Abstract
The primary purpose of this article is to document whether demographic, clinical, regimen-related, intrapersonal, and interpersonal factors predict medication non-adherence for vasculitis patients. A secondary purpose is to explore whether adherence varies by medication type and whether patients experienced drug-related side effects. Vasculitis patients (n=228) completed online baseline and 3-month follow-up surveys. Demographic (age, gender, education, race, marital status, and insurance status), clinical (perceived vasculitis severity, disease duration, vasculitis type, and relapse/remission status), regimen-related (experience of side effects), intrapersonal (depressive symptoms), and interpersonal (adherence-related support from family and friends) factors were measured at baseline. Medication non-adherence was assessed at follow-up using the Vasculitis Self-Management Survey medication adherence sub-scale (α=0.89). Variables that significantly correlated (p<0.05) with non-adherence were included in a linear regression model to predict non-adherence. Younger age (r=−0.23, p<0.001), female sex (r=0.16, p<0.05), experience of side effects (r=0.15, p<0.05), and more depressive symptoms (r=0.22, p< 0.001) were associated with more medication non-adherence, In the regression model, younger age (β=−0.01, p=0.01) and more depressive symptoms (β=0.01 p=0.02) predicted worse adherence. For six out of eight vasculitis medication types, patients who experienced side effects were less adherent than patients who did not experience side effects. Multiple factors are associated with medication non-adherence for vasculitis patients. Providers should discuss medication adherence and drug-related side effects with vasculitis patients. Providers may want to particularly target younger patients and patients with clinical signs of depression.
Keywords: Depression, Medication adherence, Side effects, Social support, Vasculitis
Introduction
Because medication non-adherence has been linked with negative clinical sequelae across a broad range of chronic diseases [1], researchers have attempted to document what factors predict non-adherence. Overall, the results lend support for a social ecological view of adherence [2], in which patients’ medication-taking behaviors are nested within several spheres of influence, which include but are not limited to: (a) clinical characteristics of the disease, (b) aspects of the regimen, (c) intrapersonal characteristics and demographic variables [3], and (d) interpersonal influences such as social support and quality of the patient-provider relationship.
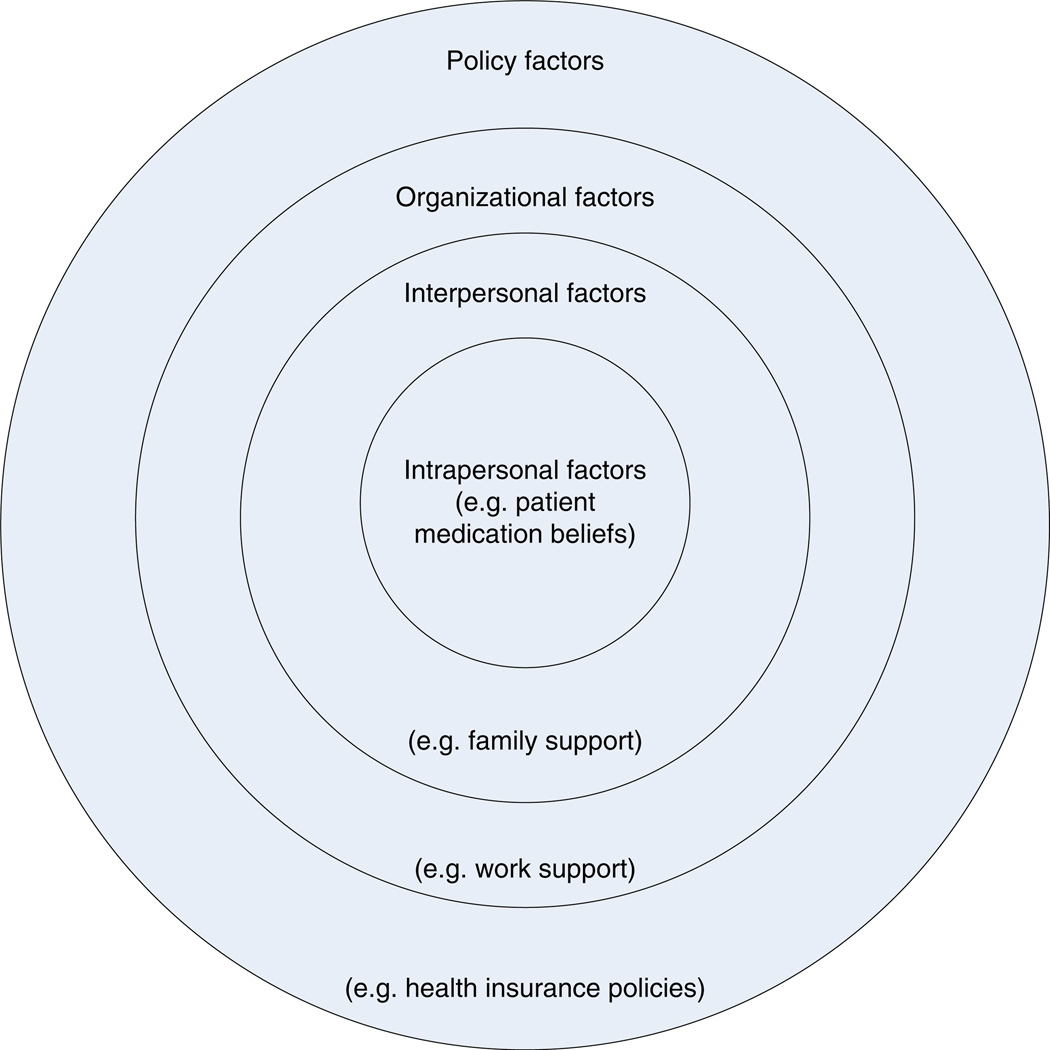
Social ecological models use concentric circles to depict the multiple levels of influence that affect patient self-management behaviors such as medication adherence (Fig. 1) [4, 5]. Patient-level factors, such as knowledge and medication beliefs, are included in the innermost circle. Interpersonal influences are typically represented by the next circle. Moving outward, each additional circle represents more macro-level influences, with the circle most distant to the patient often representing policy level influences such as health insurance policies. Ecological models provide a logical way to organize the many factors that affect patient medication adherence.
