Abstract
Introduction
Various temporal metrics of daily pollution levels have been used to examine relationships between air pollutants and acute health outcomes. However, daily metrics of the same pollutant have rarely been systematically compared within a study. In this analysis, we describe the variability of effect estimates attributable to the use of different temporal metrics of daily pollution levels.
Methods
We obtained hourly measurements of ambient particulate matter (PM2.5), carbon monoxide (CO), nitrogen dioxide (NO2), and ozone (O3) from air monitoring networks in 20-county Atlanta for the time period 1993–2004. For each pollutant we created: 1) a daily 1-hour maximum; 2) a 24-hour average; 3) a commute average; 4) a day-time average; 5) a night-time average; and a daily 8-hour maximum (only for O3). Using Poisson generalized linear models, we examined associations between daily counts of respiratory emergency department visits and the previous day’s pollutant metrics.
Results
Variability was greatest across O3 metrics, with the 8-hour maximum, 1-hour maximum, and day-time metrics yielding strong positive associations and the night-time O3 metric yielding a negative association (likely reflecting confounding by air pollutants oxidized by O3). With the exception of the day-time metric, all of the CO and NO2 metrics were positively associated with respiratory emergency department visits.
Discussion
Differences in observed associations with respiratory emergency room visits among temporal metrics of the same pollutant were influenced by the diurnal patterns of the pollutant, spatial representativeness of the metrics, and correlation between each metric and copollutant concentrations. Overall, the use of metrics based on the US National Ambient Air Quality Standards (e.g., the use of a daily 8-hour maximum O3 as opposed to a 24-hour average metric) was supported by this analysis. Comparative analysis of temporal metrics also provided insight into underlying relationships between specific air pollutants and respiratory health.
Keywords: air pollution, respiratory disease, emergency department visits, exposure assessment, criteria pollutants, time-series
INTRODUCTION
Studies of the acute health effects of ambient air pollution have used various temporal metrics to characterize daily carbon monoxide (CO), nitrogen dioxide (NO2), ozone (O3), and fine particulate matter (PM2.5) concentrations. Commonly examined metrics include 24-hour average concentrations, daily 1-hour maximum concentrations, and daily 8-hour maximum concentrations. Bell and colleagues demonstrated that variation in O3 between cities and over time, differed by averaging time used to characterize the O3 concentrations (i.e., 24-hour average, 1-hour maximum, 8-hour maximum) (Bell et al., 2005). The choice of daily pollutant averaging time could likewise affect risk estimates observed in epidemiologic studies. Some pollutant averaging times may show stronger associations with health outcomes because they reflect a more biologically relevant exposure (e.g., peak vs. average exposure) or because they more strongly correlate with average population exposures compared to other temporal metrics. In addition, certain metrics could act as better surrogates for other, unmeasured pollutants responsible for the adverse health effects, such as certain metrics of CO and NO2 acting as surrogates of particles from traffic sources (Sarnat et al., 2001).
Temporal metrics that reflect peak pollution levels (e.g., 1-hour maximum) may be the most biologically relevant if the health effect is triggered by a high, short-term dose rather than a steady dose throughout the day. Peak concentrations, however, are frequently associated with episodic, local emission events, resulting in spatially heterogeneous concentrations across an urban area and thus prone to measurement error when using fixed site concentrations as the estimate of exposure. As a result, a 24-hour average concentration metric may often be more representative of average population exposures.
It is also possible that the most appropriate temporal metric for an epidemiologic analysis is determined by exposure factors related to population time-activity patterns; some metrics may better capture average population exposures because they include hours when the population is most likely to be exposed to ambient air. A relevant exposure time window for assessing health effects of traffic pollution, for example, may be during heavy commuting hours, when pollutant concentrations are highest and people are more directly exposed to ambient air. Alternatively, if centrally located monitoring stations, which are often used in epidemiologic studies to characterize exposure, only reflect downtown concentrations, day-time hours might correlate best with personal exposures given the influx of people into the city center during the day and exodus at night. Conversely, night-time exposure metrics incorporate hours when people are likely to be in their homes and less likely to be outdoors and exposed directly to ambient air, thus increasing exposure measurement error. Because exposure measurement error can lead to attenuated effect estimates, if some pollutant metrics approximate population exposures better than other metrics, we would expect to see variation in epidemiologic results according to choice of metric. Furthermore, if associations between night-time concentrations and health outcomes are dramatically different from associations between day-time concentrations and health outcomes, this may indicate that a 24-hour average metric is inappropriate, as it inherently combines both day and night concentrations into one metric. Lastly, if the use of different temporal metrics of air pollution leads to different results, metric choice could potentially explain differences in observed epidemiologic associations across studies.
In our previous analyses of ambient air pollution levels and respiratory emergency department visits in Atlanta (Peel et al., 2005; Metzger et al., 2004; Tolbert et al., 2008; Sarnat et al., 2008; Sarnat et al., in press), we presented results using a priori exposure metrics chosen for each pollutant of interest, based on the National Ambient Air Quality Standards and previous studies of air pollution and acute health effects (Sunyer et al., 1997; Ostro et al., 2001; Zmirou et al., 1998). These a priori exposure metrics included the daily 1-hour maximum concentration for CO and NO2, the daily 8-hour maximum concentration for O3, and a 24-hour average for PM2.5. In the present study, we describe the variability of epidemiologic results attributable to the use of different daily metrics of the same pollutant, comparing the results using our a priori pollutant metrics to those obtained using alternative temporal metrics of pollutant concentrations. The degree of sensitivity to the choice of metric is relevant to future investigations of air pollution and respiratory health, can offer clues to biological mechanisms, and ultimately can be used to inform regulatory policy.
METHODS
Air Quality Data
We obtained hourly ambient concentrations of CO, NO2, O3 and PM2.5 from the US Environmental Protection Agency’s Air Quality System as well as the Aerosol Research Inhalation Epidemiology Study (ARIES) monitor located near downtown Atlanta (Van Loy, 2000; Hansen et al. 2006). Data from all monitoring stations in the study area that provided hourly measurements were used to assess the spatial heterogeneity of each metric (described below). However, for the epidemiologic models, we used measurements from a single, centrally located monitor for each pollutant. We obtained daily meteorological data from the National Climatic Data Center at Hartsfield-Atlanta International Airport. We chose to examine CO, NO2 and O3 because these pollutants were associated with respiratory emergency room visits in our previous analyses (Peel et al., 2005). We also assessed PM2.5 because our previous analyses were suggestive of an effect in spite of limited sample size (Peel et al., 2005). Furthermore, a motivation of the study was to explore whether alternative temporal metrics would yield stronger associations than our a priori daily metrics, which are metrics commonly used in air pollution studies. For each pollutant, we created the following temporal metrics of daily pollutant concentrations: a daily1-hour maximum, a 24-hour average, an average of commute hours (‘commute,’ 7:00–10:00am and 4:00–7:00pm), a day-time average (‘day-time,’8:00am–7:00pm), a night-time average (‘night-time,’ 12:00am–6:00am) and, for ozone only, a daily 8-hour maximum. Within a pollutant, the study days included in the analysis were the same across metrics. The analytic time period differed by pollutant depending on the period of monitoring available at the central monitoring station: CO was examined from January 1, 1993 through June 30, 2003; NO2 was examined between March 1, 1994 and December 31, 2004; O3 was examined March through October of every year between 1993 and 2004; PM2.5 was examined from August 1, 1998 through December 31, 2004‥
Emergency Department Data
Individual-level data from computerized billing records were obtained from 41 of 42 acute-care hospitals in the 20-county Atlanta area (50 mile radius). We examined daily counts of selected respiratory-related emergency department visits for patients living within any one of the 225 ZIP codes located wholly or partially in the 20-county Atlanta study area. Emergency department visits with a primary International Classification of Diseases 9th Revision (ICD-9) diagnostic code for asthma and wheeze (493, 786.09), chronic obstructive pulmonary disease (491, 492, 496), upper respiratory infection (460–466, 477), and pneumonia (480–486) were classified as respiratory emergency department visits. We excluded repeat visits by patients visiting the same hospital within a single day. There were 1,068,525 respiratory emergency department visits between 1993 and 2004, with an average of 244 visits per day.
