Abstract
Objectives
The absence of pathophysiologically relevant diagnostic markers of bipolar disorder (BD) leads to its frequent misdiagnosis as unipolar depression (UD). We aimed to determine whether whole brain white matter connectivity differentiated BD from UD depression.
Methods
We employed a three-way analysis of covariance, covarying for age, to examine whole brain fractional anisotropy (FA), and corresponding longitudinal and radial diffusivity, in currently depressed adults: 15 with BD-type I (mean age 36.3 years, SD 12.0 years), 16 with recurrent UD (mean age 32.3 years, SD 10.0 years), and 24 healthy control adults (HC) (mean age 29.5 years, SD 9.43 years). Depressed groups did not differ in depression severity, age of illness onset, and illness duration.
Results
There was a main effect of group in left superior and inferior longitudinal fasciculi (SLF and ILF) (all F ≥ 9.8; p ≤ .05, corrected). Whole brain post hoc analyses (all t ≥ 4.2; p ≤ .05, corrected) revealed decreased FA in left SLF in BD, versus UD adults in inferior temporal cortex and, versus HC, in primary sensory cortex (associated with increased radial and decreased longitudinal diffusivity, respectively); and decreased FA in left ILF in UD adults versus HC. A main effect of group in right uncinate fasciculus (in orbitofrontal cortex) just failed to meet significance in all participants but was present in women. Post hoc analyses revealed decreased right uncinate fasciculus FA in all and in women, BD versus HC.
Conclusions
White matter FA in left occipitotemporal and primary sensory regions supporting visuospatial and sensory processing differentiates BD from UD depression. Abnormally reduced FA in right fronto-temporal regions supporting mood regulation, might underlie predisposition to depression in BD. These measures might help differentiate pathophysiologic processes of BD versus UD depression.
Keywords: Depression, diffusion tensor imaging, inferior longitudinal fasciculus, mood disorders, superior longitudinal fasciculus, uncinate fasciculus
Bipolar disorder (BD) is one of the top 10 most debilitating of all illnesses. Yet, the absence of biologically relevant diagnostic markers of BD results in misdiagnosis of the illness as recurrent UD in 60% of BD individuals seeking treatment for depression, leading to inadequate treatment that promotes switching to mania and worsens illness outcome (1), a 15% suicide rate, and direct annual costs in the US of $7.6 billion (2). It is therefore crucial to society and the well-being of all individuals with BD that objective markers of BD are identified to help distinguish BD from UD as early as possible in the lifetime of these individuals. A first stage toward this ultimate goal is the identification of objective biological markers reflecting pathophysiologic processes that might differ between BD and UD depression.
Examination in BD adults of structural and functional measures of neural circuitry supporting emotion regulation and the extent to which these measures might distinguish BD from UD depression is one promising study that has potential to increase understanding of pathophysiologic processes that might differ between BD and UD depression. We previously found in BD adults, with diffusion tensor imaging (DTI) and fractional anisotropy (FA), a measure of the structural integrity of brain white matter (WM), abnormal fiber alignment in right and left WM tracts connecting orbitomedial prefrontal cortical and limbic regions implicated in mood regulation (3) that parallels findings of previous studies demonstrating abnormal FA in prefrontal cortical regions in BD adults and adolescents (4–12). We also recently showed different patterns of abnormal functional (effective) connectivity between bilateral orbitomedial prefrontal cortical and limbic regions during positive emotion processing in BD and UD depressed adults (13), with BD depressed showing abnormal bilateral connectivity and UD depressed showing abnormal left-sided effective connectivity between these regions. Although previous studies reported abnormal prefrontal cortical-limbic WM connectivity in UD depression across the lifespan (14–23), the extent to which patterns of WM connectivity between prefrontal cortical and limbic regions can differentiate BD from UD depression remains unknown.
A further consideration is that interpretation of FA changes in pathological groups can be challenging, because these changes can be interpreted as one of several possibilities, including alterations in longitudinally/obliquely oriented fibers ratio, in axonal integrity, in tightness of axonal packing, in permeability of myelin sheaths, or abnormality of one set of fibers in a large group of intersecting fiber pathways. Recently, it was suggested that eigenvalues might represent more specific relationships to WM pathology. For example, radial diffusivity seems to be modulated by myelin in WM, whereas longitudinal diffusivity is more sensitive to axonal degeneration (24–26).
In the present study we therefore aimed to compare whole brain WM connectivity—with FA, longitudinal and radial diffusivity measures—in the same BD and UD depressed adults as in the aforementioned effective connectivity study as a first step toward identifying WM connectivity abnormalities that might help differentiate BD from UD depression. This would then allow future studies to examine the extent to which the combination of effective and WM connectivity measures could be used to discriminate BD from UD depression in independent samples of BD and UD depressed individuals. Our previous findings allowed us to hypothesize that BD and UD depressed adults would be distinguished by patterns of amygdala-orbitomedial prefrontal WM connectivity, specifically with BD depressed adults showing abnormal bilateral WM connectivity and UD depressed adults showing abnormal left-sided, WM connectivity between these regions. Our whole brain analytic approach also allowed us to examine the extent to which WM connectivity in other regions over the whole brain distinguished BD from UD depression.
