Abstract
Context
Varenicline, an effective smoking cessation medication, functions as an α4β2 nicotinic acetylcholine receptor partial agonist. It indirectly affects the dopaminergic reward system by reducing withdrawal symptoms during abstinence and by decreasing the reinforcement received from nicotine while smoking. We hypothesize that varenicline would have a third mechanism to blunt responses to smoking cues in the reward-related ventral striatum and medial orbitofrontal cortex and would be associated with a reduction in smoking cue–elicited craving.
Design
A laboratory model of conditioned responding and arterial spin-labeled perfusion functional magnetic resonance imaging, a biomarker of regional brain activity, was used to test our hypothesis. Perfusion functional magnetic resonance imaging is quantitative and stable across time, facilitating the measurement of medication-induced neural modifications in the brain in response to a challenge (smoking cue exposure) and in the brain in the resting condition (without provocation). Smokers were imaged during rest and during smoking cue exposure before and after a 3-week randomized placebo-controlled medication regimen. Subjects were nonabstinent to explicitly examine the effects of varenicline on cue reactivity independent of withdrawal.
Setting
Center for the Study of Addictions, University of Pennsylvania, Philadelphia.
Subjects
Subjects were nicotine-dependent smokers who responded to advertisements placed on local radio and Listservs to participate in a medication-related research study that specifically stated “this is not a Quit Smoking Study” and “smokers may be contemplating but not currently considering quitting.”
Results
Prerandomization smoking cues vs nonsmoking cues activated the ventral striatum and medial orbitofrontal cortex (t=3.77) and elicited subjective reports of craving (P=.006). Craving reports correlated with increased activity in the posterior cingulate (t=4.11). Administration of varenicline diminished smoking cue– elicited ventral striatum and medial orbitofrontal cortex responses (t values from −3.75 to −5.63) and reduced self-reported smoking cue–elicited craving, whereas placebo-treated subjects exhibited responses similar to those observed prior to randomization. Varenicline-induced activation of lateral orbitofrontal cortex in the brain at rest (t=5.63) predicted blunting of smoking cue responses in the medial orbitofrontal cortex (r=−0.74).
Conclusions
Varenicline’s reciprocal actions in the reward-activated medial orbitofrontal cortex and in the reward-evaluating lateral orbitofrontal cortex underlie a diminished smoking cue response, revealing a distinctive new action that likely contributes to its clinical efficacy.
Numerous factors are involved in the motivation to smoke and are associated with relapse, including stress, peer pressure, availability, menstrual cycle phase, and even weight management.1–5 However, smoking cue–induced and withdrawal-induced cravings are 2 of the major contributors to relapse.6–9 Inability to combat withdrawal-induced craving, which declines within a month,10 plays a role in early relapse. Nevertheless, smokers report that smoking cues (eg, seeing a pack of cigarettes, socializing with others who smoke, and even internal mood states repeatedly associated with smoking) can trigger relapse months or even years after quitting. Some smokers who are thought to possess high “cue re-activity” are especially vulnerable and have an increased probability of relapse initiated by exposure to smoking cues.11,12 Therefore, treatments that target cue reactivity are important, particularly for cue-vulnerable individuals, but the effect of existing smoking cessation medications on smoking cue reactivity has not been thoroughly investigated. Thus far, research has focused on reduction of withdrawal and nicotine reward, which are known mechanisms underlying the effectiveness of first-line smoking cessation agents, such as varenicline, nicotine replacement therapy, and bupropion hydrochloride.13–15
Varenicline is a first-line smoking cessation agent16,17 that acts as a partial agonist at α4β2 acetylcholine nicotinic receptors, with indirect agonist and antagonist actions on the mesolimbic dopamine system. During the absence of nicotine, as in a quit attempt, it acts as an agonist to mildly increase dopaminergic tone and reduce withdrawal-induced craving. When nicotine is available, as in a relapse, it acts as an antagonist, preventing nicotine-evoked dopamine release and effectively blocking the reward usually received from nicotine while smoking.15 It is thought that the dual agonist-antagonist properties of varenicline are key mechanisms underlying its clinical effectiveness.
Various imaging modalities have observed a consistent neural substrate for cocaine, heroin, cigarette, and sexual cues.3,18–21 In studies of smoking cue reactivity, we characterized a neuroanatomical brain signature in response to exposure to smoking cues, independent of withdrawal, wherein the most profound effects were found in the interconnected ventral striatum (VS) and medial orbitofrontal cortex (mOFC).22–24 Our findings are in accordance with the substantial preclinical literature.25–27 Based on the evidence supporting a role for the medial ventral aspects of the mesolimbic system in drug cue reactivity, and varenicline’s actions to manipulate dopamine release, we hypothesized that chronic varenicline administration would suppress these responses; specifically, we hypothesized that varenicline would modulate activity in the mOFC, which is involved in sensory integration (representing the affective value of reinforcers) and decision making (for emotional rewards).28,29 Furthermore, we suspected that varenicline might diminish ventral striatal responses to cues because this region exerts strong control over emotional and motivational behavior, including craving.30,31 Preliminary data from our laboratory showed that varenicline selectively activated the lateral OFC (LOFC) in the brain at rest. Based on our data and the literature demonstrating that lateral pre-frontal regions are involved in regulating impulses, in reevaluating previously rewarded behavior, and in modulating downstream limbic regions involved in motivated behavior,29,32,33 we suspected that varenicline might enhance activity in lateral prefrontal regions. We predicted that varenicline-induced activation of the LOFC in the brain at rest would correlate with diminished neural responses during exposure to smoking cues.
To quantify the effects of long-term administration of varenicline on the resting brain and on the brain’s responses during exposure to smoking cues, we implemented a laboratory model of conditioned responding and the technique of continuous arterial spin-labeled (CASL) perfusion functional magnetic resonance imaging (fMRI) to image nonabstinent smokers before and after a 3-week double-blind randomized placebo-controlled medication regimen. The importance of using nontreatment-seeking smokers in our paradigm is 2-fold. First, our goal was to determine if and how varenicline affected smoking cue reactivity independent of withdrawal (which can persist for up to a month) because it has been shown that withdrawal itself can affect brain activity.34 Second, it was important that varenicline-treated and placebo-treated groups had similar smoking characteristics because differences in smoking behavior modulate brain activity.35 Thus, issues related to withdrawal and quitting smoking, which might obviate accurate interpretation of the effects of varenicline on exposure to smoking cues, were minimized.
