Abstract
Many synaptic plasticity-related signaling pathways have been identified as important regulators of the pathogenesis of chronic pain in animal models. However, their relevance to human pathological pain is rarely confirmed rigorously. Recent studies suggest that Wnt signaling plays critical roles in synaptic plasticity and is dysregulated in the spinal cord dorsal horn (SDH) of different mouse pain models. In this study, we compared the protein levels of Wnt ligands, Wnt receptors and their downstream effector proteins in the SDH from non-HIV patients, HIV patients who developed chronic pain (‘pain-positive’ HIV patients), and HIV patients who did not develop chronic pain (‘pain-negative’ HIV patients). Our results indicate that many Wnt ligands and downstream effector proteins were specifically up-regulated in the SDH of ‘pain-positive’ HIV patients but not in the ‘pain-negative’ HIV patients. These findings describe an HIV pain-associated activation of Wnt signaling in the SDH of human patients. Given the established role of Wnt signaling in the regulation of synaptic plasticity, these results suggest that the activated Wnt signaling might contribute to the expression of the synaptic plasticity in the SDH during the pathogenesis of HIV-associated chronic pain.
Keywords: HIV-1, pain, Wnt signaling, spinal cord dorsal horn
Introduction
Pathological (chronic, neuropathic) pain is a major neurological complication suffered by over 50% of HIV-1/AIDS patients (Hewitt et al., 1997; Mirsattari et al., 1999; Evers et al., 2000). However, there is currently no effective therapy to this devastating condition that severely deteriorates the patients’ life quality. The HIV-associated pain disorder is thought to be caused by HIV-1 infection, opportunistic infections, anti-HIV therapies, or a combination of them (Dalakas, 2001; Verma et al., 2005). The molecular mechanism of HIV-associated pain is largely unknown.
While some HIV patients develop chronic pain, others do not. The differential development of chronic pain among HIV patients indicates that host factors play a critical role in determining the transition to the chronic pain state. Identification of these factors in the neural pathway of pain transmission will not only provide a firm basis to elucidate the molecular process of this transition. Importantly, such host factors would be strong candidates of pharmacological targets for preventing or even reversing the transition to HIV-associated chronic pain.
Peripheral neuropathy is a common neurological phenotype often observed in many HIV patients who develop chronic pain (Polydefkis et al., 2002; Morgello et al., 2004; Simpson et al., 2006). Several animal models have been generated to mimic this neurological defect (Keswani et al., 2006; Melli et al., 2006; Wallace et al., 2007). Extensive studies on different animal models have clearly established that the abnormal plasticity of pain processing neurons in the CNS, especially central sensitization in the spinal cord dorsal horn (SDH), plays a key role in the development of chronic pain (Melzack et al., 2001; Latremoliere and Woolf, 2009; Kuner, 2010; Woolf, 2011). Currently, little is known about the pathogenic changes, especially at a molecular level, in the SDH that contribute to the development of HIV-associated chronic pain.
Wnts are secreted proteins that elicit canonical and non-canonical intracellular signaling pathways (after binding to their Frizzled receptors and other co-receptors) to regulate diverse cellular processes (Logan and Nusse, 2004; Speese and Budnik, 2007). In neurons, the synthesis and secretion of Wnt proteins appear to be regulated by synaptic activity (Chen et al., 2006; Tang, 2007; Ataman et al., 2008; Li et al., 2012). The activity-regulated Wnt signaling is known to play an important role in the regulation of long-term potentiation (LTP), a major type of synaptic plasticity involved in memory formation (Chen et al., 2006). Wnt signaling is known to modulate synaptic transmission and NMDA receptor activity (Farias et al., 2009; Schmeisser et al., 2009; Avila et al., 2010; Wan et al., 2012; Neuberger, 2013). Deregulation of Wnt signaling is implicated in the development of Alzheimer’s disease, an aging-related memory disorder (Maguschak and Ressler, 2008, 2011). More recently, we have observed that Wnt signaling is dysregulated by nociceptive inputs in the SDH of various rodent models (Shi et al., 2012b).
We report here the up-regulation of Wnt signaling in the SDH of ‘pain-positive’ HIV/AIDS patients but not in the ‘pain-negative’ patients. The findings identify the Wnt signaling pathways as potential candidates of the host factors that contribute to the HIV-1/AIDS chronic pain. These observations provide the first piece of clinically relevant evidence for a potential role of Wnt signaling in regulating the development of pathological pain.
Materials and methods
Human postmortem tissues
Fifteen subjects were selected from the autopsy archive of the Texas NeuroAIDS Research Center, which is one unit of The National NeuroAIDS Tissue Consortium (NNTC) (Morgello et al., 2001). Among them, five were HIV-negative subjects, who had no known history of peripheral neuropathy, myelopathy or chronic pain (HIV− Pain−); five were HIV-positive subjects, who had neuropathologically normal distal sural nerves and did not have definitive clinical evidence of HIV-associated distal sensory neuropathy (HDSPN), clinical or neuropathological myelopathy, or clinical pain syndrome (‘pain− negative’ HIV patients; HIV+ Pain−); and another five were HIV-positive subjects, who developed HDSPN and a clinically documented pain syndrome (‘pain-positive’ HIV patients; HIV+ Pain+). For all 15 subjects, a complete autopsy was performed, including removal of the spinal cord and the distal sural nerve. The spinal cord specimens were sectioned (1.0 mm) and stored at −80°C for immunoblotting analysis. Paraffin sections (5 µm) were also collected for immunostaining. All of the 10 HIV-infected subjects developed AIDS and received antiretroviral therapy. No accurate information regarding pain treatment was available. Detailed patient information has been described elsewhere (Shi et al., 2012a).
