Abstract
A marked increase in hospitalization for patients with AF has previously been noted. Whether this increase was related to a change in the prevalence of AF, or change in the pattern of practice with respect to the management of AF remains unclear. To determine the trends in hospital utilization after first atrial fibrillation (AF) in a community-based setting, Olmsted County, Minnesota residents diagnosed with first AF during 1980-2000 were identified and followed until 2004. The primary outcome of interest was hospital admission for cardiovascular reasons. Of a total of 4,498 subjects (73±14 years old, 51% men), 2,503 (56%) were admitted to the hospital for cardiovascular causes for at least once during a mean follow-up of 5.5±5.0 years. The risk of first hospitalization was greatest during the first year of AF [cumulative incidence 31%, 95% confidence interval (CI) 30-32%]. First hospitalization was strongly related to age (P<0.0001), but not with sex (P=0.38). During 1980-2000, the age-and sex-adjusted rate of first hospitalization increased, on average, by 2.5% a year (95% CI 1.8-3.2%, P<0.0001), even after multivariable adjustment for comorbidities. When we excluded all hospital admissions for the purposes of AF management, the increase in hospitalization was only 0.8% per year (95% CI 0.05-1.6%, P=0.04), which was no longer significant after multivariable adjustment for comorbidities (P=0.25). In conclusion, the marked increase in hospitalization after first AF diagnosis during 1980-2000 appeared to be largely driven by the changing practice pattern in AF management.
Keywords: atrial fibrillation, hospitalization, epidemiology
Introduction
Based on the National Hospital Discharge Survey, there has been a 3-fold increase in hospitalization for patients with a diagnosis of atrial fibrillation (AF) over the period 1985 through 1999 1. Similar increases were evident in Canada 2, and in Europe 3-5. Whether the increase in hospitalization was due to an increase in prevalence of AF, or changes in the pattern of practice 6,7with respect to AF management remains unclear. The aim of this study was to determine the trends in incidence of hospital admission after incurring the diagnosis of first AF, and the contributing factors for hospital admission for these patients in a community-based setting.
Methods
Following the approval from the Mayo Foundation Institutional Review Board, we conducted a community-based study within Olmsted County, Minnesota. In this study, Olmsted County residents who were diagnosed with first AF in 1980-2000 were identified and followed to 2004. Olmsted County is well suited for the conduct of studies with long-term follow-up because of a number of unique features 8. Geographically, the community is relatively isolated from other urban centers, and medical care is delivered by only a few health care providers, principally, the Mayo Clinic and its associated hospitals. The majority of Olmsted County residents return to the Mayo Clinic on a regular basis, allowing capture of health-related events. A previous study has shown that 96% of Olmsted County women residents aged 65-74 years returned to the Mayo Clinic within a 3-year period 8. For each patient at the Mayo Clinic, a unified medical record containing details of all inpatient and outpatient encounters is maintained. Within each medical record, diagnoses made during office visits, clinic consultations, emergency room visits, hospital admissions, nursing home care, autopsy examinations, as well as surgical procedures, are listed on a master sheet and subsequently coded. Coded diagnoses are electronically entered into a medical diagnostic index, allowing easy identification of all patients with a diagnosis of interest.
The details with respect to the study population and design, as well as definitions of the covariates have previously been reported 9. Briefly, the original study population consisted of persons who were diagnosed with, or documented to have, AF for the first time in 1980-2000. To qualify for the study, the defining AF event had to be verified as the first documented AF episode for the patient, and the AF event had to be confirmed by a 12-lead electrocardiogram. For the purpose of this study, persons who died during the initial AF event in the hospital were excluded. Patients with atrial flutter alone, without any evidence of AF, were not included in the study population.