Methods and Materials
Participants
We recruited 15 currently depressed individuals with BD and 16 currently depressed individuals with recurrent UD (mean age [SD] = 36.3 [12.0] years and 32.9 [10.0] years, respectively; age range 18–54 years) on the basis of standardized diagnostic criteria for these illnesses (27) and a 25-item Hamilton Rating Scale for Depression (28) (25-HDRS) (score ≥ 13; mean 25-HDRS [SD] = 21.9 [6.3] and 25.1 [5.5], respectively). Each depressed group had previously participated in our effective connectivity study (13); these two depressed groups were matched for depression severity (t = –1.5, p = .137), illness duration (t = .1, p = .994), illness onset age (t = .8, p = .397), age (t = .9, p = .392), gender ratio (χ2 = 1.9, p = .165), and lifetime history of drug and/or alcohol abuse/dependence (χ2 = 2.3, p = .311). Total medication load (29) differed between groups (t = 3.1, p = .004), because individuals were being treated specifically for either BD or UD depression (Table 1). Twenty-four healthy control adults (HC) (mean age [SD] = 27.7 [8.6] years, M/F = 9/15) with no previous psychiatric history (on the basis of Structured Clinical Interview for DSM-IV—Patient Edition criteria) or psychiatric history in first- and second-degree relatives participated in the study. The HC were not significantly different in gender-ratio but were younger than BD and UD depressed adults (t = 2.4, p = .023 and t = 1.7, p = .089, respectively) (Table 1). Sixteen of these HC had previously participated in our effective connectivity study (13). All participants were right-handed. The BD and UD depressed adults were alcohol and substance abuse/dependent-free for a minimum of 2 months before participation in the study. Lifetime history and/or current alcohol and illicit substance abuse (determined by saliva and urine screen, respectively) were exclusion criteria for HC. All participants gave informed consent after explanation of the nature and possible consequences of the study.
Table 1.
Demographic and Clinical Variables
| Group (n) | Mean (SD) | Statistics | df | p (2-tailed) | |
|---|---|---|---|---|---|
| Age at Scan (yr) | BD = 15 | 36.3 (12.0) | F = 3.6a | 2,52 | .034b |
| UD = 16 | 32.9 (10.0) | ||||
| HC = 24 | 27.7 (8.6) | ||||
| Gender (M/F) | BD = 1/14 | χ2 = 4.6 | 2 | .099 | |
| UD = 4/12 | |||||
| HC = 9/15 | |||||
| Age of Illness Onset (yr) | BD = 15 | 21.6 (10.3) | t = .8 | 29 | .397 |
| UD = 16 | 18.9 (7.2) | ||||
| Illness Duration (yr) | BD = 15 | 14.7 (9.5) | t = .1 | 29 | .994 |
| UD = 16 | 14.7 (10.0) | ||||
| HRSD-25 | BD = 15 | 21.9 (6.3) | t = –1.5 | 29 | .137 |
| UD = 16 | 25.1 (5.5) | ||||
| Medication Load | BD = 15 | 4.3 (2.8) | t = 3.1 | 29 | .004b |
| UD = 16 | 1.9 (1.1) | ||||
| Mood Stabilizer (on/off) | BD = 10/5 | χ2 = 15.7 | 1 | <.00b | |
| UD = 0/16 | |||||
| Antipsychotic Medications (on/off) | BD = 10/5 | χ2 = 12.3 | 1 | <.001b | |
| UD = 1/15 | |||||
| Antidepressants (on/off) | BD = 8/7 | χ2 = 4.4 | 1 | .036b | |
| UD = 14/2 | |||||
| Benzodiazepines (on/off) | BD = 6/9 | χ2 = .3 | 1 | .611 | |
| UD = 5/11 | |||||
| Lifetime History of Alcohol/Substance Abuse/Dependence (on/off) | BD = 2/11 | χ2 = 2.3 | 2c | .311 | |
| UD = 3/13 |
BD, depressed bipolar disorder individuals; UD, depressed unipolar disorder individuals; HC, healthy control individuals; HRSD-25, Hamilton Rating Scale for Depression 25-item score.
Independent sample t test between groups were, respectively: BD vs. UD: t(29) = .9, p = .392; BD vs. HC: t(37) = 2.4, p = .023; UD vs. HC: t(38) = 1.7, p = .089.
p < .05 (2-tailed); statistics refer to between-group differences.
Missing information in BD 2 (women; 41 and 28 year-old) about lifetime history of drug/alcohol abuse/dependence.
DTI Data Acquisition and Analyses
Magnetic resonance imaging (MRI) scans were acquired with a 3T Siemens Magnetom Allegra syngo MR-2004A (Siemens, Malvern, Pennsylvania). Diffusion tensor data were acquired with an axial diffusion weighted single-shot spin-echo planar imaging sequence, parallel to the anterior–posterior commissure line (repetition time = 4400 msec, echo time = 76 msec, bandwidth 1860 Hz/Px, flip angle = 90, field of view = 200 × 200 mm2, 33 3-mm-thick slices, no-gaps, matrix size = 80 × 128, echo planar imaging factor = 128; acquisition: 72-inch/16-inch). Two b values were used: one b = 0 (no-diffusion weighting) image and six non-coplanar b = 850 sec/mm2 (diffusion-weighting b-value) images were acquired.