Western blotting analysis
The dorsal horn gray matter of the human postmortem lumbar spinal cord was dissected from frozen sections on dry ice and homogenized in RIPA lysis buffer (1% Nonidet P-40, 50 mM Tris-HCl pH 7.4, 1% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA pH 8.0) containing the protease inhibitor cocktail (Sigma). After centrifugation (12,000 g), the supernatant was collected and the protein concentration was detected using the BCA Protein Assay Kit (Pierce). Equal amounts of protein (50 µg) were loaded and separated on SDS-PAGE, followed by transferring to nitrocellulose membranes. The membranes were then blocked and incubated with anti-Wnt3a (1:1000, Millipore: 09162), anti-Wnt5a (1:1000, Abcam: ab72583), anti-Wnt4 (1:1000, R&D: MAB 475), anti-Wnt9b (1:1000, R&D: AF3669), anti-Fzd3 (1:1000, R&D: AF1001), anti-Fzd5 (1:2000, Abcam: ab75234), anti-Ror2 (1:1000, a gift from Dr. Roel Nusse, Stanford University School of Medicine (Mikels et al., 2009), anti-phospho-GSK3β (pY216; 1:3000, BD: 612312), anti-GSK3β (1:3000, BD: 610201), anti-β-catenin (1:5000, BD: 610153), anti-Axin2 (1:1000, Cell Signaling: 2151) or anti-Actin (1:1000, Santa Cruz: sc1616R) primary antibodies. Protein bands were detected with an Enhanced Chemiluminescence kit (Pierce).
Immunohistochemistry
Paraffin sections (5 µm) of human lumbar spinal cord tissue were baked overnight at 37°C, followed by three de-paraffin steps in xylene for 5 min each at room temperature (RT). After that, the sections were rehydrated in a series of ethanol washes (100%, 95%, 80% and 70%) and PBS. This was followed by incubation in 3% H2O2 for 5–10 min and biotin blocking buffer for 20 min to quench endogenous peroxidase and endogenous biotin activity, respectively. Subsequently, the slides were boiled in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween-20, pH 6.0) for 20 min for antigen retrieval. After cooling down at room temperature, the slides were blocked for 60 min at RT in blocking buffer (5% BSA, 0.05% Tween-20 in TBS). After blocking, the sections were incubated with anti-Wnt3a (1:200), anti-Wnt5a (1:200), anti-NeuN (1:200, Millipore: MAB377) or normal control IgG (used as a negative control) overnight at 4°C. After five washes with PBS buffer, the sections were incubated with FITC- or Cy3-conjugated secondary antibody (1:200, Jackson Immunoresearch), followed by incubation with DAPI for nuclear staining (Sigma). Images were captured using a laser confocal microscope (Zeiss). For neutralization control experiment, Wnt3a or Wnt5a antibody (1µg) was incubated with the corresponding peptide (1µg, RnD: 1324-WN-002/CF and 645-WN-010/CF) overnight at 4°C prior to being used for immunostaining.
Data analysis and statistics
The immunoblotting results were quantified using ImageJ (NIH) and normalized with β-actin loading controls. Quantitative summary data (mean ± SEM) were from at least 3 independent experiments. One-way ANOVA was performed using Prism 5 (GraphPad) software (p<0.05 was considered significant).
Results
Up-regulation of Wnt ligands in ‘pain-positive’ HIV patients
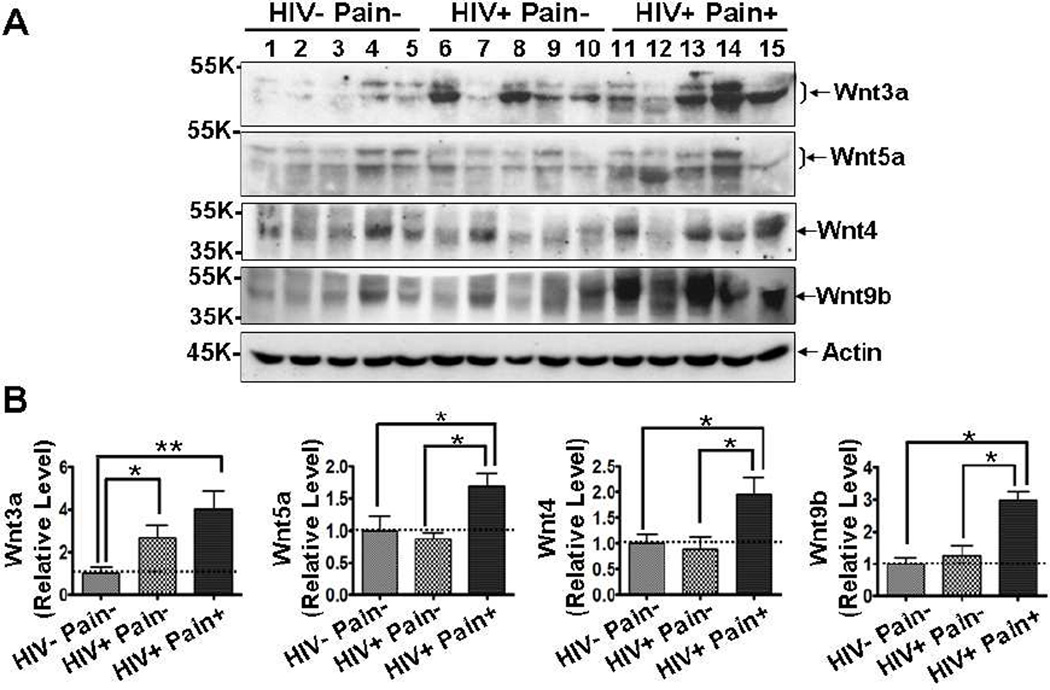
Accumulating evidence reveals that Wnt signaling plays important roles in the regulation of synaptic plasticity (Chen et al., 2006; Beaumont et al., 2007; Ataman et al., 2008; Avila et al., 2010). Because long-term potentiation (LTP)-like synaptic plasticity is implicated in the establishment of central sensitization in chronic pain (Basbaum et al., 2009), we wanted to know if Wnt signaling pathways are involved in the HIV-related chronic pain. As an initial step toward this goal, we performed Western blotting analysis of postmortem spinal cord dorsal horn (SDH) to determine the expression levels of Wnt ligands. We found that Wnt3a, a prototypic Wnt ligand that activates the Wnt canonical pathway, was significantly increased in 4 of the 5 ‘pain-positive’ HIV patients, compared with the levels in HIV-negative subjects. The mean of Wnt3a protein levels in ‘painpositive’ HIV patients is about 4-fold higher than that in HIV-negative patients (p<0.01) (Fig. 1). Among HIV patients, the Wnt3a level appeared to be higher in ‘pain-positive’ HIV patients than that in ‘pain-negative’ HIV patient, although the difference did not reach statistical significance (Fig. 1). Wnt5a was significantly up-regulated in ‘pain-positive’ HIV patients, compared with either HIV-negative or ‘pain-negative’ HIV patients (Fig. 1). Similarly, we also observed significant up-regulation of Wnt4 and Wnt9b specifically in the ‘pain-positive’ but not in ‘pain-negative’ HIV patients (Fig. 1). These findings reveal that Wnt ligands are up-regulated in the SDH of HIV patients who developed chronic pain.
