Abstract
Residual bone marrow damage (RBMD) persists for years following exposure to radiation and is believed to be due to decreased self-renewal potential of radiation-damaged hematopoietic stem cells (HSC). Current literature has examined primarily sub-lethal doses of radiation and time points within a few months of exposure. In this study, we examined RBMD in mice surviving lethal doses of total body ionizing irradiation (TBI) in a murine model of the Hematopoietic Syndrome of the Acute Radiation Syndrome (H-ARS). Survivors were analyzed at various time points up to 19 months post-TBI for hematopoietic function. The competitive bone marrow (BM) repopulating potential of 150 purified c-Kit+ Sca-1+ lineage- CD150+ cells (KSLCD150+) remained severely deficient as long as 16months post-TBI compared to KSLCD150+ cells from non-TBI age-matched controls. The minimal engraftment from these TBI HSC is predominantly myeloid, with minimal production of lymphocytes both in vitro and in vivo. All classes of blood cells as well as BM cellularity were significantly decreased in TBI mice, especially at later time points as mice aged. Primitive BM hematopoietic cells (lin-, KSL, KSLCD150+) displayed significantly increased cell cycling in TBI mice at all time points, which may be a physiological attempt to maintain HSC numbers in the post-irradiation state. Taken together, these data suggest that the increased cycling among primitive hematopoietic cells in survivors of lethal radiation may contribute to long-term HSC exhaustion and subsequent RBMD, exacerbated by the added insult of aging at later time points.
Keywords: Bone marrow, Blood, Mice, Radiation
INTRODUCTION
The growing threat of terrorist events involving radiation, as well as the potential for radiation accidents, underscores the need for medical preparedness for management of radiation victims. Depending on absorbed radiation dose and type of exposure, treatment strategies and immediate and long-term clinical outcomes will differ among victims. While the lethal dose for 50% of humans at 60 days post-exposure (LD50/60) is estimated to be 3.5-4.5Gy (Lushbaugh 1969, Vriesendorp and Van Bekkum 1984), this can be extended to up to 6-7Gy when antibiotics and fluids are provided as supportive care (Dainiak 2002). Exposures of more than 10Gy result in rapid death due to extensive neurological and gastrointestinal damage. Exposures between 1 and 10Gy affect primarily the hematopoietic system, one of the most highly proliferative and thus radiosensitive tissues in the human body. Radiation damage to the hematopoietic system results in what is known as the Hematopoietic Syndrome of the Acute Radiation Syndrome (H-ARS), with rather benign symptoms at the lower end of the range, but probable death due to severe neutropenia and thrombocytopenia at the higher end.
Hematopoietic stem cells (HSC) are a specialized group of rare bone marrow (BM) cells which possess two unique qualities distinguishing them from other BM cells: 1) the ability to differentiate into hematopoietic progenitor cells (HPC) which give rise to mature cells belonging to all the lineages comprising the formed elements of the blood (neutrophils, platelets, erythrocytes, etc), and 2) the ability to self-renew, a process by which HSC divide and give rise to daughter HSC with similar hematopoietic potential as parent HSC (Till and McCulloch 1961, Abramson et al. 1977, Visser et al. 1984, Jones et al. 1989, Jones et al. 1990). It is believed that through these two processes, HSC are capable of sustaining life-long hematopoiesis and of restoring normal BM functions following transplantation, exposure to radio- or chemotherapy, or unwanted high dose ionizing radiation (Till and McCulloch 1961, Visser et al. 1984, Jones et al. 1990, Keller and Snodgrass 1990). Primitive marrow hematopoietic progenitor cells (HPC) also give rise to thymic progenitors which leave the BM and seed the thymus to initiate immune reconstitution (Kondo et al. 1997, Perry et al. 2003, Perry et al. 2004). The loss of HSC and HPC are the ultimate causes of morbidity and mortality in H-ARS.
Although the radiosensitivity of HSC has been debated (McCulloch and Till 1962, van Bekkum 1991, Inoue et al. 1995, Wagemaker 1995, Belkacemi et al. 2003), at least some HSC appear to be radioresistant, either intrinsically or via physical shielding during a radiation accident. Despite their degree of radioresistance, HSC are likely susceptible to damage by radiation, which is believed to lead to a latent condition termed “residual bone marrow damage” (RBMD). RBMD is a situation whereby personnel exposed to sublethal or lethal radiation are faced with a depressed hematopoietic system evident under times of stress for years after exposure. RBMD is characterized by decreased self-renewal potential of HSC and is believed to be due to induction of HSC senescence (Hellman and Botnick 1977, Botnick et al. 1979, Mauch et al. 1988, Meng et al. 2003, Wang et al. 2006). Although RBMD has been described for several years, the specific cell types and mechanisms involved have remained largely undefined. In addition, murine model systems of RBMD have largely examined sublethal radiation exposure or have carried out analyses for only a few months post-exposure. Few, if any, studies have examined RBMD in survivors of lethal radiation exposure and/or for the lifetime of the model system. It therefore remains unknown whether the hematopoietic system and/or HSC population ever recover from ionizing radiation damage, or the ramifications of RBMD in aged irradiated victims. Given the current environment and threat of terrorist use of radiation, such information on the long term effects and consequences of RBMD are of increasing concern.
The aim of the current studies is to determine the extent and nature of long-term damage to the hematopoietic system and HSC compartment in survivors of lethal irradiation, and to what extent this damage contributes to RBMD. It is anticipated that findings will lead to better understanding of the mechanisms behind RBMD and the ramifications of RBMD as radiation victims age, with the important goal of development of potential medical countermeasures to alleviate this debilitating condition.
METHODS
Mice, radiation, dosimetry, and husbandry
Information on irradiated (TBI) and age-matched non-irradiated (non-TBI) control C57Bl/6 mice (CD45.2+) analyzed in these studies, as well as husbandry, irradiation, dosimetry and health status monitoring are all described in the accompanying article by Plett et al (in press). Recipient mice and donors of competitor cells used in bone marrow transplantation studies were male or female congenic PtrcaPep3b/BoyJ (B6.BoyJ; CD45.1+) or the F1 hybrid of C57Bl/6 and B6.BoyJ mice (CD45.2+/CD45.1+). B6.BoyJ and F1 hybrids were bred in-house and used between 8-12 weeks of age. All studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Experimental design
TBI mice had been used as controls in other studies where they were exposed, at 12 weeks of age, to the estimated LD50/30 to LD70/30 dose of radiation (range: 7.76 Gy to 7.97 Gy). At the end of those studies, surviving control group mice (TBI mice) were transferred to the current studies, along with age-matched non-irradiated controls (non-TBI mice). Since the biological effects on the hematopoietic system and RBMD after exposure to lethal radiation within this dose range were found to be indistinguishable, TBI mice exposed to any dose between 7.76 Gy to 7.97 Gy were grouped together and used in the current studies, regardless of exposure dose. TBI and non-TBI mice were assessed at various times post-exposure (1.5, 2, 3.5, 4, 6, 8, 10, 13, 14, 15, 16, and 19 months post-exposure) for body weight, CBC analyses by tail-bleeding, and various bone marrow (BM) hematopoietic stem and progenitor cell analyses. BM cells were flushed from femurs, tibias, pelvis, and humeri, then fractionated by ficol separation to yield low density bone marrow (LDBM, 1.077 g/mL or less). LDBM were enumerated and processed for flow cytometric analyses of primitive hematopoietic phenotype, cell cycle position, stem cell transplantation, or progenitor assays, as described below. BM cellularity was adjusted to the number of cells in the whole mouse according to the methods of Boggs (Boggs 1984).
