Abstract
Background
Interventions using sustained aerobic exercise programs to aid smoking cessation have resulted in modest, short-term cessation rates comparable to conventional cessation methods. No smoking cessation trial to date has prescribed intermittent bouts of exercise in response to nicotine cravings.
Objectives
This pilot randomized controlled trial examined the feasibility and efficacy of an Internet-based smoking cessation program alone (CON) vs. the same Internet-based program + intermittent exercise in response to cigarette cravings (EX).
Study population
Participants (N = 38; mean age = 43.6 [SD = 11.5]; 60.5% women) were generally healthy, inactive adult smokers who desired to quit.
Results
The overall retention rate was 60.5% (n = 23), and no significant retention rate differences were found between groups (EX vs. CON). Although retained participants achieved a higher cessation rate (26.1%) than all enrolled participants (15.8%), adjusted intent-to-treat and per-protocol binary logistic regression analyses revealed no significant cessation rate differences between EX and CON groups. Linear regression results indicated that additional days of self-reported exercise on the study website during the intervention phase predicted significantly higher reduction rates among EX group participants, F(2, 16) = 31.08, p < .001.
Conclusions
Results were mixed with regard to the incremental benefit of exercise in the presence of the apparently valuable Internet-based smoking cessation program. The results support findings from related research and underscore the need for additional investigation into both the mechanisms underlying the effect of exercise on cigarette cravings and the challenges of poor adherence in the context of exercise-based smoking cessation interventions.
Keywords: Smoking cessation, Exercise, Internet, Intervention, Pilot, Randomized controlled trial
1. Intermittent exercise in the context of an Internet-based smoking cessation program
Tobacco use and physical inactivity are the leading causes of preventable death in the United States, accounting for approximately one-third of annual deaths (Centers for Disease Control and Prevention, Department of Health and Human Services, & National Center for Chronic Disease Prevention and Health Promotion, 2007). They also tend to co-exist and apparently influence each other, as persistent smokers are more likely to be physically inactive (Nagaya, Yoshida, Takahashi, & Kawai, 2007).
Despite the widespread dangers associated with tobacco use, the declining trend in the U.S. smoking rate appears to have stalled in recent years, consistently hovering around 20–21% of adults (Dube et al., 2010). Although an estimated 39.8% of adult every day smokers attempt to quit each year (Centers for Disease Control and Prevention, 2008), only a small percentage succeeds (Fiore et al., 2008). Recent research has demonstrated that cessation rates in Internet-based programs are comparable to those reported in more traditional types of programs, such as in-person groups and telephone counseling sessions (Myung, McDonnell, Kazinets, Seo, & Moskowitz, 2009; Walters, Wright, & Shegog, 2006), particularly when the programs are tailored to the study population (Strecher et al., 2008). However, another recent review of Internet-based smoking cessation trials concluded that high attrition and low adherence rates may reduce the efficacy of web-based programs (Hutton et al., 2011).
In 2008, only 30.7% of the adult population reportedly engaged in regular leisure-time physical activity (Pleis, Lucas, & Ward, 2009). Just as a majority of smokers desire to quit but struggle to do so, most adults desire to exercise but struggle to maintain a regular exercise regimen (Barnes, 2007). Research has shown equal health benefits (Murphy, Blair, & Murtagh, 2009) regardless of whether exercise is obtained in one prolonged session (e.g., 30+ min moderate or 20+ min of vigorous exercise) or multiple shorter sessions (e.g., 5–15 min each), with no evidence of consistent differences between adherence rates (Linke, Gallo, & Norman, 2011).
In addition to boosting physical health, exercise also improves mood and well-being (Daley, 2008). Furthermore, exercise may be habit-forming for some individuals through brain reward pathways similar to those implicated in other forms of addiction (Hausenblas & Symons Downs, 2002). Specifically, increased activity of certain neurotransmitters, including beta-endorphins (Boecker et al., 2008; Heitkamp, Schmid, & Scheib, 1993), epinephrine, norepinephrine, and dopamine (Bortz et al., 1981; Mathes et al., 2010), appears to be at least partially responsible (Adams, 2009). These neurotransmitters, especially dopamine, have also been implicated in nicotine addiction (Ikemoto, 2007; Zhang et al., 2009). Changes in these pathways may help individuals maintain regular exercise habits by increasing the physical, physiological, and psychological rewards associated with exercise.
1.1. Short-term effects of exercise on smoking characteristics
A review of 14 studies that examined the relationships among short bouts of exercise, nicotine withdrawal symptoms, cravings, affect, and subsequent smoking behavior found that exercise reduces cravings and improves mood among nicotine-dependent but temporarily abstinent (typically overnight for 12–15 h) smokers (Taylor, Ussher, & Faulkner, 2007). The studies’ protocols incorporated various durations, intensities, and types of exercise. Altogether, results suggested that short bouts of moderate to intense, but not very light exercise (Daniel, Cropley, Ussher, & West, 2004), decrease cigarette cravings among temporarily abstinent smokers. Some evidence suggests that a point of diminishing returns may be reached when exercise becomes too challenging for typically inactive smokers (Daley, Oldham, & Townson, 2004; Pomerleau et al., 1987); although another study prescribing intense exercise indicated otherwise (Bock, Marcus, King, Borrelli, & Roberts, 1999), its participants were attempting cessation and thus may have been qualitatively different from those enrolled in the other trials investigating the short-term effects of exercise among abstaining smokers.