Fig. 1.
Up-regulation of Wnt ligands in the SDH of ‘pain-positive’ HIV patients. (A) Western blotting results of Wnt3a, Wnt5a, Wnt4 and Wnt9b in the postmortem lumbar spinal cord dorsal horn (SDH). (B) Quantitative summaries of the results of (A). Wnt ligands were increased in the SDH of ‘pain-positive’ HIV patients (mean ± SEM; * p<0.05; ** p<0.01; one-way ANOVA).
Spatial distribution of Wnt3a and Wnt5a in human spinal cord
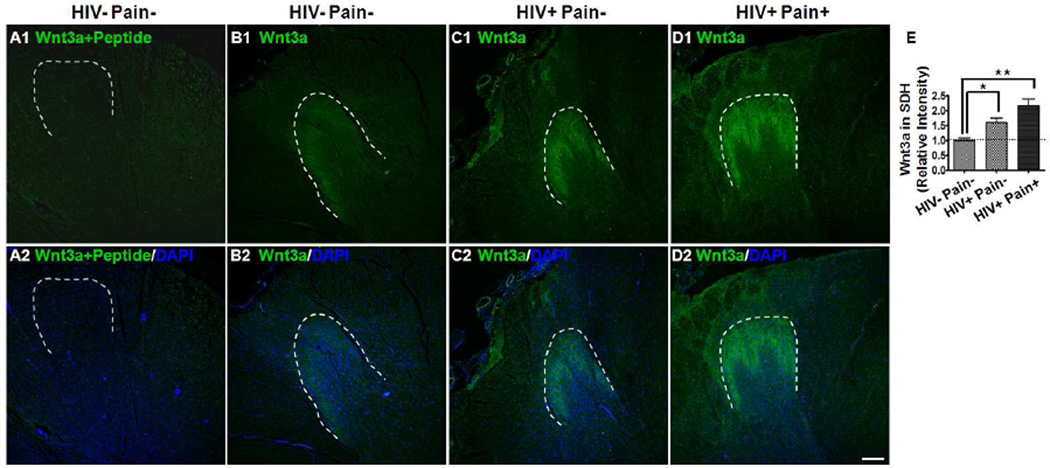
The immunoblotting data described above indicates that Wnt ligands, including Wnt3a, Wnt5a, Wnt4 and Wnt9b, are up-regulated in the SDH of ‘pain-positive’ HIV patients. Next, we performed immunostaining experiments to determine the distribution of Wnt3a and Wnt5a in the human spinal cord and identify the spatial domain where Wnt ligands increased. As shown in Fig. 2 B1–B2, in HIV-negative controls, Wnt3a signals were predominantly restricted to the superficial layers of the dorsal horn. Wnt3a signals in the ventral horn and the white matter of the spinal cord were low and barely detectable. If the antibody had been pre-incubated with Wnt3a peptide, no characteristic Wnt3a staining was observed (Fig. 2 A1–A2). The spatial distribution pattern of Wnt3a in the human spinal cord revealed here is similar to what we have observed in the rodent spinal cord (Shi et al., 2012b; Yuan et al., 2012). In addition, we found that Wnt3a signals were markedly increased in the HIV-infected subjects, especially in those who developed chronic pain (Fig. 2 C1–C2, D1–D2, and E). The increase of Wnt3a staining was confined in the superficial layers of the dorsal horn.
Fig. 2.
Spatial distribution of Wnt3a in the SDH. (A1–A2) Immunostaining results (HIV− pain− patients) with the antibody pre-blocked with Wnt3a peptide. (B–D) Immunostaining results of Wnt3a in the SDH of an HIV-negative subject (B1–B2), a ‘pain-negative’ HIV patient (C1–C2), and a ‘pain-positive’ HIV patient (D1–D2). Wnt3a signals formed a predominant band in the SDH, and the signals were hard to detect outside of the dorsal horn. (E) Intensity of Wnt staining in the SDH. The Wnt signal was markedly increased in the SDH of ‘pain-positive’ HIV patients, compared with ‘pain-negative’ HIV patients or HIV-negative subjects (mean ± SEM; * p<0.05; ** p<0.01; one-way ANOVA). DAPI (Blue) staining was included to visualize all cells. Dashed lines were drawn to indicate the SDH regions. Scale bar: 400 µm.