Statistical analysis
We modeled the association between air pollution and respiratory emergency department visits using a case-crossover framework, a special case of the time-series approach (Lu and Zeger, 2007). Using this time-stratified approach, referent days were chosen within the same calendar month and within the same maximum temperature category as the day of the respiratory emergency department visit (Schwartz, 2004; Schwartz, 2005; Zanobetti and Schwartz, 2006). For example, if the visit occurred in March 2000 on a day with a maximum temperature of 72 degrees (the case day), we selected all other days in March 2000 with a maximum temperature between 70 and 75 degrees as the control days. Maximum temperature categories were in five-degree increments and three degree increments at the extremes: < 35° F or >89° F. Counts were then pooled across individuals within a hospital to create a time series of counts for each hospital. We chose to match on temperature rather than day-of-week because temperature effects are non-linear and can be challenging to adequately control in regression models compared to day-of week, which can be controlled using indicator variables.
We analyzed the data using Poisson generalized linear models, scaling the variance estimates to account for overdispersion. The model included indicator variables for day-of-week and holidays, cubic terms for two-day moving average (lag 1–2) minimum temperature (same-day temperature was accounted for by matching) and three-day moving average (lag 0–2) of dew point temperature (cubic terms). We also repeated the analysis using the time-series models from our previously published work (Peel et al., 2005) to evaluate whether the observed patterns were sensitive to modeling approach. Briefly, our previous time-series models included cubic splines with monthly knots to control for temporal trends, seasonal indicator variables and cubic splines to control for temperature and dew point temperature. All analyses were performed using SAS, version 9.2, statistical software (SAS Institute, Inc., Cary, North Carolina).
Metrics comparison
We calculated partial correlations (i.e., correlations after controlling for the covariates included in the time-series models) between all of the metrics, both within and across pollutants. We compared the spatial heterogeneity of the metrics for each pollutant to assess whether some metrics might better reflect population-wide exposures (Ito et al., 2001); metrics that are more spatially representative (i.e., more correlated across space) might better reflect personal exposures in the study population, thus reducing bias due to measurement error relative to other metrics. Thus, comparing the spatial heterogeneity of the metrics may shed light on any observed differences in strength of association with respiratory emergency room visits. To compare the spatial heterogeneity of the different averaging times for a given pollutant, we created the metrics at every air quality monitoring station in the study area that measured hourly concentrations. Because these additional monitoring stations were located at various distances from the central monitor, we were able to assess the degree of correlation at various distances for each of the metrics.
We examined associations between daily respiratory emergency department visits and metrics of CO, NO2, O3 and PM2.5 concentrations on the previous day, lag 1. In the primary analyses we chose to focus on previous day (lag 1) pollution concentrations, as this lag was consistently associated with the outcome in previous analyses (Peel et al., 2005). Due to the uncertainty of the relevant lag period of exposure for the pollutants of interest, in sensitivity analyses we also examined alternative lags of each metric (lag days 0, 2, 3). Lag 0 was defined as the period from midnight to midnight on the day of the visit; lag 1 was defined as the period from midnight to midnight on the day preceding the visit, and so on. To compare the magnitude of effect across different metrics of the same pollutant, we calculated risk ratios for both an interquartile range (IQR) increase in concentration, which differed across metrics, and for a standard unit increase, which was the same for all metrics of a given pollutant. The risk ratios for an IQR increase allowed for a comparison of effects for a similar degree of increase relative to each metric’s distribution of concentrations, whereas the risk ratios for a standard unit increase allowed us to compare the magnitude of effect for an absolute increase (e.g., 10 ppb) in pollutant concentration. Chi-square values and corresponding p-values, which are not unit dependent, were calculated to compare the strength of statistical association for each pollutant metric. Chi-square and p-values, which are highly influenced by sample size, could be compared because the number of days included in the analysis was the same across metrics for a given pollutant.
RESULTS
Descriptive statistics for each of the pollutant metrics examined are presented in Table 1. For a given pollutant, many of the metrics were highly correlated (Table 2). As expected, correlations were generally higher between overlapping temporal metrics; for example, the night-time and day-time metrics were less correlated with each other than with the 24-hour average, which encompassed both night-time and day-time hours. For CO, correlations among the metrics ranged from 0.48 to 0.91, with the weakest correlation observed between the day-time and night-time values. Correlations among NO2 metrics ranged from 0.44 to 0.90, with relatively weak correlations between the night-time and day-time metrics and the 1-hour maximum and day-time metrics (r = 0.45 and 0.44, respectively). The O3 metrics were more strongly correlated with one another (r=0.68–0.95) with the exception of the night-time metric, which was uncorrelated with the other O3 metrics except for the 24-hour average metric (r=0.46). Correlations among PM2.5 metrics ranged from 0.60 to 0.94. Similar to CO and NO2, the weakest correlation among the PM2.5 metrics was between day-time and night-time.
Table 1.