Fig. 1.
Ecological model of medication adherence. Figure adapted from Clark 2003 [4]
Relatively few studies have examined medication adherence for chronic inflammatory rheumatic diseases [6–12], Only two studies, the Vasculitis Self-Management (VSM) study and the Accessing Social Support in Symptom Treatment (ASSIST) study, have focused specifically on vasculitis [13–15]. The VSM study used a cross-sectional study design to examine barriers to performing various self-management behaviors, including medication adherence, in a sample (n=202) of patients with small vessel vasculitis [e.g., Granulomatosis with polyangiitis (GPA, formerly known as Wegener’s), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA, formerly Churg-Strauss)]. Five barriers were associated with worse medication adherence. The first two barriers were disruptions to the patient’s daily routine and forgetting to take medication. The other three barriers were related to the complexity of the medication regimen and included: (1) a large number of medications, (2) difficult medication instructions, and (3) a complicated dosing schedule. The second vasculitis-specific study, ASSIST, used a longitudinal study design to examine whether social support and conflicting medication information from different sources (e.g., physicians and the Internet) was associated with worse medication adherence. Data were collected in 2008, and the ASSIST sample (n=228) included patients with any form of vasculitis. Carpenter and colleagues [15] documented that vasculitis patients who received conflicting information about their medications from different sources at baseline were less adherent at 3-month follow-up. The study did not collect data about which sources provided patients with conflicting information; thus, it is unknown whether professional sources, such as two physicians, or informal sources, such as the Internet or other vasculitis patients, provided patients with conflicting medication information. Regarding support, Carpenter and colleagues demonstrated that physician support increased vasculitis patients’ adherence self-efficacy, and consequently predicted better medication adherence [15]. The positive relationship between physician support, self-efficacy, and medication adherence was confirmed by Pepper and associates using an alternative measure of medication adherence [14]. Pepper et al. also found that adherence-related support from vasculitis patients’ partners was significantly associated with greater adherence; however, the relationship between partner support and adherence became insignificant after adjusting for various demographic and clinical factors. Taken together, the results from VSM and ASSIST suggest that both regimen-related (i.e., treatment complexity) and interpersonal (i.e., social support) factors are related to medication adherence for vasculitis patients; yet, no study has taken a more holistic approach to examining the medication adherence of vasculitis patients.
Studies with other rheumatic conditions, including gout, lupus, and rheumatoid arthritis (RA), offer some guidance about additional factors that may affect vasculitis medication adherence. In a review of RA patient adherence to disease-modifying anti-rheumatic drugs, Salt and colleagues concluded that age, patient self-efficacy, patient beliefs about medications, and interpersonal factors, including patient–provider relationship and social support, influence medication adherence [7]. Additionally, experience of side effects [12, 16, 17], non-White race [17, 18], fewer years of education [17], taking fewer medications [8], fewer comorbid conditions [19, 20], medication type [6, 20], and fewer visits to a health care provider [12, 20] have been associated with worse adherence.
The objective of this study is to provide a more comprehensive understanding of predictors of medication adherence for vasculitis patients. A broad range of factors, including demographic, clinical, regimen-related, intrapersonal, and interpersonal influences, are examined in relation to patient medication adherence at 3-month follow-up. Specific factors were chosen based on their association with medication adherence in previous empirical studies and represent multiple levels (intrapersonal, interpersonal) of the social ecological framework. Additionally, we examine whether medication adherence varies as a function of medication type and experience of medication side effects.
