:100 (Kogan et al., 2009) and the heightened awareness of autism symptoms by parents and pediatricians (Johnson & Myers, 2007), earlier diagnosis of FXS may be facilitated through the autism diagnostic process. The current study informs this process by demonstrating that in the first year of life, infants with FXS demonstrate atypical behavioral and physiological features that are associated with autism. Thus, rather than an emphasis on more traditional screening tools that measure language, motor, and cognitive skills, a targeted focus on social-communicative behaviors and, perhaps, specific processes such as visual attention might result in infants with FXS being detected as “at risk” for autism resulting in an earlier onset of intervention and subsequent testing for FXS. Indeed, current policy of the American Academy of Pediatricians (AAP) is to conduct developmental screening at 9, 18 and 30 month well-child visits (Visootsak, Warren, Anido, & Graham, 2005) with heightened sensitivity to early recognition of autism spectrum disorders (Johnson & Myers, 2007). Fragile X syndrome is noted as the most common known genetic cause for autism in AAP policy statements and referral for FXS testing is routine.
While this is the first study to examine visual attention as an early predictor of autism in FXS, and there are a number of strengths to this work including multiple measures, a prospective longitudinal design, and inclusion of a comparison group, there are a number of limitations to this work that reduce its impact. Most notably, the sample size is small for both groups (FXS and TD). Other limitations include the use of the CARS rather than gold-standard DSM- IV measures such as the ADOS and ADI-R. Also, unlike the group with FXS, the comparison group was only assessed at one time rather than across multiple ages. Finally, we did not include a third group of infants with developmental delay (e.g., Down syndrome) matched on mental and chronological age to allow us to identify features that are common or unique to FXS compared to other developmental disorders.
Acknowledgements
This study was supported by the National Institute of Child Health and Human Development (P30-HD003110-35S1) and the office of Special Education Programs, U.S. Department of Education (H324C990042).
Footnotes
Authors’ Current Affiliations
Jane E. Roberts is now at: The University of South Carolina Department of Psychology Columbia, SC 29201
Deborah D. Hatton is now at: Vanderbilt University's Peabody College Nashville, TN 37203-5721
Don B. Bailey is now at: RTI International Research Triangle Park, NC 27709
Anna C.J. Long is now at: 3-C Family Services 1901 N. Harrison Ave Cary, NC 27513
Contributor Information
Jane E. Roberts, The University of North Carolina at Chapel Hill FPG Child Development Institute Chapel Hill, NC 27514
Deborah D. Hatton, The University of North Carolina at Chapel Hill FPG Child Development Institute Chapel Hill, NC 27514
Donald Bailey, The University of North Carolina at Chapel Hill FPG Child Development Institute Chapel Hill, NC 27514.
Anna C. J. Long, The University of North Carolina at Chapel Hill FPG Child Development Institute Chapel Hill, NC 27514
Vittoria Anello, The University of South Carolina Department of Psychology Columbia, SC 29201.
John Colombo, The University of Kansas Department of Psychology 1415 Jayhawk Boulevard Lawrence, KS 66045.
References
- Bailey DB, Jr, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders. 1998;28:499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr., Raspa M, Holiday D, Bishop E, Olmsted M. Functional skills of individuals with fragile X syndrome: A lifespan cross-sectional analysis. American Journal on Intellectual and Developmental Disabilities. 2009;14:289–303. doi: 10.1352/1944-7558-114.4.289-303. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Danko CD, Skinner ML, Bailey DB, Hatton DD, Roberts JE, Mirrett PL. Video analysis of sensory-motor features in infants with fragile X syndrome at 9-12 months of age. Journal of Autism and Developmental Disorders. 2005;35:645–656. doi: 10.1007/s10803-005-0008-7. [DOI] [PubMed] [Google Scholar]
- Bornstein MH. Stability in mental development from early life: Methods, measures, models, meanings, and myths. In: Simion F, Butterworth G, editors. The development of sensory, motor and cognitive capacities in early infancy: From perception to cognition. Psychology Press/Erlbaum (UK) Taylor & Francis; Hove, England: 1998. pp. 301–332. [Google Scholar]
- Bryson SE, Czapinski P, Landry R, McDonnel B, Rombough V, Wainwright A. Autistic spectrum disorders: Causal mechanisms and recent findings on attention and emotion. Internaltional Journal of Special Education. 2004;19:14–22. [Google Scholar]
- Bryson SE, Rogers SJ, Fombonne E. Autism spectrum disorders: Early detection, intervention, education, and psychopharmacological management. Canadian Journal of Psychiatry. 2003;48:506–516. doi: 10.1177/070674370304800802. [DOI] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders. 2007;37:738–47. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, Blaga OM, Carlson SE. Maternal DHA and the development of attention in infancy and toddlerhood. Child Development. 2004;75:1254–1267. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- Colombo J, Shaddy DJ, Richman WA, Maikranz JM, Blaga OM. The developmental course of habituation in infancy and preschool outcome. Infancy. 2004;5:1–38. [Google Scholar]
