Abstract
OBJECTIVE
The National Lung Screening Trial (NLST) is a multicenter randomized controlled trial comparing low-dose helical CT with chest radiography in the screening of older current and former heavy smokers for early detection of lung cancer. Recruitment was launched in September 2002 and ended in April 2004, when 53,454 participants had been randomized at 33 screening sites. The objective of this study was to determine the effective radiation dose associated with individual chest radiographic screening examinations.
SUBJECTS AND METHODS
A total of 73,733 chest radiographic examinations were performed with 92 chest imaging systems. The entrance skin air kerma (ESAK) of participants’ chest radiographic examinations was estimated and used in this analysis. The effective dose per ESAK for each examination was determined with a Monte Carlo–based program. The examination effective dose was calculated as the product of the examination ESAK and the Monte Carlo estimate of the ratio of effective dose per ESAK.
RESULTS
This study showed that the mean effective dose assessed from 66,157 posteroanterior chest examinations was 0.052 mSv. Additional findings were a median effective dose of 0.038 mSv, a 95th percentile value of 0.136 mSv, and a fifth percentile value of 0.013 mSv.
CONCLUSION
The effective dose for participant NLST chest radiographic examinations was determined and is of specific interest in relation to that associated with the previously published NLST low-dose CT examinations conducted during the trial.
Keywords: chest radiographs, CT, lung cancer screening, Monte Carlo, radiation dose
The National Lung Screening Trial (NLST) is a multicenter randomized controlled trial comparing low-dose helical CT with chest radiography for screening older current and former heavy smokers for early detection of lung cancer [1]. Enrollment began in September 2002 and ended in April 2004, when 53,454 participants had been randomized at 33 sites to either screening low-dose helical CT or screening chest radiography in equal proportions. The NLST is a collaborative effort of the U.S. National Cancer Institute Division of Cancer Prevention, which funds and administers the NLST Lung Screening Study, and the American College of Radiology Imaging Network, which administers the NLST American College of Radiology Imaging Network. The primary endpoint of the NLST is lung cancer mortality [2]. Participants agreed to a baseline imaging procedure and two annual follow-up evaluations. The age range of the participants was 55–74 years at the date of entry to the study. Participants had a significant smoking history of 30 or more pack-years of cigarette smoking, and former smokers must have stopped within the previous 15 years. The trial enrolled 26,722 participants in the low-dose CT screening arm and 26,732 participants in the chest radiographic screening arm. Examination data were obtained from chest radiographic examinations performed with all NLST site imaging systems. The purpose of this study was to assess the effective dose associated with individual NLST chest radiographic examinations. This information is needed to compare the radiation risk of the two screening methods used in the NLST.
Subjects and Methods
During the NLST screening period (2002–2007), a total of 73,733 chest radiographic examinations were performed with NLST chest imaging systems; 66,157 of the examinations were included in this assessment. Each NLST screening center received institutional review board approval before starting recruitment. Eligible participants underwent a consent process with institutional review board–approved informed consent materials. Chest radiographic quality control data were collected annually from 92 imaging systems at NLST screening sites. The Medical Physics Working Group performed equipment quality control monitoring during the trial. Specific details regarding the parameters and methods used to standardize the chest imaging systems are presented in an earlier publication [3]. Annual measurements of source-image distance, radiation output (milliroentgens/milliampere-seconds), and half-value layer (HVL) for the nominal tube potential of the chest radiograph were performed on the radiographic systems used at each of the sites.
Participant Examinations
Participants underwent imaging upright at suspended maximal inspiration with scapulae positioned outside the lung fields if possible. Both lung apices and both costophrenic angles were included in the image. Adequate definition of the vertebral bodies, the left retrocardiac pulmonary vessels, lateral wall of the descending aorta, and left hemidiaphragm was required. Use of the specified technical parameters resulted in an image presenting the lung fields at a midgray level (optical density range, 1.4–1.8) for film-screen systems or with acceptable degrees of noise without overexposure of the participant in the case of computed radiography or digital radiography.