Materials and methods
Overview
All data were collected as part of the ASSIST Study, which was a longitudinal, observational study that evaluated medication management issues for vasculitis patients. The ASSIST Study consisted of two on-line questionnaires administered 3 months apart. Because vasculitis patients’ medication regimens frequently change due to medication intolerance or ineffectiveness [21], we limited our follow-up period to 3 months in order to minimize the number of potential medication regimen changes. Eligible patients had a self-reported diagnosis of vasculitis, were at least 18 years of age, were able to read and write in English, had Internet access, and were taking at least one medication to treat their vasculitis. This Institutional Review Board at the University of North Carolina at Chapel Hill approved this study.
Sample
A total of 253 patients were eligible. Of these, we recruited 106 participants for the ASSIST Study by distributing study information to attendees at a vasculitis patient conference (n=39) and mailing physician-diagnosed vasculitis patients who were part of the Glomerular Disease Collaborative Network (n=38) and prior vasculitis studies (n=29). We also contacted members of vasculitis support groups and posted announcements on vasculitis websites, in patient newsletters, and on patient list serves, which yielded an additional 147 eligible and interested participants. Participants recruited through the vasculitis conference, support groups, newsletters, and list serves had a self-reported vasculitis diagnosis. Carpenter and colleagues [15] provide a more thorough description of recruitment procedures.
Of the 253 eligible patients, 232 (91.7 %) completed the 1-h baseline questionnaire. Reasons for non-completion included never responding to study correspondence (n=7), technical issues (n=7), or being too sick (n=4) or too busy (n=3) to participate. When compared with completers, non-completers were not significantly different in terms of gender or self-reported vasculitis type.
Only 4 of the 232 participants were lost to follow-up, resulting in a retention rate of 98.2 %. Participants who completed both questionnaires received a $10 gift card.
Measures
The baseline survey contained measures of demographic, regimen-related, clinical, intrapersonal and interpersonal variables. The 3-month follow-up questionnaire contained the medication non-adherence measure.
Demographic characteristics
Participants answered one item each about gender, race (recoded as white vs. nonwhite), age, education (in years), health insurance status (insured vs. not insured), and marital status (recoded as married vs. unmarried).
Regimen factors
Patients indicated whether they were currently taking any of the following eight medications: cyclophosphamide (Cytoxan), steroids (Prednisone, Methylprednisolone), co-trimoxazole (Bactrim), azathioprine (Imuran), cyclosprin, methotrexate (Trexall, Rheumatrex), mycophenolate mofeitil (Cellcept), and rituximab (Rituxan). For each medication, patients indicated whether they had experienced any side effects related to that medication (yes/no). A total side effects score was calculated by summing the number of yes responses; responses could range from 0 to 8.
Clinical factors
Patients responded to four clinical questions: (1) vasculitis type [recoded as granulomatosis with polyangiitis (GPA) vs. other], (2) disease status (recoded as currently experiencing a flare/relapse or not currently experiencing a flare/relapse), (3) disease duration or how long they had been diagnosed with vasculitis, and (4) perceived disease severity, which was measured on a scale from 1=“not at all severe” to 10=“extremely severe.”
Intrapersonal factors
Depressive symptoms were measured with the 20-item Center for Epidemiologic Studies Depression Scale (CES-D) [22]. Relevant items were reverse-scored so that higher overall scale scores represent higher levels of depressive symptomatology. Scale scores can range from 0 to 60, with a total score of 16 or higher indicative of clinically significant depressive symptoms [8]. We treated depressive symptoms as a continuous variable. Cronbach’s alpha was 0.92 for the current study.
Interpersonal factors
Participants completed one item about perceived medication-related adherence support from their family and their friends. Specifically, participants indicated how often their family and friends provided support for taking their vasculitis medications as prescribed. Responses ranged from 1=“does not do this” to 4=“does this a lot.” Responses of “not applicable” were recoded as 1 to indicate that family or friends did not provide that type of support. Higher scores reflected greater perceived adherence support.
Medication non-adherence
The Vasculitis Self-Management Survey (VSMS) medication adherence scale, which was developed specifically for use with vasculitis populations, was used to measure non-adherence [23]. The VSMS asks respondents to describe their medication-taking behavior during the past 4 weeks. The scale consists of seven items measured on a five-point Likert scale; the response scale for six items ranges from 1=“none of the time” to 5=“all of the time,” while the seventh item (percentage of medication doses taken exactly as directed) ranges from 1=“0–24 %” to 5=“100 %.” The VSMS medication adherence scale has demonstrated acceptable internal consistency (Cronbach’s α=0.77) and test-retest reliability of 0.60 in a previous study of vasculitis patients [23, 24]. Higher summary scores indicate greater average non-adherence for all medications the patient was taking to treat their vasculitis. Cronbach’s alpha was 0.89 in this study.
