Abstract
Primary varicella-zoster virus (VZV) infection causes varicella (chickenpox), after which VZV becomes latent in ganglionic neurons along the entire neuraxis. A decline in cell-mediated immunity to VZV in elderly and immunocompromised individuals results in zoster (shingles). Within the first year after herpes zoster, there is a 30% increased risk of stroke.1–2 Approximately one-third of patients with VZV vasculopathy do not have zoster rash and diabetic patients are at greater risk for both zoster3 and stroke; therefore, we examined the cerebral arteries of 4 diabetic subjects for the presence of VZV DNA and antigen.
Methods
Cerebral arteries obtained from 4 subjects with diabetes less than 24 hours after they died were analyzed. All subjects had diabetes and no history of zoster infection, transient ischemic attacks, stroke, or immunosuppression. Subject 1 was a 51-year-old woman with hypertension who died of drug overdose; subject 2 was a 60-year-old man with systemic lupus erythematosus who died of asphyxia; subject 3 was a 51-year-old woman who died of myocardial infarction; and subject 4 was a 60-year-old woman with melanoma, hypertension, and hyperlipidemia who died of a drug overdose. An average of 17 pieces of cerebral arteries (about 10 mm/piece) from each subject were analyzed (total of 68). Each piece was cut in half. DNA was extracted from the first half and analyzed by real-time polymerase chain reaction for the presence of VZV DNA using VZV gene 63 specific primers (gene 63-forward 5′-GCTTACGCGCTACTTTAATGGAA-3′ and gene 63-reverse 5′-GCCTCAATGAACCCGTCTTC-3′) and probes (gene 63 5′-TGTCCCATCGACCCCCTCGG-3′).4 Positive controls were provided by amplification of serial dilutions of known quantities of VZV DNA. Negative controls were provided by omission of VZV DNA from the polymerase chain reaction, which was performed 3 times. The other half was formalin-fixed and paraffin-embedded. If VZV DNA was detected, immunohistochemical analysis was performed on the remaining formalin-fixed, paraffin-embedded arterial sections for VZV gene 63 protein and leukocyte CD45 antigen. Limited arterial tissue precluded a search for additional antigens. Slides were viewed by Nikon Eclipse E800 microscopy with AxioVision digital imaging software (Carl Zeiss MicroImaging GmbH).
Results
Multiple cerebral arteries from 4 diabetic subjects were analyzed for the presence of VZV DNA. Because 1 of 17 arterial pieces from subject 3 was found to contain 3740 copies of VZV DNA per microgram of total DNA, multiple 6-μm sections were prepared from the corresponding formalin-fixed, paraffin-embedded piece for histological and immunohistochemical analysis.
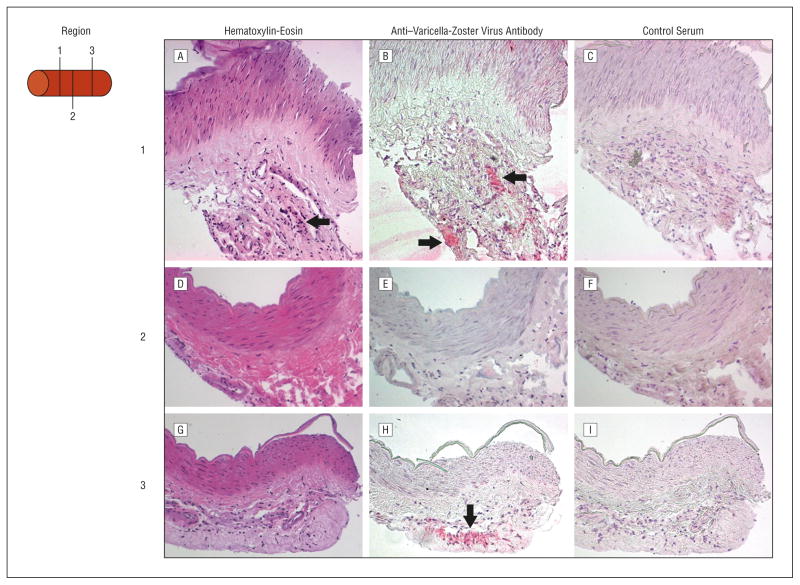
The 1 piece was then analyzed histologically by hematoxylin-eosin staining and for VZV gene 63 protein and CD45 antigen expression. Hematoxylin-eosin staining revealed inflammation in the arterial adventitia (Figure 1A, D, and G), mostly in region 1. Cells containing VZV antigen were seen in noncontiguous regions 1 and 3 separated by 300 μm (Figure 1B and H); the intervening region 2 did not contain VZV antigen (Figure 1E). Staining with control normal rabbit serum was negative in all 3 regions (Figure 1C, F, and I). Leukocytes in region 1 that expressed CD45 antigen (Figure 2B) were associated with cells containing VZV antigen (Figure 2A). Varicella-zoster virus antigen and cells expressing CD45 antigen were also found in region 3 but not in region 2 (Figure 2C and D).
Figure 1.