Flow cytometric analyses
Bone marrow cells expressing the cell surface phenotype c-Kit+, Sca-1+, lineage-, CD150+ (KSLCD150+ cells) represent a population of cells highly enriched for HSC potential (Kiel et al. 2005, Chen et al. 2008). We thus chose to use this phenotype as our putative HSC population in these studies.
For analysis of hematopoietic populations using SLAM markers (Kiel et al. 2005, Chen et al. 2008) and cell cycle status, LDBM cells from TBI or non-TBI mice were stained for hematopoietic markers using CD3-FITC, Gr1-FITC, B220-FITC, Sca-1-PE, c-Kit-APC, and CD150-PerCPCy5-5 antibodies. Cells were fixed overnight in 1% (v/v) formaldehyde and then stained with DAPI (Invitrogen, Eugene, OR) in some experiments.
The content of reactive oxygen species (ROS) in primitive hematopoietic cells was determined by pre-incubating LDBM with C-DFDA (Invitrogen), followed by staining with CD3-APC, Gr1-APC, B220-APC, Sca-1-PECy7, c-kit-PE, and CD150-PerCPCy5-5 antibodies.
To determine donor chimerism in transplant recipients, peripheral blood (PB) was obtained by tail snips and subject to ammonium chloride lysis (0.16M NH4Cl, 0.01M KHCO3). Lysed PB was stained with CD45.1-PE antibodies to detect white blood cells of recipient origin (B6.BoyJ or the F1 hybrid of C57Bl/6 and B6.BoyJ mice) and CD45.2-FITC antibodies to detect white blood cells of donor origin (TBI or non-TBI C57Bl/6 mice). To determine multi-lineage reconstitution in transplant recipients at 6 month post-transplantation, PB was stained with CD45.1-PE, CD45.2-PerCPCy5.5, CD8a-Pacific Blue, CD4-Alexa Fluor 647, B220-PE Texas Red and Gr1-FITC.
All antibody staining protocols were carried out for 30 mins at 4°C, followed by washing (1% bovine calf serum/PBS) by centrifugation at 2000 rpm at 4°C. Flow cytometric analysis was carried out using a FACScan or LSRII systems [Becton Dickinson Immunocytometry Systems (BDIS), San Jose, CA]. All antibodies were purchased from BD Biosciences (San Jose, CA) except CD150-PerCPCy5.5 (Biolegend, San Diego, CA) and Sca-PECy7 (eBiosciences, San Diego, CA). Approximately 1.0 to 2.0 × 105 events were acquired for chimerism and lineage analysis, and 0.2 to 1.0 × 106 events were acquired for primitive hematopoietic cell phenotyping and cell cycle analysis.
Flow cytometric cell sorting
LDBM (3 to 20 × 106) from TBI or non-TBI mice was stained with CD3-FITC, B220-FITC, Gr1-FITC, Sca-1-PE, c-Kit-APC and CD150-PerCPCy5.5 antibodies and sorted for KSLCD150+ cells using a FACSVantage SE or FACSAria (Becton Dickinson, Franklin Lakes, NJ) or Reflection (iCyt, Champaign, IL) systems. A primary light scatter gate was constructed and used to visualize cells expressing lineage markers. A second gate was constructed around lineage-negative cells and used to visualize c-Kit+ and Sca-1+ cells [c-Kit+Sca-1+lin- (KSL) cells]. A final gate defining KSL cells was used to examine and sort on CD150+ cells. KSLCD150+ cells were collected in IMDM (Lonza, Walkersville, MD) containing 10% (v/v) fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA).
Long term competitive transplantation
Purified KSLCD150+ cells from TBI or non-TBI mice (C57Bl/6) were obtained by flow cytometric cell sorting, and 150 of these cells were transplanted intravenously along with 1.0 × 105 congenic competitor LDBM (B6.BoyJ or the F1 hybrid of C57Bl/6 and B6.BoyJ mice) into congenic recipients (B6.BoyJ or the F1 hybrid of C57Bl/6 and B6.BoyJ mice) that had been lethally irradiated with a split-dose of 700cGy followed by 400cGy 4 hours later, for a total of 1100cGy. Use of congenic C57Bl/6, B6.BoyJ, and F1 hybrids in competitive BM transplantation studies has been previously described by us (Plett et al. 2002, Plett et al. 2003) and others (Hoggatt et al. 2009). Recipients were provided doxycycline chow and neomycin water beginning one week prior to transplantation and continuing for one month thereafter. PB was analyzed at monthly intervals to 6 months for donor chimerism and for lineage reconstitution at 6 months post-transplant.
Hematopoietic progenitor cell assays
1.0×105 LDBM cells were suspended in duplicate in 1 mL methylcellulose media containing the hematopoietic growth factors murine stem cell factor, murine interleukin-3, recombinant human interleukin-6, and recombinant human erythropoietin (Methocult M3434, Stem Cell Technologies, Vancouver, BC, Canada). Cultures were incubated in 100% humidified 5% CO2 in air at 37°C, and enumerated 13 days later for CFU-GM, BFU-E, and CFU-GEMM colonies under a light microscope utilizing a benzidine solution to visualize BFU-E colonies (Clarke and Housman 1977).
Pre-B Lymphoid colony assays
1.0×105 LDBM cells were suspended in duplicate in 1 mL methylcellulose media containing recombinant human IL-7 (MethocultvM3630, Stem Cell Technologies, Vancouver, BC, Canada). Cultures were incubated in 100% humidified 5% CO2 in air at 37°C, and enumerated 7-8 days later for pre-B lymphoid colonies.
Statistical Analyses
Two-way ANOVA models were used to investigate the association of treatment group and months post irradiation with weight, donor chimerism, BM cellularity, CBC parameters, progenitor frequency and number per mouse, percent and number per mouse of BM primitive hematopoietic cells (lin-, KSL, and KSLCD150+) and %G0G1 of lin-, KSL, and KSLCD150+. For each parameter, this analysis compares TBI and non-TBI mice after adjusting for time. Thus, the comparisons involved all the mice rather than multiple comparisons between small groups of mice at individual time points. The interaction between treatment group and months post-irradiation was also examined in all models.
