Summary
Women suffering from breast cancer often succumb to incurable recurrent disease resulting from therapy-resistant cancer cells. In this issue of Cancer Cell, Alvarez and colleagues identify downregulation of the tumor suppressor Par-4 as the key determinant in apoptosis evasion that leads to tumor recurrence in breast cancer.
Keywords: Breast cancer, Par-4, recurrence, apoptosis
Breast cancer is the second leading cause of cancer deaths in women. It is estimated that in the US alone over 200,000 women will be diagnosed and over 20% of them will die of breast cancer in 2013 (Siegel et al., 2013). Surgical intervention in conjunction with chemotherapeutic agents generally provides distinct benefit to patients with localized primary tumors, especially those that have been detected early. Breast cancer patients with positive estrogen receptor (ER), progesterone receptor (PR) and/or human epidermal growth factor receptor 2 (HER2) status are also responsive to targeted therapeutics, given as monotherapy, such as trastuzumab, bevacizumab or cetuximab, or in combination with chemotherapy (Alvarez et al., 2010). Moreover, polychemotherapy that includes cyclophosphamide, methotrexate and fluorouracil, or anthracycline-based regimens combined with taxanes has met with considerable success in prolonging survival and delaying breast cancer recurrence (Martin et al., 2013). However, 1 in 5 patients exhibit relapse of the disease within 10 years after treatment (Brewster et al., 2008). In addition, about 20% of newly diagnosed breast cancer cases present with triple-negative status, which is defined by loss of ER, PR and HER2, (Metzger-Filho et al., 2012) and over 50% of primary tumors change their hormone receptor status from ER-positive, PR-positive to ER-negative, PR-negative at the time of recurrence (Thompson et al., 2010). Breast cancers with triple-negative or basal-like characteristics are often associated with a high risk of metastasis and both local and distant recurrence compared to receptor positive tumors (Ahmad, 2013). Treatment of such recurrent breast tumors is challenging owing to their aggressive nature as they tend to be resistant to the ‘standard of care’ adjuvant or neo-adjuvant systemic therapies. Consequently, patients with recurrent disease have low median survival. Despite the alarming statistics on recurrent breast tumors, the development of effective treatment modalities has been severely hampered by the paucity of data on the therapy-resistant traits of such tumors. There exists an urgent need for more effective breast cancer prognosis to aid the judicious treatment of tumors that are most likely to recur.
The report by Alvarez et al. (2013) in this issue of Cancer Cell transcends many of these foregoing limitations and provides timely insight into the molecular basis of breast cancer recurrence. In this seminal paper, the authors present compelling evidence that down-regulation of the pro-apoptotic, tumor suppressor Prostate apoptosis response-4 (Par-4) (Hebbar et al., 2012) is a major determinant underlying breast cancer recurrence. Although Par-4 was first described in prostate cancer (Sells et al., 1994), it is ubiquitously expressed and serves as a tumor suppressor in diverse tumor types (Hebbar et al., 2012). Based on a highly refined analysis of gene expression records from human breast cancer datasets and the I-SPY 1 trial, Alvarez et al. (2013) conclude that low levels Par-4 expression results in significantly decreased recurrence-free survival.
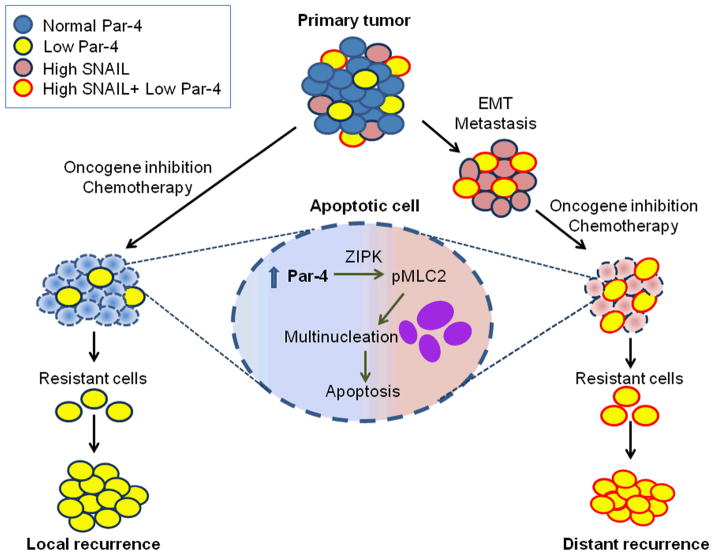
Elegantly designed cell culture and mouse tumor experiments by Alvarez et al. (2013) indicate that primary breast tumors consist of a heterogeneous population of cancer cells, especially with regard to Par-4 expression (Figure 1). Tumor cells expressing high levels of Par-4 are eliminated by apoptosis following oncogene-inhibition or chemotherapy, whereas those expressing low levels of Par-4 show an increased propensity to recur at both local and distant metastatic sites. Par-4 down-regulation was particularly associated with all breast cancer traits that confer poor prognosis, such as ER-negative status, basal-like subtype, and high grade (grade III). By using primary and recurrent murine tumors driven by HER2/neu, MYC, or WNT1 oncogenic signaling, the authors demonstrate that tumors that relapse after oncogene-inhibition or chemotherapy exhibit significantly diminished levels of Par-4 RNA and protein. Moreover, using orthotopic tumors with either low levels of Par-4 caused by short hairpin-RNA knockdown or overexpressed Par-4 via retroviral transduction, the authors demonstrate that downregulation of Par-4 is essential for tumor recurrence. By contrast, Par-4 levels do not influence primary tumor formation. The observations on the three distinct clinically relevant mouse models therefore recapitulate the findings of the meta-analysis on human breast cancer gene expression profiles. Thus, oncogene-inhibition or chemotherapeutic regimens select for pre-existing tumor cells that express low levels of Par-4, which are below the critical threshold for induction of apoptosis, and such cells constitute the therapy-resistant population that emerges as a recurrent tumor.
