Abstract
Objective
Childhood adverse experiences are known to engender persistent changes in stress-related systems and brain structures involved in mood, cognition, and behavior in animal models. Uncertainty remains about the causal effect of early stressful experiences on physiological response to stress in human beings, as the impact of these experiences has rarely been investigated while controlling for both genetic and shared environmental influences.
Method
We tested whether bullying victimization, a repeated adverse experience in childhood, influences cortisol responses to a psychosocial stress test (PST) using a discordant monozygotic (MZ) twin design. Thirty pairs (43.3% males) of 12-year-old MZ twins discordant for bullying victimization were identified in the Environmental Risk (E-Risk) Longitudinal Twin Study, a nationally representative 1994–1995 cohort of families with twins.
Results
Bullied and nonbullied MZ twins showed distinct patterns of cortisol secretion after the PST. Specifically, bullied twins exhibited a blunted cortisol response compared with their nonbullied MZ co-twins, who showed the expected increase. This difference in cortisol response to stress could not be attributed to children's genetic makeup, their familial environments, pre-existing and concomitant individual factors, or the perception of stress and emotional response to the PST.
Conclusion
Results from this natural experiment provide support for a causal effect of adverse childhood experiences on the neuroendocrine response to stress.
Keywords: early-life stress, cortisol, HPA axis, discordant MZ twin design, bullying
Severe abuse and neglect experienced early in life are associated with poor physical and mental health.1 Harmful effects of other forms of stress experienced in childhood, such as bullying victimization, are also increasingly recognized, but their consequences for health are less studied. Bullying is present when children or adolescents are exposed to repeated harassment and humiliation from peers between whom there is an imbalance of power whereby it is difficult for the victims to defend themselves. Evidence indicates that emotional problems in bullied children were not merely due to the victims’ genetic background or pre-existing characteristics,2 supporting an environmentally mediated effect of bullying victimization on emotional problems. Considering that approximately 13% of children are victims of bullying worldwide,3 and that its adverse impacts are not entirely explained by genetic factors, identifying the mechanisms by which bullying victimization gets “under the skin” is pressing.4 This is the focus of the present study.
It has been hypothesized that early-life stress may alter physical and mental health through hypothalamic–pituitary–adrenocortical (HPA) axis activity.5,6 The HPA axis underlies both adaptive and maladaptive responses to stress. Adaptive responses are characterized by a relatively rapid increase in cortisol, the end-product of the HPA axis, followed by a progressive decline. Conversely, persistently increased or blunted cortisol secretion may signal maladaptive responses to stress and are hypothesized to increase vulnerability to stress-related diseases.7 Whether bullying victimization, a repeated stress commonly experienced during childhood, is associated with disrupted cortisol secretion remains unclear.8-10
Early-life stress, such as maternal depression and maltreatment, has been associated with disrupted HPA axis activity, showing both high and low cortisol secretion in childhood.5,11 A study reported that 12- to 16-year-old females with a history of maltreatment showed blunted cortisol responses to the Trier Social Stress Test (TSST) in comparison to typical increases exhibited by controls matched for age and neighborhood.12 Studies examining the association between early-life stress and cortisol activity, however, often rely on retrospective reports of childhood adversity, which may include bias and substantial measurement error. In addition, because most studies are cross-sectional, it is difficult to test the cumulative cost of repeated adverse experiences. Finally, time delay between early-life stress and cortisol measurements leaves the possibility that intermediate events obscure the presumed effects of early-life stress on HPA axis activity.
Animal models have demonstrated that early-life stress causes persistent changes in HPA axis activity that can not be explained by genetic factors.11,13,14 In human studies, the effect of early-life stress on the HPA axis, controlling for both genetic and shared environmental influences, has rarely been investigated.15 Disrupted cortisol secretion in bullied children could be explained by inherited factors affecting both cortisol activity and exposure to adversity because cortisol reactivity and bullying victimization are partly heritable.16-19 In the absence of experimental designs involving random assignment of participants to different early stress conditions, uncertainty remains about the causal effect of early-life stress on cortisol secretion in human beings.