Descriptive statistics for air pollution data
| Percentiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pollutant | Na | Time period | Metric | Mean | SD | 25th | 50th | 75th | Max |
| CO (ppm) | 3486 | 1/1/1993-6/30/2003 | 1-hr max | 1.6 | 1.1 | 0.8 | 1.3 | 2.2 | 7.7 |
| 24-hr average | 0.7 | 0.4 | 0.5 | 0.6 | 0.9 | 3.7 | |||
| Commute (7–10am, 4–7pm) | 0.7 | 0.4 | 0.4 | 0.6 | 0.9 | 4.0 | |||
| Day-time (8am–7pm) | 0.5 | 0.3 | 0.4 | 0.5 | 0.7 | 2.6 | |||
| Night-time (12am–6am) | 0.8 | 0.7 | 0.3 | 0.5 | 1.0 | 5.2 | |||
| NO2 (ppb) | 3635 | 3/1/1994-12/31/2004 | 1-hr max | 43 | 18 | 30 | 41 | 53 | 181 |
| 24-hr average | 22 | 10 | 15 | 21 | 28 | 74 | |||
| Commute (7–10am, 4–7pm) | 21 | 11 | 13 | 20 | 27 | 97 | |||
| Day-time (8am–7pm) | 17 | 9 | 10 | 16 | 22 | 82 | |||
| Night-time (12am–6am) | 25 | 16 | 13 | 22 | 35 | 97 | |||
| O3 (ppb) | 2883 | March-October, 1993–2004 | 8-hr max | 53 | 22 | 38 | 51 | 67 | 148 |
| 1-hr max | 62 | 25 | 45 | 59 | 76 | 180 | |||
| 24-hr average | 30 | 12 | 21 | 29 | 37 | 81 | |||
| Commute (7–10am, 4–7pm) | 35 | 16 | 24 | 35 | 45 | 106 | |||
| Day-time (8am–7pm) | 45 | 20 | 32 | 44 | 58 | 123 | |||
| Night-time (12am–6am) | 14 | 12 | 3 | 11 | 22 | 64 | |||
| PM2.5 (µg/m3) | 1660 | 8/1/1998-12/31/2004 | 1-hr max | 29 | 16 | 18 | 26 | 36 | 188 |
| 24-hr average | 16 | 9 | 10 | 14 | 21 | 72 | |||
| Commute (7–10am, 4–7pm) | 17 | 9 | 10 | 15 | 21 | 76 | |||
| Day-time (8am–7pm) | 15 | 8 | 8 | 13 | 19 | 71 | |||
| Night-time (12am–6am) | 17 | 11 | 5 | 9 | 14 | 88 | |||
Number of days used in analysis
PM2.5 was measured continuously using a tapered element oscillating microbalance (TEOM) operated at 30°C to minimize volatilization. Gaseous pollutants were measured using standard approaches (NO2 and O3 by chemiluminescence and CO by infrared analyzer).
Table 2.
Partiala spearman correlation coefficients for all air pollutant metrics
| CO | NO2 | O3 | PM2.5 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metric* | 1-hr | 24-hr | com | day | night | 1-hr | 24-hr | com | day | night | 8-hr | 1-hr | 24-hr | com | day | night | 1-hr | 24-hr | com | day | |
| CO | 1-hr | 1 | |||||||||||||||||||
| 24-hr | 0.87 | 1 | |||||||||||||||||||
| com | 0.65 | 0.85 | 1 | ||||||||||||||||||
| day | 0.53 | 0.76 | 0.91 | 1 | |||||||||||||||||
| night | 0.53 | 0.71 | 0.59 | 0.48 | 1 | ||||||||||||||||
| NO2 | 1-hr | 0.61 | 0.55 | 0.38 | 0.28 | 0.38 | 1 | ||||||||||||||
| 24-hr | 0.62 | 0.66 | 0.55 | 0.44 | 0.51 | 0.79 | 1 | ||||||||||||||
| com | 0.47 | 0.56 | 0.57 | 0.49 | 0.41 | 0.55 | 0.84 | 1 | |||||||||||||
| day | 0.41 | 0.50 | 0.54 | 0.53 | 0.34 | 0.44 | 0.76 | 0.90 | 1 | ||||||||||||
| night | 0.47 | 0.53 | 0.44 | 0.31 | 0.66 | 0.59 | 0.78 | 0.56 | 0.45 | 1 | |||||||||||
| O3 | 8-hr | 0.15 | 0.11 | 0.02 | −0.06 | 0.14 | 0.34 | 0.24 | 0.12 | 0.02 | 0.24 | 1 | |||||||||
| 1-hr | 0.21 | 0.19 | 0.10 | 0.03 | 0.19 | 0.40 | 0.33 | 0.21 | 0.13 | 0.30 | 0.93 | 1 | |||||||||
| 24-hr | −0.17 | −0.22 | −0.22 | −0.22 | −0.15 | 0.02 | −0.15 | −0.17 | −0.22 | −0.11 | 0.78 | 0.68 | 1 | ||||||||
| com | 0.01 | −0.07 | −0.20 | −0.23 | −0.02 | 0.20 | 0.01 | −0.16 | −0.21 | 0.05 | 0.83 | 0.74 | 0.88 | 1 | |||||||
| day | 0.12 | 0.06 | −0.06 | −0.14 | 0.11 | 0.31 | 0.18 | 0.04 | −0.07 | 0.22 | 0.95 | 0.89 | 0.84 | 0.91 | 1 | ||||||
| night | −0.43 | −0.50 | −0.38 | −0.24 | −0.63 | −0.35 | −0.52 | −0.37 | −0.31 | −0.66 | 0.04 | −0.04 | 0.46 | 0.22 | 0.07 | 1 | |||||
| PM2.5 | 1-hr | 0.46 | 0.48 | 0.37 | 0.31 | 0.39 | 0.50 | 0.53 | 0.46 | 0.43 | 0.42 | 0.39 | 0.43 | 0.15 | 0.22 | 0.32 | −0.23 | 1 | |||