Data analysis
All analyses were conducted using SAS v. 9.2. After calculating descriptive statistics (means, standard deviations, skewness, kurtosis), bivariate Pearson correlations and independent samples t tests, as appropriate, were used to determine whether the relationship between medication non-adherence and demographic, clinical, regimen-related, intrapersonal, and interpersonal factors was significant. We also report the percentage of patients experiencing side effects for each type of medication as well as average medication non-adherence for each type of medication (e.g., mycophenolate mofeitil, steroids). Due to small cell sizes (i.e., low numbers of patients taking particular medications), we present descriptive statistics rather than trying to determine whether there were statistically significant differences in adherence for patients who experienced side effects versus did not experience side effects.
Next, a linear regression model that included all significant (p<0.05) correlates of medication adherence was examined to determine which baseline variables were significant predictors of medication non-adherence at 3-month follow-up. Beta coefficients were considered significant if p<0.05. We used linear regression, rather than logistic regression, because the dependent variable (i.e., medication non-adherence summary score) was continuous and had reasonable skewness and kurtosis values. Missing data were handled with listwise deletion and, for multi-item scales, summary scores were treated as missing if more than 25 % of the scale’s items were missing.
Results
Sample characteristics
Table 1 summarizes the characteristics of the final study sample (n=228). A majority of participants were female (70 %), white (91 %), married or partnered (82 %), and had health insurance (93 %). On average, participants were middle-aged and reported some college education. Most patients had a diagnosis of GPA (59 %), but EGPA, microscopic polyangiitis, and Takayasu arteritis patients were also represented in the sample. Patients had been living with vasculitis for an average of 6.4 years. Regarding relapse/-flare status, 28 % of patients were experiencing a relapse or flare at the time of the baseline survey, and 13 % reported never experiencing a relapse or flare. An additional 29 % of patients had experienced a relapse or flare within 1 year of taking the survey, while more than a year had passed since experiencing a relapse for the remaining 29 % of patients. Patients perceived their vasculitis as moderately severe and viewed family and friends as moderately supportive. The average CES-D score was over 16.0, indicating depressive symptomology with 55 % (n=126) having a score of 16 or greater. Overall, patients reported low levels of medication non-adherence. Patient responses to individual medication non-adherence scale items are presented in Table 2.
Table 1.
Participant characteristics (n=228)
| Characteristic | Mean (SD) or % | Range | Skewness | Kurtosis |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age | 51.0 (13.3) | 21.0–82.0 | −0.1 | −0.7 |
| Male | 30.3 % | |||
| White | 91.3 % | |||
| Education (in years) | 15.6 (2.8) | 4.0–22.0 | −0.2 | 1.7 |
| Married or partnered | 81.6 % | |||
| Have health insurance | 93.4 % | |||
| Clinical | ||||
| Self-reported vasculitis type | ||||
| GPA (formerly Wegener’s) | 59.2 % | |||
| EGPA (formerly Churg-Strauss) | 12.7 % | |||
| Microscopic polyangiitis (MPA) | 7.9 % | |||
| Takayasu arteritis (TA) | 4.8 % | |||
| Other | 15.4 % | |||
| Years with vasculitis | 6.4 (6.2) | 0.5–36.5 | −2.0 | 5.2 |
| Currently experiencing flare/relapse | 28.4 % | |||
| Perceived vasculitis severity | 4.5 (2.3) | 1.0–10.0 | 0.1 | −0.6 |
| Regimen-related | ||||
| Number of medication side effects | 1.5 (1.1) | 0.0–6.0 | 0.9 | 1.7 |
| Perceived regimen complexity | 3.0 (2.4) | 1.0–10.0 | 1.2 | 0.8 |
| Intrapersonal | ||||
| Depressive symptoms | 16.8 (12.2) | 0.0–51.6 | 0.7 | −0.4 |
| Interpersonal | ||||
| Family adherence-related supporta (n=186) | 2.1 (1.1) | 10–4.0 | 0.3 | −1.3 |
| Friend adherence-related supporta (n=173) | 2.1 (1.2) | 10–4.0 | 0.5 | −1.3 |
| Outcome | ||||
| Medication non-adherenceb | 1.7 (0.7) | 10–44 | 1.2 | 1.6 |
Possible score range=1 to 4, higher scores reflect greater medication-related support
Possible score range=1 to 5, higher scores reflect greater non-adherence
Table 2.