Randomly assigning children to adverse environments is unethical in human beings for obvious reasons, hence limiting causal inferences relating to early-life stress. Alternatively, rigorous control for confounders could be achieved by contrasting genetically identical individuals drawn from the same family environment but who are exposed to distinct naturally occurring experiences. The discordant monozygotic (MZ) twin design offers this possibility. Differences in cortisol activity within MZ (genetically identical) twins who grow up in the same family but who are exposed to different experiences such as bullying victimization would not be attributable to the children's genetic makeup or their familial environments.15,20
The objective of this study was to examine the impact of bullying victimization on cortisol reactivity. More specifically, we examined whether cortisol response to a psychosocial stress test (PST) differed between bullied and nonbullied children controlling for the confounding effect of genetic and familial environmental factors using a discordant MZ twin design. Based on previous findings suggesting that early-life stress is associated with abnormal cortisol secretion, we hypothesized that bullied twins would have impaired cortisol responses to the PST whereas their nonbullied MZ co-twins will show an increase in cortisol secretion after exposure to this experimental stress task. We also explored the association between cortisol response and a continuously distributed index of bullying in bullied twins to investigate whether children exposed to more frequent, chronic, and severe bullying experiences showed greater cortisol disruption during the PST.
METHOD
Sample
Participants were recruited from the E-Risk Longitudinal Twin Study, which tracks the development of a nationally representative birth cohort of 2,232 British children.21 The sample was drawn from a larger birth register of twins born in England and Wales in 1994–1995. The E-Risk sample was constructed in 1999–2000, when 1,116 families with same-gender 5-year-old twins (93% of those eligible) participated in home-visit assessments. Follow-up home visits were conducted when the children were aged 7 (98% participation), 10 years (96% participation), and 12 years (96% participation). Twins’ zygosity was determined with a standardized zygosity questionnaire that has been shown to have 95% accuracy.22 Ambiguous cases were zygosity typed using DNA. Ethical approval was granted by the Joint South London and Maudsley and the Institute of Psychiatry NHS Ethics Committee (UK). Parents gave informed consent and children gave assent to participate in the study.
Based on prior investigations conducted by our research group, 27% of the variance in bullying victimization was due to unique environments or random experiences.19 These factors may explain why genetically identical individuals were differently exposed to bullying experiences. For example, British twins are routinely separated into different classrooms in secondary schools, which may randomly place them at distinct risk for bullying victimization. From the total E-Risk sample, we identified twin pairs eligible to participate in this study of cortisol if they met the following five criteria: 1) they were MZ twins; 2) one twin was bullied at least occasionally; 3) bullying was reported by both mothers and children at age 12; 4) bullying incidents involved harm, either psychological or physical; and 5) co-twins never experienced bullying victimization (as measured prospectively when they were 7, 10, and 12 years). This study sample comprised 30 12-year-old MZ twin pairs (mean [SD] = 12.53 [0.52]) discordant on bullying victimization and with valid cortisol data (43.3% males). Hence, this substudy sample included one twin who had been the victim of bullying (n = 30 children) while their co-twin had not (n = 30). Most twins were Caucasian (93.7%) and one in four families came from a low socioeconomic background (26.7%). These children had an IQ within the normal range when they were 5 years (from 76 to 135; mean [SD] = 104.26 [13.65]). Furthermore, the bullied and nonbullied MZ twins were similar on a series of pre-existing, child-specific family environments and concomitant individual factors such as birth weight, IQ, and maltreatment experiences (Table 1; also Supplement 1, available online, for detailed description of measures). E-Risk discordant MZ twins did not differ from concordant bullied MZ twins on SES, IQ, or birth complications.
TABLE 1.
