Abstract
Aims/hypothesis
This paper presents a rationale for the selection of intermediate endpoints to be used in the design of type 1 diabetes prevention clinical trials.
Methods
Relatives of individuals diagnosed with type 1 diabetes were enrolled on the TrialNet Natural History Study and screened for diabetes-related autoantibodies. Those with two or more such autoantibodies were analysed with respect to increased HbA1c, decreased C-peptide following an OGTT, or abnormal OGTT values as intermediate markers of disease progression.
Results
Over 2 years, a 10% increase in HbA1c, and a 20% or 30% decrease in C-peptide from baseline, or progression to abnormal OGTT, occurred with a frequency between 20% and 41%. The 3- to 5-year risk of type 1 diabetes following each intermediate endpoint was high, namely 47% to 84%. The lower the incidence of the endpoint being reached, the higher the risk of diabetes. A diabetes prevention trial using these intermediate endpoints would require a 30% to 50% smaller sample size than one using type 1 diabetes as the endpoint.
Conclusions/interpretation
The use of an intermediate endpoint in diabetes prevention is based on the generally held view of disease progression from initial occurrence of autoantibodies through successive immunological and metabolic changes to manifest type 1 diabetes. Thus, these markers are suitable for randomised phase 2 trials, which can more rapidly screen promising new therapies, allowing them to be subsequently confirmed in definitive phase 3 trials.
Keywords: Clinical trial, C-peptide, Dysglycaemia, HbA1c, Intermediate endpoints, Prevention, Type 1 diabetes
Introduction
The notion of alternative type 1 diabetes prevention strategies is consistent with a concept of diabetes as a continuum, ranging from normal glycaemic control to the need for exogenous insulin therapy. The process is thought to begin with an unknown initiating event, followed by: (1) an immunological host response (T cell, B cell and islet cell autoantibodies [ICAs]); (2) metabolic changes (impaired glucose tolerance) after an OGTT; (3) loss of first-phase insulin response to an intravenous glucose tolerance test; (4) loss of C-peptide; and (5) elevated HbA1c. Dysglycaemia, and eventually increased fasting or postprandial glucose levels, meet the definition of diabetes and warrant exogenous insulin therapy [1]. However, in light of the continuum of disease progression, type 1 diabetes should be diagnosed at the beginning of this process, i.e. at the first indication of an autoimmune response, rather than at its end when glucose levels reach a threshold for intervention. Disease progression within the continuum follows definable and measurable steps, which, as individuals progress from one step to the next, reach the point when insulin replacement therapy is warranted to prevent acute and long-term health effects [2]. Previous studies have shown that the accumulation of these markers of disease progression leads to an increased risk of type 1 diabetes [3, 4].
A prevention strategy is designed to prevent or delay disease progression from one step to the next. When designing prevention strategies, a step should be defined as a measurable change in an immunological or metabolic measure that is associated with a significant increase in the risk of requiring exogenous therapy. In terms of clinical trial design, the transition to an intermediate step should occur with reasonable frequency (incidence) in a relatively short period of time, if the step is to be a valuable alternative to the planning of studies with type 1 diabetes (as currently defined) as the endpoint.
Current prevention strategies use the diagnosis of diabetes as their endpoint. The problem of finding a population in which the incidence of type 1 diabetes is high enough for the effect of a preventative intervention to be observed and tested means that prevention strategies are often limited to the study of individuals with high-risk genetic characteristics or individuals who have already transitioned to the first step in disease progression, i.e. the presence of multiple diabetes-related autoantibodies. In the former case, the highest genetic risk (HLA-DR3/DR4) is associated with a 10 year estimated type 1 diabetes incidence of 10% and the trial sample size would be >2000 to detect a 40% effect [5]. In the latter example, the presence of two or more diabetes-related autoantibodies is associated with a 5 year estimated type 1 diabetes incidence of 30 to 50% and the sample size would be >330 to detect the same 40% effect [6]. To find participants with the required genetic or autoimmune characteristics, the study populations would be limited to those identified by screening of first- or second-degree relatives of individuals with established type 1 diabetes, even though they represent only 15% of those affected with the disease.