RESULTS
Body weights and CBCs
Visually, TBI and non-TBI did not differ in appearance other than the fur of TBI mice was much lighter and grayer in color compared to that of non-TBI mice. Prior to euthanasia for HSC and HPC analyses at the various times post-exposure, TBI and non-TBI mice were weighed and blood collected by tail snips for CBC analyses. While body weights (Fig. 1a) and all CBC parameters (Figs. 1b-1f) of TBI mice appeared similar to those of non-TBI mice at early time points post-exposure, differences in all these parameters became quite apparent at time points more than 10 to 13 months post-TBI (p≤0.01). Whereas body weight and CBC parameters tended to increase in non-TBI mice as they aged, all parameters tended to decrease in TBI mice with age.
Figure 1. Body weight and CBC profiles in TBI and non-TBI mice.
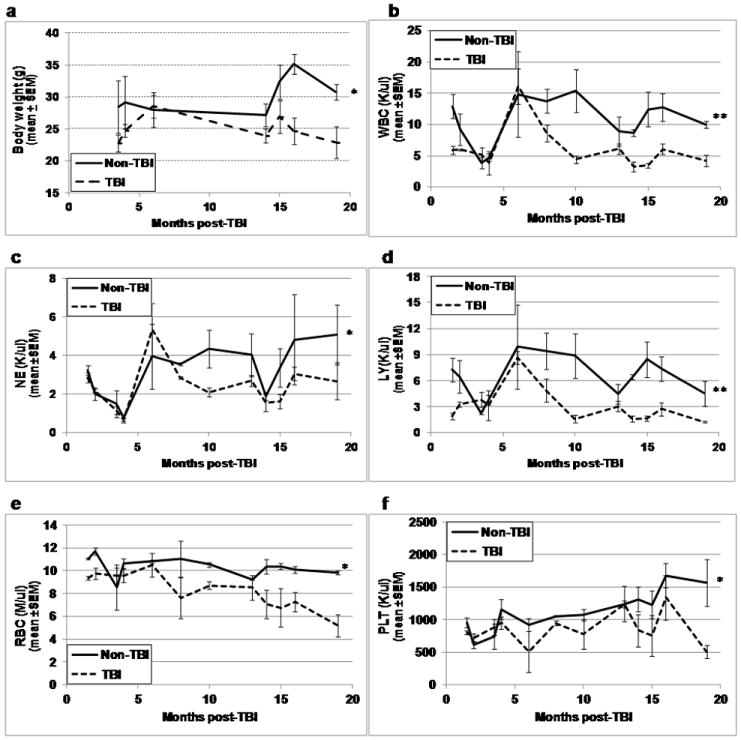
At various times between 1.5 and 19 months post-exposure, TBI and non-TBI mice were assessed for body weight (panel a), peripheral blood white blood cells (WBC, panel b), neutrophils (NE, panel c), lymphocytes (LY, panel d), red blood cells (RBC, panel e), and platelets (PLT, panel f). Lines represent mean±SEM; *p≤0.01 comparing non-TBI to TBI, **p<0.05 for significant interaction indicating greater difference between TBI and non-TBI at later time points. n=2-3 mice per group per time point.
Although the absolute neutrophil (NE) count was significantly less in TBI mice compared to non-TBI mice (Fig. 1c), the percentage of NE was greater (overall mean±SEM: 41.75±2.38 versus 30.37±2.45, respectively, p<0.001). The absolute lymphocyte (LY) count (Fig. 1d), as well as the percentage of LY, was significantly less in TBI mice on average over time as compared to non-TBI mice (overall mean %LY±SEM: 49.43±2.79 versus 62.37±2.44, respectively, p<0.001), illustrating the significant impact that radiation exposure poses on the immune system. Together, these data suggest that compensatory mechanisms supplying hematopoietic support in TBI mice cannot match the “second hit” that aging imparts on these irradiated survivors.
Bone marrow (BM) cellularity and primitive hematopoietic phenotypes
Similar to the WBC and other CBC parameters, BM cellularity (defined as the absolute numbers of LDBM per mouse) was consistently decreased in TBI mice compared to non-TBI mice (Fig. 2a, p = 0.008), especially at later time points (13-19 months post-TBI). Although BM cellularity was decreased, the percentage of primitive hematopoietic lineage-negative (lin-) cells was significantly increased in TBI LDBM compared to non-TBI LDBM (Fig. 2b, p = 0.021), allowing for maintenance of absolute numbers of lin- cells in TBI mice (Fig. 2c). Expression of Sca-1 and c-Kit on lin- cells was significantly increased in non-TBI mice (Fig. 2d, p = 0.009), resulting in significantly higher numbers of KSL cells in non-TBI mice compared to TBI mice (Fig. 2e, p = 0.044). No consistent differences were evident in the percentage or absolute number of KSLCD150+ cells between TBI and non-TBI mice (Fig. 2f and 2g). There were, however, apparent increases in both percentage and absolute number of these cells in non-TBI mice as they aged (Figs. 2f and 2g), as previously reported for aged HSC (Morrison et al. 1996, Sudo et al. 2000). Similar to data from Simonnet et al. (Simonnet et al. 2009), the percentage of KSL cells expressing the SLAM marker CD150 was significantly increased in TBI mice (Fig 2h, p < 0.001).
Figure 2. Bone marrow (BM) cellularity and primitive hematopoietic phenotypes in TBI and non-TBI mice.
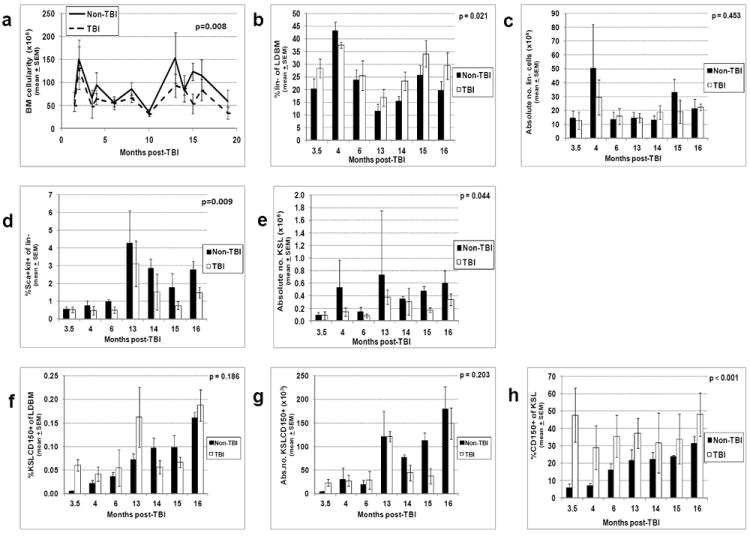
TBI and non-TBI mice were sacrificed at various times post-exposure and LDBM isolated. LDBM was enumerated and absolute numbers of LDBM cells per mouse (BM cellularity) were calculated as described in Materials and Methods (panel a). The percentage of different primitive hematopoietic cell phenotypes was determined by flow cytometry as described in Materials and Methods, and multiplied by BM cellularity in panel a to give the absolute number of these different cells per mouse. The following data are shown: panel b, the percentage of lineage-negative cells; panel c, the absolute number of lineage-negative cells per mouse; panel d, the percentage of Sca-1+ c-Kit+ cells on lin- cells; panel e, the absolute number of KSL per mouse; panel f, the percentage of KSLCD150+ cells; panel g, the absolute number of KSLCD150+ cells per mouse; panel h, the percentage of CD150+ cells within the KSL population. Phenotyping of bone marrow cells at month 19 post-TBI could not be performed due to limiting numbers of cells isolated at this time point. Bars represent mean±SEM; p values comparing non-TBI to TBI are given on each figure. n=3 mice per group per time point.