One problem for DTI is that analysis is compromised by the use of standard registration algorithms, so that there is no satisfactory solution to the question of how to align FA images from multiple subjects in voxelwise analysis (30). A recent advance is the development of tract-based spatial statistics (TBSS), an automated observer-independent method of aligning FA images from multiple subjects to allow groupwise comparisons of DTI data (30–31). The TBSS also focuses upon the WM skeleton (i.e., the most compact whole brain WM).
Data were transferred to a Unix-based workstation. Diffusion-weighted images (DWI) were analyzed with the FMRIB (Functional Magnetic Resonance Imaging of the Brain) Software Library (FSL) (http://www.fmrib.ox.ac.uk/fsl). First, data were inspected for motion artifacts, then DWI were registered to the b = 0 image, as a reference, by affine transformations to minimize distortions due to eddy currents and reduce simple head motion, with Eddy Current Correction. Images were extracted with the Brain Extraction Tool (32), part of the FSL package (33). A diffusion tensor model was fitted at each voxel, providing a voxelwise calculation of FA (34). Whole brain voxelwise analysis of FA data were performed by first aligning each subject's FA-image into a higher-resolution FA standard space (Montreal Neurological Institute [MNI] atlas), according to a nonlinear registration algorithm, implemented in TBSS v. 1.2 (30,31). The derived mean FA image was minimized to generate a template-skeleton embodying the center of all tracts derived from the whole group. An FA ≥ .20 threshold was set to exclude peripheral tracts that might lead to erroneous interpretations due to anatomic intersubject variability and/or partial volume effects with gray matter. To examine between-group differences in FA, the preprocessed data were entered into a whole brain voxelwise analysis (Randomize vs. 2.1; http://www.fmrib.ox.ac.uk/fsl/randomise/index.html). This is a permutation program enabling modeling and inference with standard “general linear model” design and nonparametric independent t tests (permutation method, n = 5000, no smoothing factor, t and F ≥ 2). Independent and simultaneous whole brain voxelwise tests increase the precision of findings in terms of clusters but also lead to increase in chance that at least one test is incorrect: the probability-α-of having at least one error (Type I) greater than the probability of an error on an individual test. Therefore, traditional cluster thresholding and threshold-free cluster enhancement (TFCE) were also employed (p < .05). If between-group differences did not reach statistical threshold with traditional cluster thresholding or TFCE, a Monte Carlo simulation with the αSim approach (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) was conducted on uncorrected F and t statistical maps (p ≤ .001), obtaining a dual thresholding of both Type I error (α; p ≤ .05) and cluster-size thresholding (CST). The αSim first generates a random image having spatially uncorrelated voxels and convolves this image with a Gaussian function, simulating the effect of voxel correlation. Then, by examining the whole brain WM-skeleton defined in the preceding text, it scales the resulting image to provide the specified voxel probability threshold and to identify which activated voxels belong to clusters. With the αSim approach, we were therefore able to obtain a reasonable correction (p < .05) for multiple tests and greatly enhanced the power of multiple statistical tests.
The most probable anatomical localization of each cluster showing significant between-group differences in FA was determined with the FSL atlas tool (http://www.fmrib.ox.ac.uk/fsl/fslview/atlas-descriptions.html), with all anatomical templates (Harvard-Oxford cortical and subcortical structural atlases, Jülich histological atlas, JHU DTI-based white-matter atlases, Oxford thalamic connectivity atlas, Talairach atlas, and MNI structural atlas).
Whole Brain Between-Group FA Analyses and Longitudinal and Radial Diffusivity Measures
The primary whole brain FA analysis focused on a 3-way analysis of covariance (age as covariate) among BD depressed adults, UD depressed adults, and HC. Post hoc whole brain analyses were performed, directly contrasting BD depressed and UD depressed adults as well as BD depressed adults and HC and UD depressed adults and HC, to determine the extent to which the main effect of group upon FA reflected FA abnormalities between each depressed group and each depressed group and HC.
We also examined eigenvalues as validated measures of longitudinal and radial diffusivity (24,25) in WM regions showing significant between-group differences in FA. We did not perform whole brain analyses of these two diffusivity measures, because the specific purpose of examining these additional analyses was to inform interpretation of our main findings regarding between-group differences in FA. These measures provide information regarding likely alterations in the proportion of longitudinally-versus obliquely-aligned myelinated fibers. Increased longitudinal diffusivity in one group would suggest greater number of longitudinally aligned and decreased radial diffusivity and reduced number of obliquely aligned fibers.
