Summary
The naked mole-rat (NMR, Heterocephalus glaber) is a long-lived mammal in which spontaneous cancer has not been observed. In order to investigate possible mechanisms for cancer resistance in this species, we studied the properties of skin fibroblasts from the NMR following transduction with oncogenes that cause cells of other mammalian species to form malignant tumors. NMR fibroblasts were transduced with a retrovirus encoding SV40 large T antigen and oncogenic RasG12V. Following transplantation of transduced cells into immunodeficient mice, cells rapidly entered crisis, as evidenced by the presence of anaphase bridges, giant cells with enlarged nuclei, multinucleated cells, and cells with large number of chromosomes or abnormal chromatin material. In contrast, similarly transduced mouse and rat fibroblasts formed tumors that grew rapidly without crisis. Crisis was also observed after >40 population doublings in SV40 TAg/Ras-expressing NMR cells in culture. Crisis in culture was prevented by additional infection of the cells with a retrovirus encoding hTERT (telomerase reverse transcriptase). SV40 TAg/Ras/hTERT-expressing NMR cells formed tumors that grew rapidly in immunodeficient mice without evidence of crisis. Crisis could also be induced in SV40 TAg/Ras-expressing NMR cells by loss of anchorage, but after hTERT transduction cells were able to proliferate normally following loss of anchorage. Thus, rapid crisis is a response of oncogene-expressing NMR cells to growth in an in vivo environment, which requires anchorage independence, and hTERT permits cells to avoid crisis and to achieve malignant tumor growth. The unique reaction of NMR cells to oncogene expression may form part of the cancer resistance of this species.
Keywords: Naked mole-rat, longevity, cancer resistance, oncogenes, crisis, DNAdamage
Introduction
The naked mole-rat (Heterocephalus glaber) is an exceptionally long-lived rodent of considerable interest to biomedical gerontology (Sherman and Jarvis, 2002; Buffenstein and Jarvis, 2002; Salmon et al., 2008; Perez et al., 2009; Sedivy, 2009). In a colony of 1500 animals in captivity, the oldest individuals are more than 30 years of age and animals routinely maintain good health well into their third decade (Buffenstein, 2008). Animals aged over 24 years show evidence of frailty, and apparently die of age-related causes. Nevertheless, death due to cancer has never been observed, and necropsies have not revealed incidental tumors (Buffenstein, 2005). Thus the NMR appears to be a cancer-resistant mammal. Cancer is a disease that generally affects most mammalian species and is usually believed to be an unavoidable accompaniment of aging (Campisi, 2003; Hasty et al., 2003; Lombard et al., 2005). Mechanisms of cancer resistance are not well understood. Understanding the basis for cancer resistance in the NMR is not only of importance in defining causes of cancer susceptibility and resistance among mammalian species but also provides insights into mechanisms of extraordinary longevity.
Previously published studies on NMR cells in culture have indicated that the cells may react to signals in the culture environment that cause them to express unusually high levels of the tumor suppressor p16INK4A (Seluanov et al., 2009). As anticipated, expression of the combination of the oncogenes SV40 large T antigen (TAg) and RasG12V reduced the level of p16, thereby permitting the cells to grow well in culture, but did not confer on the cells the ability to grow as colonies in soft agarose, a surrogate assay for tumorigenicity (Seluanov et al., 2009). This oncogene combination has long been known to be sufficient for full malignant transformation of mouse and rat cells (Livingston and Bradley, 1987; Weinberg, 1989; Fanning, 1992).
In the present experiments we directly investigated the tumorigenicity of NMR cells expressing this oncogene combination, SV40 TAg and Ras. We show that cells expressing these genes rapidly enter crisis when transplanted into immunodeficient mice. Ectopic expression of hTERT in these cells rescues this phenotype. Rapid crisis, acting as a tumor suppressor mechanism, may represent an important component of cancer resistance in this species.
Results
Preparation and growth of SV40 TAg/Ras-expressing NMR cells
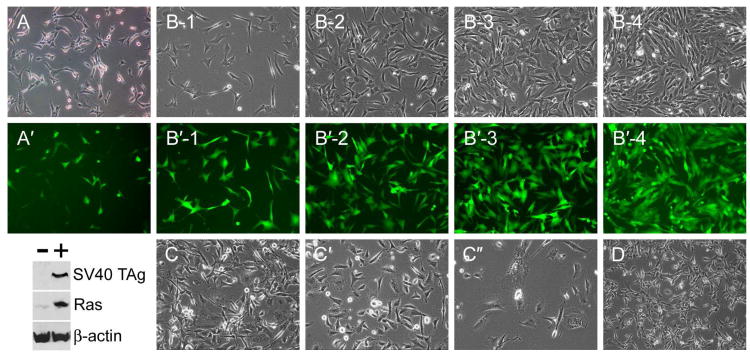
Fibroblasts were isolated by enzymatic digestion of the skin of newborn naked mole-rats. Primary skin fibroblast cultures were infected with a retrovirus encoding SV40 TAg, RasG12V, and GFP (Sun et al., 2004). Successful infection was observed by expression of GFP (Fig. 1). At 2 days post-infection ∼25% of cells in the population were GFP+; after 2 weeks of growth following infection, the population was 90% GFP+, indicating that retrovirally transduced cells grew at a higher rate than nontransduced cells. Consistent with a previous report on SV40 TAg/Ras-expressing NMR cells (Seluanov et al., 2009), retrovirally infected cells grew well in culture. They proliferated at a rate equivalent to an average doubling time of ∼24 hours and reached high cell densities (Fig. 1). When the cells were passaged continuously, they were observed to proliferate at a constant rate for ∼40 population doublings (PDs) following retroviral infection. After this time, slower growth was noted, accompanied by the appearance of cells with the characteristics of crisis (Fig. 1). As we observed previously in human and bovine cells expressing SV40 TAg/Ras, cells in crisis are enlarged, have multiple nuclei or abnormal nuclear shapes, and cannot divide properly (Sun et al., 2004; Sun et al., 2005a). NMR cells in crisis were often observed to enter cytokinesis but failed to successfully complete cell division (Fig. 1C″). By 50 PDs post-infection, few cells remained in the culture and all that did were abnormal. Such cultures could be maintained for many weeks but eventually all cells were lost from the culture. No escape (i.e. regrowth of a minority population) was observed.