These current strategies require the screening of relatively large numbers of individuals to identify the few with increased type 1 diabetes risk (40% with high-risk HLA or 1–2% with multiple diabetes-related antibodies in relatives of individuals diagnosed with type 1 diabetes [5, 7]); these must then be enrolled in a prevention trial and followed for 5 to 10 years after enrolment. It generally takes 4 to 7 years to identify the requisite target population size, necessitating a time commitment of up to 15 years from study outset to the end of follow-up for current diabetes prevention studies. Thus a design requiring fewer participants and with an endpoint that can be reached in a shorter time would be more efficient.
This paper describes the use of intermediate endpoints for diabetes prevention trials, based on changes in HbA1c or C-peptide and glucose following an OGTT in a population with the following characteristics: (1) relatives of type 1 diabetes patients; (2) presence of several diabetes-related autoantibodies and a normal OGTT. Each intermediate endpoint was evaluated according to its incidence within 2 years of baseline and the associated risk of diabetes calculated once that endpoint had been observed.
Methods
The data used for this analysis come from the TrialNet Natural History Study [7]. The study was approved by the responsible Ethics Committees (Institutional Review Boards) and all study participants provided informed consent according to institutional policy.
First-degree (age 1-45 years) and second-degree (age 1-20 years) relatives of individuals diagnosed with type 1 diabetes were screened for diabetes-related ICAs and the following biochemical autoantibodies: GADA, insulin autoantibodies, insulinoma-associated protein 2 (islet antigen-2) antibodies and zinc transporter autoantibodies (ZnT8A). ICA and ZnT8A were tested in participants who were positive for one of the other autoantibodies. Individuals who tested positive for two or more of the antibodies were screened every 6 months by OGTT and HbA1c determination. Blood samples were sent to TrialNet core laboratories (University of Colorado, Aurora, CO, USA; University of Washington, Seattle, WA, USA; University of Florida, Gainesville, FL, USA) for central analysis. C-peptide concentrations were measured from frozen plasma using a two-site immunoenzymometeric assay (Tosoh Bioscience, South San Francisco, CA, USA). HbA1c was measured by ion-exchange HPLC (Variant II; Bio-Rad Diagnostics, Hercules, CA, USA). Details of the protocol and the antibody assays, along with their coefficients of variation and performance in external quality control programmes, have been previously published [7].
The proposed intermediate endpoints are relative changes from baseline in: (1) HbA1c (≥10% relative increase); (2) C-peptide (decrease of ≥20% and ≥30%); and (3) glycaemia, i.e. dysglycaemia following an OGTT (impaired fasting glucose, indeterminate glucose tolerance [at least one glucose measurement of ≥11.1 mmol/l at 30, 60 or 90 min] or elevated 2 h postprandial glucose according to the American Diabetes Association (ADA) criteria [2]). Their incidence and the incidence of diabetes from the time that the intermediate endpoint was observed were estimated using survival methods (Kaplan–Meier [8] with Greenwood estimates of their standard errors [9]). Because of the known glucose level variability following an OGTT, dysglycaemia had to be confirmed by a subsequent OGTT for this endpoint to be considered to have been met. Confirmation of the other endpoints was not required in this analysis. The C-peptide AUC was computed using the trapezoidal rule, which is a weighted sum of the fasting and the 30, 60, 90 and 120 min values.
Results
The data set for this analysis is based on the cumulative screening of 93,877 individuals in the TrialNet Natural History Study, of whom 1151 (1.2%) were found to have two or more diabetes-related autoantibodies at screening, and had baseline and follow-up OGTTs.[7] Their demographic characteristics and antibody status are described in Table 1.
Table 1.