BM hematopoietic progenitor cells (HPC) and pre-B lymphoid colonies
Given that various PB CBC parameters and primitive BM hematopoietic phenotypes are significantly decreased in TBI mice compared to non-TBI mice, it stood to reason that BM hematopoietic progenitors may also be compromised in TBI mice. To test this, LDBM from TBI and non-TBI mice was assayed for HPC in methylcellulose progenitor and pre-B lymphoid assays. Bone marrow from non-TBI mice was found to contain a significantly higher frequency and absolute number of both HPC (panels a and b) and pre-B lymphoid colonies (panels c and d) than TBI BM.
Competitive Transplantation and Lineage Reconstitution
When assessed in competitive BM transplantation assays, 150 purified, sorted KSLCD150+ cells, isolated from TBI mice at all time points post-exposure, were found to contain <2% of the repopulating potential of similar cells isolated from non-TBI age-matched controls (Fig. 4). The inability of this highly purified HSC phenotype to reconstitute the hematopoietic system of suitably-conditioned recipients remained severely deficient up to 16mo post-TBI, the latest time point that TBI and non-TBI mice have been analyzed to date. Comparing the generally higher level of chimerism from non-TBI HSC at early time points to the lower values observed at later time points, the reported loss of HSC potential that comes with age becomes evident (Chambers et al. 2007).
Figure 4. Long term engraftment potential of TBI and non-TBI HSC in competitive transplantation assays.
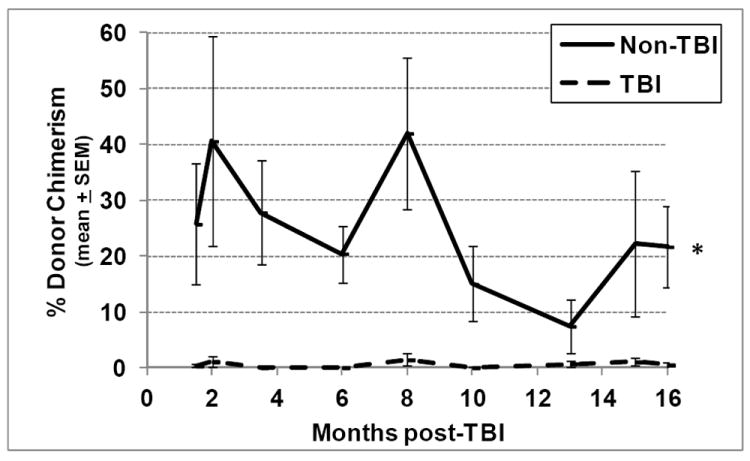
Lethally irradiated congenic murine recipients were transplanted with 150 KSLCD150+ cells isolated from either TBI or non-TBI donors, along with 1.0 × 105 LDBM competitor cells of congenic origin. Peripheral blood from tail snips was obtained from transplanted recipients at monthly intervals and was analyzed by flow cytometry to determine donor chimerism using antibodies against CD45.1 and CD45.2. Lines represent mean±SEM donor chimerism at 6 months post-transplant (except data at months 3.5, 15 and 16, which represent chimerism in ongoing experiments analyzed at 4, 3 and 2 months post-transplant, respectively). *p<0.001 comparing non-TBI to TBI, n=4-14 recipient mice per group per time point.
Recipients that received TBI KSLCD150+ cells with at least 0.04% donor-derived peripheral blood reconstitution at 6 months post-transplant were assayed for donor-derived lineage reconstitution of peripheral blood CD8+ T cells, CD4+ T cells, B220+ B cells, and Gr-1+ granulocytes by flow cytometry. Remarkably, recipients of KSLCD150+ cells from TBI mice were severely deficient in donor lymphoid reconstitution, producing negligible levels of T and B cells up to at least 13 months post-TBI (Fig. 5a), whereas KSLCD150+ cells from non-TBI mice (Fig. 5b), or competitor cells co-transplanted with either TBI (Fig 5c) or non-TBI (Fig. 5d) KSLCD150+ cells, provided a normal pattern of lineage reconstitution in transplant recipients. These data indicate that TBI HSC likely never regain full HSC potential during the lifetime of the irradiated survivor. Of interest and in agreement with others (Morrison et al. 1996, Kim et al. 2003), HSC from aged non-TBI mice displayed skewing toward the myeloid lineage, as evidenced by a greater percentage of donor-derived granulocytes at month 13 compared to earlier months (Fig. 5b).
Figure 5. Lineage reconstitution of TBI and non-TBI HSC in competitive transplantation assays.
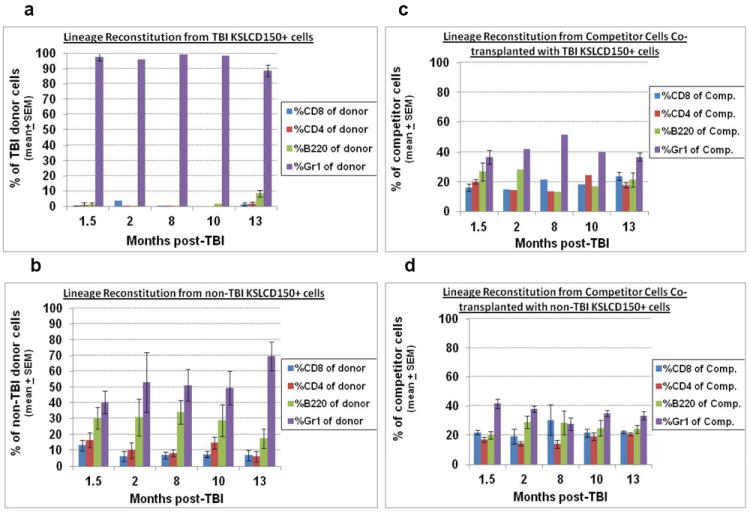
Mice transplanted with TBI or non-TBI KSLCD150+ cells in Figure 4 were assayed for donor-derived lineage reconstitution at 6 months post-transplantation. Peripheral blood from tail snips was stained with fluorescently tagged antibodies to CD45.1, CD45.2, CD4, CD8, B220, and Gr1, and analyzed by flow cytometry for donor- or competitor-derived CD4+ and CD8+ T cells, B cells, and granulocytes. Panel a depicts the lineage reconstitution of 150 KSLCD150+ cells from TBI donors (expressed as a percentage of total TBI donor cells). Data on months 2, 8, and 10 in Panel a were n=1 since mice transplanted with TBI KSLCD150+ cells often did not exhibit high enough donor chimerism to allow further phenotyping for lineage cells. Panel b shows the lineage reconstitution of 150 KSLCD150+ cells from non-TBI donors (expressed as a percentage of total non-TBI donor cells). Panels c and d show the lineage reconstitution by the congenic competitor cells (expressed as a percentage of total competitor cells) that were co-transplanted with KSLCD150+ from TBI or non-TBI donors, respectively, which acts as an internal standard for normal lineage reconstitution. Bars represent mean±SEM; n=1-7 recipient mice per group per time point.