Fig. 1.
Characteristics of NMR fibroblasts after introduction of SV40 TAg and Ras. (A, A′) Phase contrast and fluorescence images of NMR cells 4 days after infection with a retrovirus encoding SV40 TAg, Ras and GFP. Below, presence of SV40 TAg and Ras in infected cells (+) shown by immunoblotting. (B, B′) A series of images of SV40 TAg/Ras-expressing NMR fibroblasts (phase contrast and fluorescence) obtained two weeks after retroviral infection, 1 to 4 days following plating of the cells. (C, C′, C″) Stages of crisis of SV40 TAg/Ras-expressing cells; 40, 45, and 50 population doublings following retroviral infection. (D) SV40 TAg/Ras-expressing cells that additionally had been infected with a retrovirus encoding hTERT, 50 population doublings following infection.
Crisis is characteristic of cells with massive DNA damage, often, but not exclusively, resulting from telomere dysfunction (Counter et al., 1992; Shay et al., 1993a; Lustig, 1999; Chang et al., 2003). Oncogenes such as SV40 TAg stimulate cell proliferation but block the intracellular pathways required for senescent growth arrest and apoptosis (i.e. p53 and pRB; Shay et al., 1991; Beausejour et al., 2003); thus they permit DNA damage to accumulate, and such cells may eventually undergo crisis. Based on the ability of human telomerase reverse transcriptase (hTERT) to rescue human and bovine cells from crisis (Sun et al., 2004; Sun et al., 2005a; Sun et al., 2005b) we investigated the effects of ectopic hTERT in NMR cells. Whereas hTERT may act on telomeres in target cells, it is also well established that hTERT has a variety of effects that do not depend on an action at telomeres, and may not even require reverse transcriptase activity (Chung et al., 2005; Choi et al., 2008). We re-infected SV40 TAg/Ras-expressing NMR cells with a retrovirus encoding hTERT. This was performed two weeks after infection with the SV40 TAg/Ras/GFP retrovirus, when 95% of the cells in the population expressed the introduced genes as judged by GFP fluorescence (Fig. 1B′). hTERT-transduced NMR cells proliferated at the same rate as the SV40 TAg/Ras cells without hTERT, but eventually, when the no-hTERT cell population entered crisis, hTERT+ cells continued to divide rapidly (Fig. 1D). They continued to divide for at least 90 PDs post-infection without a decrease in the rate of cell proliferation. hTERT conferred increased telomerase activity in SV40 TAg/Ras-expressing NMR cells, despite the evolutionary distance between the two species, NMR and humans (Supplementary Figure 1).
DNA damage in SV40 TAg/Ras-expressing NMR fibroblasts
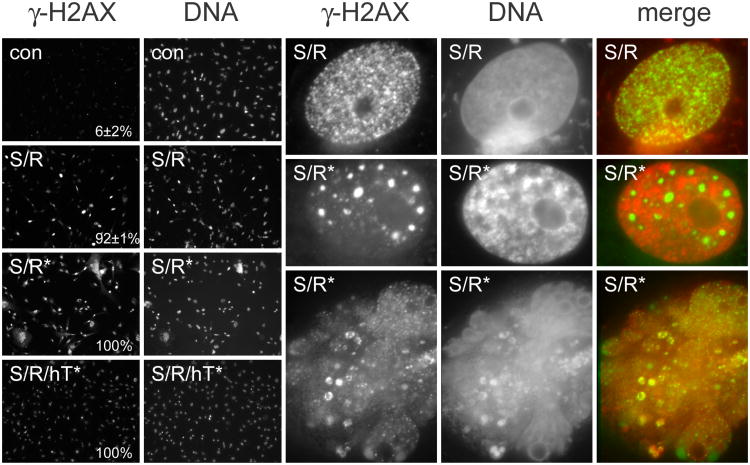
While stimulating cellular processes such as increased cell proliferation, activated oncogenes also directly damage DNA by several partially understood mechanisms (d'Adda di Fagagna, 2008). We investigated whether SV40 TAg/Ras-expressing NMR cells showed evidence of DNA damage and whether the level of damage was greater in cells in crisis. Figure 2 shows that SV40 TAg/Ras-expressing NMR fibroblasts have variable levels of DNA damage, as evidenced by γ-H2AX immunoreactivity. While almost all cells had γ-H2AX foci, the number of such foci varied greatly from cell to cell. In late passage SV40 TAg/Ras-expressing NMR cells, cells had high very levels of γ-H2AX immunoreactivity. At high magnification, γ-H2AX in early passage SV40 TAg/Ras-expressing cells was observed as multiple small foci; in late passage cultures, γ-H2AX could be seen as large amorphous masses within the nucleus, as well as in discrete foci. Moreover, in cells with morphological evidence of crisis, such as multiple nuclei, there was extensive DNA damage as evidenced by a variety of γ-H2AX staining patterns. In late passage SV40 TAg/Ras-expressing cells that had previously been transduced with hTERT, γ-H2AX foci were still clearly observable in all cells, but cells did not show the unusual patterns of staining seen in late-passage no-hTERT cells.
Fig. 2.
DNA damage foci in NMR cells, detected by immunoreactivity with an antibody against γ-H2AX. The low power images on the left show γ-H2AX (immunofluorescence) and DNA (DNA-binding dye) in control NMR fibroblasts before transduction with SV40 TAg and Ras (con); in NMR cells following infection with a retrovirus encoding SV40 TAg and Ras (S/R); in late passage SV40 TAg/Ras-expressing NMR cells (S/R*); and in late passage SV40 TAg/Ras-expressing NMR cells further infected with a retrovirus encoding hTERT (S/R/hT*). Magnification × 100. The γ-H2AX immunofluorescence images were each obtained with a 2-second exposure; the DNA binding dye images were each obtained with a 0.5-second exposure. The fraction of γ-H2AX positive cells is indicated in each case. The high power images on the right (γ-H2AX immunofluorescence, DNA-binding dye fluorescence, and merged images) show an early-passage NMR cell expressing SV40 TAg and Ras (S/R); and two examples of late-passage SV40 TAg/Ras-expressing NMR cells (S/R*). The bottom image shows a cell in crisis with a large abnormal nuclear mass. Magnification × 4000.