| 24-hr | 0.36 | 0.45 | 0.37 | 0.34 | 0.45 | 0.42 | 0.52 | 0.47 | 0.45 | 0.45 | 0.46 | 0.49 | 0.25 | 0.31 | 0.41 | −0.19 | 0.82 | 1 | |||
| com | 0.31 | 0.40 | 0.38 | 0.34 | 0.40 | 0.36 | 0.47 | 0.46 | 0.45 | 0.42 | 0.42 | 0.46 | 0.21 | 0.26 | 0.37 | −0.19 | 0.75 | 0.94 | 1 | ||
| day | 0.21 | 0.31 | 0.31 | 0.33 | 0.33 | 0.27 | 0.37 | 0.38 | 0.41 | 0.33 | 0.40 | 0.44 | 0.24 | 0.26 | 0.36 | −0.10 | 0.68 | 0.91 | 0.93 | 1 | |
| night | 0.28 | 0.37 | 0.31 | 0.26 | 0.55 | 0.33 | 0.45 | 0.41 | 0.37 | 0.52 | 0.31 | 0.32 | 0.15 | 0.18 | 0.28 | −0.22 | 0.62 | 0.79 | 0.69 | 0.60 | |
1-hr= 1hour maximum 24-hr=24 hour average com=commute hours:7–10 am and 4–7pm day=workday hours 8am=7pm night=night hours 12 am –6 am
controlling for covariates included in the Poisson regression model (month-year-maximum temperature strata, lag 1–2 moving average minimum temperature, lag 0-1-2 moving average of dew point temperature, day of week, holidays)
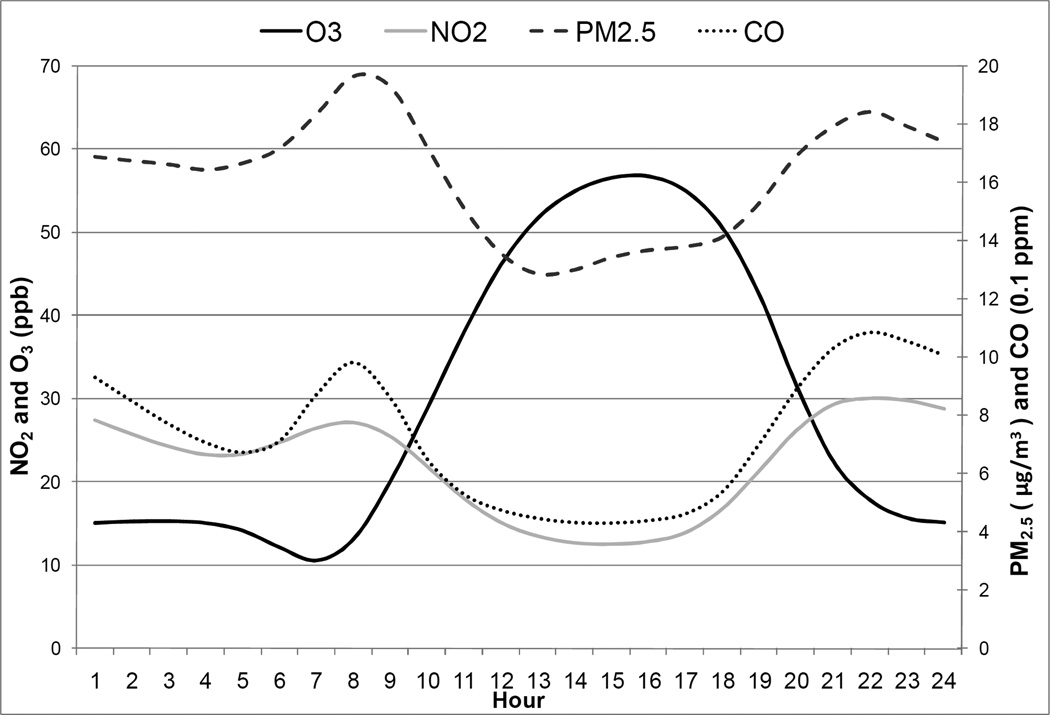
Diurnal patterns for the traffic-related pollutants (CO, NO2, PM2.5) were bimodal, with peaks during the morning and evening rush hours (Figure 1). Hourly maxima for CO and NO2 typically occurred at night between 9 and 11 pm. Concentrations of these pollutants remain elevated during much of the overnight period due to meteorology. Ozone also exhibited a typical diurnal trend, with peaks occurring in the mid- to late afternoon and minima occurring during the night.
Figure 1.
Diurnal pattern* for selected pollutants
*Average of hourly values over study period, hour 1 refers to the hour between 12:00am and 1:00am.
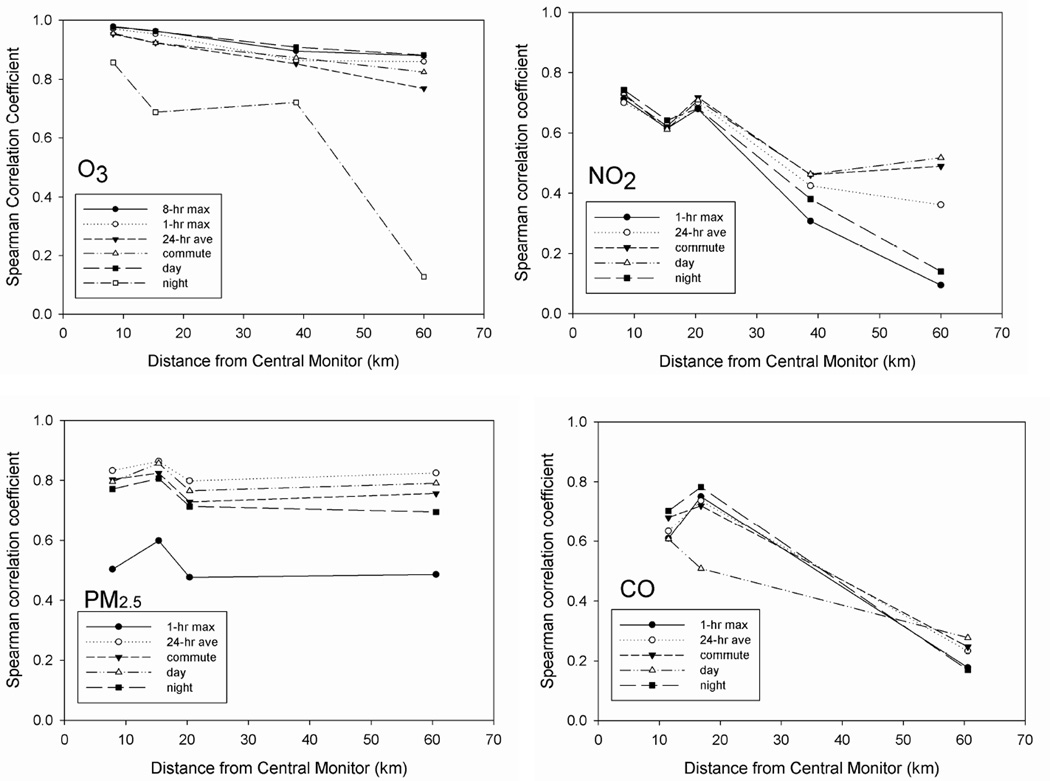
Spatial Correlations of the Metrics
We examined the spatial correlation of the various metrics to assess whether differences in spatial correlation between the metrics could explain differences in the observed associations. The spatial correlations between CO, NO2 , O3 and PM2.5 metrics at the various monitoring station distances are shown in Figure 2. Night-time O3 was the most spatially heterogeneous of the O3 metrics; all of the other O3 metrics showed strong spatial correlations even for long distances between monitors. Spatial correlations for NO2 were fairly similar across metrics for distances less than 20 km. However, in comparisons of monitors more than 38 km apart, the NO2 daily 1-hour maximum was considerably more spatially heterogeneous than the day-time metric. Generally, metrics that included hours when NO2 concentrations were highest exhibited greater spatial heterogeneity (note that the monitoring station located at 15 km is impacted by a nearby freeway). Similarly, the PM2.5 metrics showed strong spatial correlations (r > 0.7 for all distances) with the exception of the 1-hour maximum, which was more spatially heterogeneous (0.5 < r < 0.6 between distances of 10 and 60 km). The 24-hour average PM2.5 was the most spatially homogeneous of the PM2.5 metrics examined.
Figure 2.
Spatial correlations for O3 and NO2 metrics
Comparing Statistical Significance of Association
Risk ratios, 95% confidence intervals, chi-square values and corresponding p-values from the regression models are shown in Table 3. Based on the chi-square values, the night-time metrics for both CO and NO2 were the most strongly associated with respiratory emergency department visits. The day-time metric for CO and NO2, which corresponds to hours of lower concentrations but also periods of time when people are more likely to be exposed, showed the weakest associations for these pollutants. With the exception of the day-time metrics, associations for the CO and NO2 metrics were all statistically significant.
Table 3.