Figure 1. Par-4 expression is a key determinant of breast cancer recurrence.
Primary breast tumors display heterogeneity in expression profiles for Par-4 and SNAIL. Breast tumor cells with higher SNAIL transcription factor activity undergo EMT and metastasize. Oncogene-inhibition or chemotherapy of primary or metastatic breast tumors causes Par-4-dependent apoptosis for cancer cells expressing normal levels of Par-4 via multinucleation, which is dependent on ZIP kinase and MLC2 activation. On the other hand, primary or metastatic breast tumor cells expressing low levels of Par-4 (i.e., below a critical threshold) evade apoptosis; such cells constitute recurrent tumors at both local and distant sites.
It is noteworthy that the expression of endogenous Par-4, as well as ectopic Par-4, is suppressed in recurrent tumors. Similar observations were previously made in the prostate of mice co-expressing oncogenic SV40 antigens and the SAC-transgene, which represent the killer domain of Par-4 (Zhao et al., 2007). Zhao et al. found that when the SAC transgene was spontaneously eliminated from benign areas of the prostate prostatic adenocarcinoma developed, while prostatic epithelial cells that continued to express the SAC domain remained normal or benign. These findings not only reiterate the tumor suppressor potential of Par-4 and but reaffirm that Par-4 induces apoptosis specifically in malignant tumor cells, not normal or benign cells.
The findings of Alvarez et al. (2013) on tumor recurrence also shed light on the distinct roles for Par-4 and other key proteins WNT and SNAIL, which have been previously shown to regulate EMT and metastasis. Unlike SNAIL and WNT that have been reported to be overexpressed or activated in breast cancer EMT, metastasis, and recurrence (Ahmad, 2013), low Par-4 levels do not cause EMT or metastasis per se, permitting breast cancer recurrence independent of these traits by evading apoptosis. Importantly, the authors show that downregulation of Par-4 is necessary and sufficient for recurrence and occurs independently of SNAIL. Future studies on the mechanism by which Par-4 is down-regulated in breast tumors may further unravel the molecular network involved in Par-4 regulation and breast cancer recurrence.
Mechanistically, increased Par-4 expression in tumor cells in response to oncogene-inhibition or treatment with chemotherapeutic agents was shown to enhance ZIP kinase-induced phosphorylation of MLC2 and multinucleation, ultimately leading to tumor cell apoptosis. As induction of tetraploidy is tumorigenic in a p53-deficient background, an intact p53 pathway is most likely required for this response. Although ZIP kinase has been previously shown to interact with Par-4, it is plausible that direct binding to Par-4 may not be necessary to provoke the ZIP kinase-dependent MLC2 phosphorylation and cytokinesis failure in breast cancer. Consequently, unlike tumor cells expressing basal levels of Par-4 that are readily eliminated by chemotherapy or oncogene-inhibition, tumors expressing low Par-4 levels fail to elicit the ZIP kinase-driven apoptotic response, and therefore constitute local or distant recurrent disease. Although the role of Par-4 in inducing apoptosis has been well characterized in the broader cellular context (Hebbar et al., 2012), this manuscript demonstrates an atypical mechanism for apoptosis by Par-4 in oncogene-addicted cells. These findings offer a new mechanistic link between oncogene-addiction, Par-4 regulation, and tumor recurrence.
In summary, Alvarez et al. (2013) provide crucial insights into the heterogeneity of breast cancer and suggest that Par-4 is a viable prognostic marker for breast cancer recurrence. Their findings may provide the basis for the development of novel treatment strategies for breast cancer, such as nanotechnology to deliver recombinant Par-4 to tumors, in order to replenish intracellular Par-4 levels and sensitize the tumors to the action of therapeutics that may prolong disease-free survival in breast cancer patients.
Acknowledgments
This work was supported by KLCR grant and NIH/NCI grant CA060872 (to VMR).
References
- Ahmad A. ISRN Oncol, 290568. 2013 doi: 10.1155/2013/290568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JV, Pan T, Ruth J, Feng Y, Zhou A, Pant D, Grimley JS, Wandless TJ, DeMichele A, Chodosh LA the I-SPY 1 TRIAL Investigators. Cancer Cell. 2013;xx:xxx–xxx. doi: 10.1016/j.ccr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RH, Valero V, Hortobagyi GN. J Clin Oncol. 2010;28:3366–3379. doi: 10.1200/JCO.2009.25.4011. [DOI] [PubMed] [Google Scholar]
- Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, Buzdar AU, Booser DJ, Valero V, Bondy M, Esteva FJ. J Natl Cancer Inst. 2008;100:1179–1183. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbar N, Wang C, Rangnekar VM. J Cell Phys. 2012;227:3715–3721. doi: 10.1002/jcp.24098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Ruiz A, Borrego MR, Barnadas A, Gonzalez S, Calvo L, Vila MM, Anton A, Rodriguez-Lescure A, Segui-Palmer MA, et al. J Clin Oncol. 2013 Epub ahead of print. [Google Scholar]
- Metzger-Filho O, Tutt A, de Azambuja E, Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA, Jr, Ellis P, et al. J Clin Oncol. 2012;30:1879–1887. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, Purdie CA Breast Recurrence in Tissues Study G. Breast Cancer Res BCR. 2010;12:R92. doi: 10.1186/bcr2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Burikhanov R, Qiu S, Lele SM, Jennings CD, Bondada S, Spear B, Rangnekar VM. Cancer Res. 2007;67:9276–9285. doi: 10.1158/0008-5472.CAN-07-2124. [DOI] [PubMed] [Google Scholar]