Abstract
BACKGROUND
This prospective, single-center study assesses progression-free survival (PFS) and overall survival (OS) in patients with recurrent glioblastoma multiforme (GBM) treated with a single dose of superselective intra-arterial cerebral infusion (SIACI) of bevacizumab (BV) after blood-brain barrier disruption (BBBD). Patients were initially enrolled in our phase I study, for which the primary end point was to determine the safety and maximum tolerated dose of SIACI BV.
METHODS
Fourteen patients with recurrent GBM were recruited between August 2009 and November 2010 after failing the standard treatment with radiation therapy and temozolomide. None of these patients were previously treated with BV. After receiving a single dose of IA BV (2 to 15 mg/kg), standard IV BV chemotherapy was continued in 12 of 14 patients (86%). The recently updated Response Assessment in Neuro-Oncology Working Group (RANO) criteria were used to evaluate PFS, and the Kaplan-Meier estimator was used to evaluate PFS and OS.
RESULTS
Using RANO criteria, the median PFS in these patients was 10 months. The median OS estimation for this cohort was 8.8 months. The OS was less than the PFS because 4 patients died without progressing. Toxicity attributed to the IA BV treatment was present in 2 patients (wound dehiscence and rash). Another patient suffered from seizures 1 week after the SIACI procedure; however, this patient had epilepsy before and seizure type/frequency were similar before and after therapy.
CONCLUSIONS
Our study shows that for patients naïve to BV, a single dose of SIACI BV after BBBD followed by IV BV offers an encouraging outcome in terms of PFS when compared with previous trials using IV BV with and without concomitant irinotecan (CPT-11). Larger phase II trials are warranted to determine whether repeated IA BV alone is superior to IV BV for recurrent GBM.
Keywords: Bevacizumab, Glioblastoma, Intra-arterial chemotherapy, Overall survival, Progression-free survival
INTRODUCTION
Glioblastoma multiforme (GBM) is the most frequent and aggressive malignant primary brain tumor, with an incidence of approximately 5 per 100,000 (4, 18). A published phase III randomized trial showed a 5-year overall survival rate of 9.8% for patients treated with surgery plus adjuvant radiation and temozolomide (14). For recurrent GBM, the U.S. Food and Drug Administration has approved the humanized monoclonal antibody bevacizumab (BV) (6), which directly binds to vascular endothelial growth factor (VEGF) that is released by endothelial cells, brain tumor stem-like cells, and other bulk tumor cells (1, 15, 17). Both intravenous (IV) and intra-arterial (IA) BV have been proven to be safe in human trials (2, 12, 13, 16). Our recently completed phase I trial was the first to use superselective IA cerebral infusion (SIACI) of BV after blood-brain barrier disruption (BBBD) for recurrent GBMs. Therefore, data regarding outcome such as progression-free survival (PFS) for SIACI BV after BBBD is lacking in the literature (2). IV BV alone or in combination with other chemotherapeutical drugs such as irinotecan (CPT-11) showed PFS and overall survival (OS) rates of up to 5.6 months and 9.8 months, respectively, in patients with recurrent malignant gliomas (8, 16). This report presents the long-term follow-up data from our previous phase I SIACI BV trial, which tested a single escalating dose of SIACI BV after BBBD and subsequent IV BV therapy.
PATIENTS AND METHODS
Patient Eligibility
This study is a follow-up to our previous phase I trial that investigated the safety and maximum tolerated dose (MTD) of SIACI of BV after osmotic BBBD (2). Fourteen patients were recruited from August 2009 until November 2010. Inclusion criteria were: 1) age older than 18 years, 2) histopathological diagnosed glioblastoma, 3) Karnofsky score >60, 4) failed combined radiation and temozolomide, and 5) no previous treatment with BV. Patients with pathologies other than glioblastoma were excluded from this study. All patients were self-selected, and therefore do not represent a randomized cohort or a consecutive series.