Resistance of SV40 TAg/Ras-expressing NMR fibroblasts to experimental tumor formation in immunodeficient mice, and reversal by hTERT
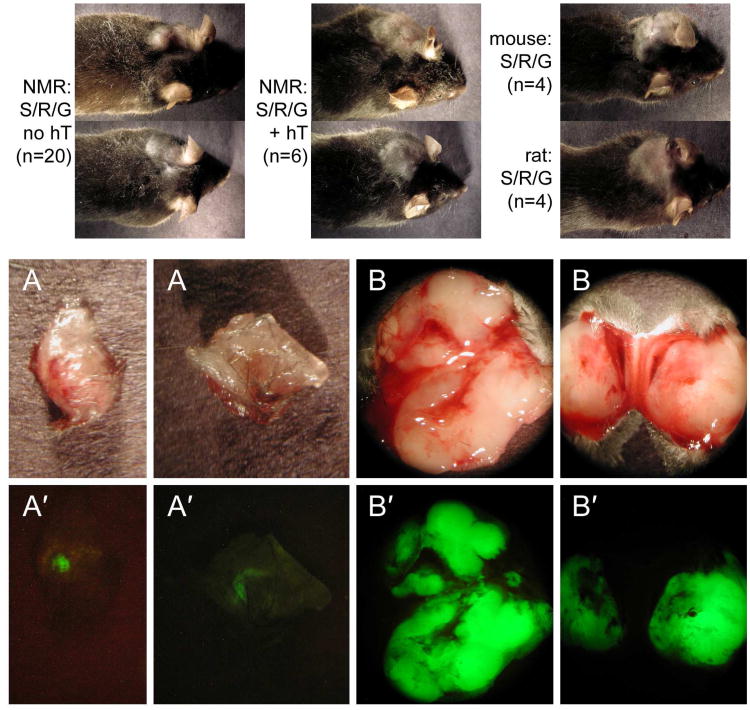
Previously it was reported that SV40 TAg/Ras-expressing NMR fibroblasts grow well in culture; a block to cell division that otherwise occurs at lower cell densities was absent in SV40 TAg/Ras-expressing cells (Seluanov et al., 2009). In SV40 TAg/Ras-expressing cells both p53 and pRb pathways are interrupted, as expected for the action of SV40 TAg (Seluanov et al., 2009). Elevated p16INK4A, which was observed in control NMR cells, was not observed in SV40 TAg/Ras-expressing NMR cells, consistent with the action of SV40 TAg on the pRB pathway (Livingston and Bradley, 1987; Weinberg, 1989; Fanning, 1992). However, SV40 TAg/Ras-expressing NMR cells did not grow as colonies in soft agarose, raising the possibility that this combination of oncogenes is insufficient to cause tumorigenicity in this species. In order to test this hypothesis directly, we transplanted SV40 TAg/Ras-expressing and SV40 TAg/Ras/hTERT-expressing NMR cells subcutaneously in immunodeficient mice. As controls, we also prepared SV40 TAg/Ras-expressing mouse and rat skin fibroblasts and transplanted these cell populations. Within 2-4 weeks, mouse and rat cells produced locally expanding and invasive tumors (Fig. 3). In contrast, NMR cells produced small nodules that did not expand even after 8 weeks (Fig. 3). Nodules exhibited faint green fluorescence, indicating the presence of retrovirally infected cells. Using fluorescence as a guide, it was possible to estimate the size of the nodules; the largest observed in 20 mice was ∼0.09 cm3 but most were much smaller. In contrast, SV40 TAg/Ras/hTERT-expressing NMR cells produced locally expanding and invasive tumors (Fig. 3). Tumor volume was 3.1±1.0 cm3 in 6 mice. We also transplanted SV40 TAg/Ras-expressing NMR cells into the subrenal capsule, as described earlier for testing oncogene-expressing human and bovine cells (Sun et al., 2004; Sun et al., 2005a), but cells did not survive as well in this site as they did in subcutaneous transplantation. We therefore made further investigations of tumorigenicity only in the subcutaneous site. We note that when precautions are taken to avoid nonspecific loss of cells during subcutaneous injections, by placing the cells in collagen gel, as performed here, results obtained with subcutaneous injection are not inferior to the subrenal capsule transplantation in xenograft studies (Popnikolov and Hornsby, 1999; Liu and Hornsby, 2007).
Fig. 3.
Tumorigenicity of NMR, mouse and rat cells expressing SV40 TAg, Ras, and hTERT. (top) NMR fibroblasts were infected with a retrovirus encoding SV40 TAg, Ras and GFP (S/R/G no hT) or were additionally infected with a retrovirus encoding hTERT (S/R/G + hT). Cells were injected subcutaneously in immunodeficient mice; site of injection was behind the right ear. After 60 days (no hTERT) or 45 days (+ hTERT), animals were sacrificed to allow examination of the nodules/tumors formed. (A, A′) Gross appearance and fluorescence of the nodules formed from no-hTERT cells; (B, B′) gross appearance and fluorescence of the tumors formed from hTERT+ cells. Additionally, the same retrovirus encoding SV40 TAg, Ras and GFP was used to infect primary mouse fibroblasts and primary rat fibroblasts. Tumor formation following subcutaneous injection in immunodeficient mice is shown for mouse cells at 25 days and for rat cells at 10 days.