Characteristics of study population
| Characteristic Age at screening |
n 1143 |
Mean (SD) or n (%) 15.2 (11.8) |
Median 11.3 |
|---|---|---|---|
| Sex | |||
| Male, n (%) | 554 (48.7) | ||
| Female, n (%) | 583 (51.3) | ||
| Data missing | 14 | ||
| Race | |||
| White, n (%) | 997 (87.1) | ||
| Black, n (%) | 34 (3.0) | ||
| Other, n (%) | 83 (7.2) | ||
| Unknown, n (%) | 31 (2.7) | ||
| Data missing | 6 | ||
| Ethnicity | |||
| Hispanic or Latino, n (%) | 122 (10.9) | ||
| Non-Hispanic/Latino, n (%) | 982 (87.8) | ||
| Unknown, n (%) | 14 (1.2) | ||
| Data missing | 33 | ||
| Participants with autoantibody positivity | |||
| Positive for two autoantibodies, n (%) | 503 (43.7) | ||
| Positive for three autoantibodies, n (%) | 343 (29.8) | ||
| Positive for four autoantibodies, n (%) | 220 (19.1) | ||
| Positive for five autoantibodies, n (%) | 85 (7.4) | ||
| HbA1c at baseline (%) | 1141 | 5.2 (0.5) | 5.2 |
| HbA1c at baseline (mmol/mol) | 1141 | 33.3 (5.1) | 33.3 |
| Mean C-peptide AUC (pmol/ml) at baseline | 1143 | 1.6 (0.8) | 1.5 |
| Glucose at baseline OGTT | |||
| Time 0 min (mmol/l) | 1151 | 5.0 (1.0) | 4.9 |
| Time 120 min (mmol/l) | 1151 | 7.5 (3.3) | 6.8 |
Relative to the HbA1c value at baseline, increasing values were associated with an increased risk of diabetes. Figure 1a shows that the 2 year incidence of a 10% increase in HbA1c was 20% and the 3 year incidence of type 1 diabetes from the time of the change in HbA1c (Fig. 1b) was 84%. The incidence of diabetes continued to increase with time, but the number of individuals followed for a longer period of time was too small to make a reliable estimate.
Fig. 1.
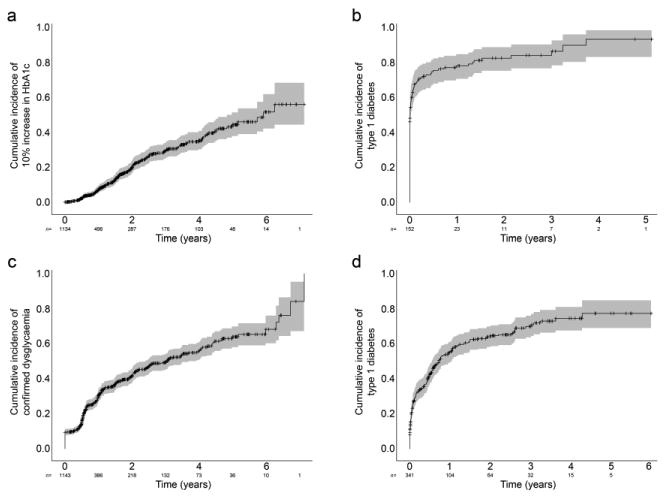
(a) The 2 year incidence of a 10% increase in HbA1c from baseline was 20% (standard error 2%, grey shading) and was associated (b) with an 84% (standard error 4%) risk of type 1 diabetes at 3 years from that time-point. (c) The 2 year risk of progressing to confirmed dysglycaemia was 41% (standard error 2%) and was associated (d) with a 74% (standard error 3%) risk of type 1 diabetes at 4 years from the time dysglycaemia was confirmed
Another possible intermediate endpoint is the occurrence of dysglycaemia as defined by an abnormal OGTT confirmed on two occasions. The 2 year risk of progressing from normal OGTT to dysglycaemia in participants with two or more diabetes-related autoantibodies was 41% (Fig. 1c). The type 1 diabetes risk from the time of the confirmed abnormal glucose tolerance test was 74% at 4 years (Fig. 1d).
As shown in Fig. 2a, the estimated 2 year incidence of a 20% decline in C-peptide AUC was 39%; that of a 30% decline was 20% (Fig. 2c). The corresponding 4 year type 1 diabetes risk for the respective decrease in C-peptide was 47% (Fig. 2b) and 50% (Fig. 2d), respectively. Generally, the larger the change from baseline, the greater the risk of diabetes. However, the incidence of that change declines as the magnitude of the change increases (Fig. 3). In addition, the increase in the risk of diabetes associated with an increasing loss of C-peptide is not nearly as steep as that associated with relative increases in HbA1c. Thus a 20% increase in HbA1c was associated with a nearly 100% risk of type 1 diabetes over 3 to 5 years, but occurred with only a 10% incidence compared with a smaller, 5% increase in HbA1c, which had a lower (60%) risk of type 1 diabetes, while occurring at a much higher (40%) incidence. This dramatic rise in type 1 diabetes risk was not seen with increasing loss of C-peptide (the curve is much flatter). This suggests that raising that intermediate endpoint (i.e. greater loss of C-peptide as endpoint) would not change the diabetes risk, but would reduce the incidence of that endpoint being reached.