Descriptive Statistics of Pre-existing, Child-Specific Family Environments, Concomitant Individual Factors, and Psychosocial Stress Test–Related Measures
| Measure | Bullied MZ Twins (n = 30) Mean (SD) or % | Nonbullied MZ Twins (n = 30) Mean (SD) or % | t or χ2 (df) | p |
|---|---|---|---|---|
| Pre-existing individual factors (age 5 y) | ||||
| Birth weight, g | 2,230.99 (459.65) | 2,354.11 (487.41) | 0.97(54) | .34 |
| IQ | 102.54 (13.47) | 105.92 (13.83) | 0.95 (57) | .35 |
| Externalizing problems | 18.10 (12.51) | 16.42 (10.66) | –0.56 (58) | .58 |
| Internalizing problems | 9.97 (6.74) | 8.74 (7.50) | –0.67 (58) | .51 |
| Child-specific family environments | ||||
| Lifetime maltreatment (%) | 20.0 | 20.0 | <0.001 (1) | >.999 |
| Maternal warmth (age 5 y) | 3.41 (0.97) | 3.41 (1.19) | <0.001 (52) | >.999 |
| Lifetime stressful life events | 13.27 (4.51) | 11.37 (4.98) | –1.55 (58) | .13 |
| Concomitant individual factors (age 12 y) | ||||
| Body mass index | 20.33 (3.58) | 19.96 (3.61) | –0.40 (58) | .69 |
| Pubertal maturity | 7.60 (3.79) | 7.80 (4.06) | 0.20 (58) | .84 |
| Bullying perpetration (%) | 23.3 | 36.7 | 1.27 (1) | .26 |
| PST-related measures (age 12 y) | ||||
| Perceived Stress | 8.83 (4.68) | 7.30 (4.83) | –1.25 (58) | .22 |
| Post-Pre Negative Affective Scale | 2.29 (4.52) | 1.74 (3.70) | –0.52 (58) | .60 |
Note: No differences were detected between the bullied and nonbullied monozygotic (MZ) co-twins. Maternal warmth information was missing for three families (six twins). PST = psychosocial stress test.
Bullying Victimization
We prospectively assessed bullying victimization for all E-Risk participants during the interviews conducted with mothers when children were 7, 10, and 12 years and with the children themselves at age 12. Before asking questions related to bullying victimization, we explained that “Someone is being bullied when another child: says mean and hurtful things, makes fun or calls a person mean and hurtful names; completely ignores or excludes someone from their group of friends; hits, kicks, or shoves a person, or locks them in a room; tells lies or spreads rumors about them; and other hurtful things like these. We call it bullying when these things happen often, and when it is difficult to make it stop. We do not call it bullying when it is done in a friendly or playful way.” We asked mothers whether each twin has been bullied by another child, responding “never” (0), “yes” (1), or “frequently” (2). We further asked mothers who reported instances of bullying victimization whether the twin suffered physical harm (e.g., bruise, cut, burn) or psychological distress (e.g., bad dreams or school avoidance) as a consequence of bullying, responding “never” (0), “yes” (1), or “frequently” (2). During private interviews, we asked children “Have you been bullied by another person,” responding “never” (0), “sometimes” (1), or “a lot” (2). An independent rater further reviewed all descriptions of the bullying events recorded by the interviewers to confirm instances of current bullying by looking for evidence of repeated harmful actions, between children, where there was a power imbalance between the bully and the victim. A test–retest reliability of 0.87 was noted for 30 parents randomly selected from the total E-Risk sample and who were interviewed 3 to 6 weeks apart. Our findings indicate that both mothers and children are valid and reliable informants of bullying victimization, and that they tended to agree with one another (60% of mothers agreed with twins’ reports of bullying victimization; kappa coefficient = 0.29) (see Shakoor et al.23 for an extended investigation of the psychometric proprieties of the measures).
We derived a cumulative index of frequent, chronic, and severe bullying victimization by summing the scores to the three questions asked to mothers described above across age 7, 10, and 12 assessments. The victims of frequent bullying who experienced frequent psychological and physical harm had a maximum score of 6 at each time point (7, 10, and 12 years). The scale could thus vary from 0 to 18, but ranged from 0 to 12 in this substudy sample (mean [SD] = 2.72 [3.08]). The internal consistency of the scale was 0.70 (Cron-bach's alpha).