Histological examination of subcutaneous nodules/tumors revealed sharp differences among the species and between no-hTERT and hTERT+ NMR cells. As anticipated from prior studies, tumors formed from mouse or rat cells comprised uniform populations of cells that invaded the subcutaneous tissues, including fat and muscle (not shown). On the other hand, the nodules formed from no-hTERT NMR cells exhibited an unusual structure (Supplementary Figure 2). To reveal the NMR cells, we used an anti-SV40 TAg antibody. This immunostaining showed that a large percentage of the cells within the nodule were not transplanted NMR cells but presumably comprised reactive stroma of mouse origin. The SV40 TAg+ cells were distributed among nonstained cells. SV40 TAg+ cells were frequently enlarged, and nuclei were often of bizarre shapes. Some cells lacked nuclei but included chromosomes in various configurations. Occasional SV40 TAg+ cells were found outside of the main nodule within adjacent fat, but there was no gross invasion of the subcutaneous tissues. The relatively low number of NMR cells in the nodules is consistent with weak fluorescence observed in the gross nodules when first excised from the host mice (Fig. 3).
These results contrasted strongly with the histological appearance of SV40 TAg/Ras/hTERT-expressing NMR cells (Supplementary Figure 2). Tumors formed from cells expressing this combination of genes were expansive and invasive. SV40 TAg immunoreactivity showed masses of small cells, which invaded the neighboring tissues. A few cells showed bizarre nuclear morphology, as observed in the no-hTERT cells, but most cells were of a uniform morphology. Consistent with the large number of NMR cells in the tumor, the gross tumors excised from the subcutaneous tissue exhibited strong fluorescence (Fig. 3).
NMR cells transplanted into immunodeficient mice exhibit a high incidence of crisis
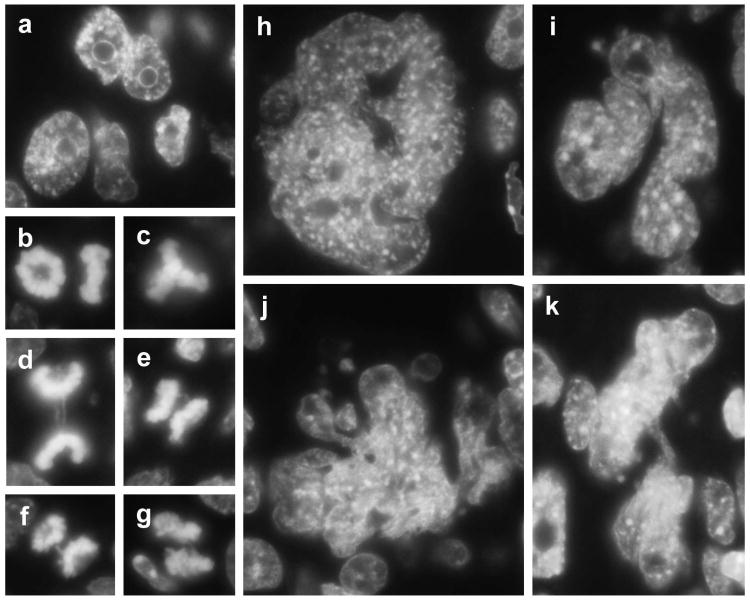
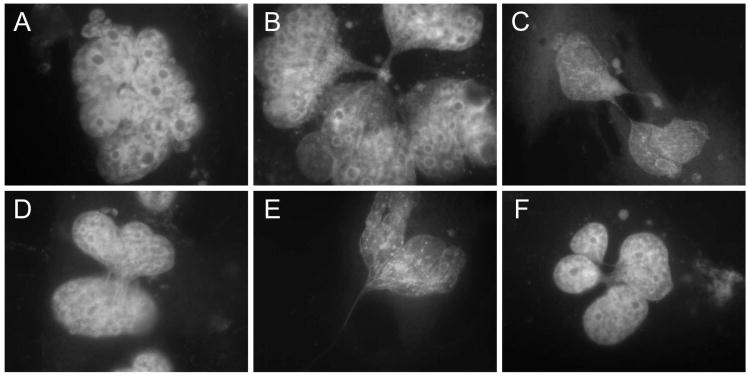
In culture after 40 PDs, and immediately when transplanted into immunodeficient mice, SV40 TAg/Ras-expressing NMR cells appeared to enter a nongrowing state caused by crisis. We investigated whether transplanted cells and late-passage cultured cells exhibited similar nuclear morphology characteristic of crisis. In crisis cells, evidence of DNA damage and chromosome dysfunction is observed as anaphase bridges, endoreduplication, failure of cytokinesis, multinucleation, and nuclear enlargement (Hornsby, 2007). Sections of nodules formed from SV40 TAg/Ras-expressing NMR cells were deparaffinized and stained with the fluorescent DNA binding dye DAPI (Fig. 4). Many NMR cells exhibited crisis as evidenced by giant bizarre nuclei, multinucleated cells, cells with abnormal chromosomes, and anaphase bridges (Fig. 4). Very similar abnormal nuclear morphologies were also observed in late-passage SV40 TAg/Ras-expressing NMR cells in culture (Fig. 5). In contrast, very few nuclei exhibiting abnormal morphology were observed in hTERT+ NMR cells, either in tumors or in cells in culture.
Fig. 4.
Nuclear abnormalities in SV40 TAg/Ras-expressing NMR cells in transplants. Cells were transplanted in immunodeficient mice. After 60 days, nodules were removed, sectioned, and examined for surviving NMR cells. Sections were stained with an anti-SV40 TAg antibody to locate NMR cells and adjacent serial sections were stained with DAPI to examine the nuclear morphology of NMR cells by fluorescence microscopy. (a) NMR cells were recognizable as large nuclei with prominent nucleoli. (b) Prometaphase rosettes observed en face and side. (c) Triradial. (d-g) Anaphases showing longer chromatin bridges (d) and various amounts of chromatin between the chromosome sets (e-g). (h-k) Examples of bizarre giant nuclear material showing multiple fused nuclei and briges between nuclei.
Fig. 5.