1.2. Long-term effects of exercise on smoking cessation
Another systematic review summarized 13 unique randomized controlled smoking cessation trials that examined exercise as the primary or supplementary intervention (Ussher, Taylor, & Faulkner, 2008). Overall, these exercise-based smoking cessation interventions resulted in modest, short-term success comparable to conventional cessation methods but insufficient evidence supporting the efficacy of exercise (Ussher et al., 2008). However, methodological issues of existing studies and a lack of interventions using short, frequent bouts of exercise to cope with cravings prevent definitive conclusions in this area.
1.3. Limitations of existing research
Despite generally positive findings of studies examining the acute effects of exercise on cravings and mood/affect after a period of smoking abstinence (Taylor et al., 2007), no known exercise-based smoking cessation intervention trial has incorporated short bouts of exercise in response to acute cigarette cravings into the prescribed exercise routines. In addition, although self-monitoring of many health behaviors, including smoking (Manske, Miller, Moyer, Phaneuf, & Cameron, 2004), physical activity/exercise (Dishman & Buckworth, 1996), and mood (Febbaro & Clum, 1998), frequently increases overall efficacy when used in combination with other behavioral intervention techniques (van Achterberg et al., 2010), it has been underutilized in previous exercise-based smoking cessation trials. Moreover, most of the previous exercise-based smoking cessation studies also included extensive multi-component interventions that may not be feasible in real-world settings (Jonsdottir & Jonsdottir, 2001). Incorporating web-based interventions that align with evidence-based smoking cessation efforts (Myung et al., 2009; Strecher et al., 2008; Walters et al., 2006) and modern conveniences that allow individuals to participate according to their own schedules (Keefe & Blumenthal, 2004) may increase the effectiveness of future smoking cessation/exercise studies.
1.4. Purpose
The “Walk Away from the Habit: Overcoming Nicotine Dependence through Exercise” pilot, randomized controlled trial examined the feasibility and efficacy of an Internet-based smoking cessation program alone vs. the same Internet-based program + exercise. We hypothesized that participants randomized to the Exercise condition would achieve significantly greater mean smoking cessation and reduction rates, measured by self-reported point prevalence cessation and daily smoking rate reductions from baseline to post-intervention assessment, than those randomized to the Control condition.
2. Methods
2.1. Overview of procedures
The entire study, including the 12-week intervention phase and 3-month follow-up visit (6 months post-baseline), is described in detail in the following sections. Social Cognitive Theory (SCT) constructs (Bandura, 1986) and other behavior change principles, which have demonstrated ability to influence health behavior change (Glanz, Rimer, & Lewis, 2002), guided the intervention’s content. Specifically, the following constructs were applied to the program design and implementation: behavioral capacity, outcome expectations, reciprocal determinism, reinforcement, self-control, self-efficacy, goal-setting, coping, and relapse prevention. An outline of the assessment schedule is located in Table 1. The study was approved by the Institutional Review Boards (IRB) at two academic institutions as well as by the Research & Development Committee at the Veterans Affairs Medical Center (VAMC).
Table 1.
Overview of assessment schedule.
| Assessment components & timeline | Base | Daily | Post | F/U |
|---|---|---|---|---|
| Psychiatric History (Diagnostic Interview Schedule) | X | |||
| Center for Epidemiological Studies-Depression (CES-D) | X | X | X | |
| Positive & Negative Affect Scale (PANAS) | X | X | X | |
| Daily Mood Record | X | |||
| Mood & Physical Symptoms Scale (MPSS) | X | X | X | |
| Fagerstrom Test for Nicotine Dependence (FTND) | X | X | X | |
| Smoking Cessation Self-efficacy Scale | X | X | X | |
| Daily Smoking Record | X | |||
| Daily Cigarette Cravings Record | X | |||
| Leisure Time Exercise Questionnaire (LTEQ) | X | X | X | |
| Exercise Self-efficacy Scale | X | X | X | |
| Daily Exercise Recorda | X | |||
| Physical Fitness Testing | X | X | X | |
| Body weight/BMI measurement | X | X | X |
Note: Base = baseline visit; Daily = daily records (completed every day on the study website); Post = post-intervention assessment (12-weeks post-baseline); F/U = follow-up assessment visit (6 months post-baseline).
Exercise (EX) group only.
2.2. Study website design/creation
With the help of information technology (IT) and web-design consultants, a secure website was built for the study. Only the researchers, IT consultant, and participants had access to the internal website.
2.3. Recruitment
Recruitment primarily took place via web-based advertisements (e.g., Craigslist) and emails to university employees in attempt to enroll individuals who were already using the Internet. In addition, smoking history questionnaires previously completed by female veterans at the VAMC were screened.
2.4. Eligibility/screening
A scripted phone screen determined whether individuals who called to inquire about the study met eligibility criteria (listed in Table 2); those who met criteria were scheduled for an in-person visit, at which time they provided written informed consent.
Table 2.
Eligibility criteria for study participation.