Acquisition parameters necessary to calculate the tube current–exposure time product (milliampere-seconds) were available from 68,836 examinations of the 73,733 chest radiographic examinations performed during the NLST. Two of the total 33 sites were technically unable to access the radiographic machine parameter necessary to calculate tube current–time product and accounted for 4897 examinations. The body heights and weights necessary to calculate the participant body mass index (BMI, weight in kilograms divided by the square of height in meters) were self-reported by participants and available for 24,397 participants of the 24,529 enrolled at 31 sites for which valid tube current–time values were obtained. For some participants, both valid tube current–time product and valid BMI were not available. Consequently, the effective dose was calculated for the techniques from 67,643 chest radiographic examinations. Review of the quality assurance data resulted in elimination of one imaging system from the analysis, and consequently, 1486 participant examinations were additionally eliminated. The final assessment was derived from 66,157 examinations among the 73,733 chest radiographic examinations performed during the NLST.
Technical Parameters
Table 1 shows the protocol specifications and typical technical parameters used in the NLST chest radiography arm of the trial. The technical parameters varied slightly for the specific chest radiographic systems used in the trial. The systems were required to include a rotating anode machine with a tube filtration sufficient to achieve an HVL greater than 3 mm of aluminum at 100 kVp. The recommended nominal focal spot size range was 0.6–1.2 mm and not to exceed 2.0 mm. The systems had a beam-limiting device for rectangular collimation. Automatic processing was required for film-screen systems. Collection of acquisition parameter data on all chest examinations was specified in the NLST protocol. The parameters included site-specific x-ray machine identifier, tube potential (kilovolts), tube current (milliamperes), exposure time, tube current–exposure time product (milliampere-seconds), and detector system (film-screen, photostimulable phosphor [computed radiography], or flat-panel receptors [digital radiography]). During enrollment, heights and weights reported by the participants were recorded.
TABLE 1.
Protocol Specifications and Typical Technical Chest Radiographic Parameters Used in the National Lung Screening Trial
| Parameter | Film-Screen | Computed Radiography | Digital Radiography |
|---|---|---|---|
|
| |||
| Tube voltage (kV) | 120–150 | 100–140 | 110–150 |
| Maximum skin entrance exposure (mGy)a | 0.3 | 0.4 | 0.3 |
| Maximum exposure time (ms) | 40 | 40 | 40 |
| Source-image distance (in)a | ≥ 72 | ≥ 72 | ≥ 72 |
| Antiscatter device (grid) | ≥ 10:1 at 103 lines/in (stationary) or 80 lines/in (reciprocating) | Optimal for system | Optimal for system |
| Minimum collimation | To image receptor | To image receptor | To image receptor |
Note—72 in = 182.88 cm, 1 in = 2.54 cm.
Skin entrance exposure may exceed these guidelines in large individuals.
Effective Dose Assessment
The examination effective dose was calculated as the product of the examination entrance skin air kerma (ESAK) and the Monte Carlo estimate of the ratio of effective dose per ESAK. The examination ESAK was calculated as the product of the tube current–exposure time product and the average x-ray tube output measured annually by the NLST site physicist, corrected according to the inverse square law (x-ray tube output measurements were acquired 100 cm from the focal spot). The subject’s entrance skin surface was 25 cm from the receptor. The effective dose per ESAK was determined with a PC-based Monte Carlo program (PCXMC [PC program for x-ray Monte Carlo]) developed at the Medical Radiation Laboratory of the Finnish Radiation and Nuclear Safety Authority.
PCXMC is commercially available special-purpose Monte Carlo code designed to calculate patients’ organ doses and effective doses in diagnostic medical radiographic examinations [4–7]. The Monte Carlo calculations are based on stochastic mathematic simulation of interactions between photons and matter. The program models interactions on the assumption that the photons are emitted from a point source into the solid angle determined by the x-ray field dimension and the focal-to-skin entrance distance. Beam shape, position, and orientation are determined with the data provided by the user, which include focus-to-skin distance, field size, and patient orientation.