Exploratory Analyses
To evaluate the extent to which diffusivity measures were associated with clinical variables in those clusters showing significant between-group differences in FA, we examined relationships among age, age of illness onset, illness duration, depression severity, and medication load in BD and UD depressed adults and all DTI measures. In each depressed group, we further examined relationships between individuals taking versus individuals not taking each of the four main psychotropic medication subclasses and individuals with versus individuals without a history of substance abuse. We used Spearman correlations for the first five and Mann–Whitney U tests for the remaining five tests, with a statistical threshold of p = .05/10 = .005, to control for multiple tests in BD and UD depressed adults. We also examined relationships between age and DTI measures in HC, with a statistical threshold of p = .05.
Because most of our study participants were female, we also examined whole brain FA differences and corresponding longitudinal and radial diffusivity measures in female participants only.
Results
Whole Brain Analyses of FA
Main Effect of Group
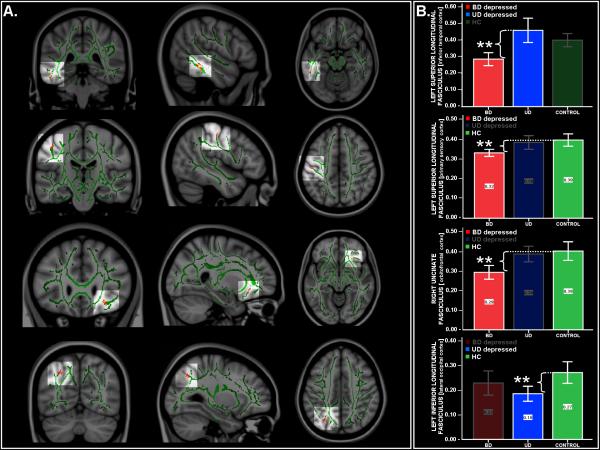
All the following main effect of group findings were αSim-corrected (p < .05), with a resulting CST of ≥10 voxels (Table 2 and Figure 1A) (FA findings and corresponding λ ∥∥ and λ⊥ statistics are reported in Tables 2 and 3 and Tables S1, S3A, and S3B in Supplement 1). Covarying for age, there was a significant main effect of group in the region of the left superior longitudinal fasciculus (SLF) (inferior temporal cortex; MNI x, y, z: –50, –38, –15, fmax = 9.8), in the region of the left SLF (primary sensory cortex; MNI x, y, z: –47, –19, 47, fmax = 19.9), and in the region of the left inferior longitudinal fasciculus (ILF) (lateral occipital cortex; MNI x, y, z: –28, –66, 40, fmax = 14.4). There was also a main effect of group in the right uncinate fasciculus (UF) (orbitofrontal cortex, MNI x, y, z: 26, 26, –10, fmax = 11.1) that just failed to meet our cluster-size threshold criterion (cluster-size = 9/10 voxels).
Table 2.
Main Effect of Group in Whole Brain Analysis of Diffusivity Measures
| 95%CI |
FA |
λ
∥
|
λ
⊥
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Brain Regions | Voxels | EMM FAa | Lower | Higher | F-Testb | p | F-Testa | p | F-Testa | p | ||
| Left SLF | (inferior temporal cortex) | 13 | BD | .31 | .26 | .37 | 9.8c | <.05 | 2.7 | .079 | 4.2c | .020c |
| (MNI coordinates x, y, z: –50, –38, –15) | UD | .48 | .43 | .53 | ||||||||
| HC | .42 | .38 | .47 | |||||||||
| Left SLF | (primary sensory cortex) | 11 | BD | .26 | .23 | .29 | 19.9c | <.05c | 3.8c | .029c | 2.8 | .072 |
| (MNI coordinates x, y, z: –47, –19, 47) | UD | .34 | .31 | .37 | ||||||||
| HC | .27 | .24 | .29 | |||||||||
| Left ILF | (lateral occipital cortex) | 10 | BD | .23 | .18 | .28 | 14.4c | <.05c | 3.0 | .060 | .1 | .870 |
| (MNI coordinates x, y, z: –28, –66, 40) | UD | .17 | .12 | .21 | ||||||||
| HC | .24 | .20 | .28 | |||||||||
| Right Uncinate Fasciculus | (orbitofrontal cortex) | 9 | BD | .28 | .23 | .33 | 11.1 | <.05c | 1.8 | .194 | .2 | .699 |
| (MNI coordinates x, y, z: 26, 26, –11) | UD | .38 | .33 | .42 | ||||||||
| HC | .39 | .35 | .43 | |||||||||
Main effect of group in whole brain analysis of diffusivity measures: BD (n = 15), UD (n = 16) and HC (n = 24).
CI, confidence interval; FA, fractional anisotropy; SLF, superior longitudinal fasciculus; MNI, Montreal Neurological Institute; ILF, inferior longitudinal fasciculus; other abbreviations as in Table 1.
Estimated marginal means (EMM) are evaluated at the covariate value of age = 31.6.
Monte Carlo simulation with αSim correction was run on uncorrected f statistical maps (p < .001), obtaining a dual thresholding of both Type I error (αSim p < .05, corrected) and cluster-size thresholding (f-tests cluster-size thresholding (CST) ≥ 10 voxels). The cluster in the right uncinate fasciculus just failed to meet our CST (9/10 voxels) but fully met criteria in whole brain analysis of female participants only (Table S3 in Supplement 1).