|
18–64 years old |

|
Nicotine-dependent |

|
Regularly smoking for at least 3 years |

|
Currently smoke at least 10 cigarettes per day |

|
Current desire to quit smoking |

|
Body mass index (BMI) < 35 |

|
No current suicidal ideation or untreated psychiatric disorders (current major depressive episode, actively psychotic, bipolar disorder, substance abuse or dependence, eating disorder) |

|
No untreated medical problems that would prevent or limit training (cardiovascular disease, chronic obstructive pulmonary disease, severe arthritis, certain disabilities) |

|
Cleared by physician to exercise moderately and complete exercise testing |

|
Sedentary: does not meet ACSM criteria for regular aerobic, strengthening, or stretching activity per week |

|
Must report daily dosages if concurrently using NRT or pharmacotherapy |

|
Cannot be or plan to become pregnant within six months of enrollment |

|
Must speak, read, and write fluently in English |

|
Must have daily Internet access readily available |

|
Must be technology-literate enough to use the website after receiving brief training |
Note: ACSM = American College of Sports Medicine; NRT = Nicotine replacement therapy.
2.5. Baseline visit
The baseline visit was conducted at the Clinical Research Center (CRC). After completing a battery of psychosocial questionnaires and physical fitness assessments, participants were randomly assigned (via coin toss) to the Internet + Exercise (EX) or the Internet-only Control (CON) group. They were then enrolled on the study website, provided with secure login information, guided through the website, and given opportunities to practice completing the daily records and ask questions about the study. Additional information about exercise was given to EX group participants via verbal summary and written information on the study website.
Participants were encouraged to set a target quit date (TQD) for approximately one week after the baseline visit. However, the TQD was flexible in that participants could choose between quitting altogether and beginning to gradually reduce their smoking rate (at a flexible but recommended 20% reduction rate per week) on the TQD. They were also given the option of supplementing the program with nicotine replacement therapy (NRT) or other pharmacotherapy, but this type of treatment was not provided. They were instructed to keep the researchers informed about their use of NRT or other pharmacotherapy by responding to the daily email each day if they utilized any other form of treatment with the type and dose utilized. All enrolled participants received a monetary incentive upon completion of the baseline visit.
2.6. Web-based tutorial
The smoking cessation tutorial consisted of twelve modules, which were divided into daily lessons and handouts. Participants were prompted on their homepage to click on the next sequential lesson/handout after viewing each daily lesson/handout, but all lesson/handouts were available to participants at any given time to allow them to be viewed multiple times.
2.7. Daily records
All participants were instructed to complete the self-report section of the website on a daily basis. This section served as an assessment tool as well as a form of self-monitoring. Participants were asked to report the following variables each day: the number of cigarettes they smoked; their average and highest cigarette craving levels; and their average mood rating. EX participants were asked to complete an additional daily record, comprised of each exercise bout they completed as well as their mood and cigarette cravings immediately before and after their most recent exercise bout.
2.8. Daily emails
All participants received a daily email reminding them to login to the website in order to complete their daily records and read their daily lesson/handout. The emails also always contained an encouraging message (e.g., “You can quit smoking for good!”). The emails were uniquely written every day throughout the program, but the same email was sent to all currently enrolled participants each day.
2.9. Weekly phone calls
All participants received a brief (approximately 0–10 min) weekly phone call consisting of checking in, addressing questions or concerns about the study, and encouraging them to continue their smoking cessation efforts. Although the themes of the phone calls were consistent, the conversations were flexible to best address individual questions and concerns. Answering or returning the weekly phone calls was optional; only one phone call attempt was made per week unless a participant returned a missed phone call and requested another attempt.
2.10. Exercise information
An additional section of the website with information about exercise (e.g., starting an exercise program, exercising safely, exercise examples) was accessible only to EX group participants. They were encouraged to read this section during the first week of the intervention phase, prior to beginning the exercise program, which started in Week 2.
2.11. Weekly exercise emails
EX participants were instructed when and how to increase their exercise during the intervention phase via weekly emails, which were tailored on a weekly basis according to each participant’s adherence and feedback.
2.12. Exercise prescription
EX participants were instructed to engage in a short bout of exercise each time they craved a cigarette and would have otherwise smoked. They were encouraged to engage in a variety of different types of exercise and to incorporate a balance of aerobic, strengthening, and stretching exercises each day in order to prevent boredom and burnout as well as to increase overall physical fitness. The exercise instructions provided general guidance in terms of total exercise time per day (approximately 60 min per day throughout the program); duration per bout (starting with 5 min per bout and gradually increasing to 15+ min per bout); intensity (moderate intensity, approximated by a “moderately difficult” rating of 12e14 on the rating of perceived exertion [RPE] scale, throughout the entire program); and type (a combination of aerobic, strengthening, and stretching exercises). However, participants selected their own daily frequency of exercise throughout the program, allowing them the flexibility of engaging in the frequency of exercise bouts personally needed to align with the number of cigarette cravings they experienced on a daily basis. They were encouraged to remain on track with the standard exercise protocol, which increased the number of minutes per bout and decreased the bouts per day each week. This standard protocol assumed three inter-related things: 1) participants quit smoking or consistently reduced their smoking rate each week; 2) their daily cigarette cravings decreased in frequency as time passed; and 3) their exercise tolerance increased as time passed. It also allowed for individual tailoring (e.g., not increasing the dose of exercise as intended for participants who struggled to keep up with the program). Adherent EX participants who denied lingering cigarette cravings were encouraged to transition from intermittent (5–15 min per bout) to sustained (30+ min per bout) daily exercise that was not dependent on their cigarette cravings.