Cell cycle analysis
Mitotic quiescence is a key characteristic of HSC, and believed to be essential to maintenance of HSC self renewal and differentiation potential (Verfaillie and Miller 1995, Peters et al. 1996, Traycoff et al. 1996, Ladd et al. 1997, Nilsson et al. 1997, Gothot et al. 1998, Habibian et al. 1998). We have demonstrated that HSC from mice exposed to lethal TBI are severely diminished in their ability to provide long term multi-lineage hematopoietic reconstitution in suitable recipients, and that this ability does not recover for nearly the lifetime of the TBI mouse. To determine if HSC quiescence is compromised in TBI mice, the percentage of TBI KSLCD150+ cells in G0/G1 at different times post-exposure was determined, and found to be significantly lower at each time point compared to non-TBI KSLCD150+ cells (Fig. 6, p<0.001), indicating significantly increased cell cycling. A similar pattern of increased cycling was found for TBI lin- cells and TBI KSL cells (p<0.001, data not shown). Taken together with data in Fig. 2 showing decreased cellularity of TBI BM, the increased percentage of TBI primitive hematopoietic phenotypes in active phases of cell cycle may be a physiological attempt to maintain HSC numbers in the post-irradiation state, albeit at the expense of HSC potential.
Figure 6. Cell cycle analysis of TBI and non-TBI HSC.
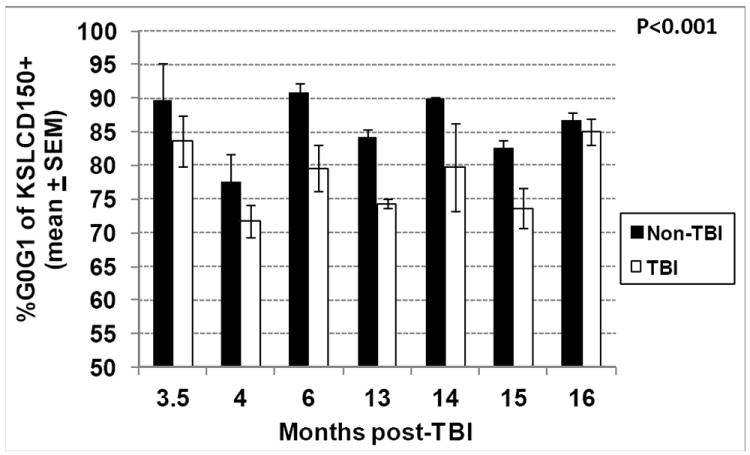
TBI and non-TBI mice were sacrificed at various times between 3.5 and 16 months post-exposure and bone marrow KSLCD150+ cells were analyzed by flow cytometry for cell cycle position using the DNA stain DAPI. Bars represent mean±SEM; *p<0.001 comparing non-TBI to TBI, n=3 mice per group per time point.
Reactive oxygen species (ROS)
Radiation-induced chronic oxidative stress has been postulated to be a contributing factor to RBMD (Wang et al. 2006, Wang et al. 2010). In support of this, increased levels of ROS, which may contribute to chronic oxidative stress via DNA damage, have been documented in HSC populations 1-2 months post exposure to sub-lethal doses of irradiation (Wang et al. 2006). ROS have also been shown to limit HSC lifespan through activation of p38 MAPK (Ito et al. 2006). However, later time points after radiation exposure, or ROS content following higher, potentially lethal doses of radiation, have not been examined. To further examine the possible contribution of ROS to RBMD, ROS levels were measured in KSL cells from TBI and non-TBI mice at various times post-exposure. While ROS content of KSL cells from TBI mice was found to be increased at 1.5mo post-exposure (in agreement with Wang et al., 2006), values at later time points were similar to those from non-TBI mice (Fig. 7).
Figure 7. Content of reactive oxygen species (ROS) in TBI and non-TBI bone marrow KSL cells.
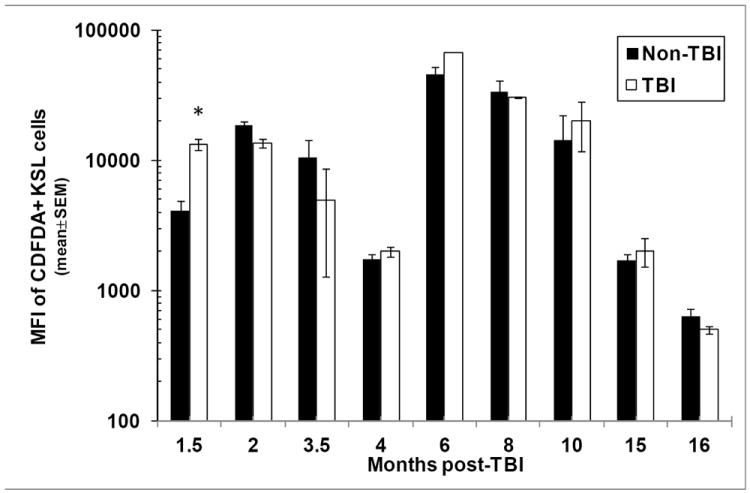
TBI and non-TBI mice were sacrificed at various times between 1.5 and 16 months post-exposure and bone marrow KSL cells were analyzed by flow cytometry for ROS content using C-DFDA. Bars represent the mean±SEM of the mean fluorescence intensity (MFI) of C-DFDA on C-DFDA+ KSL cells; *p<0.05 comparing TBI and non-TBI C-DFDA+ KSL cells at 1.5 months post-exposure. n=2-3 mice per group per time point.
Other assays
Results of experiments assessing HSC homing potential, chemotaxis to SDF-1a, mitochondrial activity, and marrow osteoblast content were similar in TBI and non-TBI mice at various time points between 3.5 and 16 months post-exposure (data not shown), indicating that none of these parameters appears to be directly responsible for radiation-induced HSC dysfunction.