Psychosocial Stress Test
Each twin was individually interviewed by a research assistant with a psychology background during a visit to a research laboratory. All twins arrived early in the afternoon (mean [SD] = 1:41 [19 minutes]). One hour after arrival, twins took part in an adapted version of the TSST for children, which includes a social stressor (speaking in front of judges) and a cognitive stressor (mental arithmetic).24 A video camera was installed in a room to record the cognitive and public-speaking tasks. The cognitive task was first administered using the Children's Paced Auditory Serial Addition Task,25 a serial-addition task used to assess sustained attention, rate of information processing, and working memory. Children heard a random series of 61 numbers ranging from 1 to 9 and were instructed to add the numbers in pairs such that each number was added to the previous number. The time interval between each number was 2.4 and 2.0 seconds for the first and second series of numbers. Before the task started, children were told to make as few mistakes as possible because they were in competition against their co-twin and the winner would get a prize. The interviewer did not offer support and avoided eye contact to enhance the stressful aspect of the challenge. The public speaking task immediately followed. Children were told to stand and were asked to recall their most unpleasant experience at school in front of an unknown and inexpressive judge and the interviewer. Children had 2 minutes to prepare in silence, standing in front of the camera, and were then asked to speak for 5 minutes. This stress paradigm was selected because a combination of public speaking and cognitive tasks has been shown to elicit reliable cortisol responses in laboratory settings.24,26
Cortisol Measurements
We collected five samples of saliva to measure the cortisol response to the PST. Saliva was collected by asking children to use a straw to pass through 1 ml of saliva into the cryovials. The first samples were collected 20 and 2 minutes before the PST. A third sample was collected immediately at the end. A fourth and a fifth samples were collected 25 and 35 minutes after the start of the tasks. Twins were asked to refrain from doing any vigorous exercise in the morning, to eat a light lunch before midday, avoiding dairy products and red meat. Saliva samples were stored in a –20°C freezer.
After thawing, saliva samples were centrifuged at 3,500 revolutions per minute for 10 minutes, which resulted in a clear supernatant of low viscosity. Saliva cortisol concentrations were determined using the “Inmmulite”– DPC's Immunoassay analyzer (www.diagnostics.siemens.com). The assay had an analytical sensitivity of 0.2 nmol/L and inter-/intra-assay precision of less than 10%. All samples from the same participant were analyzed together. Cortisol measures were skewed and were normalized using a log10 transformation.
Individual Risk Factors and Covariates
A full description of the instruments and procedures used to assess the pre-existing, child-specific family environments, concomitant, and stress-related individual factors is available online (Supplement 1, available online).
Statistical Analyses
The presence of distinct cortisol responses to the PST between bullied and nonbullied MZ twins was tested using repeated-measures analyses of variance (ANOVAs). All five measures of cortisol sampled before and after the PST constituted the within-subjects factor (time), whereas the groups defining bullying victimization was included in the model as a between-subjects factor (bullying). Distinct patterns of cortisol responses to the PST between the bullied and nonbullied twins were tested through the interaction between the within- and between-subjects factors (time × bullying). Two cortisol confounders were also included in the model (dairy consumption and histaminic medication). Greenhouse–Geisser corrections for repeated measures were reported when data violated the sphericity assumption. For a significant time × bullying interaction, we tested the distinct cortisol responses separately for the bullied and nonbullied twins using repeated-measures ANOVAs. We then tested whether the bullied and nonbullied MZ twins differed on a series of individual risk factors and covariates using t-tests and χ2 analyses. We explored the association between the bullied twins’ continuous index of bullying victimization and cortisol secretion during the PST using Pearson correlation (cortisol secretion was indexed using the standardized [Z] residuals of the area under the curve (AUC) calculated using the five cortisol measures,27 controlling for dairy consumption and histaminic medication).
RESULTS
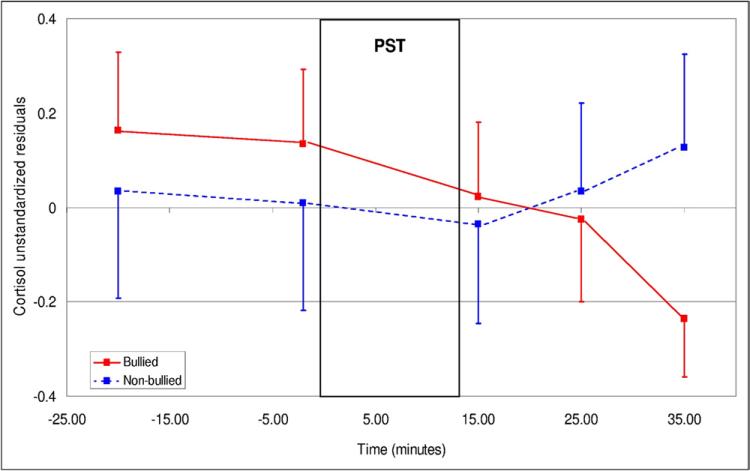
Repeated-measures ANOVA showed distinct patterns of cortisol secretion over time between bullied and nonbullied MZ twins (time × bullying: F2.2,122.9 = 3.82, P = .02) (Figure 1). More specifically, both groups had similar levels of cortisol prior to the PST (F1,56 = 1.26, P = .27), but distinct patterns of secretion emerged subsequently as a function of time (+15 minutes vs. later; Helmert time × bullying (within-subjects) contrast: F1,56 = 6.18, P = .02). We explored further this interaction using repeated measures ANOVAs conducted for each group separately. While nonbullied twins showed the expected cortisol increase after the PST (F2.0,55.0 = 5.09, P = .009), bullied twins did not exhibit this increase (F2.3,61.7 = 1.24, P = .30). Analyses took into account the confounding effect of two covariates shown to be associated with cortisol in this substudy sample: consumption of dairy product before the visit (F2.2,123.6 = 4.78, P = .008), and histaminic medication (F1,57 = 8.47, P = .005). Gender did not affect cortisol levels (gender: F1,54 = 1.74, P = .19; time × gender: F2.2,119.7 = 1.24, P = .30) or interact with bullying victimization (gender × bullying: F1,54 = 1.00, P = .32; time × gender × bullying: F2.2,119.7 = 0.47, P = .64).