2.13. Subsequent assessments
At two additional time points (post-intervention three months after baseline and follow-up six months after baseline), participants reported to the CRC for additional assessments. They completed the same battery of questionnaires and physical fitness assessments that they had completed at the baseline visit (Table 1). They also reported their current daily smoking rate and described any and all changes in their daily smoking rate since their previous assessment (baseline or post-intervention), using a calendar to anchor their memories. Participants were compensated for completing these assessments.
2.14. Statistics
2.14.1. Smoking
Binary logistic regression was used to test the effect of group (EX vs. CON) on smoking cessation, with self-reported point prevalence abstinence at the post-intervention assessment as the dependent variable. Participants who did not complete the post-intervention assessment were counted as smokers. Baseline daily smoking rate was included as a covariate.
Paired t-tests were conducted to examine individual changes in smoking rates from baseline to post-intervention among all enrolled participants (intent-to-treat), retained participants only (per-protocol), and each group (EX and CON, both intent-to-treat and per-protocol). A univariate analysis of variance (ANOVA) was conducted to examine the effect of group (EX vs. CON) on changes in self-reported daily smoking rate from baseline to post-intervention. Baseline smoking rates were carried over for participants who did not complete the post-intervention assessment. Quit status (to control for the 100% rate reductions among participants who had quit) and baseline daily smoking rate were included as covariates.
2.14.2. Exercise
Linear regression was used to test the effect of exercise dose on smoking reduction rates. The total number of days during the intervention phase with any self-reported exercise, extracted from the study website’s daily exercise records, served as the independent variable. Specifically, all days with 1+ exercise bouts recorded were counted as exercise compliant days. Days with missing exercise record data were treated as exercise non-compliant days. Point prevalence self-reported daily smoking rate (“0” for participants who were quit) at the post-intervention assessment served as the dependent variable. Participants who did not complete a post-intervention assessment were conservatively assumed to be smoking at their baseline rate at the time that their post-intervention assessment should have occurred. Baseline daily smoking rate was included as a covariate.
2.14.3. Self-efficacy
The Smoking Cessation Self-efficacy Scale and the Exercise Self-efficacy Scale were completed at the baseline and post-intervention visits to measure these two specific types of self-efficacy. Paired t-tests were conducted to assess changes in these scores over time.
2.14.4. Power analysis
Power analyses were conducted using G-power software (Faul, Erdfelder, Lang, & Buchner, 2007). Based on a design with two groups, two time points (baseline, post-intervention), a medium effect size of .25, a power level of .80, an alpha value of .05, and two tails, analyses indicated that the study was underpowered to find between group differences in the binary logistic regression analysis examining smoking cessation rates. Furthermore, 55 participants would be required to detect group differences in daily smoking rate (i.e., mean number of cigarettes per day) changes from baseline to post-intervention in a univariate ANOVA with an effect size of .15, a power level of .80, an alpha value of .05, and one predictor variable. The study was originally designed with a goal of 60 randomized participants, which would have given it adequate power to detect group differences in daily smoking rates but not smoking cessation rates. All statistical analyses were conducted using SPSS, Version 19.0. Tests were conducted with two tails, and significance levels were set at p < .05 for all analyses.
3. Results
3.1. Recruitment
A total of 99 individuals called to inquire about the study. Of those, 14 were unable to be reached upon attempt to return their voicemail inquiry, 14 were not interested in participating after hearing the study description, and 14 were deemed ineligible based upon the phone screening. The reasons for ineligibility based upon the phone screening included the following: physical disability limiting ability to exercise (2); uncontrolled, serious medical problems (1); lack of access to a medical provider for medical clearance (1); lack of daily access to Internet (2); non-proficient English skills (1); daily smoking less than three years (1); already quit/not currently smoking (2); currently engaging in regular exercise meeting or exceeding ACSM recommendations (2); psychiatric reasons (1); and body mass index > 35 (1).
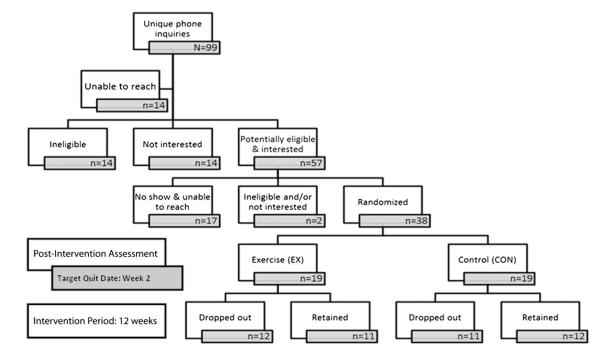
A total of 57 individuals who were deemed potentially eligible based upon the phone screening and expressed interest in participating were scheduled for an in-person screening/baseline visit. Of those, 17 did not show and were unable to be reached to reschedule, 1 was deemed ineligible based upon the in-person screening (for uncontrolled, serious psychiatric problems), and 1 was no longer interested after attending the in-person screening. A total of 38 eligible and interested individuals were randomized to either the EX (n = 19) or CON (n = 19) group. A diagram depicting the recruitment, screening, and enrollment process is provided in Fig. 1.
Fig. 1.
Flow diagram of the recruitment/enrollment procedure and study timing/duration.
3.2. Baseline characteristics
Enrolled participants’ (N = 38) mean age was 43.6 (SD = 11.5) years, ranging from 20 to 60 years, and 60.5% (n = 23) of all participants were women. At baseline, the mean smoking rate was 15.9 (SD = 7.2) cigarettes per day, and the mean FTND score was 5.2 (SD = 2.3), together suggesting that participants were moderate to heavy smokers with substantial nicotine dependence. Participants’ mean weighted score on the Leisure Time Exercise Questionnaire (LTEQ; 29.6 [SD = 18.7]) suggested that they led relatively inactive lives. These and other baseline characteristics are listed in Table 3. No significant group differences were found on any baseline characteristics.