Significant between-group comparisons.
Figure 1.
(A) Fractional anisotropy (FA) maps showing three orthogonal (coronal, sagittal, and axial) views of the main effect of group on FA in bipolar disorder (BD) depressed adults, unipolar disorder (UD) depressed adults, and healthy control adults (HC) (from top to bottom): left superior longitudinal fasciculus in inferior temporal cortex; left superior longitudinal fasciculus in primary sensory cortex; right uncinate fasciculus in orbitofrontal cortex, and left inferior longitudinal fasciculus in lateral occipital cortex. Background: Montreal Neurological Institute (MNI)152 brain and white matter skeleton used for randomized analysis (in green). The images represent findings (in red-yellow) projected onto the white matter skeleton. The red-yellow spectrum represents a significance range: 2 < f < 20. Monte Carlo simulation with the αSim approach was conducted on uncorrected F and t statistical maps (p ≤ .001), obtaining a dual thresholding of both Type I error (α; p ≤ .05) and cluster-size thresholding (CST). (B) Bar graphs show mean FA values and 95% confidence intervals for each group for each of the aforementioned four regions in which there was a main effect of group on FA. **Highlighted is the significant pairwise between-group post hoc comparison that resulted in the main effect of group in each of these four regions (BD depressed adults in red, UD depressed adults in blue, and HC in green). For each region, the third group that did not contribute to the main effect of group is also graphically represented (bar obscured) From top to bottom: pairwise comparison between BD depressed adults relative to UD depressed adults in the left superior longitudinal fasciculus in inferior temporal cortex (MNI x, y, z = –51, –38, –17); pairwise comparison between BD depressed adults relative to HC in the left superior longitudinal fasciculus in primary sensory cortex (MNI x, y, z = –40, –27, 50) and the right uncinate fasciculus in orbitofrontal cortex (MNI x, y, z = 26, 26, –11); and pairwise comparison between UD depressed adults relative to HC in the left inferior longitudinal fasciculus in lateral occipital cortex (MNI x, y, z = –28, –76, 40).
Table 3.
Post Hoc Comparisons in Whole Brain Analysis of Diffusivity Measures
| 95%CI |
FA |
λ
∥
|
λ
⊥
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Brain Regions | Voxels | EMM FAa | Lower | Higher | t Testb | p | t Testa | p | t Testa | p | ||
| BD < UD | ||||||||||||
| Left SLF | (inferior temporal cortex) | 33 | BD | .29 | .23 | .34 | –4.5c | <.05c | –2.7 | .113 | 12.8c | .001c |
| (MNI coordinates x, y, z: –51,–38,–17) | UD | .45 | .40 | .51 | ||||||||
| BD < HC | ||||||||||||
| Left SLF | (primary sensory cortex) | 19 | BD | .32 | .29 | .36 | –4.2c | <.05c | –4.2c | .047c | 2.8 | .104 |
| (MNI coordinates x, y, z: –40,–27,50) | HC | .39 | .36 | .42 | ||||||||
| Right Uncinate Fasciculus | (orbitofrontal cortex) | 19 | BD | .29 | .24 | .34 | –4.7c | <.05c | –4.0c | .053c | 4.2c | .048c |
| (MNI coordinates x, y, z: 26,26,–11) | HC | .39 | .35 | .43 | ||||||||
| UD < HC | ||||||||||||
| Left ILF | (lateral occipital cortex) | 21 | UD | .19 | .14 | .23 | –5.4c | <.05c | .2 | .646 | .3 | .581 |
| (MNI coordinates x, y, z: –28,–67,40) | HC | .26 | .23 | .30 | ||||||||
Post hoc comparisons in whole brain analysis of diffusivity measures: BD (n = 15), UD (n = 16), and HC (n = 24). The λ∥ and λ⊥ extracted only in white matter (WM) regions showing significant between-group differences upon FA; whole brain analyses and/or corrections of these two diffusivity measures were not performed. λ∥ , longitudinal diffusivity; λ⊥ , radial diffusivity; other abbreviations as in Tables 1 and 2.
The EMM are evaluated at the covariate value of age = 34.5 for SLF (inferior temporal cortex), age = 31.0 for SLF (primary sensory cortex) and Uncinate Fasciculus, and age = 29.8 for ILF.
MonteCarlo simulation with αSim correction was run on uncorrected t statistical maps (p < .001), obtaining a dual thresholding of both Type I error (αSim p < .05, corrected) and cluster-size thresholding (t tests CST ≥ 18 voxels).
Significant between-group comparisons.
Post Hoc Comparisons
All the following post hoc findings were αSim-corrected (p < .05) with a resulting CST of ≥ 18 voxels (Table 3 and Figure 1B).
BD Versus UD Depressed Adults
The BD relative to UD depressed adults showed, covarying for age, significantly decreased FA in the region of left SLF (inferior temporal cortex; MNI x, y, z: –51, –38, –17, tmax. = 4.3), associated with increased radial diffusivity (t = 12.8, p = .001).