FIGURE 1.
Cortisol response to the psychosocial stress test (± standard error of the mean (SEM)) in bullied and nonbullied monozygotic twins (N = 60 children). Note: Adjusted means took into account the effects of dairy products and histaminic medication. PST = psychosocial stress test.
Bullied and nonbullied MZ twins showed distinct cortisol responses to the PST. Yet, it is possible that individual factors other than bullying victimization could explain this group difference in stress reactivity. We considered four alternative explanations: 1) individual factors pre-existing bullying victimization, 2) child-specific family environments, 3) individual factors concomitant with bullying victimization, and 4) differences in PST-related measures. First, bullied and nonbullied MZ twins did not differ on pre-existing individual factors that could have affected their risk of being bullied; bullied and nonbullied twins had comparable birth weight, IQ, internalizing and externalizing problems at 5 years, suggesting that these characteristics cannot explain cortisol differences within the discordant MZ twin pairs. Second, bullied and nonbul-lied twins experienced similar levels of maternal warmth before bullying victimization (age 5 years), similar lifetime stressful life events, and, in families where maltreatment occurred (n = 6 families, 20%), both twins were targeted. Third, bullied and nonbullied twins were comparable according to their body mass index, pubertal maturity, and bullying perpetration assessed at 12 years. Fourth, bullied and nonbullied MZ twins reported comparable levels of stress during the PST and similar increases in negative affect, indicating that different cortisol responses are not due to distinct perceptions of the PST. These findings show that individual or uniquely experienced family factors can not account for cortisol differences between the MZ twins.
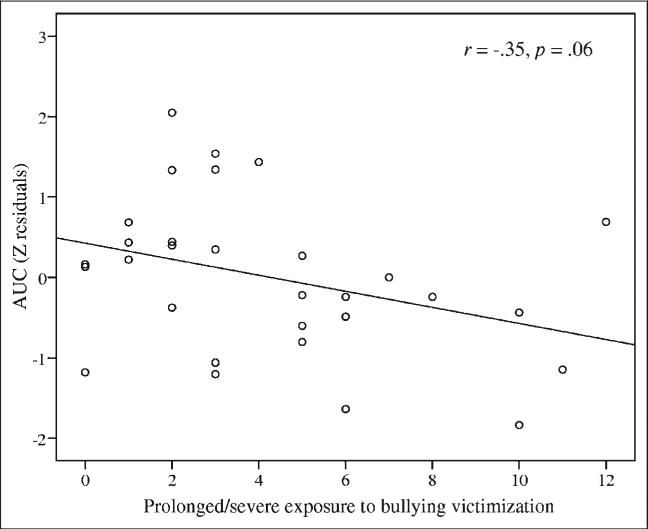
These findings were further strengthened by an association between cortisol reactivity and a continuous index of bullying in victimized twins. Twins exposed to more frequent, chronic, and severe bullying experiences showed a trend toward lower cortisol secretion during the PST (r = –0.35, P = .06) (Figure 2).
FIGURE 2.
Cortisol secretion before and during the psychosocial stress test in bullied twins according to a measure of frequency, chronicity, and severity of bullying victimization (n = 30 children). Note: Standardized residuals were used to take into account the covariates. AUC = area under the curve; Z = standardized.