Table 3.
Baseline characteristics.
| Variablea,b | EX (n = 19) | CON (n = 19) | Total (N = 38) |
|---|---|---|---|
| Female (%) | 52.6% | 68.4% | 60.5% |
| Age | 45.7 (9.8) | 41.4 (12.9) | 43.6 (11.5) |
| Body mass index (BMI) | 28.7 (5.0) | 29.0 (5.1) | 28.8 (5.0) |
| Number of cigarettes per day |
16.8 (8.6) | 15 (5.5) | 15.9 (7.2) |
| FTND score | 5.6 (2.5) | 4.7 (2.2) | 5.2 (2.3) |
| LTEQ weighted score | 33.8 (15.1) | 25.3 (20.3) | 29.6 (18.7) |
| Smoking Cessation | 29.3 (8.9) | 31.2 (8.7) | 30.3 (8.7) |
| Self-Efficacy | 49% (14.9%) | 52% (14.5%) | 50.5% (14.6%) |
| Exercise Self-Efficacy | 949 (370) | 1073 (301) | 1013 (337) |
| 52.8% (20.5%) | 59.6% (16.7%) | 56.3% (18.7%) | |
| CES-D score | 15.4 (10.4) | 11.0 (8.5) | 13.1 (9.6) |
Note: EX = Internet + Exercise Group; CON = Internet-only Control Group; FTND = Fagerstrom Test of Nicotine Dependence; LTEQ = Leisure Time Exercise Questionnaire; CES-D = Center for Epidemiological Studies-Depression Scale.
Results are reported as mean (standard deviation) unless otherwise noted.
No significant differences observed between groups on any baseline variables.
3.3. Feasibility
The overall retention rate (i.e., percentage of enrolled participants who completed a post-intervention assessment) was 60.5% (n = 23), and no significant retention rate differences were found between groups (EX = 57.9%, CON = 63.2%). A sex difference in retention rate barely reached statistical significance, indicating that men (80%) were more likely than women (48%) to complete a post-intervention assessment, F(1, 36) = 4.2, p = .05. No other significant differences in participant characteristics were found according to retention status.
Throughout the intervention phase, the 23 retained participants clicked on an average of 19.7 (SD = 12.9) of the 36 available daily lesson website pages (median = 16, range = 1–36) at least once. Clicks on the 24 action-oriented handouts that comprised the rest of the smoking cessation tutorial were not tracked due to backend website limitations. Retained EX participants (n = 11) reported 1+ bouts of exercise on the exercise daily records approximately 33% of the days they were enrolled in the intervention phase of the study (mean = 32.3 [SD = 21.8]). LTEQ scores of both groups significantly increased from baseline to post-intervention (all p’s < .05). These and other variables often associated with quit status in smoking cessation trials are presented in Table 4. Only two participants (one per group) reported utilizing NRT during the intervention; they used it irregularly at less than the recommended therapeutic dose, and both reported smoking at their baseline rate at the post-intervention assessment.
Table 4.
Post-intervention variables among retained participants.
| Variablea | EX (n = 11) | CON (n = 12) | Total (N = 23) |
|---|---|---|---|
| Body mass index (BMI) | 31 (11) | 27.5 (3.9) | 29.1 (7.9) |
| Number of cigarettes per day |
13 (14.2)b | 6.5 (8.6)b | 9.6 (11.8)b |
| FTND score | 4.1 (3.8)b | 3.3 (3.1)b | 3.7 (3.4)b |
| LTEQ weighted score | 42.4 (25.9)b | 27.1 (21.5)b | 34.1 (24.3)b |
| Smoking Cessation | 38 (7.1) | 41.4 (12.4)b | 40 (10.4)b |
| Self-Efficacy | 63.3% (11.8%) | 69% (20.7%) | 66.6% (17.3%) |
| Exercise Self-Efficacy | 797 (393) | 782 (351) | 788 (360) |
| 44.3% (21.8%) | 43.4% (19.5%) | 43.8% (20%) | |
| CES-D score | 13.2 (10.6) | 16.2 (10.2)b | 14.8 (10.3) |
| Unique page views | 20 (11.9) | 19.5 (14.4) | 19.7 (12.9) |
| 55.6% (33%) | 54.2% (39.9%) | 54.8% (35.9%) | |
| Exercise days | 32.3 (21.8) | n/a | n/a |
| 35.1% (23.7%) |
Note: EX = Internet + Exercise Group; CON = Internet-only Control Group; FTND = Fagerstrom Test of Nicotine Dependence; LTEQ = Leisure Time Exercise Questionnaire; CES-D = Center for Epidemiological Studies-Depression Scale.
Results are reported as mean (standard deviation) unless otherwise labeled.
Denotes significant change (p < .05) from baseline to post-intervention, via paired t-test.
3.4. Smoking cessation/reduction
The adjusted intent-to-treat binary logistic regression analysis revealed that the EX and CON groups’ post-intervention point prevalence smoking cessation rates were identical at 15.8% each, β = −.07, S.E. = .90, p = .93. Although retained participants (n = 23) achieved a higher cessation rate (26.1%) than all enrolled participants (15.8%), an adjusted per-protocol binary logistic regression analysis revealed no significant cessation rate difference between the EX (27.3%) and CON (25%) groups, β = −.50, S.E. = 1.02, p = .62.
Paired t-tests examining individual changes in daily cigarettes smoked from baseline to post-intervention resulted in statistically significant changes among all sub-groups of participants examined, including: all enrolled participants (t = 4.6, p < .001), all retained participants (t = 5.7, p < .001), all enrolled CON group participants (t = 3.5, p = .002), all retained CON group participants (t = 4.5, p = .001), all enrolled EX group participants (t = 2.9, p = .011), and all retained EX group participants (t = 3.4, p = .007). Mean numbers of cigarettes at baseline and post-intervention associated with these analyses are presented in Table 5.
Table 5.
Mean self-reported daily smoking rates among participant sub-groups.
| Participant sub-group |
Exercise | Control | All participants | |
|---|---|---|---|---|
| Baseline | All enrolled | 16.8 [8.6] | 15 [5.5] | 15.9 [7.2] |
| Post-intervention | 13 [10.8] | 9.7 [8.5] | 11.4 [9.7] | |
| Percent changea | 26.7% [40.0] | 38% [41.3] | 32.4% [40.4] | |
| Baseline | Retained | 19.5 [10.3] | 14.8 [6.1] | 17.1 [8.5] |
| Post-intervention | only | 13 [14.2] | 6.5 [8.6] | 9.6 [11.8] |
| Percent changea | 46.2% [43.0] | 60.2% [36.5] | 53.5% [39.5] |
Changes from baseline to post-intervention were statistically significant (all p’s < .01) among all sub-groups reported, via paired t-tests.
Results from the ANOVA, adjusted for quit status and baseline daily smoking rate, revealed no significant between group difference on percent reduction in daily smoking rate from baseline to post-intervention, F(1, 34) = 1.32, p = .26, partial η2 = .037.
3.5. Exercise
Linear regression results, adjusted for baseline daily smoking rate, indicated that additional days of self-reported exercise on the study website during the intervention phase predicted significantly higher reduction rates at the post-intervention assessment among EX group participants, F(2, 16) = 31.08, p < .001, adjusted R2 = .77. Specifically, each additional day of at least one bout of exercise recorded on the study website was associated with a 1.6% greater reduction rate from baseline to post-intervention.