Treatment Plan
This study was approved by both the Weill Cornell Medical College Institutional Review Board and the U.S. Food and Drug Administration (Investigational New Drug 107,402). All patients had to sign a written informed consent before entering into the study. At baseline, patients were subjected to complete neurological and physical examinations as well as magnetic resonance (MR) imaging of the brain with contrast. All patients received a single SIACI dose of mannitol (1.4 M mannitol at 10 mL per 120 seconds) followed by BV with dose escalation from 2 mg/kg to 15 mg/kg as previously described (16, 20). The patients were then monitored one month post-operatively for dose limiting toxicity. At end of the one-month observational period, the patients underwent follow-up brain MR imaging and repeat physical and neurological examinations. If no adverse effects or toxicities were seen, patients were then started on IV BV (10 mg/kg) on a biweekly basis except for 2 patients (no. 7 and no. 9) (Figure 1). These 2 patients received only repeated IA therapy until progression because they refused IV treatment. MR imaging after 3 months, 6 months, and 12 months as well as continuous clinical follow-up examinations were obtained. Long-term adverse side effects and toxicities related to BV were documented and analyzed.
Figure 1.
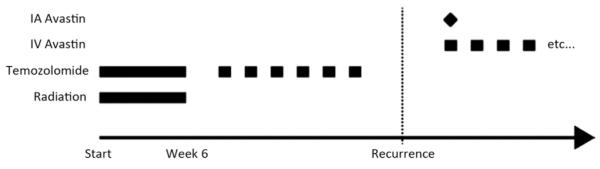
Treatment plan of the included patients: all patients failed combined radiation and temozolamide and were naïve to BV before IA BV treatment. After a single IA BV administration of IV BV was continued in 12 of 14 patients (86%). BV, bevacizumab; IA, intra-arterial; IV, intravenous.
Evaluation of Response
The primary end point of the phase I trial was safety, and those results have been published previously (2). The secondary end points of interest were PFS and OS, respectively, after a single SIACI BV treatment with BBBD. The PFS was determined as the time between IA BV administration and progression. The OS was defined as the time between IA BV administration and death. For progression assessment, the updated Macdonald criteria were utilized as outlined by the Response Assessment in Neuro-oncology Working Group (RANO) (19). The updated assessment criteria, which included radiological as well as clinical findings, account more accurately for pseudoresponses that have been associated with antiangiogenic drugs such as BV. Progression of disease was assigned by a senior board-certified neuroradiologist (A.J.T.) when one or more of the following criteria were demonstrated: (1) increasing enhancement on post-gadolinium T1-weighted images (T1-WI), (2) increasing mass-like hyperintensity on T2-weighted FLAIR (fluid attenuated inversion recovery) images, and/or (3) appearance of new lesions. If a patient died without progression, the last MR image before death was used as the last censored follow-up for the PFS analysis. The slice of greatest cross-sectional area of the enhanced tumor (MR imaging T1 sequence) was chosen for size analysis.
Statistical Analysis
Descriptive statistics and nonparametric tests were used to describe the radiological outcomes and determine statistical significance, as appropriate. All P values are 2-sided, and statistical significance was evaluated at the 0.05 alpha level. All statistical analyses were performed in SPSS (PASW) version 18.0 (SPSS Inc., Chicago, IL) and STATA version 11.0 (StataCorp, College Station, TX).
RESULTS
Patient Demographics
Fourteen patients (9 men and 5 women) with recurrent glioblastoma were included in this analysis (Table 1). The mean age was 52 years (range 29 to 70), with a median Karnofsky performance status score of 80 (range 60 to 100), and the mean follow-up time was 6.5 months (range 3 to 14.7). Four patients received more than 1 IA BV infusion (patient no. 6: 2; patients no. 7, 9, and 13: 3). In the 5 patients who received more than 1 IA BV, MR imaging assessment was performed only after the initial SIACI BV treatment.
Table 1.