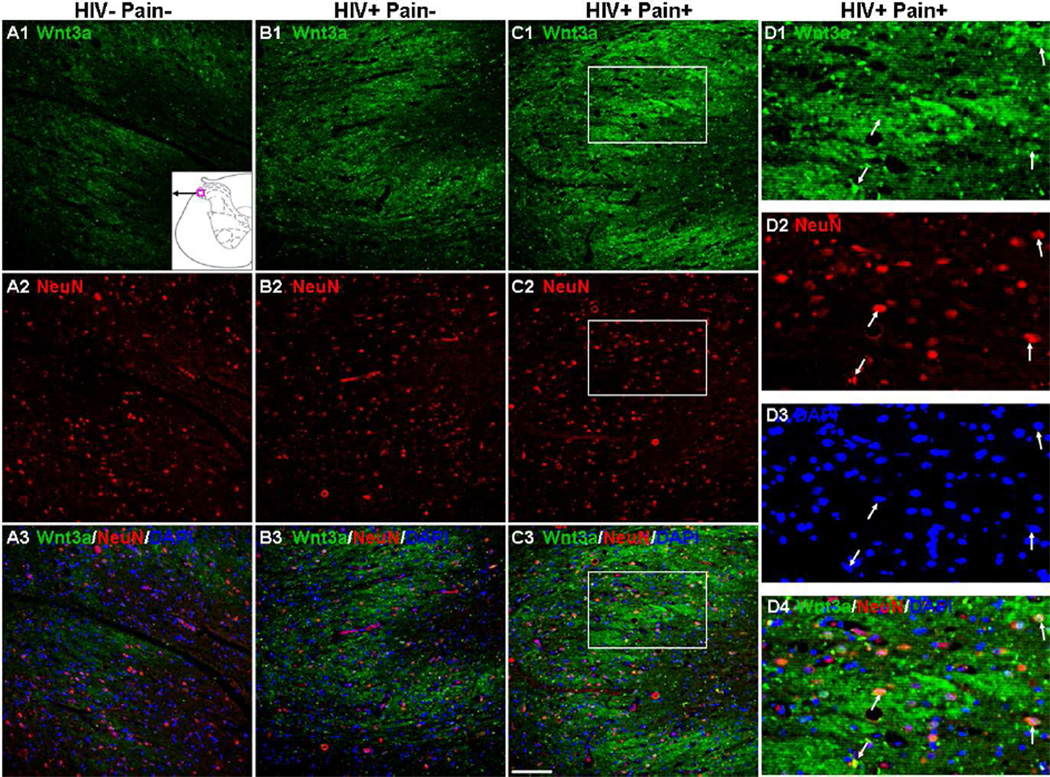
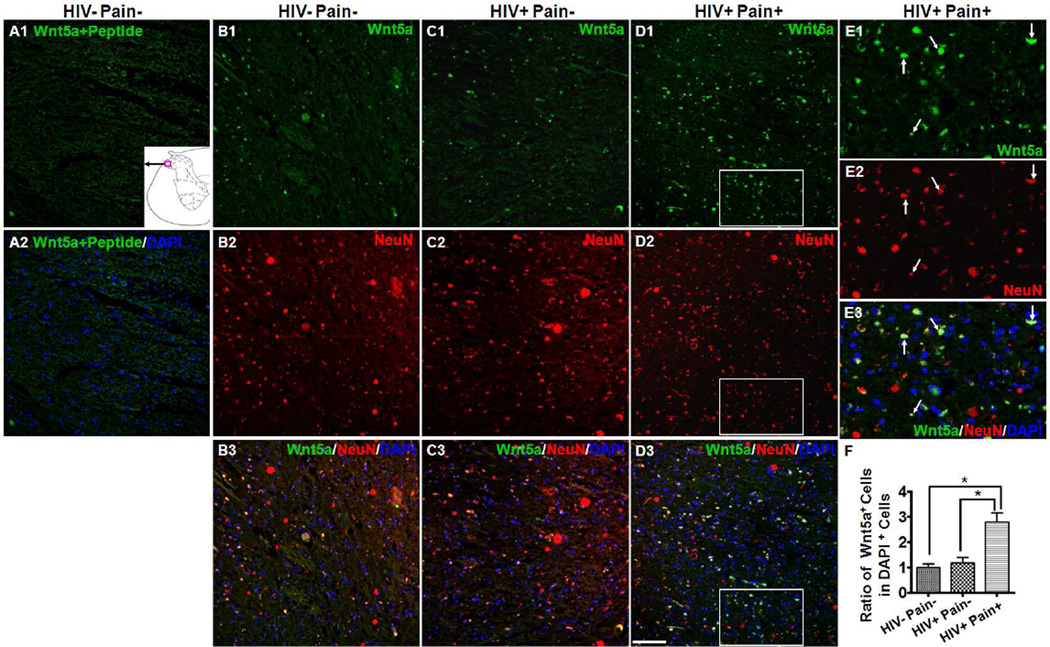
To identify the cell types that express Wnt3a protein in the SDH, we performed double-staining experiments with NeuN, a neuronal marker. As shown in Fig. 3, most of the Wnt3a-expressing cells (~90%) in the superficial layers of the dorsal horn were NeuN-positive. Additionally, Wnt3a staining was observed in cell bodies and regions surrounding the cell bodies (Fig. 3), which was consistent with previous studies that Wnt3a was localized in the cell bodies and dendrites in hippocampal neurons (Chen et al., 2006; Avila et al., 2010; Liu et al., 2011). Thus, Wnt3a is predominantly expressed in neurons in the superficial layer of the human SDH. We also determined the cell distribution of Wnt5a in the SDH and found that Wnt5a was more evenly distributed in the gray matter of the spinal cord, including the dorsal horn (Fig. 4) and ventral horn (data not shown), than Wnt3a. In the dorsal horn, ~95% of Wnt5a-positive cells were neurons (NeuN-positive). Unlike Wnt3a, Wnt5a was more restricted to neuronal cell bodies (Fig. 4 B1–E3). Wnt5a-positive cells increased 2.8 fold in the SDH of ‘pain-positive’ HIV patients, compared with the number in ‘pain-negative’ HIV patients or HIV-negative subjects (p<0.05) (Fig. 4F). The predominant expression of Wnt3a and Wnt5a in the human SDH neurons were similar to those in rodent SDH (Shi et al., 2012b). Staining was largely diminished when the antibody had been blocked with Wnt5a peptide (Fig. 4 A1–A2).
Fig. 3.