Nuclear abnormalities in SV40 TAg/Ras-expressing NMR cells in vitro. Cells were cultured for 45 population doublings after infection by a retrovirus encoding SV40 TAg, Ras, and GFP. Cells were fixed and nuclear morphology was examined by staining with DAPI. (A) Example of multiple fused nuclei. (B) Complex of multiple fused nuclei linked by chromatin material. (C-F) Examples of nuclei bridged by various numbers of thin chromatin strings.
Accelerated crisis induced by loss of anchorage in NMR cells: Rescue by hTERT
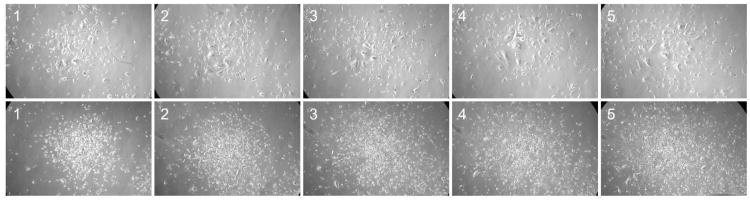
The ability of cells to survive the loss of cell anchorage is a key characteristic of tumorigenicity. We noted that SV40 TAg/Ras-expressing NMR cells apparently entered crisis much more rapidly following transplantation into immunodeficient mice than they did during long-term growth in culture, although they did not die, as evidenced by the fact that the transplanted cells were present in the subcutaneous nodules. We hypothesized that the more rapid entry into crisis could be the result of the fact that tumorigenic growth of cells is associated with anchorage independence; subcutaneous cell transplantation may be experienced by the cells as a form of loss of anchorage. It was previously shown that SV40 TAg/Ras-expressing cells do not form colonies in suspension in soft agarose (Seluanov et al., 2009). Here we tested the effects of loss of anchorage by placing cells in suspension in normal growth medium over a surface to which the cells are incapable of attachment. We performed the experiments in this way so as to be able to determine the properties of the cells after the treatment, which is much more difficult when cells are prevented from attachment by placement in semi-solid media. SV40 TAg/Ras-expressing NMR cells were held in suspension for 10 days, after which they were then replated on a normal culture substratum. Even though this was performed in early-passage cells, which are not close to a population doubling level at which crisis would normally occur, the reattached cells entered crisis (Fig. 6). These cells were unable to resume cell division. After several weeks, these cells died via the typical nonspecific death of crisis cells, which involves a gradual detachment from the culture substratum and loss from the culture. In parallel, we performed the same experiment on cells that had been transduced with hTERT following transduction with SV40 TAg/Ras. When replated, some of these cells appeared not to be capable of cell proliferation, but many of them were able to form proliferating colonies of cells (Fig. 6). The population of hTERT+ cells showed a progressive overall increase in growth rate, and following this recovery period could then be cultured indefinitely.
Fig. 6.
Effect of hTERT on re-growth of NMR cells after being without anchorage. SV40 TAg/Ras-expressing NMR cells were held in suspension for 10 days and were then replated under regular growth conditions. Cells were photographed under phase-contrast at intervals following replating. Upper row, cells that were not additionally transduced with hTERT. Lower row, cells additionally expressing hTERT. The numbers indicate the number of days after replating following suspension.
Discussion
To investigate the cellular basis for the apparent cancer resistance of the naked mole-rat, we directly tested the tumor-forming capacity of NMR cells expressing a combination of oncogenes, SV40 TAg and Ras. This gene combination efficiently transforms other rodent cells to aggressive cancers, an observation well established previously (Livingston and Bradley, 1987; Weinberg, 1989; Fanning, 1992) and confirmed here. Moreover, we previously showed that these oncogenes also transform human and bovine cells to aggressive cancers that are able to invade, metastasize and kill their hosts (Sun et al., 2004; Sun et al., 2005a). The form of SV40 T antigen used here encodes only the large T antigen and not small t antigen. The small t antigen is not necessary for oncogenic transformation of rodent cells (Rangarajan and Weinberg, 2003). It is also not required in nonrodent cells when cells are implanted into host immunodeficient mice using methods that avoid initial cell death (Sun et al., 2004; Sun et al., 2005a). We conclude that uniquely among cells from this evolutionarily diverse group of mammals, including both rodents and nonrodents, NMR cells were not susceptible to malignant transformation by SV40 TAg/Ras. Instead, NMR cells expressing this oncogene combination rapidly entered crisis when transplanted into immunodeficient mice.
In previous studies, crisis has been shown to be a terminal state of cells resulting from DNA damage and chromosome dysfuction. In a classical model of crisis caused by telomere dysfunction, short dysfunctional telomeres cause end-to-end chromosome fusions; in cells with disrupted checkpoints this results in (i) breakage-fusion-bridge cycles, leading to increasing aneuploidy; and (ii) mitotic catastrophe, a failure of cytokinesis, resulting in multiploidy, multipolar cell division, and gross aberrations in chromosome number (Roninson et al., 2001; Guiducci et al., 2001; Gisselsson et al., 2001a; Gisselsson et al., 2001b; Maser and DePinho, 2002; DePinho and Wong, 2003; Gisselsson, 2003). Mitotic catastrophe leads to arrest in mitosis, or alternatively to the formation of cells with multiple nuclei or a single giant nucleus (Gisselsson et al., 2001a; Gisselsson et al., 2001b; Gisselsson, 2003). While telomere dysfunction is a classic cause of crisis, the same constellation of characteristics (chromosomal and nuclear abnormalities and mitotic catastrophe) may result from other forms of DNA damage (Maser and DePinho, 2002; DePinho and Wong, 2003).
The fact that SV40 TAg/Ras caused NMR cells to enter crisis, rather than causing tumorigenic conversion, could have been because these gene products are inactive in NMR cells. This possibility is rendered very unlikely because (a) there is direct biochemical evidence for their activity in NMR cells (Seluanov et al., 2009) and (b) when a third gene was added, hTERT, the cells formed expanding and invasive tumors. The fact that SV40 TAg/Ras/hTERT-expressing NMR cells are aggressively tumorigenic indicates that the failure of SV40 TAg/Ras-expressing cells to form malignant tumors is not because SV40 TAg and/or Ras lack activity in NMR cells. We conclude that while this oncogene combination is insufficient for malignant transformation of NMR cells, cells of this species are nevertheless capable of tumorigenic behavior when hTERT is added to the combination.