Descriptive statistics for individual medication non-adherence scale items
| Item | Mean (SD) |
|---|---|
| I skipped a dose of my medicine. | 1.49 (0.7) |
| I did not follow specific instructions for taking my medicines (for example, taking medicine with meals, drinking a certain amount of water, timing of doses). |
1.55 (0.9) |
| I could have done a better job following my health professionals’ recommendations for taking my medicine. | 1.62 (1.0) |
| During the past 4 weeks, how often did you FAIL to take all of your recommended medicine(s) EXACTLY as directed? | 1.67 (0.8) |
| During the past 4 weeks, how often did you find it easy to take your medicine(s) exactly as directed?a | 4.32 (0.8) |
| During the past 4 weeks, how often did you have a hard time taking your medicine(s) exactly as directed? | 1.60 (0.9) |
| During the past 4 weeks, what percentage of your recommended medicine(s) did you take exactly as directed?a,b | 3.96 (1.2) |
Responses range from 1=none of the time to 5=all of the time
Item was reverse-scored
Responses range from 1=0–24 % to 5=100 %
With regard to medications, patients most commonly took steroids (n=173; 75.9 %), co-trimoxazole (n=83; 36.4 %), azathioprine (n=74; 32.5 %), and cyclophosphamide (n=63; 27.6 %). An overwhelming majority of patients (97.7 %) who were taking steroids reported experiencing side effects. Similarly, 79.4 % of patients reported side effects from cyclophosphamide. Approximately half of patients (47.3 %) reported azathioprine-related side effects, and only 25.3 % of patients reported side effects due to co-trimoxazole (Table 3).
Table 3.
Medication non-adherence mean scores for vasculitis patients who did and did not experience side effects by medication type
| Total sample |
Did not experience side effects |
Experienced side effects |
||||
|---|---|---|---|---|---|---|
| Medication type | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) |
| Steroids | 173 | 1.67 (0.7) | 4 | 1.29 (0.6) | 169 | 1.68 (0.7) |
| Azathioprine | 74 | 1.65 (0.7) | 39 | 1.51 (0.7) | 35 | 1.80 (0.6) |
| Methotrexate | 58 | 1.68 (0.7) | 26 | 1.58 (0.7) | 32 | 1.78 (0.7) |
| Rituximab | 27 | 1.68 (0.7) | 13 | 1.65 (0.7) | 14 | 1.70 (0.8) |
| Mycophenolate mofetil | 42 | 1.74 (0.7) | 20 | 1.66 (0.9) | 22 | 1.81 (0.6) |
| Cyclophosphamide | 63 | 1.77 (0.7) | 13 | 1.82 (0.7) | 50 | 1.75 (0.7) |
| Cyclosporin | 11 | 1.94 (0.6) | 7 | 1.88 (0.7) | 4 | 2.04 (0.3) |
| Co-trimoxazole | 83 | 1.88 (0.8) | 62 | 1.88 (0.8) | 21 | 1.88 (0.8) |
Table 3 descriptively presents the mean medication non-adherence scores by type of medication. On average, patients were least adherent to co-trimoxazole and cyclosprin and were most adherent to steroids and azathioprine. With the exception of co-trimoxazole and cyclophosphamide, patients who experienced drug-related side effects reported greater non-adherence, on average, than patients who did not experience drug-related side effects.
Predictors of medication non-adherence
Younger age (r=−0.23, p<0.001), female gender (t(225)=−2.50, p=0.01), experiencing more drug-related side effects (r=0.15,p=.02), and more depressive symptomatology (r=0.22, p<0.001) were significantly associated with greater non-adherence at 3-month follow-up. The relationship between greater perceived vasculitis severity and adherence trended toward significance (r=0.12,p=0.07). Support variables and clinical characteristics were not significantly associated with adherence.
In a linear regression model (Table 4) that included the significant correlates of non-adherence described above (i.e., age, gender, side effects, depressive symptoms), only age and depressive symptoms were significantly associated with medication non-adherence. Specifically, younger patients and patients with more depressive symptoms were more non-adherent.
Table 4.
Beta coefficients and p values for linear regression predicting medication non-adherence for vasculitis patient (n=227)
| Variable | B(SE) | p value |
|---|---|---|
| Age | −0.01 (0.00) | 0.01 |
| Gender | 0.14 (0.10) | 0.16 |
| Experienced drug-related side effects | 0.03 (0.04) | 0.44 |
| Depressive symptomatology | 0.01 (0.00) | 0.02 |
Discussion
Adherence to vasculitis medications is complex and associated with a variety of factors, including demographic, regimen-related, and intrapersonal factors. Overall, vasculitis patients reported a high level of medication adherence. But even among this highly adherent sample, patients who were younger and had more depressive symptoms reported worse adherence to therapy at 3-month follow-up. Given that previous research has found that perceived regimen complexity and interpersonal factors, such as adherence-related support from physicians, predict medication adherence for vasculitis patients, attempts to improve patient adherence should take a multifaceted ecological approach [13–15] by: (1) addressing patients’ medication beliefs and behaviors, (2) enlisting partner support, and (3) improving patient–provider communication about medications and medication side effects during medical visits.