Summary of GBM Patients Treated with IA BV after BBBD and Subsequent IV BV
| No. | Gender | Age | KPS | IA Avastin Dose (mg/kg) |
Complications | Progression (Y/N) |
Time to Progression after IA Treatment or Last Follow-up if not Progressed (days) |
|---|---|---|---|---|---|---|---|
| 1 | M | 50 | 100 | 2 | Wound dehiscence | Y | 299 |
| 2 | F | 44 | 60 | 6 | – | N | 104 |
| 3 | M | 61 | 70 | 10 | – | N | 132 |
| 4 | M | 66 | 70 | 11 | – | N | 102 |
| 5 | M | 52 | 80 | 11 | – | N | 8 |
| 6 | F | 52 | 90 | 13 | – | Y | 89 |
| 7 | F | 38 | 80 | 13 | – | N | 276 |
| 8 | M | 66 | 66 | 14 | Seizures | Y | 122 |
| 9 | M | 46 | 70 | 14 | – | N | 63 |
| 10 | F | 29 | 100 | 15 | Rash in trunk region | N | 126 |
| 11 | F | 59 | 100 | 15 | – | Y | 69 |
| 12 | M | 70 | 80 | 15 | – | N | 117 |
| 13 | M | 55 | 90 | 15 | – | Y | 67 |
| 14 | M | 44 | 90 | 15 | – | N | 91 |
BBBD, blood-brain barrier disruption; BV, bevacizumab; F, female; GBM, glioblastoma; IA, intra-arterial; IV intravenous; KPS, Karnofsky performance status scale; M, male; N, no; Y, yes.
Treatment-Associated Toxicity
Neuroradiological and clinical follow-up were performed at close intervals to identify early signs of toxicity related to IA BV treatment. Overall, our adverse events were comparable with previous studies (Table 1). None of the patients suffered an intervention-related intracerebral hemorrhage or stroke. One patient suffered from seizures 1 week after the SIACI procedure; however, this patient had epilepsy before IA BV treatment, and seizure type/frequency were similar before and after therapy. Therefore, a causal relation to the procedure or drug is unclear. One patient presented with a truncal rash 3 weeks after the IA BV treatment. None of the complications led to death in the patient series.
Response Rate, Progression-Free Survival, and Overall Survival
All 14 patients had a radiographic response within 1 month after a single SIACI BV treatment by means of partial response (8 patients) or stable disease (6 patients), respectively, by the updated RANO criteria. The 8 patients (57%) with partial response showed at least a 50% decrease in the cross-sectional area of the enhanced tumor on postgadolinium T1-weighted images. Additionally, patients with partial response demonstrated no new lesions, a decrease or stable hyperintensity on T2-weighted FLAIR images, and no clinical deterioration or increase in the corticosteroid dose. Three patients who initially responded showed radiological progression 3 months after SIACI despite IV BV. A single SIACI BV treatment showed a statistically significant cross-sectional area reduction of the enhanced tumor at 1 month (P = 0.001) (Figure 2). The return to growth of cross-sectional area occurred at 3 to 6 months. Using the RANO criteria, the median PFS was 10 months in this series (Figure 3). The median OS for this cohort was 8.8 months (Figure 4). The OS was less than the progression-free survival because 4 patients died before progressing. At the time of analysis, 6 patients (43%) died and 8 (57%) were alive.
Figure 2.
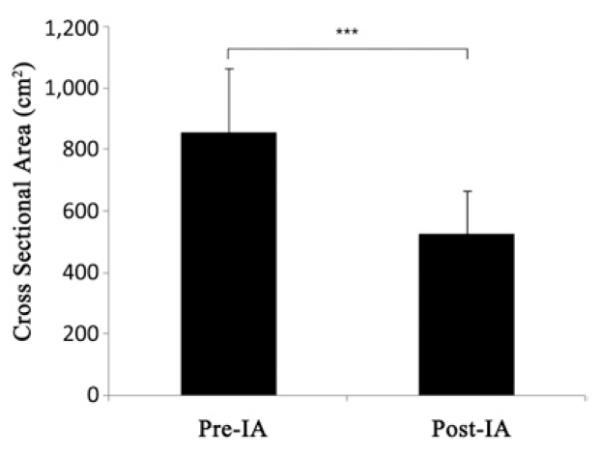
Cross-sectional volume calculation of the enhanced tumor (magnetic resonance imaging T1 sequence) after IA BV showed a significant decrease after 1 month in the patient cohort (P = 0.001). BV, bevacizumab; IA, intra-arterial.
Figure 3.