The assessment in our study was conducted with a patient orientation without arms during the effective dose calculation. The receptor field size was set to 35.6 × 43.2 cm for all calculations. The focal-to-skin entrance distance was set at 160 cm for all calculations. The program simulates photon interactions based on the bremsstrahlung spectrum, which corresponds to the input peak kilo-voltage (up to 150 keV), the x-ray tube anode angle, and the total filtration of the x-ray tube (two filters are allowed with atomic number and thickness input parameters to match the characteristics of a specific x-ray tube). Spectra are produced by the Monte Carlo algorithm in 10-keV increments with 10 lots per increment and a specified number of photons per lot equal to 10,000 (default value), representing a total of 100,000 photon histories in each kiloelectron volt bin [4–6].
The effective dose calculation and organ doses are available for 29 organs and tissues. A mathematic hermaphrodite phantom model based on that described by Cristy [8] is used by the program, which also incorporates adjustable-size pediatric and adult patient models and allows a free choice of the x-ray examination technique. In a limited manner, the standard phantom can be adapted to represent patient-specific parameters such as height and weight. The effective dose is determined with the revised tissue weighting factors of International Commission of Radiological Protection (ICRP) publication 103 [9]. A constant field size is used in the PCXMC model, and therefore phantom length (patient height) is not an influential factor in determining the ratio effective dose to ESAK. The PCXMC procedure used to assess the variations of the effective dose per ESAK caused by patient height variations was determined to be simplistic and not appropriate. If only the subject’s height is varied, that is, the field size is fixed and the phantom weight is fixed, the posteroanterior thickness of the phantom is unchanged, and the portions of each organ in the radiation field are similar. Thus similar energy is deposited in each organ. Consequently, the calculated effective dose per ESAK depends only on phantom weight. A linear fit function between ESAK and body mass is used to obtain the effective dose per examination.
Results
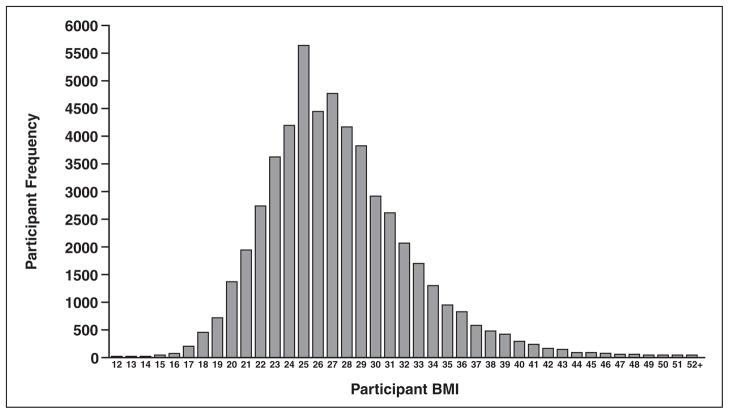
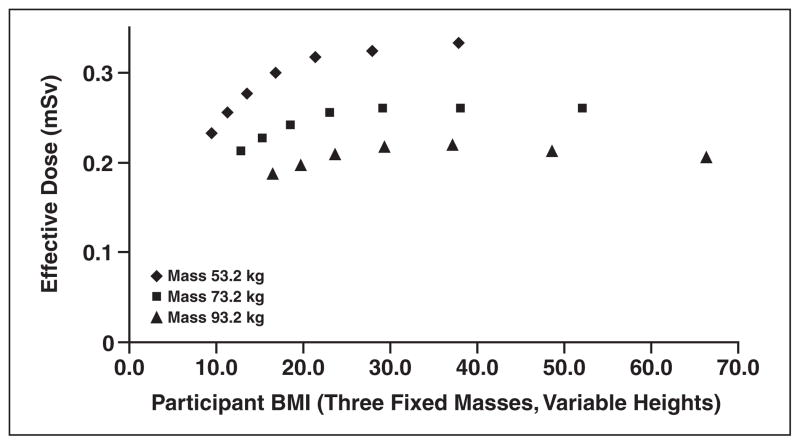
This study showed that the mean effective dose assessed for the posteroanterior chest examinations derived from 66,157 examinations of the 73,733 chest radiographic examinations performed during the NLST was 0.052 mSv. Approximately 59.1% of the patient examinations were of men and 40.9% of women. Additional findings include a median effective dose of 0.038 mSv, a 95th percentile value of 0.136 mSv, and a fifth percentile value of 0.013 mSv. Figure 1 shows the distribution of BMI values for all the participants evaluated in this trial (participants who provided BMI data). Figure 2, which plots the effective dose versus BMI, shows that few participants had a BMI less than 20. Findings on effective dose assessments stratified by receptor system (computed radiography, digital radiography, and film-screen) are shown in Table 2. The PCXMC code provides organ-specific dose calculations made with the revised tissue weighting factors of ICRP publication 103 [9]. The top 10 normalized (ratio of ESAK to effective dose) organ dose estimates for the parameters most commonly used in the trial (120 kV, filtration 3.5-mm aluminum and 0.1-mm copper) are shown in Table 3.