“Paroxysmal” AF was defined by i) ECG evidence of AF followed by subsequent ECG showing sinus rhythm; and ii) clinical documentation by physician as having paroxysmal or intermittent AF. Persistent/permanent AF (or chronic AF) was defined by the presence of all the following: i) serial ECGs (at least 2) showing AF only and no interim evidence of sinus rhythm; ii) documentation by physician as having “chronic” or “permanent” AF from clinical assessment. Because the definitions for persistent and permanent AF were established years after the start of the study period 10, the 2 terms were considered interchangeable for the purpose of this study. Myocardial infarction was defined by at least 2 of the 3 diagnostic criteria: compatible clinical presentation, diagnostic cardiac enzyme levels, and consistent electrocardiographic changes. Coronary revascularization referred to coronary artery by-pass grafting or percutaneous coronary intervention. Heart failure was defined by the presence of 2 major, or 1 major and 2 minor Framingham criteria 11. Valvular heart disease was defined by presence of a murmur on physical examination, with or without echocardiographic confirmation. Carotid artery disease was defined by the presence of at least 50% stenosis based on neurovascular imaging, or prior intervention. Stroke included development of any type of stroke, as defined by clinical documentation of the diagnosis with or without confirmatory findings on imaging studies. Systemic hypertension was defined by a physician's diagnosis, need for antihypertensive therapy, systolic blood pressure>140 mmHg, or diastolic blood pressure >90 mmHg on at least 2 occasions that were not associated with acute illness or injury. Diabetes mellitus was defined by physician's diagnosis, and treatment with insulin or oral hypoglycemic agents. Smoking history was classified as past (over 6 months prior) or current smoker. Regular alcohol use was defined by self-reported consumption of >1 drink per day regularly. Chronic renal disease, chronic obstructive pulmonary disease, obstructive sleep apnea, and hyperthyroidism were defined by physician's diagnosis as documented in the medical records.
The primary outcome of interest was hospitalization for any cardiovascular reasons. The ascertainment of outcomes was accomplished through comprehensive review of the medical records in addition to cross-referencing the multiple administrative databases for identification of any inconsistencies. The cardiovascular causes of hospitalization were broadly classified into the following categories: 1) AF-related, 2) other arrhythmia-related causes, 3) syncope that cannot be directly attributed to arrhythmic reasons, 4) congestive heart failure, 5) thromboembolic events, 6) bleeding events, 7) coronary or peripheral arterial events, and 8) other cardiovascular reasons. AF-related causes included any admissions related to AF management, such as initiation of antiarrhythmic or anticoagulation therapy, electrical cardioversion, device therapy (implantation of pacemaker or cardioverter-defibrillator), or catheter or surgical (MAZE) ablation procedures. Syncope was considered as the cause for admission if the spell of loss of consciousness was indeterminate and could not be attributed to arrhythmic reasons. Thromboembolic events referred to transient ischemic attack, ischemic stroke, or peripheral thromboembolism. Bleeding events included any bleeding as well as hemorrhagic stroke. Coronary or peripheral arterial events included angina, myocardial infarction, coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), or events related to peripheral artery disease, such as claudication and peripheral revascularization.
Baseline characteristics were stratified by four time periods (1980 through 1984, 1985 through 1989, 1990 through 1994, and 1995 through 2000) and summarized by means and standard deviations or frequency percents. The trends in baseline characteristics across calendar-year of AF diagnosis were assessed using linear regression analyses for continuous variables, and logistic regression analyses for binary variables, adjusting for age and sex. Calendar-year of AF diagnosis was treated as a continuous variable. Only linear or linear logistic trends in calendar-year of AF diagnosis were considered. The overall cumulative incidence of first hospitalization after first AF diagnosis was estimated using Kaplan-Meier method. Incidence of first hospitalization was assessed as a function of age and sex, using Cox proportional hazards modeling for time to first hospitalization. Calendar-year trends were assessed by adding calendar-year of AF diagnosis to the proportional hazards model, as well as all 3 possible 2-way interactions among the 3 variables with only significant interactions being retained. Cox models based on clinical variables at the time of first AF were also developed using backward stepwise selection. The clinical covariates considered included body mass index, systolic blood pressure, diastolic blood pressure, heart rate at AF diagnosis, type of AF (paroxysmal versus persistent/permanent) at initial diagnosis, history of myocardial infarction, coronary revascularization, cardiac surgery, valvular heart disease, congestive heart failure, peripheral or carotid vascular disease, stroke, systemic hypertension, diabetes mellitus, smoking, regular alcohol use, chronic renal disease, chronic obstructive pulmonary disease, obstructive sleep apnea, hyperthyroidism, and malignancy.
To assess the temporal changes in the primary indication of hospital admission, we examined all admissions for cardiovascular causes during the first 3 years of follow-up in all study subjects. The rate of hospitalization was modeled using Poisson regression on the number of hospital admissions, using a log link function, and log (follow-up time) as an offset, with age, sex, calendar-year, and follow-up period (year 1, 2, or 3) as predictor variables. All tests of significance were two-tailed, and P value<0.05 was considered statistically significant.