DISCUSSION
This study provides evidence for a causal effect of early-life stress on cortisol reactivity in human beings. Our psychosocial stress test elicited a cortisol response in nonbullied children compared with a blunted response in bullied children. Cortisol differences between bullied and nonbullied children were observed in a stringent discordant MZ twin design, in which distinct cortisol responses to stress could not be attributable to either the children's genetic makeup or their shared familial environments. Our findings thus offer additional support for the hypothesized impact of early-life stress on cortisol activity in childhood.
Our findings are consistent with experiments showing that early-life stress alters HPA axis activity in rodents and nonhuman primates11,14,28 and with theories about human beings.5 Moreover, lower cortisol response to a psychological challenge in bullied children is consistent with accumulating evidence suggesting that lower HPA axis activity is not restricted to persons with post-traumatic stress disorder, but is also detected in healthy adults who experience chronic stress29,30 and who report childhood adverse experiences.12 Our study's discordant MZ twin design further suggests that the effect of bullying victimization is environmentally mediated. Moreover, the strength of our association was comparable to those observed in relation to other adverse childhood experiences31,32 and to depression,33 suggesting that the distinct cortisol profiles shown in our study could have biological relevance to psychopathology.
The effect of early-life stress on cortisol secretion not only was shown through group differences between bullied and nonbullied children, but a trend was observed for a dose–response relationship between a continuous index of bullying victimization and cortisol secretion. More frequent, chronic, and severe bullying victimization experiences measured repeatedly in childhood showed lower cortisol secretion during the PST in bullied children. This matches findings in rodent studies showing that the magnitude of prior exposure to chronic stress reduced the HPA axis response to acute stressors.34,35 Characterization of stress experiences using repeated prospective measures appears to be valuable for investigating the impact of cumulative stress on HPA axis functioning.
The mechanisms by which early-life stress leads to lower cortisol responses to psychosocial stress are not fully understood, but likely to arise from structural and circuitry changes in the corticolimbic brain regions and the HPA axis.36 First, Heim et al. outlined several molecular pathways leading to lower cortisol secretion, including reduced biosynthesis of cortisol or increased negative feed-back sensitivity of the HPA axis.30 For example, lower cortisol levels in the context of normal adrenocorticotropic hormone responses to the dexamethasone/corticotrophin-releasing hormone test is consistent with an increased negative feed-back sensitivity of the HPA axis shown in adults who experienced parental desertion and low parental care.37 Second, intertwined with these molecular pathways, rodent models have also demonstrated that low maternal care can modify the HPA axis response to stress through epigenetic programming of the GR gene promoter expression in the hippocampus.38 Third, early-life stress could affect stress-related systems through psychological factors associated with cortisol secretion such as defensive coping strategies39 or consolidation of aversive memories.40 Prospective studies designed to control for genetic and familial influences are needed to document the molecular and psychological mechanisms linking early-life stress to lower cortisol reactivity.
Research emerging from animal models and studies conducted with human beings has yet to clarify whether low cortisol response to psychosocial stress is adaptive or detrimental for children. On the one hand, exposure to mildly stressful events that are not overwhelming but are stressful enough to elicit a cortisol response may induce stress resistance.41,42 For example, monkeys exposed to mild early stress paradigms exhibited lower HPA axis reactivity to stress than controls.43 Independent of maternal care, this difference was associated with species-specific signs of positive adjustment (enhanced inhibitory control, emotion regulation, and exploration). Furthermore, down-regulation of the HPA axis may protect the developing brain from the harmful effects of prolonged cortisol elevations and facilitate adaptation to anticipated environmental challenges.44 On the other hand, enhanced resistance to stress may come with long-term costs. Alterations in primary stress mediators (e.g., cortisol, catecholamines) promote adaptation to changing environments. However, persistent low cortisol secretion may increase future risk of poor physical and psychological health.7 Low cortisol levels have been associated to reduced attention, impaired working memory, and reduced responsiveness to reinforcement and punishment.45 Children secreting low levels of cortisol may thus be least apt to benefit from resources present in their environment, engage optimally in complex social interactions or achieve adequately at school compared with higher secretors.31,44 Moreover, because cortisol moderates the amplitude and length of proinflammatory responses, persistent low cortisol levels may result in sustained overactivity of the immune system and increased risk of autoimmune disorders.46 This idea is consistent with increased inflammation levels noted in individuals who have experienced childhood maltreatment.47,48