3.6. Self-efficacy
Paired t-tests examining changes in self-efficacy from baseline to post-intervention revealed significant increases in smoking cessation self-efficacy scores among all participants combined (t = −3.2, p = .005); in separate group analyses, the increase among CON (t = −2.4, p = .036) but not EX (t = −2.03, p = 0.77) reached statistical significance. Exercise self-efficacy scores decreased non-significantly among both EX and CON participants, and neither group’s change reached statistical significance when analyzed separately (all p’s > .05). Mean scores and percentages of total possible self-efficacy scores at post-intervention are listed in Table 4.
4. Discussion
This randomized controlled trial examined the efficacy of an Internet-based smoking cessation program alone (CON) vs. the same Internet-based program + exercise (EX) among generally healthy, sedentary adult smokers who desire to quit. The smoking cessation program, guided by SCT constructs, was delivered primarily over the Internet, via email and a study website that included extensive self-monitoring as well as daily lessons/handouts with smoking cessation related information.
4.1. Feasibility
The overall retention rate of 60.5% was slightly lower than the expected 65% but not entirely surprising considering the difficulties associated with behavior change in general, let alone the specific health behavior changes addressed in this study. The Internet-based intervention may have made dropping out especially convenient because of the sense of anonymity associated with the Internet (Hutton et al., 2011). Tracking data suggested that website usage was comparable to that reported in other pilot, Internet-based smoking cessation studies (Lenert et al., 2003). Furthermore, participant burden for EX participants, who were asked not only to exercise but also to record their exercise in detail every day on the study website, may have been excessive. This high level of burden may have counteracted the otherwise engaging exercise-enhanced intervention. Future iterations of this study should attempt to reduce this burden by streamlining the exercise records. Efforts should also be made to balance intervention-related time requirements between groups to control for the potential confounding effects of contact time. Nevertheless, participant satisfaction, assessed via a standardized survey at the post-intervention assessment visit, was high. Participants particularly appreciated the daily emails, weekly phone calls, and constant accountability of the program.
4.2. Smoking
Results were mixed with regard to the incremental benefit of exercise in the presence of the apparently valuable smoking cessation program offered to CON and EX participants alike. Although no group differences were found in terms of post-intervention smoking cessation and reduction rates according to randomization, EX group participants who recorded more exercise days on the study website throughout the intervention phase experienced greater reduction rates at the post-intervention assessment. Furthermore, the mean daily smoking rate among all participants decreased significantly from baseline to post-intervention, with even greater reductions apparent among retained compared to all enrolled participants. These results support those reported in the exercise-based smoking cessation intervention literature, which has consistently found significant within participant smoking cessation and reduction rates but a lack of group differences between exercise and control groups (Ussher et al., 2008). However, the results should be considered in light of the fact that this was a pilot study underpowered to detect treatment differences between groups. Moreover, cessation rates were comparable to those reported in other Internet-based smoking cessation studies, which are in turn comparable to those reported in intensive programs that require additional resources and time (Myung et al., 2009).
4.3. Exercise
Based on the number of days of 1+ bouts of exercise reported on the study website, adherence to the exercise prescription appeared poor. However, because the daily exercise records were quite time-consuming, many EX participants may not have completed them every day they exercised. Exercise intervention studies that require participants to complete exercise assessments regardless of whether or not they exercised typically assume that participants actually engage in less exercise than they report (i.e., participants over-report exercise completed). Although the daily exercise records on the study website allowed participants to complete them even if they did not exercise on any given day (i.e., by selecting “0” bouts of exercise for that day), completion of daily exercise records on non-exercise days was not emphasized. Thus, we surmise that participants under-reported rather than over-reported the number of days they exercised on the daily exercise records. Moreover, some EX participants who quit or significantly reduced their smoking rate reported at the post-intervention assessment that they had stopped recording their exercise on the study website because they had developed a regular exercise routine and felt that they no longer needed to self-monitor their exercise. Future studies should utilize objective measures of physical activity (e.g., pedometers or accelerometers) and/or ecological momentary assessment methods of exercise data collection (e.g., smart phones) to increase the validity and reliability of self-reported exercise.
Regardless, the linear regression analysis revealed that participants who reported more exercise days also reported greater reductions in daily smoking rates. The effect was small but statistically significant, and we suspect it may have been substantially larger if adherence to exercise self-monitoring had been better. Although self-reported exercise adherence was quite low, both groups’ LTEQ scores significantly increased over time. Self-directed increases in exercise among CON participants may have diluted the between group analyses, preventing the exercise effect from appearing. Individuals responding to an advertisement about using exercise to aid smoking cessation were obviously interested in attempting this type of intervention, so exercise increases among CON participants is not particularly surprising.
4.4. Self-Efficacy