Similar to positron emission tomography, perfusion fMRI is quantitative, providing a measure of cerebral blood flow in milliliters of blood per 100 g of tissue per minute,36 which facilitates the measurement of medication-induced neural modifications in the brain in response to tasks (cue exposure)23 and in the brain in the resting condition (without provocation)37 at successive time points. A pharmacological manipulation can have profound effects on the brain that cannot be observed using a relative measure such as blood oxygen level–dependent fMRI, which can only accurately examine changes that occur within a scanning session during a task or other provocation. Perfusion fMRI is reliable and reproducible following intervals as long as 7 weeks and is therefore ideal for longitudinal studies examining brain modifications induced by pharmacological agents.37
METHODS
SUBJECTS
Demographic characteristics and smoking history are listed in Table 1. Subjects were recruited through radio advertisements and Internet-based local Listservs that specifically stated that the study was intended for smokers who may be contemplating but who were not currently considering quitting. Severity of nicotine dependence was ascertained from the Fagerstrom Test for Nicotine Dependence.38 Subjects were screened, tested on study knowledge, and consented to participate prior to psychological and physical evaluations. The Mini International Neuropsychiatric Interview39 was used to determine current DSM-IV diagnosis of substance dependence (other than nicotine) and current severe psychiatric symptoms. Individuals with other current substance dependence, current Axis I DSM-IV psychiatric diagnoses, significant medical conditions, an intellectual ability estimate score of 80 or less on the Weschler Abbreviated Scale of Intelligence,40 an abnormal structural MRI, or a history of head trauma or injury causing loss of consciousness lasting longer than 3 minutes or associated with skull fracture or intercranial bleeding or who had irremovable magnetically active objects on or within their body were excluded. Smokers were compensated $233 for successful participation in all study procedures. The study, approved by the University of Pennsylvania institutional review board, adhered to the Declaration of Helsinki.
Table 1.
General Baseline Characteristics
| Characteristic | Both Groups (n=22) | Varenicline Group (n=11) | Placebo Group (n=11) | P Value |
|---|---|---|---|---|
| Male sex, No. | 16 | 9 | 7 | |
| Race, No. | ||||
| African American | 7 | 5 | 2 | |
| European American | 14 | 5 | 9 | |
| Mixed | 1 | 1 | 0 | |
| Handedness,a No. | ||||
| Right | 18 | 9 | 9 | |
| Left | 3 | 2 | 1 | |
| Both | 1 | 0 | 1 | |
| Age, mean (SEM), y | 36.1 (2.2) | 37.9 (3.4) | 34.4 (2.9) | .44 |
| Education, mean (SEM), y | 14.2 (0.4) | 14.4 (0.6) | 14.1 (0.6) | .74 |
| Cigarettes smoked per day, mean (SEM) [range], No. | ||||
| At start of study | 17.5 (1.6) [6–30] | 19.1 (2.4) [6–30] | 15.8 (2.0) [9–20] | .31 |
| At end of study | 11.9 (1.6) [1–30] | 11.8 (2.6) [2–30] | 12.0 (2.0) [1–24] | .96 |
| Pack yearsb | 12.6 (2.1) | 16.4 (3.5) | 8.7 (1.9) | .07 |
| FTND score | 4.7 (0.4) | 5.1 (0.7) | 4.3 (0.3) | .31 |
| Desire to quit,c % | 86.3 | 85.5 | 87.2 | .84 |
Abbreviation: FTND, Fagerstrom Test for Nicotine Dependence.
As assessed by the Edinburgh Handedness Inventory.
Calculated as the number of cigarettes smoked per day divided by number of cigarettes in a pack multiplied by the number of years smoking.
Assessed by asking subjects to rate their agreement with the phrase “I want to quit smoking.” Scores ranged from 0 to 100, where 0 indicates strongly disagree and 100 indicates strongly agree.
MEDICATION
Study medication was manufactured and provided by Pfizer Pharmaceuticals Inc. Study medication was prepared and maintained by the Investigational Drug Service located at the hospital of the University of Pennsylvania, in capsules containing 0.5 or 1 mg of varenicline or matching placebo. Medication was distributed in blister packs containing 1 weeks’ worth of medication. Varenicline was administered at a dosage of 0.5 mg twice a day for 3 days and 1.0 mg twice a day for the subsequent 18 days. The study physician dispensed the first week of medication at time 1 following the baseline scanning session. Adverse events, adherence to the dosing schedule, and cigarette smoking behaviors were monitored by the subjects using a daily diary, by study staff during biweekly telephone calls, and by a certified nurse practitioner under the direction of the study physician at weekly medication monitoring appointments.
STUDY DESIGN
Smokers were randomized in our double-blind study to receive either varenicline or placebo. Medication was prescribed to nonabstinent, nontreatment-seeking smokers. Two scanning sessions were administered: one prior to randomization at time 1 and the other on the 21st day of medication administration at time 2. Just prior to each scanning session, subjects smoked one of their own cigarettes to satiety.
Smoking preceded acquisition of imaging data by 35 minutes to ensure dissipation of the acute cardiovascular effects of smoking.41 Following a period of approximately 5 minutes during which subjects were made comfortable and placed in the scanner, images were acquired during a scanning session that included, in sequence, a 1-minute localizer scan, a 5-minute CASL resting-baseline scan, a 10-minute nonsmoking cue CASL scan, a 5-minute high-resolution structural scan, and a 10-minute smoking cue CASL scan. Nonsmoking cues were shown before smoking cue videos to minimize interference in “carryover” arousal initiated when drug cues are shown first, which can potentially affect responses to nondrug cues.42–44 The Shiffman-Jarvik (S-J) withdrawal scale was administered prior to exposure to smoking cues and again immediately afterward to assess cue-elicited or time-dependent changes in craving and withdrawal.45
Stimuli consisted of 10-minute audiovisual clips that featured either smoking-related or nonsmoking-related cues. Both videos featured actors who differed in race, age, and sex. Actors in the smoking video smoked while using explicit language designed to induce appetitive desire for a cigarette (eg, “The cigarette I enjoy most is the first cigarette of the day.”). The nonsmoking video was similar in content, except the actors related short stories that did not include cigarette smoking or smoking reminders. Two smoking cue and nonsmoking cue sets that were similarly valenced were used to control for habituation. Cue sets were counterbalanced such that half the subjects were exposed to the first cue set at time 1 and half were exposed to the second cue set at time 1. Procedures for imaging acquisition and processing have been previously published22,23 and are available in the eAppendix (http://www.archgenpsychiatry.com).