Results
A total of 4,618 subjects (mean age 73±14 years, 51% men) were confirmed to have incurred the diagnosis of first AF between 1980 and 2000. Of these, 113 were excluded because first AF was diagnosed during hospitalization and died during the same admission, and 7 were excluded from analyses because follow-up data were unavailable after hospital dismissal. Thus, the study population constituted the remaining 4,498 subjects (mean age 73±14 years; range 18-107 years, 51% men). The baseline characteristics of study population, stratified by calendar-year of AF diagnosis, are displayed in Table 1.
Table 1. Baseline Characteristics of Study Population, Stratified by Four Time Periods.
| Calendar-Year of AF Diagnosis | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | Overall (n=4,498) | 1980-1984 (n=808) | 1985-1989 (n=913) | 1990-1994 (n=1,181) | 1995-2000 (n=1,596) | P-value* |
| Age (years) | 73.0±14.4 | 72.9±14.5 | 72.6±14.2 | 72.9±14.7 | 73.4±14.3 | 0.07 |
| Men | 2,298 (51%) | 403 (50%) | 457 (50%) | 616 (52%) | 822 (52%) | 0.14 |
| Body mass index (kg/m2) | 27.1 ±6.2 | 25.8±5.3 | 26.3±5.6 | 27.3±6.3 | 28.0±6.6 | <0.0001 |
| Systolic blood pressure (mmHg) | 138±20 | 137±22 | 143±21 | 140±20 | 135±19 | <0.0001 |
| Diastolic blood pressure (mmHg) | 78±11 | 79±11 | 80±10 | 79±11 | 75±11 | <0.0001 |
| Heart rate at AF (bpm) | 112±32 | 113±32 | 115±32 | 111±31 | 112±32 | 0.15 |
| Paroxysmal AF | 3,311 (74%) | 572 (71%) | 676 (74%) | 864 (73%) | 1,199 (75%) | <0.01 |
| Prior myocardial infarction | 916 (20%) | 186 (23%) | 182 (20%) | 234 (20%) | 314 (20%) | 0.05 |
| Coronary revascularization | 559 (12%) | 44 (5.4%) | 80 (8.8%) | 151 (13%) | 284 (18%) | <0.0001 |
| Prior cardiac surgery | 523 (12%) | 62 (7.7%) | 89 (10%) | 136 (12%) | 236 (15%) | <0.0001 |
| Prior heart failure | 439 (10%) | 59 (7.3%) | 82 (9.0%) | 127 (11%) | 171 (11%) | <0.01 |
| Valvular heart disease | 1,095 (24%) | 129 (16%) | 209 (23%) | 297 (25%) | 460 (29%) | <0.0001 |
| Peripheral artery disease | 584 (13%) | 104 (13%) | 131 (14%) | 157 (13%) | 192 (12%) | 0.14 |
| Carotid artery disease | 200 (4.4%) | 25 (3.1%) | 41 (4.5%) | 55 (4.7%) | 79 (4.9%) | 0.05 |
| Stroke history | 423 (9.4%) | 86 (11%) | 82 (9.0%) | 123 (10%) | 132 (8.3%) | 0.06 |
| Systemic hypertension | 3,591 (80%) | 567 (70%) | 731 (80%) | 958 (81%) | 1,335 (84%) | <0.0001 |
| Diabetes mellitus | 812 (18%) | 145 (18%) | 143 (16%) | 220 (19%) | 304 (19%) | 0.18 |
| Smoker | 2,522 (56%) | 423 (52%) | 518 (57%) | 667 (56%) | 914 (57%) | 0.08 |
| Regular alcohol use | 532 (12%) | 88 (11%) | 118 (13%) | 151 (13%) | 175 (11%) | 0.75 |
| Chronic renal disease | 747 (17%) | 143 (18%) | 133 (15%) | 184 (16%) | 287 (18%) | 0.42 |
| Chronic obstructive pulmonary disease | 971 (22%) | 189 (23%) | 224 (25%) | 237 (20%) | 321 (20%) | <0.01 |
| Obstructive sleep apnea | 86 (1.9%) | 0 (0.0%) | 4 (0.4%) | 15 (1.3%) | 67 (4.2%) | <0.0001 |
| Hyperthyroidism | 47 (1.0%) | 10 (1.2%) | 18 (2.0%) | 8 (0.7%) | 11 (0.7%) | <0.05 |
| Malignancy history | 1,201 (27%) | 168 (21%) | 235 (26%) | 306 (26%) | 492 (31%) | <0.0001 |
Values are given as mean ± SD or number (percentage).