Collective and group increases in Smoking Cessation Self-Efficacy aligned with smoking cessation results. Although both groups’ scores increased from baseline to post-intervention, only CON group participants’ increases reached statistical significance, in line with that group’s greater daily cigarette reduction rate. The non-significant declines in Exercise Self-Efficacy may seem counterintuitive considering the significant increases in LTEQ scores within both groups. However, decreased self-efficacy may reflect greater awareness of exercise behaviors and more realistic self-assessments of one’s ability to exercise despite less than ideal circumstances. Participants’ smoking cessation self-efficacy assessments were likely much more accurate than their exercise self-efficacy assessments at baseline simply because they had more frequent and recent experiences with smoking cessation attempts than regular exercise behavior at that point. Self-efficacy ratings in both realms were salient and thus more accurate at post-intervention.
4.5. Limitations
Numerous limitations prevented this pilot study from reaching its full potential. Budget constraints, inherent with dissertation research, posed the biggest challenge. A larger budget would have facilitated a more visually appealing, technologically advanced study website with additional features; objective physical activity measures (e.g., accelerometers); and nicotine replacement therapy for participants who desired to use it, among other things. Sample size was also limited in part by the budget; a larger budget would have supported additional methods of recruitment; staff support; and greater participant reimbursements/incentives, which may have improved retention rates and adherence to the protocol. Weekly check-in phone calls were added to the protocol in attempt to increase retention, adherence, and motivation. Unfortunately, the phone calls’ value is unknown because all participants received them; furthermore, they inhibited our ability to draw conclusions about the efficacy of a strictly web-based intervention (i.e., without phone contact). On the other hand, the three in-person assessment visits also took away from the otherwise almost entirely web-based study, so the phone calls’ negative impact on our ability to assess a purely web-based intervention may not have been relevant.
Another limitation of this study was an over-reliance on self-report data. Objective measures, such as breath carbon monoxide (CO) level monitors and/or saliva or urine cotinine levels to verify self-reported smoking status and pedometers or accelerometers to verify self-reported exercise, would have provided criterion validity. Similarly, because the self-report measures used were not comprehensive, some data that may have revealed important moderators and mediators were not collected. For example, nicotine withdrawal and cravings were not assessed with one of the field’s most preferred, established scales (Shiffman, West, & Gilbert, 2004; West & Hajek, 2004; West, Ussher, Evans, & Rashid, 2006).
Finally, because participants were required to have daily access to the Internet and the ability to read, write, and understand English fluently, the study sample was limited to individuals with relatively higher socioeconomic status; therefore, the results may not accurately reflect a large percentage of current smokers, who tend to have lower socioeconomic status. Likewise, other eligibility criteria limit the generalizability of the study’s results to individuals with few medical and psychiatric problems, among other characteristics.
5. Summary
Outcomes from this pilot study were comparable to more resource- and time-consuming smoking cessation programs, suggesting that future iterations of this program may be promising. Results were mixed with regard to the incremental benefit of exercise in the presence of the apparently valuable study website. Although no group differences were found in terms of post-intervention smoking cessation and reduction rates according to random assignment, reduction rates were statistically significant among all enrolled participants and comparatively greater among retained participants. Furthermore, EX group participants who recorded exercise on the study website more frequently experienced higher reduction rates than those who recorded exercise less frequently. Results from this study support findings from previous research and underscore the need for additional investigation into both the mechanisms behind exercise-based smoking cessation interventions and the challenges of poor exercise adherence.
References
- van Achterberg T, Huisman-de Waal GGJ, Ketelaar NABM, Oostendorp RA, Jacobs JE, Wollersheim HCH. How to promote healthy behaviours in patients? An overview of evidence for behaviour change techniques. Health Promotion International. 2010 doi: 10.1093/heapro/daq050. doi:10.1093/heapro/daq050, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. Understanding exercise dependence. Journal of Contemporary Psychotherapy. 2009;39:231–240. doi:10.1007/s10879-009-9117-5. [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice-Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- Barnes P. Physical activity among adults: United States, 2000 and 2005. US Department of Health and Human Services, CDC; Hyattsville, MD: 2007. [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, et al. The runner’s high: opioidergic mechanisms in the human brain. Cerebral Cortex. 2008;18:2523–2531. doi: 10.1093/cercor/bhn013. doi:10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- Bortz WM, Angwin P, Mefford IN, Border MR, Noyce N, Barchas JD. Catecholamines, dopamine, and endorphin levels during extreme exercise. New England Journal of Medicine. 1981;305:466–467. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Cigarette smoking among adults-United States, 2007. Morbidity and Mortality Weekly Report (MMWR) 2008;57(45):1221–1226. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Department of Health and Human Services. National Center for Chronic Disease Prevention and Health Promotion Division for heart disease and stroke prevention: Addressing the nation’s leading killers, at a glance 2007. 2007 Retrieved from. http://www.cdc.gov/nccdphp/publications/AAG/dhdsp.htm.
- Daley AJ. Exercise and depression: a review of reviews. Journal of Clinical Psychology in Medical Settings. 2008;15:140–147. doi: 10.1007/s10880-008-9105-z. doi:10.1007/s10880-008-9105-z. [DOI] [PubMed] [Google Scholar]
- Daley AJ, Oldham ARH, Townson M. The effects of acute exercise on affective responses and desire to smoke in sedentary temporarily abstaining smokers: a preliminary study. Journal of Sports Sciences. 2004;22:303–304. [Google Scholar]