BD Depressed Adults Versus HC
The BD depressed adults relative to HC showed, covarying for age, significantly decreased FA in the region of left SLF (primary sensory cortex; MNI x, y, z: –40, –27, 50, tmax. = 4.2), associated with decreased longitudinal diffusivity (t = 4.2, p = .047) and in the region of right UF (orbitofrontal cortex; MNI x, y, z: 26, 26, –11, tmax. = 4.7), associated with decreased longitudinal and increased radial diffusivity, respectively (t = 4.0, p = .053, and t = 4.2, p = .048).
UD Depressed Adults Versus HC
The UD depressed adults relative to HC showed, covarying for age, significantly decreased FA in the region of left ILF (lateral occipital cortex; MNI x, y, z: –28, –67, 40, tmax. = 5.4), not associated with any significant change in longitudinal and/or radial diffusivity.
Additional Regions Showing Significant Changes in FA in Post Hoc Comparisons
The BD relative to UD depressed adults showed increased FA in the region of right UF (subgenual cortex; MNI x, y, z: 14, 13, –10, tmax. = 3.8), not associated with any significant change in longitudinal and/or radial diffusivity.
The BD depressed adults relative to HC showed significantly decreased FA in the region of right SLF (primary sensory cortex; MNI x, y, z: 40, –27, 51, tmax. = 3.9), associated with increased radial diffusivity (tmax. = 4.7, p = .037) (Table S1 in Supplement 1).
Exploratory Analyses Between Clinical and Demographic Variables and DTI Measures in Regions That Differentiated BD and UD Depressed Adults
No relationships among diffusivity measures and age, age of illness onset, depression severity, medication load, psychotropic medication subclasses, or lifetime history of substance abuse/dependence reached our conservative threshold for statistical significance (Tables S2A and S2B in Supplement 1).
Female Participants Only
Whole brain FA differences and corresponding longitudinal and radial diffusivity measures in female participants only mostly confirmed our findings in the aforementioned analyses of all participants. All the following main effect of group and post hoc findings were αSim-corrected (p < .05) and cluster-size threshold (CST ≥ 10 and 18 voxels, respectively) selected (Tables S3A and S3B in Supplement 1). These analyses showed a main effect of group in the region of left SLF. Post hoc analyses confirmed the decreased FA in the inferior and middle temporal cortices in BD relative to UD depressed women and in the primary sensory cortex in BD depressed relative to HC women. The main effect of group in the right UF that just failed to meet the CST (9/10 voxels) in whole brain analysis in all participants fully met our significance criteria in whole brain analyses of women only and confirmed the decreased FA in BD depressed relative to HC women (fmax = 13.4 and tmax. = 5.1, respectively). Additionally, post hoc analyses revealed a decreased FA in the left UF (entorhinal cortex, MNI coordinates x, y, z: –31, –24, –28), in UD depressed adults relative to HC women (tmax. = –4.0, not associated with any significant change in longitudinal and/or radial diffusivity) and increased FA in the region of left SLF, in the inferior temporal cortex (tmax. = –4.8, associated with significant decreased in radial diffusivity).
Discussion
To our knowledge, this is the first study to compare whole brain FA and corresponding longitudinal and radial diffusivity measures of WM connectivity in BD and UD depressed adults and HC in BD and UD depressed adults with a similar clinical presentation, in terms of illness duration, age of illness onset, and depression severity. Our findings indicate a significant main effect of group in left-sided sensory and visuospatial information processing regions, namely in the region of left SLF, in the inferior temporal and primary sensory cortices, and in the region of the left ILF, in the lateral occipital cortex. Here, BD depressed adults showed significantly decreased FA in left SLF in the region of inferior temporal cortex, relative to UD depressed adults, and in the region of primary sensory cortex relative to HC, whereas UD depressed adults showed significantly decreased FA in the region of the left ILF relative to HC. Post hoc whole brain comparisons further revealed in BD depressed adults—relative to both UD depressed adults and HC—significant differences in FA in the region of right UF, a key WM tract connecting fronto-temporal cortices, supporting emotion processing and regulation. Here, BD depressed adults showed significantly reduced right UF FA in the region of orbitofrontal cortex relative to HC but significantly greater right UF FA in the region of subgenual cortex relative to UD depressed adults. Furthermore, in women only comparisons, there was a significant main effect of group in the right UF FA, where BD depressed women showed significantly decreased FA relative to healthy women. These decreases in FA in between-group comparisons were paralleled predominantly by decreases in longitudinal and increases in radial diffusivity.