DISCUSSION
Our results define severe, life-long HSC damage in survivors of lethal doses of ionizing radiation in a murine model of H-ARS. The long term deleterious effects of lethal TBI on HSC was evidenced by significantly lower donor competitive repopulation potential up to at least 19 months post-exposure (22 months of age) and abnormal multi-lineage differentiation potential marked by dramatic skewing towards the myeloid lineage of TBI HSC compared to HSC from non-TBI age-matched control mice. Similar findings have been reported by others, but at earlier time points post-exposure and/or after sub-lethal radiation (Simonnet et al. 2009). The significant loss of BM hematopoietic and lymphoid progenitors and peripheral blood cells illustrates a dramatic loss of HSC progeny, further suggesting severe deficits in HSC potential. The long term loss of self renewal and differentiation potential, the two hallmark properties of HSC, indicates true loss of HSC function that is unrecoverable for the life of the mouse.
These data also show that hematopoietic stem and progenitor cell dysfunction from TBI mice becomes more pronounced compared to non-TBI age-matched controls beyond 10 months post exposure. It is well known that compensatory mechanisms in place following HSC damage allow for normal levels of blood elements and apparent hematopoietic support provided largely by the progenitor pool in the bone marrow. However, in times of stress, compensatory mechanisms cannot sustain the increased hematopoietic demand, and hematopoietic failure becomes apparent as evidenced by an inability to maintain production of normal levels of blood cells (Testa et al. 1985, Mauch et al. 1988, Mauch and Hellman 1989, Mauch et al. 1995). This loss of hematopoietic support can be traced to an insufficiency of the HSC pool, which feeds the HPC pool and finally mature blood elements (Fliedner et al. 1986, Mauch et al. 1988). It is likely in our studies that we have gone beyond the time line for normal compensatory hematology and the required renewal of HSC is lost and the system cannot maintain normal production of blood cells. As it is well known that age is a stressor to the hematopoietic and immune systems (Morrison et al. 1996, Sudo et al. 2000, Liang et al. 2005, Rossi et al. 2005, Chambers et al. 2007, Nijnik et al. 2007, Rossi et al. 2007, Pang et al. 2011), it follows that hematopoietic failure in our TBI mice would be more apparent at later time points, in our case, beyond 10 months from exposure. It may not be coincidental that effects in our H-ARS model occur temporally with those observed in the gastrointestinal and pulmonary systems and are part of the delayed effects of acute radiation exposure (DEARE) syndrome described elsewhere in this issue.
The HSC niche provides both cell contact and humoral mechanisms which influence proliferative decisions of HSC (Wilson and Trumpp 2006). It is possible that radiation damage to the HSC niche may have contributed to our observed long-term HSC dysfunction. While we found no differences in number of marrow osteoblasts in our studies, radiation-damage to these or other niche cell types, such as endothelial cells, adipocytes, smooth muscle cells, reticular cells, or stromal fibroblasts, or changes in the humoral factors produced by these cells, may have affected HSC function. A detailed examination of the HSC niche/ microenvironment was beyond the scope of the current studies.
In our studies, impaired HSC function in TBI mice does not appear to be due directly to homing defects [as suggested by others (Morrison et al. 1996, Liang et al. 2005, Simonnet et al. 2009)], differences in chemotaxis to SDF-1a, mitochondrial activity, nor to persistent differences in ROS content of HSC. We found significantly increased levels of ROS in HSC up to 1.5 months post-TBI [in agreement with Wang et al. (Wang et al. 2006)], but lack of significant differences in ROS at time points up to 16 months post-exposure. It has been suggested that chronic oxidative stress induced in HSC by radiation contributes to RBMD, as evidenced by increased ROS in HSC populations within 1.5 months of exposure (Wang et al. 2006, Wang et al. 2010).
Whether the significantly increased ROS content in HSC early after TBI contributes to persistent HSC dysfunction more than one year post-exposure, requires further investigation. ROS-low HSC isolated from steady state bone marrow reportedly possess higher self-renewal potential, whereas ROS-high are prone to HSC exhaustion (Jang and Sharkis 2007). ROS-low HSC may reside in the low-oxygenic osteoblastic niche (Jang and Sharkis 2007) and possibly comprise the purported radioresistant HSC population (van Bekkum 1991) due to protection from radiation-induced oxidative stress. It is therefore possible that ROS-high HSC, evident early post-TBI, eventually undergo apoptosis, leaving ROS-low HSC the task of providing life-long hematopoiesis in the mouse. However, the radiation-damaged ROS-low HSC lack full HSC potential and thus cannot provide adequate hematopoietic support, resulting in RBMD.
The task of repopulating the depleted marrow by the radiation-damaged ROS-low HSC likely forces the normally quiescent HSC population to undergo unscheduled proliferation. Our data indeed document increased cell cycling of TBI lin-, KSL, and KSLCD150+ cells, all BM cell populations highly enriched for classes of primitive HPC. As enhanced cycling of HSC is believed to lead to loss of self renewal potential and has been shown by our group to be detrimental to engraftment potential (Traycoff et al. 1996), it seems likely that the enhanced cycling of HSC from TBI mice up to 16 months post-exposure is a major contributor to RBMD in these mice. Although it seems plausible that TBI HSC would eventually “catch up” from the initial radiation insult and return to steady state levels of proliferation, TBI mice suffer the added stress of aging, which in itself leads to enhanced cycling (Morrison et al. 1996) and loss of self renewal and differentiation potential (Morrison et al. 1996, Kim et al. 2003, Kamminga et al. 2005). Therefore, aging may be viewed as a “second hit” to the TBI HSC.
Aberrant multilineage differentiation was marked by lack of production of donor-derived lymphocytes in vivo as well as in lymphocyte-directed in vitro assays, accompanied by a concomitant increase in the number of myeloid cells, as described by others (Morrison et al. 1996, Kim et al. 2003, Pang et al. 2011). These data may help explain the depressed immune function observed for years following radiation exposure, and suggest that radiation-induced long-term immunosuppression may be due to direct effects on BM HSC that compromise their multi-lineage repopulating potential, rather that direct effects on immune cells and/or their environment.
The percentage of KSL cells expressing CD150 was found to be increased up to 10-fold in TBI mice from 1.5 to 16 months post-exposure (Fig. 2h). Increased expression of CD150 on HPC from irradiated mice has been reported by others (Simonnet et al. 2009), albeit at earlier time points post-exposure. Although the function of CD150 on HSC is unknown, CD150 is known to be upregulated by cytokines and LPS in lymphoid cells (Sidorenko and Clark 1993), and regulates AKT and/or ERK1/2 (Yurchenko and Sidorenko 2010), suggesting possible roles in cell survival (Yurchenko et al. 2011). It is possible that chronic upregulation of CD150 in HSC from irradiated mice may reflect the post-exposure chronic inflammatory state and may be a protective mechanism to attempt to control apoptosis of cells damaged by irradiation.