P value for trends across calendar-year of AF diagnosis, considered as a continuous variable, by linear regression analysis for continuous variables and logistic regression analysis for binary variables, with adjustment for age and sex.
During a mean follow-up of 5.5±5.0 years, 2,503 subjects (56%; mean age 73±13years, 50% men) were admitted to the hospital for cardiovascular reasons for at least once after the initial diagnosis of AF, totaling 6,993 admissions. The likelihood of hospitalization was greatest during the first year of AF [cumulative incidence 31%, 95% confidence interval (CI) 30-32%]. The cumulative incidence was 48% (95%CI, 46-49%) at 3 years, and 59% (95% CI, 57-61%) at 5 years. After adjustment for age and sex, hospital admission was strongly related to advancing age [hazard ratio (HR) per 10 years, 1.2, 95% CI, 1.1-1.2, P<0.0001], but did not vary with sex (P=0.38).
The age-and sex-adjusted incidence of first hospital admission for cardiovascular reasons increased, on average, by 2.5% a year (95% CI 1.8-3.2%, P<0.0001)(Figure 1). The rate of increase was significant for both men (2.6% per year, 95% CI 1.5-3.6%, P<0.0001) and women (2.4% per year, 95% CI 1.4-3.4%, P<0.0001), and did not differ between the sexes (P=0.94). There was a slight, but statistically significant (P=0.018), quantitative interaction between age and calendar-year of AF diagnosis in the Cox model for the prediction of hospital admission with older age having a smaller calendar-year-related percent increase than younger age [the averaged increase in hospitalization was 4.0% per year at age 50 years, versus 2.1% per year at age 80 years). There were no significant interactions in the model otherwise.
Figure 1.
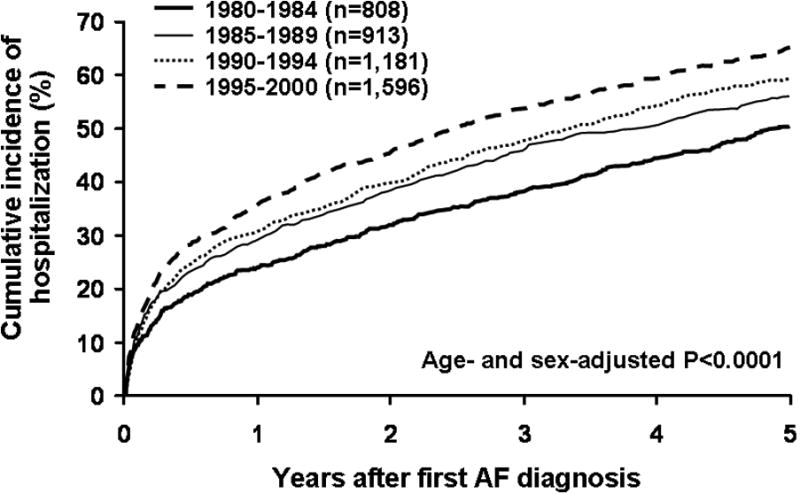
Time trends of cumulative incidence of hospitalization for any cardiovascular causes following first AF in 1980-2000.
In a multivariable Cox model for the prediction of admission to the hospital, advancing age, higher body mass index, higher systolic blood pressure, paroxysmal AF (as opposed to permanent/persistent AF) at initial diagnosis, and history of myocardial infarction, valvular heart disease, peripheral or carotid artery disease, stroke, systemic hypertension, diabetes mellitus, chronic renal disease, and chronic obstructive pulmonary disease were all significant independent predictors. The calendar-year of AF diagnosis remained a significant positive predictor of hospital admission, even after multivariable adjustment including all relevant comorbidities (2.1% increase per year on average, 95% CI 1.3-2.9%, P<0.0001)(Table 2).
Table 2. Multivariable Model for the Prediction of Hospitalization after First Atrial Fibrillation Diagnosis.