Our findings of decreased FA, together with decreased longitudinal diffusivity and greater radial diffusivity—in the region of right UF in orbitofrontal cortex BD but not UD depressed adults relative to HC—are in partial support of our main hypothesis of abnormal right-sided amygdala-orbitomedial prefrontal WM connectivity in BD depressed but not UD depressed adults. These findings also parallel our previous findings of greater WM radial diffusivity in right orbitomedial prefrontal cortex in BD depressed adults relative to HC (35) and abnormally reduced right-sided orbitomedial prefrontal cortical-limbic effective connectivity to positive emotional stimuli in BD depressed but not UD depressed adults (13). Our present findings in the UF in BD depressed adults are also consistent with other reports in BD of reduced FA in BD adolescents (8,36) and BD adults (10–11) in frontal cortical regions relative to age-matched healthy individuals. Interestingly, our findings further suggest that BD and UD depressed adults might be distinguished by FA in the right UF in the region of subgenual cortex. The pattern of greater FA in this region in BD depressed adults relative to UD depressed adults might suggest greater WM connectivity between amygdala and subgenual cingulate gyrus in the former group, although the absence of previous studies directly comparing BD and UD depression make further interpretation of this finding difficult. Together, these findings indicate, however, that BD and UD depression are distinguished by FA in right UF that in turn might reflect different pathophysiologic processes associated with emotion dysregulation in the two types of depression.
In UD, many previous studies focused on late-life UD depression and employed a region of interest rather than a whole brain approach. These studies reported mainly decreases in FA in frontal WM regions (14–16,18–20). Similarly, whole brain DTI studies in UD depression reported decreased FA in older UD depressed adults relative to HC in prefrontal regions (21,23). Previous studies also reported decreased FA in prefrontal WM regions in young UD depressed adults relative to HC (17). These findings are consistent with our present finding of decreased FA in the region of left UF in UD depressed adults relative to HC, although our finding was specific to UD depressed women. Our finding is, however, in support of our hypothesis that UD depressed adults would show left- but not right-sided abnormalities in amygdala-orbitomedial prefrontal cortical WM connectivity and further suggests that differential patterns of abnormal right and left orbitomedial prefrontal cortical-limbic WM connectivity might underlie the predisposition to depression in female BD and UD depression.
Our whole brain analyses allowed us to examine WM connectivity in regions other than orbitomedial prefrontal cortex. We showed a main effect of group in left SLF in the region of inferior temporal and primary sensory cortices, resulting from BD depressed adults showing significantly decreased FA in these regions relative to UD depressed adults and HC, respectively, and in left ILF, in lateral occipital cortex, resulting from UD depressed adults showing significantly decreased FA in this region relative to HC. The BD depressed adults also showed significantly decreased FA in right SLF in the region of primary sensory cortex. These findings of decreased FA in between-group comparisons were paralleled predominantly by patterns of deceased longitudinal diffusivity and increased radial diffusivity. Furthermore, analyses of female participants only revealed similar findings of significantly decreased FA in left SLF in both depressed groups relative to HC and in BD depressed relative to UD depressed adults. Our findings in BD depressed adults are consistent with previous findings of decreased FA in left SLF in BD adults (37) and of bilateral decreases in FA in SLF in adolescents with BD and those at high-risk of BD (38). Our findings of decreased FA in left ILF in all UD depressed adults relative to HC and of decreased left SLF FA in female UD depressed adults relative to healthy women support findings of previous DTI studies showing decreased FA in UD depressed adults relative to HC in temporal WM (18,20) and occipital and subcortical WM (21). Our specific finding of decreased left ILF FA in UD depressed adults relative to HC supports that of a previous whole brain DTI study (22). This latter study reported decreased FA in UD depressed adults relative to HC in a temporo-occipitoparietal region similar in location to the left ILF region in lateral occipital cortex showing decreased FA in UD depressed adults relative to HC in the present study.
Our present findings therefore add to the extant literature indicating that abnormalities in WM in left-sided temporo-occipitoparietal and primary sensory cortical regions underling visuospatial and sensory processing, respectively, are evident in BD and UD depression. These abnormalities might be associated with previously reported patterns in BD adults of abnormally elevated left medial occipital cortical activity during attention and executive function tasks (38–39) and an attentional bias to salient material (40) and in UD of abnormally decreased left occipital cortical activity to positive emotional stimuli (41). Examination of relationships between abnormal WM and abnormal activity in cortical regions supporting visuospatial processing—particularly in response to emotionally salient stimuli—should be the focus of further study in both BD and UD depression.
One potential limitation of the study was that both BD and UD depressed adults were taking psychotropic medication at the time of neuroimaging assessment, as was necessary because both groups required treatment for depression. The BD depressed adults were taking more antipsychotic and mood stabilizer medications, but fewer BD depressed were taking antidepressant medications than UD depressed adults. There were no relationships between medications and DTI measures that distinguished groups, which met the threshold for statistical significance after correction for multiple tests. The relatively small number of participants taking versus not taking each psychotropic medication subclass did not, however, allow the exclusion of a false negative finding. Additionally, most of our participants were female. Findings from analyses restricted to women, however, largely supported our findings for the entire sample of male and female participants. Further studies of WM connectivity should be conducted in larger numbers of men and women with BD and UD depression to determine whether gender differences in WM connectivity are present in these illnesses. These studies should also include further examination of relationships between WM connectivity and specific psychotropic medication subclasses in these individuals. Furthermore, studies with advanced DWI acquisitions and algorithms are needed to define the probability of an affected WM region to belong to a specific tract. With the αSim approach, we were able to obtain a reasonable correction (p < .05) for multiple tests and greatly enhanced the power of multiple statistical tests, but these findings did not achieve familywise cluster correction or TFCE and therefore require replication.