Cellular localization of Wnt3a in the SDH. (A1–C3) Double staining of Wnt3a (Green) and NeuN (Red). Staining in the SDH of HIV-negative subjects (A1–A3), ‘pain-negative’ HIV patients (B1–B3), and ‘pain-positive’ HIV patients (C1–C3). Wnt3a staining was mainly observed in NeuN-labeled cell bodies and regions surrounding the cell bodies. The signals were increased in ‘pain-positive’ HIV patients. The region of the micrograph in the SDH is indicated by the red box located at the bottom in A1. (D1–D4) Higher power images of the box regions in (C1–C3). Wnt3a signals partly overlapped with NeuN in the neuronal cell bodies (arrow). Scale bar: 100 µm.
Fig. 4.
Cellular localization of Wnt5a in the SDH. (A1–A2) Immunostaining results with Wnt5a antibody pre-blocked with Wnt5a peptide (HIV− pain− subjects). (B–D) Double staining of Wnt5a (Green) and NeuN (Red) in the SDH of an HIV-negative subject (B1–B3), ‘pain-negative’ HIV patients (C1–C3), and ‘pain-positive’ HIV patients (D1–D3). Wnt5a signals formed bright spots in the SDH and were found in NeuN-labeled cell bodies. (E1–E3) Higher power images of the box regions in (D1–D3). Most Wnt5a signals were co-localized with NeuN in the neuronal cell bodies (arrows). (F) Quantitative Wnt5a-postive cells in the SDH. Wnt5a-positive cells were significantly up-regulated in the SDH of ‘pain-positive’ HIV patients (mean ± SEM; * p<0.05; one-way ANOVA). Scale bar: 100 µm.
Expression levels of Wnt receptors in the SDH
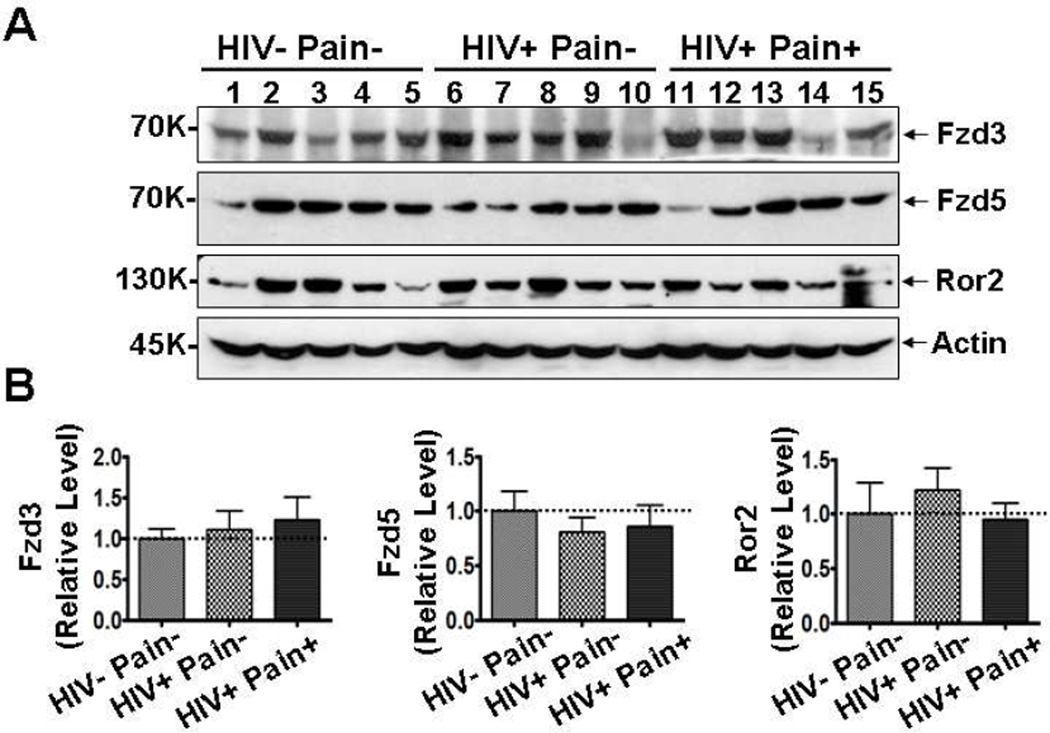
Next, we sought to determine if Wnt receptors were also dysregulated in the SDH of ‘pain-positive’ HIV patients. Immunoblotting analysis showed that the levels of Fzd3 and Fzd5 proteins, two Wnt receptors belonging to the Frizzled (Fzd) family, were not significantly different in the SDH of either ‘pain-negative’ or ‘pain-positive’ HIV patients than in the non-HIV patients (Fig. 5). In addition, Ror2, a Wnt5a receptor tyrosine kinase that activates the Wnt non-canonical pathway (Mikels et al., 2009; Ho et al., 2012), was also not significantly different among the three groups of human patients (Fig. 5). These data indicate that the expression of many, if not all, Wnt receptors are not affected during the development of HIV-associated pain.
Fig. 5.
Receptors of Wnt ligands are not up-regulated in ‘pain-positive’ HIV patients. (A) Western blotting results of Fzd3, Fzd5 and Ror2 in the postmortem lumbar spinal cord dorsal horn (SDH). (B) Quantitative summaries of the results of (A). Protein levels of Fzd3, Fzd5 and Ror2 were not significantly different in the three groups (mean ± SEM; one-way ANOVA).