Fig. 2.
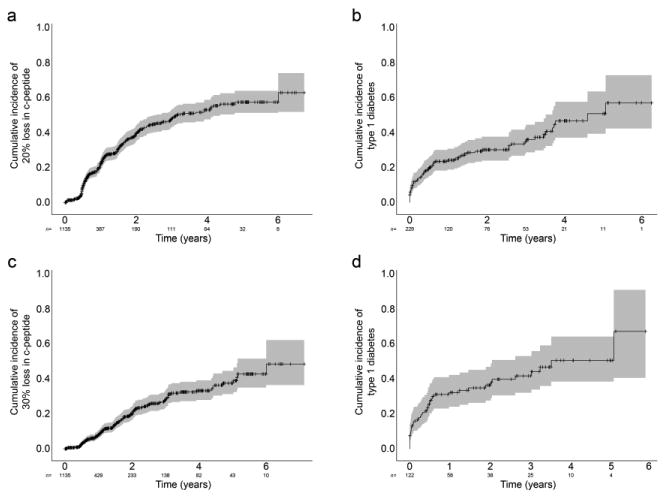
(a) The 2 year incidence of a 20% and (c) 30% decrease in C-peptide from baseline was 39% (standard error 2%, grey shading) and 20% (standard error 2%), respectively. (b,d) The risk of type 1 diabetes at 4 years from the time of the respective C-peptide decreases was (b) 47% (standard error 5%) based on data in (a), and (d) 50% (standard error 7%) based on data in (c)
Fig. 3.
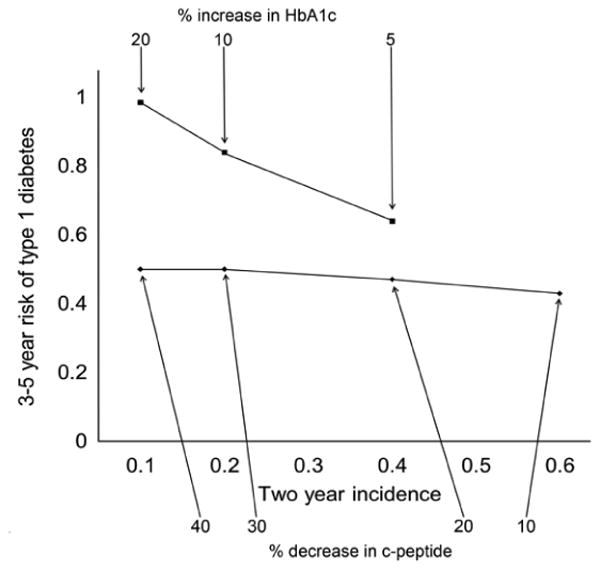
The risk of type 1 diabetes as a function of the magnitude of the change from baseline in C-peptide or HbA1c. A larger increase in HbA1c (which occurred at a lower incidence) was associated with a dramatic rise in type 1 diabetes risk (steeper slope), a development not seen with C-peptide loss (flatter slope)
In terms of the positive predictive value of each intermediate endpoint for subsequent diagnosis of type 1 diabetes, 98% and 96% of those who reached the endpoints of a 10% increase in HbA1c and confirmed dysglycaemia in 2 years were indeed subsequently diagnosed with type 1 diabetes by year 5. Of those who reached the 2 year endpoint of a 20% or 30% decrease in C-peptide, 78% and 76%, respectively, were diagnosed with type 1 diabetes within 5 years.