Finally, it is appreciated that TBI models do not mimic the nuclear terrorist scenario or other uncontrolled nuclear events. Shielding at the radiation accident scene will likely occur due to physical obstacles between the victim and the source, heavy clothing, or body tissues. This shielding will allow sparing of some HSC that may aid recovery from the acute effects of radiation. The protective effects of shielding a portion of the hematopoietic system on acute lethality have been reported as early as 1949, when Jacobsen et. al. (Jacobsen et al. 1949) showed that the LD50 in mice could be increased from 5.50Gy to 9.75Gy by shielding the spleen. Other studies showed protection by shielding a single leg or tail in mice (Robinson et al. 1965, Carsten and Bond 1968, Carsten and Bond 1969). Thus, shielding may also impact the manifestations of RBMD. Such studies were beyond the scope of the current paper, but will be addressed in ongoing “partial body irradiation” (PBI) studies where 5% of the murine BM is shielded from irradiation. The impact of PBI in other model systems has been reported in other papers elsewhere in this issue (in press refs).
CONCLUSIONS
Taken together, these data suggest that the increased cycling among primitive hematopoietic cells in survivors of lethal radiation may contribute to hematopoietic stem cell exhaustion, prolonged immune suppression, and subsequent RBMD. These data document that hematopoietic stem cell function remains severely depressed in lethally-irradiated mice for up 19 months post-exposure, and the “second hit” of aging further compromises the already-deficient HSC function in these mice.
Figure 3. Hematopoietic and pre-B lymphoid progenitors in TBI and non-TBI mouse BM.
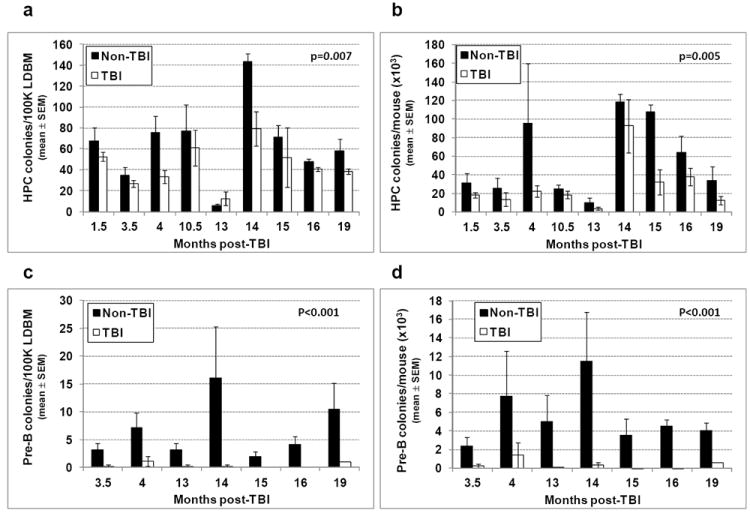
1.0 × 105 LDBM from TBI and non-TBI mice isolated between 1.5 and 19 months post-exposure was suspended in duplicate in 1 mL methylcellulose media containing muSCF, muIL-3, rhuIL-6, and rhuEPO (for hematopoietic progenitors), or in 1 mL methylcellulose media containing recombinant human IL-7 (for pre-B lymphoid colonies). Cells were incubated in 100% humidified 5% CO2 in air at 37°C and enumerated 13 days later for CFU-GM, BFU-E, and CFU-GEMM colonies, or 7 days later for pre-B lymphoid colonies. The frequency of CFU-GM, BFU-E, and CFU-GEMM combined and total number of these per mouse are shown in panels a and b, respectively. Panels c and d give the frequency and total number of pre-B lymphoid colonies per mouse, respectively. Bars represent mean±SEM; p values comparing non-TBI to TBI are given on each figure. n=1-3 mice per group per time point.
Acknowledgments
Funding:
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases (Contract numbers HHSN266200500043C and HHSN272201000046C) and from the National Heart, Lung, and Blood Institute (Award Number R01HL075660 to CMO), National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
No financial conflict of interest was declared by any of the authors.
LITERATURE
- Abramson S, Miller RG, Phillips RA. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. Journal of Experimental Medicine. 1977;145:1567. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkacemi Y, Bouchet S, Frick J, Huchet A, Pene F, Aigueperse J, Gourmelon P, Lopez M, Gorin NC. Monitoring of residual hematopoiesis after total body irradiation in humans as a model for accidental x-ray exposure: dose-effect and failure of ex vivo expansion of residual stem cells in view of autografting. Int J Radiat Oncol Biol Phys. 2003;57:500–7. doi: 10.1016/s0360-3016(03)00596-0. [DOI] [PubMed] [Google Scholar]
- Boggs D. The total marrow mass of the mouse: A simplified method of measurement. Am J Hematol. 1984;16:277–286. doi: 10.1002/ajh.2830160309. [DOI] [PubMed] [Google Scholar]
- Botnick LE, Hannon EC, Hellman S. A long lasting proliferative defect in the hematopoietic stem cell compartment following cytotoxic agents. Int J Radiat Oncol Biol Phys. 1979;5:1621–5. doi: 10.1016/0360-3016(79)90785-5. [DOI] [PubMed] [Google Scholar]
- Carsten A, Bond V. CFU content of the x-ray exposed and shielded mouse femur. Exptl Hematology. 1968;15:95–103. [Google Scholar]
- Carsten A, Bond V. Colony forming units in the bone marrow of partial body irradiated mice. Normal and Malignant Cell Growth. 1969 [Google Scholar]
- Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. Plos Biology. 2007;5:1750–1762. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ellison FM, Keyvanfar K, Omokaro SO, Desierto MJ, Eckhaus MA, Young NS. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol. 2008;36:1236–43. doi: 10.1016/j.exphem.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke BJ, Housman D. Characterization of an erythroid precursor cell of high proliferative capacity in normal human peripheral blood. Proc Natl Acad Sci U S A. 1977;74:1105–9. doi: 10.1073/pnas.74.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- Fliedner TM, Nothdurft W, Calvo W. The development of radiation late effects to the bone marrow after single and chronic exposure. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:35–46. doi: 10.1080/09553008514552211. [DOI] [PubMed] [Google Scholar]
- Gothot A, van der Loo J, Clapp W, Srour E. Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34+ cells in non-obese diabetic/severe combined immune-deficient mice. Blood. 1998;92:2641–2649. [PubMed] [Google Scholar]
- Habibian HK, Peters SO, Hsieh CC, Wuu J, Vergilis K, Grimaldi CI, Reilly J, Carlson JE, Frimberger AE, Stewart FM, Quesenberry PJ. The fluctuating phenotype of the lymphohematopoietic stem cell with cell cycle transit. Journal of Experimental Medicine. 1998;188:393–8. doi: 10.1084/jem.188.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman S, Botnick LE. Stem cell depletion: an explanation of the late effects of cytotoxins. Int J Radiat Oncol Biol Phys. 1977;2:181–4. doi: 10.1016/0360-3016(77)90028-1. [DOI] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hirabayashi Y, Mitsui H, Sasaki H, Cronkite EP, Bullis JE, Jr, Bond VP, Yoshida K. Survival of spleen colony-forming units (CFU-S) of irradiated bone marrow cells in mice: evidence for the existence of a radioresistant subfraction. Exp Hematol. 1995;23:1296–300. [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Medicine. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Marks E, Robson M, Gaston E, Zirkle R. The effect of spleen protection on mortality following x-irradiation. J Lab Clin Med. 1949;34:1538–1543. [Google Scholar]
- Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–63. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RJ, Celano P, Sharkis SJ, Sensenbrenner LL. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood. 1989;73:397–401. [PubMed] [Google Scholar]
- Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming units. Nature. 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- Kamminga LM, Van Os R, Ausema A, Noach EJK, Weersing E, Dontje B, Vellenga E, De Haan G. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells. 2005;23:82–92. doi: 10.1634/stemcells.2004-0066. [DOI] [PubMed] [Google Scholar]
- Keller G, Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990;171:1407–18. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kim M, Moon H-B, Spangrude GJ. Major Age-Related Changes Of Mouse Hematopoietic Stem/Progenitor Cells. Annals of the New York Academy of Sciences. 2003;996:195–208. doi: 10.1111/j.1749-6632.2003.tb03247.x. [DOI] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Ladd A, Pyatt R, Gothot A, Rice S, McMahel J, Traycoff C, Srour E. Orderly process of sequential cytokine stimulation is required for activation and maximal proliferation of primitive human bone marrow CD34+ hematopoietic progenitor cells residing in G0. Blood. 1997;90:658–668. [PubMed] [Google Scholar]
- Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushbaugh C. Reflections on some recent progress in human radiobiology. Advances in Radiation Biology. 1969;277 [Google Scholar]
- Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, Deeg HJ. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–39. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- Mauch P, Hellman S. Loss of hematopoietic stem cell self-renewal after bone marrow transplantation. Blood. 1989;74:872–5. [PubMed] [Google Scholar]
- Mauch P, Rosenblatt M, Hellman S. Permanent loss in stem cell self renewal capacity following stress to the marrow. Blood. 1988;72:1193–6. [PubMed] [Google Scholar]
- McCulloch E, Till J. The sensitivity of cells from normal mouse bone marrow to radiation in vitro and vivo. Radiation Res. 1962;16:822. [PubMed] [Google Scholar]
- Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–9. [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nature Medicine. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, Enver T, Bell JI, Slijepcevic P, Goodnow CC, Jeggo PA, Cornall RJ. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–U9. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Dooner MS, Quesenberry PJ. Synchronized cell-cycle induction of engrafting long-term repopulating stem cells. Blood. 1997;90:4646–50. [PubMed] [Google Scholar]
- Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S, Pierce L, Slayton W, Spangrude G. Characterization of Thymic Progenitors in Adult Mouse Bone Marrow. The Journal of Immunology. 2003;170:1877–1886. doi: 10.4049/jimmunol.170.4.1877. [DOI] [PubMed] [Google Scholar]
- Perry SS, Wang H, Pierce LJ, Yang AM, Tsai S, Spangrude GJ. L-selectin defines a bone marrow analog to the thymic early T-lineage progenitor. Blood. 2004;103:2990–6. doi: 10.1182/blood-2003-09-3030. [DOI] [PubMed] [Google Scholar]
- Peters SO, Kittler ELW, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- Plett PA, Frankovitz SM, Orschell-Traycoff CM. In vivo trafficking, cell cycle activity, and engraftment potential of phenotypically defined primitive hematopoietic cells after transplantation into irradiated or nonirradiated recipients. Blood. 2002;100:3545–3552. doi: 10.1182/blood.V100.10.3545. [DOI] [PubMed] [Google Scholar]
- Plett PA, Frankovitz SM, Orschell CM. Distribution of marrow repopulating cells between bone marrow and spleen early after transplantation. Blood. 2003;102:2285–2291. doi: 10.1182/blood-2002-12-3742. [DOI] [PubMed] [Google Scholar]
- Robinson CV, Commerford SL, Bateman JL. EVIDENCE FOR THE PRESENCE OF STEM CELLS IN THE TAIL OF THE MOUSE. Proc Soc Exp Biol Med. 1965;119:222–6. doi: 10.3181/00379727-119-30142. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–U15. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorenko SP, Clark EA. Characterization of a Cell-Surface Glycoprotein Ipo-3, Expressed on Activated Human B-Lymphocytes and T-Lymphocytes. Journal of Immunology. 1993;151:4614–4624. [PubMed] [Google Scholar]
- Simonnet AJ, Nehme J, Vaigot P, Barroca V, Leboulch P, Tronik-Le Roux D. Phenotypic and functional changes induced in hematopoietic stem/progenitor cells after gamma-ray radiation exposure. Stem Cells. 2009;27:1400–9. doi: 10.1002/stem.66. [DOI] [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. Journal of Experimental Medicine. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa NG, Hendry JH, Molineux G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res. 1985;5:101–10. [PubMed] [Google Scholar]
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Research. 1961;14:213. [PubMed] [Google Scholar]
- Traycoff C, Yoder M, Hiatt K, Srour E. Cell cycle stage-specific expression of adhesion molecules may augment engraftment potential of quiescent but not mitotically active hematopoietic progenitor cells. Blood. 1996;88:475. abstract. [Google Scholar]
- van Bekkum DW. Radiation sensitivity of the hemopoietic stem cell. Radiat Res. 1991;128:S4–8. [PubMed] [Google Scholar]
- Verfaillie CM, Miller JS. A novel single-cell proliferation assay shows that long-term culture-initiating cell (LTC-IC) maintenance over time results from the extensive proliferation of a small fraction of LTC-IC. Blood. 1995;86:2137–2145. [PubMed] [Google Scholar]
- Visser JWM, Bauman JGJ, Mulder AH, Eliason JF, DeLeeuw AM. Isolation of murine pluripotent hematopoietic stem cells. Journal of Experimental Medicine. 1984;159:1576. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriesendorp H, Van Bekkum D. Susceptibility to total-body irradiation. Response of Different Species to Total Body Irradiaton. 1984 [Google Scholar]
- Wagemaker G. Heterogeneity of Radiation Sensitivity of Hematopoietic Stem-Cell Subsets. Stem Cells. 1995;13:257–260. doi: 10.1002/stem.5530130731. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–56. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–66. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- Yurchenko M, Shlapatska LM, Romanets OL, Ganshevskiy D, Kashuba E, Zamoshnikova A, Ushenin YV, Snopok BA, Sidorenko SP. CD150-mediated Akt signalling pathway in normal and malignant B cells. Experimental Oncology. 2011;33:9–18. [PubMed] [Google Scholar]
- Yurchenko M, Sidorenko SP. Hodgkin’s lymphoma: the role of cell surface receptors in regulation of tumor cell fate. Experimental Oncology. 2010;32:214–23. [PubMed] [Google Scholar]