The most direct explanation for the requirement for hTERT is that the cellular properties conferred by this gene permitted the cells to avoid the rapid crisis observed when the cells are transplanted. In support of this possibility, we found that SV40 TAg/Ras-expressing NMR cells also eventually enter crisis when grown in culture, although only after a large number of population doublings; under these circumstances hTERT also enables NMR cells to avoid crisis. While it is possible that hTERT acts to confer tumorigenicity by telomere maintenance, this possibility must be evaluated with respect to the very rapid crisis that occurs when NMR cells are transplanted, compared to the much more extended period required for crisis to occur in cell culture. We hypothesized that cell transplantation might be experienced by the cells as a form of removal of anchorage, and that hTERT might then enable the cells to overcome the deleterious effects of anchorage removal. To test this, we placed SV40 TAg/Ras-expressing cells in suspension, a condition under which they do not divide (Seluanov et al., 2009); some cell death occurs, but large number of cells survive 10 days in suspension and are able to re-attach to a normal culture substratum. However, the cells that survive uniformly entered crisis and no proliferating cells emerged from the recovered cells. The expression of hTERT did not affect the ability of cells to grow in suspension, but within the cell population that survived the 10 days of suspension a subpopulation was capable of regrowth and of restoring a long-term proliferating culture. This experiment suggests that hTERT may both confer resistance to the adverse effects of cell transplantation, which may resemble the adverse effects of loss of anchorage.
These results raise the possibility that hTERT may act on NMR cells via one or more of the partially characterized extratelomeric effects of this protein (Chung et al., 2005). A clear demonstration of an effect of hTERT that is independent of telomere maintenance is its action to permit the tumorigenic growth of ALT+ cells (ALT = alternative lengthening of telomeres), which are telomerase-negative (Sun et al., 2005b). Recent experiments have begun to place the extratelomeric effects of TERT on a firm molecular basis (Choi et al., 2008).
SV40 TAg/Ras-expressing NMR cells, both in culture and in vivo, exhibited features of crisis similar to those previously observed in SV40 TAg/Ras-expressing human and bovine cells (Sun et al., 2004; Sun et al., 2005a). These features included anaphase bridges, grossly enlarged and bizarre nuclei, multinucleation, chromatin bridges between nuclei, and collections of large numbers of mitotic chromosomes in cells that did not appear to be dividing. As discussed in more detail elsewhere (Hornsby, 2007), crisis acts as a reliable tumor suppressor on the cell population as a whole, which fails to be able to increase in cell number. The ultimate fate of cells in crisis is not well established. The subcutaneous nodules formed from SV40 TAg/Ras-expressing NMR cells persisted for 120 days and cells in crisis were found within them at time-points over this period. In tumors with large numbers of cells in crisis that were formed from SV40 TAg/Ras-expressing human and bovine cells, we observed that their eventual fate is nonspecific massive necrosis. In any case, the failure of the cell population to be able to expand shows that crisis acts as a reliable tumor suppressor mechanism. It is of interest that in more than 100 xenografts of hTERT-negative SV40 TAg/Ras-expressing human cells we never observed an escape from crisis, as is occasionally observed in culture (at a reported frequency of 3 × 10-7 in SV40 TAg-expressing human fibroblasts: Shay et al., 1993). Although our observations on NMR xenografts have not been as extensive, we also observed no escape from crisis in ∼30 xenografts. We conclude that the rapid entry into crisis in oncogene-expressing NMR cells provides one mechanism that may account for cancer resistance in this species and thereby contribute to its extreme longevity.
Materials and Methods
Growth of NMR fibroblasts and retroviral transduction
Primary NMR fibroblasts were obtained by enzymatic digestion of skin from newborn animals. Cells were cultured in low glucose Dulbecco's Modified Eagle's Medium (DME) and 10% Cosmic Calf Serum (Hyclone Laboratories, Logan, UT) in a 35 °C incubator with a gas phase of 3% O2, 5% CO2, 92% N2. Culture dishes used were Biocoat collagen I-coated polystyrene (BD Bioscience, San Jose, CA). We cultured NMR cells at 35 °C rather than at 32 °C (Seluanov et al., 2009) because pilot experiments showed that NMR cell growth was faster at 35 °C, and because other rodent (mouse and rat) cells also grow well at this temperature, permitting us to use a single temperature for all species in these studies.
The retrovirus used has the structure LTR-Ras-CMV-SV40T-CMV-EGFP-LTR and has been described previously (Sun et al., 2004). The SV40T gene is intronless and encodes SV40 TAg but not SV40 small t antigen. The hTERT vector used was pBabe-puro-hTERT (Rubio et al., 2002). The Phoenix cell line (amphotropic) was used for production of retroviral particles (Pear et al., 1993). Supernatant medium from the packaging cells was passed through a 0.45 μm filter and added to the target cells. Infection was performed twice for 24 hours each. Following infection, the cell population was expanded without selection. For long-term growth studies and determination of cumulative population doublings (Hornsby and Harris, 1987) cells were cultured in the growth medium described above and were subcultured using trypsin at a 1:3 ratio when cells reached confluence.
In vitro assays of cell behavior
In order to test the effects of loss of cell anchorage, cells were trypsinized and placed in normal growth medium in a polystyrene dish that had been precoated with Pluronic F-68 (Sigma-Aldrich, St. Louis, MO) (1 mg/ml in water for 1 hour). Every 2 days during the period of suspension, the medium was carefully removed, to avoid disturbing the cells, and was replaced with fresh medium. After 10 days, cells were replated on Biocoat dishes in regular growth medium.
Telomerase activity was assessed using the telomerase repeat amplification (TRAP) assay, which was performed as previously described (Sun et al., 2004).