Risk ratios, 95% confidence intervals and chi-square values for associations between lag 1 air pollution metrics and respiratory emergency department visits
| Pollutant | N | Metric | IQR a | RR (95% CI) per IQR | Standard unit | RR (95% CI) per std unit | Χ2 | P value |
|---|---|---|---|---|---|---|---|---|
| CO (ppm) | 3486 | 1-hr maximum | 1.40 | 1.014 (1.009, .1.019) | 0.5 | 1.005 (1.003, 1.007) | 30.6 | <0.001 |
| 24-hr average | 0.45 | 1.015 (1.010, 1.019) | 0.5 | 1.016 (1.011, 1.021) | 39.4 | <0.001 | ||
| Commute (7–10am, 4–7pm) | 0.43 | 1.007 (1.003, 1.011) | 0.5 | 1.008 (1.003, 1.013) | 10.6 | 0.001 | ||
| Day-time (8am–7pm) | 0.30 | 1.004 (0.999, 1.008) | 0.5 | 1.006 (0.998, 1.014) | 2.0 | 0.156 | ||
| Night-time (12am–6am) | 0.62 | 1.011 (1.008, 1.015) | 0.5 | 1.009 (1.007, 1.012) | 46.8 | <0.001 | ||
| NO2 (ppb) | 3635 | 1-hr maximum | 23.0 | 1.011 (1.006, 1.016) | 10 | 1.005 (1.003, 1.007) | 22.1 | <0.001 |
| 24-hr average | 13.3 | 1.012 (1.007, 1.017) | 10 | 1.009 (1.005, 1.013) | 21.8 | <0.001 | ||
| Commute (7–10am, 4–7pm) | 13.8 | 1.008 (1.003, 1.013) | 10 | 1.006 (1.002, 1.010) | 11.1 | 0.001 | ||
| Day-time (8am–7pm) | 11.5 | 1.003 (0.998, 1.008) | 10 | 1.002 (0.998, 1.007) | 1.2 | 0.282 | ||
| Night-time (12am–6am) | 22.2 | 1.016 (1.011, 1.021) | 10 | 1.007 (1.005, 1.009) | 42.4 | <0.001 | ||
| O3 (ppb) | 2883 | 8-hr maximum | 28.9 | 1.020 (1.012, 1.028) | 25 | 1.017 (1.010, 1.024) | 23.9 | <0.001 |
| 1-hr maximum | 31.0 | 1.018 (1.010, 1.025) | 25 | 1.014 (1.008, 1.020) | 22.7 | <0.001 | ||
| 24-hr average | 16.2 | 1.007 (0.999, 1.015) | 25 | 1.011 (0.999, 1.024) | 3.4 | 0.067 | ||
| Commute (7–10am, 4–7pm) | 21.3 | 1.006 (0.998, 1.015) | 25 | 1.007 (0.998, 1.017) | 2.2 | 0.139 | ||
| Day-time (8am–7pm) | 26.7 | 1.018 (1.010, 1.026) | 25 | 1.017 (1.009, 1.025) | 18.3 | <0.001 | ||
| Night-time (12am–6am) | 19.0 | 0.991 (0.985, 0.997) | 25 | 0.988 (0.980, 0.996) | 8.6 | 0.003 | ||
| PM2.5 (μg/m3) | 1660 | 1-hr maximum | 17.4 | 1.004 (0.999, 1.009) | 10 | 1.002 (1.000, 1.005) | 2.9 | 0.089 |
| 24-hr average | 10.9 | 1.004 (0.998, 1.010) | 10 | 1.004 (0.998, 1.010) | 1.9 | 0.171 | ||
| Commute (7–10am, 4–7pm) | 11.5 | 1.005 (0.999, 1.011) | 10 | 1.004 (0.999, 1.009) | 2.5 | 0.113 | ||
| Day-time (8am–7pm) | 10.8 | 1.003 (0.997, 1.009) | 10 | 1.003 (0.997, 1.008) | 0.8 | 0.380 | ||
| Night-time (12am–6am) | 13.6 | 1.004 (0.999, 1.010) | 10 | 1.003 (0.999, 1.007) | 2.3 | 0.133 | ||
IQR=interquartile range of pollutant metric
The daily 1-hour and 8-hour maximums yielded the strongest associations for O3. The day-time metric, which captured the hours of peak O3 concentrations, was also strongly associated with respiratory emergency department visits. The commute and the 24-hour average metrics for O3, however, were only weakly associated with respiratory emergency department visits (p>0.05), and the night-time metric of O3 was inversely associated with respiratory emergency department visits. Ozone was the only pollutant for which the choice of metric affected the direction of association. Given the negative correlations between the night-time O3 metric and the CO and NO2 metrics (Table 2), we suspected this negative association might be confounded by the positive associations observed with the various metrics of CO and NO2. In multipollutant models, when any of the CO and NO2 metrics were included in the model as covariates (with the exception of day-time CO), night-time O3 was not negatively (or positively) associated with respiratory emergency department visits. There were no observed associations between any of the PM2.5 metrics and respiratory emergency department visits. However, the sample size was more limited for PM2.5 and all point estimates were above the null; chi-square values and risk ratios were comparable across metrics.
Table 4 displays the chi-square values and risk ratios (per inter-quartile range) for models using alternative lags of each metric (lag days 0, 2 and 3), in addition to the lag 1 day chi-square values as presented in Table 3. Based on the chi-square values, at shorter lags (0 and 1 days) the night-time metrics for CO and NO2 were the strongest predictors of respiratory emergency department visits, whereas at longer lags (2 and 3 days) the chi-square values were similar between the night-time, daily 1-hour maximum and 24-hour average metrics. This result suggests that strong associations with the lag 1 night-time metric may reflect associations with a longer lag of pollutant concentrations. For example, the night-time metric included hours (12am–6am) closest in time to the previous day compared to our other metrics of interest. For CO, NO2 and PM2.5 the night-time metrics were the most strongly correlated to the previous day’s concentrations regardless of previous day-time metric chosen (supplementary information Table A).
Table 4.