Previous research with patients who have diseases such as RA and gout have yielded mixed results regarding the effects of age on medication adherence [7, 10]. To be specific, some studies have found that older patients are more adherent than younger patients [9, 19, 20], while others found no significant effects for age [10, 18]. In our sample, which had a mean age of 51, younger patients were less adherent to their medications than older patients. There are several possible explanations for this finding. First, younger patients have less experience managing vasculitis medication regimens. Because adherence may improve as patients develop and refine their medication management routines, younger patients may be more at risk of non-adherence. Briesacher and associates found that a history of medication use did not influence adherence for gout patients, so greater experience with taking medications may not be the most likely explanation for the relationship between older age and better adherence [19]. Alternatively, younger patients may have busier lifestyles that interfere with medication-taking regimens; Park and colleagues partially attributed worse adherence with younger RA patients to busy lifestyles [9]. Regardless of the potential mediating mechanisms between age and adherence, providers should discuss medication-related issues with younger patients to assess whether they are having difficulties managing their medications.
Interestingly, clinical characteristics were not significantly correlated with adherence. Vasculitis patients are probably aware that their medications are critical to survival; hence; they may have been adherent to their medications regardless of perceived disease severity or relapse/remission status. For example, patients in remission may be just as motivated as relapsing patients to take their medications. In the first case, patients in remission take maintenance medications to avoid future relapse, whereas, in the second case, relapsing patients take medications to induce remission. Either way, medications are generally associated with positive outcomes (avoiding relapse or inducing remission). It is possible that more objective measures of disease severity, such as disease activity measures (e.g., Birmingham vasculitis activity score [25]), may have been significantly associated with patient adherence. Because objective and subjective measures of disease severity have been associated with patient adherence in a large meta-analysis [26], both should be assessed during medical visits. Additionally, future adherence studies should measure both objective and patient-reported disease severity.
Over 97 % of patients who took steroids reported experiencing drug-related side effects. Experience of drug-related side effects on the initial survey was significantly associated with worse adherence at 3-month follow-up; however, this relationship did not remain significant when adjusting for other factors. Side effects have been associated with worse adherence in previous studies [2, 12, 16, 17, 27]; thus, providers should assess patients’ experiences with medication side effects. We observed that patients who experienced side effects were less adherent, on average, to six of eight vasculitis medication types than patients who did not experience side effects. These findings suggest that providers should assist patients with managing drug-related side effects since this may ultimately improve adherence. Additionally, future research should obtain patient ratings for side effect severity as this variable may be more strongly related to adherence than whether any side effect was experienced.
In the multivariable regression model, baseline depressive symptoms predicted worse adherence at 3-month follow-up. On average, patients in our sample reported a clinically significant level of depressive symptoms [22], which has been documented in other samples of vasculitis patients [28–30]. Given that depression also has been strongly associated with non-adherence in the past [3], we believe that providers should evaluate patients for depressive symptoms and specifically discuss adherence-related issues with vasculitis patients who show clinical signs of depression.
Although patients received moderate amounts of adherence-related support from their family and friends, support was not significantly associated with medication adherence at 3-month follow-up. This finding contradicts previous studies that have found that regimen-related support from friends and family is associated with better adherence [31]. We have previously documented that adherence-related support from physicians has been associated with better medication adherence [14, 15]; hence, vasculitis patients may prefer to receive medication-related support from knowledgeable health professionals rather than less knowledgeable friends and family [32]. Because many vasculitis patients believe that their friends and family do not understand their illness, patients may be more likely to rely on these sources for emotional and companionship support and turn to health providers for disease-specific support [33].
This study possesses several limitations. First, we used a self-report measure of medication adherence. Although the measure had been validated previously with vasculitis patients, it is likely that patients overestimated their medication adherence [34]. This overestimation may have biased our results toward the null; thus, we may not have been able to detect all significant predictors of medication non-adherence. Additionally, patients reported overall adherence for all medications they were taking to treat their vasculitis; hence, we were unable to look at medication-specific adherence. The use of a one-item measure of social support is also a limitation. Future studies should use more comprehensive, validated measures of social support. Second, generalizability was limited by our sample’s lack of diversity, which was predominantly older, female, and white. Because the study surveys were completed online, our sample may have been more motivated, better educated, and had greater resources than the general vasculitis population; thus, our sample may have been more adherent than the general population. Moreover, because some study participants were recruited at patient conferences and from pools of patients who completed other vasculitis studies, it is likely that our sample was more motivated and adherent than the general population of vasculitis patients. Although our sample was highly adherent, it may be impossible to recruit samples that include large numbers of non-adherent patients because most vasculitis patients are probably aware that medication non-adherence is associated with major morbidity and mortality. Third, self-reported diagnoses of vasculitis may not be completely accurate; no studies have established the validity of self-reported vasculitis diagnosis. For our analyses, we grouped multiple types of vasculitis patients together (e.g., Takayasu’s arteritis, EGPA, and MPA) in order to compare them to those with GPA; thus, we cannot be sure if there were between-group differences in adherence. Fourth, we did not control for the number of vasculitis medications that patients were taking. Future studies should account for whether patients were taking multiple medications.