STATISTICAL ANALYSES OF DEMOGRAPHICS AND BEHAVIORS
Continuous demographic variables were summarized by calculating means and standard errors. Nominal demographic variables were summarized by calculating proportions and were compared across groups using χ2 analyses. Inadvertently, the S-J withdrawal scale was not administered to 3 subjects. Thus, analyses of items on the S-J withdrawal scale included 19 smokers (11 smokers receiving varenicline and 8 smokers receiving placebo). A repeated-measures analysis of variance (ANOVA) was used to assess the effect of medication (varenicline vs placebo), time (time 1 vs time 2), and the medication × time interaction on the S-J withdrawal scale and on the number of cigarettes smoked per day. Additional post hoc analyses were conducted to examine specific group differences. Analyses were conducted in Excel version 2008 (Microsoft, Redmond, Washington) and SPSS version 16.0 (SPSS Inc, Chicago, Illinois).
IMAGING ANALYSES
Voxel-wise analyses of the whole-brain cerebral blood flow (CBF) data were conducted for each subject, using a general linear model. Global CBF time course was included in the model as a covariate to examine the effects of varenicline on absolute regional blood flow. Analyses were conducted on absolute (resting baseline) and relative (during exposure to cue) CBF. No temporal filtering or smoothing was applied. Contrasts between conditions (smoking cue vs nonsmoking cue at each time point and resting baseline at time 1 vs time 2) were defined in the general linear model to assess the voxel-by-voxel CBF difference. Individual contrast images (β maps) were normalized into canonical space (Montreal Neurological Institute standard brain).
With the corresponding parametric maps of this contrast (β maps), random-effects analysis was used to test for a significant main effect of condition with a statistical parametric map of the t statistic at each voxel for population inference for each scan or session (second-level analysis). This step is equivalent to comparing CBF values between corresponding experimental conditions within each subject.
For comparisons between conditions and/or groups and regression analyses, only clusters with voxels having a height threshold exceeding P < .001 (uncorrected) and an extent threshold of 20 contiguous voxels are reported. Coordinates are in Montreal Neurological Institute standard brain as provided by SPM5 (Wellcome Trust Centre for Neuroimaging, London, England) and are those chosen from the suprathreshold voxel of each cluster using the The Human Brain by Duvernoy46 and the Atlas of the Human Brain by Mai et al47 as references.
RESULTS
SUBJECT CHARACTERISTICS
General demographic characteristics and smoking behaviors were not different between the varenicline group and the placebo group; however, there was a trend of fewer number of packs per year in the varenicline group. There was no difference in desire to quit smoking and cigarette dependence, and number of cigarettes smoked per day was not different at either time point (Table 1).
Repeated-measures ANOVA showed a significant time effect (reduction in number of cigarettes smoked per day) (F1,20=8.698; P=.008); however, there were no main effects of medication group or of medication × time interactions. In post hoc analyses, paired t tests showed that from time 1 to time 2, the number of cigarettes smoked per day was significantly reduced in the varenicline group (t10 = 2.654; P = .02) but not in the placebo group (t10=1.435; P=.18). The small sample sizes of our study are not powered to detect adverse events associated with varenicline administration; however, the eTable provides a summary of adverse events according to medication group.
BEHAVIORAL RESULTS
S-J Withdrawal Scale
The S-J withdrawal scale contains 5 items related to withdrawal: craving, psychological discomfort, physical discomfort, stimulation or sedation, and appetite (Table 2). It was administered prior to and immediately after exposure to smoking cues at time 1 and time 2.
Table 2.
Shiffman-Jarvik Withdrawal Scalea
| Item | Mean (SEM) Change Score at Time 1
|
Mean (SEM) Change Score at Time 2
|
||||||
|---|---|---|---|---|---|---|---|---|
| Varenicline Group | P Value | Placebo Group | P Value | Varenicline Group | P Value | Placebo Group | P Value | |
| Craving | 0.95 (0.38) | .03 | 2.03 (0.55) | .008 | 0.85 (0.51) | .13 | 1.65 (0.49) | .01 |
| Psychological discomfort | 0.15 (0.33) | .66 | 0.13 (0.44) | .78 | −0.06 (0.35) | .88 | −0.00 (0.49) | .99 |
| Physical symptoms | 0.03 (0.14) | .83 | 0.46 (0.59) | .47 | 0.24 (0.27) | .39 | −0.21 (0.24) | .42 |
| Stimulation or sedation | −1.21 (0.38) | .009 | −0.17 (0.39) | .69 | −0.52 (0.21) | .03 | −0.25 (0.32) | .46 |
| Appetite | −0.27 (0.41) | .52 | 0.13 (0.44) | .79 | 0.23 (0.30) | .47 | 0.38 (0.35) | .32 |
Paired samples’ t test between pre- and postscan scores at time 1 (prerandomization) and at time 2 (on the 21st day of a 3-week medication regimen). At time 1, only craving was affected by exposure to smoking cues in all subjects and when subjects were grouped by medication type. At time 2, craving decreased significantly from before to after exposure to smoking cues in the varenicline group, but no significant change was observed in the placebo group. The varenicline group reported a decrease in stimulation at both time points, whereas the other items remained unchanged in both groups. P values in bold indicate statistical significance at an α level of less than .05.
Time 1: Prerandomization
Repeated-measures ANOVA yielded no significant main effects of medication assignment at time 1 on all 5 items of the S-J withdrawal scale (ie, no differences between groups at baseline). There was a significant main effect of exposure to cue (before vs after exposure to smoking cues) on craving (F1,17=20.90; P=.001). There were no significant cue-induced changes in any other items, including psychological discomfort and physical discomfort, which are typically associated with nicotine withdrawal. In post hoc analyses, we observed significant increases in craving within the varenicline group (t10=2.47; P=.03) and the placebo group (t7=3.66; P=.008). There were no significant differences between before and after exposure to smoking cues in other items of the scale, except for stimulation or sedation, which decreased in the varenicline group (t10=3.21; P=.009).
Time 2: On Medication
Repeated-measures ANOVA yielded no significant main effects of medication group (varenicline vs placebo) or medication × time interactions on all items of the S-J withdrawal scale at time 2. The main effect of before vs after exposure to smoking cues in the craving item was significant (F1,17=11.65; P=.003). In post hoc analyses, craving was significantly increased from before to after exposure to smoking cues for the placebo group (t7=3.36; P=.01), whereas the varenicline group did not show a statistically significant increase from before to after exposure to smoking cues. As at time 1, the varenicline group reported a significant decrease in stimulation or sedation (t10= −2.48; P=.03), evincing that the effect was not related to medication.
Brain Imaging Results
Notes
Varenicline had numerous effects on the brain in both the resting state and during cue exposure, some of which likely contribute to its actions to blunt smoking cue reactivity and underlie its effectiveness in smoking cessation. The brain imaging results reported are those from hypotheses-driven a priori regions. Complete data on all brain activations and brain-behavioral associations are given to provide hypothesis-generating information to the field. An interactive visual display of all brain data in all 3 planes can be found at http://franklinbrainimaging.com. All comparisons and associations related to exposure smoking cues are made relative to exposure to nonsmoking cues.