Activation of Wnt signaling pathways in the SDH of ‘pain-positive’ HIV patients
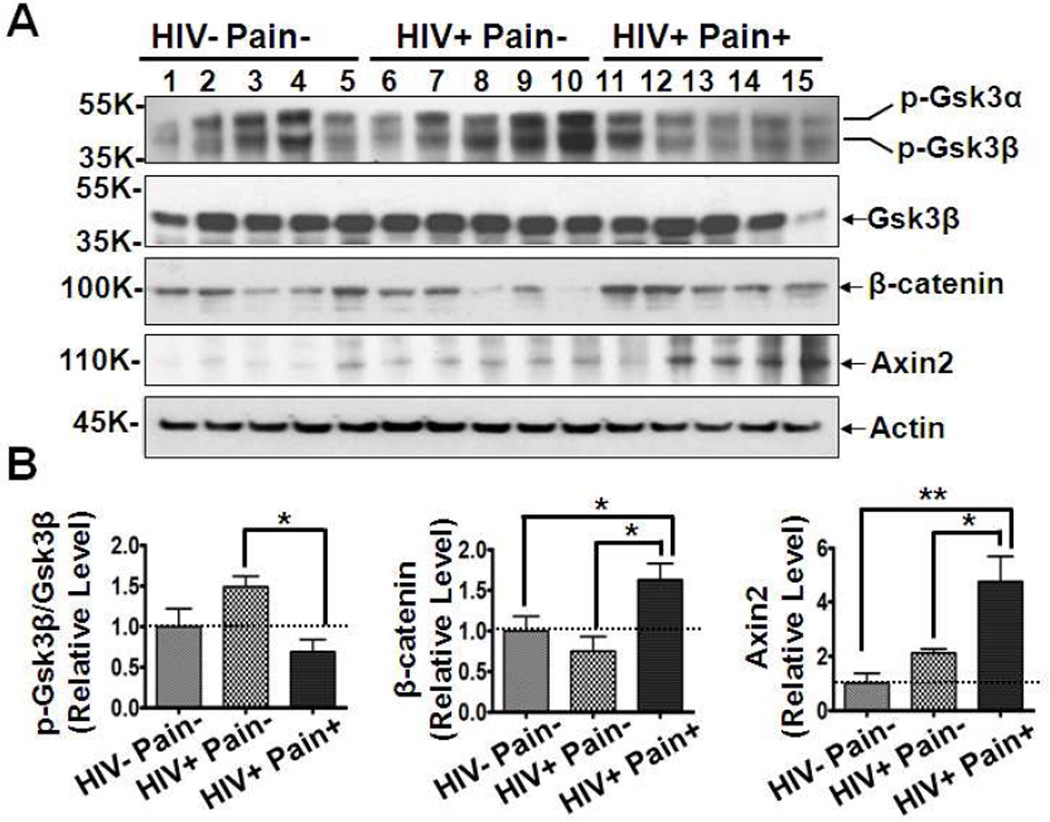
The up-regulation of Wnt ligands suggests aberrant activation of Wnt signaling in SDH of ‘pain-positive’ HIV patients. To directly test this hypothesis, Western blotting analysis showed that the phosphorylated Gsk3β (pY216) level was significant decreased in the SDH of ‘pain-positive’ HIV patients while total Gsk3β protein levels in the three groups remained constant, indicating that the activity of Gsk3β is down-regulated in the SDH of ‘pain-positive’ HIV patients (Fig. 6). Conversely, β-catenin protein (total), a key protein in the Wnt canonical pathway, was found to be remarkably accumulated in ‘pain-positive’ HIV patients compared to levels in ‘pain-negative’ HIV patients or HIV-negative subjects. Next, we selected Axin2, a well-known target gene of the Wnt canonical pathway (Jho et al., 2002; Lustig et al., 2002), to confirm the activation. As shown in Fig. 6, Axin2 protein was significantly increased in 4 of the 5 ‘pain-positive’ HIV patients compared with levels in ‘pain-negative’ HIV patients (2.2 fold, p<0.05) or HIV-negative subjects (4.7 fold, p<0.01). These data showed that the Wnt canonical pathway is specifically activated in the SDH of ‘pain-positive’ HIV patients, suggesting that Wnts should play important roles in the process of HIV-associated pain.
Fig. 6.
Activation of Wnt canonical signaling pathway in ‘pain-positive’ HIV patients. (A) Western blotting results of phosphorylated Gsk3β (at Y216), Gsk3β, β-catenin and Axin2. (B) Quantitative summaries of the results of (A). Protein levels of p-Gsk3β (pY216, active form) were significantly decreased in the SDH of ‘pain-positive’ HIV patients. Conversely, β-catenin and Axin2 were highly increased in ‘pain-positive’ HIV patients (mean ± SEM; * p<0.05; ** p<0.01; one-way ANOVA).
Discussion
In this study, we observed the up-regulation of Wnt proteins, including Wnt3a, Wnt5a, Wnt4 and Wnt9b, in the spinal cord dorsal horn of HIV patients who developed chronic pain. Strikingly, except for Wnt3a, most of them did not increase in the patients who did not manifest chronic pain (Fig. 1). Consistent with the up-regulation of the Wnt ligands, we also observed the up-regulation of β-catenin, a key effector protein in the canonical Wnt signaling pathway, and Axin2, a well-established target gene of the Wnt/β-catenin pathway. These findings indicate that the Wnt signaling activation in the SDH is specifically associated with the development of HIV-related pain. The differential activity of Wnt signaling in the SDH of ‘pain-positive’ and ‘pain-negative’ HIV patients suggests that Wnt signaling is a potential candidate for host factors that help the transition to, the establishment of, or the maintenance of the chronic pain state in HIV patients. In support of this interpretation, we found that Wnt signaling is activated in the SDH of various rodent pain models (Shi et al., 2012b). It is important to note that the data presented in this paper, by themselves, do not allow one to establish a causal role of Wnt signaling deregulation in the pathogenesis of HIV-associated pain. Interestingly, recent studies started showing that inhibition of Wnt signaling pathways impaired the development of pathological pain in animal models (Yuan et al., 2012) (Zhang et al., 2013).
HIV-1 viral loads and CD4 counts varied greatly within either ‘pain-positive’ or ‘pain-negative’ HIV-1 patient groups. We did not observe significant differences of viral loads and CD4 counts between the cohorts, with the caveat of a small patient number. In addition, we did not detect significant correlations between the levels of Wnt ligands and either the viral loads or CD4 counts in HIV-1 patients. These observations appear to argue against the possibility that the viral loads and CD4 counts are causally linked to the activation of Wnt signaling in the SDH of ‘pain-positive’ patients.
Both the ‘pain-negative’ and the ‘pain-positive’ HIV-1 patients received long-term antiretroviral therapy (Shi et al., 2012a). Because Wnt signaling is specifically up-regulated in the ‘pain-positive’ HIV-1 patients, it is unlikely caused solely by the antiretroviral therapy.