| Hazard ratio | ||
|---|---|---|
| Variable | (95% CI) | P-value |
| Calendar-year of AF diagnosis | 1.02 (1.01-1.03) | <0.0001 |
| Age (per 10 years) | 1.08 (1.05-1.12) | <0.0001 |
| Male sex | 0.98 (0.90-1.07) | 0.65 |
| Body mass index (per 5 kg/m2) | 1.04 (1.01-1.08) | 0.016 |
| Systolic blood pressure (per 5 mmHg) | 1.02 (1.01-1.03) | 0.003 |
| Paroxysmal AF | 1.16 (1.05-1.27) | 0.002 |
| Prior myocardial infarction | 1.46 (1.33-1.62) | <0.0001 |
| Valvular heart disease | 1.43 (1.30-1.57) | <0.0001 |
| Peripheral artery disease | 1.27 (1.13-1.44) | <0.001 |
| Carotid artery disease | 1.28 (1.06-1.54) | 0.010 |
| Stroke history | 1.17 (1.02-1.35) | 0.026 |
| Systemic hypertension | 1.29 (1.14-1.47) | <0.0001 |
| Diabetes mellitus | 1.18 (1.06-1.31) | 0.002 |
| Chronic renal disease | 1.31 (1.17-1.47) | <0.0001 |
| Chronic obstructive pulmonary disease | 1.14 (1.04-1.26) | 0.008 |
To examine the trends in the primary indication for hospital admission following first AF, we examined all hospital admissions for cardiovascular causes within 3 years of first AF diagnosis. A total of 3,504 admissions were reported for 4,498 study subjects. The most common cardiovascular causes listed as the primary indications for hospital admission within 3 years of first AF diagnosis were 1) AF-related (26.4%), 2) congestive heart failure (21.7%), 3) coronary or peripheral arterial causes (21.6%), and 4) thromboembolic events (10.5%). The proportion of AF-related causes increased significantly during the period 1980 to 2000 (16% in 1980-1984 to 36% in 1995-2000, P<0.0001) (Table 3).
To assess the rate of increase in first AF-related hospital admission, we also examined the Cox proportional hazards model for the prediction of first AF-related hospitalization. Of 4,498 subjects, 920 (20%) were admitted at least once to the hospital for AF-related cause (totaling 1,633 hospitalization admissions), with an averaged age-and sex-adjusted rate of 7.6% per year (95% CI, 6.3-9.0%, P<0.0001 for trends; P=0.48 for interaction between men and women), even after multivariable adjustment including comorbid conditions (7.5% increase per year, 95% CI 6.0-8.9%, P<0.0001).
We also assessed the impact of changing practice pattern regarding AF management on the trends of increase in hospitalization. If we excluded AF-related hospitalization (N=1,633), the increase in hospital admission was only 0.8% per year (95% CI 0.05-1.6%, P=0.04) (Figure 2), which was no longer significant after multivariable adjustment for age, sex, and comorbidities (P=0.25).
Figure 2.
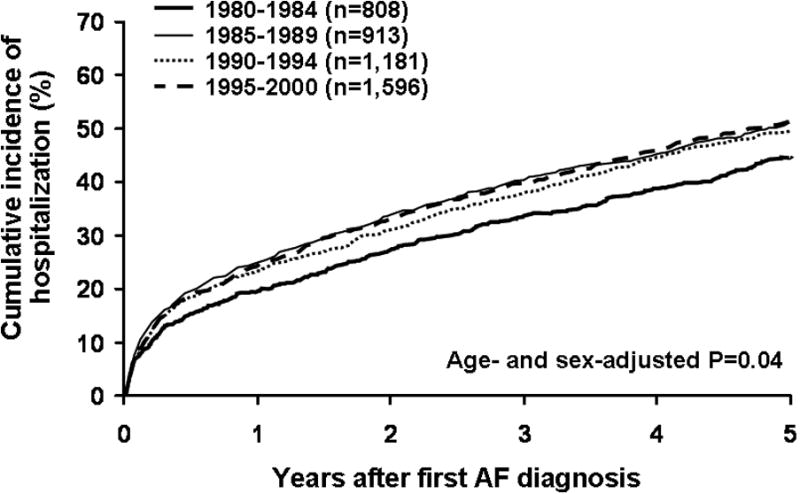
Time trends of cumulative incidence of hospital admissions for cardiovascular causes, excluding those related to management of AF, in 1980-2000.
Discussion
For persons newly diagnosed with AF in our tertiary care center, 1 in 3 was admitted to the hospital for cardiovascular reasons for at least once within the first year of diagnosis. During the period of 1980-2000, the age-and sex-adjusted rate of hospital admission for cardiovascular causes after first AF diagnosis increased significantly in both men and women, and was comparable for the 2 sexes. The increase in hospital admission appeared to be largely related to the changing practice pattern with AF management, independent of the changes over time in the distribution of age, sex, and comorbid conditions.