Smoking Cue Responses at Time 1
As expected from our previous work,23,24 at time 1 and prior to randomization, exposure to smoking cues elicited activation in a large region of the VS and the interconnected mOFC, supporting hypotheses that these medial, reward-relevant structures play a key role in smoking cue reactivity,26,27 even in the absence of withdrawal (Figure 1; Table 3). Craving correlated with activation of the posterior cingulate cortex (coordinates x, y, z, respectively, in Montreal Neurological Institute space: 12, −40, 40; t=4.81). Although t values were not significant at P < .001, when applying a relaxed threshold (P values from .001 to .006), craving correlated with increased activity in the mOFC and the VS (Table 3).
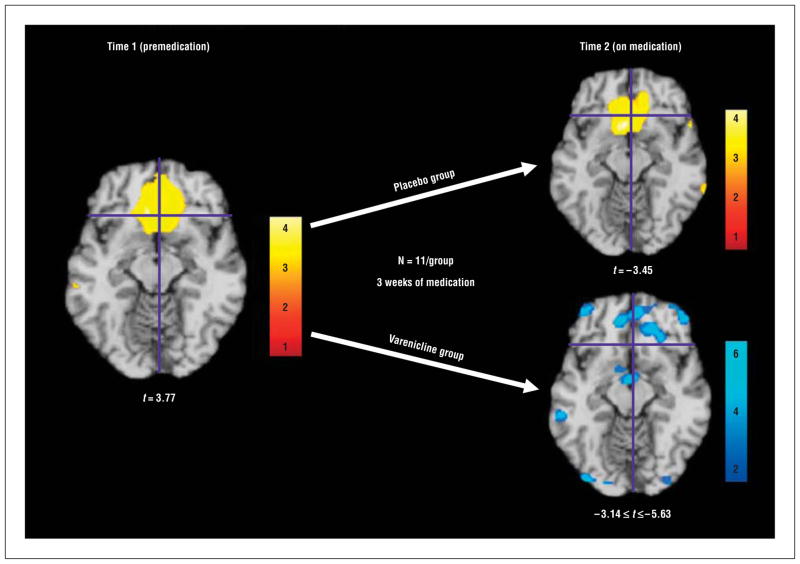
Figure 1.
Following the 3-week medication regimen, subjects receiving varenicline had reduced brain activity to smoking cues in the medial orbitofrontal cortex (mOFC). Illustrated are magnetic resonance imaging axial slices of a Montreal Neurological Institute (MNI) standard brain at times 1 and 2. At time 2, smoking cue–induced vs nonsmoking cue–induced increases in activity in the ventral striatum (VS) and mOFC illustrate varenicline’s action to reduce smoking cue–induced activity in several clusters within the mOFC. The placebo group exhibited similar VS and mOFC responses at both time points. Data are analyzed and displayed neurologically (left is left) in SPM5 at P=.01 and are overlaid on the MNI standard brain. Activations are significant at P < .001 (uncorrected at the cluster level). Axial slices are shown. “Hot” colors (shades of yellow, orange, and red) represent increases in brain activity, and “cool” colors (shades of blue) represent decreases in brain activity. Crosshairs for all conditions are centered on the peak voxel activated during exposure to smoking cues at time 1 (coordinates x, y, z, respectively, in MNI space: 2, 32, −12). Note the different t value scale in the varenicline image.
Table 3.
Brain Perfusion During Smoking vs Nonsmoking Cuesa
| Region | Both Groups at Time 1
|
Varenicline Group at Time 2
|
Placebo Group at Time 2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t | x | y | z | t | x | y | z | t | |
| VS and medial OFC bilateralb | −6 | 28 | 18 | 3.77 | −2 | 18 | −14 | 3.45 | ||||
| Medial OFCb | −10 | 60 | −20 | −4.21 | ||||||||
| 4 | 52 | −14 | −5.63 | |||||||||
| −8 | 48 | −14 | −3.58 | |||||||||
| −10 | 26 | −20 | −3.75 | |||||||||
| Superior frontal gyrus | −14 | 70 | 6 | −6.84 | ||||||||
| Dorsolateral prefrontal cortex | 36 | 54 | 18 | −5.04 | ||||||||
| Amygdala | 44 | 12 | −22 | −3.02 | ||||||||
| Superior temporal | −56 | −36 | −8 | −3.13 | 48 | 28 | −22 | 3.47 | ||||
| Anterior cingulate | −8 | −14 | 40 | 4.40 | ||||||||
| Cingulate sulcus | 2 | −44 | 52 | −4.02 | 14 | 58 | 2 | 4.65 | ||||
| Posterior cingulate | 0 | −68 | 16 | 4.73 | 0 | −36 | 42 | 3.25 | ||||
| −12 | −44 | 14 | 4.13 | |||||||||
| −14 | −40 | 36 | 6.88 | |||||||||
| Insula (ventral posterior) | 38 | −4 | −8 | 4.07 | 34 | 16 | −6 | 3.38 | ||||
| Dorsal posterior insula | 62 | −26 | 14 | −5.10 | ||||||||
| −60 | −14 | 2 | −4.69 | |||||||||
| Middle occipital | −30 | −78 | −2 | 5.80 | ||||||||
| Inferior temporal gyrus | −60 | −54 | −20 | 4.38 | ||||||||
| Medial temporal gyrus | −62 | −28 | 40 | 5.07 | ||||||||
| −58 | −14 | −2 | 3.24 | |||||||||
| 58 | −2 | −8 | 6.86 | |||||||||
| Inferior frontal gyrus | 60 | 18 | 14 | 15.65 | ||||||||
| Lateral (extreme) OFC | 44 | 28 | −20 | 4.75 | ||||||||
| Dorsolateral prefrontal cortex | −26 | 44 | 6 | 4.20 | ||||||||
| −26 | 44 | −4 | 4.39 | |||||||||
| −38 | 38 | 4 | 3.63 | |||||||||
| Precentral gyrus | −46 | −10 | 50 | 15.83 | ||||||||
| −26 | −34 | 66 | 4.68 | |||||||||
| 12 | −6 | 64 | 4.18 | |||||||||
| 34 | −32 | 66 | 5.12 | |||||||||
| Cerebellum | 0 | −48 | −10 | 9.71 | ||||||||
Abbreviations: OFC, orbitofrontal cortex; VS, ventral striatum.