Histological examination of the varicella-zoster virus (VZV)–positive cerebral artery from neurologically asymptomatic diabetic subject 3. A, A hematoxylin-eosin–stained section of region 1 of VZV DNA–positive artery showed inflammation restricted to the arterial adventitia (arrow). B, Incubation of the adjacent section with rabbit anti-VZV gene 63 antibodies6 revealed VZV antigen in the adventitia of 5 consecutive arterial sections spanning 30 μm (arrows) that was not seen with control normal rabbit serum (C). D, A hematoxylin-eosin–stained section of region 2 approximately 150 μm downstream from region 1 of the VZV DNA–positive artery revealed minimal inflammation and no staining with rabbit anti-VZV IgG antibody (E) or control normal rabbit serum (F). G, A hematoxylin-eosin–stained section of region 3 approximately 150 μm downstream from region 2 again showed inflammation in the adventitia. Region 3 was positive for VZV antigen staining in all 10 sections spanning 60 μm (arrow) but not with control normal rabbit serum (I), indicating that VZV infection in the artery was not contiguous (original magnification x200).
Figure 2.
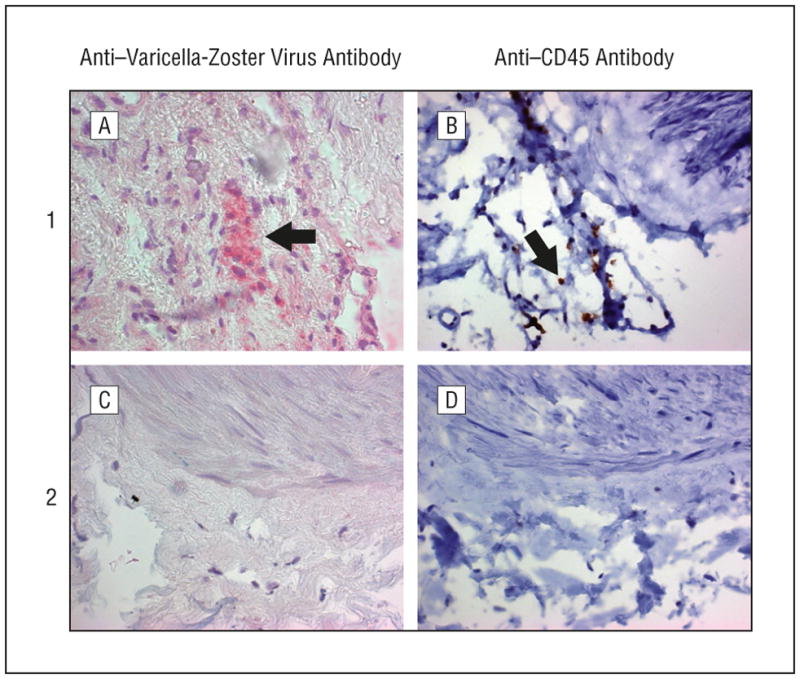
Detection of cells containing varicella-zoster virus and cells expressing CD45 antigen in noncontiguous regions of the arterial adventitia. Arterial regions 1 and 2 after incubation with a 1:400 dilution of mouse monoclonal anti-CD45 antibody (Dako). Region 1 contained varicella-zoster virus antigen (A, arrow) as well as cells expressing CD45 antigen (B, arrow). Region 2 did not contain varicella-zoster virus antigen (C) or cells expressing CD45 antigen (D). Region 3 was positive for varicella-zoster virus antigen and also contained CD45-positive cells (not shown) (original magnification x600).
Comment
Varicella-zoster virus infection of a cerebral artery in the absence of neurologic symptoms related to that artery was first found in the temporal artery of a patient with VZV vasculopathy.5 Extension of asymptomatic VZV infection of a cerebral artery in a diabetic subject raises several issues. First, because strokes are common in people with diabetes and attributed to atherosclerosis, it may be prudent to consider VZV vasculopathy as an additional cause of stroke in people with diabetes, particularly since VZV vasculopathy is treatable with antiviral drugs. Second, VZV antigen was found in noncontiguous arterial regions, underlining the need to examine multiple areas of a single artery to identify virus, just as multiple areas are examined to diagnose giant cell arteritis. Third, the detection of VZV exclusively in arterial adventitia supports earlier observations that VZV vasculopathy begins in the adventitia, most likely after transaxonal spread of virus from ganglionic afferent fibers.5–6 Finally, while more diabetic subjects require study, identification of cells expressing CD45 in proximity to cells containing VZV antigen points to the need for further analysis of the immune repertoire in cerebrovascular remodeling and stroke.
Acknowledgments
Funding/Support: This study was supported by National Institutes of Health grants NS067070 (Dr Nagel), AG032958, and AG006127 (Dr Gilden). Ms Rempel’s work was supported by National Institutes of Health grant 5R25GM083333.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: We thank Marina Hoffman, BA, for editorial assistance and Cathy Allen for manuscript preparation.
Author Contributions: Study concept and design: Nagel and Gilden. Acquisition of data: Nagel, Traktinskiy, Choe, Rempel, and Gilden. Analysis and interpretation of data: Nagel and Gilden. Drafting of the manuscript: Nagel and Gilden. Critical revision of the manuscript for important intellectual content: Nagel, Traktinskiy, Choe, and Rempel. Obtained funding: Nagel and Gilden. Administrative, technical, and material support: Nagel, Traktinskiy, Choe, and Rempel. Study supervision: Nagel and Gilden.
References
- 1.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40(11):3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 2.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74(10):792–797. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 3.Heymann AD, Chodick G, Karpati T, et al. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection. 2008;36(3):226–230. doi: 10.1007/s15010-007-6347-x. [DOI] [PubMed] [Google Scholar]
- 4.Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol. 2007;81(6):2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salazar R, Russman AN, Nagel MA, et al. Varicella zoster virus ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol. 2011;68:517–520. doi: 10.1001/archneurol.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77(4):364–370. doi: 10.1212/WNL.0b013e3182267bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]