Fig. 1.
Graph shows National Lung Screening Trial body mass index (BMI) distribution for all participants (total number of participants in distribution, 53,090).
Fig. 2.
Graph shows National Lung Screening Trial chest radiography effective dose versus body mass index (BMI) (120 kVp, 3.5-mm aluminum added filter, 35 × 43 cm FOV).
TABLE 2.
Chest Radiographic Effective Dose Assessment Stratified by Image Receptor System
| Image Receptor System | Mean Effective Dose (mSv) | SD (mSv) |
|---|---|---|
|
| ||
| Computed radiography | 0.078 | 0.056 |
| Digital radiography | 0.035 | 0.031 |
| Film-screen | 0.033 | 0.022 |
TABLE 3.
Normalized Organ Doses for an Average National Lung Screening Trial Participant (Body Mass Index, 27.3)
| Organ | Ratio of Organ Dose to Effective Dose (mGy/mSv) |
|---|---|
|
| |
| Spine, thoracic | 7.21 |
| Scapulae | 6.76 |
| Ribs | 6.04 |
| Adrenal glands | 3.59 |
| Kidneys | 3.17 |
| Spine, lumbar | 2.98 |
| Spleen | 2.91 |
| Lungs | 2.84 |
| Esophagus | 1.73 |
| Pancreas | 1.69 |
Discussion
Diagnostic radiographic imaging procedures constitute the largest human-made source of radiation exposure of the general population [10]. We report the technical parameters for 66,157 chest radiographs obtained from 2002 to 2007 in a large multicenter screening trial. Chest radiographic screening was performed with a significantly lower dose than that of standard chest CT or low-dose NLST chest CT. The purpose of this study was to assess the effective dose associated with individual NLST chest radiographic examinations.
Effective Dose Assessment Comparisons
We found that the mean effective dose assessed from NLST posteroanterior chest radiographic examinations was 0.052 mSv (single-view examination). Unique to this report is the standardization and scale of participants involved in this clinical trial. Results of several clinical studies of the effective dose for chest radiographic examinations are compared with our results in Table 4. The mean effective dose assessed in our study is within the range reported in the literature (0.30–0.016 mSv). We report the mean effective dose for a single (posteroanterior) view. Many of the comparative study results show a mean effective dose for a two-view (posteroanterior and lateral) examination. The clinical and technical data necessary to explain differences in mean effective dose across studies are not always available, and therefore a detailed discussion of such differences is not feasible. The mean effective dose assessed in this study stratified by image receptor system (computed radiography, digital radiography, and film-screen) resulted in an observed higher mean effective dose for systems operating with photostimulable phosphor (computed radiography) technology.
TABLE 4.