Listed are the coordinates x, y, z from the suprathreshold voxel within a cluster and the t value from regions activated during exposure to smoking cues relative to exposure to nonsmoking cues at time 1 in all subjects and at time 2 in the varenicline group and the placebo group, separately. Note that the VS and medial OFC are still activated in the placebo group at time 2. Varenicline administration reduced activity in reward-related circuitry, including several areas in the VS and medial OFC and in the amygdala. Additionally, varenicline administration increased activity in several regions. Activations are significant at P < .001 (uncorrected at the cluster level). Left-sided brain responses are indicated by negative x-coordinate values.
A priori regions.
Smoking Cue Responses at Time 2
The smoking cue– elicited neural responses in reward-related circuits observed at time 1 were blunted after 3 weeks of varenicline administration, including within several clusters of the mOFC (Figure 2; Table 3). Three weeks of varenicline administration increased activity in several brain regions (including the anterior and posterior cingulate, the inferior, medial, and superior frontal gyri, the LOFC, and the dorsolateral prefrontal cortex) during exposure to smoking cues (Table 3). Subjects treated with placebo exhibited mOFC and VS responses that were similar to those observed at time 1. Craving correlated with increased activity in the posterior cingulate and the mOFC in the placebo group but not in the varenicline group (Table 4). Because the item stimulation or sedation on the S-J withdrawal scale was significantly different in the varenicline group at both time points, it was included in the final contrasts as a covariate of no interest, which did not statistically affect the results.
Figure 2.
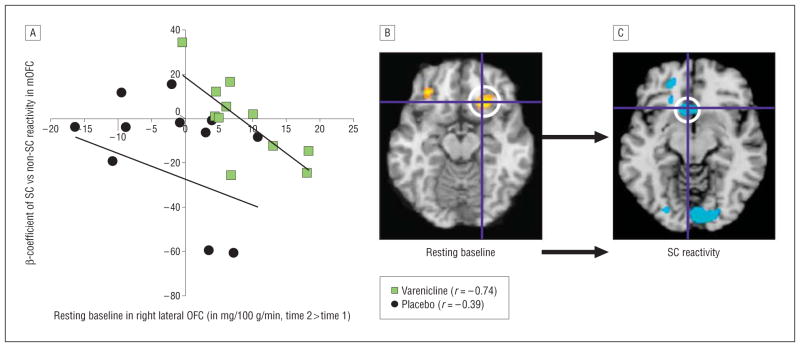
Resting baseline activity in right lateral orbitofrontal cortex (LOFC) predicted diminished cue responses in medial OFC (mOFC). A, Change scores from time 1 to time 2 of individual perfusion functional magnetic resonance imaging responses (β coefficients) during exposure to smoking cues (SCs) vs non-SCs in the mOFC (x axis) as a function of change scores in resting brain activity in the right LOFC (y axis). Individual “subject” values selected for quantifying LOFC effects were acquired by calculating a mean value from a sphere with a 6-mm radius, its center located at the resting-baseline group data suprathreshold voxel of the activated cluster (coordinates x, y, z, respectively, in Montreal Neurological Institute [MNI] standard brain: 22, 26, −14). B, Three weeks of varenicline resulted in an increase in activity in the right LOFC. Crosshairs are centered on the coordinates of the peak voxel of the cluster. C, Effect of varenicline-induced activation of the right LOFC on the mOFC during SC exposure (coordinates: −6, 20, −10). Data are analyzed and displayed neurologically (left is left) in SPM5 at P=.01 and are overlaid on the MNI standard brain. Activations are significant at P < .001 (uncorrected at the cluster level). Axial slices are shown. “Hot” colors (shades of yellow and orange) represent increases in brain activity, and “cool” colors (shades of blue) represent decreases in brain activity.
Table 4.
Correlations Between Brain Perfusion During Smoking Cue Exposure and Smoking Cue–Elicited Cravinga
| Region | Both Groups at Time 1
|
Varenicline Group at Time 2
|
Placebo Group at Time 2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t | x | y | z | t | x | y | z | t | |
| Medial OFCb | 2 | 32 | −12 | 2.67 | 8 | 60 | −22 | 3.77 | ||||
| −6 | 62 | −18 | 3.37 | |||||||||
| 16 | 54 | −20 | 4.00 | |||||||||
| VSb | 26 | 16 | −2 | 2.93 | ||||||||
| VS and medial OFCb | 24 | 14 | −14 | 2.42 | ||||||||
| Lateral OFC | 30 | 36 | −20 | −3.27 | ||||||||
| −48 | 16 | 46 | −6.12 | |||||||||
| 44 | 48 | −8 | −3.84 | |||||||||
| Dorsolateral prefrontal cortex | −30 | 34 | 12 | −6.84 | ||||||||
| Ventromedial prefrontal cortex | 12 | 54 | −6 | 3.00 | ||||||||
| Parahippocampus | 18 | −22 | −8 | 3.94 | ||||||||
| Anterior dorsal insula | −38 | −26 | 26 | 6.42 | ||||||||
| −48 | 18 | 50 | 3.66 | |||||||||
| Posterior dorsal insula | 40 | −10 | 2 | 2.94 | 40 | −6 | 2 | 3.02 | ||||
| Posterior cingulate | 12 | −40 | 40 | 4.71 | 8 | −44 | 46 | 3.05 | ||||
| Precentral gyrus | 46 | −12 | 36 | 5.76 | ||||||||
| 62 | 2 | 24 | 4.88 | |||||||||
| Superior parietal (precuneus) | 2 | −60 | 58 | 3.84 | ||||||||
| Superior temporal gyrus | 60 | 16 | −18 | 3.80 | ||||||||
| Anterior occipital sulcus | −38 | −70 | −4 | 3.53 | ||||||||
| Supramarginal gyrus | −54 | −46 | 34 | −3.89 | ||||||||
| Middle frontal gyrus | ||||||||||||
| Inferior temporal cortex | 54 | −24 | −22 | −3.44 | ||||||||
| Cerebellum | −30 | −48 | −26 | 3.27 | ||||||||
Abbreviations: OFC, orbitofrontal cortex; VS, ventral striatum.
Listed are the coordinates x, y, z from the suprathreshold voxel within a cluster and t values from the regions that correlated with smoking cue–elicited craving as assessed by the “craving” item of the Shiffman-Jarvik withdrawal scale in all groups at both time points. Activations are significant at P < .001 (uncorrected at the cluster level). Left-sided brain responses are indicated by negative x-coordinate values. Negative t values are regions wherein activation was reduced. (Note that lateral OFC activity decreased in the placebo group at time 2.)
Regions that were a priori and that approached significance.