Discussion
These analyses demonstrate that changes in metabolic markers occurring within 2 years of a baseline visit, at which participants were identified as being multiple autoantibody-positive, are associated with a dramatically increased risk of type 1 diabetes. The implication for type 1 diabetes prevention trial designs is that smaller sample sizes are required to test the efficacy of a diabetes prevention intervention and that trials can be completed in a shorter time. Thus the larger the incidence rate of the proposed intermediate endpoint (not the magnitude of the change itself), the smaller the target sample size needed to observe the same effect size in a clinical trial.
Depending on the intermediate outcome chosen, the sample size required to see a 40% effect with only 2 years of recruitment and 2 years of follow-up from the date the last person is recruited can be as low as 179, or nearly half that of current diabetes prevention trials, with trial completion being concluded in much less time (Table 2). Moreover, trials could be designed to enrol even fewer participants by following those enrolled for a longer period of time. Enrolment rates and pre-specified follow-up periods determine the target sample size. Importantly, the choice of an intermediate endpoint reduces the screening burden required to identify the target population of multiple autoantibody-positive participants to be enrolled in a prevention trial, given that, on average, 50 to 100 individuals need to be screened to identify one with multiple autoantibodies. [7].
Table 2.
Illustrative sample sizes for clinical trials designed to detect a 40% difference in risk for each intermediate endpoint
| Variable | Intermediate endpoint | 2 year incidence (%)a | Recruitment and follow-up, 2 years eachb | Recruitment and follow-up, 1 and 3 years, respectivelyb |
|---|---|---|---|---|
| HbA1c | ≥10% increase | 20 | 400 | 348 |
| AUC C-peptide | ≥20% decrease | 39 | 189 | 168 |
| AUC C-peptide | ≥30% decrease | 20 | 400 | 348 |
| OGTT | Dysglycaemia | 41 | 179 | 159 |
Data was computed using PASS 11 (NCSS, Kaysville, UT, USA [www.ncss.com])
Standard error 2% for each
In participant numbers
The advantage of an intermediate marker is that it occurs relatively quickly and is associated with a significant increase in the incidence of type 1 diabetes, hence the focus on 2 year endpoint incidence and type 1 diabetes risk at 3 to 5 years. The use of intermediate endpoints in type 1 diabetes prevention trials is only helpful if the endpoint has clinical validity as an accepted marker of disease progression. The intermediate endpoints suggested in this analysis do not meet the high standard of a surrogate endpoint [10]; that is, these intermediate endpoints do not have sufficiently high sensitivity and specificity to replace a diagnosis of diabetes as endpoint. Nonetheless, they do enable assessment of the ability of an experimental intervention to limit progression from one diabetes risk level to a higher risk level. Contrary to the thrust of discussion in the literature, the intermediate endpoints used here do have a biological significance based on our understanding of the pathogenesis of type 1 diabetes, and it is this that makes them appealing in this setting. Furthermore, while they cannot replace definitive phase 3 trials, they can serve as acceptable endpoints for randomised phase 2 trials designed to permit early indication of treatment efficacy. Indeed, a randomised phase 2 study using the proposed intermediate endpoints is conceivable, in which, as in a phase 3 design, the participants would be followed until diagnosed with type 1 diabetes, if the phase 2 stage suggested that the intervention was effective. The follow-up period could be extended to compensate somewhat for the lower statistical power due to the smaller sample size. This could be an acceptable strategy, as power would be less of a concern if the phase 2 portion of the study were positive.
This approach leads naturally to the identification of subpopulations at different risk levels, within which there may be alternative intervention opportunities with alternative endpoints (e.g. the reversal of dysglycaemia, progression of dysglycaemia to diabetes and even the prevention of multiple diabetes-related antibodies in a population in which only one antibody has been found to be positive). Thus the future of diabetes prevention strategies may be built round the recognition that individuals transition from one immunological or metabolic state to another as they progress towards loss of the ability to produce sufficient insulin for normal glycaemic control. As a result, intervention strategies could be geared to reducing the probability of transitioning from one state to another, leading to the consideration of new treatment strategies and alternative endpoints that can be addressed relatively quickly compared with current strategies focused on the prevention of type 1 diabetes as endpoint.
The analysis presented is not without limitations. Although the TrialNet population is very large and was studied in a systematic way, with central processing of samples and careful attention to quality control, the follow-up is relatively short and the number of participants followed for up to 4 years is still small in some cases. Hence, the 3 and 4 year estimates of diabetes risk may be subject to some variability. The strength of our analysis is that all of the intermediate endpoints discussed are associated with diabetes pathogenesis and have already demonstrated increased risk.