Chi-square values and risk ratios* for associations between various lags of each pollutant metric and respiratory emergency department visits
| POLLUTANT | Metric | χ2 lag 0 | χ2 lag1 | χ2 lag2 | χ2 lag3 |
|---|---|---|---|---|---|
| CO | 1-hr max | 36.8 (1.018) | 30.6 (1.014) | 27.3 (1.013) | 26.8 (1.013) |
| 24-hr ave | 44.6 (1.019) | 39.4 (1.015) | 29.4 (1.012) | 21.8 (1.011) | |
| commute | 15.6 (1.011) | 10.6 (1.007) | 10.1 (1.007) | 4.1 (1.004) | |
| day-time | 4.6 (1.007) | 2.0 (1.004) | 4.2 (1.005) | 0.9 (1.002) | |
| night-time | 60.6 (1.020) | 46.8 (1.011) | 23.9 (1.008) | 24.7 (1.008) | |
| NO2 | 1-hr max | 48.7 (1.020) | 22.1 (1.011) | 45.9 (1.014) | 50.9 (1.015) |
| 24-hr ave | 50.4 (1.021) | 21.8 (1.012) | 47.2 (1.016) | 49.6 (1.017) | |
| commute | 34.3 (1.017) | 11.1 (1.008) | 32.3 (1.013) | 23.9 (1.012) | |
| day-time | 7.4 (1.009) | 1.2 (1.003) | 9.3 (1.008) | 10.2 (1.008) | |
| night-time | 97.1 (1.027) | 42.4 (1.016) | 59.1 (1.018) | 48.1 (1.016) | |
| O3 | 8-hr max | 28.7 (1.027) | 23.9 (1.020) | 33.2 (1.021) | 40.1 (1.022) |
| 1-hr max | 28.4 (1.026) | 22.7 (1.018) | 35.1 (1.020) | 37.0 (1.020) | |
| 24-hr ave | 3.4 (1.009) | 3.4 (1.007) | 3.1 (1.007) | 9.3 (1.011) | |
| commute | 1.8 (1.007) | 2.2 (1.006) | 6.1 (1.009) | 14.5 (1.014) | |
| day-time | 19.5 (1.024) | 18.3 (1.018) | 23.7 (1.019) | 28.3 (1.019) | |
| night-time | 4.3 (0.993) | 8.6 (0.991) | 3.6 (0.994) | 1.7 (0.996) | |
| PM2.5 | 1-hr max | 1.3 (0.996) | 2.9 (1.004) | 0.1 (0.999) | 2.3 (1.004) |
| 24-hr ave | 0.9 (0.996) | 0.2 (1.004) | 1.2 (1.003) | 3.4 (1.006) | |
| commute | 0.7 (0.996) | 2.5 (1.005) | 1.1 (1.003) | 5.7 (1.007) | |
| day-time | 2.6 (0.993) | 0.8 (1.003) | 0.4 (1.002) | 3.8 (1.006) | |
| night-time | 0.3 (0.998) | 2.3 (1.004) | 1.7 (1.004) | 1.4 (1.003) | |
RR per interquartile increase in pollutant metric
Comparing Magnitude of Association
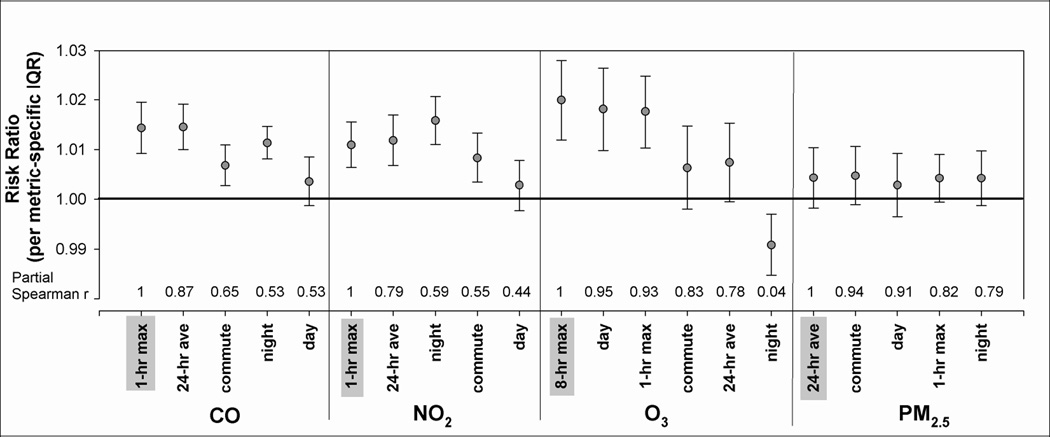
Figure 3 displays the risk ratios and 95% confidence intervals scaled to each metric’s IQR; correlations between our a priori metric (shaded) and alternative metrics are shown on the x-axis. For an IQR increase in each metric, risk ratios ranged from 1.004 to 1.015 for CO, 1.003 to 1.016 for NO2, 0.991 to 1.020 for O3 and 1.003 to 1.005 for PM2.5. When comparing the magnitudes of association, interpretation differed slightly according to how the regression coefficients were scaled: standard unit or IQR (Table 3). For example, the risk ratio estimate for night-time NO2 was highest when effects were scaled to the IQR, but the 24-hour average risk ratio was highest when effects were scaled to the standard unit (10 ppb). For CO, the 24-hour average had the highest risk ratio for both scaling approaches, but the daily 1-hour maximum was second highest when scaled to its IQR (1.4 ppm), and was lowest when scaled to the standard unit (0.5 ppm). The 1-hour maximum, 8-hour maximum and day-time O3 metrics yielded higher risk ratios than the 24-hour average, commute and night-time O3 metrics, regardless of scaling. In Figure 3 and Table 4 we only present the results scaled to each metric’s IQR so that risk ratios can be compared for the same relative degree of variability.
Figure 3.
Risk ratios and 95% confidence intervals* for associations between lag 1 pollutant metrics and respiratory emergency department visits. Partial spearman correlations between a priori metrics (shaded in grey) and other pollutant metrics shown above x-axis.
* RRs scaled to the interquartile range of each metric
In sensitivity analyses using our previous time-series modeling approach (Peel et al., 2005), we observed similar patterns in risk ratios and chi-square values across the metrics. However, using this approach, the commute metrics for CO and NO2 were not significantly associated with respiratory emergency room visits.
DISCUSSION
In this time-series analysis, we compared associations between various temporal metrics of daily ambient air pollution levels and respiratory health using a large dataset of more than one million respiratory emergency department visits. Our motivation was to explore the implications of choice of pollutant temporal averaging time on health risk estimates within a time-series framework.
For a given pollutant, many of the metrics were strongly correlated and yielded similar magnitude and statistical significance of associations with daily respiratory visits. As expected, pollutant metrics that were less correlated with each other exhibited larger differences in epidemiologic associations than correlated metrics. Differences in epidemiologic results between metrics of the same pollutant may be due to: (a) differences in biological relevance of the dose for the measured pollutant (e.g., peak vs. average exposures) (b) differences in metric spatial heterogeneity and corresponding representativeness of population exposures (exposure measurement error) (c) differences in correlation with personal exposures due to time-activity patterns (exposure measurement error) (d) differences in representing the true etiologic agent (related to surrogacy) (e) differences in representing the relevant lag period of the pollutant (misspecified lag) (f) confounding by other pollutants during certain time-windows (e.g., night-time O3 with the NO2 and CO metrics) (g) model misspecification (e.g., violation of linearity assumptions) or (h) random variation. While some of these possible explanations were not directly testable in our study, we discuss them in the context of our findings below.
In general, variability in the observed results reflected pollutant diurnal patterns, with temporal metrics that included peak pollutant hours tending to show the strongest associations with respiratory emergency department visits and metrics capturing hours of low concentrations showing weaker associations. For example, O3 is formed during the daylight hours and is depleted at night; metrics incorporating the peak afternoon hours of O3 were correspondingly most strongly associated with our outcome. Conversely, NO2 is lowest during the daylight hours when it is being more rapidly dispersed and oxidized; during the evening and overnight hours NO2 is oxidized more slowly and the mixing height decreases, so concentrations increase. NO2 and CO metrics that included the hours of higher concentrations (including the 1-hour maximum and night-time metrics) showed stronger associations than models using metrics that included concentration minima for these pollutants, despite the typical hours of peak concentration for these pollutants being late evening hours, when people are less likely to be outside.