- Daniel J, Cropley M, Ussher M, West R. Acute effects of a short bout of moderate versus light intensity exercise versus inactivity on tobacco withdrawal symptoms in sedentary smokers. Psychopharmacology. 2004;174:320–326. doi: 10.1007/s00213-003-1762-x. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Buckworth J. Increasing physical activity: a quantitative synthesis. Medicine and Science in Sports and Exercise. 1996;28:706–719. doi: 10.1097/00005768-199606000-00010. [DOI] [PubMed] [Google Scholar]
- Dube S, McClave A, James C, Caraballo R, Kaufmann R, Pechacek T, et al. Vital signs: current cigarette smoking among adults aged ≥18 years e United States, 2009. Morbidity and Mortality Weekly Report (MMWR) 2010;59:1–6. (Early Release) [PubMed] [Google Scholar]
- Febbaro G, Clum G. Meta-analytic investigation of the effectiveness of self-regulatory components in the treatment of adult problem behaviors. Clinical Psychology Review. 1998;18:143–161. doi: 10.1016/s0272-7358(97)00008-1. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Quick Reference Guide for Clinicians. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: 2008. [Google Scholar]
- Glanz K, Rimer BK, Lewis FM, editors. Health behavior and health education: Theory, research, and practice. 3rd ed Jossey-Bass; San Francisco: 2002. [Google Scholar]
- Hausenblas HA, Symons Downs D. Exercise dependence: a systematic review. Psychology of Sport and Exercise. 2002;3(2):89–123. doi:10.1016/s1469-0292(00)00015-7. [Google Scholar]
- Heitkamp H, Schmid K, Scheib K. Beta-endorphin and adrenocorticotropic hormone production during marathon and incremental exercise. European Journal of Applied Physiology and Occupational Physiology. 1993;66:269–274. doi: 10.1007/BF00235105. [DOI] [PubMed] [Google Scholar]
- Hutton HE, Wilson LM, Apelberg BJ, Avila Tang E, Odelola O, Bass EB, et al. A systematic review of randomized controlled trials: web-based interventions for smoking cessation among adolescents, college students, and adults. Nicotine & Tobacco Research. 2011;13:227–238. doi: 10.1093/ntr/ntq252. doi:10.1093/ntr/ntq252. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbenseolfactory tubercle complex. Brain Research Reviews. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir D, Jonsdottir H. Does physical exercise in addition to a multicomponent smoking cessation program increase abstinence rate and suppress weight gain? An intervention study. Scandinavian Journal of Caring Sciences. 2001;15:275–282. doi: 10.1046/j.1471-6712.2001.00039.x. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Blumenthal JA. Health psychology: what will the future bring? Health Psychology. 2004;23(2):156–157. doi: 10.1037/0278-6133.23.2.156. [DOI] [PubMed] [Google Scholar]
- Lenert L, Muñoz RF, Stoddard J, Delucchi K, Bansod A, Skoczen S, et al. Design and pilot evaluation of an Internet smoking cessation program. Journal of the American Medical Informatics Association. 2003;10(1):16–20. doi: 10.1197/jamia.M1128. doi:10.1197/jamia.M1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke S, Gallo L, Norman G. Attrition and adherence rates of sustained vs. intermittent exercise interventions. Annals of Behavioral Medicine. 2011 doi: 10.1007/s12160-011-9279-8. doi:10.1007/s12160-011-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manske S, Miller S, Moyer C, Phaneuf MR, Cameron R. Best practice in group-based smoking cessation: results of a literature review applying effectiveness, plausibility, and practicality criteria. American Journal of Health Promotion. 2004;18:409–423. doi: 10.4278/0890-1171-18.6.409. [DOI] [PubMed] [Google Scholar]
- Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T, Jr., Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behavioural Brain Research. 2010;210:155–163. doi: 10.1016/j.bbr.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MH, Blair SN, Murtagh EM. Accumulated versus continuous exercise for health benefit: a review of empirical studies. Sports Medicine. 2009;39:29–43. doi: 10.2165/00007256-200939010-00003. [DOI] [PubMed] [Google Scholar]
- Myung S-K, McDonnell DD, Kazinets G, Seo HG, Moskowitz JM. Effects of web- and computer-based smoking cessation programs: metaanalysis of randomized controlled trials. Archives of Internal Medicine. 2009;169(10):929–937. doi: 10.1001/archinternmed.2009.109. doi:10.1001/archinternmed.2009.109. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Yoshida H, Takahashi H, Kawai M. Cigarette smoking weakens exercise habits in healthy men. Nicotine & Tobacco Research. 2007;9:1027–1032. doi: 10.1080/14622200701591575. [DOI] [PubMed] [Google Scholar]
- Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: national health interview survey, 2008. Vital Health Statistics. 2009;242:1–157. [PubMed] [Google Scholar]
- Pomerleau OF, Scherzer HH, Grunberg NE, Pomerleau CS, Judge J, Fetig JB. The effects of acute exercise on subsequent cigarette smoking. Journal of Behavior Medicine. 1987;10:117–127. doi: 10.1007/BF00846420. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ, Gilbert DG. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research. 2004;6(4):599–614. doi: 10.1080/14622200410001734067. doi:10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, McClure JB, Alexander GL, Chakraborty B, Nair VN, Konkel JM, et al. Web-based smoking-cessation programs: results of a randomized trial. American Journal of Preventive Medicine. 2008;34:373–381. doi: 10.1016/j.amepre.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102(4):534–543. doi: 10.1111/j.1360-0443.2006.01739.x. doi:10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2008;4:CD002295. doi: 10.1002/14651858.CD002295.pub3. doi:10.1002/14651858.CD002295.pub3. [DOI] [PubMed] [Google Scholar]
- Walters ST, Wright JA, Shegog R. A review of computer and Internetbased interventions for smoking behavior. Addictive Behaviors. 2006;31:264–277. doi: 10.1016/j.addbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology. 2004;177(1):195–199. doi: 10.1007/s00213-004-1923-6. doi:10.1007/s00213-004-1923-6. [DOI] [PubMed] [Google Scholar]
- West R, Ussher M, Evans M, Rashid M. Assessing DSM-IV nicotine withdrawal symptoms: a comparison and evaluation of five different scales. Psychopharmacology. 2006;184(3):619–627. doi: 10.1007/s00213-005-0216-z. doi:10.1007/s00213-005-0216-z. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou F-M, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. The Journal of Neuroscience. 2009;29:4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009. doi:10.1523/jneurosci.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]