How is Wnt signaling up-regulated in the SDH of HIV human patients? HIV gp120 is a potential inducer of Wnt expression. We recently observed that intrathecal injection of gp120 rapidly up-regulated Wnt ligands, including Wnt5a, in the SDH of mice (Li et al., 2013). This observation bears interesting clinical relevance because, compared with the ‘pain-negative’ HIV patients, the gp120 levels in the SDH of ‘pain-positive’ HIV patients are about 10 fold higher (manuscript in preparation). HIV gp120 is known to activate neurons by stimulating NMDA receptors and/or chemokine receptors CXCR4 or CCR5 (Catani et al., 2000; Gemignani et al., 2000; Oh et al., 2001). We recently described that the activation of NMDA receptors causes rapid synthesis and secretion of Wnt ligands (Chen et al., 2006; Li et al., 2012; Wan et al., 2012). Thus, one potential way by which gp120 up-regulates Wnt expression is to stimulate neurons.
One potential mechanism by which Wnt signaling activation facilitates the development of pathological pain is to regulate neuronal plasticity of the pain transmission circuit at the SDH. LTP-like central sensitization is a well-established cellular substrate in the SDH in animals that have had chronic pain induced (Sandkuhler and Liu, 1998; Rygh et al., 1999). We and others have revealed a critical role of Wnt signaling in the expression of LTP (Chen et al., 2006; Avila et al., 2010; Cerpa et al., 2011). Wnt signaling plays important roles in the regulation of synaptic structure and function (Chen et al., 2006; Tang, 2007; Ataman et al., 2008; Varela-Nallar et al., 2010; Ciani et al., 2011; Li et al., 2012). Because Wnt signaling can increase miniature activity of presynaptic termini (Ahmad-Annuar et al., 2006; Davis et al., 2008), the activation of Wnt signaling may facilitate the quantum release of afferent fibers of nociceptive neurons.
Another potential mechanism that we have conceived is that Wnt signaling may facilitate the development of HIV-related chronic pain by regulating neuroinflammation. Persistent neuroinflammation in the SDH, including prominent activation of glia and the expression of pro-inflammatory mediators, is the etiologically relevant hallmark of the establishment of chronic pain in animal models (Raghavendra et al., 2002; Milligan and Watkins, 2009). We recently found pronounced neuroinflammation in the SDH of HIV patients who developed chronic pain (Shi et al., 2012a). Interestingly, Wnt signaling pathways have lately been implicated in the regulation of peripheral inflammation (Sen et al., 2000; Christman et al., 2008; Pereira et al., 2008; Camilli and Weeraratna, 2010; Suarez-Farinas et al., 2011). Our studies in mixed neuron cultures revealed a critical role of Wnt5a signaling in regulation of TNF-α and IL-1β (Li et al., 2011). In addition, we also found that Wnt5a/CaMKII and Wnt5a/JNK signaling pathways are critical for HIV gp120 to elicit proinflammatory cytokine expression in the mouse SDH (Li et al., 2013). Based on these findings, we propose that Wnt signaling isa potential molecular gateway that controls HIV pain-related neuroinflammation in the SDH of human patients.
In summary, this is the first study that shows an association of dysregulated Wnt signaling with the manifestation of chronic pain in HIV human patients. This finding may provide a clue to elucidate the host factor-mediated molecular processes in the SDH that facilitate the transition to HIV-associated chronic pain. Our results indicate that Wnt signaling and its downstream cellular and molecular pathways could be valuable drug targets to block the transition.
Acknowledgements
This work was supported by the UTMB start-up funds, the Whitehall Foundation and National Institutes of Health Grants R01-NS079166 to SJT, and U01-MH-083507 and R24-NS-045491 to BBG.
Footnotes
Conflict of interest disclosure
There is no conflict of interest for the authors.
REFERENCES
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila ME, Sepulveda FJ, Burgos CF, Moraga-Cid G, Parodi J, Moon RT, Aguayo LG, Opazo C, De Ferrari GV. Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons. J Biol Chem. 2010;285:18939–18947. doi: 10.1074/jbc.M110.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont V, Thompson SA, Choudhry F, Nuthall H, Glantschnig H, Lipfert L, David GR, Swain CJ, McAllister G, Munoz-Sanjuan I. Evidence for an enhancement of excitatory transmission in adult CNS by Wnt signaling pathway modulation. Mol Cell Neurosci. 2007;35:513–524. doi: 10.1016/j.mcn.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Camilli TC, Weeraratna AT. Striking the target in Wnt-y conditions: intervening in Wnt signaling during cancer progression. Biochem Pharmacol. 2010;80:702–711. doi: 10.1016/j.bcp.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani MV, Corasaniti MT, Navarra M, Nistico G, Finazzi-Agro A, Melino G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem. 2000;74:2373–2379. doi: 10.1046/j.1471-4159.2000.0742373.x. [DOI] [PubMed] [Google Scholar]
- Cerpa W, Gambrill A, Inestrosa NC, Barria A. Regulation of NMDA-receptor synaptic transmission by Wnt signaling. J Neurosci. 2011;31:9466–9471. doi: 10.1523/JNEUROSCI.6311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Christman MA, 2nd, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, Benencia F, Silver MJ, Kohn LD, Malgor R. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008;294:H2864–H2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- Ciani L, Boyle KA, Dickins E, Sahores M, Anane D, Lopes DM, Gibb AJ, Salinas PC. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca(2)(+)/Calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 2011;108:10732–10737. doi: 10.1073/pnas.1018132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas MC. Peripheral neuropathy and antiretroviral drugs. Journal of the peripheral nervous system : JPNS. 2001;6:14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- Davis EK, Zou Y, Ghosh A. Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Dev. 2008;3:32. doi: 10.1186/1749-8104-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers S, Wibbeke B, Reichelt D, Suhr B, Brilla R, Husstedt I. The impact of HIV infection on primary headache. Unexpected findings from retrospective, cross-sectional, and prospective analyses. Pain. 2000;85:191–200. doi: 10.1016/s0304-3959(99)00266-3. [DOI] [PubMed] [Google Scholar]
- Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemignani A, Paudice P, Pittaluga A, Raiteri M. The HIV-1 coat protein gp120 and some of its fragments potently activate native cerebral NMDA receptors mediating neuropeptide release. Eur J Neurosci. 2000;12:2839–2846. doi: 10.1046/j.1460-9568.2000.00172.x. [DOI] [PubMed] [Google Scholar]
- Hewitt DJ, McDonald M, Portenoy RK, Rosenfeld B, Passik S, Breitbart W. Pain syndromes and etiologies in ambulatory AIDS patients. Pain. 1997;70:117–123. doi: 10.1016/s0304-3959(96)03281-2. [DOI] [PubMed] [Google Scholar]
- Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, Kuruvilla R, Greenberg ME. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci U S A. 2012;109:4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Jack C, Zhou C, Hoke A. Establishment of a rodent model of HIV-associated sensory neuropathy. J Neurosci. 2006;26:10299–10304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhong L, Yang X, Andersson T, Huang M, Tang SJ. WNT5A signaling contributes to A beta-induced neuroinflammation and neurotoxicity. PLoS One. 2011;6:e22920. doi: 10.1371/journal.pone.0022920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Shi Y, Shu J, Gao J, Wu P, Tang SJ. Wingless-type mammary tumor virus integration site family, member 5A (Wnt5a) regulates human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120)-induced expression of pro-inflammatory cytokines via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) signaling pathways. J Biol Chem. 2013 doi: 10.1074/jbc.M112.381046. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li B, Wan X, Zhang W, Zhong L, Tang SJ. NMDA receptor activation stimulates transcriptionin-dependent rapid wnt5a protein synthesis via the MAPK signaling pathway. Molecular brain. 2012;5:1. doi: 10.1186/1756-6606-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JX, Pinnock SB, Herbert J. Novel control by the CA3 region of the hippocampus on neurogenesis in the dentate gyrus of the adult rat. PLoS One. 2011;6:e17562. doi: 10.1371/journal.pone.0017562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Wnt signaling in amygdala-dependent learning and memory. J Neurosci. 2011;31:13057–13067. doi: 10.1523/JNEUROSCI.3248-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–1338. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Melzack R, Coderre TJ, Katz J, Vaccarino AL. Central neuroplasticity and pathological pain. Ann N Y Acad Sci. 2001;933:157–174. doi: 10.1111/j.1749-6632.2001.tb05822.x. [DOI] [PubMed] [Google Scholar]
- Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem. 2009;284:30167–30176. doi: 10.1074/jbc.M109.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsattari SM, Power C, Nath A. Primary headaches in HIV-infected patients. Headache. 1999;39:3–10. doi: 10.1046/j.1526-4610.1999.3901003.x. [DOI] [PubMed] [Google Scholar]
- Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, Cavert W, Marra C, Grant I, Singer EJ. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathology and applied neurobiology. 2001;27:326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, Ryan E, Yakoushina T, Khan S, Mahboob R, Naseer M, Dorfman D, Sharp V, Manhattan HIVBB. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61:546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- Neuberger J. Transplantation: Assessment of liver allograft steatosis. Nature reviews Gastroenterology & hepatology. 2013;283:5918–5927. doi: 10.1038/nrgastro.2013.74. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Yiannoutsos CT, Cohen BA, Hollander H, Schifitto G, Clifford DB, Simpson DM, Katzenstein D, Shriver S, Hauer P, Brown A, Haidich AB, Moo L, McArthur JC. Reduced intraepidermal nerve fiber density in HIV-associated sensory neuropathy. Neurology. 2002;58:115–119. doi: 10.1212/wnl.58.1.115. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygh LJ, Svendsen F, Hole K, Tjolsen A. Natural noxious stimulation can induce long-term increase of spinal nociceptive responses. Pain. 1999;82:305–310. doi: 10.1016/S0304-3959(99)00056-1. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci. 1998;10:2476–2480. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- Schmeisser MJ, Grabrucker AM, Bockmann J, Boeckers TM. Synaptic cross-talk between N-methyl-D-aspartate receptors and LAPSER1-beta-catenin at excitatory synapses. J Biol Chem. 2009;284:29146–29157. doi: 10.1074/jbc.M109.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Gelman BB, Lisinicchia JG, Tang SJ. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci. 2012a;32:10833–10840. doi: 10.1523/JNEUROSCI.5628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yuan S, Li B, Wang J, Carlton SM, Chung K, Chung JM, Tang SJ. Regulation of Wnt signaling by nociceptive input in animal models. Molecular pain. 2012b;8:47. doi: 10.1186/1744-8069-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, Goodkin K, Gerschenson M, So Y, Marra CM, Diaz-Arrastia R, Shriver S, Millar L, Clifford DB, Group AAS. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, Krueger JG. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol. 2011;131:391–400. doi: 10.1038/jid.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ. The synaptic Wnt signaling hypothesis. Synapse. 2007;61:866–868. doi: 10.1002/syn.20434. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci U S A. 2010;107:21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Estanislao L, Simpson D. HIV-associated neuropathic pain: epidemiology, pathophysiology and management. CNS Drugs. 2005;19:325–334. doi: 10.2165/00023210-200519040-00005. [DOI] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice AS. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007;133:47–63. doi: 10.1016/j.pain.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan XZ, Li B, Li YC, Yang XL, Zhang W, Zhong L, Tang SJ. Activation of NMDA receptors upregulates a disintegrin and metalloproteinase 10 via a Wnt/MAPK signaling pathway. J Neurosci. 2012;32:3910–3916. doi: 10.1523/JNEUROSCI.3916-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Shi Y, Tang SJ. Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. J Neuroimmune Pharmacol. 2012;7:904–913. doi: 10.1007/s11481-012-9370-3. [DOI] [PubMed] [Google Scholar]
- Zhang YK, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013;123:2268–2286. doi: 10.1172/JCI65364. [DOI] [PMC free article] [PubMed] [Google Scholar]