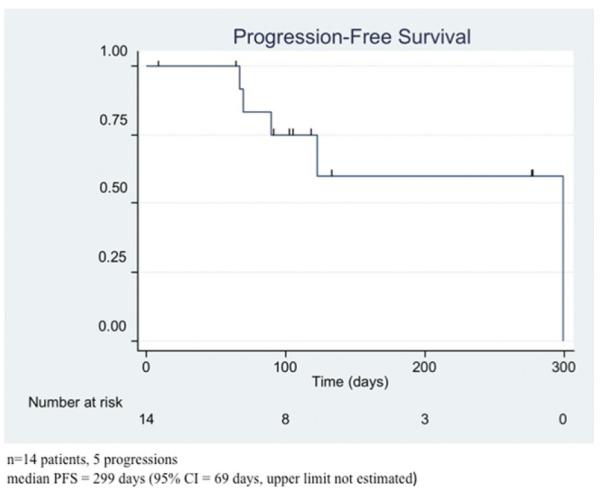
Median progression-free survival (PFS) probability of the included patients with a median PFS of 10 months.
Figure 4.
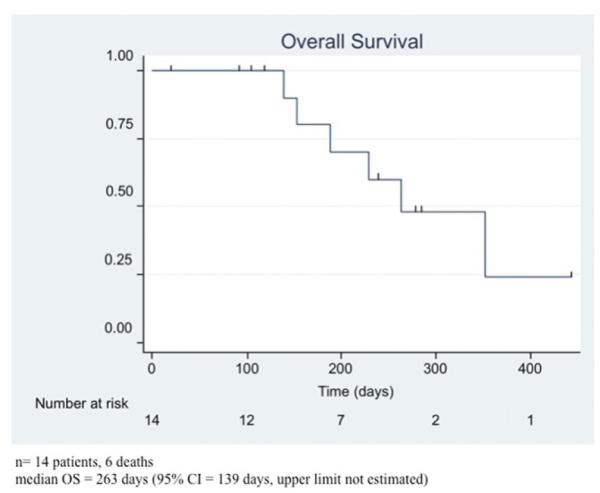
Median overall survival (OS) probability of the included patients with a median OS of 8.8 months.
DISCUSSION
Glioblastomas are highly challenging to treat due to their aggressive behavior. Early invasion of tumor cells into normal brain tissue usually results in subtotal resections. Additionally, drug resistance or limitation of drug delivery through the BBB impedes adjuvant treatment (3, 5). Although the introduction of temozolomide in combination with radiation therapy has led to a significantly higher PFS in newly diagnosed GBMs compared with previous chemotherapeutic regiments, there are very few effective agents for the treatment of recurrent malignant gliomas. For instance, alkylating agents such as nitrosureas or carboplatin have a response rate of <20% in patients already treated with radiation therapy (15).IV BV alone or in combination with irinotecan is well established and is one of the currently used treatment regiments for progressive malignant gliomas.
We have recently published data that showed that IA BV is safe for the treatment of recurrent malignant gliomas (2, 12, 13). In this study, we analyzed the patient outcome of BV-naïve GBM patients from our phase I trial who received 1 dose of IA SIACI BV after BBBD with subsequent IV BV alone. By MR imaging, we saw a statistically significant decrease in tumoral enhancement on T1-weighted images after 1 SIACI BV in all patients (P = 0.001). In this patient series, we found a median PFS of 10 months, which compares very favorably with past studies, in which progression may have been underestimated (11). For example, Kreisl et al. reported a median PFS of 3.7 months for patients with glioblastomas treated with IV BV, and Friedman et al. showed a median PFS of only 4.2 months with IV BV alone (7, 9). Considering that we used the updated RANO response criteria, which seek to eliminate false pseudoresponses, our treatment responses were encouraging but not directly comparable with previous studies that used the outdated version of the Macdonald criteria. Our findings are also comparable with other studies using IV BV in combination with irinotecan (CPT-11), although none of our patients received CPT-11. In previous studies using IV BV and irinotecan, a median PFS of 5.6 months was found (7).