Differences among metrics were most pronounced for O3, where three metrics were strongly associated with the outcome (1-hour maximum, 8-hour maximum, day-time), two metrics were weakly associated with the outcome (commute, 24-hour average) and one metric was inversely associated with the outcome (night-time). Night-time O3 concentrations were negatively correlated with all of the CO, NO2 and PM2.5 metrics, likely due to the depletion of O3 by reaction with NO; when vehicle emission pollutant concentrations (i.e., CO and NOx) are elevated at night, O3 depletion is high as well. We found when we controlled for CO and NO2 concentrations in multipollutant models, night-time O3 was no longer inversely associated with respiratory emergency department visits. The night-time concentrations of O3 may serve as an inverse surrogate of traffic-related pollutants such as NOx. Fresh NO emissions scavenge ozone at night without the reverse process of NO2 photolysis that leads to O3 formation. Furthermore, lower mixing heights at night tend to increase NOx, CO and PM2.5 concentrations. Because NOx emissions are spatially heterogeneous, O3 scavenging at night is also spatially heterogeneous; in the more populated urban center, the higher NOx levels result in lower O3 levels at night. The negative association observed for night-time O3 suggests that O3 is not the only pollutant linked to respiratory outcomes -- an example of biologic insights gained through assessment of alternative temporal metrics. Lastly, while investigators would be unlikely to choose a night-time metric of O3 in an epidemiologic study, this finding also argues against the use of a 24-hour average metric for O3, which itself was only weakly associated with the outcome. In our analysis, inclusion of night-time O3 concentrations within a 24-hour average not only dilutes the relevant concentrations by adding irrelevant hours (i.e., bias toward the null), but includes hours when the relationship between O3 and respiratory emergency room visits may be negatively confounded by other pollutants.
In a previous study, Bell and colleagues compared air quality under seven emissions scenarios using 1-hour maximum, 8-hour maximum and 24-hour average O3 concentrations to characterize air quality (2005). Rankings of the different emissions scenarios differed according to the metric of O3 chosen, but rankings based on the 1-hour maximum and 8-hour maximum were more similar to each other than to rankings based on the 24-hour average. In a panel study of asthma symptoms in 25 asthmatic children, associations using the 1-hour maximum O3 metric were similar to those using the 8-hour maximum (Delfino et al., 1998). Our O3 findings are consistent with these previous studies.
From an exposure standpoint, our results highlight the potential for certain pollutant metrics to act as surrogates for other pollutant metrics. Our CO and NO2 findings, for example, indicate CO or NO2 could be acting as a surrogate for the other, as shown by the correlations in Table 2 (e.g., r=0.61 for 1-hour max). Alternatively, CO and NO2 may be serving as surrogates of another pollutant, namely O3, as CO and NO2 metrics incorporating peak concentration hours were shown to be the most strongly associated with the outcome, as well as more strongly correlated with peak O3 concentrations than other CO and NO2 metrics. Thus, associations between emergency department visits and peak (1-hour maximum) NO2, a precursor of O3, may be partly confounded by O3, or vice versa.
Teasing out the effects of each pollutant through the use of multipollutant modeling is complicated by the differences in measurement error between the pollutants (Tolbert et al., 2007). Comparative analysis of temporal metrics within and across pollutants may provide an alternative approach for identifying the pollutant more likely to be the etiologic agent. For example, NO2 metrics that were more strongly correlated with 8-hour maximum O3 showed stronger associations with respiratory emergency room visits. This was also true for CO. Furthermore, these CO and NO2 metrics yielding the strongest associations were the hours when people were least likely to be exposed to ambient air (i.e., night hours), and weaker associations were observed when people were more likely to be exposed to ambient air (i.e., day-time and commute hours). If NO2 and CO were the true etiologic agents, we might expect to observe associations for metrics incorporating hours when people are more likely to be exposed to ambient air, regardless of the correlation with 8-hour maximum O3. These results suggest that the etiologic agent is more likely to be O3 than CO or NO2.
Certain metrics may also serve as surrogates of the same pollutant but for a different lag. The night-time metrics for previous day (lag 1) CO and NO2 showed some of the strongest associations with respiratory emergency room visits, despite night hours being some of the least likely hours of population exposure to ambient air. Perhaps the night-time metric at lag 1 is more predictive of respiratory emergency room visits because it acts as a better surrogate for pollution on earlier days (longer lags). Night-time NO2 and CO concentrations were the best surrogates of NO2 and CO concentrations on the previous day regardless of metric, likely because night-time hours (12am–6am) were closer in time to the previous day (supplementary Table A).
The comparison among PM2.5 metrics was less informative because we did not observe significant associations with the outcome of interest. However, the consistency of effect estimates across metrics provided reassurance that a strong association would not missed if analysis was limited to the standard 24-hour average metric. In our data, the spatial correlations of the PM2.5 metrics were also similar, except for the daily 1-hour maximum, which was more spatially heterogeneous. Although in this analysis we did not assess specific chemical components of PM2.5, it should be noted that the spatial heterogeneity of PM2.5 can vary by the chemical composition of the particles (Wade et al., 2006). Few epidemiologic studies have presented results for PM2.5 or PM10 using a temporal metric other than the 24-hour average. We noted reports of three panel studies of asthmatic children investigating the relationship between PM10 and asthma symptoms that examined more than one averaging time for PM10. One found modestly stronger associations using a 24-hour average PM10 metric compared to a 1-hour maximum (Ostro et al., 2001) and two showed slightly stronger associations using peak PM10 metrics (1-hour maximum, 8-hour maximum) compared to the 24-hour average (Delfino et al., 1998, 2002).
This analysis highlights some of the challenges involved in comparing scaled risk ratios. We presented the risk ratios scaled to each metric’s IQR; these risk ratios take into account the range of concentrations for each metric and provide a comparison for the same relative degree of variability. We also presented results for a standard unit (e.g., 0.5 ppm) so that results could be compared for the same absolute unit increase in concentration for each pollutant. However, comparisons based on absolute increases in concentration may be misleading in this setting where daily temporal metrics of the same pollutant are being compared, since a 0.5 ppm increase in day-time CO is a meaningfully greater relative increase compared to a 0.5 ppm increase in 1-hour maximum CO, for example. As a consequence of these differences among metrics in concentration variability, the metrics yielding the largest magnitude of effects often differed by the choice of scaling. In this analysis we preferred the chi-square values to identify the strongest associations since chi-square values are not affected by scaling. Comparing chi-square values across metrics was appropriate in this study because the sample size was the same for all metrics of a given pollutant.
In this analysis we focused on respiratory-related emergency department visits and did not present results for cardiovascular disease visits. In our previous work, we found same-day pollution levels (lag 0) to be most strongly associated with cardiovascular emergency room visits (Metzger et al., 2004). Same-day pollution effects can be difficult to compare across temporal metrics because some of the averaging times include hours late in the day, potentially after the bulk of emergency room visits have occurred on a given day. In the present analyses of various metrics of lag 1 pollution, while temporality issues may still play a role (e.g., the night-time metric captured hours at a longer lag than the day-time metric), all temporal metrics included concentrations before the emergency room visit occurred. These temporality and choice of lag issues are clearly important to the estimation of effects, as recently demonstrated by Lokken and colleagues (Lokken et al., 2009).