Effects of Varenicline on Resting Baseline
A CASL perfusion scan was acquired on the brain at rest prior to acquisition of cue data at both time points, to examine neural activity specifically related to the medication regimen unprovoked by task. Three weeks of varenicline administration increased activity in a priori LOFC (Figure 2B; Table 5). In addition to modulation of the LOFC, varenicline also modified the resting-baseline activity in other brain regions. Although these regional changes were not part of our a priori hypothesis, they deserve consideration as part of the circuitry underlying varenicline’s effectiveness in smoking cessation. Activity in the right amygdala, a region that is rapidly activated by salient emotional stimuli, was reduced as a result of varenicline administration. Varenicline-induced reductions in activity were also observed in the insula; however, the portion of the insula modified was posterior and dorsal to the ventral anterior portion, which has been shown to be involved in exposure to smoking cues, craving, and relapse.12,24,48 There were no differences in resting-baseline activity between times 1 and 2 in the placebo group.
Table 5.
Effects of Varenicline on Resting Baselinea
| Effect on Region | x | y | z | t |
|---|---|---|---|---|
| Increases | ||||
| Lateral OFC | 22 | 26 | −14 | 5.63 |
| −30 | 34 | 12 | 4.21 | |
| 46 | 52 | 18 | 4.24 | |
| Medial temporal cortex | −52 | −28 | −8 | 8.23 |
| Cerebellum | −46 | −66 | −26 | 7.47 |
| Occipital cortex | −6 | −92 | −10 | 5.48 |
| Gyrus rectus (extreme ventral OFC) | 4 | 56 | −20 | 4.04 |
| Middle frontal gyrus | −46 | 8 | 44 | 3.70 |
| Superior frontal cortex | 2 | 42 | 52 | 3.31 |
| Decreases | ||||
| Amygdala | 22 | 4 | −28 | −5.53 |
| Dorsal middle insula | −38 | 10 | 8 | −3.56 |
| Parahippocampus | −30 | −30 | −2 | −7.52 |
| 24 | −34 | 4 | −4.41 | |
| Superior frontal cortex | 0 | 6 | 58 | −3.77 |
Abbreviation: OFC, orbitofrontal cortex.
Listed are the coordinates x, y, z from the suprathreshold voxel within a cluster and t values in the brain at rest that were affected by 3 weeks of varenicline administration (time 2 vs time 1). Activations are significant at P < .001 (uncorrected at the cluster level). Left-sided brain responses are indicated by negative x-coordinate values. There were no effects of placebo on the brain at rest during the 3-week regimen.
Effects of Varenicline-Induced Resting-Baseline Activation in LOFC on Smoking Cue Responses
Next, we examined the effects of varenicline’s activation of resting baseline in the LOFC on smoking cue–induced neural responses. The individual subject’s perfusion fMRI responses in the LOFC in the brain at rest were extracted and entered as a covariate into the SPM5 smoking cue vs nonsmoking cue contrast at time 2. The values used for LOFC activity were acquired by calculating a mean value from a sphere with a 6-mm radius, its center located at the resting-baseline group data suprathreshold voxel (coordinates x, y, z, respectively, in Montreal Neurological Institute standard brain: 22, 26, −14; t=5.63). We observed an inverse relationship between resting-baseline activation in the LOFC and smoking cue responses in the mOFC; that is, increased LOFC activity in the brain at rest predicted decreased reward-related responses during exposure to smoking cues (Figure 2; Table 6). Although both the left and right LOFC were activated in the resting-baseline condition in the varenicline group (but not in the placebo group), only right OFC activation predicted blunted smoking cue responses in the mOFC and in additional non–a priori brain regions.
Table 6.
Varenicline-Induced Activation of the Lateral Orbitofrontal Cortex in the Brain at Rest Correlate With Its Reductions in Neural Responses to Smoking Cuesa
| Effect on Region | Both Groups at Time 1
|
Varenicline Group at Time 2
|
Placebo Group at Time 2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t | x | y | z | t | x | y | z | t | |
| Increases | ||||||||||||
| Anterior cingulate | 2 | 38 | 4 | 4.46 | ||||||||
| Thalamus | −22 | −6 | −6 | 3.80 | ||||||||
| −12 | −4 | 0 | 4.31 | |||||||||
| 8 | −16 | −6 | 3.03 | |||||||||
| Parahippocampus | 16 | −56 | −2 | 3.10 | 30 | −44 | −6 | 3.63 | ||||
| −34 | −64 | −8 | 4.53 | |||||||||
| Hippocampus | 24 | −14 | −12 | 3.44 | ||||||||
| Precuneus | 0 | −58 | 16 | 3.92 | ||||||||
| Decreases | ||||||||||||
| VS and mOFC | −6 | 20 | −10 | −3.31 | ||||||||
| Postcentral gyrus | 4 | −30 | 62 | −4.64 | ||||||||
| Superior frontal cortex | 2 | 24 | 40 | −4.21 | ||||||||
| Ventral lateral PFC | −38 | 44 | −4 | −3.23 | ||||||||
| 40 | 42 | −10 | −3.35 | |||||||||
| Amygdala | 20 | 6 | −20 | −3.26 | ||||||||
| Lingual gyrus | 8 | −88 | −16 | −5.59 | ||||||||
Abbreviations: mOFC, medial orbitofrontal cortex; PFC, prefrontal cortex; VS, ventral striatum.
Listed are the coordinates x, y, z from the suprathreshold voxel within a cluster and t values from regions wherein resting-baseline activation in lateral OFC correlated with activity in the brain during exposure to smoking cues. Note that in the varenicline group only, enhanced activation in the lateral OFC correlated with reductions in activity in the VS and mOFC. Individual “subject” values selected for measuring lateral OFC effects were acquired by calculating a mean value from a sphere with a 6-mm radius, its center located at the resting-baseline group data suprathreshold voxel (coordinates x, y, z, respectively: 22, 26, −14). The t values listed are from the suprathreshold voxel within the cluster. Activations are significant at P < .001 (uncorrected at the cluster level). Left-sided brain responses are indicated by negative x-coordinate values.
COMMENT
We report herein that neural responses to smoking cues in the anatomically connected VS and mOFC are diminished after 3 weeks of varenicline administration and that subjective craving responses to smoking cues are also diminished. Furthermore, we show that varenicline-induced activation of the LOFC in the brain at rest predicts the blunted smoking cue–induced activity in the mOFC. Varenicline’s reciprocal actions in the reward-relevant mOFC and the reward-evaluating LOFC underlie a diminished smoking cue response and may reveal a distinctive new action that likely contributes to its clinical efficacy in smoking cessation.