Although 8 patients (57%) were still alive at the time of analysis, we calculated a median OS estimation of 8.8 months using the Kaplan-Meier method. Our results are comparable with previous studies that treated patients with IV BV alone or in combination with irinotecan. For instance, Kreisl et al. and Friedman et al. showed a median OS of 7.2 and 9.2 months in IV BV–treated patients, respectively (7, 9). In patients treated with IV BV plus irinotecan, Friedman et al. and Vredenburgh et al. demonstrated a median OS of 8.7 and 9.8 months, respectively. Because 57% of our patients are still alive at the time of analysis, the final actual OS might vary from our Kaplan-Meier estimated OS.
Overall, our data indicate that a single treatment with SIACI BV after BBBD followed by IV BV shows promising results for PFS and comparable results for OS when compared with IV BV studies for recurrent glioblastoma (8, 10, 20). However, one caveat of the RANO criteria is demonstrated by the fact that 4 of our patients died without progressing because death is not defined as progression. This should be further addressed in the next update of the RANO working group. Although the lack of an adequate control group limits the value of this comparison, we believe that the favorable PFS observed in our patient cohort warrant further investigation for the following reasons: (1) none of these patients received CPT-11 after IA BV, (2) our study used the stricter updated response assessment criteria for progression (RANO criteria), which helps to eliminate false pseudoresponses, (3) some of our patients received a very low dose of SIACI BV (i.e., 2 mg/kg).
CONCLUSIONS
Our study suggests that patients naïve to BV who receive a single SIACI BV dose after BBBD followed by IV BV have an encouraging outcome in terms of progression-free survival using the updated RANO response criteria. Based on these data, larger phase II trials are warranted to determine whether repeated IA BV alone is superior to IV BV for recurrent GBM.
Abbreviations and Acronyms
- BBBD
Blood-brain barrier disruption
- BV
Bevacizumab
- FLAIR
Fluid attenuated inversion recovery
- GBM
Glioblastoma multiforme
- IA
Intra-arterial
- IV
Intravenous
- MR
Magnetic resonance
- MTD
Maximum tolerated dose
- OS
Overall survival
- PFS
Progression-free survival
- SIACI
Superselective intra-arterial cerebral infusion
- TMZ
Temozolomide
- VEGF
Vascular endothelial growth factor
Footnotes
Conflict of interest statement: Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-RR024996).
REFERENCES
- 1.Abrey LE. Bevacizumab in recurrent malignant glioma. Curr Neurol Neurosci Rep. 2008;8:233–234. doi: 10.1007/s11910-008-0035-9. [DOI] [PubMed] [Google Scholar]
- 2.Boockvar JA, Tsiouris AJ, Hofstetter CP, Kovanlikaya I, Fralin S, Kesavabhotla K, Seedial SM, Pannullo SC, Schwartz TH, Stieg P, Zimmerman RD, Knopman J, Scheff RJ, Christos P, Vallabhajosula S, Riina HA. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. J Neurosurg. 2011;114:624–632. doi: 10.3171/2010.9.JNS101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles N, Holland EC. The perivascular niche microenvironment in brain tumor progression. Cell Cycle. 2010;9:3012–3021. doi: 10.4161/cc.9.15.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 7.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto FM, Fine HA. Bevacizumab for malignant gliomas. Arch Neurol. 2010;67:285–288. doi: 10.1001/archneurol.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, Robins HI, Lieberman FS, Fine HA, Fink KL, Junck L, Abrey L, Gilbert MR, Mehta M, Kuhn JG, Aldape KD, Hibberts J, Peterson PM, Prados MD. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 12.Riina HA, Fraser JF, Fralin S, Knopman J, Scheff RJ, Boockvar JA. Superselective intraarterial cerebral infusion of bevacizumab: a revival of interventional neuro-oncology for malignant glioma. J Exp Ther Oncol. 2009;8:145–150. [PubMed] [Google Scholar]
- 13.Riina HA, Knopman J, Greenfield JP, Fralin S, Gobin YP, Tsiouris AJ, Souweidane MM, Boockvar JA. Balloon-assisted superselective intra-arterial cerebral infusion of bevacizumab for malignant brainstem glioma. A technical note. Interv Neuroradiol. 2010;16:71–76. doi: 10.1177/159101991001600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 15.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 16.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 17.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 18.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 19.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 20.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]