Furthermore, while the observed rates of progression in the TrialNet study may differ by age of the participant, genetic predisposition (i.e. HLA), the titres of specific autoantibodies present (as opposed to the number of autoantibodies) or other demographic characteristics (e.g. family relationship to the proband with diabetes), these variables can nevertheless be taken into account in a prior or subsequent stratification. Their relative frequency in a proposed study may alter somewhat the exact target numbers of a planned trial.
This paper contends that the intermediate endpoints used describe the progression from normal to type 1 diabetes and that the transition from one level of risk to another enables the selection of specific targeted interventions and subpopulations in prevention trial design. The 10%, 20% and 30% changes in HbA1c and/or C-peptide, and even the definition of dysglycaemia, are all rather arbitrary and future research should determine whether other thresholds are better for the purpose of trial design. Inspection of the cumulative incidence curves and the trade-off between marker change and diabetes risk (Fig. 3) would suggest that the relationship is continuous and there is no special threshold. But, we leave that problem to future studies.
Supplementary Material
Acknowledgments
Jeffrey P. Krischer is the guarantor of this work, had full access to all the data and takes full responsibility for the integrity of data and the accuracy of data analysis.
Funding The sponsor of the Natural History Study is the Type 1 Diabetes TrialNet Study Group. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded: (1) by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Allergy and Infectious Diseases; and (2) by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The above funding was through the cooperative agreements U01 DK061010, U01 DK061016, U01 DK061034, U01 DK061036, U01 DK061040, U01 DK061041, U01 DK061042, U01 DK061055, U01 DK061058, U01 DK084565, U01 DK085453, U01 DK085461, U01 DK085463, U01 DK085466, U01 DK085499, U01 DK085505 and U01 DK085509, and through contract HHSN267200800019C. The National Center for Advancing Translational Sciences also provided funding through Clinical Translational Science Awards UL1 RR024131, UL1 RR024139, UL1 RR024153, UL1 RR024975, UL1 RR024982, UL1 RR025744, UL1 RR025761, UL1 RR025780, UL1 RR029890 and UL1 RR031986, and through the General Clinical Research Center Award M01 RR00400. Other funding came from JDRF and the ADA. The contents of this Article are solely the responsibility of the author and do not necessarily represent the official views of the NIH, JDRF or ADA.
Abbreviations
- ICAs
Islet cell autoantibodies
- ZnT8A
Zinc transporter autoantibodies
Footnotes
Duality of interest JRK is Director of the TrialNet Coordinating Center, which is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH. Otherwise, the authors declare that there is no duality of interest associated with this manuscript.
Contribution statement JPK researched the data, contributed to the discussion, and wrote, reviewed and edited the manuscript.
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:62–69. [Google Scholar]
- 3.Sosenko JM, Palmer JP, Greenbaum CJ, et al. Patterns of metabolic progression to type 1 diabetes in the Diabetes prevention trial–type 1. Diabetes Care. 2006;29:643–649. doi: 10.2337/diacare.29.03.06.dc05-1006. [DOI] [PubMed] [Google Scholar]
- 4.Sosenko JM, Palmer JP, Rafkin-Mervis L, et al. Glucose and C-peptide changes in the perionset period of type 1 diabetes in the Diabetes prevention trial-type 1. Diabetes Care. 2008;31:2188–2192. doi: 10.2337/dc08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TRIGR Investigators. Study design of the trial to reduce IDDM in the genetically at risk (TRIGR) Pediatr Diabetes. 2007;8:117–137. doi: 10.1111/j.1399-5448.2007.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes prevention trial-type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 7.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet natural history study of the development of type 1 diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10:97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 9.Greenwood M. Reports on public health and medical subjects. Vol. 33. Her Majesty's Stationery Office; London: 1926. The natural duration of cancer; pp. 1–26. [Google Scholar]
- 10.Fleming TR. Surrogate endpoints and FDA's accelerated approval process. Health Aff (Millwood) 2005;24:67–78. doi: 10.1377/hlthaff.24.1.67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