We hypothesize that varenicline’s antagonistic action to block the reinforcement normally received during smoking might suppress smoking cue–elicited responses in the VS and mOFC. In support of our hypothesis, we demonstrate that varenicline significantly reduced prerandomization smoking cue–elicited craving and diminished smoking cue–induced neural responses in the VS and mOFC and in other reward-related regions. Placebo-treated smokers displayed smoking cue–induced craving at both time points and had brain responses similar to those observed prior to randomization.
Varenicline induced bilateral activation of the LOFC in the brain in the resting condition. Several lines of evidence support the theory that the OFC is involved in assimilating information on the reward value of incoming stimuli to determine the appropriate course of action, with its medial and lateral aspects subserving diverse functions.29,49 Evidence suggests a functional segregation of the OFC’s role in goal-directed behavior such that when reward value is low, as in a sated condition, processing is evident in the LOFC. In contrast, when reward value is high, as in a deprived condition, processing in the medial portion of the OFC is prominent.32,33 The results of our study extend hypotheses of 2 separate motivational systems within the OFC: the medial-portion– orchestrating approach and the lateral-portion avoidance behaviors.32 Given that varenicline reduces the reinforcement received from smoking and that subjects had smoked immediately prior to scanning, the role of the LOFC here may be to reevaluate and devalue the previous appetitive conditioned motivational properties of smoking-associated stimuli.
Given the role of the LOFC in reward-processing and decision making,49 we hypothesize that the varenicline-induced increased activity in the LOFC in the resting condition may account for the attenuated cue-induced responses in the mOFC. We observed an inverse relationship between resting-baseline activation in the LOFC and cue responses in the mOFC (ie, increased LOFC activity in the brain at rest predicted decreased mOFC responses during exposure to smoking cues). These results are important in and of themselves as they elucidate the neural correlates underlying varenicline’s action to diminish smoking cue reactivity. They also have broader implications, because the perfusion fMRI cue-reactivity pre- and postmedication model employed here may potentially be used as a screening tool to examine the likelihood of the effectiveness of promising candidate smoking cessation medications in reducing cue reactivity, prior to investing in arduous and expensive treatment trials. Although varenicline is clinically effective in preventing relapse, it aids only a subgroup of individuals; thus, knowledge of the underlying mechanism is crucial because it will guide the development of future, more effective interventions for cue-vulnerable individuals.
Because varenicline is an effective smoking cessation agent, one might speculate that the varenicline-treated group would either spontaneously quit smoking or reduce the number of cigarettes they smoked per day. No subjects in either group quit smoking, and the level of cigarette dependence and the number of cigarettes smoked per day were not different between varenicline- and placebo-treated groups at time 2. However, the varenicline group did show a significant reduction in number of cigarettes smoked per day from time 1 to time 2. Furthermore, there is considerable variability in human smoking behavior (ie, puff duration, puff volume, puff interval, and vent blocking), and it is highly probable that varenicline- and placebo-treated smokers differed in the actual amount of nicotine and other tobacco constituents that were consumed over the course of the medication regimen. Because all subjects smoked a cigarette prior to the scanning sessions, we feel that any potential differences in smoking behavior were minimized and that any residual effects are unrelated to our major finding of varenicline-induced reductions in smoking cue reactivity. Smoking immediately prior to scanning also served to minimize withdrawal, which facilitated achieving our overarching goal: to examine varenicline’s effects on cue reactivity independent of its known effects on withdrawal reduction. Given the absence of differences between the items of the S-J withdrawal scale, administered before and after exposure to smoking cues (at both time points), we assert that the observed effects of varenicline were specific to exposure to smoking cues and not to either withdrawal or changes in smoking behavior.
We observed correlative relationships between craving and brain activity in the VS and mOFC, which may be expected given the role of these regions in reward-related behavior. And, as in our first study,23 at both time points, we observed a correlative relationship between craving and activation in the posterior cingulate cortex, a region that is beginning to attract attention in the addiction literature, albeit its role is still unclear.3 The congruence between our earlier studies and this one implicating the posterior cingulate as a substrate mediating subjective craving may be fortuitous, because studies demonstrating correlative relationships between craving induced by drug cue exposure and brain activity are often in opposition18,22,50 or have not observed these relationships.51,52 Alternatively, as evinced by Franklin et al,22 wherein correlative relationships between brain and craving responses were found to be dopamine transporter–dependent, genetic variance may underlie the discordant and/or negative findings in the literature. Additionally, because there are caveats associated with reliance on subjective measures, the use of objective markers such as cognitive bias and/or attentional tasks to examine brain-behavioral relationships may be important.
Although brain-craving correlates vary across studies, including our own, the overall “brain” finding of enhanced responses to smoking cues in reward-relevant interconnected medial ventral aspects of the mesolimbic system is consistent across our 3 studies. These regions have also been consistently observed in cocaine, heroin, and other nicotine and sexual cues using a variety of imaging modalities.3,8–21 This congruence across studies emphasizes the power of a direct assay of brain physiology, such as fMRI, to study addiction processes. Because the brain’s response to emotionally laden processes is not completely under subjective control, and may be confounded by the ability to identify and communicate one’s emotional state, neurophysiological measures may be more direct and more sensitive than subjective measures of the same processes.53–56
Because drug cues play a key role in cigarette and other drug addiction processes, the action of varenicline to reduce cue reactivity may help explain its efficiency in reducing relapse. Varenicline is known to mitigate withdrawal symptoms and the reinforcement received during smoking.15 These actions may be more beneficial for smokers whose relapse is influenced to a greater degree by the absence of the pharmacological effects of nicotine on the brain (withdrawal). Conversely, medications that reduce the effect of exposure to smoking cues may be more beneficial to cue-vulnerable smokers. Unsuccessful smoking cessation is more prevalent in individuals with psychiatric illness,57,58 suggesting that they have greater difficulty quitting. Varenicline and other medications that can reduce both withdrawal and cue reactivity may be of special benefit to these subgroups.
As stated, varenicline’s action to blunt cue-induced reward-related activity in the medial OFC was predicted by its activation of the LOFC in the brain at rest. Although there is a large body of literature demonstrating 2 separate motivational systems within the OFC in response to natural rewards, to our knowledge, none of the studies have attempted to dissect their diverse roles in drug reward. The results of our study reveal a distinctive new action of varenicline that may contribute to its clinical efficacy. Furthermore, the discovery of the neural correlates underlying its action to diminish cue reactivity has relevance for the use of neuroimaging in the development of improved treatment strategies in cigarette and other drug addictions.
Acknowledgments
Funding/Support: Funding for this study was provided by an Investigator Initiated Research grant from Pfizer and grants NIH NIDA 5-P60-DA-005186-20 and NIH NIDA R21DA025882-02 from the National Institutes of Health.