The present findings provide support for causality although further tests are needed. Our study controlled for a wide range of confounders (including genetic and familial influences), and we subsequently tested for twin differences on individual factors known to increase bullying victimization or affect cortisol secretion. However, we were not able to control for pre-existing twin differences in de novo mutations, epigenetic states, and differences in latent unique environments. Nevertheless, our study design allowed us to control for epigenetic variations that may have been inherited or due to shared familial influences, and we tested for differences in a series of unique environments or individual characteristics that could have increased the risk of being bullied or the emergence of cortisol disruptions. Furthermore, we measured cortisol reactivity only once in our longitudinal study. Support for temporal priority would have necessitated longitudinal measures of cortisol to rule out the alternative explanation of pre-existing differences in HPA axis activity. However, because we controlled for a wide range of confounders, we speculate that this alternative is unlikely. Rigorous criteria were applied to ascertain clear discordance of bullying victimization between MZ twins, limiting the number of eligible twin pairs, which could jeopardize the generalizability of the results. However, in comparison to case-control design studies, the selection of discordant MZ twin pairs from a population-based cohort implies that our sample was not tainted by sampling bias associated with treatment referral. Finally, our assessment of cortisol reactivity would have benefited from an additional post-test saliva sample, which would also have allowed a better characterization of the distinct patterns of cortisol reactivity in bullied and nonbullied twins. However, the trend for a dose–response association between bullying victimization and cortisol secretion consolidated this finding.
In conclusion, our findings offer support for a causal effect of early-life stress on cortisol reactivity in human beings. The evidence of an environmentally mediated influence of early-life stress in general, and bullying victimization in particular, has implications for research and intervention. The question of whether lower cortisol reactivity resulting from exposure to early-life stress is adaptive or harmful should be studied using broader indices of general functioning and well-being.
Supplementary Material
Acknowledgments
The E-Risk Study is funded by the Medical Research Council (MRC; G9806489). Additional support was provided by the British Academy, the Jacobs Foundation, the Economic and Social Research Council (ESRC; RES-177-25-0013), the Nuffield Foundation, and the National Institute of Child Health and Human Development (HD061298). Additional support was provided by the Canadian Institutes of Health Research (I.O.M.), the National Alliance for Research on Schizophrenia and Depression Young Investigator Award (A.D.), the ESRC (L.B.), the MRC (S.S.), a Career Scientist Award from the UK Department of Health (L.A.), a Royal Society Wolfson Research Merit Award (A.C.), the Lady Davis Fellowship of the Hebrew University, and the Caselberg Trust (A.C. and T.E.M.).
We are grateful to the families and the twins’ teachers. Our thanks to Michael Rutter and Robert Plomin of the Institute of Psychiatry, King's College London, UK, to Irene Papadopoulos of Bethlem Royal Hospital, UK, for technical assistance, and to members of the E-Risk team for their dedication, hard work, and insights.
Footnotes
Disclosure: Drs. Ouellet-Morin, Danese, Bowes, Pariante, Papadopoulos, Caspi, Moffitt, and Arseneault, and Ms. Shakoor, and Mr. Ambler report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Isabelle Ouellet-Morin, Institute of Psychiatry, King's College London.
Andrea Danese, Institute of Psychiatry, King's College London.
Lucy Bowes, Institute of Psychiatry, King's College London.
Sania Shakoor, Institute of Psychiatry, King's College London.
Antony Ambler, Institute of Psychiatry, King's College London.
Carmine M. Pariante, Institute of Psychiatry, King's College London.
Andrew S. Papadopoulos, Institute of Psychiatry, King's College London. Bethlem Royal Hospital.
Avshalom Caspi, Institute of Psychiatry, King's College London. Duke University, Durham.
Terrie E. Moffitt, Institute of Psychiatry, King's College London. Duke University, Durham.
Louise Arseneault, Institute of Psychiatry, King's College London.