Chest Radiographic Effective Dose Reported in Published Studies Compared With National Lung Screening Trial Assessment
| Study or Location | Mean Effective Dose (mSv) | Reference | View | Effective Dose Variation |
|---|---|---|---|---|
|
| ||||
| Dutch National Institute of Public Health (2004) | 0.016 | 16 | Posteroanterior | Coefficient of variation, 38% |
| National Radiological Protection Board, United Kingdom (1996) | 0.017 | 17 | Posteroanterior | Percentile (5th and 95th), 0.008–0.037 mSv |
| Nationwide Evaluation of X-ray Trends, chest radiography, United States (1984–2001) | 0.026 | 18 | Posteroanterior | SD, 0.001 mSv |
| National Lung Screening Trial, chest radiography, United States (2002–2007) | 0.052 | Posteroanterior | Percentile (5th and 95th), 0.013–0.136 mSv | |
| UNSCEAR, Japan (2000) | 0.057 | 19 | Not reported | Variation range, 0.01–0.4 mSv |
| Switzerland (2002) | 0.057 | 20 | Posteroanterior | Relative error, 15% |
| Taiwan (2008) | 0.06 | 21 | Posteroanterior | Variability not reported |
| UNSCEAR, Netherlands (2000) | 0.06 | 19 | Not reported | Variation range, 0.01–0.4 mSv |
| UNSCEAR, Finland (2000) | 0.10 | 19 | Not reported | Variation range, 0.01–0.4 mSv |
| UNSCEAR, Norway (2000) | 0.13 | 19 | Not reported | Variation range, 0.01–0.4 mSv |
| UNSCEAR, Sweden (2000) | 0.15 | 19 | Not reported | Variation range, 0.01–0.4 mSv |
| UNSCEAR, Germany (2000) | 0.30 | 19 | Not reported | Variation range, 0.01–0.4 mSv |
Note—UNSCEAR = United Nations Scientific Committee on the Effects of Atomic Radiation.
Limitations and Variabilities of Monte Carlo Calculations
Only intrasite variations of tube potential and filtration influence the assessed participant effective doses, because measurement of x-ray tube output accounts for the intersite variations. The calculation did not account for variations in effective dose per ESAK caused by tube potential or source filtration. With PCXMC the effective dose per ESAK at 150 kV was 30% greater than the effective dose per ESAK at 100 kV. However, accounting for the actual distribution of tube potential, average effective dose per ESAK would have been 0.4% greater.
The PCXMC program requires that the tube voltage and amount of aluminum filtration be specified. The x-ray spectrum is then computed with the Birch and Marshall algorithm [5]. The HVL of this spectrum was independently computed and compared with the site measurements. Based on this HVL, the effective dose computations were done with 3.5 mm of added aluminum filtration, for which the aluminum HVL was 5.1 mm at 120 kVp. The error in effective dose resulting from actual variations in site-specific HVL is estimated to be ±10%.
Study Observations and Summary
A risk associated with either a CT or chest radiographic examination is increased likelihood of cancer caused by the radiation exposure. Radiation risk is commonly assessed by determining the whole-body dose that is equivalent to the dose delivered to portions of the body by a radiologic procedure. This involves determination of the dose delivered to specific organs and the computation of a weighted average dose, or effective dose that accounts for the varying radiosensitivity of different organs. This study showed that the mean effective dose assessed from NLST posteroanterior chest radiographic examinations was 0.052 mSv (single-view examination). The effective dose for individual NLST chest radiographic examinations is of specific interest in relation to that associated with the NLST low-dose CT examinations conducted during the trial [11–15].
Acknowledgments
Supported by contracts from the Division of Cancer Prevention, National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services, and by grants U01 CA80098 and CA79778 to the American College of Radiology Imaging Network (ACRIN) under a cooperative agreement with Cancer Imaging Program, Division of Cancer Treatment and Diagnosis, NCI. The National Lung Screening Trial (NLST) is funded by NIH-NCI ACRIN grant CA80098 and by contracts with the Division of Cancer Prevention, NCI, NIH. This project was conducted by members of the NLST Joint Medical Physics Working Group.
We thank the screening center investigators and staff of the National Lung Screening Trial (NLST). The online staff listing can be found at www.nejm.org/doi/suppl/10.1056/NEJMoa1102873/suppl_file/nejmoa1102873_appendix.pdf. We also thank the study participants, whose contributions made this study possible.