Several studies have suggested that a high rate of hospitalization for AF patients, exacting a heavy toll on the healthcare resources 1-5,12,13. Most of these studies did not differentiate incident versus prevalent AF. Our study is unique in that we have complete longitudinal follow-up data for a community-based cohort since the first diagnosis of AF was incurred, which allowed systematic analyses of the pattern for hospital utilization from a common point of time. For persons incurring the AF diagnosis for the first time in our tertiary care center, 1 in 3 was admitted to the hospital for at least once within the first year of AF diagnosis, and nearly half of all patients were hospitalized within 3 years.
Much of this hospital utilization appeared to be related to the changing trends in AF management. These findings could be explained from the increased availability of therapeutic options, both in terms of drugs, as well as non-pharmacological interventions6,7. Over the 21 year study period, we did not see a change in mortality 14, post AF stroke mortality 15, or heart failure incidence and mortality 16. Yet, the rate of hospital admission had increased during this time, which appeared to have been driven by the changing practice in AF management.
This study focused on the experience of AF patients in Olmsted County, and reflected the practice pattern of a single tertiary care institution, thus limiting the generalizability. It was a retrospective study with its inherent biases, and misclassification of the primary cause of hospital admission remained possible. Acknowledging these limitations, we believe that the data represent the most comprehensive report of the largest study of AF patients followed from first AF diagnosis, and provide important and new insights into the contributing factors for the changing trends in hospital use amongst AF patients.
Acknowledgments
This study was supported by the American Heart Association National Scientist Development Grant.
Footnotes
There are no conflicts of interest on the part of any authors.
References
- 1.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–716. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 2.Humphries KH, Jackevicius C, Gong Y, Svensen L, Cox J, Tu JV, Laupacis A. Population rates of hospitalization for atrial fibrillation/flutter in Canada. Can J Cardiol. 2004;20:869–876. [PubMed] [Google Scholar]
- 3.Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14:666–672. doi: 10.1097/01.ede.0000091649.26364.c0. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, MacIntyre K, MacLeod MM, Bailey AE, Capewell S, McMurray JJ. Trends in hospital activity, morbidity and case fatality related to atrial fibrillation in Scotland, 1986-1996. Eur Heart J. 2001;22:693–701. doi: 10.1053/euhj.2000.2511. [DOI] [PubMed] [Google Scholar]
- 5.Frost L, Engholm G, Moller H, Husted Decrease in mortality in patients with a hospital diagnosis of atrial fibrillation in Denmark during the period 1980-1993. Eur Heart J. 1999;20:1592–1599. doi: 10.1053/euhj.1999.1713. [DOI] [PubMed] [Google Scholar]
- 6.Miyasaka Y, Barnes ME, Cha SS, Bailey KR, Seward JB, Abhayaratna WP, Gersh BJ, Tsang TSM. Pharmacological therapy following first atrial fibrillation: data from two decades (1980-2000) J Am Coll Cardiol. 2005;45:334A. abstract. [Google Scholar]
- 7.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Carlson LA, Seward JB, Tsang TSM. Utilization of non-pharmacological therapy in atrial fibrillation: changing clinical practice over 2 decades. Circulation. 2005:575. abstract. 112:II 574-II. [Google Scholar]
- 8.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 9.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota 1980-2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 10.Levy S, Camm AJ, Saksena S, Aliot E, Breithardt G, Crijns H, Davies W, Kay N, Prystowsky E, Sutton R, Waldo A, Wyse DG. International consensus on nomenclature and classification of atrial fibrillation; a collaborative project of the Working Group on Arrhythmias and the Working Group on Cardiac Pacing of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Europace. 2003;5:119–122. doi: 10.1053/eupc.2002.0300. [DOI] [PubMed] [Google Scholar]
- 11.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 12.Wolf PA, Mitchell JB, Baker CS, Kannel WB, D'Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. doi: 10.1001/archinte.158.3.229. [DOI] [PubMed] [Google Scholar]
- 13.Khairallah F, Ezzedine R, Ganz LI, London B, Saba S. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am J Cardiol. 2004;94:500–504. doi: 10.1016/j.amjcard.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 14.Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 15.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Seward JB, Bailey KR, Iwasaka T, Tsang TS. Time Trends of Ischemic Stroke Incidence and Mortality in Patients Diagnosed With First Atrial Fibrillation in 1980 to 2000. Report of a Community-Based Study. Stroke. 2005;36:2362–2366. doi: 10.1161/01.STR.0000185927.63746.23. [DOI] [PubMed] [Google Scholar]
- 16.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TS. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community-based study over two decades. Eur Heart J. 2006;27:936–941. doi: 10.1093/eurheartj/ehi694. [DOI] [PubMed] [Google Scholar]