Role of the Sponsor: Pfizer had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Franklin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: Dr Franklin has received research support from Pfizer Pharmaceuticals and served as a consultant for Pfizer Pharmaceuticals and Abbott Laboratories. Dr Detre served as a consultant for Pfizer Pharmaceuticals. Dr Detre is an inventor on the University of Pennsylvania’s patent for arterial spin labeling and entitled to institutional royalty sharing for its licensure. Dr O’Brien served as a consultant for Abbott Laboratories and Embera. Dr Childress served as a consultant for Abbott Laboratories.
Previous Presentations: This study was presented in part at the American College of Neuropsychopharmacology Annual Meetings in December 9, 2008 (Hollywood, Florida), and December 8, 2009 (Scottsdale, Arizona).
Online-Only Material: The eAppendix and eTable are available at http://www.archgenpsychiatry.com.
Additional Information: Data acquired at time 1 (pre-randomization) were combined with a larger data set examining cue reactivity with respect to genetic variance in the dopamine transporter, which resulted in a manuscript that was accepted by Addiction Biology. The data presented here are a subset of those subjects who then participated in a 3-week randomized placebo-controlled medication regimen to examine the effects of varenicline on cue reactivity.
Additional Contributions: The nursing staff at the Treatment Research Center provided the initial physical evaluations and subject medication monitoring. We thank the magnetic resonance imaging technicians at the hospital of the University of Pennsylvania for their technical assistance, Robert Fabianski, BS, for final proofreading, and Hans Rollema, PhD, formerly a Research Fellow of Pfizer Global Research and Development, for encouraging the submission of the Investigator Initiated Application.
References
- 1.Perkins KA. Smoking cessation in women: special considerations. CNS Drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- 2.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26 (1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 3.Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain Res. 2009;1293:40–48. doi: 10.1016/j.brainres.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health (Larchmt) 2008;17(2):287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins KA, Marcus MD, Levine MD, D’Amico D, Miller A, Broge M, Ashcom J, Shiffman S. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consult Clin Psychol. 2001;69(4):604–613. [PubMed] [Google Scholar]
- 6.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70(4):515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 7.Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. Nebr Symp Motiv. 1986;34:257–323. [PubMed] [Google Scholar]
- 8.Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5(2):137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- 9.Rose JE. Nicotine addiction and treatment. Annu Rev Med. 1996;47:493–507. doi: 10.1146/annurev.med.47.1.493. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 11.Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95(suppl 2):S129–S144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- 12.Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, III, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 15.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28(7):316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Wu P, Wilson K, Dimoulas P, Mills EJ. Effectiveness of smoking cessation therapies: a systematic review and meta-analysis. BMC Public Health. 2006;6:300. doi: 10.1186/1471-2458-6-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008;(3):CD006103. doi: 10.1002/14651858.CD006103.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59(12):1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 19.Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3(1):e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33(9):2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 21.Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165(3):390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- 22.Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O’Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34(3):717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32(11):2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 24.Franklin TR, Wang Z, Li Y, Suh J, Goldman M, Lohoff F, Cruz J, Hazan, Jens W, Detre JA, Berretini W, O’Brien CP, Childress AR. Dopamine transporter genotype modulation of neural responses to smoking cues: confirmation in a new cohort. Addict Biol. doi: 10.1111/j.1369-1600.2010.00277.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin TR, Druhan TP. Involvement of the nucleus accumbens and medial pre-frontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology. 2000;23(6):633–644. doi: 10.1016/S0893-133X(00)00162-7. [DOI] [PubMed] [Google Scholar]
- 26.Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107(2–3):285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 27.Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393(1–3):295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 28.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 29.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 30.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 31.Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning: role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- 32.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(pt 9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 33.Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10(3):308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27(51):14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155 (8):1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 36.Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16(6):1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Hermes M, Hagemann D, Britz P, Lieser S, Rock J, Naumann E, Walter C. Reproducibility of continuous arterial spin labeling perfusion MRI after 7 weeks. MAGMA. 2007;20(2):103–115. doi: 10.1007/s10334-007-0073-3. [DOI] [PubMed] [Google Scholar]
- 38.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 39.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–57. [PubMed] [Google Scholar]
- 40.Weschler D. Wechsler Abbreviated Scale of Intelligence Manual. San Antonio, TX: Harcourt Brace & Co; 1999. [Google Scholar]
- 41.Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422–1431. doi: 10.1016/s0735-1097(97)00079-x. [DOI] [PubMed] [Google Scholar]
- 42.Wilson SJ, Sayette MA, Fiez JA, Brough E. Carry-over effects of smoking cue exposure on working memory performance. Nicotine Tob Res. 2007;9(5):613–619. doi: 10.1080/14622200701243144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma D, Money S. Carryover effects to addiction-associated stimuli in a group of marijuana and cocaine users. J Psychopharmacol. 2010;24(9):1309–1316. doi: 10.1177/0269881109350079. [DOI] [PubMed] [Google Scholar]
- 44.Waters AJ, Sayette MA, Franken IH, Schwartz JE. Generalizability of carry-over effects in the emotional Stroop task. Behav Res Ther. 2005;43(6):715–732. doi: 10.1016/j.brat.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976;50(1):35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- 46.Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with Magnetic Resonance Imaging and Blood Supply. 2. New York, NY: Springer-Verlag Wien; 1999. [Google Scholar]
- 47.Mai JK, Voss T, Paxinos G. Atlas of the Human Brain. 3. New York, NY: Elsevier Academic Press; 2008. [Google Scholar]
- 48.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elliott R, Agnew Z, Deakin JF. Hedonic and informational functions of the human orbitofrontal cortex. Cereb Cortex. 2010;20(1):198–204. doi: 10.1093/cercor/bhp092. [DOI] [PubMed] [Google Scholar]
- 50.McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30(10):1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58(6):488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159(6):954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 53.Kirsch P, Reuter M, Mier D, Lonsdorf T, Stark R, Gallhofer B, Vaitl D, Hennig J. Imaging gene-substance interactions: the effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neurosci Lett. 2006;405(3):196–201. doi: 10.1016/j.neulet.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 54.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 56.Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimasu K, Kiyohara C. Genetic influences on smoking behavior and nicotine dependence: a review. J Epidemiol. 2003;13(4):183–192. doi: 10.2188/jea.13.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilens TE, Vitulano M, Upadhyaya H, Adamson J, Sawtelle R, Utzinger L, Biederman J. Cigarette smoking associated with attention deficit hyperactivity disorder. J Pediatr. 2008;153(3):414–419. doi: 10.1016/j.jpeds.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]