References
- 1.Aberle DR, Berg CD, et al. National Lung Screening Trial Research Team. The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle DR, Gamsu G, Henschke CI, Naidich DP, Swensen SJ. A consensus statement of the Society of Thoracic Radiology: screening for lung cancer with helical computed tomography. J Thorac Imaging. 2001;16:65–68. doi: 10.1097/00005382-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Cagnon CH, Cody DD, McNitt-Gray MF, Seibert JA, Judy PF, Aberle DR. Description and implementation of a quality control program in an imaging-based clinical trial. Acad Radiol. 2006;13:1431–1441. doi: 10.1016/j.acra.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Tapiovaara M, Siiskonen T. PCXMC: a PC-based Monte Carlo program for calculating patient doses in medical x-ray examinations. 2. Helsinki, Finland: Finnish Centre for Radiation; 2008. Report no. STUK-A231. [Google Scholar]
- 5.Tapiovaara M, Siiskonen T. PCXMC 2.0: user’s guide. Report no STUK-TR7. Helsinki, Finland: Finnish Centre for Radiation and Nuclear Safety; 2008. [Google Scholar]
- 6.Servomaa A, Tapiovaara M. Organ dose calculation in medical x-ray examinations by the program PCXMC. Radiat Prot Dosimetry. 1998;80:213–219. [Google Scholar]
- 7.Khelassi-Toutaoui N, Berkani Y, Tsapaki V, et al. Experimental evaluation of PCXMC and PREPARE codes used in conventional radiology. Radiat Prot Dosimetry. 2008;131:374–378. doi: 10.1093/rpd/ncn183. [DOI] [PubMed] [Google Scholar]
- 8.Cristy M. NUREG/CR-1159, ORNL/NUREG/TM-367. Washington, DC: U.S. Nuclear Regulatory Commission; 1980. Mathematical phantoms representing children of various ages for use in estimates of internal dose. [Google Scholar]
- 9.The 2007 recommendations of the International Commission on Radiological Protection, ICRP publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. No authors listed. [DOI] [PubMed] [Google Scholar]
- 10.Berrington de González A, Darby S. Risk of cancer from diagnostic x-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 11.Cody DD, Kim HJ, Cagnon CH, et al. Normalized CT dose index of the CT scanners used in the National Lung Screening Trial. AJR. 2010;194:1539–1546. doi: 10.2214/AJR.09.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larke FJ, Kruger RL, Cagnon CH, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR. 2011;197:1165–1169. doi: 10.2214/AJR.11.6533. [DOI] [PubMed] [Google Scholar]
- 13.Church TR. Chest radiography as the comparison for spiral CT in the National Lung Screening Trial. Acad Radiol. 2003;10:713–715. doi: 10.1016/s1076-6332(03)80095-8. [DOI] [PubMed] [Google Scholar]
- 14.Berrington de González A, Kim KP, Berg CD. Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J Med Screen. 2008;15:153–158. doi: 10.1258/jms.2008.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberle DR, Adams AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teeuwisse W, Geleijus J, Veldkamp W. An inter-hospital comparison of patient dose based on clinical indications. Eur Radiol. 2007;17:1795–1805. doi: 10.1007/s00330-006-0473-1. [DOI] [PubMed] [Google Scholar]
- 17.Wall BF, Hart D. Commentary: revised radiation doses for typical x-ray examinations (UK) Br J Radiol. 1997;70:437–439. doi: 10.1259/bjr.70.833.9227222. [DOI] [PubMed] [Google Scholar]
- 18.Spelic DC, Kaczmarek RV, Hilohi MC, Moyal AE. Nationwide surveys of chest, abdomen, lumbosacral spine radiography, and upper gastrointestinal fluoroscopy: a summary of findings. Health Phys. 2010;98:498–514. doi: 10.1097/HP.0b013e3181c182cd. [DOI] [PubMed] [Google Scholar]
- 19.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation: 2000 report to the General Assembly—with scientific annexes. New York, NY: United Nations; 2000. [Google Scholar]
- 20.Aroua A, Decka I, Burnand B, Vader JP, Valley JF. Dosimetric aspects of a national survey of diagnostic and interventional radiology in Switzerland. Med Phys. 2002;29:2247–2259. doi: 10.1118/1.1508380. [DOI] [PubMed] [Google Scholar]
- 21.Chen TR, Tyan YS, Teng PS, et al. Population dose from medical exposure in Taiwan for 2008. Med Phys. 2011;38:3139–3148. doi: 10.1118/1.3592936. [DOI] [PubMed] [Google Scholar]