In summary, we found that epidemiologic results were generally similar across different temporal metrics of the same pollutant and would have led to similar conclusions about the relationship between the pollutant and respiratory emergency room visits. Exceptions included the night-time O3 metric and the day-time metrics of CO and NO2. It would be of interest to know how well each of the time-averaged metrics correlate with measured personal exposures; studies where personal exposures have been measured longitudinally could likely address this question without additional data collection. We found that our a priori metrics for CO (1-hour maximum), NO2 (1-hour maximum), and O3 (8-hour maximum), based on the National Ambient Air Quality Standards and designed to capture peak concentrations, yielded associations that were as strong or stronger than the other metrics. Our analysis supports the use of these exposure metrics in future studies of ambient air pollution and respiratory health.
Supplementary Material
Acknowledgements
This work was supported by grants from the Electric Power Research Institute (EP-P27723/C13172), the U.S. Environmental Protection Agency (CR-83407301-0, RD833626, R82921301-0), and the National Institute of Environmental Health Sciences (R01ES11294).
REFERENCES
- Bell ML, Hobbs BF, Ellis H. Metrics matter: conflicting air quality rankings from different indices of air pollution. J Air Waste Manag Assoc. 2005;55(1):97–106. doi: 10.1080/10473289.2005.10464596. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Zeiger RS, Seltzer JM, Street DH. Symptoms in pediatric asthmatics and air pollution: differences in effects by symptom severity, anti-inflammatory medication use and particulate averaging time. Environ Health Perspect. 1998;106(11):751–761. doi: 10.1289/ehp.98106751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Zeiger RS, Seltzer JM, Street DH, McLaren CE. Association of asthma symptoms with peak particulate air pollution and effect modification by anti-inflammatory medication use. Environ Health Perspect. 2002;110(10):A607–A617. doi: 10.1289/ehp.021100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DA, Edgerton E, Hartsell B, Jansen J, Burge H, Koutrakis P, Rogers C, Suh H, Chow J, Zielinska B, McMurry P, Mulholland J, Russell A, Rasmussen R. Air quality measurements for the aerosol research and inhalation epidemiology study. J Air Waste Manag Assoc. 2006;56(10):1445–1458. doi: 10.1080/10473289.2006.10464549. [DOI] [PubMed] [Google Scholar]
- Ito K, Thurston GD, Nadas A, Lippmann M. Monito-to-monitor temporal correlation of air pollution and weather variables in the North-Central U.S. J Expo Sci Environ Epidemiol. 2001;11(1):21–32. doi: 10.1038/sj.jea.7500144. [DOI] [PubMed] [Google Scholar]
- Lokken RP, Wellenius GA, Coull BA, Burger MR, Schlaug G, Suh HH, Mittleman MA. Air pollution and risk of stroke: underestimation of effect due to misclassification of time of event onset. Epidemiology. 2009;20(1):137–142. doi: 10.1097/ede.0b013e31818ef34a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zeger SL. On the equivalence of case-crossover and time series methods in environmental epidemiology. Biostatistics. 2007;8(2):337–344. doi: 10.1093/biostatistics/kxl013. Epub 2006 Jun 29. [DOI] [PubMed] [Google Scholar]
- Metzger KB, Tolbert PE, Klein M, Peel JL, Flanders WD, Todd K, Mulholland JA, Ryan PB, Frumkin H. Ambient air pollution and cardiovascular emergency department visits. Epidemiology. 2004;15(1):46–56. doi: 10.1097/01.EDE.0000101748.28283.97. [DOI] [PubMed] [Google Scholar]
- Ostro B, Lipsett M, Mann J, Braxton-Owens H, White M. Air pollution and exacerbation of asthma in African-American children in Los Angeles. Epidemiology. 2001;12(2):200–208. doi: 10.1097/00001648-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, Mulholland JA, Ryan PB, Frumkin H. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16(2):164–174. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Marmur A, Klein M, Kim E, Russell AG, Sarnat SE, Mulholland JA, Hopke PK, Tolbert PE. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect. 2008;116(4):459–466. doi: 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat JA, Schwartz J, Catalano PJ, Suh HH. Gaseous pollutants in particulate matter epidemiology: confounders or surrogates? Environ Health Perspect. 2001;109(10):1053–1061. doi: 10.1289/ehp.011091053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat SE, Klein M, Sarnat JA, Flanders WD, Waller LA, Mulholland JA, Russell AG, Tolbert PE. An examination of exposure measurement error from air pollutant spatial variability in time-series studies. J Expo Sci Environ Epidemiol. 2009 doi: 10.1038/jes.2009.10. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. The effects of particulate air pollution on daily deaths: a multi-city case crossover analysis. Occup Environ Med. 2004 Dec;61(12):956–961. doi: 10.1136/oem.2003.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. How sensitive is the association between ozone and daily deaths to control for temperature? Am J Respir Crit Care Med. 2005 Mar 15;171(6):627–631. doi: 10.1164/rccm.200407-933OC. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Spix C, Quénel P, Ponce-de-León A, Pönka A, Barumandzadeh T, Touloumi G, Bacharova L, Wojtyniak B, Vonk J, Bisanti L, Schwartz J, Katsouyanni K. Urban air pollution and emergency admissions for asthma in four European cities: the APHEA Project. Thorax. 1997 Sep;52(9):760–765. doi: 10.1136/thx.52.9.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert PE, Klein M, Peel JL, Sarnat SE, Sarnat JA. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol. 2007;17(Suppl 2):S29–S35. doi: 10.1038/sj.jes.7500625. [DOI] [PubMed] [Google Scholar]
- Van Loy M, Bahadori T, Wyzga R, Hartsell B, Edgerton E. Aerosol Research and Inhalation Epidemiology Study (ARIES): PM2.5 mass and aerosol component concentrations and sampler intercomparisons. J AirWaste Manage Assoc. 2000;50:1446–1458. doi: 10.1080/10473289.2000.10464187. [DOI] [PubMed] [Google Scholar]
- Wade KS, Mulholland JA, Marmur A, Russell AG, Hartsell EE, Edgerton E, Klein M, Waller L, Peel JL, Tolbert PE. Effect of instrument precision and spatial variability on the assessment of the temporal variation of ambient air pollution in Atlanta, Georgia. J Air Waste Manag Assoc. 2006;56:876–888. doi: 10.1080/10473289.2006.10464499. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Air pollution and emergency admissions in Boston, MA. J Epidemiol Community Health. 2006 Oct;60(10):890–895. doi: 10.1136/jech.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmirou D, Schwartz J, Saez M, Zanobetti A, Wojtyniak B, Touloumi G, Spix C, Ponce de León A, Le Moullec Y, Bacharova L, Schouten J, Pönkä A, Katsouyanni K. Time-series analysis of air pollution and cause-specific mortality. Epidemiology. 1998 Sep;9(5):495–503. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.