This study offers an overview of factors that may influence medication adherence for vasculitis patients. Future studies should build upon these results by: (1) recruiting more diverse samples of patients, (2) using more objective measures of medication adherence like pharmacy refill records, and (3) including a broader array of predictors such as severity of medication side effects. Providers should be aware that non-adherence may be an issue for their vasculitis patients and should pay particular attention to younger patients who may have difficulty incorporating a medication regimen into their lifestyles. Because gender, side effects, and depressive symptoms were also associated with adherence, providers should attempt to engage their vasculitis patients in discussions about adherence-related issues.
Acknowledgments
We would like to thank the Vasculitis Foundation, its support group leaders, Vasculitis Foundation Canada, Wegener’s Granulomatosis Support Group Of Australia Inc, the Glomerular Disease Collaborative Network, the UNC Kidney Center (especially Ronald J. Falk, Kristen Hendrickson, and Caroline E. Jennette), and Jim Bornac for their help with recruitment. This work was supported by the Renal Epidemiology Predoctoral Traineeship at the UNC Kidney Center (T32DK007750), the Thurston Arthritis Research Center Postdoctoral Fellowship (5T32-AR007416), and the ACR REF/Abbott Health Professional Graduate Medical Student Research Preceptorship. The project described also was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000084. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures None.
Contributor Information
Delesha M. Carpenter, Division of Pharmaceutical Outcomes and Policy, CB#7573, Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC 27599, USA, dmcarpenter@unc.edu
Susan L. Hogan, UNC Kidney Center, CB#7155, School of Medicine, University of North Carolina, Chapel Hill, NC 27599, USA
Robert F. DeVellis, Thurston Arthritis Research Center, CB#7280, School of Medicine, University of North Carolina, Chapel Hill, NC 27599, USA
References
- 1.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a metaanalysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Haynes RB, Sackett DL. Compliance with therapeutic regimens. Baltimore: Johns Hopkins University Press; 1976. A critical review of “determinants” of patient compliance with therapeutic regimens; pp. 26–39. [Google Scholar]
- 3.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: metaanalysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 4.Clark NM. Management of chronic disease by patients. Annual Rev Public Health. 2003;24(1):289–313. doi: 10.1146/annurev.publhealth.24.100901.141021. [DOI] [PubMed] [Google Scholar]
- 5.Fisher EB, Brownson CA, O’Toole ML, Shetty G, Anwuri VV, Glasgow RE. Ecological approaches to self-management: the case of diabetes. Am J Public Health. 2005;95(9):1523–1535. doi: 10.2105/AJPH.2005.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrold LR, Andrade SE. Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Semin Arthritis Rheum. 2009;38(5):396–402. doi: 10.1016/j.semarthrit.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salt E, Frazier S. Adherence to disease modifying antirheumatic drugs in rheumatoid arthritis patients: a narrative review of the literature. Orthop Nurs. 2010;29(4):260–275. doi: 10.1097/NOR.0b013e3181e5c2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treharne GJ, Lyons AC, Kitas GD. Medication adherence in rheumatoid arthritis: effects of psychosocial factors. Psychol Health Med. 2004;9(3):337–349. [Google Scholar]
- 9.Park DC, Hertzog C, Leventhal H, Morrell RW, Leventhal E, Birchmore D. Medication adherence in the elderly. J Am Geriatr Soc. 1999;47:172–183. doi: 10.1111/j.1532-5415.1999.tb04575.x. [DOI] [PubMed] [Google Scholar]
- 10.Bradley LA. Adherence with treatment regimens among adult rheumatoid arthritis patients: current status and future directions. Arthritis Rheum. 1989;2(3):S33–S39. doi: 10.1002/anr.1790020312. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen LE, Saxne T, Nilsson J, Geborek P. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res Ther. 2006;8:R174. doi: 10.1186/ar2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller R, Kallikorm R, Põlluste K, Lember M. Compliance with treatment of rheumatoid arthritis. Rheumatol Int. 2012;32(10):3131–3135. doi: 10.1007/s00296-011-2162-x. [DOI] [PubMed] [Google Scholar]