- Colombo J, Richman WA, Shaddy DJ, Greenhoot AF, Maikranz JM. Heart rate-defined phases of attention, look duration, and infant performance in the paired-comparison paradigm. Child Development. 2001;72:1605–1616. doi: 10.1111/1467-8624.00368. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Spatial cognition in males with fragile-X syndrome: Evidence for a neuropsychological phenotype. Cortex. 1999;35:263–271. doi: 10.1016/s0010-9452(08)70799-8. [DOI] [PubMed] [Google Scholar]
- Courage ML, Reynolds GD, Richards JE. Infants’ attention to patterned stimuli: Developmental change from 3 to 12 months of age. Child Development. 2006;77:680–695. doi: 10.1111/j.1467-8624.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Dahlgren SO, Gillberg C. Symptoms in the first two years of life: A preliminary population study of infantile autism. European Archives of Psychiatry and Neurological Sciences. 1989;238:169–174. doi: 10.1007/BF00451006. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Developmental Psychopathology. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- De Giacomo A, Fombonne E. Parental recognition of developmental abnormalities in autism. European Child & Adolescent Psychiatry. 1998;7:131–136. doi: 10.1007/s007870050058. [DOI] [PubMed] [Google Scholar]
- Dissanayake C, Bui Q, Bulhak-Paterson D, Huggins R, Loesch DZ. Behavioural and cognitive phenotypes in idiopathic autism versus autism associated with fragile X syndrome. Journal of Child Psychology and Psychiatry. 2009;50:209–9. doi: 10.1111/j.1469-7610.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- Dombrowski C, Lévesque S, Morel ML, Rouillard P, Morgan K, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: Loss of an AAG interruption is a late event in the generation of fragile X syndrome alleles. Human Molecular Genetics. 2002;11:371–378. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, Tucker L, Krljes S, Baron-Cohen S, Bolton P, Charman T, Baird G, Johnson MH. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry. 2009;65:31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, Bolton P, Charman T, Baird G, Johnson MH. Visual orienting in the early broader autism phenotype: Disengagement and facilitation. Journal of Child Psychological Psychiatry. 2009;50:637–642. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Whitney D, Hagerman RJ, Rivera SM. Contrast detection in infants with fragile X syndrome. Vision Research. 2008;48:1471–1478. doi: 10.1016/j.visres.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Zwaigenbaum L, Smith IM, Brian J, Roberts W, Szatmari P. Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. Journal of Abnormal Child Psychology. 2009;37:59–78. doi: 10.1007/s10802-008-9258-0. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. The Laboratory Temperament Assessment Battery (LAB-TAB) University of Wisconsin; Madison, WI: 1996. [Google Scholar]
- Goldsmith HH, Rothbart MK. The Laboratory Temperament Assessment Battery (LAB-TAB, Prelocomotor Version, Edition 3.1) University of Wisconsin Press; Madison, WI: 1999. [Google Scholar]
- Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. Journal of Developmental Behavioral Pediatrics. 2006;27:63–74. doi: 10.1097/00004703-200602000-00012. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Etiology, diagnosis, and development in fragile X syndrome. In: Roberts JE, Chapman RS, Warren SF, editors. Speech and language development and intervention in Down syndrome and fragile X syndrome. Paul H Brookes Publishing; Baltimore, MD: 2008. pp. 27–49. [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Reiss AL. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. American Journal on Mental Retardation. 2008;113:44–53. doi: 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman K, Hagerman RJ. Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Archives of General Psychiatry. 2005;6:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: A longitudinal evaulation. American Journal of Medical Genetics A. 2009;149A:1125–1137. doi: 10.1002/ajmg.a.32848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, Cox C, Capone GT, Stanard P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics A. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Bertone A, Cornish K, Boutet I, Der Kaloustian VM, Andermann E, Faubert J, Chaudhuri A. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology. 2004;63:1634–1639. doi: 10.1212/01.wnl.0000142987.44035.3b. [DOI] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, Singh GK, Strickland BB, Trevathan E, van Dyck PC. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish K, Boutet I, Der Kaloustian VM, Andermann E, Faubert J, Chaudhuri A. Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain. 2004;127:591–601. doi: 10.1093/brain/awh069. [DOI] [PubMed] [Google Scholar]
- Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nature of Clinical Practice, Neurology. 2008;4:138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- Landa R, Garret-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychological Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Coock EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule- Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, Bishop S, Esler A, Hus V, Oti R, Richler J, Risi S, Lord C. The Autism Diagnostic Observation Schedule-Toddler Module: A new module of standardized diagnostic measure for autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:1305–1320. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merin N, Young GS, Ozonoff S, Rogers SJ. Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders. 2007;37:108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- [Computer software] Mini Mitter Co., Inc; Sunriver, OR: 1994. Mini-Logger 2000. [Google Scholar]
- Moldin SO, Rubenstein JL, Hyman SE. Can autism speak to neuroscience? Journal of Neuroscience. 2006;26:6893–6896. doi: 10.1523/JNEUROSCI.1944-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning: AGS Edition. Pearson; San Antonio, TX: 1995. [Google Scholar]
- MxEdit 2.21 [Computer software] Delta-Biometrics, Inc; Bethesda, MD: 1989. [Google Scholar]
- Richards JE. Infant visual sustained attention and respiratory sinus arrhythmia. Child Development. 1987;58:488–496. [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Heart rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Menon V, White CD, Glaser B, Reiss AL. Functional brain activation during arithmetic processing in females with fragile X syndrome is related to FMR1 protein expression. Human Brain Mapping. 2002;16:206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivto ER, Jorde LB, Mason-Brothers A, Freeman BJ, Pingree C, Jones MB, McMahon WM, Peterson PB, Jenson WR, Mo A. The UCLA-University of Utah epidemiologic survey of autism: Recurrence risk estimates and genetic counseling. American Journal of Psychiatry. 1989;146:1032–1036. doi: 10.1176/ajp.146.8.1032. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Boccia ML, Bailey DB, Jr., Hatton DD, Skinner M. Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology. 2001;39:107–123. doi: 10.1002/dev.1035. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Boccia ML, Hatton DD, Skinner ML, Sideris J. Temperament and vagal tone in boys with fragile X syndrome. Journal of Developmental Behavior Pediatrics. 2006;27:193–201. doi: 10.1097/00004703-200606000-00003. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Mankowski JB, Sideris J, Goldman BD, Hatton DD, Mirrett PL, Baranek GT, Reznick JS, Long AC, Bailey DB., Jr. Trajectories and predictors of the development of very young boys with fragile X syndrome. Journal of Pediatric Psychology. 2009;34:827–836. doi: 10.1093/jpepsy/jsn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ. Empirically supported comprehensive treatments for young children with autism. Journal of Child Clinical Psychology. 1998;127:168–179. doi: 10.1207/s15374424jccp2702_4. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental and Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI, Rosicky J. Orienting in normal and pathological development. Development and Psychopathology. 1994;6:635–652. [Google Scholar]
- Scerif G, Cornish K, Wilding J, Driver J, Karmiloff-Smith A. Visual search in typically developing toddlers and toddlers with Fragile X or Williams syndrome. Developmental Science. 2004;7:116–130. doi: 10.1111/j.1467-7687.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- Scerif G, Karmiloff-Smith A, Campos R, Elsabbagh M, Driver J, Cornish K. To look or not to look? Typical and atypical development of oculomotor control. Journal of Cognitive Neuroscience. 2005;17:591–604. doi: 10.1162/0898929053467523. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. Childhood autism rating scale. Los Angeles: Western Psychological Services. 1988 [Google Scholar]
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, Lee LC, Rice C, Giarelli E, Kirby R, Baio J, Pinto-Martin J, Cuniff C. Timing of identification among children with an autism spectrum disorder: findings from a population-based surveillance study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:463–464. doi: 10.1097/CHI.0b013e31819b3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, Bailey D., Jr. ADHD symptoms in children with FXS. American Journal of Medical Genetics A. 2006;140:2275–2288. doi: 10.1002/ajmg.a.31388. [DOI] [PubMed] [Google Scholar]
- Vismara LA, Colombi C, Rogers SJ. Can one hour per week of therapy lead to lasting changes in young children with autism? Autism. 2007;13:93–115. doi: 10.1177/1362361307098516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visootsak J, Warren ST, Anido A, Graham JM., Jr. Fragile X syndrome: An update and review for the primary pediatrician. Clinical Pediatrics. 2005;44:371–381. doi: 10.1177/000992280504400501. [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population based sample. Journal of Developmental Behavioral Pediatrics. 2006;27:S79–87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Signman M. The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry. 2006;47:511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: Predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingerevich C, Greiss-Hess L, Lemons-Chitwood K, Harris SW, Hessl D, Cook K, Hagerman RJ. Motor abilities of children diagnosed with fragile X syndrome with and without autism. Journal of Intellectual Disability Research. 2009;53:11–18. doi: 10.1111/j.1365-2788.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]