REFERENCES
- 1.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 2.Arseneault L, Milne BJ, Taylor A, et al. Being bullied as an environmentally mediated contributing factor to children's internalizing problems: a study of twins discordant for victimization. Arch Pediatr Adolesc Med. 2008;162:145–150. doi: 10.1001/archpediatrics.2007.53. [DOI] [PubMed] [Google Scholar]
- 3.Nansel TR, Craig W, Overpeck MD, Saluja G, Ruan WJ. Cross-national consistency in the relationship between bullying behaviors and psychosocial adjustment. Arch Pediatr Adolesc Med. 2004;158:730–736. doi: 10.1001/archpedi.158.8.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arseneault L, Bowes L, Shakoor S. Bullying victimization in youths and mental health problems: ’Much ado about nothing’? Psychol Med. 2010;40:717–729. doi: 10.1017/S0033291709991383. [DOI] [PubMed] [Google Scholar]
- 5.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 6.De Bellis MD. The psychobiology of neglect. Child Maltreat. 2005;10:150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 8.Vaillancourt T, Duku E, Decatanzaro D, Macmillan H, Muir C, Schmidt LA. Variation in hypothalamic-pituitary-adrenal axis activity among bullied and nonbullied children. Aggr Behav. 2008;34:294–305. doi: 10.1002/ab.20240. [DOI] [PubMed] [Google Scholar]
- 9.Kliewer W. Violence exposure and cortisol responses in urban youth. Int J Behav Med. 2006;13:109–120. doi: 10.1207/s15327558ijbm1302_2. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton LD, Newman ML, Delville CL, Delville Y. Physiological stress response of young adults exposed to bullying during adolescence. Physiol Behav. 2008;95:617–624. doi: 10.1016/j.physbeh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 12.MacMillan HL, Georgiades K, Duku EK, et al. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the Youth Mood Project. Biol Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Rutter M. Proceeding from observed correlation to causal inference: the use of natural experiment. Perspect Psychol Sci. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 16.Steptoe A, van Jaarsveld CH, Semmler C, Plomin R, Wardle J. Heritability of daytime cortisol levels and cortisol reactivity in children. Psychoneuroendocrinology. 2009;34:273–280. doi: 10.1016/j.psyneuen.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouellet-Morin I, Boivin M, Dionne G, et al. Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Arch Gen Psychiatry. 2008;65:211–218. doi: 10.1001/archgenpsychiatry.2007.27. [DOI] [PubMed] [Google Scholar]
- 19.Ball HA, Arseneault L, Taylor A, Maughan B, Caspi A, Moffitt TE. Genetic and environmental influences on victims, bullies and bully-victims in childhood. J Child Psychol Psychiatry. 2008;49:104–112. doi: 10.1111/j.1469-7610.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 20.Vitaro F, Brendgen M, Arseneault L. The discordant MZ-twin method: one step closer to the holy grail of causality. Int J Behav Dev. 2009;33:376–382. [Google Scholar]
- 21.Moffitt TE. Teen-aged mothers in contemporary Britain. J Child Psychol Psychiatry. 2002;43:727–742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- 22.Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Res. 2000;3:129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- 23.Shakoor S, Jaffee SR, Andreou P, et al. Mothers and children as informants of bullying victimization: results from an epidemiological cohort of children. J Abnorm Child Psychol. 2011;39:379–387. doi: 10.1007/s10802-010-9463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Dyche G, Johnson D. Development and evaluation of CHIPASAT, an attentional test for children: II. Test-retest reliability and practice effect for a normal sample. Percept Mot Skills. 1991;72:563–572. doi: 10.2466/pms.1991.72.2.563. [DOI] [PubMed] [Google Scholar]
- 26.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 27.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 28.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 29.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 30.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter LL, Carvalho JP, Tyrka AR, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- 34.Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinol. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–533. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Gunnar M, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Donald JC, editors. Developmental Psychopathology. Vol. 2. John Wiley & Sons; Hoboken, New Jersey: 2006. pp. 533–577. [Google Scholar]
- 37.Tyrka AR, Wier L, Price LH, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 39.Blackhart GC, Eckel LA, Tice DM. Salivary cortisol in response to acute social rejection and acceptance by peers. Biol Psychol. 2007;75:267–276. doi: 10.1016/j.biopsycho.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Polanczyk G, Caspi A, Williams B, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- 43.Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 44.Boyce TW, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 45.Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci. 2004;24:5492–5499. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller GE, Chen E, Fok AK, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danese A, Caspi A, Williams B, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.