- 13.Thorpe CT, DeVellis RF, Blalock SJ, Hogan SL, Lewis MA, DeVellis BM. Patient perceptions about illness self-management in ANCA-associated small vessel vasculitis. Rheumatology. 2008;47(6):881–886. doi: 10.1093/rheumatology/ken126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pepper J, Carpenter D, DeVellis R. Does adherence-related support from physicians and partners predict medication adherence for vasculitis patients? J Behav Med. 2012;35:115–123. doi: 10.1007/s10865-012-9405-5. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter DM, DeVellis RF, Hogan SL, Fisher EB, DeVellis BM, Jordan JM. The effect of conflicting medication information and physician support on medication adherence for chronically ill patients. Patient Educ Couns. 2010;81(2):169–176. doi: 10.1016/j.pec.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popa-Lisseanu MG, Greisinger A, Richardson M, O’Malley KJ, Janssen NM, Marcus DM, et al. Determinants of treatment adherence in ethnically diverse, economically disadvantaged patients with rheumatic disease. J Rheumatol. 2005;32:913–919. [PubMed] [Google Scholar]
- 17.Garcia-Gonzalez A, Richardson M, Garcia Popa-Lisseanu M, Cox V, Kallen M, Janssen N, et al. Treatment adherence in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol. 2008;27(7):883–889. doi: 10.1007/s10067-007-0816-6. [DOI] [PubMed] [Google Scholar]
- 18.Salt E, Peden A. The complexity of the treatment: the decision-making process among women with rheumatoid arthritis. Qual Health Res. 2011;21(2):214–222. doi: 10.1177/1049732310381086. [DOI] [PubMed] [Google Scholar]
- 19.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–443. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrold LR, Andrade SE, Briesacher BA, Raebal MA, Fouayzi H, Yood RA, et al. Adherence with urate-lowering therapies of the treatment of gout. Arthritis Res Ther. 2009;11(2):R46. doi: 10.1186/ar2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayne D. How to induce remission in primary systemic vasculitis. Best Practice & Res Clin Rheumatol. 2005;19(2):293–305. doi: 10.1016/j.berh.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23.Thorpe CT, Devellis RF, Lewis MA, Blalock SJ, Hogan SL, Devellis BM. Development and initial evaluation of a measure of self-management for adults with antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Arthritis Care Res. 2007;57(7):1296–1302. doi: 10.1002/art.23017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorpe C. Illness self-management among adults living with ANCA small vessel vasculitis. University of North Carolina at Chapel Hill; 2006. Dissertation. [Google Scholar]
- 25.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, et al. Birmingham Vasculitis Activity Score (BVAS) Dim system necrotizinig vasculitis. QJM. 1994;87(11):671–678. [PubMed] [Google Scholar]
- 26.DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence. Med Care. 2007;45(6):521–528. doi: 10.1097/MLR.0b013e318032937e. [DOI] [PubMed] [Google Scholar]
- 27.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clinical Epidemiol. 2001;54(12):S57–S60. doi: 10.1016/s0895-4356(01)00457-7. [DOI] [PubMed] [Google Scholar]
- 28.Hajj-Ali RA, Wilke WS, Calabrese LH, Hoffman GS, Liu X, Bena J, et al. Pilot study to assess the frequency of fibromyalgia, depression, and sleep disorders in patients with granulomatosis with polyangiitis (Wegener’s) Arthritis Care Res. 2011;63(6):827–833. doi: 10.1002/acr.20442. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman GS, Drucker Y, Cotch MF, Locker GA, Easley K, Kwoh K. Wegener’s granulomatosis: patient-reported effects of disease on health, function, and income. Arthritis Rheum. 1998;41(12):2257–2262. doi: 10.1002/1529-0131(199812)41:12<2257::AID-ART22>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 30.Boomsma MM, Bijl M, Stegeman CA, Kallenberg CG, Hoffman GS, Tervaert JW. Patients’ perceptions of the effects of systemic lupus erythmatosus on health, function, income, and interpersonal relationships: a comparison with Wegener’s granulomatosis. Arthritis Rheum. 2002;47(2):196–201. doi: 10.1002/art.10341. [DOI] [PubMed] [Google Scholar]
- 31.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23(2):207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter DM, DeVellis RF, Hogan SL, Fisher EB, DeVellis BM, Jordan JM. Use and perceived credibility of medication information sources for vasculitis patients: differences by gender. J Health Comm. 2011;16(6):629–642. doi: 10.1080/10810730.2011.551995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter DM, Meador AE, Elstad EA, Hogan SL, DeVellis RF. The impact of vasculitis on patient social participation and friendships. Clin Exp Rheumatol. 2012;30:S15–S21. [PMC free article] [PubMed] [Google Scholar]
- 34.Treharne GJ, Lyons AC, Hale ED, Douglas KMJ, Kitas GD. 'Compliance' is futile but is 'concordance' between rheumatology patients and health professionals attainable? Rheumatology. 2006;45(1):1–5. doi: 10.1093/rheumatology/kei223. [DOI] [PubMed] [Google Scholar]