In vivo assessment of tumorigenicity by transplantation of cells in immunodeficient mice
RAG2-/-,γc-/- mice were purchased from Taconic (Germantown, NY). Animals (both males and females) at an age greater than 6 weeks (∼25 g body weight) were used in these experiments. Procedures were approved by the institutional animal care committee and were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. 2 × 106 NMR cells were co-transplanted with 2 × 106 mitomycin C-treated 3T3-L1 fibroblasts in collagen gel as previously described (Popnikolov and Hornsby, 1999). Cells were injected subcutaneously close to the base of the external ear, a site previously shown to permit excellent tumor growth (Liu and Hornsby, 2007). Collagen was prepared as described previously from rat tail collagen fibers (Popnikolov and Hornsby, 1999). Before use, the ice-cold collagen solution was neutralized and adjusted to the proper osmolarity by adding a mixture of NaOH and 10 × DME. The cells to be transplanted were centrifuged. The cell pellet (∼40 μl) was mixed with 40 μl collagen solution. The cell/collagen mixture was maintained at 4 °C. Before the cells were transplanted, the cell/collagen mixture was taken up into a 200 μl pipette tip and the mixture was allowed to partially gel for 3 minutes at room temperature. An incision of about 1 - 2 mm was made in the skin at the base of the ear. A pocket beneath the skin was formed using forceps. A silk 6-O suture was introduced around the skin opening. The mixture was then injected into the subcutaneous pocket and the suture was tightened to prevent leakage of cells from the transplantation site. Animals were sacrificed at various times following transplantation, as described in Results. After excision, tumors were photographed using fluorescence (470 nm light source; Lightools Research, Encinitas, CA).
Histological, immunohistochemical and immunocytochemical analysis
The fixation, paraffin embedding, and histological examination of tissue formed from transplanted cells were carried out using standard techniques. SV40 TAg was detected with monoclonal antibody PAb416 (EMD Biosciences, La Jolla, CA) used at 1:50 dilution. A secondary antibody conjugated with biotin (Vector Laboratories, Burlingame, CA) was added to the sections; positive cells were visualized by an avidin-biotin-peroxidase complex (Vector Laboratories). Sections were counterstained with hematoxylin.
In some experiments sections were deparaffinized with xylene and nuclei/nuclear material was visualized using the DNA binding dye 4,6-diamidino-2-phenylindole (DAPI). Photography was performed using a Zeiss Axiovert fluorescence microscope.
For immunocytochemistry, cells were plated on collagen-coated glass coverslips (Biocoat, Becton Dickinson) and were grown for 2 days under regular culture conditions. They were then fixed in 4% paraformaldehyde, 30 minutes at room temperature. DNA damage foci were visualized using an antibody against γ-H2AX (mouse monoclonal clone JBW301, Millipore Corp.) at 1:100. A secondary goat anti-mouse antibody conjugated with Oregon Green (Invitrogen) was used for fluorescence visualization at 1:100. DNA was visualized with DAPI at 4 μg/ml. Photography was performed using a Zeiss Axiovert fluorescence microscope equipped with a 12-bit camera. Positive cells were counted by the following procedure. All cells were photographed using the same exposure time. The time used was chosen such that the pixel intensity within the nuclei of the most highly labeled cells was maximized, but not saturating. A background pixel intensity of 5% of that level was subtracted from all images. Positive cells were counted using the particle analysis function of the program ImageJ(version 1.43u).
Supplementary Material
Supplementary Fig. 1: Telomerase activity (TRAP assay) in NMR fibroblasts expressing hTERT. (0) Buffer only control; (con) NMR fibroblasts before retroviral infection; (S/R) NMR fibroblasts 2 weeks after infection with a retrovirus encoding SV40 TAg, Ras and GFP; (S/R[+hT]) same cells, 2 weeks after an additional infection with a retrovirus encoding hTERT; (S/R*) SV40 TAg/Ras-expressing cells after 40 additional population doublings; (S/R/hT*) SV40 TAg/Ras-expressing cells with additional infection by hTERT, after 40 additional population doublings; (+ve) hTERT-positive human cells.
Supplementary Fig. 2: Histology of nodules/tumors formed from NMR cells expressing SV40 TAg, Ras, and hTERT. (A) Nodules formed from NMR fibroblasts expressing SV40 TAg, Ras and GFP were fixed and sectioned. The transplanted NMR cells were visualized with an anti-SV40 TAg antibody. Low power image and three higher power images showing various bizarre cell morphologies. (Bars, low power = 200 μm; high power = 100 μm). (B) Tumor formed from NMR fibroblasts expressing SV40 TAg, Ras and GFP plus hTERT. Low power image showing uniform mass of cells. (Bar = 200 μm).
Acknowledgments
This work was supported by grants from the National Institute on Aging, from the Owens Medical Research Foundation, and from the Glenn Foundation for Medical Research.
Contributor Information
Sitai Liang, Email: LiangS@uthscsa.edu.
James Mele, Email: mele@uthscsa.edu.
Yuehong Wu, Email: WuY4@uthscsa.edu.
Rochelle Buffenstein, Email: buffenstein@uthscsa.edu.