Our findings indicate that BD and UD depressed adults—otherwise matched for the majority of clinical variables, including depression severity, age of illness onset, and illness duration—can be distinguished by different patterns of right orbitomedial prefrontal cortical-limbic and left corticocortical WM connectivity. Our WM connectivity measures are promising and easily obtainable objective biological markers that have the potential to be used in future studies, alongside other biological measures of prefrontal cortical-limbic effective connectivity, to help identify pathophysiologic processes that might differ between BD and UD depression.
Supplementary Material
Acknowledgments
This study was supported in part by Grant 1 R01 MH076971-01 (MLP) from the National Institutes of Health (NIH), K25 MH076981-01 (WKT) from the NIH, a National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award (Nellie Blumenthal Investigator) (MLP), DMS-0806106 from the National Science Foundation (WKT), and an NIH NARSAD Young Investigator Award (AV).
Dr. Phillips had access to all data from the study, both what is reported and what is unreported, and had complete freedom to direct analysis and reporting, without influence from any sponsors. There was no editorial direction or censorship from any sponsors.
Footnotes
The authors reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: How far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64:161–174. [PubMed] [Google Scholar]
- 2.Kleinman L, Lowin A, Flood E, Gandhi G, Edgell E, Revicki D. Costs of bipolar disorder. Pharmacoeconomics. 2003;21:601–622. doi: 10.2165/00019053-200321090-00001. [DOI] [PubMed] [Google Scholar]
- 3.Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Jackowski M, Kalmar JH, Chepenik LG, Tie K, Qiu M, et al. Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. Br J Psychiatry. 2008;193:126–129. doi: 10.1192/bjp.bp.107.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Kalmar JH, Edmiston E, Chepenik LG, Bhagwagar Z, Spencer L, et al. Abnormal corpus callosum integrity in bipolar disorder: A diffusion tensor imaging study. Biol Psychiatry. 2008;64:730–733. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haznedar MM, Roversi F, Pallanti S, Baldini-Rossi N, Schnur DB, LiCalzi EM, et al. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57:733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, et al. Increased white matter connectivity in euthymic bipolar patients: Diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12:1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 8.Kafantaris V, Kingsley P, Ardekant B, Saito E, Lencz T, Lim K, et al. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:79–86. doi: 10.1097/CHI.0b013e3181900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahon K, Wu J, Malhotra AK, Burdick KE, Derosse P, Ardekani BA, Szeszko PR. A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology. 2009;34:1590–600. doi: 10.1038/npp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntosh AM, Maniega SM, Lymer GKS, McKirdy J, Hall J, Sussmann JED, et al. White matter tractography in bipolar disorder and Schizophrenia. Biol Psychiatry. 2008;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Maniega SM, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–21. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: A preliminary study. Am J Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 15.Bae JN, MacFall JR, Krishnan KRR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Li LJ, Ma N, Li ZX, Tan LW, Liu J, Gong GL, et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: A diffusion tensor imaging study. Brain Res. 2007;1168:124–128. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 17.Ma N, Li LJ, Shu N, Liu J, Gong GL, He Z, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. 2007;164:823–826. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- 18.Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, et al. Frontal white matter anisotropy and symptom severity of late-life depression: A magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–122. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Huang XB, Hong N, Yu X. White matter microstructural abnormalities in late-life depression. Int Psychogeriatr. 2007;19:757–766. doi: 10.1017/S1041610207004875. [DOI] [PubMed] [Google Scholar]
- 21.Yuan YG, Zhang ZJ, Bai F, Yu H, Shi YM, Qian Y, et al. White matter integrity of the whole brain is disrupted in first-episode remitted geriatric depression. Neuroreport. 2007;18:1845–1849. doi: 10.1097/WNR.0b013e3282f1939f. [DOI] [PubMed] [Google Scholar]
- 22.Zou K, Huang XQ, Li T, Gong QY, Li Z, Luo OY, et al. Alterations of white matter integrity in adults with major depressive disorder: A magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:525–530. [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy CF, Gunning-Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, et al. White-matter integrity predicts Stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan KM, Narayana PA. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magn Reson Med. 2006;56:130–137. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- 25.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 26.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassel S, Almeida JRC, Kerr N, Nau S, Ladouceur CD, Fissell K, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: No associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 34.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 35.Zanetti MV, Jackowski MP, Versace A, Almeida JR, Hassel S, Duran FL, et al. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci. 2009;259:316–328. doi: 10.1007/s00406-009-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frazier JA, Breeze JL, Papadimitriou G, Kennedy DN, Hodge SM, Moore CM, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 2007;9:799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 37.Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry. 2009;194:527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- 38.Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, Strakowski SM. Abnormal frontal white matter tracts in bipolar disorder: A diffusion tensor imaging study. Bipolar Disord. 2004;6:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 39.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: A review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 40.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 41.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.