References
- Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–22. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol. 2005;60A:1369–77. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. 2008;178:439–45. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Jarvis JU. The naked mole rat--a new record for the oldest living rodent. Sci Aging Knowledge Environ. 2002;2002:7. doi: 10.1126/sageke.2002.21.pe7. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cancer and ageing: Rival demons? Nature Rev Cancer. 2003;3:339–49. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- Chang S, Khoo CM, Naylor ML, Maser RS, DePinho RA. Telomere-based crisis: functional differences between telomerase activation and ALT in tumor progression. Genes Dev. 2003;17:88–100. doi: 10.1101/gad.1029903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N, Kim SK, Artandi SE. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HK, Cheong C, Song J, Lee HW. Extratelomeric functions of telomerase. Curr Mol Med. 2005;5:233–41. doi: 10.2174/1566524053586635. [DOI] [PubMed] [Google Scholar]
- Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–22. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- DePinho RA, Wong KK. The age of cancer: telomeres, checkpoints, and longevity. J Clin Invest. 2003;111:S9–S14. [PubMed] [Google Scholar]
- Fanning P. Simian virus 40 large T antigen: The puzzle, the pieces, and the emerging picture. J Virol. 1992;66:1289–1293. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselsson D. Chromosome instability in cancer: how, when, and why? Adv Cancer Res. 2003;87:1–29. doi: 10.1016/s0065-230x(03)87164-6. [DOI] [PubMed] [Google Scholar]
- Gisselsson D, Jonson T, Petersen A, Strombeck B, Dal Cin P, Hoglund M, Mitelman F, Mertens F, Mandahl N. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc Natl Acad Sci USA. 2001a;98:12683–8. doi: 10.1073/pnas.211357798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselsson D, Bjork J, Hoglund M, Mertens F, Dal Cin P, Akerman M, Mandahl N. Abnormal nuclear shape in solid tumors reflects mitotic instability. Am J Pathol. 2001b;158:199–206. doi: 10.1016/S0002-9440(10)63958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C, Cerone MA, Bacchetti S. Expression of mutant telomerase in immortal telomerase-negative human cells results in cell cycle deregulation, nuclear and chromosomal abnormalities and rapid loss of viability. Oncogene. 2001;20:714–25. doi: 10.1038/sj.onc.1204145. [DOI] [PubMed] [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–9. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ. Senescence as an anticancer mechanism. J Clin Oncol. 2007;25:1852–1857. doi: 10.1200/JCO.2006.10.3101. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ, Harris SE. Oxidative damage to DNA and replicative life span in cultured adrenocortical cells. Exp Cell Res. 1987;168:203–217. doi: 10.1016/0014-4827(87)90429-0. [DOI] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–26. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- Livingston DM, Bradley MK. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987;4:63–80. [PubMed] [Google Scholar]
- Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Lustig AJ. Crisis intervention: The role of telomerase. Proc Natl Acad Sci USA. 1999;96:3339–3341. doi: 10.1073/pnas.96.7.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–9. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–6. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, Ward W, Richardson A, Chaudhuri A. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA. 2009;106:3059–64. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popnikolov NK, Hornsby PJ. Subcutaneous transplantation of bovine and human adrenocortical cells in collagen gel in scid mice. Cell Transplantation. 1999;8:617–625. doi: 10.1177/096368979900800608. [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Weinberg RA. Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2003;3:952–9. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat. 2001;4:303–13. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- Rubio MA, Kim SH, Campisi J. Reversible manipulation of telomerase expression and telomere length. Implications for the ionizing radiation response and replicative senescence of human cells. J Biol Chem. 2002;277:28609–17. doi: 10.1074/jbc.M203747200. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Sadighi Akha AA, Buffenstein R, Miller RA. Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, ultraviolet light, and endoplasmic reticulum stress. J Gerontol A Biol Sci Med Sci. 2008;63:232–41. doi: 10.1093/gerona/63.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedivy JM. How to learn new and interesting things from model systems based on “exotic” biological species. Proc Natl Acad Sci USA. 2009;106:19207–8. doi: 10.1073/pnas.0911232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seluanov A, Hine C, Azpurua J, Feigenson M, Bozzella M, Mao Z, Catania KC, Gorbunova V. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc Natl Acad Sci USA. 2009;106:19352–7. doi: 10.1073/pnas.0905252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- Shay JW, Van der Haegen BA, Ying Y, Wright WE. The frequency of immortalization of human fibroblasts and mammary epithelial cells transfected with SV40 large T-antigen. Exp Cell Res. 1993;209:45–52. doi: 10.1006/excr.1993.1283. [DOI] [PubMed] [Google Scholar]
- Sherman PW, Jarvis JUM. Extraordinary life spans of naked mole-rats (Heterocephalus glaber) J Zool. 2002;258:307–311. [Google Scholar]
- Sun B, Huang Q, Liu S, Chen M, Hawks CL, Wang L, Zhang C, Hornsby PJ. Progressive loss of malignant behavior in telomerase-negative tumorigenic adrenocortical cells and restoration of tumorigenicity by human telomerase reverse transcriptase. Cancer Res. 2004;64:6144–6151. doi: 10.1158/0008-5472.CAN-04-1376. [DOI] [PubMed] [Google Scholar]
- Sun B, Chen M, Hawks CL, Pereira-Smith OM, Hornsby PJ. The minimal set of genetic alterations required for conversion of primary human fibroblasts to cancer cells in the subrenal capsule assay. Neoplasia. 2005a;7:585–593. doi: 10.1593/neo.05172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Chen M, Hawks CL, Hornsby PJ. Immortal ALT+ human cells do not require telomerase reverse transcriptase for malignant transformation. Cancer Res. 2005b;65:6512–6515. doi: 10.1158/0008-5472.CAN-05-1210. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. Oncogenes, antioncogenes, and the molecular basis for multistep carcinogenesis. Cancer Res. 1989;49:3713–3721. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: Telomerase activity (TRAP assay) in NMR fibroblasts expressing hTERT. (0) Buffer only control; (con) NMR fibroblasts before retroviral infection; (S/R) NMR fibroblasts 2 weeks after infection with a retrovirus encoding SV40 TAg, Ras and GFP; (S/R[+hT]) same cells, 2 weeks after an additional infection with a retrovirus encoding hTERT; (S/R*) SV40 TAg/Ras-expressing cells after 40 additional population doublings; (S/R/hT*) SV40 TAg/Ras-expressing cells with additional infection by hTERT, after 40 additional population doublings; (+ve) hTERT-positive human cells.
Supplementary Fig. 2: Histology of nodules/tumors formed from NMR cells expressing SV40 TAg, Ras, and hTERT. (A) Nodules formed from NMR fibroblasts expressing SV40 TAg, Ras and GFP were fixed and sectioned. The transplanted NMR cells were visualized with an anti-SV40 TAg antibody. Low power image and three higher power images showing various bizarre cell morphologies. (Bars, low power = 200 μm; high power = 100 μm). (B) Tumor formed from NMR fibroblasts expressing SV40 TAg, Ras and GFP plus hTERT. Low power image showing uniform mass of cells. (Bar = 200 μm).